Enriched Environment Attenuates Enhanced Trait Anxiety in Association with Normalization of Aberrant Neuro-Inflammatory Events
Abstract
1. Introduction
2. Results
2.1. EE Housing Attenuates Anxiety Behavior in HAB
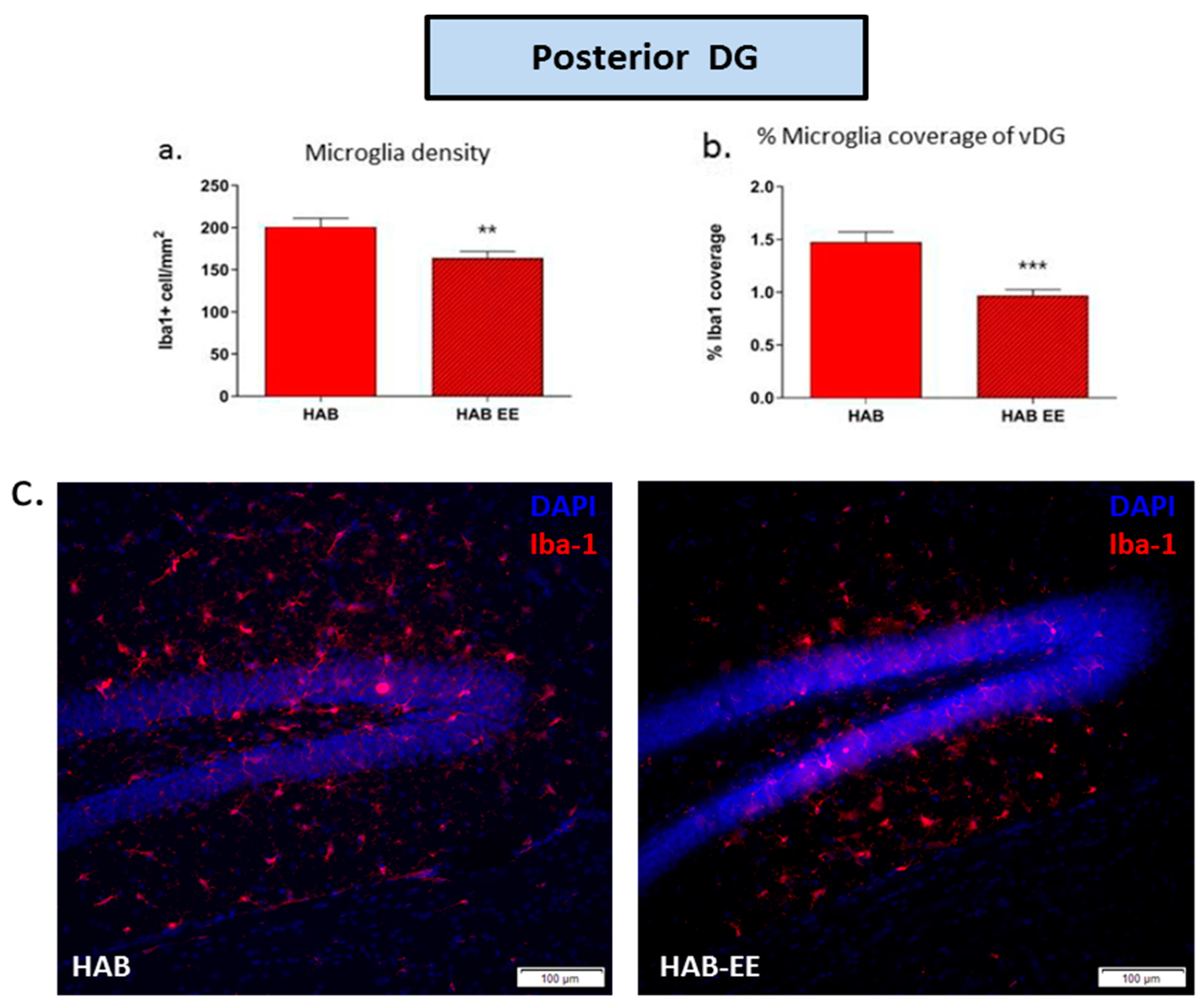
2.2. EE-Induced Anxiolysis Is Associated with Attenuation of Aberrant Microglia in HABs within the Anterior and Posterior DG
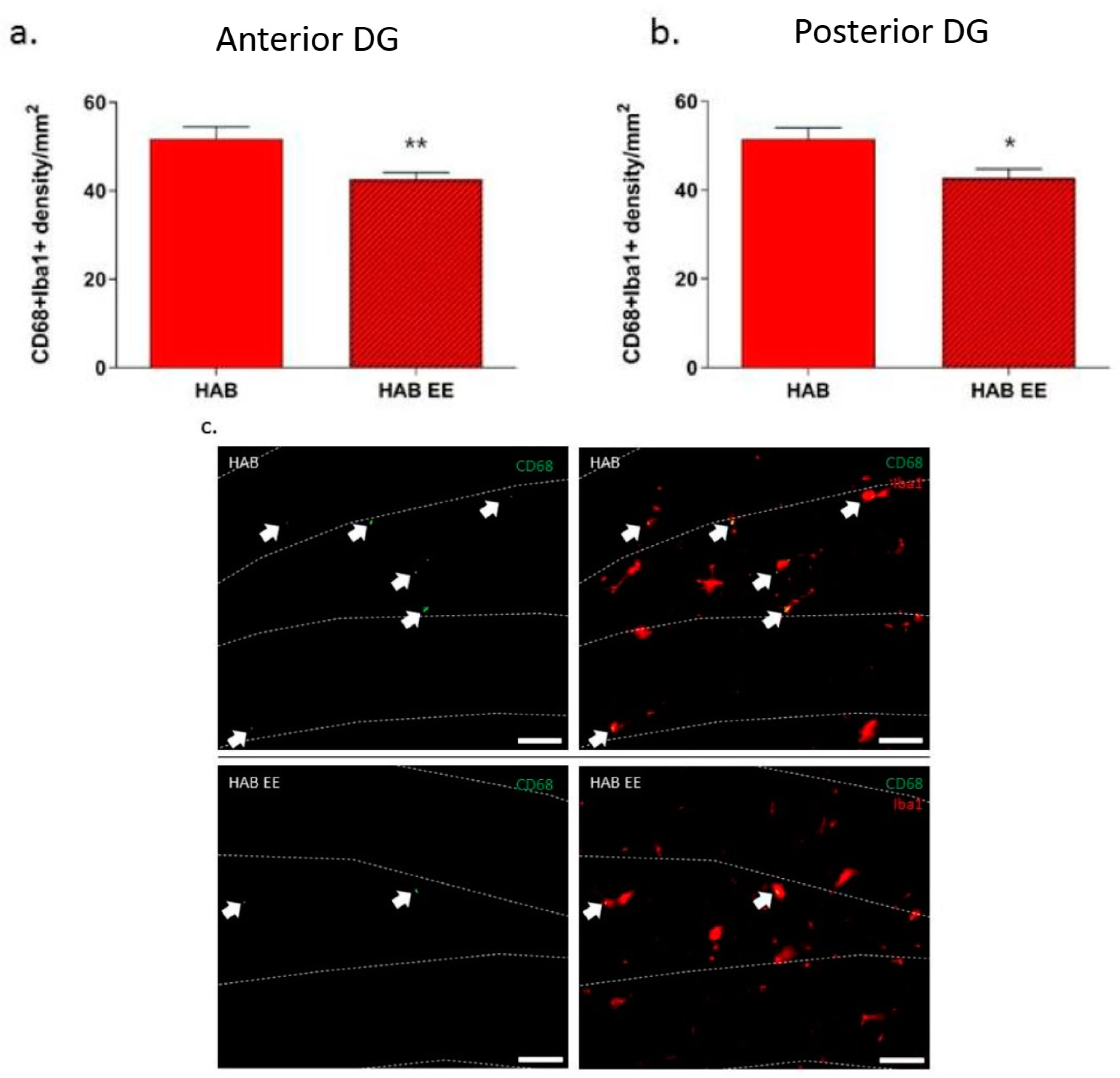
2.3. EE-Induced Anxiolysis Is Associated with Attenuation of Phagocytic Microglial Density in HABs within the DG
2.4. EE-Induced Anxiolysis Is Associated with Attenuation of Upregulated Microglia in HABs within the mPFC
3. Discussion
3.1. EE-Mediated Anxiolysis in HAB Mice
3.2. EE Attenuates Aberrant Microglial Phenotype in the HAB Mice
3.3. Effects of EE on Microglial CD68-Mediated Phagocytosis in HAB Mice
4. Materials and Methods
4.1. Animals
4.2. Enriched Environment
4.3. Open-Field Test
4.4. Light–Dark Test
4.5. Immunohistochemistry
4.6. Immunofluorescence Microscopy
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- COVID-19 Mental Disorders Collaborators. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet 2021, 398, 1700–1712. [Google Scholar] [CrossRef]
- Weger, M.; Sandi, C. High anxiety trait: A vulnerable phenotype for stress-induced depression. Neurosci. Biobehav. Rev. 2018, 87, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Sartori, S.B.; Singewald, N. Novel pharmacological targets in drug development for the treatment of anxiety and anxiety-related disorders. Pharmacol. Ther. 2019, 204, 107402. [Google Scholar] [CrossRef]
- Craske, M.G.; Stein, M.B. Anxiety. Focus (Am. Psychiatr. Publ.) 2017, 15, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Michopoulos, V.; Powers, A.; Gillespie, C.F.; Ressler, K.J.; Jovanovic, T. Inflammation in Fear- and Anxiety-Based Disorders: PTSD, GAD, and Beyond. Neuropsychopharmacology 2017, 42, 254–270. [Google Scholar] [CrossRef] [PubMed]
- Yirmiya, R.; Rimmerman, N.; Reshef, R. Depression as a Microglial Disease. Trends Neurosci. 2015, 38, 637–658. [Google Scholar] [CrossRef] [PubMed]
- Disabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The devil is in the details. J. Neurochem. 2016, 139 (Suppl. 2), 136–153. [Google Scholar] [CrossRef]
- Haroon, E.; Daguanno, A.W.; Woolwine, B.J.; Goldsmith, D.R.; Baer, W.M.; Wommack, E.C.; Felger, J.C.; Miller, A.H. Antidepressant treatment resistance is associated with increased inflammatory markers in patients with major depressive disorder. Psychoneuroendocrinology 2018, 95, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Radtke, F.A.; Chapman, G.; Hall, J.; Syed, Y.A. Modulating Neuroinflammation to Treat Neuropsychiatric Disorders. BioMed Res. Int. 2017, 2017, 5071786. [Google Scholar] [CrossRef]
