Depleted Calcium Stores and Increased Calcium Entry in Rod Photoreceptors of the Cacna2d4 Mouse Model of Cone-Rod Dystrophy RCD4
Abstract
1. Introduction
2. Results
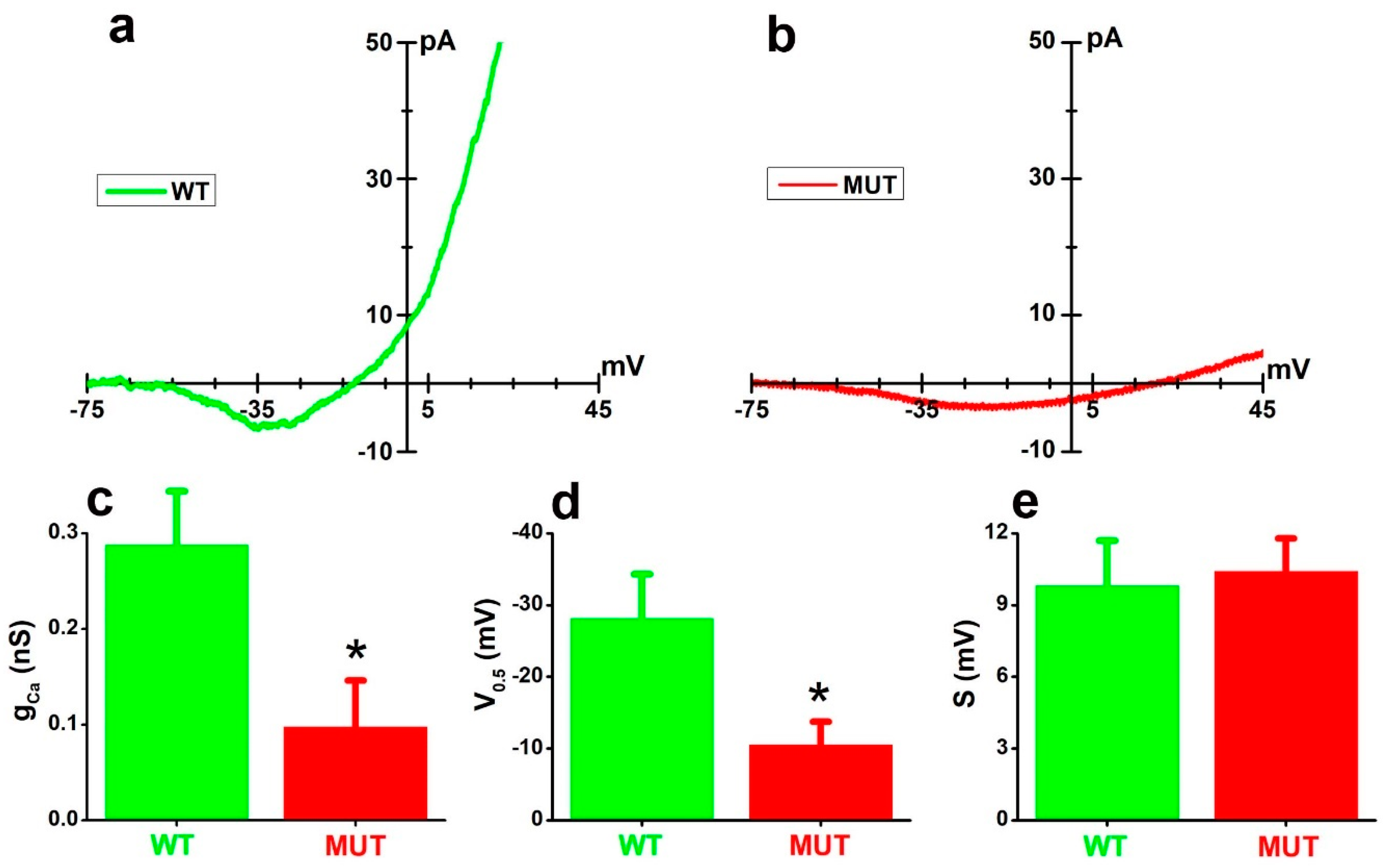
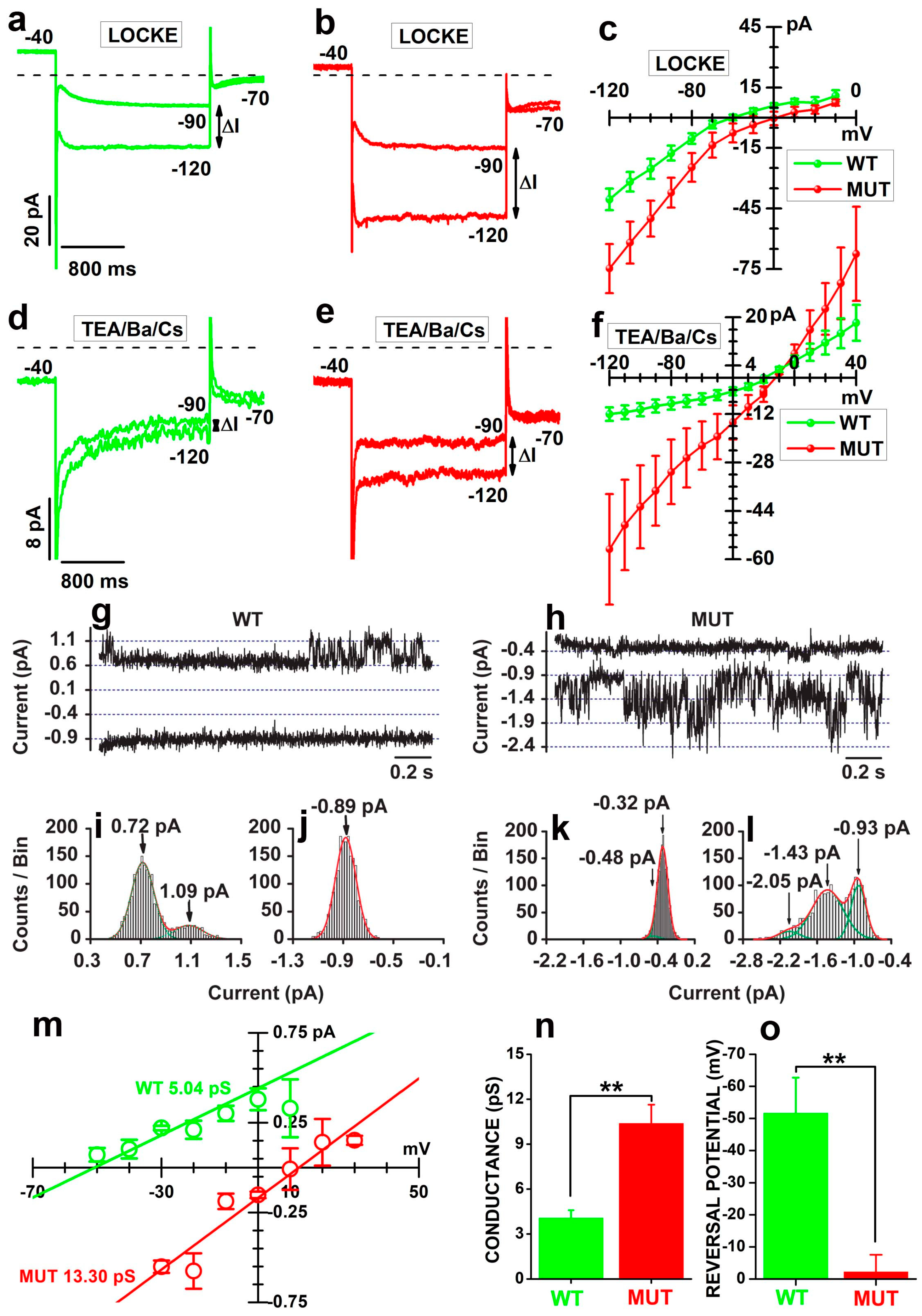
2.1. The Cacna2d4 Mutation Reduces Inward Currents through the VGCC of Mouse Rod Photoreceptors
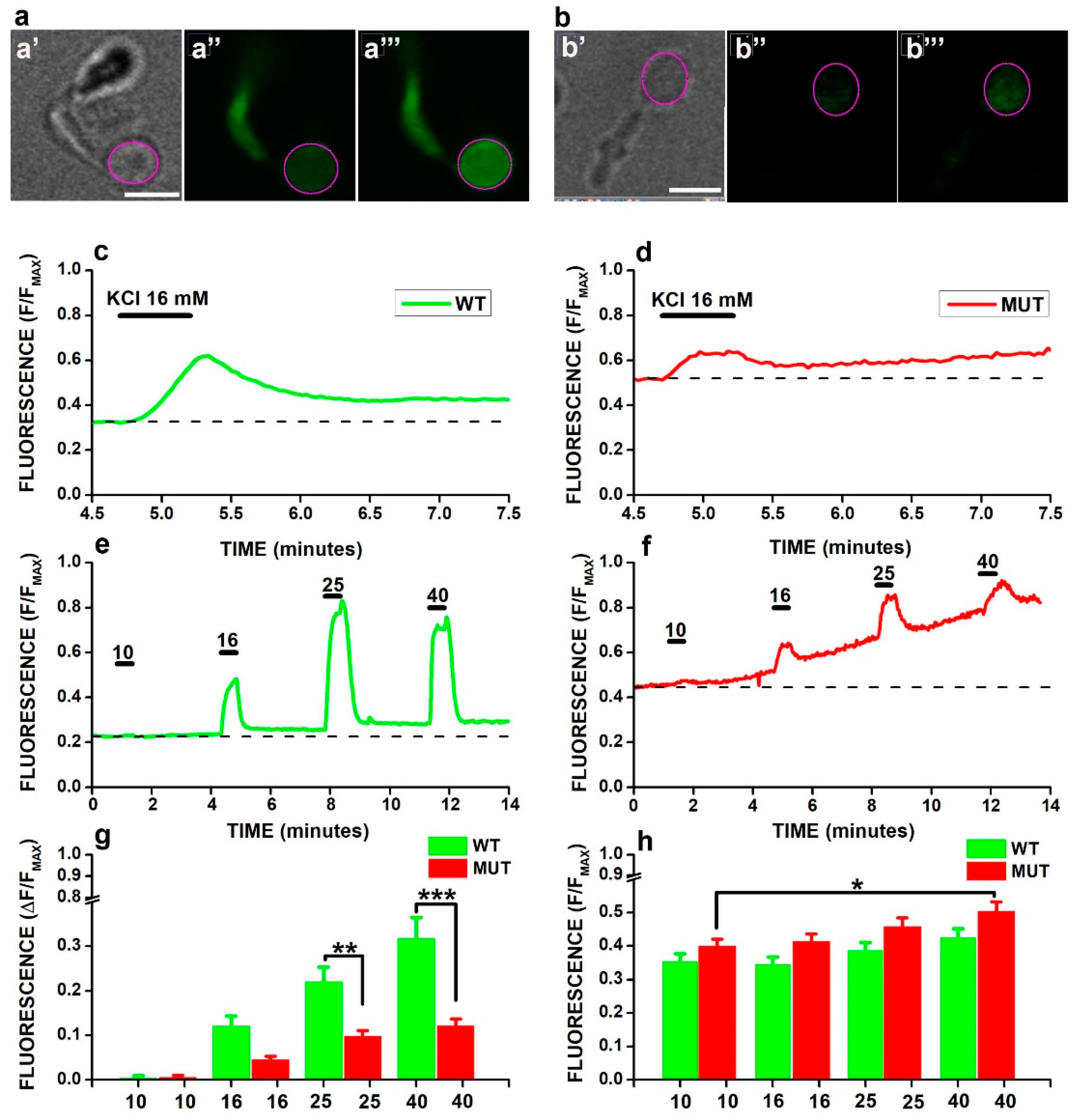
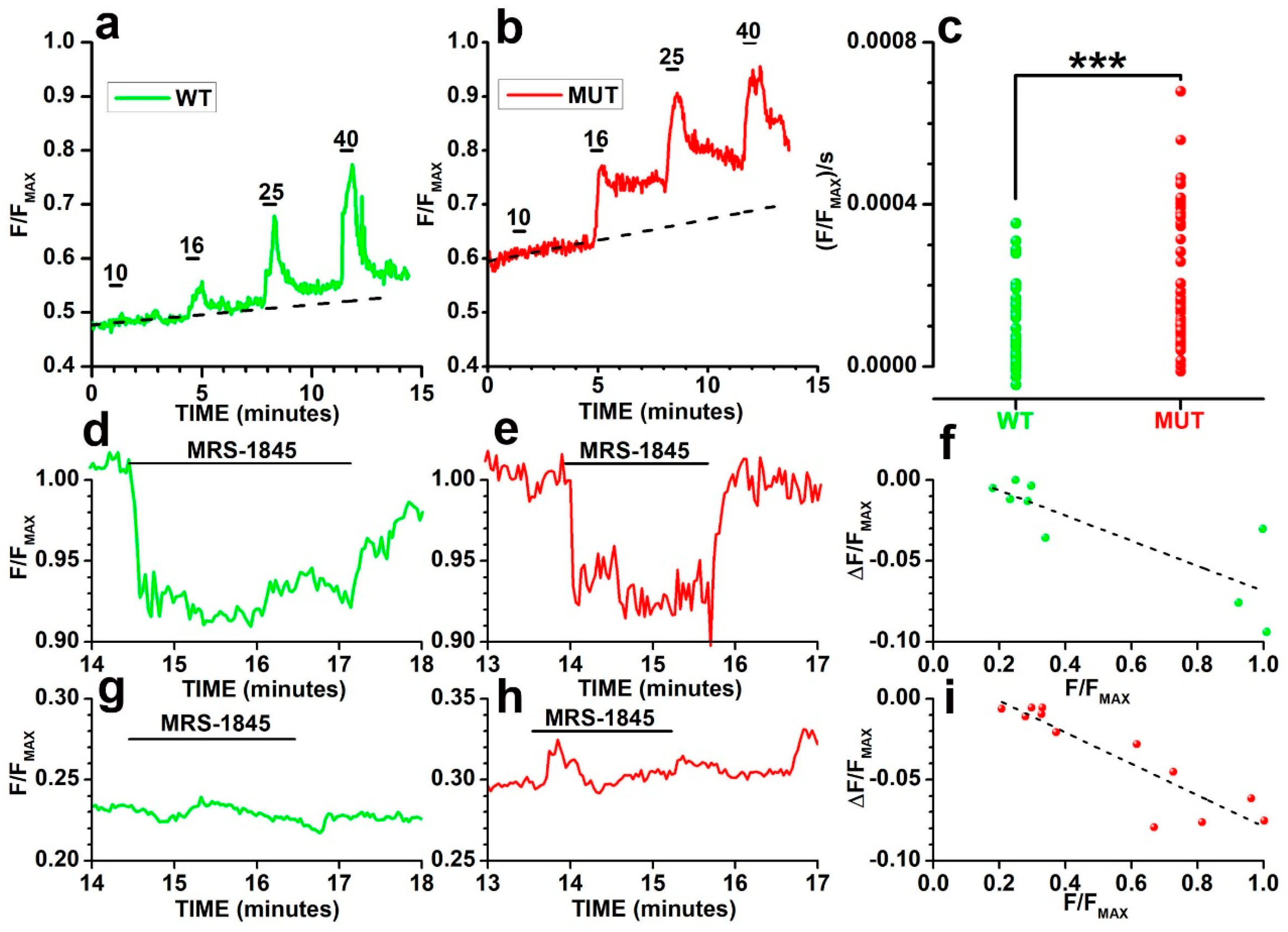
2.2. Analysis of Cacna2d4 Mutation on Calcium Homeostasis in Non-Synaptic Cell Compartments by Calcium Imaging
2.3. Multiple Mechanisms Contribute to F/FMAX Buildup in WT and MUT Rods
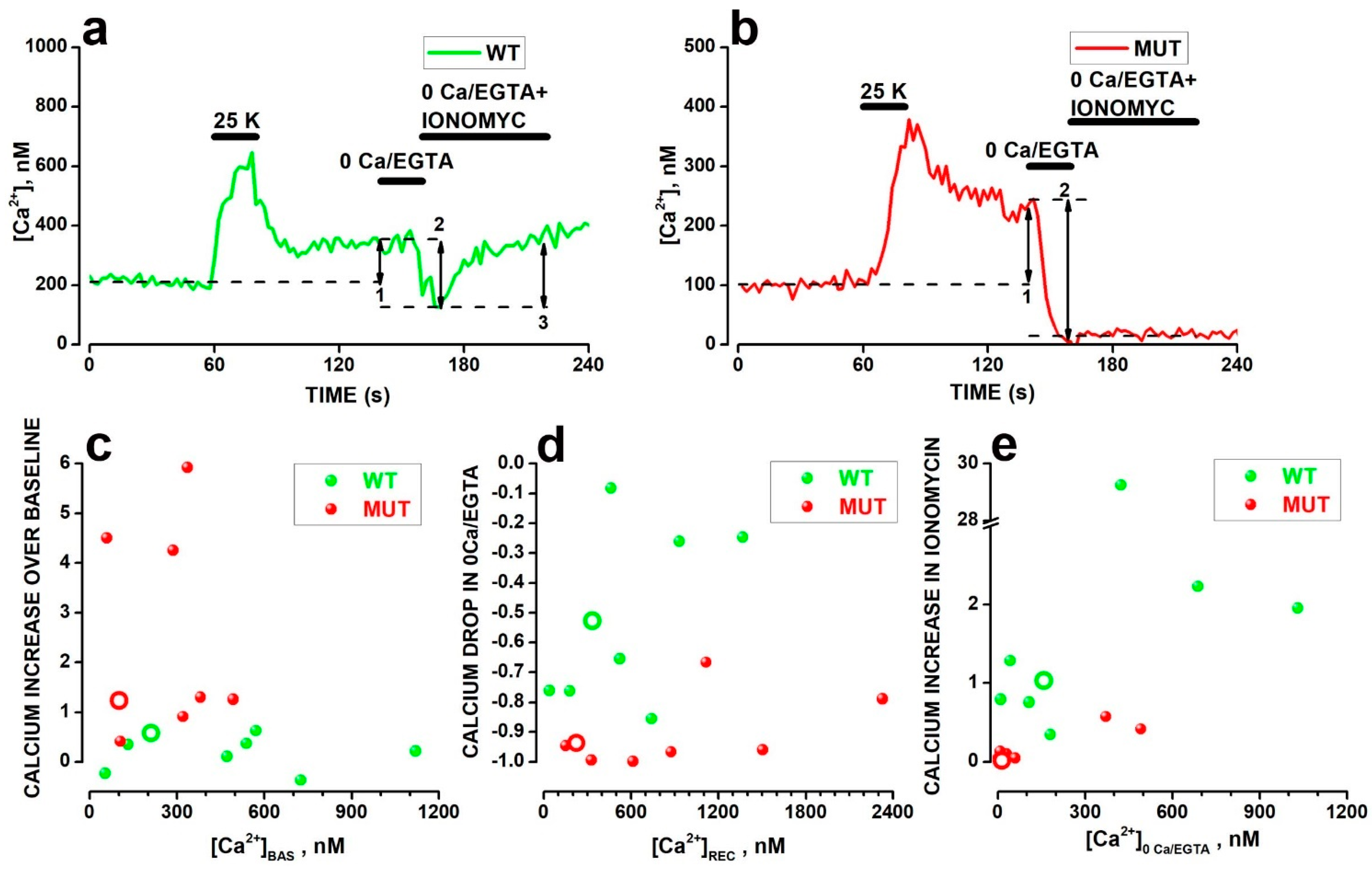
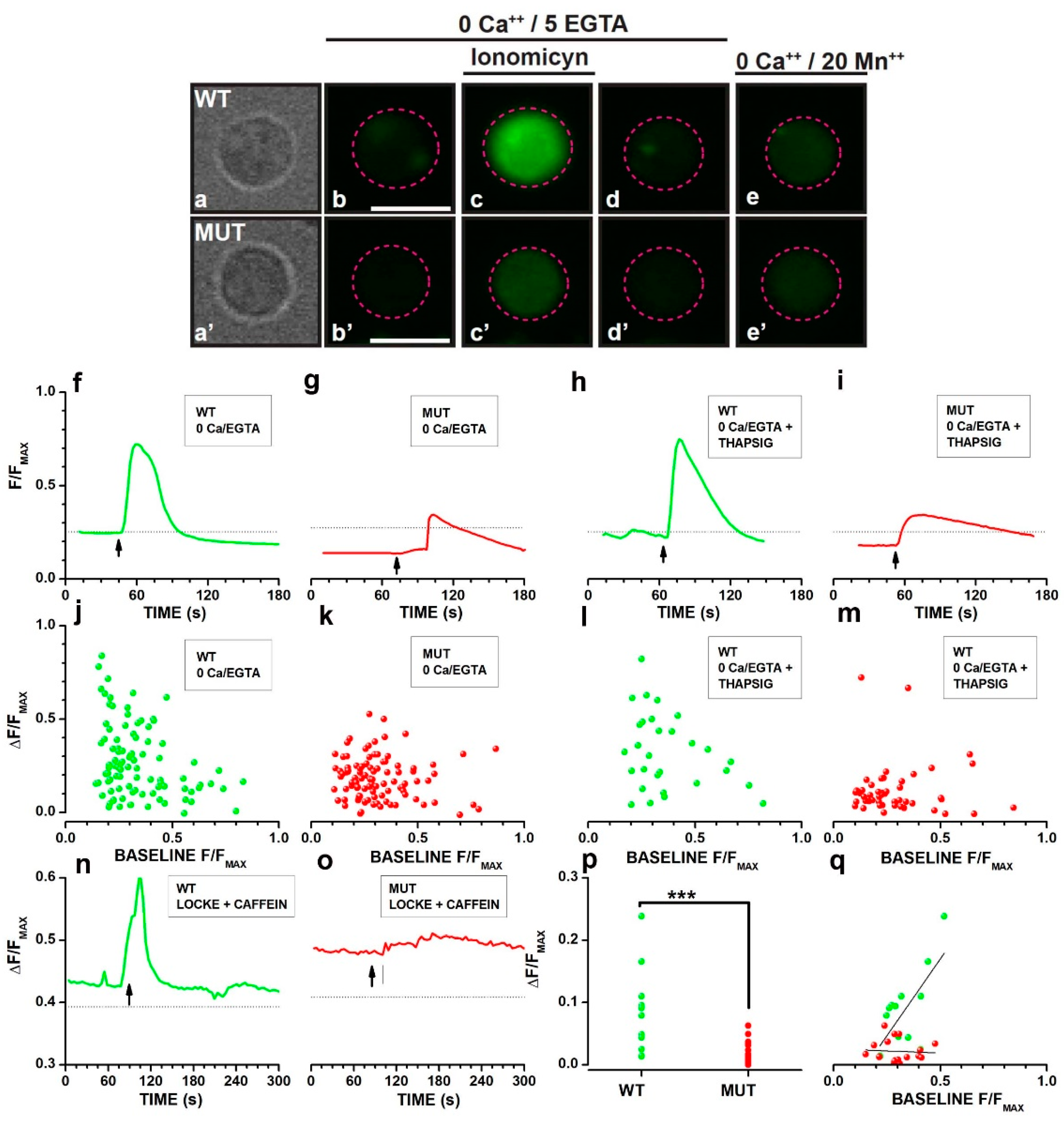
2.4. Analysis of Cacna2d4 Impact on Calcium Stores
2.5. Shaping of the Electrophysiological Profile by Cation-Selective Channels in Mutant Rods
2.6. The Ca2+ Buildup in Response to VGCC Activation Correlates with the Increased CSC Activation
3. Discussion
3.1. Effects of the Mutation on VGCC
3.2. Effects of the Mutation on the Electrophysiological Profile of Rods
3.3. Effects of the Mutation on Ca2+ Handling
3.4. Functional Relevance of [Ca2+] Homeostasis at the Cell Body Compartment and the Link between Cacna2d4 Mutation and Rod-Cone Dystrophy
4. Materials and Methods
4.1. Origin of the Animals
4.2. Access to Food and Water
4.3. Euthanasia
4.4. Patch-Clamp Recordings
4.5. Ca2+ Imaging
4.6. Data Analysis Perforated-Patch Recordings
4.7. Ca2+ Imaging Analysis
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andreazzoli, M.; Barravecchia, I.; De Cesari, C.; Angeloni, D.; Demontis, G.C. Inducible Pluripotent Stem Cells to Model and Treat Inherited Degenerative Diseases of the Outer Retina: 3D-Organoids Limitations and Bioengineering Solutions. Cells 2021, 10, 2489. [Google Scholar] [CrossRef] [PubMed]
- Pierrache, L.H.M.; Ghafaryasl, B.; Khan, M.I.; Yzer, S.; van Genderen, M.M.; Schuil, J.; Boonstra, F.N.; Pott, J.W.R.; de Faber, J.; Tjon-Fo-Sang, M.J.H.; et al. Longitudinal Study of Rpe65-Associated Inherited Retinal Degenerations. Retina 2020, 40, 1812–1828. [Google Scholar] [CrossRef] [PubMed]
- Iuliano, L.; Fogliato, G.; Corbelli, E.; Bandello, F.; Codenotti, M. Blind patients in end-stage inherited retinal degeneration: Multimodal imaging of candidates for artificial retinal prosthesis. Eye 2021, 35, 289–298. [Google Scholar] [CrossRef]
- Khramtsov, N.V.; Feshchenko, E.A.; Suslova, V.A.; Shmukler, B.E.; Terpugov, B.E.; Rakitina, T.V.; Atabekova, N.V.; Lipkin, V.M. The human rod photoreceptor cGMP phosphodiesterase beta-subunit. Structural studies of its cDNA and gene. FEBS Lett. 1993, 327, 275–278. [Google Scholar] [CrossRef]
- Farber, D.B.; Lolley, R.N. Cyclic guanosine monophosphate: Elevation in degenerating photoreceptor cells of the C3H mouse retina. Science 1974, 186, 449–451. [Google Scholar] [CrossRef] [PubMed]
- Farber, D.B.; Lolley, R.N. Enzymic basis for cyclic GMP accumulation in degenerative photoreceptor cells of mouse retina. J Cycl. Nucleotide Res. 1976, 2, 139–148. [Google Scholar]
- Farber, D.B.; Lolley, R.N. Calcium and magnesium content of rodent photoreceptor cells as inferred from studies of retinal degeneration. Exp. Eye Res. 1976, 22, 219–228. [Google Scholar] [CrossRef]
