Serum SELENBP1 and VCL Are Effective Biomarkers for Clinical and Forensic Diagnosis of Coronary Artery Spasm
Abstract
1. Introduction
2. Results
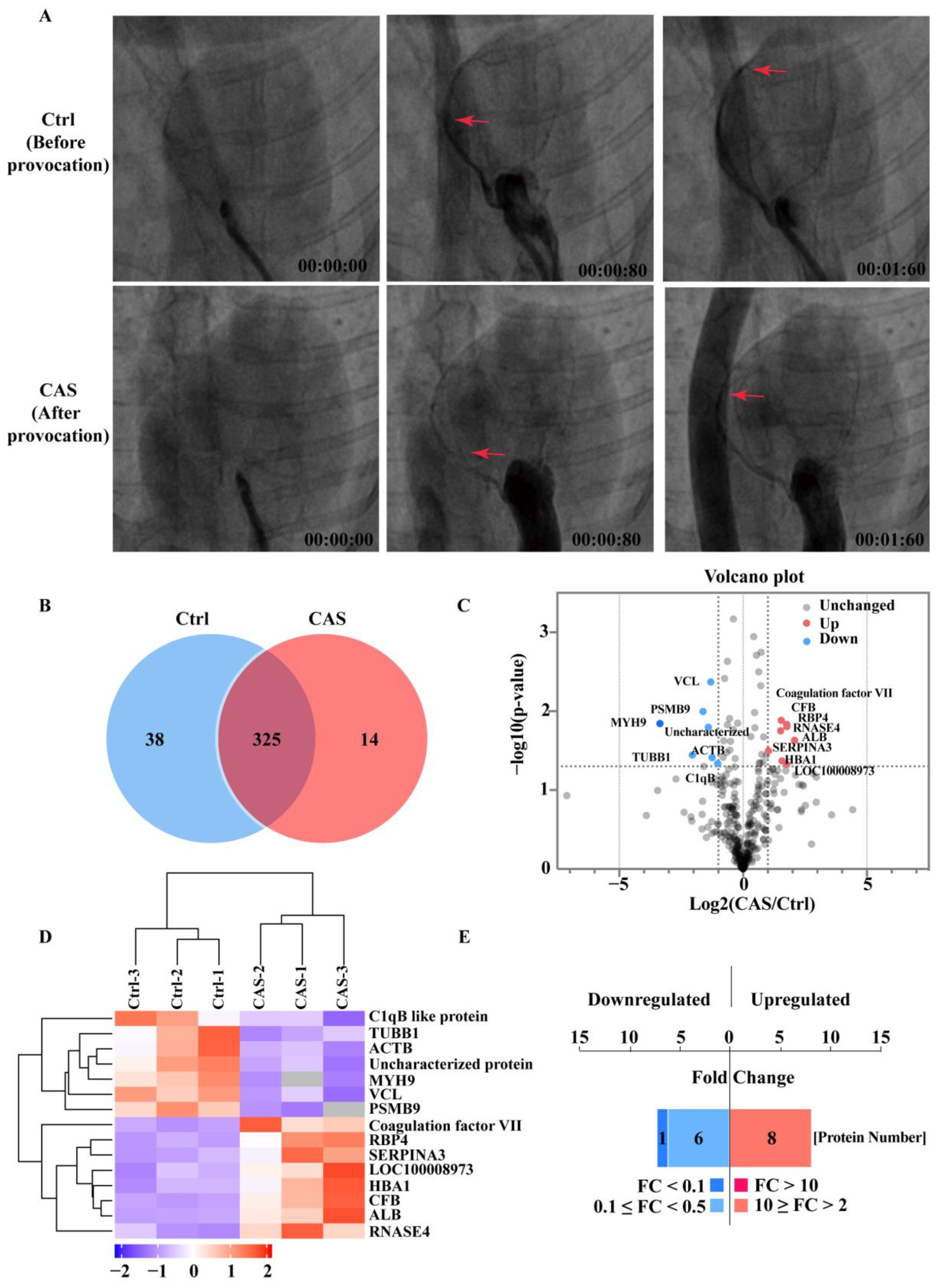
2.1. Proteins Identified with the CAS Provocation Model
2.2. PRM/MS-Based Targeted Proteome and PLS-DA Algorithm Validated SELENBP1 and VCL as Top Dysregulated Serum Proteins
2.3. Genome-Wide Association Studies (GWAS) and Phenome-Wide Association Studies (PheWAS) Revealed Association of SELENBP1 and VCL Variations with Coronary Artery Diseases
2.4. SELENBP1 and VCL Were Abundantly Enriched in Extracellular Vesicles (EVs)-Free Serum Samples
2.5. Lower Serum SELENBP1 and VCL Levels Were Due to Their Decreased Secretion from Cardiomyocytes under Contractile Conditions
2.6. Diagnostic Potential of Serum SELENBP1 and VCL in Clinical CAS Patients
2.7. Diagnostic Potential of Serum SELENBP1 and VCL for CAS-Induced SCDs
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Animal Experiments
4.3. Clinical Sample Collection
4.4. Forensic Sample Collection
4.5. Serum Sample Preparation
4.6. Protein Extraction and Enzymolysis
4.7. Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) Analysis
4.8. Protein Identification and Quantitative Analysis
4.9. Bioinformatics Analysis
4.10. LC-PRM/MS Analysis
4.11. GWAS and PheWAS Analyses
4.12. PLS-DA Algorithm
4.13. Serum EVs Isolation
4.14. ELISA
4.15. Cells and Cell Culture
4.16. Western Blot Analysis
4.17. Statistical Analysis
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prinzmetal, M.; Kennamer, R.; Merliss, R.; Wada, T.; Bor, N. Angina pectoris. I. A variant form of angina pectoris; preliminary report. Am. J. Med. 1959, 27, 375–388. [Google Scholar] [CrossRef]
- Waterbury, T.M.; Tarantini, G.; Vogel, B.; Mehran, R.; Gersh, B.J.; Gulati, R. Non-atherosclerotic causes of acute coronary syndromes. Nat. Rev. Cardiol. 2020, 17, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Beltrame, J.F.; Sasayama, S.; Maseri, A. Racial heterogeneity in coronary artery vasomotor reactivity: Differences between Japanese and Caucasian patients. J. Am. Coll. Cardiol. 1999, 33, 1442–1452. [Google Scholar] [CrossRef]
- Hung, M.J.; Cherng, W.J.; Cheng, C.W.; Li, L.F. Comparison of serum levels of inflammatory markers in patients with coronary vasospasm without significant fixed coronary artery disease versus patients with stable angina pectoris and acute coronary syndromes with significant fixed coronary artery disease. Am. J. Cardiol. 2006, 97, 1429–1434. [Google Scholar] [CrossRef] [PubMed]
