Linking Nonalcoholic Fatty Liver Disease and Brain Disease: Focusing on Bile Acid Signaling
Abstract
1. Introduction
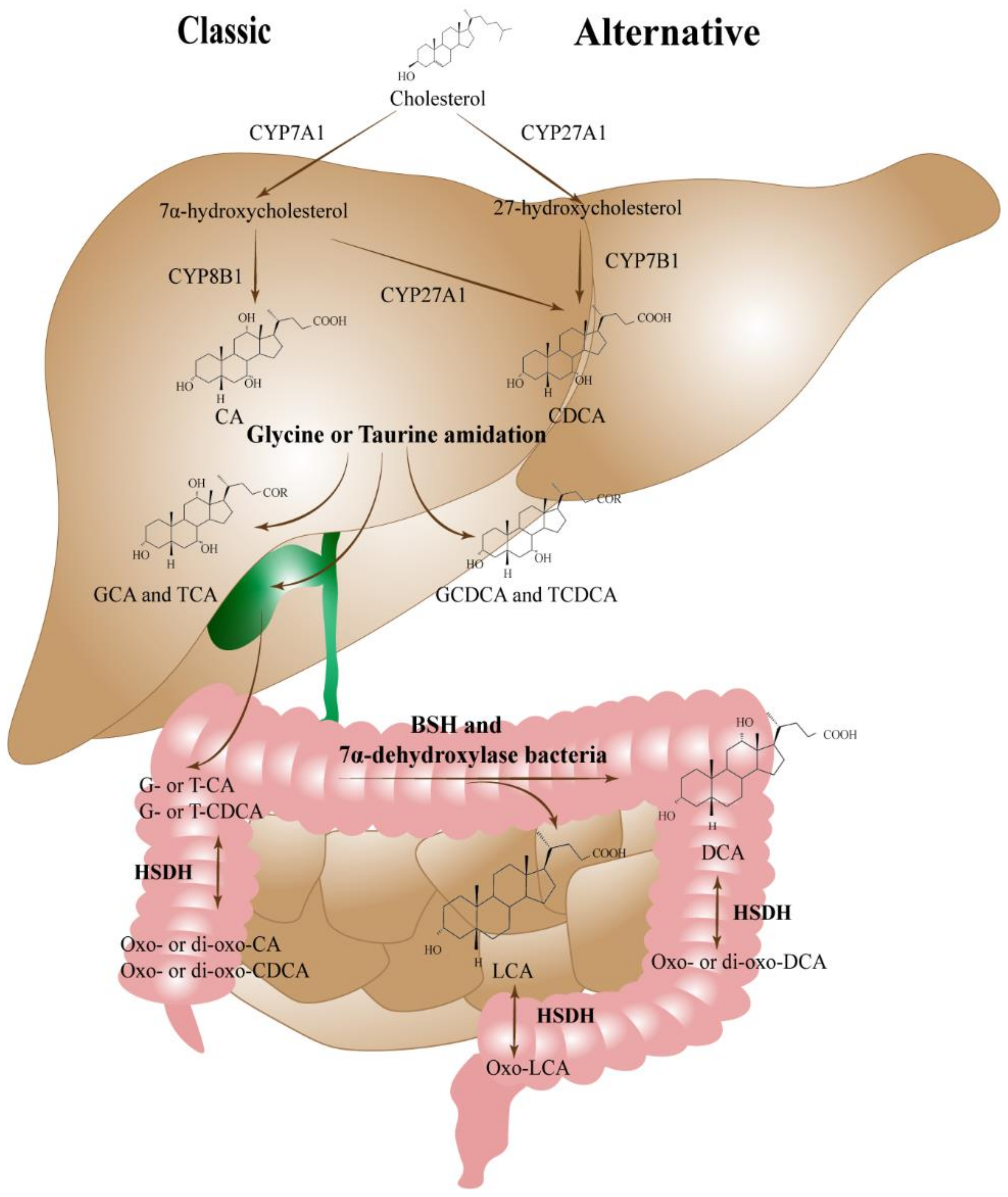
2. Bile Acid of Liver Damage in NAFLD
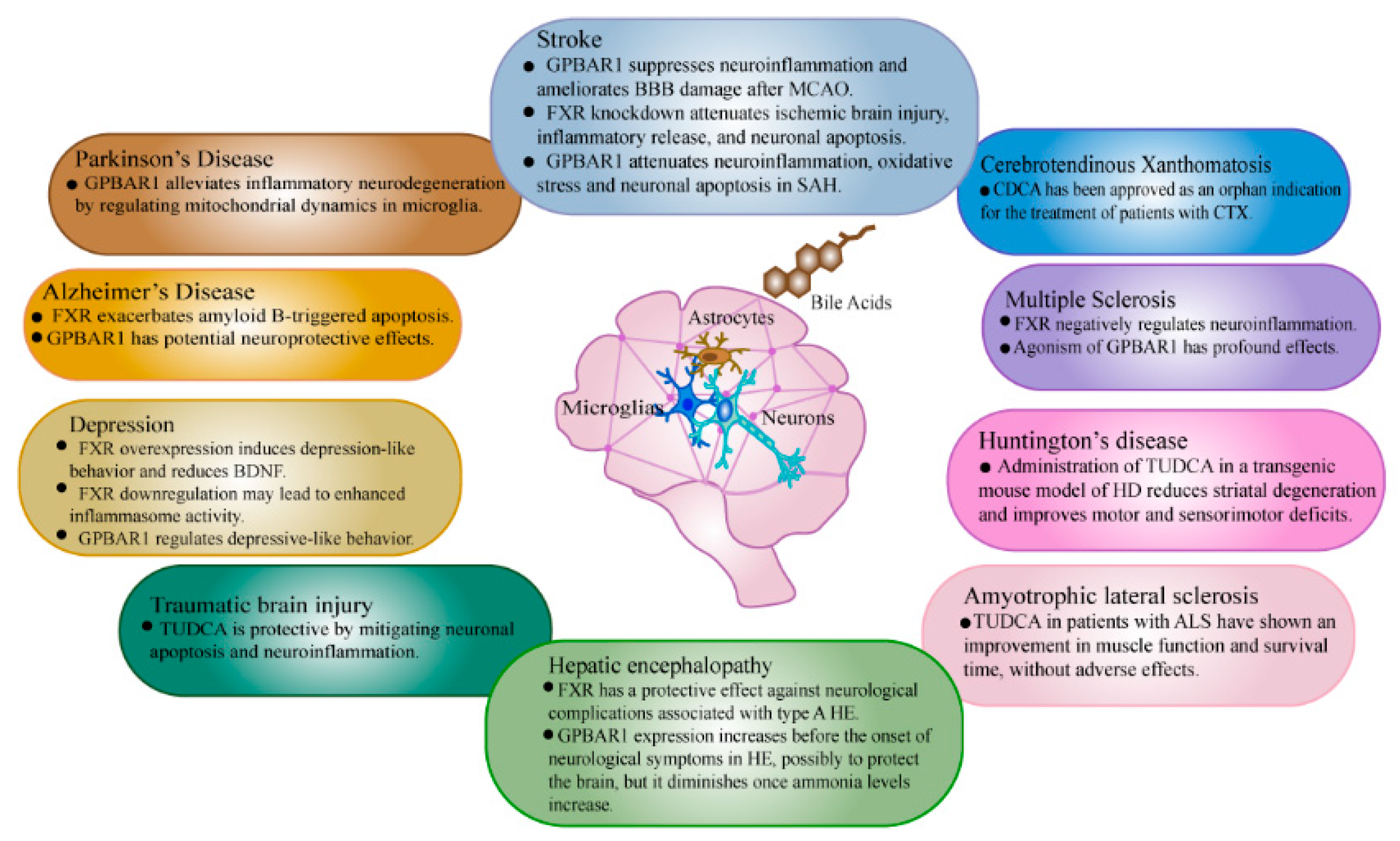
3. Bile Acid Alterations and Their Function in Brain Disease
3.1. Stroke
3.2. Depression
3.3. Alzheimer’s Disease
3.4. Parkinson’s Disease
4. Bile Acid Signaling to the Central Nervous System
4.1. Sending Bile Acid Signaling to the Central Nervous System via a Direct Pathway
| Receptor | Cellular Localization | Bile Acid Ligands | Main Function | References |
|---|---|---|---|---|
| FXR | Prefrontal cortical neurons, hippocampal neurons | CDCA, DCA, LCA, CA | The function of FXR in the brain is still paradoxical. FXR knockdown attenuated neuronal apoptosis in ischemic brain injury. FXR aggravated amyloid-β-triggered apoptosis by modulating the CREB-BDNF pathway in vitro. By causing CRTC2 to translocate into the cytoplasm and disrupt the CREB-BDNF signaling pathway in the hippocampus nucleus, FXR played a role in the pathophysiology of depression. In rats, the hippocampal BDNF expression is decreased and depression-like behavior caused by CUMS is reversed by the hippocampus FXR knockdown. FXR signaling pathway was neuroprotective in mice with depression. In the hippocampi of AlCl3-treated rats, CDCA was successful in enhancing insulin sensitivity. | [101,137,138,139,140,141] |
| GPBAR1 | Neurons, astrocytes, microglia | LCA, DCA, CDCA, CA | In a rodent model of subarachnoid hemorrhage, neuroinflammation, oxidative stress, and apoptosis, as well as BBB damage and neuroinflammation after MCAO, were all reduced by the GPBAR1 activation. The locomotor activity of mice was increased and obesity, depression, and cognitive decline were all protected by the olive leaf extract, which contains a GPBAR1 agonist. Through the hippocampal CA3 pyramidal neurons afferent to the dorsolateral septum, GPBAR1 altered depressive-like behaviors. GPBAR1 activation alleviated inflammatory neurodegeneration in a mouse model of PD by regulating the mitochondrial dynamics in microglia. | [135,136,142,143,144,145,146,147] |
| PXR | Brain endothelial cells, hippocampal neurons | LCA | Nonylphenol had neurotoxic and apoptotic effects on mouse hippocampus cells, and PXR was involved in the spread of those effects. Knockdown expression of PXR in the midbrain of Long–Evans rats lead to impaired mating behavior and reduced hippocampal BDNF levels. | [148,149] |
| VDR | Neurons, glia | LCA | In rat primary astrocytes, the VDR activation controlled the quantities of glutathione and gamma-glutamyl transpeptidase produced. VDR activation suppressed the inducible synthesis of nitric oxide and decreased the generation of pro-inflammatory cytokines by the activated microglia. The pathophysiological process of depressive-like behaviors brought on by persistent stress may involve the 25(OH) D and VDR. By increasing the hippocampus BDNF expression, the VDR signaling helped mice with post-stroke depressive symptoms. In a rat model of traumatic brain damage, the VDR activation altered the NADPH oxidase 2 activity and prevented neurological impairments and apoptosis. Brain endothelial P-glycoprotein levels were reduced in PD via a VDR-dependent pathway. | [150,151,152,153,154,155] |
| S1P2R | Cortical neurons, microglia, astrocytes, hippocampal pyramidal cells, retinal ganglion cells | GCA, GDCA TCA TDCA, TUDCA | S1P2R antagonists improved the neural progenitor cell migration near the brain infarction and reduced the hepatic encephalopathy-related neurological impairment. By weakening the adherens junctions, S1P2R may control BBB permeability. | [156,157,158,159] |
| α5β1 integrin | Cortical neurons, brain endothelial cells | TUDCA, nor UDCA (UDCA homolog) | Activation of integrin α5β1 promoted angiogenesis in brain endothelial cells under cerebral hypoxia, as well as the vascular endothelial growth factor secretion in MCAO rats. α5β1 integrin influenced the BBB permeability following an ischemic stroke. | [160,161,162] |
| GR | Neurons, microglia, cortical neurons | GCDCA TCA, TUDCA, UDCA | Inhibiting NF-κB in a glucocorticoid-dependent way throughout the middle stage of depressive-like behavior, GR was beneficial. Both too little and too much GR-mediated signaling hampered the neuronal migration. In neuron-like cells, GR suppressed the production of the brain-derived neurotrophic factor. GR-signaling in ginseng had an anti-inflammatory protective effect on neurodegenerative models. | [163,164,165,166] |
4.2. Sending Bile Acid Signaling to the Central Nervous System via an Indirect Pathway
4.2.1. FXR Signaling
4.2.2. GPBAR1 Signaling
5. Bile Acid Signaling in Brain Disease
5.1. FXR Signaling
5.2. GPBAR1 Signaling
6. Therapeutic Targeting of the Bile Acid Signaling
6.1. FXR Agonists
6.2. FXR Antagonists
6.3. GPBAR1 Agonists
6.4. Dual GPBAR1/FXR Ligands
7. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global Epidemiology of Nonalcoholic Fatty Liver Disease-Meta-Analytic Assessment of Prevalence, Incidence, and Outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global Burden of NAFLD and NASH: Trends, Predictions, Risk Factors and Prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Anstee, Q.M.; Targher, G.; Day, C.P. Progression of NAFLD to Diabetes Mellitus, Cardiovascular Disease or Cirrhosis. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 330–344. [Google Scholar] [CrossRef]
- Powell, E.E.; Wong, V.W.-S.; Rinella, M. Non-Alcoholic Fatty Liver Disease. Lancet 2021, 397, 2212–2224. [Google Scholar] [CrossRef]
- Bizino, M.B.; Sala, M.L.; de Heer, P.; van der Tol, P.; Smit, J.W.A.; Webb, A.G.; de Roos, A.; Lamb, H.J. MR of Multi-Organ Involvement in the Metabolic Syndrome. Magn. Reson. Imaging Clin. N. Am. 2015, 23, 41–58. [Google Scholar] [CrossRef]
- Mahfood Haddad, T.; Hamdeh, S.; Kanmanthareddy, A.; Alla, V.M. Nonalcoholic Fatty Liver Disease and the Risk of Clinical Cardiovascular Events: A Systematic Review and Meta-Analysis. Diabetes Metab. Syndr. Clin. Res. Rev. 2017, 11, S209–S216. [Google Scholar] [CrossRef] [PubMed]
- Celikbilek, A.; Celikbilek, M.; Bozkurt, G. Cognitive Assessment of Patients with Nonalcoholic Fatty Liver Disease. Eur. J. Gastroenterol. Hepatol. 2018, 30, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Labenz, C.; Huber, Y.; Michel, M.; Nagel, M.; Galle, P.R.; Kostev, K.; Schattenberg, J.M. Nonalcoholic Fatty Liver Disease Increases the Risk of Anxiety and Depression. Hepatol. Commun. 2020, 4, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Fan, J.-G.; Shi, J.-P.; Mao, Y.-M.; Wang, B.-Y.; Zhao, J.-M.; Lu, L.-G.; Zhong, B.-H.; Zou, Z.-S.; Xu, Y.-Q.; et al. Health-Related Quality of Life in Chinese Population with Non-Alcoholic Fatty Liver Disease: A National Multicenter Survey. Health Qual. Life Outcomes 2021, 19, 140. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.Y.L.; Ferrell, J.M. Bile Acids as Metabolic Regulators and Nutrient Sensors. Annu. Rev. Nutr. 2019, 39, 175–200. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.Y.L.; Ferrell, J.M. Bile Acid Receptors FXR and TGR5 Signaling in Fatty Liver Diseases and Therapy. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, G554–G573. [Google Scholar] [CrossRef]
- Keitel, V.; Görg, B.; Bidmon, H.J.; Zemtsova, I.; Spomer, L.; Zilles, K.; Häussinger, D. The Bile Acid Receptor TGR5 (Gpbar-1) Acts as a Neurosteroid Receptor in Brain. Glia 2010, 58, 1794–1805. [Google Scholar] [CrossRef]
- Huang, C.; Wang, J.; Hu, W.; Wang, C.; Lu, X.; Tong, L.; Wu, F.; Zhang, W. Identification of Functional Farnesoid X Receptors in Brain Neurons. FEBS Lett. 2016, 590, 3233–3242. [Google Scholar] [CrossRef]
- McMillin, M.; DeMorrow, S. Effects of Bile Acids on Neurological Function and Disease. FASEB J. 2016, 30, 3658–3668. [Google Scholar] [CrossRef]
- Kiriyama, Y.; Nochi, H. The Biosynthesis, Signaling, and Neurological Functions of Bile Acids. Biomolecules 2019, 9, 232. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, H.D.; Gerhard, G.S. Bile Acids in Neurodegenerative Disorders. Front. Aging Neurosci. 2016, 8, 263. [Google Scholar] [CrossRef]
- Fakheri, R.J.; Javitt, N.B. 27-Hydroxycholesterol, Does It Exist? On the Nomenclature and Stereochemistry of 26-Hydroxylated Sterols. Steroids 2012, 77, 575–577. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.Y.L.; Ferrell, J.M. Bile Acid Metabolism in Liver Pathobiology. Gene Expr. 2018, 18, 71–87. [Google Scholar] [CrossRef]
