Biological Cover Mitigates Disruption of the Dermal Structure in Mechanically Expanded Skin in a Porcine Model
Abstract
:1. Introduction
2. Results
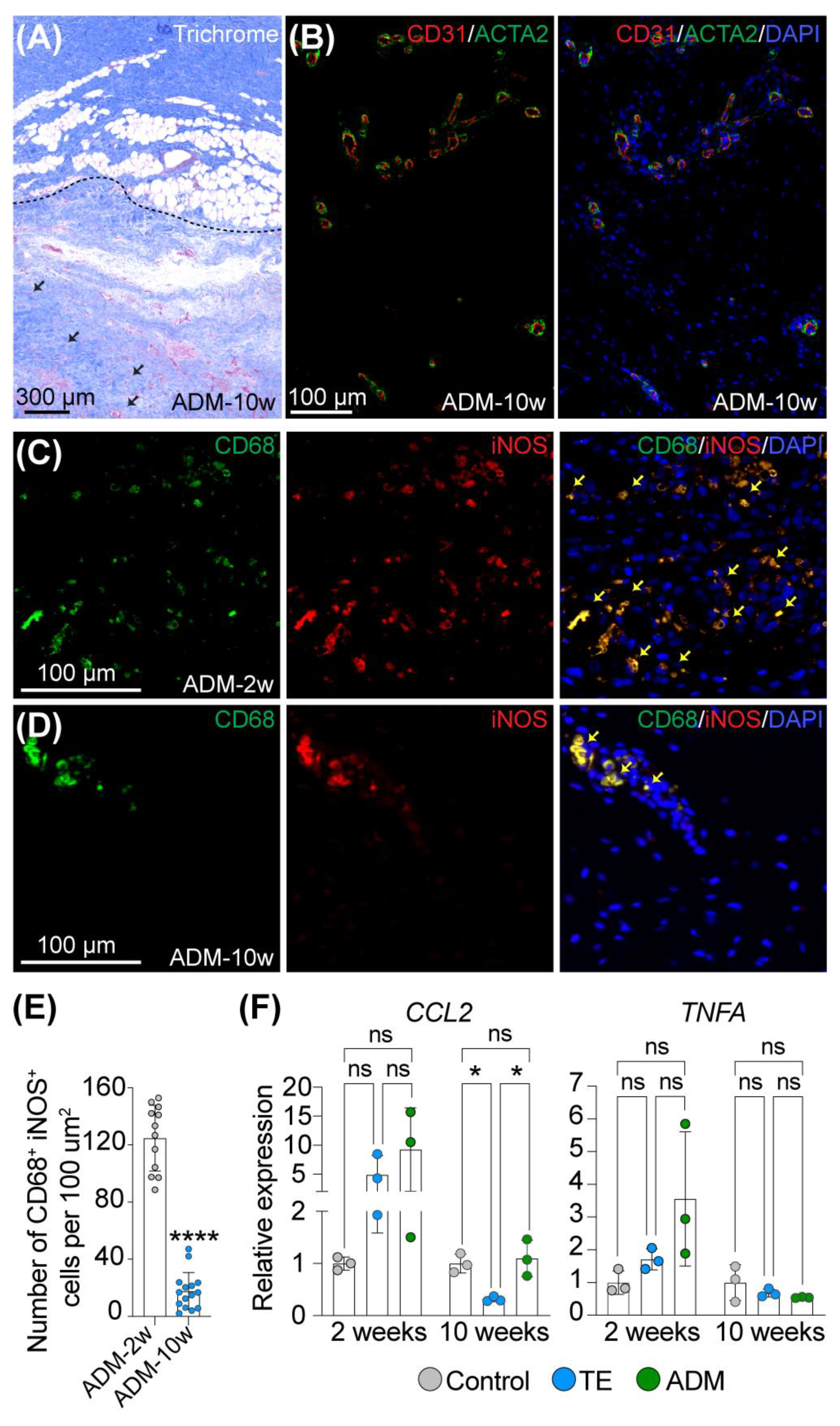
2.1. Macrophages Facilitate Integration of a Biological Cover with Host Tissue
2.2. Biological Cover Diminishes Structural Changes in the Dermis Induced by Tissue Expansion
2.3. Biological Cover Prevents Excessive Collagen Deposition in Expanded Non-Irradiated Skin
2.4. Biological Cover Prevents Capsule formation in Expanded Non-Irradiated Skin
3. Discussion
4. Material and Methods
4.1. Ethics Statement
4.2. Animal Model and Study Design
4.3. Radiotherapy Protocol
4.4. Masson Trichrome Staining
4.5. Immunofluorescence (IF) Staining
4.6. Total RNA Extraction
4.7. Quantitative Real-Time PCR Analysis (qRT-PCR)
4.8. Measurement of the Dermal Thickness
4.9. Picrosirius Red Staining
5. Conclusions
Limitation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Rolph, R.; Farhadi, J. The use of meshes and matrices in breast reconstruction. Br. J. Hosp. Med. (Lond) 2018, 79, 454–459. [Google Scholar] [CrossRef]
- Nahabedian, M.Y. Acellular dermal matrices in primary breast reconstruction: Principles, concepts, and indications. Plast. Reconstr. Surg. 2012, 130, 44s–53s. [Google Scholar] [CrossRef]
- Ho, G.; Nguyen, T.J.; Shahabi, A.; Hwang, B.H.; Chan, L.S.; Wong, A.K. A systematic review and meta-analysis of complications associated with acellular dermal matrix-assisted breast reconstruction. Ann. Plast. Surg. 2012, 68, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Sorkin, M.; Qi, J.; Kim, H.M.; Hamill, J.B.; Kozlow, J.H.; Pusic, A.L.; Wilkins, E.G. Acellular Dermal Matrix in Immediate Expander/Implant Breast Reconstruction: A Multicenter Assessment of Risks and Benefits. Plast. Reconstr. Surg. 2017, 140, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Ganesh Kumar, N.; Berlin, N.L.; Kim, H.M.; Hamill, J.B.; Kozlow, J.H.; Wilkins, E.G. Development of an evidence-based approach to the use of acellular dermal matrix in immediate expander-implant-based breast reconstruction. J. Plast. Reconstr. Aesthet. Surg. 2021, 74, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Tevlin, R.; Borrelli, M.R.; Irizarry, D.; Nguyen, D.; Wan, D.C.; Momeni, A. Acellular Dermal Matrix Reduces Myofibroblast Presence in the Breast Capsule. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2213. [Google Scholar] [CrossRef]
- Koltz, P.F.; Frey, J.D.; Langstein, H.N. The use of human acellular dermal matrix in the first stage of implant-based breast reconstruction simplifies the exchange procedure. Plast. Reconstr. Surg. 2013, 132, 691e–692e. [Google Scholar] [CrossRef]
- Basu, C.B.; Leong, M.; Hicks, M.J. Acellular cadaveric dermis decreases the inflammatory response in capsule formation in reconstructive breast surgery. Plast. Reconstr. Surg. 2010, 126, 1842–1847. [Google Scholar] [CrossRef]
