Skeletal Muscle Regeneration in Cardiotoxin-Induced Muscle Injury Models
Abstract
1. Introduction
2. The Characteristics and Positions of Injury in CTX-Induced Skeletal Muscle Injury Models
3. Skeletal Muscle Regeneration in Different Mouse Models after CTX-Induced Skeletal Muscle Injury
4. Mechanisms of Regeneration in CTX-Induced Injury Models
4.1. Inflammatory Response in CTX-Induced Injury Models
4.1.1. The Mechanisms of Inflammatory Response in CTX-Induced Skeletal Muscle Injury
4.1.2. The Mechanism of Macrophage Polarization in CTX-Induced Skeletal Muscle Injury
4.1.3. The Mechanism of Necrotic Fiber Debris Clearance in CTX-Induced Skeletal Muscle Injury
4.2. SC Activation and Myoblast Proliferation, Differentiation, and Fusion in CTX-Induced Injury Models
4.2.1. Mechanisms of SCs Activation and Myoblast Proliferation in CTX-Induced Injury Models
4.2.2. Mechanisms of Self-Renewal of SC Pool in CTX-Induced Injury Models
4.2.3. Mechanisms of Myoblast Differentiation in CTX-Induced Injury Models
4.2.4. Mechanisms of Myoblast Fusion in CTX-Induced Injury Models
4.2.5. Mechanisms of Myotube Maturation in CTX-Induced Injury Models
4.3. Fibrosis in CTX-Induced Injury Models
4.4. Calcification in CTX-Induced Injury Models
4.5. Angiopoiesis and Neurogenesis in CTX-Induced Injury Models
4.6. Other Regeneration-Related Genes in CTX-Induced Injury Models
4.7. Non-SC Stem Cells Regulate Regeneration in CTX-Induced Injury Muscle
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Shi, X.; Garry, D.J. Myogenic regulatory factors transactivate the Tceal7 gene and modulate muscle differentiation. Biochem. J. 2010, 428, 213–221. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Koh, T.J.; Bryer, S.C.; Pucci, A.M.; Sisson, T.H. Mice deficient in plasminogen activator inhibitor-1 have improved skeletal muscle regeneration. Am. J. Physiol. Cell Physiol. 2005, 289, C217–C223. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.; Manabe, I.; Suzuki, Y.; Relaix, F.; Oishi, Y. Klf5 regulates muscle differentiation by directly targeting muscle-specific genes in cooperation with MyoD in mice. eLife 2016, 5, e17462. [Google Scholar] [CrossRef] [PubMed]
- Alves, J.M.; Martins, A.H.; Lameu, C.; Glaser, T.; Boukli, N.M.; Bassaneze, V.; Dariolli, R.; Nascimento, I.C.; Martins, P.C.M.; de Souza, H.D.N.; et al. Kinin-B2 Receptor Activity in Skeletal Muscle Regeneration and Myoblast Differentiation. Stem Cell Rev. Rep. 2019, 15, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Choi, S.; Liu, X.; Zhang, M.; Schageman, J.J.; Lee, S.Y.; Hart, R.; Lin, L.; Thurmond, F.A.; Williams, R.S. Highly coordinated gene regulation in mouse skeletal muscle regeneration. J. Biol. Chem. 2003, 278, 8826–8836. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, R.; Suzuki, T.; Do, M.Q.; Ohya, Y.; Anderson, J.E.; Shibata, A.; Kawaguchi, M.; Ohya, S.; Ohtsubo, H.; Mizunoya, W.; et al. Slow-Myofiber Commitment by Semaphorin 3A Secreted from Myogenic Stem Cells. Stem Cells 2017, 35, 1815–1834. [Google Scholar] [CrossRef]
- Ramadasan-Nair, R.; Gayathri, N.; Mishra, S.; Sunitha, B.; Mythri, R.B.; Nalini, A.; Subbannayya, Y.; Harsha, H.C.; Kolthur-Seetharam, U.; Srinivas Bharath, M.M. Mitochondrial alterations and oxidative stress in an acute transient mouse model of muscle degeneration: Implications for muscular dystrophy and related muscle pathologies. J. Biol. Chem. 2014, 289, 485–509. [Google Scholar] [CrossRef]
- Ohtsubo, H.; Sato, Y.; Suzuki, T.; Mizunoya, W.; Nakamura, M.; Tatsumi, R.; Ikeuchi, Y. APOBEC2 negatively regulates myoblast differentiation in muscle regeneration. Int. J. Biochem. Cell Biol. 2017, 85, 91–101. [Google Scholar] [CrossRef]
- Parise, G.; McKinnell, I.W.; Rudnicki, M.A. Muscle satellite cell and atypical myogenic progenitor response following exercise. Muscle Nerve 2008, 37, 611–619. [Google Scholar] [CrossRef]
- Armand, A.S.; Launay, T.; Gaspera, B.D.; Charbonnier, F.; Gallien, C.L.; Chanoine, C. Effects of eccentric treadmill running on mouse soleus: Degeneration/regeneration studied with Myf-5 and MyoD probes. Acta Physiol. Scand. 2003, 179, 75–84. [Google Scholar] [CrossRef]
- Czerwinska, A.M.; Streminska, W.; Ciemerych, M.A.; Grabowska, I. Mouse gastrocnemius muscle regeneration after mechanical or cardiotoxin injury. Folia Histochem. Cytobiol. 2012, 50, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.E.; Jiang, M.S.; Gong, Q.H.; Yudkowsky, M.L.; Wieland, S.J. Effects of a cardiotoxin from Naja naja kaouthia venom on skeletal muscle: Involvement of calcium-induced calcium release, sodium ion currents and phospholipases A2 and C. Toxicon 1991, 29, 1489–1500. [Google Scholar] [CrossRef]
- Lin Shiau, S.Y.; Huang, M.C.; Lee, C.Y. Mechanism of action of cobra cardiotoxin in the skeletal muscle. J. Pharmacol. Exp. Ther. 1976, 196, 758–770. [Google Scholar] [PubMed]
- Aoki, Y.; Nagata, T.; Yokota, T.; Nakamura, A.; Wood, M.J.; Partridge, T.; Takeda, S. Highly efficient in vivo delivery of PMO into regenerating myotubes and rescue in laminin-alpha2 chain-null congenital muscular dystrophy mice. Hum. Mol. Genet. 2013, 22, 4914–4928. [Google Scholar] [CrossRef]
- Randazzo, D.; Khalique, U.; Belanto, J.J.; Kenea, A.; Talsness, D.M.; Olthoff, J.T.; Tran, M.D.; Zaal, K.J.; Pak, K.; Pinal-Fernandez, I.; et al. Persistent upregulation of the beta-tubulin tubb6, linked to muscle regeneration, is a source of microtubule disorganization in dystrophic muscle. Hum. Mol. Genet. 2019, 28, 1117–1135. [Google Scholar] [CrossRef]
- Fan, W.; Gao, X.K.; Rao, X.S.; Shi, Y.P.; Liu, X.C.; Wang, F.Y.; Liu, Y.F.; Cong, X.X.; He, M.Y.; Xu, S.B.; et al. Hsp70 Interacts with Mitogen-Activated Protein Kinase (MAPK)-Activated Protein Kinase 2 To Regulate p38MAPK Stability and Myoblast Differentiation during Skeletal Muscle Regeneration. Mol. Cell. Biol. 2018, 38, e00211-18. [Google Scholar] [CrossRef] [PubMed]
- de Boer, M.D.; Seynnes, O.R.; di Prampero, P.E.; Pisot, R.; Mekjavic, I.B.; Biolo, G.; Narici, M.V. Effect of 5 weeks horizontal bed rest on human muscle thickness and architecture of weight bearing and non-weight bearing muscles. Eur. J. Appl. Physiol. 2008, 104, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Morton, A.B.; Jacobsen, N.L.; Segal, S.S. Functionalizing biomaterials to promote neurovascular regeneration following skeletal muscle injury. Am. J. Physiol. Cell Physiol. 2021, 320, C1099–C1111. [Google Scholar] [CrossRef]
- Markert, C.; Petroski, G.F.; Childers, C.K.; McDonald, K.S.; Childers, M.K. Stretch-induced force deficits in murine extensor digitorum longus muscles after cardiotoxin injection. Muscle Nerve 2006, 34, 485–488. [Google Scholar] [CrossRef]
- Takahashi, Y.; Shimizu, T.; Kato, S.; Nara, M.; Suganuma, Y.; Sato, T.; Morii, T.; Yamada, Y.; Fujita, H. Reduction of Superoxide Dismutase 1 Delays Regeneration of Cardiotoxin-Injured Skeletal Muscle in KK/Ta-Ins2(Akita) Mice with Progressive Diabetic Nephropathy. Int. J. Mol. Sci. 2021, 22, 5491. [Google Scholar] [CrossRef]
- Vignaud, A.; Ramond, F.; Hourde, C.; Keller, A.; Butler-Browne, G.; Ferry, A. Diabetes provides an unfavorable environment for muscle mass and function after muscle injury in mice. Pathobiology 2007, 74, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.H.; Cheng, M.; Koh, T.J. Impaired muscle regeneration in ob/ob and db/db mice. Sci. World J. 2011, 11, 1525–1535. [Google Scholar] [CrossRef] [PubMed]
- Krause, M.P.; Al-Sajee, D.; D’Souza, D.M.; Rebalka, I.A.; Moradi, J.; Riddell, M.C.; Hawke, T.J. Impaired macrophage and satellite cell infiltration occurs in a muscle-specific fashion following injury in diabetic skeletal muscle. PLoS ONE 2013, 8, e70971. [Google Scholar] [CrossRef]