- Farooqui, N.; Elhence, A.; Shalimar. A Current Understanding of Bile Acids in Chronic Liver Disease. J. Clin. Exp. Hepatol. 2022, 12, 155–173. [Google Scholar] [CrossRef]
- Chow, M.D.; Lee, Y.-H.; Guo, G.L. The Role of Bile Acids in Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Mol. Asp. Med. 2017, 56, 34–44. [Google Scholar] [CrossRef]
- Lepercq, P.; Gérard, P.; Béguet, F.; Raibaud, P.; Grill, J.-P.; Relano, P.; Cayuela, C.; Juste, C. Epimerization of Chenodeoxycholic Acid to Ursodeoxycholic Acid by Clostridium Baratii Isolated from Human Feces. FEMS Microbiol. Lett. 2004, 235, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Nimer, N.; Choucair, I.; Wang, Z.; Nemet, I.; Li, L.; Gukasyan, J.; Weeks, T.L.; Alkhouri, N.; Zein, N.; Tang, W.H.W.; et al. Bile Acids Profile, Histopathological Indices and Genetic Variants for Non-Alcoholic Fatty Liver Disease Progression. Metabolism 2021, 116, 154457. [Google Scholar] [CrossRef] [PubMed]
- Caussy, C.; Hsu, C.; Singh, S.; Bassirian, S.; Kolar, J.; Faulkner, C.; Sinha, N.; Bettencourt, R.; Gara, N.; Valasek, M.A.; et al. Serum Bile Acid Patterns Are Associated with the Presence of NAFLD in Twins, and Dose-Dependent Changes with Increase in Fibrosis Stage in Patients with Biopsy-Proven NAFLD. Aliment. Pharmacol. Ther. 2019, 49, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Ye, Z.; Qin, Y.; Wu, T. Abnormal Metabolic Processes Involved in the Pathogenesis of Non-alcoholic Fatty Liver Disease (Review). Exp. Ther. Med. 2020, 20, 26. [Google Scholar] [CrossRef]
- Ferslew, B.C.; Xie, G.; Johnston, C.K.; Su, M.; Stewart, P.W.; Jia, W.; Brouwer, K.L.R.; Sidney Barritt, A. Altered Bile Acid Metabolome in Patients with Nonalcoholic Steatohepatitis. Dig. Dis. Sci. 2015, 60, 3318–3328. [Google Scholar] [CrossRef]
- Chen, T.; Zhou, K.; Sun, T.; Sang, C.; Jia, W.; Xie, G. Altered Bile Acid Glycine: Taurine Ratio in the Progression of Chronic Liver Disease. J. Gastroenterol. Hepatol. 2022, 37, 208–215. [Google Scholar] [CrossRef]
- Lake, A.D.; Novak, P.; Shipkova, P.; Aranibar, N.; Robertson, D.; Reily, M.D.; Lu, Z.; Lehman-McKeeman, L.D.; Cherrington, N.J. Decreased Hepatotoxic Bile Acid Composition and Altered Synthesis in Progressive Human Nonalcoholic Fatty Liver Disease. Toxicol. Appl. Pharmacol. 2013, 268, 132–140. [Google Scholar] [CrossRef]
- Okushin, K.; Tsutsumi, T.; Enooku, K.; Fujinaga, H.; Kado, A.; Shibahara, J.; Fukayama, M.; Moriya, K.; Yotsuyanagi, H.; Koike, K. The Intrahepatic Expression Levels of Bile Acid Transporters Are Inversely Correlated with the Histological Progression of Nonalcoholic Fatty Liver Disease. J. Gastroenterol. 2016, 51, 808–818. [Google Scholar] [CrossRef]
- Krattinger, R.; Boström, A.; Lee, S.M.L.; Thasler, W.E.; Schiöth, H.B.; Kullak-Ublick, G.A.; Mwinyi, J. Chenodeoxycholic Acid Significantly Impacts the Expression of MiRNAs and Genes Involved in Lipid, Bile Acid and Drug Metabolism in Human Hepatocytes. Life Sci. 2016, 156, 47–56. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Liu, H.-M.; Lee, T.-Y. Ursodeoxycholic Acid Regulates Hepatic Energy Homeostasis and White Adipose Tissue Macrophages Polarization in Leptin-Deficiency Obese Mice. Cells 2019, 8, 253. [Google Scholar] [CrossRef]
- Li, H.; Wang, Q.; Chen, P.; Zhou, C.; Zhang, X.; Chen, L. Ursodeoxycholic Acid Treatment Restores Gut Microbiota and Alleviates Liver Inflammation in Non-Alcoholic Steatohepatitic Mouse Model. Front. Pharmacol. 2021, 12, 788558. [Google Scholar] [CrossRef]
- Zhang, W.; Tang, Y.; Huang, J.; Ren, H.; Yang, Y.; Yang, Q.; Hu, H. Efficacy of Ursodeoxycholic Acid in Nonalcoholic Fatty Liver Disease: An Updated Meta-Analysis of Randomized Controlled Trials. Asia Pac. J. Clin. Nutr. 2019, 29, 696–705. [Google Scholar] [CrossRef]
- Nadinskaia, M.; Maevskaya, M.; Ivashkin, V.; Kodzoeva, K.; Pirogova, I.; Chesnokov, E.; Nersesov, A.; Kaibullayeva, J.; Konysbekova, A.; Raissova, A.; et al. Ursodeoxycholic Acid as a Means of Preventing Atherosclerosis, Steatosis and Liver Fibrosis in Patients with Nonalcoholic Fatty Liver Disease. World J. Gastroenterol. 2021, 27, 959–975. [Google Scholar] [CrossRef]
- George, E.S.; Sood, S.; Daly, R.M.; Tan, S.-Y. Is There an Association between Non-Alcoholic Fatty Liver Disease and Cognitive Function? A Systematic Review. BMC Geriatr. 2022, 22, 47. [Google Scholar] [CrossRef]
- Shea, S.; Lionis, C.; Kite, C.; Atkinson, L.; Chaggar, S.S.; Randeva, H.S.; Kyrou, I. Non-Alcoholic Fatty Liver Disease (NAFLD) and Potential Links to Depression, Anxiety, and Chronic Stress. Biomedicines 2021, 9, 1697. [Google Scholar] [CrossRef]
- Hadjihambi, A. Cerebrovascular Alterations in NAFLD: Is It Increasing Our Risk of Alzheimer’s Disease? Anal. Biochem. 2022, 636, 114387. [Google Scholar] [CrossRef]
- Filipović, B.; Marković, O.; Đurić, V.; Filipović, B. Cognitive Changes and Brain Volume Reduction in Patients with Nonalcoholic Fatty Liver Disease. Can. J. Gastroenterol. Hepatol. 2018, 2018, 69638797. [Google Scholar] [CrossRef]
- Li, W.; Liu, J.; Cai, J.; Zhang, X.; Zhang, P.; She, Z.; Chen, S.; Li, H. NAFLD as a Continuous Driver in the Whole Spectrum of Vascular Disease. J. Mol. Cell. Cardiol. 2022, 163, 118–132. [Google Scholar] [CrossRef]
- Saha, D.; Saha, S.; Sergeeva, E.G.; Ionova, Z.I.; Gorbach, A.V. Tissue Factor and Atherothrombosis. Curr. Pharm. Des. 2015, 21, 1152–1157. [Google Scholar] [CrossRef]
- Posadas-Sánchez, R.; Vargas-Alarcón, G. Innate Immunity in Coronary Disease. The Role of Interleukin-12 Cytokine Family in Atherosclerosis. Rev. Investig. Clín. 2018, 70, 130. [Google Scholar] [CrossRef]
- Khan, R.; Rheaume, E.; Tardif, J.-C. Examining the Role of and Treatment Directed at IL-1β in Atherosclerosis. Curr. Atheroscler. Rep. 2018, 20, 53. [Google Scholar] [CrossRef]
- Więckowska-Gacek, A.; Mietelska-Porowska, A.; Wydrych, M.; Wojda, U. Western Diet as a Trigger of Alzheimer’s Disease: From Metabolic Syndrome and Systemic Inflammation to Neuroinflammation and Neurodegeneration. Ageing Res. Rev. 2021, 70, 101397. [Google Scholar] [CrossRef]
- Chan, K.L.; Cathomas, F.; Russo, S.J. Central and Peripheral Inflammation Link Metabolic Syndrome and Major Depressive Disorder. Physiology 2019, 34, 123–133. [Google Scholar] [CrossRef]
- Chen, Y.; Wright, N.; Guo, Y.; Turnbull, I.; Kartsonaki, C.; Yang, L.; Bian, Z.; Pei, P.; Pan, D.; Zhang, Y.; et al. Mortality and Recurrent Vascular Events after First Incident Stroke: A 9-Year Community-Based Study of 0·5 Million Chinese Adults. Lancet Glob. Health 2020, 8, e580–e590. [Google Scholar] [CrossRef]
- The GBD 2016 Lifetime Risk of Stroke Collaborators. Global, Regional, and Country-Specific Lifetime Risks of Stroke, 1990 and 2016. N. Engl. J. Med. 2018, 379, 2429–2437. [Google Scholar] [CrossRef]
- Moshayedi, H.; Ahrabi, R.; Mardani, A.; Sadigetegad, S.; Farhudi, M. Association between Non-Alcoholic Fatty Liver Disease and Ischemic Stroke. Iran. J. Neurol. 2014, 13, 144–148. [Google Scholar]
- Abdeldyem, S.M.; Goda, T.; Khodeir, S.A.; Abou Saif, S.; Abd-Elsalam, S. Nonalcoholic Fatty Liver Disease in Patients with Acute Ischemic Stroke Is Associated with More Severe Stroke and Worse Outcome. J. Clin. Lipidol. 2017, 11, 915–919. [Google Scholar] [CrossRef]
- Baik, M.; Kim, S.U.; Nam, H.S.; Heo, J.H.; Kim, Y.D. The Paradoxical Protective Effect of Liver Steatosis on Severity and Functional Outcome of Ischemic Stroke. Front. Neurol. 2019, 10, 375. [Google Scholar] [CrossRef]
- Baik, M.; Kim, S.U.; Kang, S.; Park, H.J.; Nam, H.S.; Heo, J.H.; Kim, B.K.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; et al. Liver Fibrosis, Not Steatosis, Associates with Long-Term Outcomes in Ischaemic Stroke Patients. Cerebrovasc. Dis. 2019, 47, 32–39. [Google Scholar] [CrossRef]
- Mori, T.; Yoshioka, K.; Tanno, Y. Non-Alcoholic Fatty Liver Disease Frequency and Associated Factors at Admission of Acute Stroke. Hepatol. Int. 2021, 16, 81–88. [Google Scholar] [CrossRef]
- Tziomalos, K.; Giampatzis, V.; Bouziana, S.D.; Spanou, M.; Papadopoulou, M.; Pavlidis, A.; Kostaki, S.; Bozikas, A.; Savopoulos, C.; Hatzitolios, A.I. Association between Nonalcoholic Fatty Liver Disease and Acute Ischemic Stroke Severity and Outcome. World J. Hepatol. 2013, 5, 621. [Google Scholar] [CrossRef]
- Xu, J.; Dai, L.; Zhang, Y.; Wang, A.; Li, H.; Wang, Y.; Meng, X.; Wu, S.; Wang, Y. Severity of Nonalcoholic Fatty Liver Disease and Risk of Future Ischemic Stroke Events. Stroke 2021, 52, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Alexander, K.S.; Zakai, N.A.; Lidofsky, S.D.; Callas, P.W.; Judd, S.E.; Tracy, R.P.; Cushman, M. Non-Alcoholic Fatty Liver Disease, Liver Biomarkers and Stroke Risk: The Reasons for Geographic and Racial Differences in Stroke Cohort. PLoS ONE 2018, 13, e0194153. [Google Scholar] [CrossRef] [PubMed]