- Komorowska-Timek, E.; Oberg, K.C.; Timek, T.A.; Gridley, D.S.; Miles, D.A.G. The effect of AlloDerm envelopes on periprosthetic capsule formation with and without radiation. Plast. Reconstr. Surg. 2009, 123, 807–816. [Google Scholar] [CrossRef]
- Sbitany, H.; Wang, F.; Peled, A.W.; Lentz, R.; Alvarado, M.; Ewing, C.A.; Esserman, L.J.; Fowble, B.; Foster, R.D. Immediate implant-based breast reconstruction following total skin-sparing mastectomy: Defining the risk of preoperative and postoperative radiation therapy for surgical outcomes. Plast. Reconstr. Surg. 2014, 134, 396–404. [Google Scholar] [CrossRef]
- Kim, I.K.; Park, S.O.; Chang, H.; Jin, U.S. Inhibition Mechanism of Acellular Dermal Matrix on Capsule Formation in Expander-Implant Breast Reconstruction After Postmastectomy Radiotherapy. Ann. Surg. Oncol. 2018, 25, 2279–2287. [Google Scholar] [CrossRef] [PubMed]
- Park, T.H.; Chung, S.W.; Song, S.Y.; Lew, D.H.; Roh, T.S.; Lee, D.W. The use of acellular dermal matrix in immediate two-stage prosthetic breast reconstruction provides protection from postmastectomy radiation therapy: A clinicopathologic perspective. J. Mater. Sci. Mater. Med. 2018, 29, 27. [Google Scholar] [CrossRef] [PubMed]
- Straub, J.M.; New, J.; Hamilton, C.D.; Lominska, C.; Shnayder, Y.; Thomas, S.M. Radiation-induced fibrosis: Mechanisms and implications for therapy. J. Cancer Res. Clin. Oncol. 2015, 141, 1985–1994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, N.T.; Roberge, D.; Freeman, C.; Wong, C.; Hines, J.; Turcotte, R.E. Skin elasticity as a measure of radiation fibrosis: Is it reproducible and does it correlate with patient and physician-reported measures? Technol. Cancer Res. Treat. 2014, 13, 469–476. [Google Scholar] [CrossRef] [Green Version]
- Summerfield, A.; Meurens, F.; Ricklin, M.E. The immunology of the porcine skin and its value as a model for human skin. Mol. Immunol. 2015, 66, 14–21. [Google Scholar] [CrossRef]
- Debeer, S.; Le Luduec, J.B.; Kaiserlian, D.; Laurent, P.; Nicolas, J.F.; Dubois, B.; Kanitakis, J. Comparative histology and immunohistochemistry of porcine versus human skin. Eur. J. Dermatol. 2013, 23, 456–466. [Google Scholar] [CrossRef] [Green Version]
- Brown, B.N.; Ratner, B.D.; Goodman, S.B.; Amar, S.; Badylak, S.F. Macrophage polarization: An opportunity for improved outcomes in biomaterials and regenerative medicine. Biomaterials 2012, 33, 3792–3802. [Google Scholar] [CrossRef] [Green Version]
- Brown, B.N.; Londono, R.; Tottey, S.; Zhang, L.; Kukla, K.A.; Wolf, M.T.; Daly, K.A.; Reing, J.E.; Badylak, S.F. Macrophage phenotype as a predictor of constructive remodeling following the implantation of biologically derived surgical mesh materials. Acta Biomater. 2012, 8, 978–987. [Google Scholar] [CrossRef] [Green Version]