- D’Souza, D.M.; Trajcevski, K.E.; Al-Sajee, D.; Wang, D.C.; Thomas, M.; Anderson, J.E.; Hawke, T.J. Diet-induced obesity impairs muscle satellite cell activation and muscle repair through alterations in hepatocyte growth factor signaling. Physiol. Rep. 2015, 3, e12506. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Wang, H.; Lee, I.H.; Modi, S.; Wang, X.; Du, J.; Mitch, W.E. PTEN inhibition improves muscle regeneration in mice fed a high-fat diet. Diabetes 2010, 59, 1312–1320. [Google Scholar] [CrossRef] [PubMed]
- Inaba, S.; Hinohara, A.; Tachibana, M.; Tsujikawa, K.; Fukada, S.I. Muscle regeneration is disrupted by cancer cachexia without loss of muscle stem cell potential. PLoS ONE 2018, 13, e0205467. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.P.; Shin, Y.J.; Kwon, K.S. microRNA for determining the age-related myogenic capabilities of skeletal muscle. BMB Rep. 2015, 48, 595–596. [Google Scholar] [CrossRef] [PubMed]
- Mouisel, E.; Vignaud, A.; Hourde, C.; Butler-Browne, G.; Ferry, A. Muscle weakness and atrophy are associated with decreased regenerative capacity and changes in mTOR signaling in skeletal muscles of venerable (18-24-month-old) dystrophic mdx mice. Muscle Nerve 2010, 41, 809–818. [Google Scholar] [CrossRef]
- Patsalos, A.; Pap, A.; Varga, T.; Trencsenyi, G.; Contreras, G.A.; Garai, I.; Papp, Z.; Dezso, B.; Pintye, E.; Nagy, L. In situ macrophage phenotypic transition is affected by altered cellular composition prior to acute sterile muscle injury. J. Physiol. 2017, 595, 5815–5842. [Google Scholar] [CrossRef]
- Ceco, E.; Celli, D.; Weinberg, S.; Shigemura, M.; Welch, L.C.; Volpe, L.; Chandel, N.S.; Bharat, A.; Lecuona, E.; Sznajder, J.I. Elevated CO2 Levels Delay Skeletal Muscle Repair by Increasing Fatty Acid Oxidation. Front. Physiol. 2020, 11, 630910. [Google Scholar] [CrossRef]
- Ohno, Y.; Matsuba, Y.; Hashimoto, N.; Sugiura, T.; Ohira, Y.; Yoshioka, T.; Goto, K. Suppression of Myostatin Stimulates Regenerative Potential of Injured Antigravitational Soleus Muscle in Mice under Unloading Condition. Int. J. Med. Sci. 2016, 13, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Matsuba, Y.; Goto, K.; Morioka, S.; Naito, T.; Akema, T.; Hashimoto, N.; Sugiura, T.; Ohira, Y.; Beppu, M.; Yoshioka, T. Gravitational unloading inhibits the regenerative potential of atrophied soleus muscle in mice. Acta Physiol. 2009, 196, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Joanisse, S.; Nederveen, J.P.; Baker, J.M.; Snijders, T.; Iacono, C.; Parise, G. Exercise conditioning in old mice improves skeletal muscle regeneration. FASEB J. 2016, 30, 3256–3268. [Google Scholar] [CrossRef] [PubMed]
- Horii, N.; Uchida, M.; Hasegawa, N.; Fujie, S.; Oyanagi, E.; Yano, H.; Hashimoto, T.; Iemitsu, M. Resistance training prevents muscle fibrosis and atrophy via down-regulation of C1q-induced Wnt signaling in senescent mice. FASEB J. 2018, 32, 3547–3559. [Google Scholar] [CrossRef] [PubMed]
- Fujiya, H.; Ogura, Y.; Ohno, Y.; Goto, A.; Nakamura, A.; Ohashi, K.; Uematsu, D.; Aoki, H.; Musha, H.; Goto, K. Microcurrent electrical neuromuscular stimulation facilitates regeneration of injured skeletal muscle in mice. J. Sports Sci. Med. 2015, 14, 297–303. [Google Scholar]
- Jinno, N.; Nagata, M.; Takahashi, T. Marginal zinc deficiency negatively affects recovery from muscle injury in mice. Biol. Trace Elem. Res. 2014, 158, 65–72. [Google Scholar] [CrossRef]
- Morioka, S.; Goto, K.; Kojima, A.; Naito, T.; Matsuba, Y.; Akema, T.; Fujiya, H.; Sugiura, T.; Ohira, Y.; Beppu, M.; et al. Functional overloading facilitates the regeneration of injured soleus muscles in mice. J. Physiol. Sci. 2008, 58, 397–404. [Google Scholar] [CrossRef]
- Kohno, S.; Yamashita, Y.; Abe, T.; Hirasaka, K.; Oarada, M.; Ohno, A.; Teshima-Kondo, S.; Higashibata, A.; Choi, I.; Mills, E.M.; et al. Unloading stress disturbs muscle regeneration through perturbed recruitment and function of macrophages. J. Appl. Physiol. 2012, 112, 1773–1782. [Google Scholar] [CrossRef]
- Kimoloi, S.; Sen, A.; Guenther, S.; Braun, T.; Brugmann, T.; Sasse, P.; Wiesner, R.J.; Pla-Martin, D.; Baris, O.R. Combined fibre atrophy and decreased muscle regeneration capacity driven by mitochondrial DNA alterations underlie the development of sarcopenia. J. Cachexia Sarcopenia Muscle 2022, 13, 2132–2145. [Google Scholar] [CrossRef]
- Fearing, C.M.; Melton, D.W.; Lei, X.; Hancock, H.; Wang, H.; Sarwar, Z.U.; Porter, L.; McHale, M.; McManus, L.M.; Shireman, P.K. Increased Adipocyte Area in Injured Muscle With Aging and Impaired Remodeling in Female Mice. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 992–1004. [Google Scholar] [CrossRef]
- Chaiyasing, R.; Sugiura, A.; Ishikawa, T.; Ojima, K.; Warita, K.; Hosaka, Y.Z. Estrogen modulates the skeletal muscle regeneration process and myotube morphogenesis: Morphological analysis in mice with a low estrogen status. J. Vet. Med. Sci. 2021, 83, 1812–1819. [Google Scholar] [CrossRef] [PubMed]
- Rebalka, I.A.; Cao, A.W.; Raleigh, M.J.; Henriksbo, B.D.; Coleman, S.K.; Schertzer, J.D.; Hawke, T.J. Statin Therapy Negatively Impacts Skeletal Muscle Regeneration and Cutaneous Wound Repair in Type 1 Diabetic Mice. Front. Physiol. 2017, 8, 1088. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Conboy, M.J.; Conboy, I.M. Pharmacological inhibition of myostatin/TGF-beta receptor/pSmad3 signaling rescues muscle regenerative responses in mouse model of type 1 diabetes. Acta Pharmacol. Sin. 2013, 34, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- McHale, M.J.; Sarwar, Z.U.; Cardenas, D.P.; Porter, L.; Salinas, A.S.; Michalek, J.E.; McManus, L.M.; Shireman, P.K. Increased fat deposition in injured skeletal muscle is regulated by sex-specific hormones. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 302, R331–R339. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Satoh, A.; Horinouchi, Y.; Hamano, H.; Watanabe, H.; Imao, M.; Imanishi, M.; Zamami, Y.; Takechi, K.; Izawa-Ishizawa, Y.; et al. Iron accumulation causes impaired myogenesis correlated with MAPK signaling pathway inhibition by oxidative stress. FASEB J. 2019, 33, 9551–9564. [Google Scholar] [CrossRef]
- Attia, M.; Maurer, M.; Robinet, M.; Le Grand, F.; Fadel, E.; Le Panse, R.; Butler-Browne, G.; Berrih-Aknin, S. Muscle satellite cells are functionally impaired in myasthenia gravis: Consequences on muscle regeneration. Acta Neuropathol. 2017, 134, 869–888. [Google Scholar] [CrossRef] [PubMed]
- Saliu, T.P.; Kumrungsee, T.; Mitsumoto, K.; Chen, S.; Yanaka, N. Satellite cell content and muscle regeneration in a mouse model of NAFLD. Nutrition 2022, 96, 111570. [Google Scholar] [CrossRef]
- Rahman, F.A.; Angus, S.A.; Stokes, K.; Karpowicz, P.; Krause, M.P. Impaired ECM Remodeling and Macrophage Activity Define Necrosis and Regeneration Following Damage in Aged Skeletal Muscle. Int. J. Mol. Sci. 2020, 21, 4575. [Google Scholar] [CrossRef]
- Paiva-Oliveira, E.L.; da Silva, R.F.; Bellio, M.; Quirico-Santos, T.; Lagrota-Candido, J. Pattern of cardiotoxin-induced muscle remodeling in distinct TLR-4 deficient mouse strains. Histochem. Cell Biol. 2017, 148, 49–60. [Google Scholar] [CrossRef]
- Yoshioka, K.; Kitajima, Y.; Seko, D.; Tsuchiya, Y.; Ono, Y. The body region specificity in murine models of muscle regeneration and atrophy. Acta Physiol. 2021, 231, e13553. [Google Scholar] [CrossRef]