- Hung, M.J.; Cheng, C.W.; Yang, N.I.; Hung, M.Y.; Cherng, W.J. Coronary vasospasm-induced acute coronary syndrome complicated by life-threatening cardiac arrhythmias in patients without hemodynamically significant coronary artery disease. Int. J. Cardiol. 2007, 117, 37–44. [Google Scholar] [CrossRef]
- Bory, M.; Pierron, F.; Panagides, D.; Bonnet, J.L.; Yvorra, S.; Desfossez, L. Coronary artery spasm in patients with normal or near normal coronary arteries. Long-term follow-up of 277 patients. Eur. Heart J. 1996, 17, 1015–1021. [Google Scholar] [CrossRef]
- Slavich, M.; Patel, R.S. Coronary artery spasm: Current knowledge and residual uncertainties. Int. J. Cardiol. Heart Vasc. 2016, 10, 47–53. [Google Scholar] [CrossRef]
- Yasue, H.; Kugiyama, K. Coronary spasm: Clinical features and pathogenesis. Intern. Med. 1997, 36, 760–765. [Google Scholar] [CrossRef]
- Araki, H.; Koiwaya, Y.; Nakagaki, O.; Nakamura, M. Diurnal distribution of ST-segment elevation and related arrhythmias in patients with variant angina: A study by ambulatory ECG monitoring. Circulation 1983, 67, 995–1000. [Google Scholar] [CrossRef]
- Yasue, H.; Nakagawa, H.; Itoh, T.; Harada, E.; Mizuno, Y. Coronary artery spasm--clinical features, diagnosis, pathogenesis, and treatment. J. Cardiol. 2008, 51, 2–17. [Google Scholar] [CrossRef]
- Stern, S.; Bayes de Luna, A. Coronary artery spasm: A 2009 update. Circulation 2009, 119, 2531–2534. [Google Scholar] [CrossRef] [PubMed]
- Hung, M.J.; Hu, P.; Hung, M.Y. Coronary artery spasm: Review and update. Int. J. Med. Sci. 2014, 11, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Probst, S.; Seitz, A.; Martinez Pereyra, V.; Hubert, A.; Becker, A.; Storm, K.; Bekeredjian, R.; Sechtem, U.; Ong, P. Safety assessment and results of coronary spasm provocation testing in patients with myocardial infarction with unobstructed coronary arteries compared to patients with stable angina and unobstructed coronary arteries. Eur. Heart J. Acute Cardiovasc. Care 2020, 10, 2048872620932422. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Lin, X.; Zhao, X.; Xu, C.; Yu, B.; Shen, Y.; Li, L. Coronary Artery Spasm: Risk Factors, Pathophysiological Mechanisms and Novel Diagnostic Approaches. Rev. Cardiovasc. Med. 2022, 23, 175. [Google Scholar] [CrossRef]
- Watanabe, K.; Shishido, T.; Otaki, Y.; Watanabe, T.; Sugai, T.; Toshima, T.; Takahashi, T.; Yokoyama, M.; Kinoshita, D.; Murase, T.; et al. Increased plasma xanthine oxidoreductase activity deteriorates coronary artery spasm. Heart Vessels 2019, 34, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Yu, B.; Zhao, X.; Lin, X.; Tang, X.; Liu, Z.; Gao, P.; Ge, J.; Wang, S.; Li, L. Valosin Containing Protein as a Specific Biomarker for Predicting the Development of Acute Coronary Syndrome and Its Complication. Front. Cardiovasc. Med. 2022, 9, 803532. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Xu, C.; Tang, X.; Liu, Z.; Lin, X.; Meng, H.; Shi, C.; Ma, K.; Xiao, B.; Li, L. Endoplasmic reticulum stress-related secretory proteins as biomarkers of early myocardial ischemia-induced sudden cardiac deaths. Int. J. Legal. Med. 2022, 136, 159–168. [Google Scholar] [CrossRef]
- Hayashi, M.; Shimizu, W.; Albert, C.M. The spectrum of epidemiology underlying sudden cardiac death. Circ. Res. 2015, 116, 1887–1906. [Google Scholar] [CrossRef]
- Sabatasso, S.; Mangin, P.; Fracasso, T.; Moretti, M.; Docquier, M.; Djonov, V. Early markers for myocardial ischemia and sudden cardiac death. Int. J. Legal. Med. 2016, 130, 1265–1280. [Google Scholar] [CrossRef]
- Factor, S.M.; Cho, S. Smooth muscle contraction bands in the media of coronary arteries: A postmortem marker of antemortem coronary spasm? J. Am. Coll. Cardiol. 1985, 6, 1329–1337. [Google Scholar] [CrossRef]
- Lin, C.S.; Goldfischer, M.; Sicular, A.; Landais, G.; Cohen, L.B. Morphodynamics and pathology of blood vessels III--comparative morphologic study of contraction of smooth muscle cells of hollow viscera and its application to vasoconstriction and vasospasm. Angiology 1998, 49, 503–522. [Google Scholar] [CrossRef] [PubMed]
- Svendsen, E.; Tindall, A.R. The internal elastic membrane and intimal folds in arteries: Important but neglected structures? Acta Physiol. Scand. Suppl. 1988, 572, 1–71. [Google Scholar] [PubMed]
- Visona, S.D.; Benati, D.; Monti, M.C.; Galie, M.; Andrello, L.; Frontini, A.; Osculati, A. Diagnosis of sudden cardiac death due to early myocardial ischemia: An ultrastructural and immunohistochemical study. Eur. J. Histochem. 2018, 62, 2866. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, Y.; Lin, J.; Jiang, J.; He, M.; Sun, D.; Zhao, Z.; Shen, Y.; Xue, A. Phosphorylated Myosin Light Chain 2 (p-MLC2) as a Molecular Marker of Antemortem Coronary Artery Spasm. Med. Sci. Monit. 2016, 22, 3316–3327. [Google Scholar] [CrossRef][Green Version]
- Xue, A.; Lin, J.; Que, C.; Yu, Y.; Tu, C.; Chen, H.; Liu, B.; Zhao, X.; Wang, T.; Ma, K.; et al. Aberrant endoplasmic reticulum stress mediates coronary artery spasm through regulating MLCK/MLC2 pathway. Exp. Cell Res. 2018, 363, 321–331. [Google Scholar] [CrossRef]
- Lanza, G.A.; Careri, G.; Crea, F. Mechanisms of coronary artery spasm. Circulation 2011, 124, 1774–1782. [Google Scholar] [CrossRef]