- Dzhambov, A.M.; Markevych, I.; Tilov, B.; Arabadzhiev, Z.; Stoyanov, D.; Gatseva, P.; Dimitrova, D.D. Pathways linking residential noise and air pollution to mental ill-health in young adults. Environ. Res. 2018, 166, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Helbich, M. Mental Health and Environmental Exposures: An Editorial. Int. J. Environ. Res. Public Health 2018, 15, 2207. [Google Scholar] [CrossRef]
- Helbich, M. Toward dynamic urban environmental exposure assessments in mental health research. Environ. Res. 2018, 161, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Wicks, C.; Barton, J.; Orbell, S.; Andrews, L. Psychological benefits of outdoor physical activity in natural versus urban environments: A systematic review and meta-analysis of experimental studies. Appl. Psychol. Health Well-Being 2022, 14, 1037–1061. [Google Scholar] [CrossRef]
- Yao, W.; Chen, F.; Wang, S.; Zhang, X. Impact of Exposure to Natural and Built Environments on Positive and Negative Affect: A Systematic Review and Meta-Analysis. Front. Public Health 2021, 9, 758457. [Google Scholar] [CrossRef] [PubMed]
- Bobel, T.S.; Hackl, S.B.; Langgartner, D.; Jarczok, M.N.; Rohleder, N.; Rook, G.A.; Lowry, C.A.; Gundel, H.; Waller, C.; Reber, S.O. Less immune activation following social stress in rural vs. urban participants raised with regular or no animal contact, respectively. Proc. Natl. Acad. Sci. USA 2018, 115, 5259–5264. [Google Scholar] [CrossRef]
- Kim, T.-H.; Jeong, G.-W.; Baek, H.-S.; Kim, G.-W.; Sundaram, T.; Kang, H.-K.; Lee, S.-W.; Kim, H.-J.; Song, J.-K. Human brain activation in response to visual stimulation with rural and urban scenery pictures: A functional magnetic resonance imaging study. Sci. Total Environ. 2010, 408, 2600–2607. [Google Scholar] [CrossRef]
- Burrows, E.L.; Hannan, A.J. Towards environmental construct validity in animal models of CNS disorders: Optimizing translation of preclinical studies. CNS Neurol. Disord. Drug Targets 2013, 12, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Queen, N.J.; Hassan, Q.N., II; Cao, L. Improvements to Healthspan Through Environmental Enrichment and Lifestyle Interventions: Where Are We Now? Front. Neurosci. 2020, 14, 605. [Google Scholar] [CrossRef] [PubMed]
- Smail, M.A.; Smith, B.L.; Nawreen, N.; Herman, J.P. Differential impact of stress and environmental enrichment on corticolimbic circuits. Pharmacol. Biochem. Behav. 2020, 197, 172993. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Jiang, H.; Zheng, Q.; Fok, A.H.K.; Li, X.; Lau, C.G.; Lai, C.S.W. Environmental enrichment or selective activation of parvalbumin-expressing interneurons ameliorates synaptic and behavioral deficits in animal models with schizophrenia-like behaviors during adolescence. Mol. Psychiatry 2021, 26, 2533–2552. [Google Scholar] [CrossRef]