- Nagata, K.; Nakamura, T.; Fujihara, S.; Tanaka, E. Ultrasound modulates the inflammatory response and promotes muscle regeneration in injured muscles. Ann. Biomed. Eng. 2013, 41, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- LaBarge, S.; McDonald, M.; Smith-Powell, L.; Auwerx, J.; Huss, J.M. Estrogen-related receptor-alpha (ERRalpha) deficiency in skeletal muscle impairs regeneration in response to injury. FASEB J. 2014, 28, 1082–1097. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.A.; Ryan, T.E.; Lin, C.T.; Inigo, M.M.R.; Green, T.D.; Brault, J.J.; Spangenburg, E.E.; McClung, J.M. Diminished force production and mitochondrial respiratory deficits are strain-dependent myopathies of subacute limb ischemia. J. Vasc. Surg. 2017, 65, 1504–1514.e1511. [Google Scholar] [CrossRef] [PubMed]
- Hardy, D.; Besnard, A.; Latil, M.; Jouvion, G.; Briand, D.; Thepenier, C.; Pascal, Q.; Guguin, A.; Gayraud-Morel, B.; Cavaillon, J.M.; et al. Comparative Study of Injury Models for Studying Muscle Regeneration in Mice. PLoS ONE 2016, 11, e0147198. [Google Scholar] [CrossRef]
- Heredia, J.E.; Mukundan, L.; Chen, F.M.; Mueller, A.A.; Deo, R.C.; Locksley, R.M.; Rando, T.A.; Chawla, A. Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell 2013, 153, 376–388. [Google Scholar] [CrossRef]
- Dinulovic, I.; Furrer, R.; Di Fulvio, S.; Ferry, A.; Beer, M.; Handschin, C. PGC-1alpha modulates necrosis, inflammatory response, and fibrotic tissue formation in injured skeletal muscle. Skelet. Muscle 2016, 6, 38. [Google Scholar] [CrossRef]
- Shi, D.; Gu, R.; Song, Y.; Ding, M.; Huang, T.; Guo, M.; Xiao, J.; Huang, W.; Liao, H. Calcium/Calmodulin-Dependent Protein Kinase IV (CaMKIV) Mediates Acute Skeletal Muscle Inflammatory Response. Inflammation 2018, 41, 199–212. [Google Scholar] [CrossRef]
- Neves Jde, C.; Rizzato, V.R.; Fappi, A.; Garcia, M.M.; Chadi, G.; van de Vlekkert, D.; d’Azzo, A.; Zanoteli, E. Neuraminidase-1 mediates skeletal muscle regeneration. Biochim. Biophys. Acta 2015, 1852, 1755–1764. [Google Scholar] [CrossRef]
- Liao, Z.H.; Huang, T.; Xiao, J.W.; Gu, R.C.; Ouyang, J.; Wu, G.; Liao, H. Estrogen signaling effects on muscle-specific immune responses through controlling the recruitment and function of macrophages and T cells. Skelet. Muscle 2019, 9, 20. [Google Scholar] [CrossRef]
- Kohno, S.; Ueji, T.; Abe, T.; Nakao, R.; Hirasaka, K.; Oarada, M.; Harada-Sukeno, A.; Ohno, A.; Higashibata, A.; Mukai, R.; et al. Rantes secreted from macrophages disturbs skeletal muscle regeneration after cardiotoxin injection in Cbl-b-deficient mice. Muscle Nerve 2011, 43, 223–229. [Google Scholar] [CrossRef]
- Wang, H.; Melton, D.W.; Porter, L.; Sarwar, Z.U.; McManus, L.M.; Shireman, P.K. Altered macrophage phenotype transition impairs skeletal muscle regeneration. Am. J. Pathol. 2014, 184, 1167–1184. [Google Scholar] [CrossRef] [PubMed]
- Park, C.Y.; Pierce, S.A.; von Drehle, M.; Ivey, K.N.; Morgan, J.A.; Blau, H.M.; Srivastava, D. skNAC, a Smyd1-interacting transcription factor, is involved in cardiac development and skeletal muscle growth and regeneration. Proc. Natl. Acad. Sci. USA 2010, 107, 20750–20755. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Boadu, E.; Mercan, F.; Le, A.M.; Flach, R.J.; Zhang, L.; Tyner, K.J.; Olwin, B.B.; Bennett, A.M. MAP kinase phosphatase-1 deficiency impairs skeletal muscle regeneration and exacerbates muscular dystrophy. FASEB J. 2010, 24, 2985–2997. [Google Scholar] [CrossRef]
- Hu, J.; Shi, D.; Ding, M.; Huang, T.; Gu, R.; Xiao, J.; Xian, C.J.; Dong, J.; Wang, L.; Liao, H. Calmodulin-dependent signalling pathways are activated and mediate the acute inflammatory response of injured skeletal muscle. J. Physiol. 2019, 597, 5161–5177. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, P.; Song, T.; Radzyukevich, T.L.; Sadayappan, S.; Lingrel, J.B.; Heiny, J.A. KLF2 in Myeloid Lineage Cells Regulates the Innate Immune Response during Skeletal Muscle Injury and Regeneration. iScience 2019, 17, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, Y.; Guo, X.; Wu, Z.; Zhang, W. Loss of STAT1 in bone marrow-derived cells accelerates skeletal muscle regeneration. PLoS ONE 2012, 7, e37656. [Google Scholar] [CrossRef] [PubMed]
- Kozakowska, M.; Pietraszek-Gremplewicz, K.; Ciesla, M.; Seczynska, M.; Bronisz-Budzynska, I.; Podkalicka, P.; Bukowska-Strakova, K.; Loboda, A.; Jozkowicz, A.; Dulak, J. Lack of Heme Oxygenase-1 Induces Inflammatory Reaction and Proliferation of Muscle Satellite Cells after Cardiotoxin-Induced Skeletal Muscle Injury. Am. J. Pathol. 2018, 188, 491–506. [Google Scholar] [CrossRef]
- Zhang, C.; Cheng, N.; Qiao, B.; Zhang, F.; Wu, J.; Liu, C.; Li, Y.; Du, J. Age-related decline of interferon-gamma responses in macrophage impairs satellite cell proliferation and regeneration. J. Cachexia Sarcopenia Muscle 2020, 11, 1291–1305. [Google Scholar] [CrossRef]
- Yaden, B.C.; Wang, Y.X.; Wilson, J.M.; Culver, A.E.; Milner, A.; Datta-Mannan, A.; Shetler, P.; Croy, J.E.; Dai, G.; Krishnan, V. Inhibition of activin A ameliorates skeletal muscle injury and rescues contractile properties by inducing efficient remodeling in female mice. Am. J. Pathol. 2014, 184, 1152–1166. [Google Scholar] [CrossRef]
- Mothe-Satney, I.; Piquet, J.; Murdaca, J.; Sibille, B.; Grimaldi, P.A.; Neels, J.G.; Rousseau, A.S. Peroxisome Proliferator Activated Receptor Beta (PPARbeta) activity increases the immune response and shortens the early phases of skeletal muscle regeneration. Biochimie 2017, 136, 33–41. [Google Scholar] [CrossRef]
- Tanaka, Y.; Kita, S.; Nishizawa, H.; Fukuda, S.; Fujishima, Y.; Obata, Y.; Nagao, H.; Masuda, S.; Nakamura, Y.; Shimizu, Y.; et al. Adiponectin promotes muscle regeneration through binding to T-cadherin. Sci. Rep. 2019, 9, 16. [Google Scholar] [CrossRef]
- Sugihara, H.; Miyaji, K.; Yamanouchi, K.; Matsuwaki, T.; Nishihara, M. Progranulin deficiency leads to prolonged persistence of macrophages, accompanied with myofiber hypertrophy in regenerating muscle. J. Vet. Med. Sci. 2018, 80, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Lo Sicco, C.; Reverberi, D.; Balbi, C.; Ulivi, V.; Principi, E.; Pascucci, L.; Becherini, P.; Bosco, M.C.; Varesio, L.; Franzin, C.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles as Mediators of Anti-Inflammatory Effects: Endorsement of Macrophage Polarization. Stem Cells Transl. Med. 2017, 6, 1018–1028. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Sun, L.; Kawabata, Y.; Murayama, F.; Maegawa, T.; Nikawa, T.; Hirasaka, K. Balenine, Imidazole Dipeptide Promotes Skeletal Muscle Regeneration by Regulating Phagocytosis Properties of Immune Cells. Mar. Drugs 2022, 20, 313. [Google Scholar] [CrossRef]
- Cardoso, E.S.; Santana, T.A.; Diniz, P.B.; Montalvao, M.M.; Bani, C.C.; Thomazzi, S.M. Thymol accelerates the recovery of the skeletal muscle of mice injured with cardiotoxin. J. Pharm. Pharmacol. 2016, 68, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.G.; Zhu, Z.Q.; He, Z.Y.; Cheng, P.; Liang, S.; Chen, A.M.; Yang, Q. Endogenous conversion of n-6 to n-3 polyunsaturated fatty acids facilitates the repair of cardiotoxin-induced skeletal muscle injury in fat-1 mice. Aging 2021, 13, 8454–8466. [Google Scholar] [CrossRef] [PubMed]
- Chaweewannakorn, C.; Tsuchiya, M.; Koide, M.; Hatakeyama, H.; Tanaka, Y.; Yoshida, S.; Sugawara, S.; Hagiwara, Y.; Sasaki, K.; Kanzaki, M. Roles of IL-1alpha/beta in regeneration of cardiotoxin-injured muscle and satellite cell function. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R90–R103. [Google Scholar] [CrossRef]
- Nikolaidis, N.; Senf, S.M.; Howard, T.M.; Ahn, B.; Ferreira, L.F.; Judge, A.R. Loss of the Inducible Hsp70 Delays the Inflammatory Response to Skeletal Muscle Injury and Severely Impairs Muscle Regeneration. PLoS ONE 2013, 8, e62687. [Google Scholar] [CrossRef]