- Ohyama, K.; Matsumoto, Y.; Takanami, K.; Ota, H.; Nishimiya, K.; Sugisawa, J.; Tsuchiya, S.; Amamizu, H.; Uzuka, H.; Suda, A.; et al. Coronary Adventitial and Perivascular Adipose Tissue Inflammation in Patients With Vasospastic Angina. J. Am. Coll. Cardiol. 2018, 71, 414–425. [Google Scholar] [CrossRef]
- Lim, G.B. Inflammation: Perivascular inflammation in coronary spasm. Nat. Rev. Cardiol. 2018, 15, 134–135. [Google Scholar] [CrossRef]
- Ciliberti, G.; Guerra, F.; Capucci, A. Coronary Spasm and Inflammation: Can We Kill 2 Birds With 1 Stone? J. Am. Coll. Cardiol. 2018, 71, 2707–2708. [Google Scholar] [CrossRef]
- Vuckovic, D.; Bao, E.L.; Akbari, P.; Lareau, C.A.; Mousas, A.; Jiang, T.; Chen, M.H.; Raffield, L.M.; Tardaguila, M.; Huffman, J.E.; et al. The Polygenic and Monogenic Basis of Blood Traits and Diseases. Cell 2020, 182, 1214–1231.e11. [Google Scholar] [CrossRef]
- Kanai, M.; Akiyama, M.; Takahashi, A.; Matoba, N.; Momozawa, Y.; Ikeda, M.; Iwata, N.; Ikegawa, S.; Hirata, M.; Matsuda, K.; et al. Genetic analysis of quantitative traits in the Japanese population links cell types to complex human diseases. Nat. Genet. 2018, 50, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Pristipino, C.; Beltrame, J.F.; Finocchiaro, M.L.; Hattori, R.; Fujita, M.; Mongiardo, R.; Cianflone, D.; Sanna, T.; Sasayama, S.; Maseri, A. Major racial differences in coronary constrictor response between japanese and caucasians with recent myocardial infarction. Circulation 2000, 101, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Beltrame, J.F.; Crea, F.; Kaski, J.C.; Ogawa, H.; Ong, P.; Sechtem, U.; Shimokawa, H.; Bairey Merz, C.N.; Coronary Vasomotion Disorders International Study Group (COVADIS). International standardization of diagnostic criteria for vasospastic angina. Eur. Heart J. 2017, 38, 2565–2568. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, E.C.; Slagman, A.; Kuhn-Heid, E.C.D.; Seelig, J.; Schwiebert, C.; Minich, W.B.; Stoppe, C.; Mockel, M.; Schomburg, L. Circulating levels of selenium-binding protein 1 (SELENBP1) are associated with risk for major adverse cardiac events and death. J. Trace Elem. Med. Biol. 2019, 52, 247–253. [Google Scholar] [CrossRef]
- Elhodaky, M.; Diamond, A.M. Selenium-Binding Protein 1 in Human Health and Disease. Int. J. Mol. Sci. 2018, 19, 3437. [Google Scholar] [CrossRef]
- Boujemaa-Paterski, R.; Martins, B.; Eibauer, M.; Beales, C.T.; Geiger, B.; Medalia, O. Talin-activated vinculin interacts with branched actin networks to initiate bundles. Elife 2020, 9, e53990. [Google Scholar] [CrossRef]
