Decellularized Extracellular Matrix Scaffolds for Cardiovascular Tissue Engineering: Current Techniques and Challenges
Abstract
1. Introduction
2. Composition and Function of Cardiac dECM
3. Key Materials for Cardiac Tissue Engineering
3.1. Cell Sources
3.2. Naturally Derived Scaffolds
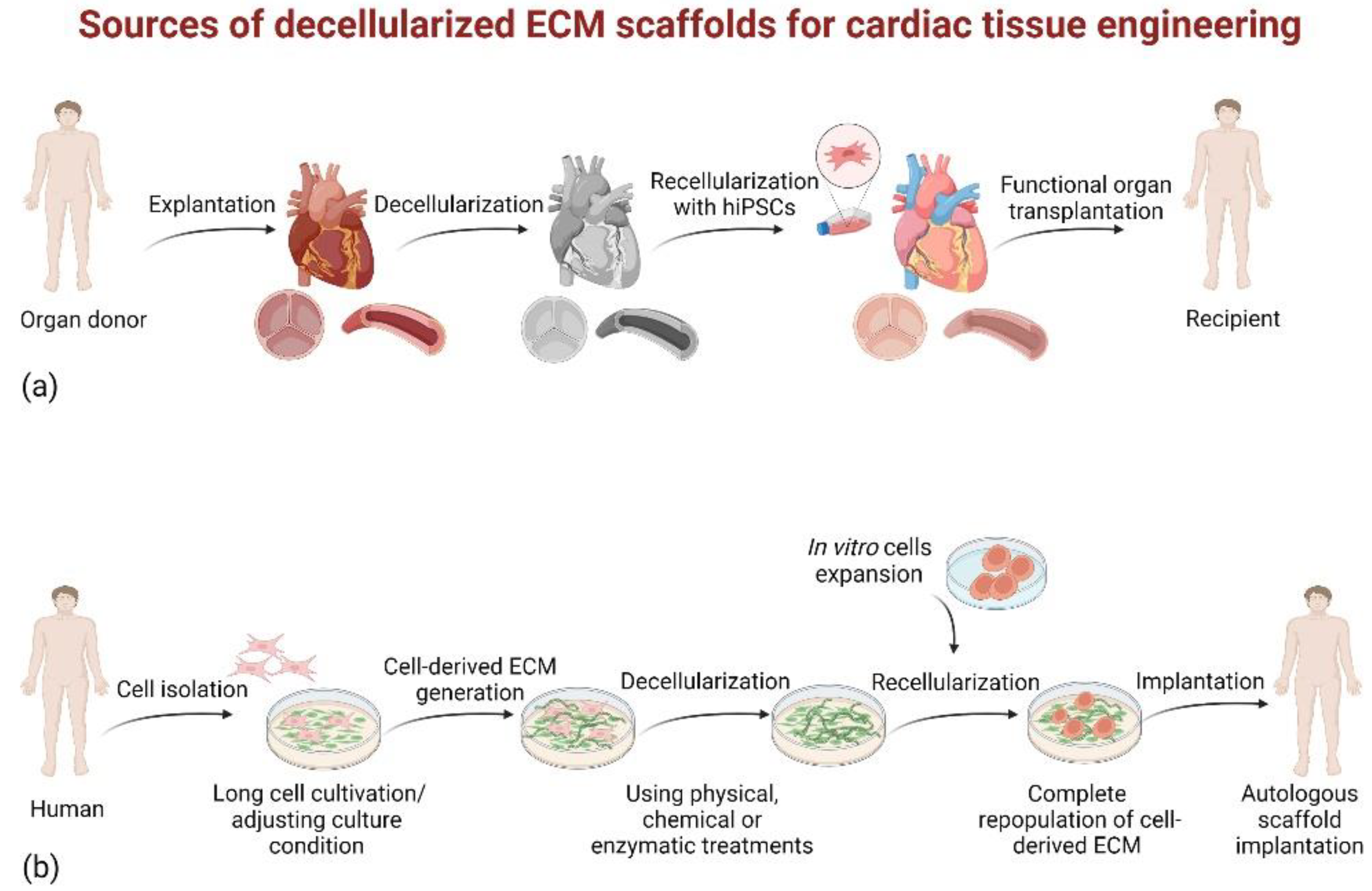
3.2.1. Native Tissue-Derived dECM Scaffolds
3.2.2. Cultured Cell-Derived dECM Scaffolds
3.3. Signals
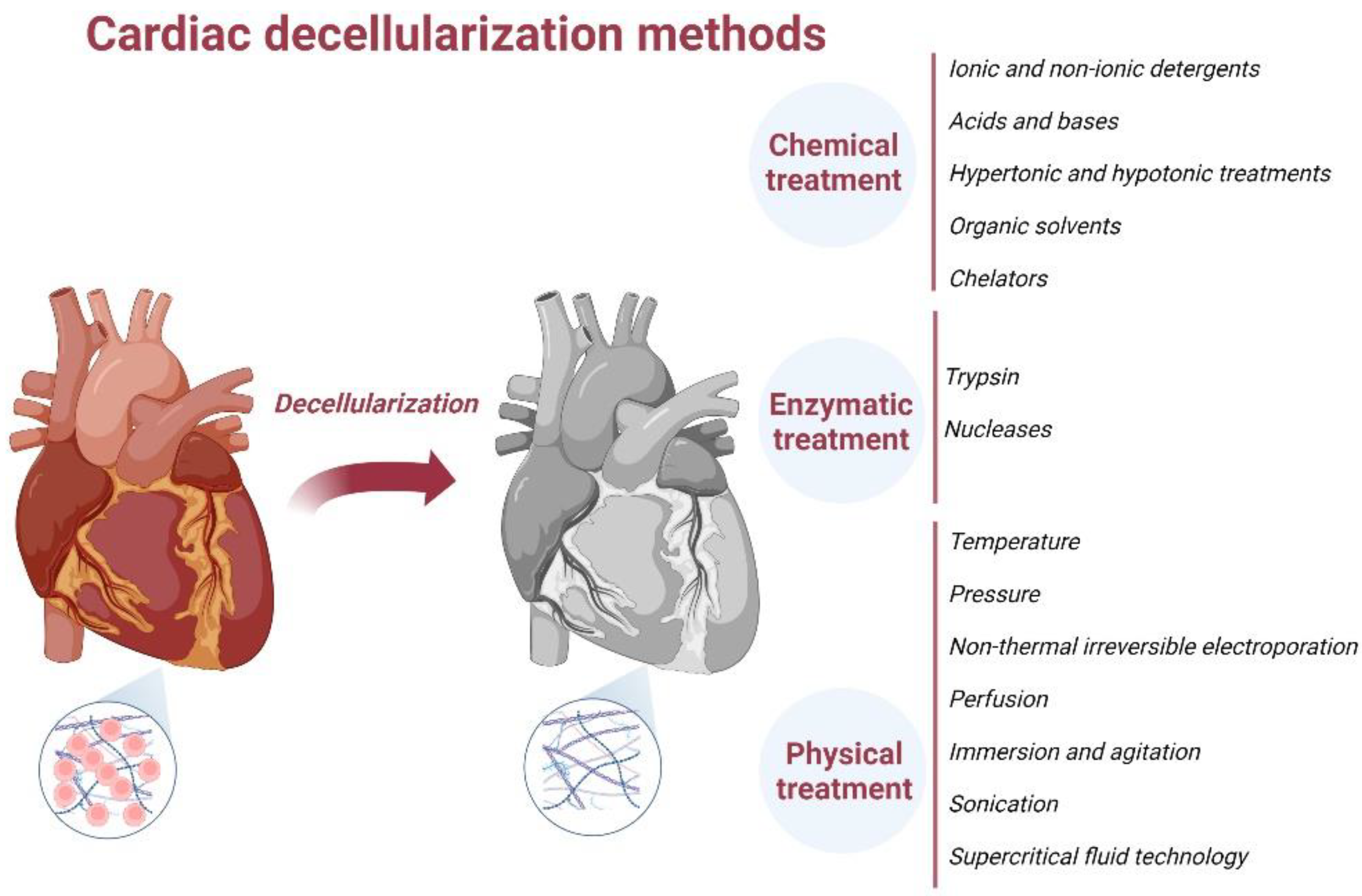
4. ECM Decellularization Methods
4.1. Chemical Treatment-Based Decellularization
4.1.1. Ionic and Non-Ionic Detergents
4.1.2. Acids and Bases
4.1.3. Hypertonic and Hypotonic Treatments
4.1.4. Organic Solvents
4.2. Enzymatic Decellularization
4.2.1. Trypsin
4.2.2. Nucleases
4.3. Physical Decellularization
4.3.1. Temperature
4.3.2. Pressure
4.3.3. Non-Thermal Irreversible Electroporation (NTIRE)
4.3.4. Perfusion
4.3.5. Immersion and Agitation
4.3.6. Sonication
4.3.7. Supercritical Fluid Technology
4.4. Combination of Chemical, Enzymatic and Physical Methods
5. Quantification of Complete Decellularization
6. Pre-Application Processing
7. Application of dECM in Regenerative Medicine
8. Challenges in Cardiac Tissue Engineering
9. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; de Ferranti, S.; Després, J.-P.; Fullerton, H.J.; Howard, V.J.; et al. Heart Disease and Stroke Statistics—2015 Update: A Report from the American Heart Association. Circulation 2015, 131, e29–e233. [Google Scholar] [CrossRef] [PubMed]
- González-Vílchez, F.; Almenar-Bonet, L.; Crespo-Leiro, M.G.; Alonso-Pulpón, L.; González-Costelo, J.; Sobrino-Márquez, J.M.; Arizón del Prado, J.M.; Sousa-Casasnovas, I.; Delgado-Jiménez, J.; Pérez-Villa, F.; et al. Spanish Heart Transplant Registry. 29th Official Report of the Spanish Society of Cardiology Working Group on Heart Failure. Rev. Española Cardiol. 2018, 71, 952–960. [Google Scholar] [CrossRef]
- Tonsho, M.; Michel, S.; Ahmed, Z.; Alessandrini, A.; Madsen, J.C. Heart Transplantation: Challenges Facing the Field. Cold Spring Harb. Perspect. Med. 2014, 4, a015636. [Google Scholar] [CrossRef]
- Söderlund, C.; Rådegran, G. Immunosuppressive Therapies after Heart Transplantation—The Balance between under- and over-Immunosuppression. Transplant. Rev. 2015, 29, 181–189. [Google Scholar] [CrossRef]
- Taylor, D.A.; Sampaio, L.C.; Ferdous, Z.; Gobin, A.S.; Taite, L.J. Decellularized Matrices in Regenerative Medicine. Acta Biomater. 2018, 74, 74–89. [Google Scholar] [CrossRef]
- Singelyn, J.M.; Christman, K.L. Injectable Materials for the Treatment of Myocardial Infarction and Heart Failure: The Promise of Decellularized Matrices. J. Cardiovasc. Trans. Res. 2010, 3, 478–486. [Google Scholar] [CrossRef]
- Sarikouch, S.; Theodoridis, K.; Hilfiker, A.; Boethig, D.; Laufer, G.; Andreas, M.; Cebotari, S.; Tudorache, I.; Bobylev, D.; Neubert, L.; et al. Early Insight Into In Vivo Recellularization of Cell-Free Allogenic Heart Valves. Ann. Thorac. Surg. 2019, 108, 581–589. [Google Scholar] [CrossRef]
- Krishnan, A.; Wang, H.; MacArthur, J.W. Applications of Tissue Decellularization Techniques in Ventricular Myocardial Biofabrication. Front. Bioeng. Biotechnol. 2022, 10, 802283. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, H.; Bai, A.; Jiang, W.; Li, X.; Wang, X.; Mao, Y.; Lu, C.; Qian, R.; Guo, F.; et al. Functional Engineered Human Cardiac Patches Prepared from Nature’s Platform Improve Heart Function after Acute Myocardial Infarction. Biomaterials 2016, 105, 52–65. [Google Scholar] [CrossRef]
- Ott, H.C.; Matthiesen, T.S.; Goh, S.-K.; Black, L.D.; Kren, S.M.; Netoff, T.I.; Taylor, D.A. Perfusion-Decellularized Matrix: Using Nature’s Platform to Engineer a Bioartificial Heart. Nat. Med. 2008, 14, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An Overview of Tissue and Whole Organ Decellularization Processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.; Saleh, T.; Yu, L.; Kwak, H.-H.; Kim, B.-M.; Park, K.-M.; Lee, Y.-S.; Kang, B.-J.; Choi, K.-Y.; Kang, K.-S.; et al. Micro and Ultrastructural Changes Monitoring during Decellularization for the Generation of a Biocompatible Liver. J. Biosci. Bioeng. 2019, 128, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Tapias, L.F.; Ott, H.C. Decellularized Scaffolds as a Platform for Bioengineered Organs. Curr. Opin. Organ Transplant. 2014, 19, 145–152. [Google Scholar] [CrossRef]
- Wainwright, J.M.; Czajka, C.A.; Patel, U.B.; Freytes, D.O.; Tobita, K.; Gilbert, T.W.; Badylak, S.F. Preparation of Cardiac Extracellular Matrix from an Intact Porcine Heart. Tissue Eng. Part C Methods 2010, 16, 525–532. [Google Scholar] [CrossRef]
- Weymann, A.; Patil, N.P.; Sabashnikov, A.; Jungebluth, P.; Korkmaz, S.; Li, S.; Veres, G.; Soos, P.; Ishtok, R.; Chaimow, N.; et al. Bioartificial Heart: A Human-Sized Porcine Model—The Way Ahead. PLoS ONE 2014, 9, e111591. [Google Scholar] [CrossRef]
- Lee, P.-F.; Chau, E.; Cabello, R.; Yeh, A.T.; Sampaio, L.C.; Gobin, A.S.; Taylor, D.A. Inverted Orientation Improves Decellularization of Whole Porcine Hearts. Acta Biomater. 2017, 49, 181–191. [Google Scholar] [CrossRef]
- Hodgson, M.J.; Knutson, C.C.; Momtahan, N.; Cook, A.D. Extracellular Matrix from Whole Porcine Heart Decellularization for Cardiac Tissue Engineering. In Decellularized Scaffolds and Organogenesis; Turksen, K., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2017; Volume 1577, pp. 95–102. [Google Scholar] [CrossRef]
- Sánchez, P.L.; Fernández-Santos, M.E.; Costanza, S.; Climent, A.M.; Moscoso, I.; Gonzalez-Nicolas, M.A.; Sanz-Ruiz, R.; Rodríguez, H.; Kren, S.M.; Garrido, G.; et al. Acellular Human Heart Matrix: A Critical Step toward Whole Heart Grafts. Biomaterials 2015, 61, 279–289. [Google Scholar] [CrossRef]
- Hong, X.; Yuan, Y.; Sun, X.; Zhou, M.; Guo, G.; Zhang, Q.; Hescheler, J.; Xi, J. Skeletal Extracellular Matrix Supports Cardiac Differentiation of Embryonic Stem Cells: A Potential Scaffold for Engineered Cardiac Tissue. Cell Physiol. Biochem. 2018, 45, 319–331. [Google Scholar] [CrossRef]
- Oberwallner, B.; Brodarac, A.; Choi, Y.-H.; Saric, T.; Anić, P.; Morawietz, L.; Stamm, C. Preparation of Cardiac Extracellular Matrix Scaffolds by Decellularization of Human Myocardium. J. Biomed. Mater. Res. 2014, 102, 3263–3272. [Google Scholar] [CrossRef]
- Oberwallner, B.; Brodarac, A.; Anić, P.; Šarić, T.; Wassilew, K.; Neef, K.; Choi, Y.-H.; Stamm, C. Human Cardiac Extracellular Matrix Supports Myocardial Lineage Commitment of Pluripotent Stem Cells. Eur. J. Cardio-Thorac. Surg. 2015, 47, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular Matrix Structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef] [PubMed]
- Brafman, D.A.; Phung, C.; Kumar, N.; Willert, K. Regulation of Endodermal Differentiation of Human Embryonic Stem Cells through Integrin-ECM Interactions. Cell Death Differ. 2013, 20, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Valiente-Alandi, I.; Schafer, A.E.; Blaxall, B.C. Extracellular Matrix-Mediated Cellular Communication in the Heart. J. Mol. Cell. Cardiol. 2016, 91, 228–237. [Google Scholar] [CrossRef]
- Chang, C.W.; Dalgliesh, A.J.; López, J.E.; Griffiths, L.G. Cardiac Extracellular Matrix Proteomics: Challenges, Techniques, and Clinical Implications. Prot. Clin. Appl. 2016, 10, 39–50. [Google Scholar] [CrossRef]