- Mojumdar, K.; Giordano, C.; Lemaire, C.; Liang, F.; Divangahi, M.; Qureshi, S.T.; Petrof, B.J. Divergent impact of Toll-like receptor 2 deficiency on repair mechanisms in healthy muscle versus Duchenne muscular dystrophy. J. Pathol. 2016, 239, 10–22. [Google Scholar] [CrossRef]
- Varga, T.; Mounier, R.; Gogolak, P.; Poliska, S.; Chazaud, B.; Nagy, L. Tissue LyC6- macrophages are generated in the absence of circulating LyC6- monocytes and Nur77 in a model of muscle regeneration. J. Immunol. 2013, 191, 5695–5701. [Google Scholar] [CrossRef]
- Ochoa, O.; Sun, D.; Reyes-Reyna, S.M.; Waite, L.L.; Michalek, J.E.; McManus, L.M.; Shireman, P.K. Delayed angiogenesis and VEGF production in CCR2-/- mice during impaired skeletal muscle regeneration. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R651–R661. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, Y.; Wu, Y.; Wang, L.; Wang, X.; Du, J. Interleukin-6/signal transducer and activator of transcription 3 (STAT3) pathway is essential for macrophage infiltration and myoblast proliferation during muscle regeneration. J. Biol. Chem. 2013, 288, 1489–1499. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xiao, Z.; Qu, C.; Cui, W.; Wang, X.; Du, J. CD8 T cells are involved in skeletal muscle regeneration through facilitating MCP-1 secretion and Gr1(high) macrophage infiltration. J. Immunol. 2014, 193, 5149–5160. [Google Scholar] [CrossRef] [PubMed]
- Martinez, C.O.; McHale, M.J.; Wells, J.T.; Ochoa, O.; Michalek, J.E.; McManus, L.M.; Shireman, P.K. Regulation of skeletal muscle regeneration by CCR2-activating chemokines is directly related to macrophage recruitment. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R832–R842. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Nguyen, M.H.; Fantuzzi, G.; Koh, T.J. Endogenous interferon-gamma is required for efficient skeletal muscle regeneration. Am. J. Physiol. Cell Physiol. 2008, 294, C1183–C1191. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, C.; Li, Y.; Miwa, T.; Liu, C.; Cui, W.; Song, W.C.; Du, J. Complement C3a signaling facilitates skeletal muscle regeneration by regulating monocyte function and trafficking. Nat. Commun. 2017, 8, 2078. [Google Scholar] [CrossRef]
- Sun, D.; Martinez, C.O.; Ochoa, O.; Ruiz-Willhite, L.; Bonilla, J.R.; Centonze, V.E.; Waite, L.L.; Michalek, J.E.; McManus, L.M.; Shireman, P.K. Bone marrow-derived cell regulation of skeletal muscle regeneration. FASEB J. 2009, 23, 382–395. [Google Scholar] [CrossRef]
- Nishimura, D.; Sakai, H.; Sato, T.; Sato, F.; Nishimura, S.; Toyama-Sorimachi, N.; Bartsch, J.W.; Sehara-Fujisawa, A. Roles of ADAM8 in elimination of injured muscle fibers prior to skeletal muscle regeneration. Mech. Dev. 2015, 135, 58–67. [Google Scholar] [CrossRef]
- Al-Zaeed, N.; Budai, Z.; Szondy, Z.; Sarang, Z. TAM kinase signaling is indispensable for proper skeletal muscle regeneration in mice. Cell Death Dis. 2021, 12, 611. [Google Scholar] [CrossRef]
- Zhang, J.; Qu, C.; Li, T.; Cui, W.; Wang, X.; Du, J. Phagocytosis mediated by scavenger receptor class BI promotes macrophage transition during skeletal muscle regeneration. J. Biol. Chem. 2019, 294, 15672–15685. [Google Scholar] [CrossRef]
- Jin, R.M.; Warunek, J.; Wohlfert, E.A. Chronic infection stunts macrophage heterogeneity and disrupts immune-mediated myogenesis. JCI Insight 2018, 3, e121549. [Google Scholar] [CrossRef] [PubMed]
- Bronisz-Budzynska, I.; Kozakowska, M.; Podkalicka, P.; Kachamakova-Trojanowska, N.; Loboda, A.; Dulak, J. The role of Nrf2 in acute and chronic muscle injury. Skelet. Muscle 2020, 10, 35. [Google Scholar] [CrossRef] [PubMed]
- Tarban, N.; Halász, H.; Gogolák, P.; Garabuczi, É.; Moise, A.R.; Palczewski, K.; Sarang, Z.; Szondy, Z. Regenerating Skeletal Muscle Compensates for the Impaired Macrophage Functions Leading to Normal Muscle Repair in Retinol Saturase Null Mice. Cells 2022, 11, 1333. [Google Scholar] [CrossRef] [PubMed]
- Dalle, S.; Poffe, C.; Hiroux, C.; Suhr, F.; Deldicque, L.; Koppo, K. Ibuprofen does not impair skeletal muscle regeneration upon cardiotoxin-induced injury. Physiol. Res. 2020, 69, 847–859. [Google Scholar] [CrossRef]
- Shen, W.; Li, Y.; Zhu, J.; Schwendener, R.; Huard, J. Interaction between macrophages, TGF-beta1, and the COX-2 pathway during the inflammatory phase of skeletal muscle healing after injury. J. Cell. Physiol. 2008, 214, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, A.S.; Murdaca, J.; Le Menn, G.; Sibille, B.; Wahli, W.; Le Garf, S.; Chinetti, G.; Neels, J.G.; Mothe-Satney, I. Invalidation of the Transcriptional Modulator of Lipid Metabolism PPARbeta/delta in T Cells Prevents Age-Related Alteration of Body Composition and Loss of Endurance Capacity. Front. Physiol. 2021, 12, 587753. [Google Scholar] [CrossRef] [PubMed]
- Mann, A.O.; Hanna, B.S.; Munoz-Rojas, A.R.; Sandrock, I.; Prinz, I.; Benoist, C.; Mathis, D. IL-17A-producing gammadeltaT cells promote muscle regeneration in a microbiota-dependent manner. J. Exp. Med. 2022, 219, e20211504. [Google Scholar] [CrossRef]
- Ding, M.; Huang, T.; Zhu, R.; Gu, R.; Shi, D.; Xiao, J.; Guo, M.; Li, J.; Hu, J.; Liao, H. Immunological Behavior Analysis of Muscle Cells under IFN-gamma Stimulation in Vitro and in Vivo. Anat. Rec. 2018, 301, 1551–1563. [Google Scholar] [CrossRef]
- Huang, T.; Huang, J.; Liao, Z.; Lan, H.; Jian, X.; Gu, R.; Ouyang, J.; Hu, J.; Liao, H. Regenerating myofiber directs Tregs and Th17 responses in inflamed muscle through the intrinsic TGF-beta signaling-mediated IL-6 production. Am J. Physiol. Endocrinol. Metab. 2022, 323, E92–E106. [Google Scholar] [CrossRef]
- Pierce, A.P.; de Waal, E.; McManus, L.M.; Shireman, P.K.; Chaudhuri, A.R. Oxidation and structural perturbation of redox-sensitive enzymes in injured skeletal muscle. Free Radic. Biol. Med. 2007, 43, 1584–1593. [Google Scholar] [CrossRef]
- Sciorati, C.; Rigamonti, E.; Manfredi, A.A.; Rovere-Querini, P. Cell death, clearance and immunity in the skeletal muscle. Cell Death Differ. 2016, 23, 927–937. [Google Scholar] [CrossRef]
- Budai, Z.; Al-Zaeed, N.; Szentesi, P.; Halasz, H.; Csernoch, L.; Szondy, Z.; Sarang, Z. Impaired Skeletal Muscle Development and Regeneration in Transglutaminase 2 Knockout Mice. Cells 2021, 10, 3089. [Google Scholar] [CrossRef] [PubMed]
- Sarang, Z.; Saghy, T.; Budai, Z.; Ujlaky-Nagy, L.; Bedekovics, J.; Beke, L.; Mehes, G.; Nagy, G.; Ruhl, R.; Moise, A.R.; et al. Retinol Saturase Knock-Out Mice are Characterized by Impaired Clearance of Apoptotic Cells and Develop Mild Autoimmunity. Biomolecules 2019, 9, 737. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Zou, X.; Wu, R.; Zhong, R.; Zhu, D.; Zhang, Y. Accelerated regeneration of the skeletal muscle in RNF13-knockout mice is mediated by macrophage-secreted IL-4/IL-6. Protein Cell 2014, 5, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.N.; Tajbakhsh, S.; Mouly, V.; Cossu, G.; Buckingham, M.; Butler-Browne, G.S. In vivo satellite cell activation via Myf5 and MyoD in regenerating mouse skeletal muscle. J. Cell Sci. 1999, 112 Pt 17, 2895–2901. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Han, Y.; Liu, J.; Liu, L.; Zheng, L.; Chen, Y.; Xia, R.; Yao, D.; Cai, X.; Xu, X. Rbm24 modulates adult skeletal muscle regeneration via regulation of alternative splicing. Theranostics 2020, 10, 11159–11177. [Google Scholar] [CrossRef]