- Capovilla, M.; Cervetto, L.; Torre, V. Antagonism between steady light and phosphodiesterase inhibitors on the kinetics of rod photoresponses. Proc. Natl. Acad. Sci. USA 1982, 79, 6698–6702. [Google Scholar] [CrossRef]
- Vallazza-Deschamps, G.; Cia, D.; Gong, J.; Jellali, A.; Duboc, A.; Forster, V.; Sahel, J.A.; Tessier, L.H.; Picaud, S. Excessive activation of cyclic nucleotide-gated channels contributes to neuronal degeneration of photoreceptors. Eur. J. Neurosci. 2005, 22, 1013–1022. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, J.A.; Choi, J.S.; Joo, C.K. Activation of caspase-3 during degeneration of the outer nuclear layer in the rd mouse retina. Ophthalmic Res. 2002, 34, 150–157. [Google Scholar] [CrossRef]
- Zeiss, C.J.; Neal, J.; Johnson, E.A. Caspase-3 in postnatal retinal development and degeneration. Investig. Ophthalmol. Vis. Sci. 2004, 45, 964–970. [Google Scholar] [CrossRef] [PubMed]
- Lohr, H.R.; Kuntchithapautham, K.; Sharma, A.K.; Rohrer, B. Multiple, parallel cellular suicide mechanisms participate in photoreceptor cell death. Exp. Eye Res. 2006, 83, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Doonan, F.; Donovan, M.; Cotter, T.G. Caspase-independent photoreceptor apoptosis in mouse models of retinal degeneration. J. Neurosci. 2003, 23, 5723–5731. [Google Scholar] [CrossRef]
- Sanges, D.; Comitato, A.; Tammaro, R.; Marigo, V. Apoptosis in retinal degeneration involves cross-talk between apoptosis-inducing factor (AIF) and caspase-12 and is blocked by calpain inhibitors. Proc. Natl. Acad. Sci. USA 2006, 103, 17366–17371. [Google Scholar] [CrossRef] [PubMed]
- Frasson, M.; Sahel, J.A.; Fabre, M.; Simonutti, M.; Dreyfus, H.; Picaud, S. Retinitis pigmentosa: Rod photoreceptor rescue by a calcium-channel blocker in the rd mouse. Nat. Med. 1999, 5, 1183–1187. [Google Scholar] [CrossRef] [PubMed]
- Pearce-Kelling, S.E.; Aleman, T.S.; Nickle, A.; Laties, A.M.; Aguirre, G.D.; Jacobson, S.G.; Acland, G.M. Calcium channel blocker D-cis-diltiazem does not slow retinal degeneration in the PDE6B mutant rcd1 canine model of retinitis pigmentosa. Mol. Vis. 2001, 7, 42–47. [Google Scholar]
- Pawlyk, B.S.; Li, T.; Scimeca, M.S.; Sandberg, M.A.; Berson, E.L. Absence of photoreceptor rescue with D-cis-diltiazem in the rd mouse. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1912–1915. [Google Scholar]
- Das, S.; Popp, V.; Power, M.; Groeneveld, K.; Yan, J.; Melle, C.; Rogerson, L.; Achury, M.; Schwede, F.; Strasser, T.; et al. Redefining the role of Ca(2+)-permeable channels in photoreceptor degeneration using diltiazem. Cell Death Dis. 2022, 13, 47. [Google Scholar] [CrossRef]
- Paquet-Durand, F.; Beck, S.; Michalakis, S.; Goldmann, T.; Huber, G.; Muhlfriedel, R.; Trifunovic, D.; Fischer, M.D.; Fahl, E.; Duetsch, G.; et al. A key role for cyclic nucleotide gated (CNG) channels in cGMP-related retinitis pigmentosa. Hum. Mol. Genet. 2011, 20, 941–947. [Google Scholar] [CrossRef]
- Paquet-Durand, F.; Hauck, S.M.; van Veen, T.; Ueffing, M.; Ekstrom, P. PKG activity causes photoreceptor cell death in two retinitis pigmentosa models. J. Neurochem. 2009, 108, 796–810. [Google Scholar] [CrossRef]