- Linde-Medina, M.; Marcucio, R. Living Tissues Are More than Cell Clusters: The Extracellular Matrix as a Driving Force in Morphogenesis. Prog. Biophys. Mol. Biol. 2018, 137, 46–51. [Google Scholar] [CrossRef]
- Hoshiba, T.; Chen, G.; Endo, C.; Maruyama, H.; Wakui, M.; Nemoto, E.; Kawazoe, N.; Tanaka, M. Decellularized Extracellular Matrix as an In Vitro Model to Study the Comprehensive Roles of the ECM in Stem Cell Differentiation. Stem. Cells Int. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Navarro-Tableros, V.; Herrera Sanchez, M.B.; Figliolini, F.; Romagnoli, R.; Tetta, C.; Camussi, G. Recellularization of Rat Liver Scaffolds by Human Liver Stem Cells. Tissue Eng. Part A 2015, 21, 1929–1939. [Google Scholar] [CrossRef]
- Silva, A.C.; Pereira, C.; Fonseca, A.C.R.G.; Pinto-do-Ó, P.; Nascimento, D.S. Bearing My Heart: The Role of Extracellular Matrix on Cardiac Development, Homeostasis, and Injury Response. Front. Cell Dev. Biol. 2021, 8, 621644. [Google Scholar] [CrossRef]
- Kato, B.; Wisser, G.; Agrawal, D.K.; Wood, T.; Thankam, F.G. 3D Bioprinting of Cardiac Tissue: Current Challenges and Perspectives. J. Mater. Sci. Mater. Med. 2021, 32, 54. [Google Scholar] [CrossRef]
- Guyette, J.P.; Charest, J.M.; Mills, R.W.; Jank, B.J.; Moser, P.T.; Gilpin, S.E.; Gershlak, J.R.; Okamoto, T.; Gonzalez, G.; Milan, D.J.; et al. Bioengineering Human Myocardium on Native Extracellular Matrix. Circ. Res. 2016, 118, 56–72. [Google Scholar] [CrossRef] [PubMed]
- Schwan, J.; Kwaczala, A.T.; Ryan, T.J.; Bartulos, O.; Ren, Y.; Sewanan, L.R.; Morris, A.H.; Jacoby, D.L.; Qyang, Y.; Campbell, S.G. Anisotropic Engineered Heart Tissue Made from Laser-Cut Decellularized Myocardium. Sci. Rep. 2016, 6, 32068. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Naranjo, J.D.; Londono, R.; Badylak, S.F. Biologic Scaffolds. Cold Spring Harb. Perspect. Med. 2017, 7, a025676. [Google Scholar] [CrossRef] [PubMed]
- Svystonyuk, D.A.; Mewhort, H.E.M.; Hassanabad, A.F.; Heydari, B.; Mikami, Y.; Turnbull, J.D.; Teng, G.; Belke, D.D.; Wagner, K.T.; Tarraf, S.A.; et al. Acellular Bioscaffolds Redirect Cardiac Fibroblasts and Promote Functional Tissue Repair in Rodents and Humans with Myocardial Injury. Sci. Rep. 2020, 10, 9459. [Google Scholar] [CrossRef]
- Di Meglio, F.; Nurzynska, D.; Romano, V.; Miraglia, R.; Belviso, I.; Sacco, A.M.; Barbato, V.; Di Gennaro, M.; Granato, G.; Maiello, C.; et al. Optimization of Human Myocardium Decellularization Method for the Construction of Implantable Patches. Tissue Eng. Part C Methods 2017, 23, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Liguori, G.R.; Liguori, T.T.A.; de Moraes, S.R.; Sinkunas, V.; Terlizzi, V.; van Dongen, J.A.; Sharma, P.K.; Moreira, L.F.P.; Harmsen, M.C. Molecular and Biomechanical Clues From Cardiac Tissue Decellularized Extracellular Matrix Drive Stromal Cell Plasticity. Front. Bioeng. Biotechnol. 2020, 8, 520. [Google Scholar] [CrossRef]
- Johnson, T.D.; Hill, R.C.; Dzieciatkowska, M.; Nigam, V.; Behfar, A.; Christman, K.L.; Hansen, K.C. Quantification of Decellularized Human Myocardial Matrix: A Comparison of Six Patients. Prot. Clin. Appl. 2016, 10, 75–83. [Google Scholar] [CrossRef]
- Higuchi, S.; Lin, Q.; Wang, J.; Lim, T.K.; Joshi, S.B.; Anand, G.S.; Chung, M.C.M.; Sheetz, M.P.; Fujita, H. Heart Extracellular Matrix Supports Cardiomyocyte Differentiation of Mouse Embryonic Stem Cells. J. Biosci. Bioeng. 2013, 115, 320–325. [Google Scholar] [CrossRef]
- Londono, R.; Badylak, S.F. Biologic Scaffolds for Regenerative Medicine: Mechanisms of In Vivo Remodeling. Ann. Biomed. Eng. 2015, 43, 577–592. [Google Scholar] [CrossRef]
- Kc, P.; Hong, Y.; Zhang, G. Cardiac Tissue-Derived Extracellular Matrix Scaffolds for Myocardial Repair: Advantages and Challenges. Regen. Biomater. 2019, 6, 185–199. [Google Scholar] [CrossRef]
- García-Gareta, E.; Abduldaiem, Y.; Sawadkar, P.; Kyriakidis, C.; Lali, F.; Greco, K.V. Decellularised Scaffolds: Just a Framework? Current Knowledge and Future Directions. J. Tissue Eng. 2020, 11, 204173142094290. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced Pluripotent Stem Cell Lines Derived from Human Somatic Cells. Science 2007, 318, 1917–1920. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Lee, C.; Skylar-Scott, M.A.; Heilshorn, S.C.; Wu, J.C. Reconstructing the Heart Using IPSCs: Engineering Strategies and Applications. J. Mol. Cell. Cardiol. 2021, 157, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.; Hsiao, C.; Wilson, G.; Zhu, K.; Hazeltine, L.B.; Azarin, S.M.; Raval, K.K.; Zhang, J.; Kamp, T.J.; Palecek, S.P. Robust Cardiomyocyte Differentiation from Human Pluripotent Stem Cells via Temporal Modulation of Canonical Wnt Signaling. Proc. Natl. Acad. Sci. USA 2012, 109, E1848–E1857. [Google Scholar] [CrossRef]
- Ong, W.K.; Sugii, S. Adipose-Derived Stem Cells: Fatty Potentials for Therapy. Int. J. Biochem. Cell Biol. 2013, 45, 1083–1086. [Google Scholar] [CrossRef]
- Lin, C.-H.; Hsia, K.; Tsai, C.-H.; Ma, H.; Lu, J.-H.; Tsay, R.-Y. Decellularized Porcine Coronary Artery with Adipose Stem Cells for Vascular Tissue Engineering. Biomed. Mater. 2019, 14, 045014. [Google Scholar] [CrossRef]
- Filova, E.; Steinerova, M.; Travnickova, M.; Knitlova, J.; Musilkova, J.; Eckhardt, A.; Hadraba, D.; Matejka, R.; Prazak, S.; Stepanovska, J.; et al. Accelerated in Vitro Recellularization of Decellularized Porcine Pericardium for Cardiovascular Grafts. Biomed. Mater. 2021, 16, 025024. [Google Scholar] [CrossRef]
- Díaz-Herráez, P.; Saludas, L.; Pascual-Gil, S.; Simón-Yarza, T.; Abizanda, G.; Prósper, F.; Garbayo, E.; Blanco-Prieto, M.J. Transplantation of Adipose-Derived Stem Cells Combined with Neuregulin-Microparticles Promotes Efficient Cardiac Repair in a Rat Myocardial Infarction Model. J. Control. Release 2017, 249, 23–31. [Google Scholar] [CrossRef]
- Wang, B.; Williams, L.N.; de Jongh Curry, A.L.; Liao, J. Preparation of Acellular Myocardial Scaffolds with Well-Preserved Cardiomyocyte Lacunae, and Method for Applying Mechanical and Electrical Simulation to Tissue Construct. In Cardiac Tissue Engineering; Radisic, M., Black III, L.D., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2014; Volume 1181, pp. 189–202. [Google Scholar] [CrossRef]
- Taylor, D.A.; Sampaio, L.C.; Gobin, A. Building New Hearts: A Review of Trends in Cardiac Tissue Engineering: Building New Hearts. Am. J. Transplant. 2014, 14, 2448–2459. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, X.; Hong, H.; Hu, R.; Liu, J.; Liu, C. Decellularized Extracellular Matrix Scaffolds: Recent Trends and Emerging Strategies in Tissue Engineering. Bioact. Mater. 2022, 10, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.A.; Elgalad, A.; Sampaio, L.C. What Will It Take before a Bioengineered Heart Will Be Implanted in Patients? Curr. Opin. Organ Transplant. 2018, 23, 664–672. [Google Scholar] [CrossRef]
- Liu, C.; Pei, M.; Li, Q.; Zhang, Y. Decellularized Extracellular Matrix Mediates Tissue Construction and Regeneration. Front. Med. 2022, 16, 56–82. [Google Scholar] [CrossRef]