- Bohac, M.; Danisovic, L.; Koller, J.; Dragunova, J.; Varga, I. What happens to an acellular dermal matrix after implantation in the human body? A histological and electron microscopic study. Eur. J. Histochem. 2018, 62, 2873. [Google Scholar] [CrossRef] [Green Version]
- Bullocks, J.M. DermACELL: A novel and biocompatible acellular dermal matrix in tissue expander and implant-based breast reconstruction. Eur. J. Plast. Surg. 2014, 37, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Julier, Z.; Park, A.J.; Briquez, P.S.; Martino, M.M. Promoting tissue regeneration by modulating the immune system. Acta Biomater. 2017, 53, 13–28. [Google Scholar] [CrossRef]
- Ding, J.; Lei, L.; Liu, S.; Zhang, Y.; Yu, Z.; Su, Y.; Ma, X. Macrophages are necessary for skin regeneration during tissue expansion. J. Transl. Med. 2019, 17, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ledwon, J.K.; Kelsey, L.J.; Vaca, E.E.; Gosain, A.K. Transcriptomic analysis reveals dynamic molecular changes in skin induced by mechanical forces secondary to tissue expansion. Sci. Rep. 2020, 10, 15991. [Google Scholar] [CrossRef] [PubMed]
- Ledwon, J.K.; Vaca, E.E.; Huang, C.C.; Kelsey, L.J.; McGrath, J.L.; Topczewski, J.; Gosain, A.K.; Topczewska, J.M. Langerhans cells and SFRP2/Wnt/beta-catenin signalling control adaptation of skin epidermis to mechanical stretching. J. Cell Mol. Med. 2022, 26, 764–775. [Google Scholar] [CrossRef] [PubMed]
- Dziki, J.L.; Huleihel, L.; Scarritt, M.E.; Badylak, S.F. Extracellular Matrix Bioscaffolds as Immunomodulatory Biomaterials. Tissue Eng. Part A 2017, 23, 1152–1159. [Google Scholar] [CrossRef]
- Pasyk, K.A.; Argenta, L.C.; Hassett, C. Quantitative analysis of the thickness of human skin and subcutaneous tissue following controlled expansion with a silicone implant. Plast. Reconstr. Surg. 1988, 81, 516–523. [Google Scholar] [CrossRef]
- Purnell, C.A.; Gart, M.S.; Buganza-Tepole, A.; Tomaszewski, J.P.; Topczewska, J.M.; Kuhl, E.; Gosain, A.K. Determining the Differential Effects of Stretch and Growth in Tissue-Expanded Skin: Combining Isogeometric Analysis and Continuum Mechanics in a Porcine Model. Dermatol. Surg. 2018, 44, 48–52. [Google Scholar] [CrossRef]
- Lin, J.; Shi, Y.; Men, Y.; Wang, X.; Ye, J.; Zhang, C. Mechanical Roles in Formation of Oriented Collagen Fibers. Tissue Eng. Part B Rev. 2020, 26, 116–128. [Google Scholar] [CrossRef] [Green Version]
- Knight, K.R.; McCann, J.J.; Vanderkolk, C.A.; Coe, S.A.; O’Brien, B.M. The redistribution of collagen in expanded pig skin. Br. J. Plast. Surg. 1990, 43, 565–570. [Google Scholar] [CrossRef]
- Johnson, T.M.; Lowe, L.; Brown, M.D.; Sullivan, M.J.; Nelson, B.R. Histology and physiology of tissue expansion. J. Dermatol. Surg. Oncol. 1993, 19, 1074–1078. [Google Scholar] [CrossRef]