- Roy, A.; Tolone, A.; Hilhorst, R.; Groten, J.; Tomar, T.; Paquet-Durand, F. Kinase activity profiling identifies putative downstream targets of cGMP/PKG signaling in inherited retinal neurodegeneration. Cell Death Discov. 2022, 8, 93. [Google Scholar] [CrossRef]
- Power, M.; Das, S.; Schutze, K.; Marigo, V.; Ekstrom, P.; Paquet-Durand, F. Cellular mechanisms of hereditary photoreceptor degeneration-Focus on cGMP. Prog. Retin. Eye Res. 2020, 74, 100772. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Chen, Y.; Yan, J.; Christensen, G.; Belhadj, S.; Tolone, A.; Paquet-Durand, F. The role of cGMP-signalling and calcium-signalling in photoreceptor cell death: Perspectives for therapy development. Pflug. Arch. 2021, 473, 1411–1421. [Google Scholar] [CrossRef] [PubMed]
- Schon, C.; Paquet-Durand, F.; Michalakis, S. Cav1.4 L-Type Calcium Channels Contribute to Calpain Activation in Degenerating Photoreceptors of rd1 Mice. PLoS ONE 2016, 11, e0156974. [Google Scholar] [CrossRef]
- Mansergh, F.; Orton, N.C.; Vessey, J.P.; Lalonde, M.R.; Stell, W.K.; Tremblay, F.; Barnes, S.; Rancourt, D.E.; Bech-Hansen, N.T. Mutation of the calcium channel gene Cacna1f disrupts calcium signaling, synaptic transmission and cellular organization in mouse retina. Hum. Mol. Genet. 2005, 14, 3035–3046. [Google Scholar] [CrossRef]
- Baumann, L.; Gerstner, A.; Zong, X.; Biel, M.; Wahl-Schott, C. Functional characterization of the L-type Ca2+ channel Cav1.4alpha1 from mouse retina. Investig. Ophthalmol. Vis. Sci. 2004, 45, 708–713. [Google Scholar] [CrossRef]
- Maddox, J.W.; Randall, K.L.; Yadav, R.P.; Williams, B.; Hagen, J.; Derr, P.J.; Kerov, V.; Della Santina, L.; Baker, S.A.; Artemyev, N.; et al. A dual role for Cav1.4 Ca(2+) channels in the molecular and structural organization of the rod photoreceptor synapse. Elife 2020, 9, e62184. [Google Scholar] [CrossRef]
- Strom, T.M.; Nyakatura, G.; Apfelstedt-Sylla, E.; Hellebrand, H.; Lorenz, B.; Weber, B.H.; Wutz, K.; Gutwillinger, N.; Ruther, K.; Drescher, B.; et al. An L-type calcium-channel gene mutated in incomplete X-linked congenital stationary night blindness. Nat. Genet. 1998, 19, 260–263. [Google Scholar] [CrossRef]
- Bech-Hansen, N.T.; Naylor, M.J.; Maybaum, T.A.; Pearce, W.G.; Koop, B.; Fishman, G.A.; Mets, M.; Musarella, M.A.; Boycott, K.M. Loss-of-function mutations in a calcium-channel alpha1-subunit gene in Xp11.23 cause incomplete X-linked congenital stationary night blindness. Nat. Genet. 1998, 19, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Simms, B.A.; Zamponi, G.W. Neuronal voltage-gated calcium channels: Structure, function, and dysfunction. Neuron 2014, 82, 24–45. [Google Scholar] [CrossRef]
- Zamponi, G.W.; Striessnig, J.; Koschak, A.; Dolphin, A.C. The Physiology, Pathology, and Pharmacology of Voltage-Gated Calcium Channels and Their Future Therapeutic Potential. Pharm. Rev. 2015, 67, 821–870. [Google Scholar] [CrossRef] [PubMed]
- Dolphin, A.C. Calcium channel auxiliary alpha2delta and beta subunits: Trafficking and one step beyond. Nat. Rev. Neurosci. 2012, 13, 542–555. [Google Scholar] [CrossRef] [PubMed]