- Taylor, D.A.; Sampaio, L.C.; Cabello, R.; Elgalad, A.; Parikh, R.; Wood, R.P.; Myer, K.A.; Yeh, A.T.; Lee, P.-F. Decellularization of Whole Human Heart Inside a Pressurized Pouch in an Inverted Orientation. JoVE 2018, 141, e58123. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Huang, S.; Ma, L. A Novel Detergent-Based Decellularization Combined with Carbodiimide Crosslinking for Improving Anti-Calcification of Bioprosthetic Heart Valve. Biomed. Mater. 2021, 16, 045022. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.E.; Baier, J.M. Acellular Vascular Tissues: Natural Biomaterials for Tissue Repair and Tissue Engineering. Biomaterials 2000, 21, 2215–2231. [Google Scholar] [CrossRef]
- Shah, M.; Kc, P.; Copeland, K.M.; Liao, J.; Zhang, G. A Thin Layer of Decellularized Porcine Myocardium for Cell Delivery. Sci. Rep. 2018, 8, 16206. [Google Scholar] [CrossRef]
- Längin, M.; Mayr, T.; Reichart, B.; Michel, S.; Buchholz, S.; Guethoff, S.; Dashkevich, A.; Baehr, A.; Egerer, S.; Bauer, A.; et al. Consistent Success in Life-Supporting Porcine Cardiac Xenotransplantation. Nature 2018, 564, 430–433. [Google Scholar] [CrossRef]
- Denner, J. Porcine Endogenous Retroviruses and Xenotransplantation, 2021. Viruses 2021, 13, 2156. [Google Scholar] [CrossRef]
- Sharma, D.; Ferguson, M.; Zhao, F. A Step-by-Step Protocol for Generating Human Fibroblast Cell-Derived Completely Biological Extracellular Matrix Scaffolds. In Methods in Cell Biology; Elsevier: Amsterdam, The Netherlands, 2020; Volume 156, pp. 3–13. [Google Scholar] [CrossRef]
- Maia, F.R.; Reis, R.L.; Oliveira, J.M. Decellularized HASCs-Derived Matrices as Biomaterials for 3D in Vitro Approaches. In Methods in Cell Biology; Elsevier: Amsterdam, The Netherlands, 2020; Volume 156, pp. 45–58. [Google Scholar] [CrossRef]
- Weber, B.; Dijkman, P.E.; Scherman, J.; Sanders, B.; Emmert, M.Y.; Grünenfelder, J.; Verbeek, R.; Bracher, M.; Black, M.; Franz, T.; et al. Off-the-Shelf Human Decellularized Tissue-Engineered Heart Valves in a Non-Human Primate Model. Biomaterials 2013, 34, 7269–7280. [Google Scholar] [CrossRef]
- Schmuck, E.G.; Mulligan, J.D.; Ertel, R.L.; Kouris, N.A.; Ogle, B.M.; Raval, A.N.; Saupe, K.W. Cardiac Fibroblast-Derived 3D Extracellular Matrix Seeded with Mesenchymal Stem Cells as a Novel Device to Transfer Cells to the Ischemic Myocardium. Cardiovasc. Eng. Tech. 2014, 5, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Aubin, H.; Rath, L.; Vey, A.; Schmidt, V.; Barth, M.; Weber, E.; Lichtenberg, A.; Akhyari, P. Ventricular Stabilization with a Customized Decellularized Cardiac ECM-Based Scaffold after Myocardial Infarction Alters Gene Expression in a Rodent LAD-Ligation Model. Front. Bioeng. Biotechnol. 2022, 10, 896269. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, S.; Pahlavan, S.; Ashtiani, M.K.; Ansari, H.; Abbasalizadeh, S.; Sayahpour, F.A.; Varzideh, F.; Kostin, S.; Aghdami, N.; Braun, T.; et al. Human Embryonic Stem Cell-Derived Cardiovascular Progenitor Cells Efficiently Colonize in BFGF-Tethered Natural Matrix to Construct Contracting Humanized Rat Hearts. Biomaterials 2018, 154, 99–112. [Google Scholar] [CrossRef]
- Marinval, N.; Morenc, M.; Labour, M.N.; Samotus, A.; Mzyk, A.; Ollivier, V.; Maire, M.; Jesse, K.; Bassand, K.; Niemiec-Cyganek, A.; et al. Fucoidan/VEGF-Based Surface Modification of Decellularized Pulmonary Heart Valve Improves the Antithrombotic and Re-Endothelialization Potential of Bioprostheses. Biomaterials 2018, 172, 14–29. [Google Scholar] [CrossRef]
- Paez-Mayorga, J.; Hernández-Vargas, G.; Ruiz-Esparza, G.U.; Iqbal, H.M.N.; Wang, X.; Zhang, Y.S.; Parra-Saldivar, R.; Khademhosseini, A. Bioreactors for Cardiac Tissue Engineering. Adv. Healthc. Mater. 2019, 8, 1701504. [Google Scholar] [CrossRef] [PubMed]
- Carrier, R.L.; Rupnick, M.; Langer, R.; Schoen, F.J.; Freed, L.E.; Vunjak-Novakovic, G. Effects of Oxygen on Engineered Cardiac Muscle. Biotechnol. Bioeng. 2002, 78, 617–625. [Google Scholar] [CrossRef]
- Liaw, N.Y.; Zimmermann, W.-H. Mechanical Stimulation in the Engineering of Heart Muscle. Adv. Drug Deliv. Rev. 2016, 96, 156–160. [Google Scholar] [CrossRef]
- Carlos-Oliveira, M.; Lozano-Juan, F.; Occhetta, P.; Visone, R.; Rasponi, M. Current Strategies of Mechanical Stimulation for Maturation of Cardiac Microtissues. Biophys. Rev. 2021, 13, 717–727. [Google Scholar] [CrossRef]
- Hernández, D.; Millard, R.; Sivakumaran, P.; Wong, R.C.B.; Crombie, D.E.; Hewitt, A.W.; Liang, H.; Hung, S.S.C.; Pébay, A.; Shepherd, R.K.; et al. Electrical Stimulation Promotes Cardiac Differentiation of Human Induced Pluripotent Stem Cells. Stem Cells Int. 2016, 2016, 1–12. [Google Scholar] [CrossRef]
- Barash, Y.; Dvir, T.; Tandeitnik, P.; Ruvinov, E.; Guterman, H.; Cohen, S. Electric Field Stimulation Integrated into Perfusion Bioreactor for Cardiac Tissue Engineering. Tissue Eng. Part C Methods 2010, 16, 1417–1426. [Google Scholar] [CrossRef]
- Wang, B.; Wang, G.; To, F.; Butler, J.R.; Claude, A.; McLaughlin, R.M.; Williams, L.N.; de Jongh Curry, A.L.; Liao, J. Myocardial Scaffold-Based Cardiac Tissue Engineering: Application of Coordinated Mechanical and Electrical Stimulations. Langmuir 2013, 29, 11109–11117. [Google Scholar] [CrossRef] [PubMed]
- Badylak, S.F.; Taylor, D.; Uygun, K. Whole-Organ Tissue Engineering: Decellularization and Recellularization of Three-Dimensional Matrix Scaffolds. Annu. Rev. Biomed. Eng. 2011, 13, 27–53. [Google Scholar] [CrossRef] [PubMed]
- Gilpin, A.; Yang, Y. Decellularization Strategies for Regenerative Medicine: From Processing Techniques to Applications. BioMed. Res. Int. 2017, 2017, 1–13. [Google Scholar] [CrossRef]
- Mendibil, U.; Ruiz-Hernandez, R.; Retegi-Carrion, S.; Garcia-Urquia, N.; Olalde-Graells, B.; Abarrategi, A. Tissue-Specific Decellularization Methods: Rationale and Strategies to Achieve Regenerative Compounds. IJMS 2020, 21, 5447. [Google Scholar] [CrossRef]
- Merna, N.; Robertson, C.; La, A.; George, S.C. Optical Imaging Predicts Mechanical Properties During Decellularization of Cardiac Tissue. Tissue Eng. Part C Methods 2013, 19, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Joyce, E.M.; Sacks, M.S. Effects of Decellularization on the Mechanical and Structural Properties of the Porcine Aortic Valve Leaflet. Biomaterials 2008, 29, 1065–1074. [Google Scholar] [CrossRef]
- Keane, T.J.; Swinehart, I.T.; Badylak, S.F. Methods of Tissue Decellularization Used for Preparation of Biologic Scaffolds and in Vivo Relevance. Methods 2015, 84, 25–34. [Google Scholar] [CrossRef]
- Bodnar, E.; Olsen, E.; Florio, R.; Dobrin, J. Damage of Porcine Aortic Valve Tissue Caused by the Surfactant Sodiumdodecylsulphate. Thorac. Cardiovasc. Surg. 1986, 34, 82–85. [Google Scholar] [CrossRef]
- Rieder, E.; Kasimir, M.-T.; Silberhumer, G.; Seebacher, G.; Wolner, E.; Simon, P.; Weigel, G. Decellularization Protocols of Porcine Heart Valves Differ Importantly in Efficiency of Cell Removal and Susceptibility of the Matrix to Recellularization with Human Vascular Cells. J. Thorac. Cardiovasc. Surg. 2004, 127, 399–405. [Google Scholar] [CrossRef]
- Dal Sasso, E.; Menabò, R.; Agrillo, D.; Arrigoni, G.; Franchin, C.; Giraudo, C.; Filippi, A.; Borile, G.; Ascione, G.; Zanella, F.; et al. RegenHeart: A Time-Effective, Low-Concentration, Detergent-Based Method Aiming for Conservative Decellularization of the Whole Heart Organ. ACS Biomater. Sci. Eng. 2020, 6, 5493–5506. [Google Scholar] [CrossRef]