- Bhagya, V.R.; Srikumar, B.N.; Veena, J.; Rao, B.S.S. Short-term exposure to enriched environment rescues chronic stress-induced impaired hippocampal synaptic plasticity, anxiety, and memory deficits. J. Neurosci. Res. 2017, 95, 1602–1610. [Google Scholar] [CrossRef]
- Lazarov, O.; Mattson, M.P.; Peterson, D.A.; Pimplikar, S.W.; van Praag, H. When neurogenesis encounters aging and disease. Trends Neurosci. 2010, 33, 569–579. [Google Scholar] [CrossRef]
- Dandi, E.; Kalamari, A.; Touloumi, O.; Lagoudaki, R.; Nousiopoulou, E.; Simeonidou, C.; Spandou, E.; Tata, D.A. Beneficial effects of environmental enrichment on behavior, stress reactivity and synaptophysin/BDNF expression in hippocampus following early life stress. Int. J. Dev. Neurosci. 2018, 67, 19–32. [Google Scholar] [CrossRef]
- Sah, A.; Sotnikov, S.; Kharitonova, M.; Schmuckermair, C.; Diepold, R.P.; Landgraf, R.; Whittle, N.; Singewald, N. Epigenetic Mechanisms Within the Cingulate Cortex Regulate Innate Anxiety-Like Behavior. Int. J. Neuropsychopharmacol. 2019, 22, 317–328. [Google Scholar] [CrossRef]
- Sotnikov, S.V.; Markt, P.O.; Malik, V.; Chekmareva, N.Y.; Naik, R.R.; Sah, A.; Singewald, N.; Holsboer, F.; Czibere, L.; Landgraf, R. Bidirectional rescue of extreme genetic predispositions to anxiety: Impact of CRH receptor 1 as epigenetic plasticity gene in the amygdala. Transl. Psychiatry 2014, 4, e359. [Google Scholar] [CrossRef]
- Kempermann, G. Environmental enrichment, new neurons and the neurobiology of individuality. Nat. Rev. Neurosci. 2019, 20, 235–245. [Google Scholar] [CrossRef]
- Planchez, B.; Surget, A.; Belzung, C. Adult hippocampal neurogenesis and antidepressants effects. Curr. Opin. Pharmacol. 2020, 50, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Gronska-Peski, M.; Goncalves, J.T.; Hebert, J.M. Enriched Environment Promotes Adult Hippocampal Neurogenesis through FGFRs. J. Neurosci. 2021, 41, 2899–2910. [Google Scholar] [CrossRef]
- Kimura, L.F.; Novaes, L.S.; Picolo, G.; Munhoz, C.D.; Cheung, C.W.; Camarini, R. How environmental enrichment balances out neuroinflammation in chronic pain and comorbid depression and anxiety disorders. Br. J. Pharmacol. 2021, 179, 1640–1660. [Google Scholar] [CrossRef]
- Singhal, G.; Jaehne, E.J.; Corrigan, F.; Baune, B.T. Cellular and molecular mechanisms of immunomodulation in the brain through environmental enrichment. Front. Cell. Neurosci. 2014, 8, 97. [Google Scholar] [CrossRef]
- Rooney, S.; Sah, A.; Unger, M.S.; Kharitonova, M.; Sartori, S.B.; Schwarzer, C.; Aigner, L.; Kettenmann, H.; Wolf, S.A.; Singewald, N. Neuroinflammatory alterations in trait anxiety: Modulatory effects of minocycline. Transl. Psychiatry 2020, 10, 256. [Google Scholar] [CrossRef] [PubMed]