- Wycisk, K.A.; Zeitz, C.; Feil, S.; Wittmer, M.; Forster, U.; Neidhardt, J.; Wissinger, B.; Zrenner, E.; Wilke, R.; Kohl, S.; et al. Mutation in the auxiliary calcium-channel subunit CACNA2D4 causes autosomal recessive cone dystrophy. Am. J. Hum. Genet. 2006, 79, 973–977. [Google Scholar] [CrossRef]
- Wycisk, K.A.; Budde, B.; Feil, S.; Skosyrski, S.; Buzzi, F.; Neidhardt, J.; Glaus, E.; Nurnberg, P.; Ruether, K.; Berger, W. Structural and functional abnormalities of retinal ribbon synapses due to Cacna2d4 mutation. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3523–3530. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fehlhaber, K.E.; Sarria, I.; Cao, Y.; Ingram, N.T.; Guerrero-Given, D.; Throesch, B.; Baldwin, K.; Kamasawa, N.; Ohtsuka, T.; et al. The Auxiliary Calcium Channel Subunit alpha2delta4 Is Required for Axonal Elaboration, Synaptic Transmission, and Wiring of Rod Photoreceptors. Neuron 2017, 93, 1359–1374.e6. [Google Scholar] [CrossRef]
- Kerov, V.; Laird, J.G.; Joiner, M.L.; Knecht, S.; Soh, D.; Hagen, J.; Gardner, S.H.; Gutierrez, W.; Yoshimatsu, T.; Bhattarai, S.; et al. alpha2delta-4 Is Required for the Molecular and Structural Organization of Rod and Cone Photoreceptor Synapses. J. Neurosci. 2018, 38, 6145–6160. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.F.; Huang, F.; Wu, K.C.; Wu, J.; Chen, J.; Pang, C.P.; Lu, F.; Qu, J.; Jin, Z.B. Genotype-phenotype correlation and mutation spectrum in a large cohort of patients with inherited retinal dystrophy revealed by next-generation sequencing. Genet. Med. 2015, 17, 271–278. [Google Scholar] [CrossRef]
- Ba-Abbad, R.; Arno, G.; Carss, K.; Stirrups, K.; Penkett, C.J.; Moore, A.T.; Michaelides, M.; Raymond, F.L.; Webster, A.R.; Holder, G.E. Mutations in CACNA2D4 Cause Distinctive Retinal Dysfunction in Humans. Ophthalmology 2016, 123, 668–671.e2. [Google Scholar] [CrossRef]
- Kurshan, P.T.; Oztan, A.; Schwarz, T.L. Presynaptic alpha2delta-3 is required for synaptic morphogenesis independent of its Ca2+-channel functions. Nat. Neurosci. 2009, 12, 1415–1423. [Google Scholar] [CrossRef]
- Hoppa, M.B.; Lana, B.; Margas, W.; Dolphin, A.C.; Ryan, T.A. alpha2delta expression sets presynaptic calcium channel abundance and release probability. Nature 2012, 486, 122–125. [Google Scholar] [CrossRef]
- D’Arco, M.; Margas, W.; Cassidy, J.S.; Dolphin, A.C. The Upregulation of alpha2delta-1 Subunit Modulates Activity-Dependent Ca2+ Signals in Sensory Neurons. J. Neurosci. 2015, 35, 5891–5903. [Google Scholar] [CrossRef] [PubMed]
- Dolphin, A.C. Voltage-gated calcium channel alpha 2delta subunits: An assessment of proposed novel roles. F1000Research 2018, 7, 1830. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Gala, U.; Zhang, Y.; Shang, W.; Nagarkar Jaiswal, S.; di Ronza, A.; Jaiswal, M.; Yamamoto, S.; Sandoval, H.; Duraine, L.; et al. A voltage-gated calcium channel regulates lysosomal fusion with endosomes and autophagosomes and is required for neuronal homeostasis. PLoS Biol. 2015, 13, e1002103. [Google Scholar] [CrossRef] [PubMed]
- Caputo, A.; Piano, I.; Demontis, G.C.; Bacchi, N.; Casarosa, S.; Della Santina, L.; Gargini, C. TMEM16A is associated with Voltage-gated Calcium Channels in mouse retina and its function is disrupted upon mutation of the auxiliary α2δ4 subunit. Front. Cell. Neurosci. 2015, 9, 422. [Google Scholar] [CrossRef]