- Gilbert, T.; Sellaro, T.; Badylak, S. Decellularization of Tissues and Organs. Biomaterials 2006, 27, 3675–3683. [Google Scholar] [CrossRef]
- Yamanaka, H.; Morimoto, N.; Yamaoka, T. Decellularization of Submillimeter-Diameter Vascular Scaffolds Using Peracetic Acid. J. Artif. Organs 2020, 23, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Novelo, B.; Avila, E.E.; Cauich-Rodríguez, J.V.; Jorge-Herrero, E.; Rojo, F.J.; Guinea, G.V.; Mata-Mata, J.L. Decellularization of Pericardial Tissue and Its Impact on Tensile Viscoelasticity and Glycosaminoglycan Content. Acta Biomater. 2011, 7, 1241–1248. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Sakakibara, S.; Terashi, H.; Hashikawa, K.; Yamaoka, T. Development of a Novel Method for Decellularizing a Nerve Graft Using a Hypertonic Sodium Chloride Solution. Int. J. Artif. Organs 2014, 37, 854–860. [Google Scholar] [CrossRef] [PubMed]
- Dahl, S.L.M.; Koh, J.; Prabhakar, V.; Niklason, L.E. Decellularized Native and Engineered Arterial Scaffolds for Transplantation. Cell Transpl. 2003, 12, 659–666. [Google Scholar] [CrossRef]
- Hu, M.; Bi, H.; Moffat, D.; Blystone, M.; DeCostanza, P.; Alayi, T.; Ye, K.; Hathout, Y.; Jin, S. Proteomic and Bioinformatic Analysis of Decellularized Pancreatic Extracellular Matrices. Molecules 2021, 26, 6740. [Google Scholar] [CrossRef]
- Moffat, D.; Ye, K.; Jin, S. Decellularization for the Retention of Tissue Niches. J. Tissue Eng. 2022, 13, 204173142211011. [Google Scholar] [CrossRef]
- Levy, R.J.; Vyavahare, N.; Ogle, M.; Ashworth, P.; Bianco, R.; Schoen, F.J. Inhibition of cusp and aortic wall calcification in ethanol- and aluminum-treated bioprosthetic heart valves in sheep: Background, mechanisms, and synergism. J. Heart Valve Dis. 2003, 12, 209–216. [Google Scholar]
- Kasimir, M.-T.; Rieder, E.; Seebacher, G.; Silberhumer, G.; Wolner, E.; Weigel, G.; Simon, P. Comparison of Different Decellularization Procedures of Porcine Heart Valves. Int. J. Artif. Organs 2003, 26, 421–427. [Google Scholar] [CrossRef]
- Al-Hejailan, R.; Weigel, T.; Schürlein, S.; Berger, C.; Al-Mohanna, F.; Hansmann, J. Decellularization of Full Heart—Optimizing the Classical Sodium-Dodecyl-Sulfate-Based Decellularization Protocol. Bioengineering 2022, 9, 147. [Google Scholar] [CrossRef]
- Yang, M.; Chen, C.-Z.; Wang, X.-N.; Zhu, Y.-B.; Gu, Y.J. Favorable Effects of the Detergent and Enzyme Extraction Method for Preparing Decellularized Bovine Pericardium Scaffold for Tissue Engineered Heart Valves. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 91, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.H.; Helms, H.R.; Nakayama, K.H. Decellularization Strategies for Regenerating Cardiac and Skeletal Muscle Tissues. Front. Bioeng. Biotechnol. 2022, 10, 831300. [Google Scholar] [CrossRef] [PubMed]
- Sajith, S. Comparative Study of Two Decellularization Protocols on a Biomaterial for Tissue Engineering. J. Clin. Exp. Cardiol. 2017, 8, 523. [Google Scholar] [CrossRef]
- Goldfracht, I.; Efraim, Y.; Shinnawi, R.; Kovalev, E.; Huber, I.; Gepstein, A.; Arbel, G.; Shaheen, N.; Tiburcy, M.; Zimmermann, W.H.; et al. Engineered Heart Tissue Models from HiPSC-Derived Cardiomyocytes and Cardiac ECM for Disease Modeling and Drug Testing Applications. Acta Biomater. 2019, 92, 145–159. [Google Scholar] [CrossRef]
- Lu, T.-Y.; Lin, B.; Kim, J.; Sullivan, M.; Tobita, K.; Salama, G.; Yang, L. Repopulation of Decellularized Mouse Heart with Human Induced Pluripotent Stem Cell-Derived Cardiovascular Progenitor Cells. Nat. Commun. 2013, 4, 2307. [Google Scholar] [CrossRef]
- Haupt, J.; Lutter, G.; Gorb, S.N.; Simionescu, D.T.; Frank, D.; Seiler, J.; Paur, A.; Haben, I. Detergent-Based Decellularization Strategy Preserves Macro- and Microstructure of Heart Valves. Interact. CardioVascular Thorac. Surg. 2018, 26, 230–236. [Google Scholar] [CrossRef]
- Wang, Z.; Long, D.W.; Huang, Y.; Chen, W.C.W.; Kim, K.; Wang, Y. Decellularized Neonatal Cardiac Extracellular Matrix Prevents Widespread Ventricular Remodeling in Adult Mammals after Myocardial Infarction. Acta Biomater. 2019, 87, 140–151. [Google Scholar] [CrossRef]
- Ramm, R.; Goecke, T.; Theodoridis, K.; Hoeffler, K.; Sarikouch, S.; Findeisen, K.; Ciubotaru, A.; Cebotari, S.; Tudorache, I.; Haverich, A.; et al. Decellularization Combined with Enzymatic Removal of N-linked Glycans and Residual DNA Reduces Inflammatory Response and Improves Performance of Porcine Xenogeneic Pulmonary Heart Valves in an Ovine in Vivo Model. Xenotransplantation 2020, 27, e12571. [Google Scholar] [CrossRef]
- Lehr, E.J.; Rayat, G.R.; Chiu, B.; Churchill, T.; McGann, L.E.; Coe, J.Y.; Ross, D.B. Decellularization Reduces Immunogenicity of Sheep Pulmonary Artery Vascular Patches. J. Thorac. Cardiovasc. Surg. 2011, 141, 1056–1062. [Google Scholar] [CrossRef]
- Pulver; Shevtsov, A.; Leybovich, B.; Artyuhov, I.; Maleev, Y.; Peregudov, A. Production of Organ Extracellular Matrix Using a Freeze-Thaw Cycle Employing Extracellular Cryoprotectants. Cryo. Lett. 2014, 35, 400–406. [Google Scholar]
- Funamoto, S.; Nam, K.; Kimura, T.; Murakoshi, A.; Hashimoto, Y.; Niwaya, K.; Kitamura, S.; Fujisato, T.; Kishida, A. The Use of High-Hydrostatic Pressure Treatment to Decellularize Blood Vessels. Biomaterials 2010, 31, 3590–3595. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.C. Cell Injury by Electric Forces. Ann. N. Y. Acad. Sci. 2005, 1066, 85–91. [Google Scholar] [CrossRef]
- Golberg, A.; Yarmush, M.L. Nonthermal Irreversible Electroporation: Fundamentals, Applications, and Challenges. IEEE Trans. Biomed. Eng. 2013, 60, 707–714. [Google Scholar] [CrossRef]
- Phillips, M.; Maor, E.; Rubinsky, B. Nonthermal Irreversible Electroporation for Tissue Decellularization. J. Biomech. Eng. 2010, 132, 091003. [Google Scholar] [CrossRef] [PubMed]
- Sano, M.B.; Neal, R.E.; Garcia, P.A.; Gerber, D.; Robertson, J.; Davalos, R.V. Towards the Creation of Decellularized Organ Constructs Using Irreversible Electroporation and Active Mechanical Perfusion. BioMed Eng. Online 2010, 9, 83. [Google Scholar] [CrossRef] [PubMed]
- Zager, Y.; Kain, D.; Landa, N.; Leor, J.; Maor, E. Optimization of Irreversible Electroporation Protocols for In-Vivo Myocardial Decellularization. PLoS ONE 2016, 11, e0165475. [Google Scholar] [CrossRef]
- Guyette, J.P.; Gilpin, S.E.; Charest, J.M.; Tapias, L.F.; Ren, X.; Ott, H.C. Perfusion Decellularization of Whole Organs. Nat. Protoc. 2014, 9, 1451–1468. [Google Scholar] [CrossRef]
- Neishabouri, A.; Soltani Khaboushan, A.; Daghigh, F.; Kajbafzadeh, A.-M.; Majidi Zolbin, M. Decellularization in Tissue Engineering and Regenerative Medicine: Evaluation, Modification, and Application Methods. Front. Bioeng. Biotechnol. 2022, 10, 805299. [Google Scholar] [CrossRef]
- Cebotari, S.; Tudorache, I.; Jaekel, T.; Hilfiker, A.; Dorfman, S.; Ternes, W.; Haverich, A.; Lichtenberg, A. Detergent Decellularization of Heart Valves for Tissue Engineering: Toxicological Effects of Residual Detergents on Human Endothelial Cells. Artif. Organs 2010, 34, 206–210. [Google Scholar] [CrossRef]
- Hülsmann, J.; Aubin, H.; Kranz, A.; Godehardt, E.; Munakata, H.; Kamiya, H.; Barth, M.; Lichtenberg, A.; Akhyari, P. A Novel Customizable Modular Bioreactor System for Whole-Heart Cultivation under Controlled 3D Biomechanical Stimulation. J. Artif. Organs 2013, 16, 294–304. [Google Scholar] [CrossRef]