- Avrabos, C.; Sotnikov, S.V.; Dine, J.; Markt, P.O.; Holsboer, F.; Landgraf, R.; Eder, M. Real-time imaging of amygdalar network dynamics in vitro reveals a neurophysiological link to behavior in a mouse model of extremes in trait anxiety. J. Neurosci. 2013, 33, 16262–16267. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S.; Morrison, J.H. The Brain on Stress: Vulnerability and Plasticity of the Prefrontal Cortex over the Life Course. Neuron 2013, 79, 16–29. [Google Scholar] [CrossRef]
- Shilpa, B.M.; Bhagya, V.; Harish, G.; Bharath, M.M.S.; Rao, B.S.S. Environmental enrichment ameliorates chronic immobilisation stress-induced spatial learning deficits and restores the expression of BDNF, VEGF, GFAP and glucocorticoid receptors. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 76, 88–100. [Google Scholar] [CrossRef]
- Hegde, A.; Suresh, S.; Mitra, R. Early-life short-term environmental enrichment counteracts the effects of stress on anxiety-like behavior, brain-derived neurotrophic factor and nuclear translocation of glucocorticoid receptors in the basolateral amygdala. Sci. Rep. 2020, 10, 14053. [Google Scholar] [CrossRef] [PubMed]
- Zeraati, M.; Najdi, N.; Mosaferi, B.; Salari, A.-A. Environmental enrichment alters neurobehavioral development following maternal immune activation in mice offspring with epilepsy. Behav. Brain Res. 2021, 399, 112998. [Google Scholar] [CrossRef] [PubMed]
- Crofton, E.J.; Zhang, Y.; Green, T.A. Inoculation stress hypothesis of environmental enrichment. Neurosci. Biobehav. Rev. 2015, 49, 19–31. [Google Scholar] [CrossRef]
- Taylor, C.T.; Lyubomirsky, S.; Stein, M.B. Upregulating the positive affect system in anxiety and depression: Outcomes of a positive activity intervention. Depress. Anxiety 2017, 34, 267–280. [Google Scholar] [CrossRef]
- Schmidtner, A.K.; Slattery, D.A.; Gläsner, J.; Hiergeist, A.; Gryksa, K.; Malik, V.A.; Hellmann-Regen, J.; Heuser, I.; Baghai, T.C.; Gessner, A.; et al. Minocycline alters behavior, microglia and the gut microbiome in a trait-anxiety-dependent manner. Transl. Psychiatry 2019, 9, 223. [Google Scholar] [CrossRef]
- Chabry, J.; Nicolas, S.; Cazareth, J.; Murris, E.; Guyon, A.; Glaichenhaus, N.; Heurteaux, C.; Petit-Paitel, A. Enriched environment decreases microglia and brain macrophages inflammatory phenotypes through adiponectin-dependent mechanisms: Relevance to depressive-like behavior. Brain Behav. Immun. 2015, 50, 275–287. [Google Scholar] [CrossRef]
- Ehninger, D.; Wang, L.P.; Klempin, F.; Romer, B.; Kettenmann, H.; Kempermann, G. Enriched environment and physical activity reduce microglia and influence the fate of NG2 cells in the amygdala of adult mice. Cell Tissue Res. 2011, 345, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Pusic, K.M.; Pusic, A.D.; Kemme, J.; Kraig, R.P. Spreading depression requires microglia and is decreased by their M2a polarization from environmental enrichment. Glia 2014, 62, 1176–1194. [Google Scholar] [CrossRef] [PubMed]