- Gee, K.R.; Brown, K.A.; Chen, W.N.; Bishop-Stewart, J.; Gray, D.; Johnson, I. Chemical and physiological characterization of fluo-4 Ca(2+)-indicator dyes. Cell Calcium 2000, 27, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Molnar, T.; Barabas, P.; Birnbaumer, L.; Punzo, C.; Kefalov, V.; Krizaj, D. Store-operated channels regulate intracellular calcium in mammalian rods. J. Physiol. 2012, 590, 3465–3481. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, M.L.; Sampath, A.P.; Matthews, H.R.; Krasnoperova, N.V.; Lem, J.; Fain, G.L. Measurement of cytoplasmic calcium concentration in the rods of wild-type and transducin knock-out mice. J. Physiol. 2002, 542, 843–854. [Google Scholar] [CrossRef]
- Szikra, T.; Cusato, K.; Thoreson, W.B.; Barabas, P.; Bartoletti, T.M.; Krizaj, D. Depletion of calcium stores regulates calcium influx and signal transmission in rod photoreceptors. J. Physiol. 2008, 586, 4859–4875. [Google Scholar] [CrossRef]
- Kao, J.P.; Harootunian, A.T.; Tsien, R.Y. Photochemically generated cytosolic calcium pulses and their detection by fluo-3. J. Biol. Chem. 1989, 264, 8179–8184. [Google Scholar] [CrossRef]
- Nadal, A.; Fuentes, E.; McNaughton, P.A. Albumin stimulates uptake of calcium into subcellular stores in rat cortical astrocytes. J. Physiol. 1996, 492 Pt 3, 737–750. [Google Scholar] [CrossRef]
- Krizaj, D.; Lai, F.A.; Copenhagen, D.R. Ryanodine stores and calcium regulation in the inner segments of salamander rods and cones. J. Physiol. 2003, 547, 761–774. [Google Scholar] [CrossRef] [PubMed]
- Lytton, J.; Westlin, M.; Hanley, M.R. Thapsigargin inhibits the sarcoplasmic or endoplasmic reticulum Ca-ATPase family of calcium pumps. J. Biol. Chem. 1991, 266, 17067–17071. [Google Scholar] [CrossRef]
- Pozzan, T.; Rizzuto, R.; Volpe, P.; Meldolesi, J. Molecular and cellular physiology of intracellular calcium stores. Physiol. Rev. 1994, 74, 595–636. [Google Scholar] [CrossRef] [PubMed]
- Krizaj, D.; Bao, J.X.; Schmitz, Y.; Witkovsky, P.; Copenhagen, D.R. Caffeine-sensitive calcium stores regulate synaptic transmission from retinal rod photoreceptors. J. Neurosci. 1999, 19, 7249–7261. [Google Scholar] [CrossRef] [PubMed]
- Beech, D.J.; Barnes, S. Characterization of a voltage-gated K+ channel that accelerates the rod response to dim light. Neuron 1989, 3, 573–581. [Google Scholar] [CrossRef]
- Harper, J.L.; Camerini-Otero, C.S.; Li, A.H.; Kim, S.A.; Jacobson, K.A.; Daly, J.W. Dihydropyridines as inhibitors of capacitative calcium entry in leukemic HL-60 cells. Biochem. Pharm. 2003, 65, 329–338. [Google Scholar] [CrossRef]
- Merritt, J.E.; Armstrong, W.P.; Benham, C.D.; Hallam, T.J.; Jacob, R.; Jaxa-Chamiec, A.; Leigh, B.K.; McCarthy, S.A.; Moores, K.E.; Rink, T.J. SK&F 96365, a novel inhibitor of receptor-mediated calcium entry. Biochem. J. 1990, 271, 515–522. [Google Scholar]
- Bacchi, N.; Messina, A.; Burtscher, V.; Dassi, E.; Provenzano, G.; Bozzi, Y.; Demontis, G.C.; Koschak, A.; Denti, M.A.; Casarosa, S. A New Splicing Isoform of Cacna2d4 Mimicking the Effects of c.2451insC Mutation in the Retina: Novel Molecular and Electrophysiological Insights. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4846–4856. [Google Scholar] [CrossRef]