- Galimov, A.; Merry, T.L.; Luca, E.; Rushing, E.J.; Mizbani, A.; Turcekova, K.; Hartung, A.; Croce, C.M.; Ristow, M.; Krutzfeldt, J. MicroRNA-29a in Adult Muscle Stem Cells Controls Skeletal Muscle Regeneration During Injury and Exercise Downstream of Fibroblast Growth Factor-2. Stem Cells 2016, 34, 768–780. [Google Scholar] [CrossRef]
- Zeng, L.; Akasaki, Y.; Sato, K.; Ouchi, N.; Izumiya, Y.; Walsh, K. Insulin-like 6 is induced by muscle injury and functions as a regenerative factor. J. Biol. Chem. 2010, 285, 36060–36069. [Google Scholar] [CrossRef]
- Hawke, T.J.; Atkinson, D.J.; Kanatous, S.B.; Van der Ven, P.F.; Goetsch, S.C.; Garry, D.J. Xin, an actin binding protein, is expressed within muscle satellite cells and newly regenerated skeletal muscle fibers. Am. J. Physiol. Cell Physiol. 2007, 293, C1636–C1644. [Google Scholar] [CrossRef]
- Al-Sajee, D.; Nissar, A.A.; Coleman, S.K.; Rebalka, I.A.; Chiang, A.; Wathra, R.; van der Ven, P.F.; Orfanos, Z.; Hawke, T.J. Xin-deficient mice display myopathy, impaired contractility, attenuated muscle repair and altered satellite cell functionality. Acta Physiol. 2015, 214, 248–260. [Google Scholar] [CrossRef]
- Nissar, A.A.; Zemanek, B.; Labatia, R.; Atkinson, D.J.; van der Ven, P.F.; Furst, D.O.; Hawke, T.J. Skeletal muscle regeneration is delayed by reduction in Xin expression: Consequence of impaired satellite cell activation? Am. J. Physiol. Cell Physiol. 2012, 302, C220–C227. [Google Scholar] [CrossRef] [PubMed]
- Hillege, M.M.G.; Shi, A.; Galli, R.A.; Wu, G.; Bertolino, P.; Hoogaars, W.M.H.; Jaspers, R.T. Lack of Tgfbr1 and Acvr1b synergistically stimulates myofibre hypertrophy and accelerates muscle regeneration. Elife 2022, 11, e77610. [Google Scholar] [CrossRef] [PubMed]
- Shelar, S.B.; Narasimhan, M.; Shanmugam, G.; Litovsky, S.H.; Gounder, S.S.; Karan, G.; Arulvasu, C.; Kensler, T.W.; Hoidal, J.R.; Darley-Usmar, V.M.; et al. Disruption of nuclear factor (erythroid-derived-2)-like 2 antioxidant signaling: A mechanism for impaired activation of stem cells and delayed regeneration of skeletal muscle. FASEB J. 2016, 30, 1865–1879. [Google Scholar] [CrossRef] [PubMed]
- Rebalka, I.A.; Monaco, C.M.F.; Varah, N.E.; Berger, T.; D’Souza, D.M.; Zhou, S.; Mak, T.W.; Hawke, T.J. Loss of the adipokine lipocalin-2 impairs satellite cell activation and skeletal muscle regeneration. Am. J. Physiol. Cell Physiol. 2018, 315, C714–C721. [Google Scholar] [CrossRef] [PubMed]
- Schaaf, G.J.; van Gestel, T.J.M.; In‘t Groen, S.L.M.; de Jong, B.; Boomaars, B.; Tarallo, A.; Cardone, M.; Parenti, G.; van der Ploeg, A.T.; Pijnappel, W. Satellite cells maintain regenerative capacity but fail to repair disease-associated muscle damage in mice with Pompe disease. Acta Neuropathol. Commun. 2018, 6, 119. [Google Scholar] [CrossRef]
- Serra, C.; Tangherlini, F.; Rudy, S.; Lee, D.; Toraldo, G.; Sandor, N.L.; Zhang, A.; Jasuja, R.; Bhasin, S. Testosterone improves the regeneration of old and young mouse skeletal muscle. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 17–26. [Google Scholar] [CrossRef]
- Liu, Q.; Pan, S.; Liu, S.; Zhang, S.; Willerson, J.T.; Martin, J.F.; Dixon, R.A.F. Suppressing Hippo signaling in the stem cell niche promotes skeletal muscle regeneration. Stem Cells 2021, 39, 737–749. [Google Scholar] [CrossRef]
- Zeng, P.; Han, W.; Li, C.; Li, H.; Zhu, D.; Zhang, Y.; Liu, X. miR-378 attenuates muscle regeneration by delaying satellite cell activation and differentiation in mice. Acta Biochim. Biophys. Sin. 2016, 48, 833–839. [Google Scholar] [CrossRef]
- Lagalice, L.; Pichon, J.; Gougeon, E.; Soussi, S.; Deniaud, J.; Ledevin, M.; Maurier, V.; Leroux, I.; Durand, S.; Ciron, C.; et al. Satellite cells fail to contribute to muscle repair but are functional in Pompe disease (glycogenosis type II). Acta Neuropathol. Commun. 2018, 6, 116. [Google Scholar] [CrossRef]
- Mizbani, A.; Luca, E.; Rushing, E.J.; Krutzfeldt, J. MicroRNA deep sequencing in two adult stem cell populations identifies miR-501 as a novel regulator of myosin heavy chain during muscle regeneration. Development 2016, 143, 4137–4148. [Google Scholar] [CrossRef]
- Fiore, P.F.; Benedetti, A.; Sandona, M.; Madaro, L.; De Bardi, M.; Saccone, V.; Puri, P.L.; Gargioli, C.; Lozanoska-Ochser, B.; Bouche, M. Lack of PKCtheta Promotes Regenerative Ability of Muscle Stem Cells in Chronic Muscle Injury. Int. J. Mol. Sci. 2020, 21, 932. [Google Scholar] [CrossRef] [PubMed]
- Fortier, M.; Figeac, N.; White, R.B.; Knopp, P.; Zammit, P.S. Sphingosine-1-phosphate receptor 3 influences cell cycle progression in muscle satellite cells. Dev. Biol. 2013, 382, 504–516. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Zhu, Q.; Guo, C.; Yuan, R.; Zhang, X.; Nie, Y.; Chen, L.; Fang, Y.; Chen, K.; Zhang, J.; et al. MLL1 promotes myogenesis by epigenetically regulating Myf5. Cell Prolif. 2020, 53, e12744. [Google Scholar] [CrossRef] [PubMed]
- Sincennes, M.C.; Brun, C.E.; Lin, A.Y.T.; Rosembert, T.; Datzkiw, D.; Saber, J.; Ming, H.; Kawabe, Y.I.; Rudnicki, M.A. Acetylation of PAX7 controls muscle stem cell self-renewal and differentiation potential in mice. Nat. Commun. 2021, 12, 3253. [Google Scholar] [CrossRef] [PubMed]
- Naito, T.; Goto, K.; Morioka, S.; Matsuba, Y.; Akema, T.; Sugiura, T.; Ohira, Y.; Beppu, M.; Yoshioka, T. Administration of granulocyte colony-stimulating factor facilitates the regenerative process of injured mice skeletal muscle via the activation of Akt/GSK3alphabeta signals. Eur. J. Appl. Physiol. 2009, 105, 643–651. [Google Scholar] [CrossRef]
- Price, F.D.; von Maltzahn, J.; Bentzinger, C.F.; Dumont, N.A.; Yin, H.; Chang, N.C.; Wilson, D.H.; Frenette, J.; Rudnicki, M.A. Inhibition of JAK-STAT signaling stimulates adult satellite cell function. Nat. Med. 2014, 20, 1174–1181. [Google Scholar] [CrossRef]
- Angione, A.R.; Jiang, C.; Pan, D.; Wang, Y.X.; Kuang, S. PPARdelta regulates satellite cell proliferation and skeletal muscle regeneration. Skelet. Muscle 2011, 1, 33. [Google Scholar] [CrossRef]
- Nishizawa, S.; Koya, T.; Ohno, Y.; Goto, A.; Ikuita, A.; Suzuki, M.; Ohira, T.; Egawa, T.; Nakai, A.; Sugiura, T.; et al. Regeneration of injured skeletal muscle in heat shock transcription factor 1-null mice. Physiol. Rep. 2013, 1, e00071. [Google Scholar] [CrossRef]
- Ahrens, H.E.; Huettemeister, J.; Schmidt, M.; Kaether, C.; von Maltzahn, J. Klotho expression is a prerequisite for proper muscle stem cell function and regeneration of skeletal muscle. Skelet. Muscle 2018, 8, 20. [Google Scholar] [CrossRef]
- Sakamoto, K.; Furuichi, Y.; Yamamoto, M.; Takahashi, M.; Akimoto, Y.; Ishikawa, T.; Shimizu, T.; Fujimoto, M.; Takada-Watanabe, A.; Hayashi, A.; et al. R3hdml regulates satellite cell proliferation and differentiation. EMBO Rep. 2019, 20, e47957. [Google Scholar] [CrossRef]
- Bye, A.J.H.; Pugazhendhi, D.; Woodhouse, S.; Brien, P.; Watson, R.; Turner, M.; Pell, J. The RNA-binding proteins Zfp36l1 and Zfp36l2 act redundantly in myogenesis. Skelet. Muscle 2018, 8, 37. [Google Scholar] [CrossRef] [PubMed]
- Tonami, K.; Hata, S.; Ojima, K.; Ono, Y.; Kurihara, Y.; Amano, T.; Sato, T.; Kawamura, Y.; Kurihara, H.; Sorimachi, H. Calpain-6 deficiency promotes skeletal muscle development and regeneration. PLoS Genet. 2013, 9, e1003668. [Google Scholar] [CrossRef] [PubMed]
- Accornero, F.; Kanisicak, O.; Tjondrokoesoemo, A.; Attia, A.C.; McNally, E.M.; Molkentin, J.D. Myofiber-specific inhibition of TGFbeta signaling protects skeletal muscle from injury and dystrophic disease in mice. Hum. Mol. Genet. 2014, 23, 6903–6915. [Google Scholar] [CrossRef] [PubMed]