- Parikh, N.S.; Koh, I.; VanWagner, L.B.; Elkind, M.S.V.; Zakai, N.A.; Cushman, M. Liver Fibrosis Is Associated with Ischemic Stroke Risk in Women but Not Men: The REGARDS Study. J. Stroke Cerebrovasc. Dis. 2021, 30, 105788. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Xu, Y.; He, Z.; Zhang, H.; Lian, X.; Zhu, T.; Liang, C.; Li, J. Increased Risk of Cerebrovascular Accident Related to Non-Alcoholic Fatty Liver Disease: A Meta-Analysis. Oncotarget 2018, 9, 2752–2760. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Zhou, L.; Shi, Y.; Li, Z.; Liu, L.; Zuo, L.; Zhang, J.; Liang, S.; Kang, J.; Du, S.; et al. Neuroprotective Effects of Danshen Chuanxiongqin Injection Against Ischemic Stroke: Metabolomic Insights by UHPLC-Q-Orbitrap HRMS Analysis. Front. Mol. Biosci. 2021, 8, 630291. [Google Scholar] [CrossRef]
- Nizamutdinov, D.; DeMorrow, S.; McMillin, M.; Kain, J.; Mukherjee, S.; Zeitouni, S.; Frampton, G.; Bricker, P.C.S.; Hurst, J.; Shapiro, L.A. Hepatic Alterations Are Accompanied by Changes to Bile Acid Transporter-Expressing Neurons in the Hypothalamus after Traumatic Brain Injury. Sci. Rep. 2017, 7, 40112. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yuan, J.; Zhao, J.; Zhang, L.; Wang, Q.; Wang, G. Serum Metabolomic Patterns in Young Patients with Ischemic Stroke: A Case Study. Metabolomics 2021, 17, 24. [Google Scholar] [CrossRef]
- Li, C.; Wang, X.; Yan, J.; Cheng, F.; Ma, X.; Chen, C.; Wang, W.; Wang, Q. Cholic Acid Protects In Vitro Neurovascular Units against Oxygen and Glucose Deprivation-Induced Injury through the BDNF-TrkB Signaling Pathway. Oxidative Med. Cell. Longev. 2020, 2020, 1201624. [Google Scholar] [CrossRef]
- Rodrigues, C.M.P.; Solá, S.; Nan, Z.; Castro, R.E.; Ribeiro, P.S.; Low, W.C.; Steer, C.J. Tauroursodeoxycholic Acid Reduces Apoptosis and Protects against Neurological Injury after Acute Hemorrhagic Stroke in Rats. Proc. Natl. Acad. Sci. USA 2003, 100, 6087–6092. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Gao, C.; Yan, Y.; Wang, T.; Luo, C.; Zhang, M.; Chen, X.; Tao, L. Inhibiting ER Stress Weakens Neuronal Pyroptosis in a Mouse Acute Hemorrhagic Stroke Model. Mol. Neurobiol. 2020, 57, 5324–5335. [Google Scholar] [CrossRef]
- Rodrigues, C.M.P.; Spellman, S.R.; Solá, S.; Grande, A.W.; Linehan-Stieers, C.; Low, W.C.; Steer, C.J. Neuroprotection by a Bile Acid in an Acute Stroke Model in the Rat. J. Cereb. Blood Flow Metab. 2002, 22, 463–471. [Google Scholar] [CrossRef]
- Bian, K.-Y.; Jin, H.-F.; Sun, W.; Sun, Y.-J. DCA Can Improve the ACI-Induced Neurological Impairment through Negative Regulation of Nrf2 Signaling Pathway. Eur. Rev. Med. Pharm. Sci. 2019, 23, 343–351. [Google Scholar] [CrossRef]
- Yanguas-Casás, N.; Barreda-Manso, M.A.; Nieto-Sampedro, M.; Romero-Ramírez, L. Tauroursodeoxycholic Acid Reduces Glial Cell Activation in an Animal Model of Acute Neuroinflammation. J. Neuroinflamm. 2014, 11, 50. [Google Scholar] [CrossRef] [PubMed]
- Kaltenboeck, A.; Harmer, C. The Neuroscience of Depressive Disorders: A Brief Review of the Past and Some Considerations about the Future. Brain Neurosci. Adv. 2018, 2, 2398212818799269. [Google Scholar] [CrossRef]
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 354 Diseases and Injuries for 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Choi, J.M.; Chung, G.E.; Kang, S.J.; Kwak, M.-S.; Yang, J.I.; Park, B.; Yim, J.Y. Association Between Anxiety and Depression and Nonalcoholic Fatty Liver Disease. Front. Med. 2021, 7, 585618. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Lim, L.K.E.; Ng, C.H.; Tan, D.J.H.; Lim, W.H.; Ho, C.S.H.; Tan, E.X.X.; Sanyal, A.J.; Muthiah, M.D. Is Fatty Liver Associated With Depression? A Meta-Analysis and Systematic Review on the Prevalence, Risk Factors, and Outcomes of Depression and Non-Alcoholic Fatty Liver Disease. Front. Med. 2021, 8, 691696. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.Y.; Park, S.K.; Oh, C.-M.; Chung, P.-W.; Ryoo, J.-H. Non-Alcoholic Fatty Liver Disease and Its Association with Depression in Korean General Population. J. Korean Med. Sci. 2019, 34, e199. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Yoo, E.R.; Li, A.A.; Tighe, S.P.; Cholankeril, G.; Harrison, S.A.; Ahmed, A. Depression Is Associated with Non-Alcoholic Fatty Liver Disease among Adults in the United States. Aliment. Pharmacol. Ther. 2019, 50, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Zelber-Sagi, S.; Toker, S.; Armon, G.; Melamed, S.; Berliner, S.; Shapira, I.; Halpern, Z.; Santo, E.; Shibolet, O. Elevated Alanine Aminotransferase Independently Predicts New Onset of Depression in Employees Undergoing Health Screening Examinations. Psychol. Med. 2013, 43, 2603–2613. [Google Scholar] [CrossRef]
- Kinder, L.S.; Carnethon, M.R.; Palaniappan, L.P.; King, A.C.; Fortmann, S.P. Depression and the Metabolic Syndrome in Young Adults: Findings from the Third National Health and Nutrition Examination Survey. Psychosom. Med. 2004, 66, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Roriz-Cruz, M.; Rosset, I.; Wada, T.; Sakagami, T.; Ishine, M.; Roriz-Filho, J.S.; Cruz, T.R.S.; Rodrigues, R.P.; Resmini, I.; Sudoh, S.; et al. Stroke-Independent Association between Metabolic Syndrome and Functional Dependence, Depression, and Low Quality of Life in Elderly Community-Dwelling Brazilian People. J. Am. Geriatr. Soc. 2007, 55, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Youssef, N.A.; Abdelmalek, M.F.; Binks, M.; Guy, C.D.; Omenetti, A.; Smith, A.D.; Diehl, A.M.E.; Suzuki, A. Associations of Depression, Anxiety and Antidepressants with Histological Severity of Nonalcoholic Fatty Liver Disease. Liver Int. 2013, 33, 1062–1070. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Liu, C.; Yu, L.; Zhou, J.; Li, Y.; Xiong, Y.; Guo, A.; Chao, L.; Qu, Q.; Wei, G.; et al. Melatonin Alleviates Neuroinflammation and Metabolic Disorder in DSS-Induced Depression Rats. Oxidative Med. Cell. Longev. 2020, 2020, 1241894. [Google Scholar] [CrossRef]
- Xu, E.; Wang, B.; Lu, S.; Zhang, C.; Zhu, L.; Liu, X.; Bai, M.; Li, Y. Tandem Mass Tag-Based Quantitative Proteomic Analysis of the Liver Reveals Potential Protein Targets of Xiaochaihutang in CUMS Model of Depression. J. Chromatogr. B 2021, 1181, 122898. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Yu, M.; Ma, L.-Y.; Zhang, H.; Zou, Z. Chaihu-Shu-Gan-San Regulates Phospholipids and Bile Acid Metabolism against Hepatic Injury Induced by Chronic Unpredictable Stress in Rat. J. Chromatogr. B 2017, 1064, 14–21. [Google Scholar] [CrossRef]
- Jia, H.; Li, Q.; Zhou, C.; Yu, M.; Yang, Y.; Zhang, H.; Ding, G.; Shang, H.; Zou, Z. Chronic Unpredictive Mild Stress Leads to Altered Hepatic Metabolic Profile and Gene Expression. Sci. Rep. 2016, 6, 23441. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Gao, X.; Liang, M.; Fang, Y.; Jia, J.; Tian, J.; Li, Z.; Qin, X. Dose-Effect/Toxicity of Bupleuri Radix on Chronic Unpredictable Mild Stress and Normal Rats Based on Liver Metabolomics. Front. Pharmacol. 2021, 12, 627451. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Jung, Y.-H.; Jin, Y.; Kang, S.; Jang, C.-G.; Lee, J. A Comprehensive Metabolomics Investigation of Hippocampus, Serum, and Feces Affected by Chronic Fluoxetine Treatment Using the Chronic Unpredictable Mild Stress Mouse Model of Depression. Sci. Rep. 2019, 9, 7566. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Huang, C.; Chen, Z. Tauroursodeoxycholic Acid Ameliorates Lipopolysaccharide-Induced Depression Like Behavior in Mice via the Inhibition of Neuroinflammation and Oxido-Nitrosative Stress. Pharmacology 2019, 103, 93–100. [Google Scholar] [CrossRef]
- Lu, X.; Yang, R.-R.; Zhang, J.-L.; Wang, P.; Gong, Y.; Hu, W.-F.; Wu, Y.; Gao, M.-H.; Huang, C. Tauroursodeoxycholic Acid Produces Antidepressant-like Effects in a Chronic Unpredictable Stress Model of Depression via Attenuation of Neuroinflammation, Oxido-Nitrosative Stress, and Endoplasmic Reticulum Stress. Fundam. Clin. Pharm. 2018, 32, 363–377. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Khaoustov, V.I.; Xie, Q.; Pan, T.; Le, W.; Yoffe, B. Interferon-Alpha-Induced Modulation of Glucocorticoid and Serotonin Receptors as a Mechanism of Depression. J. Hepatol. 2005, 42, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Lane, C.A.; Hardy, J.; Schott, J.M. Alzheimer’s Disease. Eur. J. Neurol. 2018, 25, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Seeger, D.; Golovko, S.; Golovko, M.; Combs, C.K. Liver Bile Acid Changes in Mouse Models of Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 7451. [Google Scholar] [CrossRef]