- Pomares, E.; Bures-Jelstrup, A.; Ruiz-Nogales, S.; Corcostegui, B.; Gonzalez-Duarte, R.; Navarro, R. Nonsense-mediated decay as the molecular cause for autosomal recessive bestrophinopathy in two unrelated families. Investig. Ophthalmol. Vis. Sci. 2012, 53, 532–537. [Google Scholar] [CrossRef]
- Permanyer, J.; Navarro, R.; Friedman, J.; Pomares, E.; Castro-Navarro, J.; Marfany, G.; Swaroop, A.; Gonzalez-Duarte, R. Autosomal recessive retinitis pigmentosa with early macular affectation caused by premature truncation in PROM1. Investig. Ophthalmol. Vis. Sci. 2010, 51, 2656–2663. [Google Scholar] [CrossRef]
- Kadurin, I.; Alvarez-Laviada, A.; Ng, S.F.; Walker-Gray, R.; D’Arco, M.; Fadel, M.G.; Pratt, W.S.; Dolphin, A.C. Calcium currents are enhanced by alpha2delta-1 lacking its membrane anchor. J. Biol. Chem. 2012, 287, 33554–33566. [Google Scholar] [CrossRef] [PubMed]
- Felix, R.; Gurnett, C.A.; De Waard, M.; Campbell, K.P. Dissection of functional domains of the voltage-dependent Ca2+ channel alpha2delta subunit. J. Neurosci. 1997, 17, 6884–6891. [Google Scholar] [CrossRef]
- Barclay, J.; Balaguero, N.; Mione, M.; Ackerman, S.L.; Letts, V.A.; Brodbeck, J.; Canti, C.; Meir, A.; Page, K.M.; Kusumi, K.; et al. Ducky mouse phenotype of epilepsy and ataxia is associated with mutations in the Cacna2d2 gene and decreased calcium channel current in cerebellar Purkinje cells. J. Neurosci. 2001, 21, 6095–6104. [Google Scholar] [CrossRef] [PubMed]
- Haeseleer, F.; Imanishi, Y.; Maeda, T.; Possin, D.E.; Maeda, A.; Lee, A.; Rieke, F.; Palczewski, K. Essential role of Ca2+-binding protein 4, a Cav1.4 channel regulator, in photoreceptor synaptic function. Nat. Neurosci. 2004, 7, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Babai, N.; Thoreson, W.B. Horizontal cell feedback regulates calcium currents and intracellular calcium levels in rod photoreceptors of salamander and mouse retina. J. Physiol. 2009, 587, 2353–2364. [Google Scholar] [CrossRef]
- Morgans, C.W.; Bayley, P.R.; Oesch, N.W.; Ren, G.; Akileswaran, L.; Taylor, W.R. Photoreceptor calcium channels: Insight from night blindness. Vis. Neurosci. 2005, 22, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Gilliam, J.C.; Wensel, T.G. TRP channel gene expression in the mouse retina. Vis. Res. 2011, 51, 2440–2452. [Google Scholar] [CrossRef]
- Zitt, C.; Zobel, A.; Obukhov, A.G.; Harteneck, C.; Kalkbrenner, F.; Luckhoff, A.; Schultz, G. Cloning and functional expression of a human Ca2+-permeable cation channel activated by calcium store depletion. Neuron 1996, 16, 1189–1196. [Google Scholar] [CrossRef]
- Strubing, C.; Krapivinsky, G.; Krapivinsky, L.; Clapham, D.E. TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron 2001, 29, 645–655. [Google Scholar] [CrossRef]
- Trebak, M.; Lemonnier, L.; Smyth, J.T.; Vazquez, G.; Putney, J.W., Jr. Phospholipase C-coupled receptors and activation of TRPC channels. Handb. Exp. Pharm. 2007, 179, 593–614. [Google Scholar] [CrossRef]