- Van Ry, P.M.; Minogue, P.; Hodges, B.L.; Burkin, D.J. Laminin-111 improves muscle repair in a mouse model of merosin-deficient congenital muscular dystrophy. Hum. Mol. Genet. 2014, 23, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Hosoyama, T.; Nishijo, K.; Prajapati, S.I.; Li, G.; Keller, C. Rb1 gene inactivation expands satellite cell and postnatal myoblast pools. J. Biol. Chem. 2011, 286, 19556–19564. [Google Scholar] [CrossRef] [PubMed]
- Rion, N.; Castets, P.; Lin, S.; Enderle, L.; Reinhard, J.R.; Ruegg, M.A. mTORC2 affects the maintenance of the muscle stem cell pool. Skelet. Muscle 2019, 9, 30. [Google Scholar] [CrossRef]
- Yoshida, T.; Galvez, S.; Tiwari, S.; Rezk, B.M.; Semprun-Prieto, L.; Higashi, Y.; Sukhanov, S.; Yablonka-Reuveni, Z.; Delafontaine, P. Angiotensin II inhibits satellite cell proliferation and prevents skeletal muscle regeneration. J. Biol. Chem. 2013, 288, 23823–23832. [Google Scholar] [CrossRef]
- Armand, A.S.; Laziz, I.; Djeghloul, D.; Lecolle, S.; Bertrand, A.T.; Biondi, O.; De Windt, L.J.; Chanoine, C. Apoptosis-inducing factor regulates skeletal muscle progenitor cell number and muscle phenotype. PLoS ONE 2011, 6, e27283. [Google Scholar] [CrossRef]
- Milanesi, A.; Lee, J.W.; Yang, A.; Liu, Y.Y.; Sedrakyan, S.; Cheng, S.Y.; Perin, L.; Brent, G.A. Thyroid Hormone Receptor Alpha is Essential to Maintain the Satellite Cell Niche During Skeletal Muscle Injury and Sarcopenia of Aging. Thyroid 2017, 27, 1316–1322. [Google Scholar] [CrossRef]
- Castets, P.; Bertrand, A.T.; Beuvin, M.; Ferry, A.; Le Grand, F.; Castets, M.; Chazot, G.; Rederstorff, M.; Krol, A.; Lescure, A.; et al. Satellite cell loss and impaired muscle regeneration in selenoprotein N deficiency. Hum. Mol. Genet. 2011, 20, 694–704. [Google Scholar] [CrossRef]
- Fujimaki, S.; Seko, D.; Kitajima, Y.; Yoshioka, K.; Tsuchiya, Y.; Masuda, S.; Ono, Y. Notch1 and Notch2 Coordinately Regulate Stem Cell Function in the Quiescent and Activated States of Muscle Satellite Cells. Stem Cells 2018, 36, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Johnston, A.P.; Bellamy, L.M.; Lisio, M.D.; Parise, G. Captopril treatment induces hyperplasia but inhibits myonuclear accretion following severe myotrauma in murine skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R363–R369. [Google Scholar] [CrossRef] [PubMed]
- Jaafar Marican, N.H.; Cruz-Migoni, S.B.; Borycki, A.G. Asymmetric Distribution of Primary Cilia Allocates Satellite Cells for Self-Renewal. Stem Cell Rep. 2016, 6, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.; Murad, K.B.A.; Tan, A.L.T.; Yada, S.; Sagiraju, S.; Bode, P.K.; Barker, N. Lgr5 Marks Adult Progenitor Cells Contributing to Skeletal Muscle Regeneration and Sarcoma Formation. Cell Rep. 2020, 33, 108535. [Google Scholar] [CrossRef] [PubMed]
- Buono, R.; Vantaggiato, C.; Pisa, V.; Azzoni, E.; Bassi, M.T.; Brunelli, S.; Sciorati, C.; Clementi, E. Nitric oxide sustains long-term skeletal muscle regeneration by regulating fate of satellite cells via signaling pathways requiring Vangl2 and cyclic GMP. Stem Cells 2012, 30, 197–209. [Google Scholar] [CrossRef]
- Urciuolo, A.; Quarta, M.; Morbidoni, V.; Gattazzo, F.; Molon, S.; Grumati, P.; Montemurro, F.; Tedesco, F.S.; Blaauw, B.; Cossu, G.; et al. Collagen VI regulates satellite cell self-renewal and muscle regeneration. Nat. Commun. 2013, 4, 1964. [Google Scholar] [CrossRef]
- Alexeev, V.; Arita, M.; Donahue, A.; Bonaldo, P.; Chu, M.L.; Igoucheva, O. Human adipose-derived stem cell transplantation as a potential therapy for collagen VI-related congenital muscular dystrophy. Stem Cell Res. Ther. 2014, 5, 21. [Google Scholar] [CrossRef]
- Brien, P.; Pugazhendhi, D.; Woodhouse, S.; Oxley, D.; Pell, J.M. p38alpha MAPK regulates adult muscle stem cell fate by restricting progenitor proliferation during postnatal growth and repair. Stem Cells 2013, 31, 1597–1610. [Google Scholar] [CrossRef]
- Hawke, T.J.; Meeson, A.P.; Jiang, N.; Graham, S.; Hutcheson, K.; DiMaio, J.M.; Garry, D.J. p21 is essential for normal myogenic progenitor cell function in regenerating skeletal muscle. Am. J. Physiol. Cell Physiol. 2003, 285, C1019–C1027. [Google Scholar] [CrossRef]
- Cortez-Toledo, O.; Schnair, C.; Sangngern, P.; Metzger, D.; Chao, L.C. Nur77 deletion impairs muscle growth during developmental myogenesis and muscle regeneration in mice. PLoS ONE 2017, 12, e0171268. [Google Scholar] [CrossRef]
- Wu, S.L.; Li, G.Z.; Chou, C.Y.; Tsai, M.S.; Chen, Y.P.; Li, C.J.; Liou, G.G.; Chang, W.W.; Chen, S.L.; Wang, S.H. Double homeobox gene, Duxbl, promotes myoblast proliferation and abolishes myoblast differentiation by blocking MyoD transactivation. Cell Tissue Res. 2014, 358, 551–566. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Melton, D.W.; Gelfond, J.A.; McManus, L.M.; Shireman, P.K. MiR-351 transiently increases during muscle regeneration and promotes progenitor cell proliferation and survival upon differentiation. Physiol. Genom. 2012, 44, 1042–1051. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.; Sinha, K.; Pan, H.; Cui, Y.; Guo, P.; Lin, C.Y.; Yang, F.; Deng, Z.; Eltzschig, H.K.; Lu, A.; et al. Markers of Accelerated Skeletal Muscle Regenerative Response in Murphy Roths Large Mice: Characteristics of Muscle Progenitor Cells and Circulating Factors. Stem Cells 2019, 37, 357–367. [Google Scholar] [CrossRef]
- Jia, Y.; Suzuki, N.; Yamamoto, M.; Gassmann, M.; Noguchi, C.T. Endogenous erythropoietin signaling facilitates skeletal muscle repair and recovery following pharmacologically induced damage. FASEB J. 2012, 26, 2847–2858. [Google Scholar] [CrossRef] [PubMed]
- Shibasaki, H.; Imamura, M.; Arima, S.; Tanihata, J.; Kuraoka, M.; Matsuzaka, Y.; Uchiumi, F.; Tanuma, S.I.; Takeda, S. Characterization of a novel microRNA, miR-188, elevated in serum of muscular dystrophy dog model. PLoS ONE 2019, 14, e0211597. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Shaikh, S.; Ahmad, K.; Ahmad, S.S.; Lim, J.H.; Park, S.; Yang, H.J.; Cho, W.K.; Park, S.J.; Lee, Y.H.; et al. Isolation and Characterization of Compounds from Glycyrrhiza uralensis as Therapeutic Agents for the Muscle Disorders. Int. J. Mol. Sci. 2021, 22, 876. [Google Scholar] [CrossRef] [PubMed]
- Armand, A.-S.; Launay, T.; Pariset, C.; Della Gaspera, B.; Charbonnier, F.; Chanoine, C. Injection of FGF6 accelerates regeneration of the soleus muscle in adult mice. Biochim. Biophys. Acta Mol. Cell Res. 2003, 1642, 97–105. [Google Scholar] [CrossRef]
- Pessemesse, L.; Tintignac, L.; Blanchet, E.; Cortade, F.; Jublanc, E.; Demangel, R.; Py, G.; Sar, C.; Cabello, G.; Wrutniak-Cabello, C.; et al. Regulation of mitochondrial activity controls the duration of skeletal muscle regeneration in response to injury. Sci. Rep. 2019, 9, 12249. [Google Scholar] [CrossRef]
- Ye, C.; Zhang, D.; Zhao, L.; Li, Y.; Yao, X.; Wang, H.; Zhang, S.; Liu, W.; Cao, H.; Yu, S.; et al. CaMKK2 Suppresses Muscle Regeneration through the Inhibition of Myoblast Proliferation and Differentiation. Int. J. Mol. Sci. 2016, 17, 1695. [Google Scholar] [CrossRef]
- Minetti, G.C.; Feige, J.N.; Bombard, F.; Heier, A.; Morvan, F.; Nurnberg, B.; Leiss, V.; Birnbaumer, L.; Glass, D.J.; Fornaro, M. Galphai2 signaling is required for skeletal muscle growth, regeneration, and satellite cell proliferation and differentiation. Mol. Cell. Biol. 2014, 34, 619–630. [Google Scholar] [CrossRef]
- Zhang, C.C.; Han, Y.C.; Cheng, N.X.; DU, J. Effect of arachidonic acid cytochrome P450ω hydroxylase Cyp4a14 gene knockout on skeletal muscle regeneration after injury. Sheng Li Xue Bao 2021, 73, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Chen, S.; Pan, X.; Dai, X.; Pan, G.; Li, Z.; Mai, X.; Tian, Y.; Zhang, S.; Liu, B.; et al. Transferrin receptor 1 ablation in satellite cells impedes skeletal muscle regeneration through activation of ferroptosis. J. Cachexia Sarcopenia Muscle 2021, 12, 746–768. [Google Scholar] [CrossRef] [PubMed]