- Piazza, F.V.; Segabinazi, E.; Centenaro, L.A.; Nascimento, P.D.; Achaval, M.; Marcuzzo, S. Enriched environment induces beneficial effects on memory deficits and microglial activation in the hippocampus of type 1 diabetic rats. Metab. Brain Dis. 2014, 29, 93–104. [Google Scholar] [CrossRef]
- Ziv, Y.; Ron, N.; Butovsky, O.; Landa, G.; Sudai, E.; Greenberg, N.; Cohen, H.; Kipnis, J.; Schwartz, M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat. Neurosci. 2006, 9, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Williamson, L.L.; Chao, A.; Bilbo, S.D. Environmental enrichment alters glial antigen expression and neuroimmune function in the adult rat hippocampus. Brain Behav. Immun. 2012, 26, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Alboni, S.; Poggini, S.; Garofalo, S.; Milior, G.; El Hajj, H.; Lecours, C.; Girard, I.; Gagnon, S.; Boisjoly-Villeneuve, S.; Brunello, N.; et al. Fluoxetine treatment affects the inflammatory response and microglial function according to the quality of the living environment. Brain Behav. Immun. 2016, 58, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Ruitenberg, M.J.; Wells, J.; Bartlett, P.F.; Harvey, A.R.; Vukovic, J. Enrichment increases hippocampal neurogenesis independent of blood monocyte-derived microglia presence following high-dose total body irradiation. Brain Res. Bull. 2017, 132, 150–159. [Google Scholar] [CrossRef]
- Wang, Y.L.; Han, Q.Q.; Gong, W.Q.; Pan, D.H.; Wang, L.Z.; Hu, W.; Yang, M.; Li, B.; Yu, J.; Liu, Q. Microglial activation mediates chronic mild stress-induced depressive- and anxiety-like behavior in adult rats. J. Neuroinflamm. 2018, 15, 21. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, J.; Zhou, Q.; Wang, J.; Liu, C.; Tian, Y.; Huang, D.; Ye, H.; Jin, Y. Exposure to a real traffic environment impairs brain cognition in aged mice. Environ. Res. 2022, 215 Pt 1, 114181. [Google Scholar] [CrossRef]
- Almeida-Suhett, C.P.; Graham, A.; Chen, Y.; Deuster, P. Behavioral changes in male mice fed a high-fat diet are associated with IL-1beta expression in specific brain regions. Physiol. Behav. 2017, 169, 130–140. [Google Scholar] [CrossRef]
- Jeong, M.-Y.; Jang, H.-M.; Kim, D.-H. High-fat diet causes psychiatric disorders in mice by increasing Proteobacteria population. Neurosci. Lett. 2019, 698, 51–57. [Google Scholar] [CrossRef]