- Methe, K.; Bäckdahl, H.; Johansson, B.R.; Nayakawde, N.; Dellgren, G.; Sumitran-Holgersson, S. An Alternative Approach to Decellularize Whole Porcine Heart. BioResearch Open Access 2014, 3, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Starnecker, F.; König, F.; Hagl, C.; Thierfelder, N. Tissue-Engineering Acellular Scaffolds-The Significant Influence of Physical and Procedural Decellularization Factors: Tissue-Engineering Acellular Scaffolds. J. Biomed. Mater. Res. 2018, 106, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Syazwani, N.; Azhim, A.; Morimoto, Y.; Furukawa, K.S.; Ushida, T. Decellularization of Aorta Tissue Using Sonication Treatment as Potential Scaffold for Vascular Tissue Engineering. J. Med. Biol. Eng. 2015, 35, 258–269. [Google Scholar] [CrossRef]
- Hazwani, A.; Sha’Ban, M.; Azhim, A. Characterization and in Vivo Study of Decellularized Aortic Scaffolds Using Closed Sonication System. Organogenesis 2019, 15, 120–136. [Google Scholar] [CrossRef]
- Lin, C.-H.; Hsia, K.; Su, C.-K.; Chen, C.-C.; Yeh, C.-C.; Ma, H.; Lu, J.-H. Sonication-Assisted Method for Decellularization of Human Umbilical Artery for Small-Caliber Vascular Tissue Engineering. Polymers 2021, 13, 1699. [Google Scholar] [CrossRef]
- Hennessy, R.S.; Jana, S.; Tefft, B.J.; Helder, M.R.; Young, M.D.; Hennessy, R.R.; Stoyles, N.J.; Lerman, A. Supercritical Carbon Dioxide–Based Sterilization of Decellularized Heart Valves. JACC Basic Transl. Sci. 2017, 2, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Sawada, K.; Terada, D.; Yamaoka, T.; Kitamura, S.; Fujisato, T. Cell Removal with Supercritical Carbon Dioxide for Acellular Artificial Tissue. J. Chem. Technol. Biotechnol. 2008, 83, 943–949. [Google Scholar] [CrossRef]
- Casali, D.M.; Handleton, R.M.; Shazly, T.; Matthews, M.A. A Novel Supercritical CO 2 -Based Decellularization Method for Maintaining Scaffold Hydration and Mechanical Properties. J. Supercrit. Fluids 2018, 131, 72–81. [Google Scholar] [CrossRef]
- Guler, S.; Aslan, B.; Hosseinian, P.; Aydin, H.M. Supercritical Carbon Dioxide-Assisted Decellularization of Aorta and Cornea. Tissue Eng. Part C Methods 2017, 23, 540–547. [Google Scholar] [CrossRef]
- Topuz, B.; Günal, G.; Guler, S.; Aydin, H.M. Use of Supercritical CO2 in Soft Tissue Decellularization. In Methods in Cell Biology; Elsevier: Amsterdam, The Netherlands, 2020; Volume 157, pp. 49–79. [Google Scholar] [CrossRef]
- Gafarova, E.R.; Grebenik, E.A.; Lazhko, A.E.; Frolova, A.A.; Kuryanova, A.S.; Kurkov, A.V.; Bazhanov, I.A.; Kapomba, B.S.; Kosheleva, N.V.; Novikov, I.A.; et al. Evaluation of Supercritical CO2-Assisted Protocols in a Model of Ovine Aortic Root Decellularization. Molecules 2020, 25, 3923. [Google Scholar] [CrossRef]
- Halfwerk, F.R.; Rouwkema, J.; Gossen, J.A.; Grandjean, J.G. Supercritical Carbon Dioxide Decellularized Pericardium: Mechanical and Structural Characterization for Applications in Cardio-Thoracic Surgery. J. Mech. Behav. Biomed. Mater. 2018, 77, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Aubin, H.; Kranz, A.; Hülsmann, J.; Lichtenberg, A.; Akhyari, P. Decellularized Whole Heart for Bioartificial Heart. In Cellular Cardiomyoplasty; Kao, R.L., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2013; Volume 1036, pp. 163–178. [Google Scholar] [CrossRef]
- Zubarevich, A.; Osswald, A.; Amanov, L.; Schmack, B.; Kleinbongard, P.; Ruhparwar, A.; Weymann, A. Development and Evaluation of a Novel Combined Perfusion Decellularization Heart-Lung Model for Tissue Engineering of Bioartificial Heart-Lung Scaffolds. J. Heart Lung Transplant. 2022, 41, S240–S241. [Google Scholar] [CrossRef]
- Remlinger, N.T.; Wearden, P.D.; Gilbert, T.W. Procedure for Decellularization of Porcine Heart by Retrograde Coronary Perfusion. J. Vis. Exp. 2012, e50059. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, P.L.; Fernández-Santos, M.E.; Espinosa, M.A.; González-Nicolas, M.A.; Acebes, J.R.; Costanza, S.; Moscoso, I.; Rodríguez, H.; García, J.; Romero, J.; et al. Data from Acellular Human Heart Matrix. Data Brief. 2016, 8, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Barbulescu, G.I.; Bojin, F.M.; Ordodi, V.L.; Goje, I.D.; Buica, T.P.; Gavriliuc, O.I.; Baderca, F.; Hoinoiu, T.; Paunescu, V. Innovative Biotechnology for Generation of Cardiac Tissue. Appl. Sci. 2021, 11, 5603. [Google Scholar] [CrossRef]
- Akhyari, P.; Aubin, H.; Gwanmesia, P.; Barth, M.; Hoffmann, S.; Huelsmann, J.; Preuss, K.; Lichtenberg, A. The Quest for an Optimized Protocol for Whole-Heart Decellularization: A Comparison of Three Popular and a Novel Decellularization Technique and Their Diverse Effects on Crucial Extracellular Matrix Qualities. Tissue Eng. Part C Methods 2011, 17, 915–926. [Google Scholar] [CrossRef]
- Johnson, T.D.; DeQuach, J.A.; Gaetani, R.; Ungerleider, J.; Elhag, D.; Nigam, V.; Behfar, A.; Christman, K.L. Human versus Porcine Tissue Sourcing for an Injectable Myocardial Matrix Hydrogel. Biomater. Sci. 2014, 2, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Massaro, M.S.; Pálek, R.; Rosendorf, J.; Červenková, L.; Liška, V.; Moulisová, V. Decellularized Xenogeneic Scaffolds in Transplantation and Tissue Engineering: Immunogenicity versus Positive Cell Stimulation. Mater. Sci. Eng. C 2021, 127, 112203. [Google Scholar] [CrossRef]
- Mesquita, F.C.P.; Morrissey, J.; Lee, P.-F.; Monnerat, G.; Xi, Y.; Andersson, H.; Nogueira, F.C.S.; Domont, G.B.; Sampaio, L.C.; Hochman-Mendez, C.; et al. Cues from Human Atrial Extracellular Matrix Enrich the Atrial Differentiation of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Biomater. Sci. 2021, 9, 3737–3749. [Google Scholar] [CrossRef]
- Song, J.J.; Ott, H.C. Organ Engineering Based on Decellularized Matrix Scaffolds. Trends Mol. Med. 2011, 17, 424–432. [Google Scholar] [CrossRef]
- Wang, B.; Tedder, M.E.; Perez, C.E.; Wang, G.; de Jongh Curry, A.L.; To, F.; Elder, S.H.; Williams, L.N.; Simionescu, D.T.; Liao, J. Structural and Biomechanical Characterizations of Porcine Myocardial Extracellular Matrix. J. Mater. Sci. Mater. Med. 2012, 23, 1835–1847. [Google Scholar] [CrossRef] [PubMed]
- Tenreiro, M.F.; Almeida, H.V.; Calmeiro, T.; Fortunato, E.; Ferreira, L.; Alves, P.M.; Serra, M. Interindividual Heterogeneity Affects the Outcome of Human Cardiac Tissue Decellularization. Sci. Rep. 2021, 11, 20834. [Google Scholar] [CrossRef] [PubMed]
- Delgado AL, J.; Carreira AC, O.; de Carvalho HJ, C.; da Palma, R.K.; de Castro Sasahara, T.H.; de Carvalho CM, F.; León, M.; Miglino, M.A. Development of a new decellularization protocol for the whole porcine heart. J. Clin. Transl. Res. 2021, 7, 563–574. [Google Scholar] [CrossRef]
- Hsieh, D.-J.; Srinivasan, P.; Yen, K.-C.; Yeh, Y.-C.; Chen, Y.-J.; Wang, H.-C.; Tarng, Y.-W. Protocols for the Preparation and Characterization of Decellularized Tissue and Organ Scaffolds for Tissue Engineering. BioTechniques 2021, 70, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Keane, T.J.; Londono, R.; Turner, N.J.; Badylak, S.F. Consequences of Ineffective Decellularization of Biologic Scaffolds on the Host Response. Biomaterials 2012, 33, 1771–1781. [Google Scholar] [CrossRef] [PubMed]