- Tan, P.C.; Zhou, S.B.; Ou, M.Y.; He, J.Z.; Zhang, P.Q.; Zhang, X.J.; Xie, Y.; Gao, Y.M.; Zhang, T.Y.; Li, Q.F. Mechanical stretching can modify the papillary dermis pattern and papillary fibroblast characteristics during skin regeneration. J. Investig. Dermatol. 2022, 9, 2386–2394.e8. [Google Scholar] [CrossRef] [PubMed]
- Wells, R.G. Tissue mechanics and fibrosis. Biochim. Biophys. Acta 2013, 1832, 884–890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siggelkow, W.; Faridi, A.; Spiritus, K.; Klinge, U.; Rath, W.; Klosterhalfen, B. Histological analysis of silicone breast implant capsules and correlation with capsular contracture. Biomaterials 2003, 24, 1101–1109. [Google Scholar] [CrossRef]
- Headon, H.; Kasem, A.; Mokbel, K. Capsular Contracture after Breast Augmentation: An Update for Clinical Practice. Arch. Plast. Surg. 2015, 42, 532–543. [Google Scholar] [CrossRef] [Green Version]
- Baker, J.L., Jr.; Chandler, M.L.; LeVier, R.R. Occurrence and activity of myofibroblasts in human capsular tissue surrounding mammary implants. Plast. Reconstr. Surg. 1981, 68, 905–912. [Google Scholar] [CrossRef]
- Wang, J.; Zohar, R.; McCulloch, C.A. Multiple roles of alpha-smooth muscle actin in mechanotransduction. Exp. Cell Res. 2006, 312, 205–214. [Google Scholar] [CrossRef]
- Jayasinghe, R.T.; Ruseckaite, R.; Gartoulla, P.; Elder, E.; Hopper, I. Patient Reported Outcome Measures After Breast Augmentation—Using the BREAST-Q IS. Patient. Relat. Outcome Meas. 2022, 13, 1–8. [Google Scholar] [CrossRef]
- Logan Ellis, H.; Asaolu, O.; Nebo, V.; Kasem, A. Biological and synthetic mesh use in breast reconstructive surgery: A literature review. World J. Surg. Oncol. 2016, 14, 121. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, J.R.; Salinas, H.M.; Broelsch, G.F.; McCormack, M.C.; Meppelink, A.M.; Randolph, M.A.; Redmond, R.W.; Austen, W.G., Jr. Prevention of capsular contracture with photochemical tissue passivation. Plast. Reconstr. Surg. 2014, 133, 571–577. [Google Scholar] [CrossRef]
- Leong, M.; Basu, C.B.; Hicks, M.J. Further evidence that human acellular dermal matrix decreases inflammatory markers of capsule formation in implant-based breast reconstruction. Aesthet. Surg. J. 2015, 35, 40–47. [Google Scholar] [CrossRef]
- Yu, D.; Hanna, K.R.; LeGallo, R.D.; Drake, D.B. Comparison of Histological Characteristics of Acellular Dermal Matrix Capsules to Surrounding Breast Capsules in Acellular Dermal Matrix-Assisted Breast Reconstruction. Ann. Plast. Surg. 2016, 76, 485–488. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.; Sim, H.B.; Huan, F.; Kim, D.J. Myofibroblasts and capsular tissue tension in breast capsular contracture. Aesthetic. Plast. Surg. 2010, 34, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Tepole, A.B.; Gart, M.; Gosain, A.K.; Kuhl, E. Characterization of living skin using multi-view stereo and isogeometric analysis. Acta Biomater. 2014, 10, 4822–4831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barton, M. Tables of equivalent dose in 2 Gy fractions: A simple application of the linear quadratic formula. Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 371–378. [Google Scholar] [CrossRef]