- Yun, K.H.; Oh, S.K.; Park, E.M.; Kim, H.J.; Shin, S.H.; Lee, E.M.; Rhee, S.J.; Yoo, N.J.; Kim, N.H.; Jeong, J.W.; et al. An increased monocyte count predicts coronary artery spasm in patients with resting chest pain and insignificant coronary artery stenosis. Korean J. Intern. Med. 2006, 21, 97–102. [Google Scholar] [CrossRef]
- Hawley, M.H.; Almontashiri, N.; Biesecker, L.G.; Berger, N.; Chung, W.K.; Garcia, J.; Grebe, T.A.; Kelly, M.A.; Lebo, M.S.; Macaya, D.; et al. An assessment of the role of vinculin loss of function variants in inherited cardiomyopathy. Hum. Mutat. 2020, 41, 1577–1587. [Google Scholar] [CrossRef]
- Mazzarotto, F.; Tayal, U.; Buchan, R.J.; Midwinter, W.; Wilk, A.; Whiffin, N.; Govind, R.; Mazaika, E.; de Marvao, A.; Dawes, T.J.W.; et al. Reevaluating the Genetic Contribution of Monogenic Dilated Cardiomyopathy. Circulation 2020, 141, 387–398. [Google Scholar] [CrossRef]
- Sugiishi, M.; Takatsu, F. Cigarette smoking is a major risk factor for coronary spasm. Circulation 1993, 87, 76–79. [Google Scholar] [CrossRef]
- Chen, J.H.; Inamori-Kawamoto, O.; Michiue, T.; Ikeda, S.; Ishikawa, T.; Maeda, H. Cardiac biomarkers in blood, and pericardial and cerebrospinal fluids of forensic autopsy cases: A reassessment with special regard to postmortem interval. Leg. Med. 2015, 17, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Michiue, T.; Ishikawa, T.; Zhu, B.L.; Maeda, H. Combined analyses of creatine kinase MB, cardiac troponin I and myoglobin in pericardial and cerebrospinal fluids to investigate myocardial and skeletal muscle injury in medicolegal autopsy cases. Leg. Med. 2011, 13, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Pang, B.; Zhu, Y.; Ni, J.; Thompson, J.; Malouf, D.; Bucci, J.; Graham, P.; Li, Y. Extracellular vesicles: The next generation of biomarkers for liquid biopsy-based prostate cancer diagnosis. Theranostics 2020, 10, 2309–2326. [Google Scholar] [CrossRef] [PubMed]
- Moller, A.; Lobb, R.J. The evolving translational potential of small extracellular vesicles in cancer. Nat. Rev. Cancer 2020, 20, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, N.; Whiteside, T.L.; Reichert, T.E. Challenges in Exosome Isolation and Analysis in Health and Disease. Int. J. Mol. Sci. 2019, 20, 4684. [Google Scholar] [CrossRef]
- Macdonald, R.L.; Zhang, J.; Han, H. Angioplasty reduces pharmacologically mediated vasoconstriction in rabbit carotid arteries with and without vasospasm. Stroke 1995, 26, 1053–1059; discussion 1059–1060. [Google Scholar] [CrossRef]
- Li, L.; Jin, Y.P.; Xia, S.D.; Feng, C. The Biochemical Markers Associated with the Occurrence of Coronary Spasm. Biomed Res. Int. 2019, 2019, 4834202. [Google Scholar] [CrossRef]