- Dumitrescu, L.; Mahoney, E.R.; Mukherjee, S.; Lee, M.L.; Bush, W.S.; Engelman, C.D.; Lu, Q.; Fardo, D.W.; Trittschuh, E.H.; Mez, J.; et al. Genetic Variants and Functional Pathways Associated with Resilience to Alzheimer’s Disease. Brain 2020, 143, 2561–2575. [Google Scholar] [CrossRef]
- Kim, D.-G.; Krenz, A.; Toussaint, L.E.; Maurer, K.J.; Robinson, S.-A.; Yan, A.; Torres, L.; Bynoe, M.S. Non-Alcoholic Fatty Liver Disease Induces Signs of Alzheimer’s Disease (AD) in Wild-Type Mice and Accelerates Pathological Signs of AD in an AD Model. J. Neuroinflamm. 2016, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- MahmoudianDehkordi, S.; Arnold, M.; Nho, K.; Ahmad, S.; Jia, W.; Xie, G.; Louie, G.; Kueider-Paisley, A.; Moseley, M.A.; Thompson, J.W.; et al. Altered Bile Acid Profile Associates with Cognitive Impairment in Alzheimer’s Disease—An Emerging Role for Gut Microbiome. Alzheimer’s Dement. 2019, 15, 76–92. [Google Scholar] [CrossRef]
- Baloni, P.; Funk, C.C.; Yan, J.; Yurkovich, J.T.; Kueider-Paisley, A.; Nho, K.; Heinken, A.; Jia, W.; Mahmoudiandehkordi, S.; Louie, G.; et al. Metabolic Network Analysis Reveals Altered Bile Acid Synthesis and Metabolism in Alzheimer’s Disease. Cell Rep. Med. 2020, 1, 100138. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wei, R.; Xie, G.; Arnold, M.; Kueider-Paisley, A.; Louie, G.; Mahmoudian Dehkordi, S.; Blach, C.; Baillie, R.; Han, X.; et al. Peripheral Serum Metabolomic Profiles Inform Central Cognitive Impairment. Sci. Rep. 2020, 10, 14059. [Google Scholar] [CrossRef]
- Marksteiner, J.; Blasko, I.; Kemmler, G.; Koal, T.; Humpel, C. Bile Acid Quantification of 20 Plasma Metabolites Identifies Lithocholic Acid as a Putative Biomarker in Alzheimer’s Disease. Metabolomics 2018, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, W.J.; Abdel-Khalik, J.; Yutuc, E.; Roman, G.; Warner, M.; Gustafsson, J.-Å.; Wang, Y. Concentrations of Bile Acid Precursors in Cerebrospinal Fluid of Alzheimer’s Disease Patients. Free Radic. Biol. Med. 2019, 134, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Varma, V.R.; Wang, Y.; An, Y.; Varma, S.; Bilgel, M.; Doshi, J.; Legido-Quigley, C.; Delgado, J.C.; Oommen, A.M.; Roberts, J.A.; et al. Bile Acid Synthesis, Modulation, and Dementia: A Metabolomic, Transcriptomic, and Pharmacoepidemiologic Study. PLoS Med. 2021, 18, e1003615. [Google Scholar] [CrossRef] [PubMed]
- Ballestri, S.; Nascimbeni, F.; Baldelli, E.; Marrazzo, A.; Romagnoli, D.; Lonardo, A. NAFLD as a Sexual Dimorphic Disease: Role of Gender and Reproductive Status in the Development and Progression of Nonalcoholic Fatty Liver Disease and Inherent Cardiovascular Risk. Adv. Ther. 2017, 34, 1291–1326. [Google Scholar] [CrossRef]
- Bell, S.M.; Barnes, K.; Clemmens, H.; Al-Rafiah, A.R.; Al-Ofi, E.A.; Leech, V.; Bandmann, O.; Shaw, P.J.; Blackburn, D.J.; Ferraiuolo, L.; et al. Ursodeoxycholic Acid Improves Mitochondrial Function and Redistributes Drp1 in Fibroblasts from Patients with Either Sporadic or Familial Alzheimer’s Disease. J. Mol. Biol. 2018, 430, 3942–3953. [Google Scholar] [CrossRef]
- Joo, S.S.; Won, T.J.; Lee, D.I. Potential Role of Ursodeoxycholic Acid in Suppression of Nuclear Factor Kappa B in Microglial Cell Line (BV-2). Arch. Pharm. Res. 2004, 27, 954–960. [Google Scholar] [CrossRef]
- Solá, S.; Castro, R.E.; Laires, P.A.; Steer, C.J.; Rodrigues, C.M.P. Tauroursodeoxycholic Acid Prevents Amyloid-β Peptide-Induced Neuronal Death Via a Phosphatidylinositol 3-Kinase-Dependent Signaling Pathway. Mol. Med. 2003, 9, 226–234. [Google Scholar] [CrossRef]
- Nunes, A.F.; Amaral, J.D.; Lo, A.C.; Fonseca, M.B.; Viana, R.J.S.; Callaerts-Vegh, Z.; D’Hooge, R.; Rodrigues, C.M.P. TUDCA, a Bile Acid, Attenuates Amyloid Precursor Protein Processing and Amyloid-β Deposition in APP/PS1 Mice. Mol. Neurobiol. 2012, 45, 440–454. [Google Scholar] [CrossRef]
- Dionísio, P.A.; Amaral, J.D.; Ribeiro, M.F.; Lo, A.C.; D’Hooge, R.; Rodrigues, C.M.P. Amyloid-β Pathology Is Attenuated by Tauroursodeoxycholic Acid Treatment in APP/PS1 Mice after Disease Onset. Neurobiol. Aging 2015, 36, 228–240. [Google Scholar] [CrossRef]
- Zangerolamo, L.; Vettorazzi, J.F.; Solon, C.; Bronczek, G.A.; Engel, D.F.; Kurauti, M.A.; Soares, G.M.; Rodrigues, K.S.; Velloso, L.A.; Boschero, A.C.; et al. The Bile Acid TUDCA Improves Glucose Metabolism in Streptozotocin-Induced Alzheimer’s Disease Mice Model. Mol. Cell. Endocrinol. 2021, 521, 111116. [Google Scholar] [CrossRef]
- Bazzari, F.H.; Abdallah, D.M.; El-Abhar, H.S. Chenodeoxycholic Acid Ameliorates AlCl3-Induced Alzheimer’s Disease Neurotoxicity and Cognitive Deterioration via Enhanced Insulin Signaling in Rats. Molecules 2019, 24, 1992. [Google Scholar] [CrossRef] [PubMed]
- Beitz, J.M. Parkinson’s Disease: A Review. Front. Biosci. Sch. 2014, 6, 65–74. [Google Scholar] [CrossRef]
- Kalia, L.V.; Lang, A.E. Parkinson’s Disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Shao, Y.; Li, T.; Liu, Z.; Wang, X.; Xu, X.; Li, S.; Xu, G.; Le, W. Comprehensive Metabolic Profiling of Parkinson’s Disease by Liquid Chromatography-Mass Spectrometry. Mol. Neurodegener. 2021, 16, 4. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Killinger, B.A.; Ensink, E.; Beddows, I.; Yilmaz, A.; Lubben, N.; Lamp, J.; Schilthuis, M.; Vega, I.E.; Woltjer, R.; et al. Gut Microbiota Dysbiosis Is Associated with Elevated Bile Acids in Parkinson’s Disease. Metabolites 2021, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Hertel, J.; Harms, A.C.; Heinken, A.; Baldini, F.; Thinnes, C.C.; Glaab, E.; Vasco, D.A.; Pietzner, M.; Stewart, I.D.; Wareham, N.J.; et al. Integrated Analyses of Microbiome and Longitudinal Metabolome Data Reveal Microbial-Host Interactions on Sulfur Metabolism in Parkinson’s Disease. Cell Rep. 2019, 29, 1767–1777.e8. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, C.; Zhao, N.; Li, W.; Yang, Z.; Liu, X.; Le, W.; Zhang, X. Potential Biomarkers of Parkinson’s Disease Revealed by Plasma Metabolic Profiling. J. Chromatogr. B 2018, 1081–1082, 101–108. [Google Scholar] [CrossRef]
- Griffiths, W.J.; Abdel-Khalik, J.; Moore, S.F.; Wijeyekoon, R.S.; Crick, P.J.; Yutuc, E.; Farrell, K.; Breen, D.P.; Williams-Gray, C.H.; Theofilopoulos, S.; et al. The Cerebrospinal Fluid Profile of Cholesterol Metabolites in Parkinson’s Disease and Their Association With Disease State and Clinical Features. Front. Aging Neurosci. 2021, 13, 685594. [Google Scholar] [CrossRef]
- Yilmaz, A.; Ugur, Z.; Ustun, I.; Akyol, S.; Bahado-Singh, R.O.; Maddens, M.; Aasly, J.O.; Graham, S.F. Metabolic Profiling of CSF from People Suffering from Sporadic and LRRK2 Parkinson’s Disease: A Pilot Study. Cells 2020, 9, 2394. [Google Scholar] [CrossRef]
- Yakhine-Diop, S.M.S.; Morales-García, J.A.; Niso-Santano, M.; González-Polo, R.A.; Uribe-Carretero, E.; Martinez-Chacon, G.; Durand, S.; Maiuri, M.C.; Aiastui, A.; Zulaica, M.; et al. Metabolic Alterations in Plasma from Patients with Familial and Idiopathic Parkinson’s Disease. Aging 2020, 12, 16690–16708. [Google Scholar] [CrossRef]
- Huang, A.; Martin, E.R.; Vance, J.M.; Cai, X. Detecting Genetic Interactions in Pathway-Based Genome-Wide Association Studies: Pathway-Based GWAS by the Group Empirical Bayesian Lasso. Genet. Epidemiol. 2014, 38, 300–309. [Google Scholar] [CrossRef]
- Mendes, M.O.; Rosa, A.I.; Carvalho, A.N.; Nunes, M.J.; Dionísio, P.; Rodrigues, E.; Costa, D.; Duarte-Silva, S.; Maciel, P.; Rodrigues, C.M.P.; et al. Neurotoxic Effects of MPTP on Mouse Cerebral Cortex: Modulation of Neuroinflammation as a Neuroprotective Strategy. Mol. Cell. Neurosci. 2019, 96, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.I.; Duarte-Silva, S.; Silva-Fernandes, A.; Nunes, M.J.; Carvalho, A.N.; Rodrigues, E.; Gama, M.J.; Rodrigues, C.M.P.; Maciel, P.; Castro-Caldas, M. Tauroursodeoxycholic Acid Improves Motor Symptoms in a Mouse Model of Parkinson’s Disease. Mol. Neurobiol. 2018, 55, 9139–9155. [Google Scholar] [CrossRef]
- Cuevas, E.; Burks, S.; Raymick, J.; Robinson, B.; Gómez-Crisóstomo, N.P.; Escudero-Lourdes, C.; Lopez, A.G.G.; Chigurupati, S.; Hanig, J.; Ferguson, S.A.; et al. Tauroursodeoxycholic Acid (TUDCA) Is Neuroprotective in a Chronic Mouse Model of Parkinson’s Disease. Nutr. Neurosci. 2020, 25, 1374–1391. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.I.; Fonseca, I.; Nunes, M.J.; Moreira, S.; Rodrigues, E.; Carvalho, A.N.; Rodrigues, C.M.P.; Gama, M.J.; Castro-Caldas, M. Novel Insights into the Antioxidant Role of Tauroursodeoxycholic Acid in Experimental Models of Parkinson’s Disease. Biochim. Biophys. Acta BBA—Mol. Basis Dis. 2017, 1863, 2171–2181. [Google Scholar] [CrossRef]