- Bruyneel, A.A.N.; Carr, C.A. Ambiguity in the Presentation of Decellularized Tissue Composition: The Need for Standardized Approaches: Thoughts and Progress. Artif. Organs 2017, 41, 778–784. [Google Scholar] [CrossRef]
- Hussein, K.H.; Park, K.-M.; Kang, K.-S.; Woo, H.-M. Biocompatibility Evaluation of Tissue-Engineered Decellularized Scaffolds for Biomedical Application. Mater. Sci. Eng. C 2016, 67, 766–778. [Google Scholar] [CrossRef]
- Ma, B.; Wang, X.; Wu, C.; Chang, J. Crosslinking Strategies for Preparation of Extracellular Matrix-Derived Cardiovascular Scaffolds. Regen. Biomater. 2014, 1, 81–89. [Google Scholar] [CrossRef]
- Yao, Q.; Zheng, Y.-W.; Lan, Q.-H.; Kou, L.; Xu, H.-L.; Zhao, Y.-Z. Recent Development and Biomedical Applications of Decellularized Extracellular Matrix Biomaterials. Mater. Sci. Eng. C 2019, 104, 109942. [Google Scholar] [CrossRef]
- Wang, X.; Zhai, W.; Wu, C.; Ma, B.; Zhang, J.; Zhang, H.; Zhu, Z.; Chang, J. Procyanidins-Crosslinked Aortic Elastin Scaffolds with Distinctive Anti-Calcification and Biological Properties. Acta Biomater. 2015, 16, 81–93. [Google Scholar] [CrossRef]
- Chang, Y.; Tsai, C.-C.; Liang, H.-C.; Sung, H.-W. In Vivo Evaluation of Cellular and Acellular Bovine Pericardia Fixed with a Naturally Occurring Crosslinking Agent (Genipin). Biomaterials 2002, 23, 2447–2457. [Google Scholar] [CrossRef]
- Lü, X.; Zhai, W.; Zhou, Y.; Zhou, Y.; Zhang, H.; Chang, J. Crosslinking Effect of Nordihydroguaiaretic Acid (NDGA) on Decellularized Heart Valve Scaffold for Tissue Engineering. J. Mater. Sci. Mater. Med. 2010, 21, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Tao, M.; Ao, T.; Mao, X.; Yan, X.; Javed, R.; Hou, W.; Wang, Y.; Sun, C.; Lin, S.; Yu, T.; et al. Sterilization and Disinfection Methods for Decellularized Matrix Materials: Review, Consideration and Proposal. Bioact. Mater. 2021, 6, 2927–2945. [Google Scholar] [CrossRef]
- Helder, M.R.K.; Hennessy, R.S.; Spoon, D.B.; Tefft, B.J.; Witt, T.A.; Marler, R.J.; Pislaru, S.V.; Simari, R.D.; Stulak, J.M.; Lerman, A. Low-Dose Gamma Irradiation of Decellularized Heart Valves Results in Tissue Injury In Vitro and In Vivo. Ann. Thorac. Surg. 2016, 101, 667–674. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, S.; Zhou, J.; Wang, J.; Zhen, M.; Liu, Y.; Chen, J.; Qi, Z. The Development of a Tissue-Engineered Artery Using Decellularized Scaffold and Autologous Ovine Mesenchymal Stem Cells. Biomaterials 2010, 31, 296–307. [Google Scholar] [CrossRef]
- Luo, J.; Korossis, S.A.; Wilshaw, S.-P.; Jennings, L.M.; Fisher, J.; Ingham, E. Development and Characterization of Acellular Porcine Pulmonary Valve Scaffolds for Tissue Engineering. Tissue Eng. Part A 2014, 20, 2963–2974. [Google Scholar] [CrossRef] [PubMed]
- Choe, J.A.; Jana, S.; Tefft, B.J.; Hennessy, R.S.; Go, J.; Morse, D.; Lerman, A.; Young, M.D. Biomaterial Characterization of Off-the-Shelf Decellularized Porcine Pericardial Tissue for Use in Prosthetic Valvular Applications. J. Tissue Eng. Regen. Med. 2018, 12, 1608–1620. [Google Scholar] [CrossRef]
- Lichtenberg, A.; Tudorache, I.; Cebotari, S.; Ringes-Lichtenberg, S.; Sturz, G.; Hoeffler, K.; Hurscheler, C.; Brandes, G.; Hilfiker, A.; Haverich, A. In Vitro Re-Endothelialization of Detergent Decellularized Heart Valves under Simulated Physiological Dynamic Conditions. Biomaterials 2006, 27, 4221–4229. [Google Scholar] [CrossRef]
- Amadeo, F.; Boschetti, F.; Polvani, G.; Banfi, C.; Pesce, M.; Santoro, R. Aortic Valve Cell Seeding into Decellularized Animal Pericardium by Perfusion-Assisted Bioreactor. J. Tissue Eng. Regen. Med. 2018, 12, 1481–1493. [Google Scholar] [CrossRef]
- Fidalgo, C.; Iop, L.; Sciro, M.; Harder, M.; Mavrilas, D.; Korossis, S.; Bagno, A.; Palù, G.; Aguiari, P.; Gerosa, G. A Sterilization Method for Decellularized Xenogeneic Cardiovascular Scaffolds. Acta Biomater. 2018, 67, 282–294. [Google Scholar] [CrossRef]
- Zouhair, S.; Aguiari, P.; Iop, L.; Vásquez-Rivera, A.; Filippi, A.; Romanato, F.; Korossis, S.; Wolkers, W.F.; Gerosa, G. Preservation Strategies for Decellularized Pericardial Scaffolds for Off-the-Shelf Availability. Acta Biomater. 2019, 84, 208–221. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.A.; Hochman-Mendez, C.; Huelsmann, J.; Elgalad, A.; Sampaio, L.C. Tissue-Engineered Cardiovascular Products. In Principles of Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1521–1536. [Google Scholar] [CrossRef]
- Assunção, M.; Dehghan-Baniani, D.; Yiu, C.H.K.; Später, T.; Beyer, S.; Blocki, A. Cell-Derived Extracellular Matrix for Tissue Engineering and Regenerative Medicine. Front. Bioeng. Biotechnol. 2020, 8, 602009. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Kc, P.; Zhang, G. In Vivo Assessment of Decellularized Porcine Myocardial Slice as an Acellular Cardiac Patch. ACS Appl. Mater. Interfaces 2019, 11, 23893–23900. [Google Scholar] [CrossRef]
- Sarig, U.; Sarig, H.; de-Berardinis, E.; Chaw, S.-Y.; Nguyen, E.B.V.; Ramanujam, V.S.; Thang, V.D.; Al-Haddawi, M.; Liao, S.; Seliktar, D.; et al. Natural Myocardial ECM Patch Drives Cardiac Progenitor Based Restoration Even after Scarring. Acta Biomater. 2016, 44, 209–220. [Google Scholar] [CrossRef]
- Perea-Gil, I.; Gálvez-Montón, C.; Prat-Vidal, C.; Jorba, I.; Segú-Vergés, C.; Roura, S.; Soler-Botija, C.; Iborra-Egea, O.; Revuelta-López, E.; Fernández; et al. Head-to-Head Comparison of Two Engineered Cardiac Grafts for Myocardial Repair: From Scaffold Characterization to Pre-Clinical Testing. Sci. Rep. 2018, 8, 6708. [Google Scholar] [CrossRef]
- Gálvez-Montón, C.; Bragós, R.; Soler-Botija, C.; Díaz-Güemes, I.; Prat-Vidal, C.; Crisóstomo, V.; Sánchez-Margallo, F.M.; Llucià-Valldeperas, A.; Bogónez-Franco, P.; Perea-Gil, I.; et al. Noninvasive Assessment of an Engineered Bioactive Graft in Myocardial Infarction: Impact on Cardiac Function and Scar Healing. Stem Cells Transl. Med. 2017, 6, 647–655. [Google Scholar] [CrossRef]
- Baraki, H.; Tudorache, I.; Braun, M.; Höffler, K.; Görler, A.; Lichtenberg, A.; Bara, C.; Calistru, A.; Brandes, G.; Hewicker-Trautwein, M. Orthotopic Replacement of the Aortic Valve with Decellularized Allograft in a Sheep Model. Biomaterials 2009, 30, 6240–6246. [Google Scholar] [CrossRef]
- Dohmen, P.M.; Lembcke, A.; Holinski, S.; Kivelitz, D.; Braun, J.P.; Pruss, A.; Konertz, W. Mid-Term Clinical Results Using a Tissue-Engineered Pulmonary Valve to Reconstruct the Right Ventricular Outflow Tract During the Ross Procedure. Ann. Thorac. Surg. 2007, 84, 729–736. [Google Scholar] [CrossRef]
- Brown, J.W.; Elkins, R.C.; Clarke, D.R.; Tweddell, J.S.; Huddleston, C.B.; Doty, J.R.; Fehrenbacher, J.W.; Takkenberg, J.J.M. Performance of the CryoValve SG Human Decellularized Pulmonary Valve in 342 Patients Relative to the Conventional CryoValve at a Mean Follow-up of Four Years. J. Thorac. Cardiovasc. Surg. 2010, 139, 339–348. [Google Scholar] [CrossRef]
- Taylor, D.A.; Frazier, O.H.; Elgalad, A.; Hochman-Mendez, C.; Sampaio, L.C. Building a Total Bioartificial Heart: Harnessing Nature to Overcome the Current Hurdles. Artif. Organs 2018, 42, 970–982. [Google Scholar] [CrossRef]