- Takagi, Y.; Yasuda, S.; Takahashi, J.; Tsunoda, R.; Ogata, Y.; Seki, A.; Sumiyoshi, T.; Matsui, M.; Goto, T.; Tanabe, Y.; et al. Clinical implications of provocation tests for coronary artery spasm: Safety, arrhythmic complications, and prognostic impact: Multicentre registry study of the Japanese Coronary Spasm Association. Eur. Heart J. 2013, 34, 258–267. [Google Scholar] [CrossRef]
- JCS Joint Working Group. Guidelines for diagnosis and treatment of patients with vasospastic angina (Coronary Spastic Angina) (JCS 2013). Circ. J. 2014, 78, 2779–2801. [Google Scholar] [CrossRef]
- Vishnevsky, A.; Julien, H.M.; Fischman, D.L.; Walinsky, P.; David Ogilby, J.; Ruggiero, N.J., 2nd; Jasti, B.; Savage, M.P. Unrecognized coronary vasospasm in patients referred for percutaneous coronary intervention: Intracoronary nitroglycerin, the forgotten stepchild of cardiovascular guidelines. Catheter. Cardiovasc. Interv. 2017, 90, 1086–1090. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Liu, Z.; Lin, X.; Zhong, F.; Li, S.; Tang, X.; Zhang, Y.; Li, L. Second-generation antipsychotics induce cardiotoxicity by disrupting spliceosome signaling: Implications from proteomic and transcriptomic analyses. Pharmacol. Res. 2021, 170, 105714. [Google Scholar] [CrossRef]
- Wilhelm, M.; Schlegl, J.; Hahne, H.; Gholami, A.M.; Lieberenz, M.; Savitski, M.M.; Ziegler, E.; Butzmann, L.; Gessulat, S.; Marx, H.; et al. Mass-spectrometry-based draft of the human proteome. Nature 2014, 509, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-D.; Xu, Q.-S.; Liang, Y.-Z. libPLS: An integrated library for partial least squares regression and linear discriminant analysis. Chemom. Intell. Lab. Syst. 2018, 176, 34–43. [Google Scholar] [CrossRef]
- Hornung, V.; Bauernfeind, F.; Halle, A.; Samstad, E.O.; Kono, H.; Rock, K.L.; Fitzgerald, K.A.; Latz, E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 2008, 9, 847–856. [Google Scholar] [CrossRef] [PubMed]






| Categories | Total | CAS | Non-CAS | p-Value |
|---|---|---|---|---|
| (n = 74) | (n = 25) | (n = 49) | ||
| Men, n (%) | 58 (78.4%) | 16 (64.0%) | 42 (85.7%) | 0.032 |
| Age (year) | 65 (55–73) | 63 (53–69) | 68 (56–76) | 0.076 |
| Smoking, n (%) | 18 (24.3%) | 6 (24.0%) | 12 (24.5%) | 0.963 |
| Drinking, n (%) | 8 (10.8%) | 1 (4.0%) | 7 (14.3%) | 0.253 |
| Hypertension, n (%) | 54 (73.0%) | 17 (68.0%) | 37 (75.5%) | 0.401 |
| DM, n (%) | 27 (36.5%) | 8 (32.0%) | 19 (38.8%) | 0.524 |
| Fast blood glucose (mmol/L) | 5.5 (4.8–7.0) | 5.3 (4.7–5.6) | 6.0 (4.9–8.5) | 0.005 |
| HbA1c (%) | 5.8 (5.5–7.2) | 5.7 (5.5–6.3) | 5.9 (5.4–7.8) | 0.072 |
| Total cholesterol (mmol/L) | 3.8 (3.0–4.5) | 3.6 (3.0–4.2) | 3.9 (3.0–4.7) | 0.139 |