- Castro-Caldas, M.; Carvalho, A.N.; Rodrigues, E.; Henderson, C.J.; Wolf, C.R.; Rodrigues, C.M.P.; Gama, M.J. Tauroursodeoxycholic Acid Prevents MPTP-Induced Dopaminergic Cell Death in a Mouse Model of Parkinson’s Disease. Mol. Neurobiol. 2012, 46, 475–486. [Google Scholar] [CrossRef]
- Moreira, S.; Fonseca, I.; Nunes, M.J.; Rosa, A.; Lemos, L.; Rodrigues, E.; Carvalho, A.N.; Outeiro, T.F.; Rodrigues, C.M.P.; Gama, M.J.; et al. Nrf2 Activation by Tauroursodeoxycholic Acid in Experimental Models of Parkinson’s Disease. Exp. Neurol. 2017, 295, 77–87. [Google Scholar] [CrossRef]
- Launay, N.; Ruiz, M.; Grau, L.; Ortega, F.J.; Ilieva, E.V.; Martínez, J.J.; Galea, E.; Ferrer, I.; Knecht, E.; Pujol, A.; et al. Tauroursodeoxycholic Bile Acid Arrests Axonal Degeneration by Inhibiting the Unfolded Protein Response in X-Linked Adrenoleukodystrophy. Acta Neuropathol. 2017, 133, 283–301. [Google Scholar] [CrossRef]
- Duan, W.-M.; Rodrigures, C.M.P.; Zhao, L.-R.; Steer, C.J.; Low, W.C. Tauroursodeoxycholic Acid Improves the Survival and Function of Nigral Transplants in a Rat Model of Parkinson’s Disease. Cell Transpl. 2002, 11, 195–205. [Google Scholar] [CrossRef]
- Abdelkader, N.F.; Safar, M.M.; Salem, H.A. Ursodeoxycholic Acid Ameliorates Apoptotic Cascade in the Rotenone Model of Parkinson’s Disease: Modulation of Mitochondrial Perturbations. Mol. Neurobiol. 2016, 53, 810–817. [Google Scholar] [CrossRef]
- Chun, H.S.; Low, W.C. Ursodeoxycholic Acid Suppresses Mitochondria-Dependent Programmed Cell Death Induced by Sodium Nitroprusside in SH-SY5Y Cells. Toxicology 2012, 292, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Shen, D.; Jiang, C.; Wang, H.; Chang, M. Ursodeoxycholic Acid Protects Dopaminergic Neurons from Oxidative Stress via Regulating Mitochondrial Function, Autophagy, and Apoptosis in MPTP/MPP+-Induced Parkinson’s Disease. Neurosci. Lett. 2021, 741, 135493. [Google Scholar] [CrossRef] [PubMed]
- Sathe, A.G.; Tuite, P.; Chen, C.; Ma, Y.; Chen, W.; Cloyd, J.; Low, W.C.; Steer, C.J.; Lee, B.; Zhu, X.; et al. Pharmacokinetics, Safety, and Tolerability of Orally Administered Ursodeoxycholic Acid in Patients With Parkinson’s Disease—A Pilot Study. J. Clin. Pharmacol. 2020, 60, 744–750. [Google Scholar] [CrossRef]
- Payne, T.; Sassani, M.; Buckley, E.; Moll, S.; Anton, A.; Appleby, M.; Maru, S.; Taylor, R.; McNeill, A.; Hoggard, N.; et al. Ursodeoxycholic Acid as a Novel Disease-Modifying Treatment for Parkinson’s Disease: Protocol for a Two-Centre, Randomised, Double-Blind, Placebo-Controlled Trial, The “UP” Study. BMJ Open 2020, 10, e038911. [Google Scholar] [CrossRef] [PubMed]
- McMillin, M.; Frampton, G.; Quinn, M.; Ashfaq, S.; de los Santos, M.; Grant, S.; DeMorrow, S. Bile Acid Signaling Is Involved in the Neurological Decline in a Murine Model of Acute Liver Failure. Am. J. Pathol. 2016, 186, 312–323. [Google Scholar] [CrossRef]
- Mertens, K.L.; Kalsbeek, A.; Soeters, M.R.; Eggink, H.M. Bile Acid Signaling Pathways from the Enterohepatic Circulation to the Central Nervous System. Front. Neurosci. 2017, 11, 617. [Google Scholar] [CrossRef] [PubMed]
- Keene, C.D.; Rodrigues, C.M.; Eich, T.; Linehan-Stieers, C.; Abt, A.; Kren, B.T.; Steer, C.J.; Low, W.C. A Bile Acid Protects against Motor and Cognitive Deficits and Reduces Striatal Degeneration in the 3-Nitropropionic Acid Model of Huntington’s Disease. Exp. Neurol. 2001, 171, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Palmela, I.; Correia, L.; Silva, R.F.M.; Sasaki, H.; Kim, K.S.; Brites, D.; Brito, M.A. Hydrophilic Bile Acids Protect Human Blood-Brain Barrier Endothelial Cells from Disruption by Unconjugated Bilirubin: An in Vitro Study. Front. Neurosci. 2015, 9, 80. [Google Scholar] [CrossRef]
- Greenwood, J.; Adu, J.; Davey, A.J.; Abbott, N.J.; Bradbury, M.W. The Effect of Bile Salts on the Permeability and Ultrastructure of the Perfused, Energy-Depleted, Rat Blood-Brain Barrier. J. Cereb. Blood Flow Metab. 1991, 11, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Quinn, M.; McMillin, M.; Galindo, C.; Frampton, G.; Pae, H.Y.; DeMorrow, S. Bile Acids Permeabilize the Blood Brain Barrier after Bile Duct Ligation in Rats via Rac1-Dependent Mechanisms. Dig. Liver Dis. 2014, 46, 527–534. [Google Scholar] [CrossRef] [PubMed]
- McMillin, M.; Frampton, G.; Quinn, M.; Divan, A.; Grant, S.; Patel, N.; Newell-Rogers, K.; DeMorrow, S. Suppression of the HPA Axis During Cholestasis Can Be Attributed to Hypothalamic Bile Acid Signaling. Mol. Endocrinol. 2015, 29, 1720–1730. [Google Scholar] [CrossRef]
- Lund, E.G.; Guileyardo, J.M.; Russell, D.W. CDNA Cloning of Cholesterol 24-Hydroxylase, a Mediator of Cholesterol Homeostasis in the Brain. Proc. Natl. Acad. Sci. USA 1999, 96, 7238–7243. [Google Scholar] [CrossRef]
- Ogundare, M.; Theofilopoulos, S.; Lockhart, A.; Hall, L.J.; Arenas, E.; Sjövall, J.; Brenton, A.G.; Wang, Y.; Griffiths, W.J. Cerebrospinal Fluid Steroidomics: Are Bioactive Bile Acids Present in Brain? J. Biol. Chem. 2010, 285, 4666–4679. [Google Scholar] [CrossRef]
- Mano, N.; Goto, T.; Uchida, M.; Nishimura, K.; Ando, M.; Kobayashi, N.; Goto, J. Presence of Protein-Bound Unconjugated Bile Acids in the Cytoplasmic Fraction of Rat Brain. J. Lipid Res. 2004, 45, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Zuo, G.; Zhang, T.; Huang, L.; Araujo, C.; Peng, J.; Travis, Z.; Okada, T.; Ocak, U.; Zhang, G.; Tang, J.; et al. Activation of TGR5 with INT-777 Attenuates Oxidative Stress and Neuronal Apoptosis via CAMP/PKCε/ALDH2 Pathway after Subarachnoid Hemorrhage in Rats. Free Radic. Biol. Med. 2019, 143, 441–453. [Google Scholar] [CrossRef]
- McMillin, M.; Frampton, G.; Tobin, R.; Dusio, G.; Smith, J.; Shin, H.; Newell-Rogers, K.; Grant, S.; DeMorrow, S. TGR5 Signaling Reduces Neuroinflammation during Hepatic Encephalopathy. J. Neurochem. 2015, 135, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Ma, H.; Guo, X.; Liu, J.; Gui, T.; Gai, Z. Farnesoid X Receptor (FXR) Aggravates Amyloid-β-Triggered Apoptosis by Modulating the CAMP-Response Element-Binding Protein (CREB)/Brain-Derived Neurotrophic Factor (BDNF) Pathway In Vitro. Med. Sci. Monit. 2019, 25, 9335–9345. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Li, H.; Jia, Y.; Xiao, Y.; Luo, S.; Zhang, D.; Han, L.; Dai, L.; Xiao, C.; Feng, L.; et al. Ganoderic Acid A Exerted Antidepressant-like Action through FXR Modulated NLRP3 Inflammasome and Synaptic Activity. Biochem. Pharmacol. 2021, 188, 114561. [Google Scholar] [CrossRef]
- Shan, H.-M.; Zang, M.; Zhang, Q.; Shi, R.-B.; Shi, X.-J.; Mamtilahun, M.; Liu, C.; Luo, L.; Tian, X.; Zhang, Z.; et al. Farnesoid X Receptor Knockout Protects Brain against Ischemic Injury through Reducing Neuronal Apoptosis in Mice. J. Neuroinflamm. 2020, 17, 164. [Google Scholar] [CrossRef]
- Hu, W.; Wu, J.; Ye, T.; Chen, Z.; Tao, J.; Tong, L.; Ma, K.; Wen, J.; Wang, H.; Huang, C. Farnesoid X Receptor-Mediated Cytoplasmic Translocation of CRTC2 Disrupts CREB-BDNF Signaling in Hippocampal CA1 and Leads to the Development of Depression-Like Behaviors in Mice. Int. J. Neuropsychopharmacol. 2020, 23, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-G.; Zheng, J.-X.; Xu, X.; Hu, Y.-M.; Ma, Y.-M. Hippocampal FXR Plays a Role in the Pathogenesis of Depression: A Preliminary Study Based on Lentiviral Gene Modulation. Psychiatry Res. 2018, 264, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Matei, N.; McBride, D.W.; Xu, Y.; Zhou, Z.; Tang, J.; Luo, B.; Zhang, J.H. TGR5 Activation Attenuates Neuroinflammation via Pellino3 Inhibition of Caspase-8/NLRP3 after Middle Cerebral Artery Occlusion in Rats. J. Neuroinflamm. 2021, 18, 40. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Matei, N.; McBride, D.W.; Xu, Y.; Tang, J.; Luo, B.; Zhang, J.H. Activation of TGR5 Protects Blood Brain Barrier via the BRCA1/Sirt1 Pathway after Middle Cerebral Artery Occlusion in Rats. J. Biomed. Sci. 2020, 27, 61. [Google Scholar] [CrossRef]
- Hu, X.; Yan, J.; Huang, L.; Araujo, C.; Peng, J.; Gao, L.; Liu, S.; Tang, J.; Zuo, G.; Zhang, J.H. INT-777 Attenuates NLRP3-ASC Inflammasome-Mediated Neuroinflammation via TGR5/CAMP/PKA Signaling Pathway after Subarachnoid Hemorrhage in Rats. Brain Behav. Immun. 2021, 91, 587–600. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tan, Y.-Z.; Mu, R.-H.; Tang, S.-S.; Liu, X.; Xing, S.-Y.; Long, Y.; Yuan, D.-H.; Hong, H. Takeda G Protein–Coupled Receptor 5 Modulates Depression-like Behaviors via Hippocampal CA3 Pyramidal Neurons Afferent to Dorsolateral Septum. Biol. Psychiatry 2021, 89, 1084–1095. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Gao, Y.; Chen, J.; Duan, Q.; He, P.; Zhang, J.; Huang, H.; Zhang, Q.; Ma, G.; Zhang, Y.; et al. TGR5 Agonist INT-777 Alleviates Inflammatory Neurodegeneration in Parkinson’s Disease Mouse Model by Modulating Mitochondrial Dynamics in Microglia. Neuroscience 2022, 490, 100–119. [Google Scholar] [CrossRef] [PubMed]