- Naito, M.; Mori, M.; Inagawa, M.; Miyata, K.; Hashimoto, N.; Tanaka, S.; Asahara, H. Dnmt3a Regulates Proliferation of Muscle Satellite Cells via p57Kip2. PLoS Genet. 2016, 12, e1006167. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.H.; Hong, M.; Jeong, H.J.; Kim, H.; Lee, S.J.; Ryu, D.; Bae, G.U.; Cho, S.C.; Lee, Y.S.; Krauss, R.S.; et al. Satellite cell-specific ablation of Cdon impairs integrin activation, FGF signalling, and muscle regeneration. J. Cachexia Sarcopenia Muscle 2020, 11, 1089–1103. [Google Scholar] [CrossRef]
- Rooney, J.E.; Gurpur, P.B.; Yablonka-Reuveni, Z.; Burkin, D.J. Laminin-111 restores regenerative capacity in a mouse model for alpha7 integrin congenital myopathy. Am. J. Pathol. 2009, 174, 256–264. [Google Scholar] [CrossRef]
- Suzuki, K.; Matsumoto, M.; Katoh, Y.; Liu, L.; Ochiai, K.; Aizawa, Y.; Nagatomi, R.; Okuno, H.; Itoi, E.; Igarashi, K. Bach1 promotes muscle regeneration through repressing Smad-mediated inhibition of myoblast differentiation. PLoS ONE 2020, 15, e0236781. [Google Scholar] [CrossRef]
- Yamashita, A.; Hatazawa, Y.; Hirose, Y.; Ono, Y.; Kamei, Y. FOXO1 delays skeletal muscle regeneration and suppresses myoblast proliferation. Biosci. Biotechnol. Biochem. 2016, 80, 1531–1535. [Google Scholar] [CrossRef]
- Girgenrath, M.; Weng, S.; Kostek, C.A.; Browning, B.; Wang, M.; Brown, S.A.; Winkles, J.A.; Michaelson, J.S.; Allaire, N.; Schneider, P.; et al. TWEAK, via its receptor Fn14, is a novel regulator of mesenchymal progenitor cells and skeletal muscle regeneration. EMBO J. 2006, 25, 5826–5839. [Google Scholar] [CrossRef]
- Martinet, C.; Monnier, P.; Louault, Y.; Benard, M.; Gabory, A.; Dandolo, L. H19 controls reactivation of the imprinted gene network during muscle regeneration. Development 2016, 143, 962–971. [Google Scholar] [CrossRef]
- Yahiaoui, L.; Gvozdic, D.; Danialou, G.; Mack, M.; Petrof, B.J. CC family chemokines directly regulate myoblast responses to skeletal muscle injury. J. Physiol. 2008, 586, 3991–4004. [Google Scholar] [CrossRef]
- Kurosaka, M.; Ogura, Y.; Funabashi, T.; Akema, T. Early Growth Response 3 (Egr3) Contributes a Maintenance of C2C12 Myoblast Proliferation. J. Cell. Physiol. 2017, 232, 1114–1122. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, N.; Nishizuka, M.; Osada, S.; Imagawa, M. Fad24, a Positive Regulator of Adipogenesis, Is Required for S Phase Re-entry of C2C12 Myoblasts Arrested in G0 Phase and Involved in p27(Kip1) Expression at the Protein Level. Biol. Pharm. Bull. 2016, 39, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Zanou, N.; Schakman, O.; Louis, P.; Ruegg, U.T.; Dietrich, A.; Birnbaumer, L.; Gailly, P. Trpc1 ion channel modulates phosphatidylinositol 3-kinase/Akt pathway during myoblast differentiation and muscle regeneration. J. Biol. Chem. 2012, 287, 14524–14534. [Google Scholar] [CrossRef] [PubMed]
- Yablonka-Reuveni, Z.; Danoviz, M.E.; Phelps, M.; Stuelsatz, P. Myogenic-specific ablation of Fgfr1 impairs FGF2-mediated proliferation of satellite cells at the myofiber niche but does not abolish the capacity for muscle regeneration. Front. Aging Neurosci. 2015, 7, 85. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Tian, X.; Yan, C.; Liu, D.; Wang, S.; Han, Y. Nicotine promotes the differentiation of C2C12 myoblasts and improves skeletal muscle regeneration in obese mice. Biochem. Biophys. Res. Commun. 2019, 511, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wang, X.; Liu, S.; Wu, Y.; Zhao, T.; Chen, X.; Zhu, L.; Wu, Y.; Ding, X.; Peng, X.; et al. Sema4C participates in myogenic differentiation in vivo and in vitro through the p38 MAPK pathway. Eur. J. Cell Biol. 2007, 86, 331–344. [Google Scholar] [CrossRef]
- Liu, N.; Williams, A.H.; Maxeiner, J.M.; Bezprozvannaya, S.; Shelton, J.M.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N. microRNA-206 promotes skeletal muscle regeneration and delays progression of Duchenne muscular dystrophy in mice. J. Clin. Invest. 2012, 122, 2054–2065. [Google Scholar] [CrossRef]
- Song, C.; Wang, J.; Ma, Y.; Yang, Z.; Dong, D.; Li, H.; Yang, J.; Huang, Y.; Plath, M.; Ma, Y.; et al. Linc-smad7 promotes myoblast differentiation and muscle regeneration via sponging miR-125b. Epigenetics 2018, 13, 591–604. [Google Scholar] [CrossRef]
- Gatta, L.; Vitiello, L.; Gorini, S.; Chiandotto, S.; Costelli, P.; Giammarioli, A.M.; Malorni, W.; Rosano, G.; Ferraro, E. Modulating the metabolism by trimetazidine enhances myoblast differentiation and promotes myogenesis in cachectic tumor-bearing c26 mice. Oncotarget 2017, 8, 113938–113956. [Google Scholar] [CrossRef]
- Gagan, J.; Dey, B.K.; Layer, R.; Yan, Z.; Dutta, A. MicroRNA-378 targets the myogenic repressor MyoR during myoblast differentiation. J. Biol. Chem. 2011, 286, 19431–19438. [Google Scholar] [CrossRef]
- Mikami, T.; Koyama, S.; Yabuta, Y.; Kitagawa, H. Chondroitin sulfate is a crucial determinant for skeletal muscle development/regeneration and improvement of muscular dystrophies. J. Biol. Chem. 2012, 287, 38531–38542. [Google Scholar] [CrossRef] [PubMed]
- Storbeck, C.J.; Al-Zahrani, K.N.; Sriram, R.; Kawesa, S.; O’Reilly, P.; Daniel, K.; McKay, M.; Kothary, R.; Tsilfidis, C.; Sabourin, L.A. Distinct roles for Ste20-like kinase SLK in muscle function and regeneration. Skelet. Muscle 2013, 3, 16. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Leong, D.; Smith, L.R.; Barton, E.R. Matrix metalloproteinase 13 is a new contributor to skeletal muscle regeneration and critical for myoblast migration. Am. J. Physiol. Cell Physiol. 2013, 305, C529–C538. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Morvan, F.; Morozzi, G.; Jourde, B.; Minetti, G.C.; Kahle, P.; Rivet, H.; Brebbia, P.; Toussaint, G.; Glass, D.J.; et al. ATP Citrate Lyase Regulates Myofiber Differentiation and Increases Regeneration by Altering Histone Acetylation. Cell Rep. 2017, 21, 3003–3011. [Google Scholar] [CrossRef]
- Yoshida, T.; Huq, T.S.; Delafontaine, P. Angiotensin type 2 receptor signaling in satellite cells potentiates skeletal muscle regeneration. J. Biol. Chem. 2014, 289, 26239–26248. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, B.; Ye, M.; Liang, P.; Zhangfang, Y.; Huang, J.; Liu, M.; Songyang, Z.; Ma, W. Ccndbp1 is a new positive regulator of skeletal myogenesis. J. Cell Sci. 2016, 129, 2767–2777. [Google Scholar] [CrossRef]
- Chen, S.E.; Jin, B.; Li, Y.P. TNF-alpha regulates myogenesis and muscle regeneration by activating p38 MAPK. Am. J. Physiol. Cell Physiol. 2007, 292, C1660–C1671. [Google Scholar] [CrossRef]
- He, S.; Fu, T.; Yu, Y.; Liang, Q.; Li, L.; Liu, J.; Zhang, X.; Zhou, Q.; Guo, Q.; Xu, D.; et al. IRE1alpha regulates skeletal muscle regeneration through Myostatin mRNA decay. J. Clin. Invest. 2021, 131, e143737. [Google Scholar] [CrossRef]
- Luca, E.; Turcekova, K.; Hartung, A.; Mathes, S.; Rehrauer, H.; Krutzfeldt, J. Genetic deletion of microRNA biogenesis in muscle cells reveals a hierarchical non-clustered network that controls focal adhesion signaling during muscle regeneration. Mol. Metab. 2020, 36, 100967. [Google Scholar] [CrossRef]
- Esteca, M.V.; Severino, M.B.; Silvestre, J.G.; Palmeira Dos Santos, G.; Tamborlin, L.; Luchessi, A.D.; Moriscot, A.S.; Gustafsson, A.B.; Baptista, I.L. Loss of Parkin Results in Altered Muscle Stem Cell Differentiation during Regeneration. Int. J. Mol. Sci. 2020, 21, 8007. [Google Scholar] [CrossRef]