| Total triglyceride (mmol/L) | 1.2 (0.9–2.1) | 1.1 (0.8–1.3) | 1.7 (0.9–2.3) | 0.116 |
| LDLC (mmol/L) | 2.0 (±0.7) | 1.9 (±0.7) | 2.0 (±0.8) | 0.348 |
| HDLC (mmol/L) | 1.2 (±0.6) | 1.2 (±0.5) | 1.2 (±0.7) | 0.665 |
| hsCRP (mg/L) | 2.45 (0.50–35.80) | 0.400 (0.150–1.95) | 7.20 (1.35–63.50) | <0.001 |
| cTnT (ng/mL) | 0.048 (0.009–0.209) | 0.009 (0.007–0.015) | 0.081 (0.033–0.427) | 0.007 |
| CK-MB (U/L) | 16.0 (12.8–22.0) | 14.0 (12.0–17.0) | 18.0 (14.0–24.0) | 0.007 |
| CK-MM (U/L) | 66.0 (41.8–116.3) | 76.0 (50.0–113.5) | 57.0 (27.5–149.8) | 0.386 |
| log10(proBNP) (pg/mL) | 2.7 (2.1–3.2) | 2.3 (1.8–2.7) | 2.8 (2.3–3.3) | 0.013 |
| LVEF (%) | 62 (52–66) | 62 (56–67) | 62 (50–66) | 0.543 |
| Hemoglobin (g/L) | 128 (113–140) | 127 (121–137) | 129.0 (106–141) | 0.504 |
| Albumin (g/L) | 40 (35–43) | 41 (39–44) | 38 (33–42) | 0.025 |
| Blood creatinine (µmol/L) | 84 (70–113) | 83 (58–104) | 85 (72–136) | 0.448 |
| SELENBP1 (ng/L) | 209.8 (161.0–272.2) | 151.1 (143.9–163.6) | 234.4 (208.5–302.9) | <0.001 |
| VCL (ng/mL) | 113.1 (90.3–136.0) | 86.7 (77.8–96.6) | 126.0 (110.5–165.7) | <0.001 |
| β-blocker, n (%) | 30 (40.5%) | 10 (40.0%) | 20 (40.8%) | 0.946 |
| CCB, n (%) | 30 (40.5%) | 9 (36.0%) | 21 (42.9%) | 0.570 |
| eGFR (mL/min/1.73 m2) | 80 (54–96) | 80 (60–96) | 79 (52–96) | 0.357 |
| urine acid, µmol/L | 352.0 (291.5–411.0) | 341.0 (291.5–406.0) | 356.0 (291.0–417.0) | 0.727 |
| Variables | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI for Odds Ratio | p Value | Odds Ratio | 95% CI for Odds Ratio | p Value | |
| Sex | 3.375 | 1.076–10.588 | 0.037 | 1.744 | 0.118–25.806 | 0.686 |
| Age | 0.970 | 0.938–1.004 | 0.081 | 0.971 | 0.877–1.075 | 0.573 |
| Smoking | 0.974 | 0.316–3.000 | 0.963 | |||
| Drinking | 0.250 | 0.029–2.156 | 0.207 | |||
| Hypertension | 0.632 | 0.215–1.854 | 0.403 | |||
| DM | 0.718 | 0.259–1.992 | 0.525 | |||
| β-blocker | 0.967 | 0.362–2.581 | 0.946 | |||
| CCBs | 0.750 | 0.278–2.026 | 0.570 | |||
| Blood glucose | 0.727 | 0.542–0.975 | 0.034 | 1.006 | 0.571–1.774 | 0.983 |
| HbA1c | 0.628 | 0.365–1.081 | 0.093 | 0.736 | 0.395–1.370 | 0.333 |
| Total cholesterol | 0.646 | 0.362–1.155 | 0.141 | |||
| Total glyceride | 0.688 | 0.418–1.134 | 0.142 | |||
| LDLC | 0.699 | 0.334–1.465 | 0.343 | |||
| HDLC | 1.216 | 0.510–2.902 | 0.659 | |||
| hs-CRP | 0.965 | 0.935–0.996 | 0.026 | 0.974 | 0.910–1.042 | 0.436 |
| cTnT | 0.059 | 0.002–1.455 | 0.084 | 1.578 | 0.091–27.400 | 0.754 |
| CK-MB | 0.930 | 0.866–0.998 | 0.045 | 0.901 | 0.780–1.040 | 0.155 |
| CK-MM | 0.999 | 0.996–1.002 | 0.409 | |||
| log10(proBNP) | 0.418 | 0.204–0.855 | 0.017 | 0.62 | 0.074–5.164 | 0.658 |