- Mikami, T.; Kim, J.; Park, J.; Lee, H.; Yaicharoen, P.; Suidasari, S.; Yokozawa, M.; Yamauchi, K. Olive Leaf Extract Prevents Obesity, Cognitive Decline, and Depression and Improves Exercise Capacity in Mice. Sci. Rep. 2021, 11, 12495. [Google Scholar] [CrossRef] [PubMed]
- Frye, C.A.; Koonce, C.J.; Walf, A.A. Involvement of Pregnane Xenobiotic Receptor in Mating-Induced Allopregnanolone Formation in the Midbrain and Hippocampus and Brain-Derived Neurotrophic Factor in the Hippocampus among Female Rats. Psychopharmacology 2014, 231, 3375–3390. [Google Scholar] [CrossRef] [PubMed]
- Litwa, E.; Rzemieniec, J.; Wnuk, A.; Lason, W.; Krzeptowski, W.; Kajta, M. RXRα, PXR and CAR Xenobiotic Receptors Mediate the Apoptotic and Neurotoxic Actions of Nonylphenol in Mouse Hippocampal Cells. J. Steroid Biochem. Mol. Biol. 2016, 156, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Garcion, E.; Sindji, L.; Leblondel, G.; Brachet, P.; Darcy, F. 1,25-Dihydroxyvitamin D3 Regulates the Synthesis of γ-Glutamyl Transpeptidase and Glutathione Levels in Rat Primary Astrocytes. J. Neurochem. 2002, 73, 859–866. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wu, Z.; Lan, T.; Wang, Y.; Tian, Y.; Chen, X.; Li, Y.; Bai, M.; Liu, J.; Gong, X.; et al. The 25(OH)D/VDR Signaling May Play a Role in Major Depression. Biochem. Biophys. Res. Commun. 2020, 523, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Garcion, E.; Sindji, L.; Montero-Menei, C.; Andre, C.; Brachet, P.; Darcy, F. Expression of Inducible Nitric Oxide Synthase during Rat Brain Inflammation: Regulation by 1,25-Dihydroxyvitamin D3. Glia 1998, 22, 282–294. [Google Scholar] [CrossRef]
- Xu, Y.; Liang, L. Vitamin D3/Vitamin D Receptor Signaling Mitigates Symptoms of Post-Stroke Depression in Mice by Upregulating Hippocampal BDNF Expression. Neurosci. Res. 2021, 170, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Shin, J.-Y.; Lee, Y.-S.; Yun, S.P.; Maeng, H.-J.; Lee, Y. Brain Endothelial P-Glycoprotein Level Is Reduced in Parkinson’s Disease via a Vitamin D Receptor-Dependent Pathway. Int. J. Mol. Sci. 2020, 21, 8538. [Google Scholar] [CrossRef]
- Cui, C.; Song, S.; Cui, J.; Feng, Y.; Gao, J.; Jiang, P. Vitamin D Receptor Activation Influences NADPH Oxidase (NOX2) Activity and Protects against Neurological Deficits and Apoptosis in a Rat Model of Traumatic Brain Injury. Oxidative Med. Cell. Longev. 2017, 2017, 9245702. [Google Scholar] [CrossRef] [PubMed]
- Kimura, A.; Ohmori, T.; Kashiwakura, Y.; Ohkawa, R.; Madoiwa, S.; Mimuro, J.; Shimazaki, K.; Hoshino, Y.; Yatomi, Y.; Sakata, Y. Antagonism of Sphingosine 1-Phosphate Receptor-2 Enhances Migration of Neural Progenitor Cells Toward an Area of Brain Infarction. Stroke 2008, 39, 3411–3417. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Orengo, L.; Daniels, B.P.; Dorsey, D.; Basak, S.A.; Grajales-Reyes, J.G.; McCandless, E.E.; Piccio, L.; Schmidt, R.E.; Cross, A.H.; Crosby, S.D.; et al. Enhanced Sphingosine-1-Phosphate Receptor 2 Expression Underlies Female CNS Autoimmunity Susceptibility. J. Clin. Investig. 2014, 124, 2571–2584. [Google Scholar] [CrossRef]
- McMillin, M.; Frampton, G.; Grant, S.; Khan, S.; Diocares, J.; Petrescu, A.; Wyatt, A.; Kain, J.; Jefferson, B.; DeMorrow, S. Bile Acid-Mediated Sphingosine-1-Phosphate Receptor 2 Signaling Promotes Neuroinflammation during Hepatic Encephalopathy in Mice. Front. Cell. Neurosci. 2017, 11, 191. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Doyle, T.M.; Luongo, L.; Largent-Milnes, T.M.; Giancotti, L.A.; Kolar, G.; Squillace, S.; Boccella, S.; Walker, J.K.; Pendleton, A.; et al. Sphingosine-1-Phosphate Receptor 1 Activation in Astrocytes Contributes to Neuropathic Pain. Proc. Natl. Acad. Sci. USA 2019, 116, 10557–10562. [Google Scholar] [CrossRef]
- Wu, C.-C.; Wang, L.-C.; Su, Y.-T.; Wei, W.-Y.; Tsai, K.-J. Synthetic A5β1 Integrin Ligand PHSRN Is Proangiogenic and Neuroprotective in Cerebral Ischemic Stroke. Biomaterials 2018, 185, 142–154. [Google Scholar] [CrossRef]
- Li, L.; Welser-Alves, J.; van der Flier, A.; Boroujerdi, A.; Hynes, R.O.; Milner, R. An Angiogenic Role for the A5β1 Integrin in Promoting Endothelial Cell Proliferation during Cerebral Hypoxia. Exp. Neurol. 2012, 237, 46–54. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.; Liu, X.; Feng, G.; Fu, Y.; Milner, R.; Li, L. Overexpression of A5β1 Integrin and Angiopoietin-1 Co-Operatively Promote Blood-Brain Barrier Integrity and Angiogenesis Following Ischemic Stroke. Exp. Neurol. 2019, 321, 113042. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.-M.; Kim, M.S.; Jo, J.; Shin, D.; Kwon, S.-H.; Seo, J.B.; Kang, D.; Lee, B.D.; Ryu, H.; Hwang, E.M.; et al. Decoding the Temporal Nature of Brain GR Activity in the NFκB Signal Transition Leading to Depressive-like Behavior. Mol. Psychiatry 2021, 26, 5087–5096. [Google Scholar] [CrossRef]
- Yu, H.; Guo, Y.; Zhao, Y.; Zhou, F.; Zhao, K.; Li, M.; Wen, J.; He, Z.; Zhu, X.; He, X. Both Insufficient and Excessive Glucocorticoid Receptor-Mediated Signaling Impair Neuronal Migration. J. Endocrinol. 2019, 242, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Lombès, M.; Le Menuet, D. Glucocorticoid Receptor Represses Brain-Derived Neurotrophic Factor Expression in Neuron-like Cells. Mol. Brain 2017, 10, 12. [Google Scholar] [CrossRef]
- Sun, X.-C.; Ren, X.-F.; Chen, L.; Gao, X.-Q.; Xie, J.-X.; Chen, W.-F. Glucocorticoid Receptor Is Involved in the Neuroprotective Effect of Ginsenoside Rg1 against Inflammation-Induced Dopaminergic Neuronal Degeneration in Substantia Nigra. J. Steroid Biochem. Mol. Biol. 2016, 155, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Houten, S.M.; Wang, L.; Moschetta, A.; Mangelsdorf, D.J.; Heyman, R.A.; Moore, D.D.; Auwerx, J. Bile Acids Lower Triglyceride Levels via a Pathway Involving FXR, SHP, and SREBP-1c. J. Clin. Investig. 2004, 113, 1408–1418. [Google Scholar] [CrossRef]
- Pineda Torra, I.; Claudel, T.; Duval, C.; Kosykh, V.; Fruchart, J.-C.; Staels, B. Bile Acids Induce the Expression of the Human Peroxisome Proliferator-Activated Receptor α Gene via Activation of the Farnesoid X Receptor. Mol. Endocrinol. 2003, 17, 259–272. [Google Scholar] [CrossRef]
- Siddiqui, M.S.; van Natta, M.L.; Connelly, M.A.; Vuppalanchi, R.; Neuschwander-Tetri, B.A.; Tonascia, J.; Guy, C.; Loomba, R.; Dasarathy, S.; Wattacheril, J.; et al. Impact of Obeticholic Acid on the Lipoprotein Profile in Patients with Non-Alcoholic Steatohepatitis. J. Hepatol. 2020, 72, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Caron, S.; Huaman Samanez, C.; Dehondt, H.; Ploton, M.; Briand, O.; Lien, F.; Dorchies, E.; Dumont, J.; Postic, C.; Cariou, B.; et al. Farnesoid X Receptor Inhibits the Transcriptional Activity of Carbohydrate Response Element Binding Protein in Human Hepatocytes. Mol. Cell Biol. 2013, 33, 2202–2211. [Google Scholar] [CrossRef]
- Kir, S.; Beddow, S.A.; Samuel, V.T.; Miller, P.; Previs, S.F.; Suino-Powell, K.; Xu, H.E.; Shulman, G.I.; Kliewer, S.A.; Mangelsdorf, D.J. FGF19 as a Postprandial, Insulin-Independent Activator of Hepatic Protein and Glycogen Synthesis. Science 2011, 331, 1621–1624. [Google Scholar] [CrossRef] [PubMed]
- Potthoff, M.J.; Boney-Montoya, J.; Choi, M.; He, T.; Sunny, N.E.; Satapati, S.; Suino-Powell, K.; Xu, H.E.; Gerard, R.D.; Finck, B.N.; et al. FGF15/19 Regulates Hepatic Glucose Metabolism by Inhibiting the CREB-PGC-1α Pathway. Cell Metab. 2011, 13, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Holt, J.A.; Luo, G.; Billin, A.N.; Bisi, J.; McNeill, Y.Y.; Kozarsky, K.F.; Donahee, M.; Wang, D.Y.; Mansfield, T.A.; Kliewer, S.A.; et al. Definition of a Novel Growth Factor-Dependent Signal Cascade for the Suppression of Bile Acid Biosynthesis. Genes Dev. 2003, 17, 1581–1591. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, T.; Choi, M.; Moschetta, A.; Peng, L.; Cummins, C.L.; McDonald, J.G.; Luo, G.; Jones, S.A.; Goodwin, B.; Richardson, J.A.; et al. Fibroblast Growth Factor 15 Functions as an Enterohepatic Signal to Regulate Bile Acid Homeostasis. Cell Metab. 2005, 2, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Hsuchou, H.; Pan, W.; Kastin, A.J. Fibroblast Growth Factor 19 Entry into Brain. Fluids Barriers CNS 2013, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Bono, B.S.; Koziel Ly, N.K.; Miller, P.A.; Williams-Ikhenoba, J.; Dumiaty, Y.; Chee, M.J. Spatial Distribution of Beta-klotho MRNA in the Mouse Hypothalamus, Hippocampal Region, Subiculum, and Amygdala. J. Comp. Neurol. 2022, 530, 1634–1657. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, L.; Bo, C.; Zhang, X.; Zhang, J.; Li, Y. MicroRNA-9-3p Aggravates Cerebral Ischemia/Reperfusion Injury by Targeting Fibroblast Growth Factor 19 (FGF19) to Inactivate GSK-3β/Nrf2/ARE Signaling. Neuropsychiatr. Dis. Treat. 2021, 17, 1989–2002. [Google Scholar] [CrossRef]