- Lee, K.M.; Kim, H.; Nemeno, J.G.; Yang, W.; Yoon, J.; Lee, S.; Lee, J.I. Natural Cardiac Extracellular Matrix Sheet as a Biomaterial for Cardiomyocyte Transplantation. Transplant. Proc. 2015, 47, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Garreta, E.; de Oñate, L.; Fernández-Santos, M.E.; Oria, R.; Tarantino, C.; Climent, A.M.; Marco, A.; Samitier, M.; Martínez, E.; Valls-Margarit, M.; et al. Myocardial Commitment from Human Pluripotent Stem Cells: Rapid Production of Human Heart Grafts. Biomaterials 2016, 98, 64–78. [Google Scholar] [CrossRef] [PubMed]
- Porzionato, A.; Stocco, E.; Barbon, S.; Grandi, F.; Macchi, V.; De Caro, R. Tissue-Engineered Grafts from Human Decellularized Extracellular Matrices: A Systematic Review and Future Perspectives. IJMS 2018, 19, 4117. [Google Scholar] [CrossRef] [PubMed]
- Iop, L.; Palmosi, T.; Dal Sasso, E.; Gerosa, G. Bioengineered Tissue Solutions for Repair, Correction and Reconstruction in Cardiovascular Surgery. J. Thorac. Dis. 2018, 10, S2390–S2411. [Google Scholar] [CrossRef] [PubMed]
- Aguiari, P.; Iop, L.; Favaretto, F.; Fidalgo, C.M.L.; Naso, F.; Milan, G.; Vindigni, V.; Spina, M.; Bassetto, F.; Bagno, A.; et al. In Vitro Comparative Assessment of Decellularized Bovine Pericardial Patches and Commercial Bioprosthetic Heart Valves. Biomed. Mater. 2017, 12, 015021. [Google Scholar] [CrossRef]
- Voges, I.; Bräsen, J.H.; Entenmann, A.; Scheid, M.; Scheewe, J.; Fischer, G.; Hart, C.; Andrade, A.; Pham, H.M.; Kramer, H.-H.; et al. Adverse Results of a Decellularized Tissue-Engineered Pulmonary Valve in Humans Assessed with Magnetic Resonance Imaging. Eur. J. Cardiothorac. Surg. 2013, 44, e272–e279. [Google Scholar] [CrossRef]
- Breitenbach, I.; El-Essawi, A.; Pahari, D.; Kappenberg, T.; Harringer, W.; Anssar, M. Early Failure of Decellularized Xenogenous Pulmonary Valve Conduit (Matrix-P-Plus) for Reconstruction of the Right Ventricular Outflow Tract in the Ross Procedure. Thorac. Cardiovasc. Surg. 2014, 62, OP123. [Google Scholar] [CrossRef]
- Taylor, D.A.; Parikh, R.B.; Sampaio, L.C. Bioengineering Hearts: Simple yet Complex. Curr. Stem Cell Rep. 2017, 3, 35–44. [Google Scholar] [CrossRef]
- Taylor, D.A.; Hochman-Mendez, C.; Sampaio, L.C. Are We Close to Bioengineering a Human-Sized, Functional Heart? J. Thorac. Cardiovasc. Surg. 2020, 159, 1357–1360. [Google Scholar] [CrossRef]
- Nicolas, J.; Magli, S.; Rabbachin, L.; Sampaolesi, S.; Nicotra, F.; Russo, L. 3D Extracellular Matrix Mimics: Fundamental Concepts and Role of Materials Chemistry to Influence Stem Cell Fate. Biomacromolecules 2020, 21, 1968–1994. [Google Scholar] [CrossRef]
- Robertson, M.J.; Dries-Devlin, J.L.; Kren, S.M.; Burchfield, J.S.; Taylor, D.A. Optimizing Recellularization of Whole Decellularized Heart Extracellular Matrix. PLoS ONE 2014, 9, e90406. [Google Scholar] [CrossRef] [PubMed]
- Hochman-Mendez, C.; Pereira de Campos, D.B.; Pinto, R.S.; Mendes, B.J.d.S.; Rocha, G.M.; Monnerat, G.; Weissmuller, G.; Sampaio, L.C.; Carvalho, A.B.; Taylor, D.A. Tissue-Engineered Human Embryonic Stem Cell-Containing Cardiac Patches: Evaluating Recellularization of Decellularized Matrix. J. Tissue Eng. 2020, 11, 204173142092148. [Google Scholar] [CrossRef] [PubMed]
- Basara, G.; Ozcebe, S.G.; Ellis, B.W.; Zorlutuna, P. Tunable Human Myocardium Derived Decellularized Extracellular Matrix for 3D Bioprinting and Cardiac Tissue Engineering. Gels 2021, 7, 70. [Google Scholar] [CrossRef] [PubMed]
- Tang-Quan, K.R.; Mehta, N.A.; Sampaio, L.C.; Taylor, D.A. Whole Cardiac Tissue Bioscaffolds. In Cardiac Extracellular Matrix; Schmuck, E.G., Hematti, P., Raval, A.N., Eds.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2018; Volume 1098, pp. 85–114. [Google Scholar] [CrossRef]
- Nguyen, D.T.; O’Hara, M.; Graneli, C.; Hicks, R.; Miliotis, T.; Nyström, A.-C.; Hansson, S.; Davidsson, P.; Gan, L.-M.; Magnone, M.C.; et al. Humanizing Miniature Hearts through 4-Flow Cannulation Perfusion Decellularization and Recellularization. Sci. Rep. 2018, 8, 7458. [Google Scholar] [CrossRef] [PubMed]
- Blazeski, A.; Kostecki, G.M.; Tung, L. Engineered Heart Slices for Electrophysiological and Contractile Studies. Biomaterials 2015, 55, 119–128. [Google Scholar] [CrossRef]
- Kc, P.; Shah, M.; Liao, J.; Zhang, G. Prevascularization of Decellularized Porcine Myocardial Slice for Cardiac Tissue Engineering. ACS Appl. Mater. Interfaces 2017, 9, 2196–2204. [Google Scholar] [CrossRef]
- Mollova, M.; Bersell, K.; Walsh, S.; Savla, J.; Das, L.T.; Park, S.-Y.; Silberstein, L.E.; dos Remedios, C.G.; Graham, D.; Colan, S.; et al. Cardiomyocyte Proliferation Contributes to Heart Growth in Young Humans. Proc. Natl. Acad. Sci. USA 2013, 110, 1446–1451. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Ueno, S.; Weidinger, G.; Osugi, T.; Kohn, A.D.; Golob, J.L.; Pabon, L.; Reinecke, H.; Moon, R.T.; Murry, C.E. Biphasic Role for Wnt/β-Catenin Signaling in Cardiac Specification in Zebrafish and Embryonic Stem Cells. Proc. Natl. Acad. Sci. USA 2007, 104, 9685–9690. [Google Scholar] [CrossRef]
- Wang, H.; Hao, J.; Hong, C.C. Cardiac Induction of Embryonic Stem Cells by a Small Molecule Inhibitor of Wnt/β-Catenin Signaling. ACS Chem. Biol. 2011, 6, 192–197. [Google Scholar] [CrossRef]
- Tsoi, C.; Deng, R.; Kwok, M.; Yan, B.; Lee, C.; Li, H.S.; Ma, C.H.Y.; Luo, R.; Leung, K.T.; Chan, G.C.-F.; et al. Temporal Control of the WNT Signaling Pathway During Cardiac Differentiation Impacts Upon the Maturation State of Human Pluripotent Stem Cell Derived Cardiomyocytes. Front. Mol. Biosci. 2022, 9, 714008. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.W.A.; Garg, P.; Yazji, J.H.; Alomari, M.; Alamouti-fard, E.; Wadiwala, I.; Jacob, S. Is a Bioengineered Heart From Recipient Tissues the Answer to the Shortage of Donors in Heart Transplantation? Cureus 2022, 14, e25329. [Google Scholar] [CrossRef] [PubMed]
- Massai, D.; Pisani, G.; Isu, G.; Rodriguez Ruiz, A.; Cerino, G.; Galluzzi, R.; Pisanu, A.; Tonoli, A.; Bignardi, C.; Audenino, A.L.; et al. Bioreactor Platform for Biomimetic Culture and in Situ Monitoring of the Mechanical Response of in Vitro Engineered Models of Cardiac Tissue. Front. Bioeng. Biotechnol. 2020, 8, 733. [Google Scholar] [CrossRef] [PubMed]
- Hochman-Mendez, C.; Mesquita, F.C.P.; Morrissey, J.; da Costa, E.C.; Hulsmann, J.; Tang-Quan, K.; Xi, Y.; Lee, P.-F.; Sampaio, L.C.; Taylor, D.A. Restoring Anatomical Complexity of a Left Ventricle Wall as a Step toward Bioengineering a Human Heart with Human Induced Pluripotent Stem Cell-Derived Cardiac Cells. Acta Biomater. 2022, 141, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, G.; Leijten, J.; Khademhosseini, A. Concise Review: Organ Engineering: Design, Technology, and Integration. Stem Cells 2017, 35, 51–60. [Google Scholar] [CrossRef]
- Liu, C.; Han, D.; Liang, P.; Li, Y.; Cao, F. The Current Dilemma and Breakthrough of Stem Cell Therapy in Ischemic Heart Disease. Front. Cell Dev. Biol. 2021, 9, 636136. [Google Scholar] [CrossRef]
- Parr, C.J.C.; Katayama, S.; Miki, K.; Kuang, Y.; Yoshida, Y.; Morizane, A.; Takahashi, J.; Yamanaka, S.; Saito, H. MicroRNA-302 Switch to Identify and Eliminate Undifferentiated Human Pluripotent Stem Cells. Sci. Rep. 2016, 6, 32532. [Google Scholar] [CrossRef]