- Demontis, G.C.; Aruta, C.; Comitato, A.; De Marzo, A.; Marigo, V. Functional and molecular characterization of rod-like cells from retinal stem cells derived from the adult ciliary epithelium. PLoS ONE 2012, 7, e33338. [Google Scholar] [CrossRef]
- Davies, A.; Kadurin, I.; Alvarez-Laviada, A.; Douglas, L.; Nieto-Rostro, M.; Bauer, C.S.; Pratt, W.S.; Dolphin, A.C. The alpha2delta subunits of voltage-gated calcium channels form GPI-anchored proteins, a posttranslational modification essential for function. Proc. Natl. Acad. Sci. USA 2010, 107, 1654–1659. [Google Scholar] [CrossRef] [PubMed]
- McMahon, D.G.; Iuvone, P.M.; Tosini, G. Circadian organization of the mammalian retina: From gene regulation to physiology and diseases. Prog. Retin. Eye Res. 2014, 39, 58–76. [Google Scholar] [CrossRef] [PubMed]
- D’Arco, M.; Dolphin, A.C. L-type calcium channels: On the fast track to nuclear signaling. Sci. Signal. 2012, 5, pe34. [Google Scholar] [CrossRef] [PubMed]
- Donato, R.; Page, K.M.; Koch, D.; Nieto-Rostro, M.; Foucault, I.; Davies, A.; Wilkinson, T.; Rees, M.; Edwards, F.A.; Dolphin, A.C. The ducky(2J) mutation in Cacna2d2 results in reduced spontaneous Purkinje cell activity and altered gene expression. J. Neurosci. 2006, 26, 12576–12586. [Google Scholar] [CrossRef]
- Demontis, G.C.; Ratto, G.M.; Bisti, S.; Cervetto, L. Effect of blocking the Na+/K+ ATPase on Ca2+ extrusion and light adaptation in mammalian retinal rods. Biophys. J. 1995, 69, 439–450. [Google Scholar] [CrossRef][Green Version]
- Cervetto, L.; Lagnado, L.; Perry, R.J.; Robinson, D.W.; McNaughton, P.A. Extrusion of calcium from rod outer segments is driven by both sodium and potassium gradients. Nature 1989, 337, 740–743. [Google Scholar] [CrossRef]
- Winkler, B.S. Glycolytic and oxidative metabolism in relation to retinal function. J. Gen. Physiol. 1981, 77, 667–692. [Google Scholar] [CrossRef]
- Linsenmeier, R.A. Effects of light and darkness on oxygen distribution and consumption in the cat retina. J. Gen. Physiol. 1986, 88, 521–542. [Google Scholar] [CrossRef]
- Demontis, G.C.; Longoni, B.; Gargini, C.; Cervetto, L. The energetic cost of photoreception in retinal rods of mammals. Arch. Ital. Biol. 1997, 135, 95–109. [Google Scholar]
- Okawa, H.; Sampath, A.P.; Laughlin, S.B.; Fain, G.L. ATP consumption by mammalian rod photoreceptors in darkness and in light. Curr. Biol. 2008, 18, 1917–1921. [Google Scholar] [CrossRef] [PubMed]
- Thapa, A.; Morris, L.; Xu, J.; Ma, H.; Michalakis, S.; Biel, M.; Ding, X.Q. Endoplasmic reticulum stress-associated cone photoreceptor degeneration in cyclic nucleotide-gated channel deficiency. J. Biol. Chem. 2012, 287, 18018–18029. [Google Scholar] [CrossRef]
- Ma, H.; Butler, M.R.; Thapa, A.; Belcher, J.; Yang, F.; Baehr, W.; Biel, M.; Michalakis, S.; Ding, X.Q. cGMP/Protein Kinase G Signaling Suppresses Inositol 1,4,5-Trisphosphate Receptor Phosphorylation and Promotes Endoplasmic Reticulum Stress in Photoreceptors of Cyclic Nucleotide-gated Channel-deficient Mice. J. Biol. Chem. 2015, 290, 20880–20892. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.R.; Ma, H.; Yang, F.; Belcher, J.; Le, Y.Z.; Mikoshiba, K.; Biel, M.; Michalakis, S.; Iuso, A.; Krizaj, D.; et al. Endoplasmic reticulum (ER) Ca(2+)-channel activity contributes to ER stress and cone death in cyclic nucleotide-gated channel deficiency. J. Biol. Chem. 2017, 292, 11189–11205. [Google Scholar] [CrossRef] [PubMed]