| LVEF | 1.015 | 0.968–1.065 | 0.538 | |||
| Hemoglobin | 1.007 | 0.987–1.028 | 0.500 | |||
| Albumin | 1.104 | 1.002–1.216 | 0.045 | 1.031 | 0.785–1.355 | 0.825 |
| Blood creatinine | 0.999 | 0.997–1.001 | 0.452 | |||
| SELENBP1 | 0.949 | 0.926–0.978 | <0.001 | 0.962 | 0.930–0.994 | 0.022 |
| VCL | 0.906 | 0.863–0.950 | <0.001 | 0.917 | 0.848–0.991 | 0.028 |
| eGFR | 1.008 | 0.991–1.025 | 0.353 | |||
| Urine acid | 0.999 | 0.995–1.004 | 0.723 | |||
| Variables | Odds Ratio | 95% CI for Odds Ratio | p-Value |
|---|---|---|---|
| SELENBP1 | 0.677 | 0.520–0.881 | 0.004 |
| VCL | 0.464 | 0.368–0.805 | 0.006 |
| Categories | Non-Cardiac Death | CAS-Induced Sudden Death |
|---|---|---|
| Cases | 11 | 12 |
| Age, years | 42.73 ± 13.36 | 52.42 ± 4.25 |
| Gender (male: female) | 6:5 | 12:0 |
| Interval from attack to death, h | <1.50 ± 0.50 | <2.58 ± 2.33 |
| Cause of death | Traumatic death/drug intoxication | Sudden cardiac death |
| Potential death mechanism | Hemorrhagic shock/respiratory suppression | Heart failure/arrhythmia |
| CK-MB (ng/mL) | 173.22 ± 139.75 | 334.41 ± 186.64 * |
| cTnI (ng/L) | 703.36 ± 309.96 | 793.88 ± 361.17 |
| Variables | Odds Ratio | 95% CI for Odds Ratio | p-Value |
|---|---|---|---|
| SELENBP1 | 0.283 | 0.104–0.775 | 0.014 |
| VCL | 0.506 | 0.265–0.968 | 0.040 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, X.; Lin, Z.; Zhao, X.; Liu, Z.; Xu, C.; Yu, B.; Gao, P.; Wang, Z.; Ge, J.; Shen, Y.; et al. Serum SELENBP1 and VCL Are Effective Biomarkers for Clinical and Forensic Diagnosis of Coronary Artery Spasm. Int. J. Mol. Sci. 2022, 23, 13266. https://doi.org/10.3390/ijms232113266
Lin X, Lin Z, Zhao X, Liu Z, Xu C, Yu B, Gao P, Wang Z, Ge J, Shen Y, et al. Serum SELENBP1 and VCL Are Effective Biomarkers for Clinical and Forensic Diagnosis of Coronary Artery Spasm. International Journal of Molecular Sciences. 2022; 23(21):13266. https://doi.org/10.3390/ijms232113266
Chicago/Turabian StyleLin, Xinyi, Zijie Lin, Xin Zhao, Zheng Liu, Chenchao Xu, Bokang Yu, Pan Gao, Zhimin Wang, Junbo Ge, Yiwen Shen, and et al. 2022. "Serum SELENBP1 and VCL Are Effective Biomarkers for Clinical and Forensic Diagnosis of Coronary Artery Spasm" International Journal of Molecular Sciences 23, no. 21: 13266. https://doi.org/10.3390/ijms232113266
APA StyleLin, X., Lin, Z., Zhao, X., Liu, Z., Xu, C., Yu, B., Gao, P., Wang, Z., Ge, J., Shen, Y., & Li, L. (2022). Serum SELENBP1 and VCL Are Effective Biomarkers for Clinical and Forensic Diagnosis of Coronary Artery Spasm. International Journal of Molecular Sciences, 23(21), 13266. https://doi.org/10.3390/ijms232113266