- Okada, M.; Tada, Y.; Seki, T.; Tohyama, S.; Fujita, J.; Suzuki, T.; Shimomura, M.; Ofuji, K.; Kishino, Y.; Nakajima, K.; et al. Selective Elimination of Undifferentiated Human Pluripotent Stem Cells Using Pluripotent State-Specific Immunogenic Antigen Glypican-3. Biochem. Biophys. Res. Commun. 2019, 511, 711–717. [Google Scholar] [CrossRef]
- Kishino, Y.; Fujita, J.; Tohyama, S.; Okada, M.; Tanosaki, S.; Someya, S.; Fukuda, K. Toward the Realization of Cardiac Regenerative Medicine Using Pluripotent Stem Cells. Inflamm. Regen. 2020, 40, 1. [Google Scholar] [CrossRef]
- Yu, J.K.; Liang, J.A.; Franceschi, W.H.; Huang, Q.; Pashakhanloo, F.; Sung, E.; Boyle, P.M.; Trayanova, N.A. Assessment of Arrhythmia Mechanism and Burden of the Infarcted Ventricles Following Remuscularization with Pluripotent Stem Cell-Derived Cardiomyocyte Patches Using Patient-Derived Models. Cardiovasc. Res. 2022, 118, 1247–1261. [Google Scholar] [CrossRef]
- Romagnuolo, R.; Masoudpour, H.; Porta-Sánchez, A.; Qiang, B.; Barry, J.; Laskary, A.; Qi, X.; Massé, S.; Magtibay, K.; Kawajiri, H.; et al. Human Embryonic Stem Cell-Derived Cardiomyocytes Regenerate the Infarcted Pig Heart but Induce Ventricular Tachyarrhythmias. Stem Cell Rep. 2019, 12, 967–981. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Novelo, B.; Castellano, L.E.; Padilla-Miranda, R.G.; Lona-Ramos, M.C.; Cuéllar-Mata, P.; Vega-González, A.; Murguía-Pérez, M.; Mata-Mata, J.L.; Ávila, E.E. The Component Leaching from Decellularized Pericardial Bioscaffolds and Its Implication in the Macrophage Response: Decellularized Pericardial Bioscaffolds. J. Biomed. Mater. Res. 2016, 104, 2810–2822. [Google Scholar] [CrossRef] [PubMed]
- Kitahara, H.; Yagi, H.; Tajima, K.; Okamoto, K.; Yoshitake, A.; Aeba, R.; Kudo, M.; Kashima, I.; Kawaguchi, S.; Hirano, A.; et al. Heterotopic Transplantation of a Decellularized and Recellularized Whole Porcine Heart. Interact. CardioVasc. Thorac. Surg. 2016, 22, 571–579. [Google Scholar] [CrossRef] [PubMed]

| Native Tissue-Derived dECM | Cultured Cell-Derived dECM | |
|---|---|---|
| Advantages |
|
|
| Disadvantages |
|
|
| Chemical Decellularization Techniques | Mechanism | General Disadvantages | Study Findings |
|---|---|---|---|
| Ionic detergents (SDS) |
|
|
|
| Non-ionic detergents (Triton X-100) |
|
|
|
| Acids and bases |
|
|
|
| Hypertonic and hypotonic treatments |
|
|
|
| Organic solvents (ethanol) |
|
|
|
| Enzymatic Decellularization Techniques | Mechanism | General Disadvantages | Study Findings |
|---|---|---|---|
| Trypsin |
|
|
|
| Nucleases |
|
|
|
| Physical Decellularization Techniques | Mechanism | General Disadvantages | Study Findings |
|---|---|---|---|
| Temperature |
|
|
|
| Pressure |
|
|
|
| Non-thermal irreversible electroporation (NTIRE) |
|
|
|
| Perfusion |
|
|
|
| Immersion and agitation |
|
|
|
| Sonication |
|
|
|
| Supercritical fluid technology |
|
|
|
| dECM Source | Formulation | Animal/Human Model | In Vitro Recellularization | In Vivo Implantation | References |
|---|---|---|---|---|---|
| Neonatal mouse heart | Injectable hydrogel | Mouse MI model | Seeding of HUVEC | Injection of decellularized nmECM hydrogel in the injured ventricle | [101] |
| Porcine myocardium slice | Acellular cardiac patch | Rat MI model | - | Implantation of the acellular patch on the infarcted myocardium | [160] |
| Porcine myocardium slice | Acellular cardiac patch | Rat MI model | - | Implantation of the acellular patch on the infarcted myocardium | [161] |
| Porcine SIS-ECM | Human CFs enriched collagen-acellular scaffold | Rat MI model/Patient diagnosed with MI within 4 weeks requiring CABG | Seeding of human CFs | Implantation of the bioscaffold on the infarcted myocardium | [35] |
| Rat CF-ECM | 3D engineered cardiac patch | Mouse MI model | Seeding of hEMSCs | Implantation of the engineered patch to the epicardial surface of the MI area | [65] |
| Rat heart | TEMS | Rat MI model | - | Epicardial implantation of TEMS | [66] |
| Rat heart | 3D engineered cardiac patch | Rat MI model | Seeding of hiPSCs differentiated into the cardiovascular lineage | Implantation of the engineered human cardiac patch on top of the infarcted area | [10] |
| Porcine myocardium/human pericardium | Acellular per/myo scaffold; per/myo-pATMSCs enriched scaffold | Pig MI model | Seeding of pATMSCs | Implantation of the engineered cardiac grafts on top of the infarcted area | [162] |
| Human pericardium | pATPCs enriched acellular human pericardium | Swine MI model | Seeding of pATPCs | Implantation of the repopulated scaffolds on the ischemic myocardium | [163] |
| Ovine aortic valve conduit | Aortic root | Juvenile sheep | - | Orthotopic replacement of the aortic valve with decellularized allograft | [164] |
| Ovine carotid artery | Tissue-engineered vascular conduits | Sheep | Seeding of autologous MSCs differentiated into ECs-like cells and SMCs-like cells | Implantation of tissue-engineered blood vessels into the carotid artery | [151] |
| Porcine coronary artery | Tissue-engineered vascular patch | Rat aorta patch repair model | Seeding of rat ASCs | Implantation of decellularized arterial scaffold patch either with or without ASCs | [48] |
| Ovine pulmonary artery | Allogeneic vascular patch | Ovine artery patch repair | - | Implantation of decellularized arterial scaffold patch into the descending thoracic aorta | [103] |
| Porcine pulmonary heart valve | Xenogeneic valve prosthesis | Ovine model | - | Orthotopic implantation | [102] |
| Human and porcine pulmonary valve | Valve prosthesis | Patient with aortic valve lesions | Seeding of autologous vascular endothelial cells | Ross procedure (the diseased aortic valve is replaced with the pulmonary valve) | [165] |
| Deceased human donor | Allogeneic valve prosthesis | Patient with valve lesions | - | Ross procedure and RVOT reconstruction | [166] |
| Porcine heart | Whole organ | Pig and calf | - | Implantation of decellularized heart in the living recipient in a heterotopic position | [167] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbulescu, G.I.; Bojin, F.M.; Ordodi, V.L.; Goje, I.D.; Barbulescu, A.S.; Paunescu, V. Decellularized Extracellular Matrix Scaffolds for Cardiovascular Tissue Engineering: Current Techniques and Challenges. Int. J. Mol. Sci. 2022, 23, 13040. https://doi.org/10.3390/ijms232113040
Barbulescu GI, Bojin FM, Ordodi VL, Goje ID, Barbulescu AS, Paunescu V. Decellularized Extracellular Matrix Scaffolds for Cardiovascular Tissue Engineering: Current Techniques and Challenges. International Journal of Molecular Sciences. 2022; 23(21):13040. https://doi.org/10.3390/ijms232113040
Chicago/Turabian StyleBarbulescu, Greta Ionela, Florina Maria Bojin, Valentin Laurentiu Ordodi, Iacob Daniel Goje, Andreea Severina Barbulescu, and Virgil Paunescu. 2022. "Decellularized Extracellular Matrix Scaffolds for Cardiovascular Tissue Engineering: Current Techniques and Challenges" International Journal of Molecular Sciences 23, no. 21: 13040. https://doi.org/10.3390/ijms232113040
APA StyleBarbulescu, G. I., Bojin, F. M., Ordodi, V. L., Goje, I. D., Barbulescu, A. S., & Paunescu, V. (2022). Decellularized Extracellular Matrix Scaffolds for Cardiovascular Tissue Engineering: Current Techniques and Challenges. International Journal of Molecular Sciences, 23(21), 13040. https://doi.org/10.3390/ijms232113040