- Hadad, I.; Johnstone, B.H.; Brabham, J.G.; Blanton, M.W.; Rogers, P.I.; Fellers, C.; Solomon, J.L.; Merfeld-Clauss, S.; DesRosiers, C.M.; Dynlacht, J.R.; et al. Development of a porcine delayed wound-healing model and its use in testing a novel cell-based therapy. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 888–896. [Google Scholar] [CrossRef]
- Archambeau, J.O.; Ines, A.; Fajardo, L.F. Response of swine skin microvasculature to acute single exposures of X rays: Quantification of endothelial changes. Radiat. Res. 1984, 98, 37–51. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Landini, G.; Martinelli, G.; Piccinini, F. Colour deconvolution: Stain unmixing in histological imaging. Bioinformatics 2022, 37, 1485–1487. [Google Scholar] [CrossRef]
- Ruifrok, A.C.; Johnston, D.A. Quantification of histochemical staining by color deconvolution. Anal. Quant. Cytol. Histol. 2001, 23, 291–299. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Bredfeldt, J.S.; Liu, Y.; Pehlke, C.A.; Conklin, M.W.; Szulczewski, J.M.; Inman, D.R.; Keely, P.J.; Nowak, R.D.; Mackie, T.R.; Eliceiri, K.W. Computational segmentation of collagen fibers from second-harmonic generation images of breast cancer. J. Biomed. Opt. 2014, 19, 16007. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ledwon, J.K.; Applebaum, S.A.; Progri, B.; Vignesh, O.; Gutowski, K.S.; Chang, A.B.; Tepole, A.B.; Gosain, A.K. Biological Cover Mitigates Disruption of the Dermal Structure in Mechanically Expanded Skin in a Porcine Model. Int. J. Mol. Sci. 2022, 23, 13091. https://doi.org/10.3390/ijms232113091
Ledwon JK, Applebaum SA, Progri B, Vignesh O, Gutowski KS, Chang AB, Tepole AB, Gosain AK. Biological Cover Mitigates Disruption of the Dermal Structure in Mechanically Expanded Skin in a Porcine Model. International Journal of Molecular Sciences. 2022; 23(21):13091. https://doi.org/10.3390/ijms232113091
Chicago/Turabian StyleLedwon, Joanna K., Sarah A. Applebaum, Bianka Progri, Oveyaa Vignesh, Kristof S. Gutowski, Alec B. Chang, Adrian B. Tepole, and Arun K. Gosain. 2022. "Biological Cover Mitigates Disruption of the Dermal Structure in Mechanically Expanded Skin in a Porcine Model" International Journal of Molecular Sciences 23, no. 21: 13091. https://doi.org/10.3390/ijms232113091
APA StyleLedwon, J. K., Applebaum, S. A., Progri, B., Vignesh, O., Gutowski, K. S., Chang, A. B., Tepole, A. B., & Gosain, A. K. (2022). Biological Cover Mitigates Disruption of the Dermal Structure in Mechanically Expanded Skin in a Porcine Model. International Journal of Molecular Sciences, 23(21), 13091. https://doi.org/10.3390/ijms232113091