- Patel, S.S.; Ray, R.S.; Sharma, A.; Mehta, V.; Katyal, A.; Udayabanu, M. Antidepressant and anxiolytic like effects of Urtica dioica leaves in streptozotocin induced diabetic mice. Metab. Brain Dis. 2018, 33, 1281–1292. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, T.C.G.; Carvalho-Paulo, D.; de Lima, C.M.; de Oliveira, R.B.; Santos Filho, C.; Diniz, D.G.; Bento Torres Neto, J.; Picanco-Diniz, C.W. Long-term environmental enrichment reduces microglia morphological diversity of the molecular layer of dentate gyrus. Eur. J. Neurosci. 2020, 52, 4081–4099. [Google Scholar] [CrossRef] [PubMed]
- Reber, S.O.; Siebler, P.H.; Donner, N.C.; Morton, J.T.; Smith, D.G.; Kopelman, J.M.; Lowe, K.R.; Wheeler, K.J.; Fox, J.H.; Hassell, J.E., Jr.; et al. Immunization with a heat-killed preparation of the environmental bacterium Mycobacterium vaccae promotes stress resilience in mice. Proc. Natl. Acad. Sci. USA 2016, 113, E3130–E3139. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, C.L.; Martinez-Cerdeno, V.; Noctor, S.C. Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J. Neurosci. 2013, 33, 4216–4233. [Google Scholar] [CrossRef] [PubMed]
- Llorens-Martin, M.; Jurado-Arjona, J.; Bolos, M.; Pallas-Bazarra, N.; Avila, J. Forced swimming sabotages the morphological and synaptic maturation of newborn granule neurons and triggers a unique pro-inflammatory milieu in the hippocampus. Brain Behav. Immun. 2016, 53, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Martinez, S.; Palla, N.; Zhang, X.; Lipman, E.; Sisodia, S.S. Deficits in Enrichment-Dependent Neurogenesis and Enhanced Anxiety Behaviors Mediated by Expression of Alzheimer’s Disease-Linked Ps1 Variants Are Rescued by Microglial Depletion. J. Neurosci. 2019, 39, 6766–6780. [Google Scholar] [CrossRef]
- Sah, A.; Schmuckermair, C.; Sartori, S.B.; Gaburro, S.; Kandasamy, M.; Irschick, R.B.; Klimaschewski, L.; Landgraf, R.; Aigner, L.; Singewald, N. Anxiety- rather than depression-like behavior is associated with adult neurogenesis in a female mouse model of higher trait anxiety- and comorbid depression-like behavior. Transl. Psychiatry 2012, 2, e171. [Google Scholar] [CrossRef]
- Kreisel, T.; Frank, M.G.; Licht, T.; Reshef, R.; Ben-Menachem-Zidon, O.; Baratta, M.V.; Maier, S.F.; Yirmiya, R. Dynamic microglial alterations underlie stress-induced depressive-like behavior and suppressed neurogenesis. Mol. Psychiatry 2014, 19, 699–709. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, W.Y.; Jeon, Y.J.; Lee, S.B.; Lee, D.S.; Son, C.G. Antidepressant-Like Activity of Myelophil via Attenuation of Microglial-Mediated Neuroinflammation in Mice Undergoing Unpredictable Chronic Mild Stress. Front. Pharmacol. 2019, 10, 683. [Google Scholar]
- Klein, B.; Mrowetz, H.; Thalhamer, J.; Scheiblhofer, S.; Weiss, R.; Aigner, L. Allergy Enhances Neurogenesis and Modulates Microglial Activation in the Hippocampus. Front. Cell. Neurosci. 2016, 10, 169. [Google Scholar] [CrossRef] [PubMed]