- Szalai, P.; Parys, J.B.; Bultynck, G.; Christensen, S.B.; Nissen, P.; Moller, J.V.; Engedal, N. Nonlinear relationship between ER Ca(2+) depletion versus induction of the unfolded protein response, autophagy inhibition, and cell death. Cell Calcium 2018, 76, 48–61. [Google Scholar] [CrossRef]
- Demontis, G.C.; Gargini, C.; Paoli, T.G.; Cervetto, L. Selective Hcn1 channels inhibition by ivabradine in mouse rod photoreceptors. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1948–1955. [Google Scholar] [CrossRef]
- Blanton, M.G.; Lo Turco, J.J.; Kriegstein, A.R. Whole cell recording from neurons in slices of reptilian and mammalian cerebral cortex. J. Neurosci. Methods 1989, 30, 203–210. [Google Scholar] [CrossRef]
- Cangiano, L.; Gargini, C.; Della Santina, L.; Demontis, G.C.; Cervetto, L. High-pass filtering of input signals by the Ih current in a non-spiking neuron, the retinal rod bipolar cell. PLoS ONE 2007, 2, e1327. [Google Scholar] [CrossRef]
- Schoenmakers, T.J.; Visser, G.J.; Flik, G.; Theuvenet, A.P. CHELATOR: An improved method for computing metal ion concentrations in physiological solutions. Biotechniques 1992, 12, 870–874+876–879. [Google Scholar]
| SALINE | NaCl | KCl | CaCl2 | MgCl2 | BaCl2 | TEACl | CsCl | HEPES | PIPES | GLUCOSE | EGTA |
|---|---|---|---|---|---|---|---|---|---|---|---|
| LOCKE’S | 140 | 3.6 | 2.0 | 2.4 | --- | --- | --- | 10 | ---- | 10 | ---- |
| TEA-Ba-Cs | 100 | 3.6 | 0 | 0 | 5 | 30 | 3 | 10 | ---- | 10 | ---- |
| INTRA | 10 | 140 | 0 | 2.4 | ---- | ---- | ---- | ---- | 10 | ---- | ---- |
| KCl 25 | 115 | 25 | 2.0 | 2.4 | ---- | ---- | ---- | 10 | ---- | 10 | ---- |
| 0 Ca/EGTA | 140 | 3.6. | 0 | 0 | ---- | ---- | ---- | 10 | ---- | 10 | 5 |
| 494 nM Ca | 140 | 3.6 | 4 | 0 | ---- | ---- | ---- | 10 | ---- | 10 | 5 |
| 30 mM Ca | ---- | 140 | 30 | 0 | ---- | ---- | ---- | ---- | 10 | ---- | ---- |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vellani, V.; Mauro, G.; Demontis, G.C. Depleted Calcium Stores and Increased Calcium Entry in Rod Photoreceptors of the Cacna2d4 Mouse Model of Cone-Rod Dystrophy RCD4. Int. J. Mol. Sci. 2022, 23, 13080. https://doi.org/10.3390/ijms232113080
Vellani V, Mauro G, Demontis GC. Depleted Calcium Stores and Increased Calcium Entry in Rod Photoreceptors of the Cacna2d4 Mouse Model of Cone-Rod Dystrophy RCD4. International Journal of Molecular Sciences. 2022; 23(21):13080. https://doi.org/10.3390/ijms232113080
Chicago/Turabian StyleVellani, Vittorio, Giovanna Mauro, and Gian Carlo Demontis. 2022. "Depleted Calcium Stores and Increased Calcium Entry in Rod Photoreceptors of the Cacna2d4 Mouse Model of Cone-Rod Dystrophy RCD4" International Journal of Molecular Sciences 23, no. 21: 13080. https://doi.org/10.3390/ijms232113080
APA StyleVellani, V., Mauro, G., & Demontis, G. C. (2022). Depleted Calcium Stores and Increased Calcium Entry in Rod Photoreceptors of the Cacna2d4 Mouse Model of Cone-Rod Dystrophy RCD4. International Journal of Molecular Sciences, 23(21), 13080. https://doi.org/10.3390/ijms232113080