- Goetz, R.; Beenken, A.; Ibrahimi, O.A.; Kalinina, J.; Olsen, S.K.; Eliseenkova, A.V.; Xu, C.; Neubert, T.A.; Zhang, F.; Linhardt, R.J.; et al. Molecular Insights into the Klotho-Dependent, Endocrine Mode of Action of Fibroblast Growth Factor 19 Subfamily Members. Mol. Cell Biol. 2007, 27, 3417–3428. [Google Scholar] [CrossRef]
- Asada, M.; Shinomiya, M.; Suzuki, M.; Honda, E.; Sugimoto, R.; Ikekita, M.; Imamura, T. Glycosaminoglycan Affinity of the Complete Fibroblast Growth Factor Family. Biochim. Biophys. Acta 2009, 1790, 40–48. [Google Scholar] [CrossRef]
- Harmer, N.J.; Pellegrini, L.; Chirgadze, D.; Fernandez-Recio, J.; Blundell, T.L. The Crystal Structure of Fibroblast Growth Factor (FGF) 19 Reveals Novel Features of the FGF Family and Offers a Structural Basis for Its Unusual Receptor Affinity. Biochemistry 2004, 43, 629–640. [Google Scholar] [CrossRef]
- Adams, A.C.; Coskun, T.; Rovira, A.R.I.; Schneider, M.A.; Raches, D.W.; Micanovic, R.; Bina, H.A.; Dunbar, J.D.; Kharitonenkov, A. Fundamentals of FGF19 & FGF21 Action in Vitro and in Vivo. PLoS ONE 2012, 7, e38438. [Google Scholar] [CrossRef]
- Wu, X.; Ge, H.; Lemon, B.; Vonderfecht, S.; Weiszmann, J.; Hecht, R.; Gupte, J.; Hager, T.; Wang, Z.; Lindberg, R.; et al. FGF19-Induced Hepatocyte Proliferation Is Mediated through FGFR4 Activation. J. Biol. Chem. 2010, 285, 5165–5170. [Google Scholar] [CrossRef] [PubMed]
- Hultman, K.; Scarlett, J.M.; Baquero, A.F.; Cornea, A.; Zhang, Y.; Salinas, C.B.G.; Brown, J.; Morton, G.J.; Whalen, E.J.; Grove, K.L.; et al. The Central Fibroblast Growth Factor Receptor/Beta Klotho System: Comprehensive Mapping in Mus musculus and Comparisons to Nonhuman Primate and Human Samples Using an Automated in Situ Hybridization Platform. J. Comp. Neurol. 2019, 527, 2069–2085. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, S.; Owen, B.M.; Song, P.; Hernandez, G.; Zhang, Y.; Zhou, Y.; Scott, W.T.; Paratala, B.; Turner, T.; Smith, A.; et al. FGF21 Regulates Sweet and Alcohol Preference. Cell Metab. 2016, 23, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Jensen-Cody, S.O.; Flippo, K.H.; Claflin, K.E.; Yavuz, Y.; Sapouckey, S.A.; Walters, G.C.; Usachev, Y.M.; Atasoy, D.; Gillum, M.P.; Potthoff, M.J. FGF21 Signals to Glutamatergic Neurons in the Ventromedial Hypothalamus to Suppress Carbohydrate Intake. Cell Metab. 2020, 32, 273–286.e6. [Google Scholar] [CrossRef] [PubMed]
- Bookout, A.L.; de Groot, M.H.M.; Owen, B.M.; Lee, S.; Gautron, L.; Lawrence, H.L.; Ding, X.; Elmquist, J.K.; Takahashi, J.S.; Mangelsdorf, D.J.; et al. FGF21 Regulates Metabolism and Circadian Behavior by Acting on the Nervous System. Nat. Med. 2013, 19, 1147–1152. [Google Scholar] [CrossRef]
- Fon Tacer, K.; Bookout, A.L.; Ding, X.; Kurosu, H.; John, G.B.; Wang, L.; Goetz, R.; Mohammadi, M.; Kuro-o, M.; Mangelsdorf, D.J.; et al. Research Resource: Comprehensive Expression Atlas of the Fibroblast Growth Factor System in Adult Mouse. Mol. Endocrinol. 2010, 24, 2050–2064. [Google Scholar] [CrossRef]
- Kawamata, Y.; Fujii, R.; Hosoya, M.; Harada, M.; Yoshida, H.; Miwa, M.; Fukusumi, S.; Habata, Y.; Itoh, T.; Shintani, Y.; et al. A G Protein-Coupled Receptor Responsive to Bile Acids. J. Biol. Chem. 2003, 278, 9435–9440. [Google Scholar] [CrossRef]
- Fiorucci, S.; Distrutti, E.; Carino, A.; Zampella, A.; Biagioli, M. Bile Acids and Their Receptors in Metabolic Disorders. Prog. Lipid Res. 2021, 82, 101094. [Google Scholar] [CrossRef]
- Wang, Y.-D.; Chen, W.-D.; Yu, D.; Forman, B.M.; Huang, W. The G-Protein-Coupled Bile Acid Receptor, Gpbar1 (TGR5), Negatively Regulates Hepatic Inflammatory Response through Antagonizing Nuclear Factor κ Light-Chain Enhancer of Activated B Cells (NF-ΚB) in Mice. Hepatology 2011, 54, 1421–1432. [Google Scholar] [CrossRef]
- Keitel, V.; Donner, M.; Winandy, S.; Kubitz, R.; Häussinger, D. Expression and Function of the Bile Acid Receptor TGR5 in Kupffer Cells. Biochem. Biophys. Res. Commun. 2008, 372, 78–84. [Google Scholar] [CrossRef]
- Fiorucci, S.; Distrutti, E. Linking Liver Metabolic and Vascular Disease via Bile Acid Signaling. Trends Mol. Med. 2022, 28, 51–66. [Google Scholar] [CrossRef]
- Arab, J.P.; Karpen, S.J.; Dawson, P.A.; Arrese, M.; Trauner, M. Bile Acids and Nonalcoholic Fatty Liver Disease: Molecular Insights and Therapeutic Perspectives. Hepatology 2017, 65, 350–362. [Google Scholar] [CrossRef] [PubMed]
- Richards, P.; Parker, H.E.; Adriaenssens, A.E.; Hodgson, J.M.; Cork, S.C.; Trapp, S.; Gribble, F.M.; Reimann, F. Identification and Characterization of GLP-1 Receptor–Expressing Cells Using a New Transgenic Mouse Model. Diabetes 2014, 63, 1224–1233. [Google Scholar] [CrossRef] [PubMed]
- Ullmer, C.; Alvarez Sanchez, R.; Sprecher, U.; Raab, S.; Mattei, P.; Dehmlow, H.; Sewing, S.; Iglesias, A.; Beauchamp, J.; Conde-Knape, K. Systemic Bile Acid Sensing by G Protein-Coupled Bile Acid Receptor 1 (GPBAR1) Promotes PYY and GLP-1 Release: Mechanism of GPBAR1-Mediated PYY/GLP-1 Release. Br. J. Pharm. 2013, 169, 671–684. [Google Scholar] [CrossRef]
- Holst, J.J. The Physiology of Glucagon-like Peptide 1. Physiol. Rev. 2007, 87, 1409–1439. [Google Scholar] [CrossRef]
- Deacon, C.F.; Pridal, L.; Klarskov, L.; Olesen, M.; Holst, J.J. Glucagon-like Peptide 1 Undergoes Differential Tissue-Specific Metabolism in the Anesthetized Pig. Am. J. Physiol. 1996, 271, E458–E464. [Google Scholar] [CrossRef] [PubMed]
- Kastin, A.J.; Akerstrom, V.; Pan, W. Interactions of Glucagon-like Peptide-1 (GLP-1) with the Blood-Brain Barrier. J. Mol. Neurosci. 2002, 18, 7–14. [Google Scholar] [CrossRef]
- Cork, S.C.; Richards, J.E.; Holt, M.K.; Gribble, F.M.; Reimann, F.; Trapp, S. Distribution and Characterisation of Glucagon-like Peptide-1 Receptor Expressing Cells in the Mouse Brain. Mol. Metab. 2015, 4, 718–731. [Google Scholar] [CrossRef] [PubMed]
- Holst, J.J.; Deacon, C.F. Glucagon-like Peptide-1 Mediates the Therapeutic Actions of DPP-IV Inhibitors. Diabetologia 2005, 48, 612–615. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, M.; Nakabayashi, H.; Uchida, K.; Nakagawa, A.; Niijima, A. The Hepatic Vagal Nerve Is Receptive to Incretin Hormone Glucagon-like Peptide-1, but Not to Glucose-Dependent Insulinotropic Polypeptide, in the Portal Vein. J. Auton. Nerv. Syst. 1996, 61, 149–154. [Google Scholar] [CrossRef]
- Llewellyn-Smith, I.J.; Reimann, F.; Gribble, F.M.; Trapp, S. Preproglucagon Neurons Project Widely to Autonomic Control Areas in the Mouse Brain. Neuroscience 2011, 180, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wei, Z.; Li, Y.; Wei, C.; Li, Y.; Cheng, P.; Xu, H.; Li, Z.; Guo, R.; Qi, X.; et al. Perturbation of Ephrin Receptor Signaling and Glutamatergic Transmission in the Hypothalamus in Depression Using Proteomics Integrated With Metabolomics. Front. Neurosci. 2019, 13, 1359. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhou, C.; Li, J.; Chen, Z.; Shi, H.; Yang, W.; Qin, Y.; Lü, L.; Zhao, L.; Fang, L.; et al. Quantitative Proteomic Study of the Plasma Reveals Acute Phase Response and LXR/RXR and FXR/RXR Activation in the Chronic Unpredictable Mild Stress Mouse Model of Depression. Mol. Med. Rep. 2017, 17, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-M.; Wang, X.; Wu, Z.-H.; Liu, H.-L.; Chen, W.; Zhang, Z.-Z.; Chen, D.; Zeng, T.-S. Beneficial Effect of Farnesoid X Receptor Activation on Metabolism in a Diabetic Rat Model. Mol. Med. Rep. 2016, 13, 2135–2142. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ge, C.; Zhou, J.; Guo, Y.; Cui, S.; Huang, N.; Yan, T.; Cao, L.; Che, Y.; Zheng, Q.; et al. Noncanonical Farnesoid X Receptor Signaling Inhibits Apoptosis and Impedes Liver Fibrosis. EBioMedicine 2018, 37, 322–333. [Google Scholar] [CrossRef]
- Wu, X.; Lv, Y.-G.; Du, Y.-F.; Chen, F.; Reed, M.N.; Hu, M.; Suppiramaniam, V.; Tang, S.-S.; Hong, H. Neuroprotective Effects of INT-777 against Aβ1–42-Induced Cognitive Impairment, Neuroinflammation, Apoptosis, and Synaptic Dysfunction in Mice. Brain Behav. Immun. 2018, 73, 533–545. [Google Scholar] [CrossRef]