- Kozareva, D.A.; Hueston, C.M.; Ó’Léime, C.S.; Crotty, S.; Dockery, P.; Cryan, J.F.; Nolan, Y.M. Absence of the neurogenesis-dependent nuclear receptor TLX induces inflammation in the hippocampus. J. Neuroimmunol. 2019, 331, 87–96. [Google Scholar] [CrossRef]
- Song, L.; Lee, C.; Schindler, C. Deletion of the murine scavenger receptor CD68. J. Lipid Res. 2011, 52, 1542–1550. [Google Scholar] [CrossRef]
- Augusto-Oliveira, M.; Verkhratsky, A. Lifestyle-dependent microglial plasticity: Training the brain guardians. Biol. Direct. 2021, 16, 12. [Google Scholar] [CrossRef] [PubMed]
- Abuelezz, S.A.; Hendawy, N.; Magdy, Y. Targeting Oxidative Stress, Cytokines and Serotonin Interactions Via Indoleamine 2, 3 Dioxygenase by Coenzyme Q10: Role in Suppressing Depressive Like Behavior in Rats. J. Neuroimmune Pharmacol. 2017, 12, 277–291. [Google Scholar] [CrossRef]
- Birch, A.M.; Kelly, A.M. Lifelong environmental enrichment in the absence of exercise protects the brain from age-related cognitive decline. Neuropharmacology 2019, 145 Pt A, 59–74. [Google Scholar] [CrossRef]
- Sartori, S.B.; Whittle, N.; Hetzenauer, A.; Singewald, N. Magnesium deficiency induces anxiety and HPA axis dysregulation: Modulation by therapeutic drug treatment. Neuropharmacology 2012, 62, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Franklin, K.; Paxinos, G. The Mouse Brain in Stereotaxic Coordinates, Compact, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2008; ISBN 9780123742445. [Google Scholar]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sah, A.; Rooney, S.; Kharitonova, M.; Sartori, S.B.; Wolf, S.A.; Singewald, N. Enriched Environment Attenuates Enhanced Trait Anxiety in Association with Normalization of Aberrant Neuro-Inflammatory Events. Int. J. Mol. Sci. 2022, 23, 13052. https://doi.org/10.3390/ijms232113052
Sah A, Rooney S, Kharitonova M, Sartori SB, Wolf SA, Singewald N. Enriched Environment Attenuates Enhanced Trait Anxiety in Association with Normalization of Aberrant Neuro-Inflammatory Events. International Journal of Molecular Sciences. 2022; 23(21):13052. https://doi.org/10.3390/ijms232113052
Chicago/Turabian StyleSah, Anupam, Sinead Rooney, Maria Kharitonova, Simone B. Sartori, Susanne A. Wolf, and Nicolas Singewald. 2022. "Enriched Environment Attenuates Enhanced Trait Anxiety in Association with Normalization of Aberrant Neuro-Inflammatory Events" International Journal of Molecular Sciences 23, no. 21: 13052. https://doi.org/10.3390/ijms232113052
APA StyleSah, A., Rooney, S., Kharitonova, M., Sartori, S. B., Wolf, S. A., & Singewald, N. (2022). Enriched Environment Attenuates Enhanced Trait Anxiety in Association with Normalization of Aberrant Neuro-Inflammatory Events. International Journal of Molecular Sciences, 23(21), 13052. https://doi.org/10.3390/ijms232113052