- Yanguas-Casás, N.; Barreda-Manso, M.A.; Nieto-Sampedro, M.; Romero-Ramírez, L. TUDCA: An Agonist of the Bile Acid Receptor GPBAR1/TGR5 With Anti-Inflammatory Effects in Microglial Cells: Anti-Inflammatory Effect of Tudca in Microglia. J. Cell. Physiol. 2017, 232, 2231–2245. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Yu, N.; Wang, X.; Yang, Y.; Liang, H. Tauroursodeoxycholic Acid Attenuates Neuronal Apoptosis via the TGR5/SIRT3 Pathway after Subarachnoid Hemorrhage in Rats. Biol. Res. 2020, 53, 56. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, J.; Hollister, K.; Sowers, L.C.; Forman, B.M. Endogenous Bile Acids Are Ligands for the Nuclear Receptor FXR/BAR. Mol. Cell 1999, 3, 543–553. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Ratziu, V.; Loomba, R.; Rinella, M.; Anstee, Q.M.; Goodman, Z.; Bedossa, P.; Geier, A.; Beckebaum, S.; Newsome, P.N.; et al. Obeticholic Acid for the Treatment of Non-Alcoholic Steatohepatitis: Interim Analysis from a Multicentre, Randomised, Placebo-Controlled Phase 3 Trial. Lancet 2019, 394, 2184–2196. [Google Scholar] [CrossRef]
- Sayin, S.I.; Wahlström, A.; Felin, J.; Jäntti, S.; Marschall, H.-U.; Bamberg, K.; Angelin, B.; Hyötyläinen, T.; Orešič, M.; Bäckhed, F. Gut Microbiota Regulates Bile Acid Metabolism by Reducing the Levels of Tauro-Beta-Muricholic Acid, a Naturally Occurring FXR Antagonist. Cell Metab. 2013, 17, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, F.J.; Jiang, C.; Patterson, A.D. An Intestinal Microbiota-Farnesoid X Receptor Axis Modulates Metabolic Disease. Gastroenterology 2016, 151, 845–859. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Sugimoto, K. Guggulsterone and Its Role in Chronic Diseases. Adv. Exp. Med. Biol. 2016, 929, 329–361. [Google Scholar] [CrossRef] [PubMed]
- Carter, B.A.; Taylor, O.A.; Prendergast, D.R.; Zimmerman, T.L.; Von Furstenberg, R.; Moore, D.D.; Karpen, S.J. Stigmasterol, a Soy Lipid-Derived Phytosterol, Is an Antagonist of the Bile Acid Nuclear Receptor FXR. Pediatr. Res. 2007, 62, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Fiorucci, S.; Distrutti, E.; Bifulco, G.; D’Auria, M.V.; Zampella, A. Marine Sponge Steroids as Nuclear Receptor Ligands. Trends Pharm. Sci. 2012, 33, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, F.J.; Jiang, C.; Xie, C.; Patterson, A.D. Intestinal Farnesoid X Receptor Signaling Modulates Metabolic Disease. Dig. Dis. 2017, 35, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Xie, C.; Li, F.; Zhang, L.; Nichols, R.G.; Krausz, K.W.; Cai, J.; Qi, Y.; Fang, Z.-Z.; Takahashi, S.; et al. Intestinal Farnesoid X Receptor Signaling Promotes Nonalcoholic Fatty Liver Disease. J. Clin. Investig. 2015, 125, 386–402. [Google Scholar] [CrossRef] [PubMed]
- Carino, A.; Marchianò, S.; Biagioli, M.; Bucci, M.; Vellecco, V.; Brancaleone, V.; Fiorucci, C.; Zampella, A.; Monti, M.C.; Distrutti, E.; et al. Agonism for the Bile Acid Receptor GPBAR1 Reverses Liver and Vascular Damage in a Mouse Model of Steatohepatitis. FASEB J. 2019, 33, 2809–2822. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki-Anzai, S.; Masuda, M.; Kohno, S.; Levi, M.; Shiozaki, Y.; Keenan, A.L.; Miyazaki, M. Simultaneous Inhibition of FXR and TGR5 Exacerbates Atherosclerotic Formation. J. Lipid Res. 2018, 59, 1709–1713. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki-Anzai, S.; Masuda, M.; Levi, M.; Keenan, A.L.; Miyazaki, M. Dual Activation of the Bile Acid Nuclear Receptor FXR and G-Protein-Coupled Receptor TGR5 Protects Mice against Atherosclerosis. PLoS ONE 2014, 9, e108270. [Google Scholar] [CrossRef] [PubMed]
- Lamers, C.; Merk, D.; Gabler, M.; Flesch, D.; Kaiser, A.; Schubert-Zsilavecz, M. SAR Studies on FXR Modulators Led to the Discovery of the First Combined FXR Antagonistic/TGR5 Agonistic Compound. Future Med. Chem. 2016, 8, 133–148. [Google Scholar] [CrossRef] [PubMed]

| Agonist | Clinical Trials | Status |
|---|---|---|
| Obeticholic acid (6-ECDCA, INT747) | NCT01265498 | Phase IIb: OCA as a FXR ligand in a NASH treatment trial (FLINT) |
| NCT03836937 | The function of OCA in NAFLD patients with elevated ALT | |
| NCT02548351 | Phase III: Randomized global study to assess the impact of OCA for fibrosis on NASH | |
| NCT03439254 | Phase III: Evaluating the OCA’s safety and effectiveness in patients who have compensated cirrhosis, as a result of NASH | |
| Tropifexor (LJN452) | NCT03681457 | Phase I: Tropifexor pharmacokinetics in patients with mild, moderate, and severe hepatic impairment |
| NCT04065841 | Phase II: Patients with NASH and hepatic fibrosis: efficacy, safety, and tolerability of the combination of Tropifexor and Licogliflozin and each single agent | |
| NCT03517540 | Phase II: Adult treatment for NASH and liver fibrosis using LJN452 and Cenicriviroc: a study of safety, tolerability, and effectiveness | |
| Cilofexor (GS-9674) | NCT02808312 | Phase I: Adults’ pharmacokinetics and pharmacodynamics of Cilofexor in those with normal and abnormal liver function |
| NCT02654002 | Phase I: Safety, tolerability, pharmacokinetics, and pharmacodynamics, as well as the impact of food on these factors of GS-9674 in healthy volunteers | |
| NCT02854605 | Phase II: Safety, tolerance, and efficacy of GS-9674 in participants with NASH | |
| NCT04971785 | Phase II: Semaglutide, Cilofexor, and Firsocostat fixed-dose combination safety and efficacy study in patients with compensated cirrhosis due to NASH | |
| NCT02781584 | Phase II: Selonsertib, Firsocostat, and Cilofexor in adults with NASH: safety, tolerability, and efficacy | |
| NCT03987074 | Phase II: In participants with NASH, the safety, tolerability, and the efficacy of monotherapy and combination regimens were examined | |
| NCT03449446 | Phase IIb: In people with compensated cirrhosis or bridging fibrosis, the effectiveness and safety of the drugs Selonsertib, Firsocostat, Cilofexor, and combinations for NASH, are being evaluated | |
| EDP-305 | NCT03748628 | Phase I: The AME (Absorption, Metabolism, and Excretion) study of [14C]EDP-305 in healthy male subjects |
| NCT02918929 | Phase I: EDP 305 research in subjects with and without presumed NAFLD | |
| NCT03207425 | Phase I: Comparison of participants in the EDP-305 study with mild and moderate hepatic impairment versus healthy people | |
| NCT03421431 | Phase II: Safety, tolerability, pharmacokinetics and effectiveness of EDP-305 in patients with NASH | |
| NCT04378010 | Phase IIb: Safety and effectiveness of EDP-305 in patients with liver-biopsy-confirmed NASH | |
| Px-102 | NCT01998672 | Phase I: Study using multiple ascending oral doses and Px-102 |
| Px-104 | NCT01999101 | Phase II: FXR agonist safety pilot study in NAFLD patients |
| Vonafexor (EYP001) | NCT03976687 | Phase I: Examination of the safety, tolerability, pharmacokinetics, and pharmacodynamics of EYP001a in NASH patients and healthy volunteers |
| NCT03812029 | Phase IIa: EYP001a’s effectiveness, tolerability, and pharmacokinetics in NASH patients | |
| MET409 | NCT04702490 | Phase IIa: In patients with type 2 diabetes and NASH, MET409 may be used alone or in combination with empagliflozin |
| TERN-101 (LY2562175) | NCT04328077 | Phase IIa: An investigation on the pharmacokinetics, efficacy, and safety of TERN-101 in people with NASH but without cirrhosis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Z.-L.; Li, C.-X.; Ma, C.-Y.; Chen, D.; Chen, J.-H.; Xu, W.-X.; Chen, C.-A.; Cheng, F.-F.; Wang, X.-Q. Linking Nonalcoholic Fatty Liver Disease and Brain Disease: Focusing on Bile Acid Signaling. Int. J. Mol. Sci. 2022, 23, 13045. https://doi.org/10.3390/ijms232113045
Ren Z-L, Li C-X, Ma C-Y, Chen D, Chen J-H, Xu W-X, Chen C-A, Cheng F-F, Wang X-Q. Linking Nonalcoholic Fatty Liver Disease and Brain Disease: Focusing on Bile Acid Signaling. International Journal of Molecular Sciences. 2022; 23(21):13045. https://doi.org/10.3390/ijms232113045
Chicago/Turabian StyleRen, Zi-Lin, Chang-Xiang Li, Chong-Yang Ma, Dan Chen, Jia-Hui Chen, Wen-Xiu Xu, Cong-Ai Chen, Fa-Feng Cheng, and Xue-Qian Wang. 2022. "Linking Nonalcoholic Fatty Liver Disease and Brain Disease: Focusing on Bile Acid Signaling" International Journal of Molecular Sciences 23, no. 21: 13045. https://doi.org/10.3390/ijms232113045
APA StyleRen, Z.-L., Li, C.-X., Ma, C.-Y., Chen, D., Chen, J.-H., Xu, W.-X., Chen, C.-A., Cheng, F.-F., & Wang, X.-Q. (2022). Linking Nonalcoholic Fatty Liver Disease and Brain Disease: Focusing on Bile Acid Signaling. International Journal of Molecular Sciences, 23(21), 13045. https://doi.org/10.3390/ijms232113045