- Ishii, A.; Lo, S.H. A role of tensin in skeletal-muscle regeneration. Biochem. J. 2001, 356, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.P.; Shin, Y.J.; Panda, A.C.; Abdelmohsen, K.; Kim, J.Y.; Lee, S.M.; Bahn, Y.J.; Choi, J.Y.; Kwon, E.S.; Baek, S.J.; et al. miR-431 promotes differentiation and regeneration of old skeletal muscle by targeting Smad4. Genes Dev. 2015, 29, 1605–1617. [Google Scholar] [CrossRef] [PubMed]
- Cerquone Perpetuini, A.; Re Cecconi, A.D.; Chiappa, M.; Martinelli, G.B.; Fuoco, C.; Desiderio, G.; Castagnoli, L.; Gargioli, C.; Piccirillo, R.; Cesareni, G. Group I Paks support muscle regeneration and counteract cancer-associated muscle atrophy. J. Cachexia Sarcopenia Muscle 2018, 9, 727–746. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Hong, Y.; Kong, G.; Lee, D.H.; Kim, M.; Tran, Q.; Cho, H.; Kim, C.; Park, S.; Kim, S.H.; et al. Yin Yang 1 is required for PHD finger protein 20-mediated myogenic differentiation in vitro and in vivo. Cell Death Differ. 2020, 27, 3321–3336. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Won, J.Y.; Yang, J.; Lee, J.; Kim, S.Y.; Lee, E.J.; Kim, H.S. AKAP6 inhibition impairs myoblast differentiation and muscle regeneration: Positive loop between AKAP6 and myogenin. Sci. Rep. 2015, 5, 16523. [Google Scholar] [CrossRef]
- Li, H.K.; Zhou, Y.; Ding, J.; Xiong, L.; Shi, Y.X.; He, Y.J.; Yang, D.; Deng, Z.L.; Nie, M.; Fei Gao, Y. LRTM1 promotes the differentiation of myoblast cells by negatively regulating the FGFR1 signaling pathway. Exp. Cell Res. 2020, 396, 112237. [Google Scholar] [CrossRef]
- Lin, Y.F.; Xiao, M.H.; Chen, H.X.; Meng, Y.; Zhao, N.; Yang, L.; Tang, H.; Wang, J.L.; Liu, X.; Zhu, Y.; et al. A novel mitochondrial micropeptide MPM enhances mitochondrial respiratory activity and promotes myogenic differentiation. Cell Death Dis. 2019, 10, 528. [Google Scholar] [CrossRef]
- Harada, A.; Maehara, K.; Ono, Y.; Taguchi, H.; Yoshioka, K.; Kitajima, Y.; Xie, Y.; Sato, Y.; Iwasaki, T.; Nogami, J.; et al. Histone H3.3 sub-variant H3mm7 is required for normal skeletal muscle regeneration. Nat. Commun. 2018, 9, 1400. [Google Scholar] [CrossRef]
- Miyake, T.; Alli, N.S.; Aziz, A.; Knudson, J.; Fernando, P.; Megeney, L.A.; McDermott, J.C. Cardiotrophin-1 maintains the undifferentiated state in skeletal myoblasts. J. Biol. Chem. 2009, 284, 19679–19693. [Google Scholar] [CrossRef]
- Paul, C.; Sardet, C.; Fabbrizio, E. The histone- and PRMT5-associated protein COPR5 is required for myogenic differentiation. Cell Death Differ. 2012, 19, 900–908. [Google Scholar] [CrossRef]
- Faralli, H.; Martin, E.; Core, N.; Liu, Q.C.; Filippi, P.; Dilworth, F.J.; Caubit, X.; Fasano, L. Teashirt-3, a novel regulator of muscle differentiation, associates with BRG1-associated factor 57 (BAF57) to inhibit myogenin gene expression. J. Biol. Chem. 2011, 286, 23498–23510. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Nelson, B.R.; Bezprozvannaya, S.; Shelton, J.M.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N. Requirement of MEF2A, C, and D for skeletal muscle regeneration. Proc. Natl. Acad. Sci. USA 2014, 111, 4109–4114. [Google Scholar] [CrossRef] [PubMed]
- Kielbasa, O.M.; Reynolds, J.G.; Wu, C.L.; Snyder, C.M.; Cho, M.Y.; Weiler, H.; Kandarian, S.; Naya, F.J. Myospryn is a calcineurin-interacting protein that negatively modulates slow-fiber-type transformation and skeletal muscle regeneration. FASEB J. 2011, 25, 2276–2286. [Google Scholar] [CrossRef] [PubMed]
- Verpoorten, S.; Sfyri, P.; Scully, D.; Mitchell, R.; Tzimou, A.; Mougios, V.; Patel, K.; Matsakas, A. Loss of CD36 protects against diet-induced obesity but results in impaired muscle stem cell function, delayed muscle regeneration and hepatic steatosis. Acta Physiol. 2020, 228, e13395. [Google Scholar] [CrossRef] [PubMed]
- Andree, B.; Fleige, A.; Arnold, H.H.; Brand, T. Mouse Pop1 is required for muscle regeneration in adult skeletal muscle. Mol. Cell. Biol. 2002, 22, 1504–1512. [Google Scholar] [CrossRef]
- Paolini, A.; Omairi, S.; Mitchell, R.; Vaughan, D.; Matsakas, A.; Vaiyapuri, S.; Ricketts, T.; Rubinsztein, D.C.; Patel, K. Attenuation of autophagy impacts on muscle fibre development, starvation induced stress and fibre regeneration following acute injury. Sci. Rep. 2018, 8, 9062. [Google Scholar] [CrossRef]
- Clow, C.; Jasmin, B.J. Brain-derived neurotrophic factor regulates satellite cell differentiation and skeltal muscle regeneration. Mol. Biol. Cell 2010, 21, 2182–2190. [Google Scholar] [CrossRef]
- Marshall, J.L.; Holmberg, J.; Chou, E.; Ocampo, A.C.; Oh, J.; Lee, J.; Peter, A.K.; Martin, P.T.; Crosbie-Watson, R.H. Sarcospan-dependent Akt activation is required for utrophin expression and muscle regeneration. J. Cell Biol. 2012, 197, 1009–1027. [Google Scholar] [CrossRef]
- Chen, S.E.; Gerken, E.; Zhang, Y.; Zhan, M.; Mohan, R.K.; Li, A.S.; Reid, M.B.; Li, Y.P. Role of TNF-{alpha} signaling in regeneration of cardiotoxin-injured muscle. Am. J. Physiol. Cell Physiol. 2005, 289, C1179–C1187. [Google Scholar] [CrossRef]
- Ravel-Chapuis, A.; Crawford, T.E.; Blais-Crepeau, M.L.; Belanger, G.; Richer, C.T.; Jasmin, B.J. The RNA-binding protein Staufen1 impairs myogenic differentiation via a c-myc-dependent mechanism. Mol. Biol. Cell 2014, 25, 3765–3778. [Google Scholar] [CrossRef]
- Langsdorf, A.; Do, A.T.; Kusche-Gullberg, M.; Emerson, C.P., Jr.; Ai, X. Sulfs are regulators of growth factor signaling for satellite cell differentiation and muscle regeneration. Dev. Biol. 2007, 311, 464–477. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Niu, A.; Chen, S.E.; Li, Y.P. Beta3-integrin mediates satellite cell differentiation in regenerating mouse muscle. FASEB J. 2011, 25, 1914–1921. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Kondo, S.; Hayasaka, M.; Hanaoka, K. Functional analysis of homeodomain-containing transcription factor Lbx1 in satellite cells of mouse skeletal muscle. J. Cell Sci. 2007, 120, 4178–4187. [Google Scholar] [CrossRef]
- Fu, D.; Lala-Tabbert, N.; Lee, H.; Wiper-Bergeron, N. Mdm2 promotes myogenesis through the ubiquitination and degradation of CCAAT/enhancer-binding protein beta. J. Biol. Chem. 2015, 290, 10200–10207. [Google Scholar] [CrossRef]
- Schroer, A.B.; Mohamed, J.S.; Willard, M.D.; Setola, V.; Oestreich, E.; Siderovski, D.P. A role for Regulator of G protein Signaling-12 (RGS12) in the balance between myoblast proliferation and differentiation. PLoS ONE 2019, 14, e0216167. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Shin, J.Y.; Jo, A.; Jyothi, K.R.; Nguyen, M.N.; Choi, T.G.; Kim, J.; Park, J.H.; Eun, Y.G.; Yoon, K.S.; et al. Carbonyl reductase 1 is an essential regulator of skeletal muscle differentiation and regeneration. Int. J. Biochem. Cell Biol. 2013, 45, 1784–1793. [Google Scholar] [CrossRef] [PubMed]
- Mammen, A.L.; Casciola-Rosen, L.A.; Hall, J.C.; Christopher-Stine, L.; Corse, A.M.; Rosen, A. Expression of the dermatomyositis autoantigen Mi-2 in regenerating muscle. Arthritis Rheum. 2009, 60, 3784–3793. [Google Scholar] [CrossRef]
- Wu, Z.; Xu, H.; Xu, Y.; Fan, W.; Yao, H.; Wang, Y.; Hu, W.; Lou, G.; Shi, Y.; Chen, X.; et al. Andrographolide promotes skeletal muscle regeneration after acute injury through epigenetic modulation. Eur. J. Pharmacol. 2020, 888, 173470. [Google Scholar] [CrossRef]
- Kurosaka, M.; Ogura, Y.; Sato, S.; Kohda, K.; Funabashi, T. Transcription factor signal transducer and activator of transcription 6 (STAT6) is an inhibitory factor for adult myogenesis. Skelet. Muscle 2021, 11, 14. [Google Scholar] [CrossRef]
- Vijayakumar, A.; Buffin, N.J.; Gallagher, E.J.; Blank, J.; Wu, Y.; Yakar, S.; LeRoith, D. Deletion of growth hormone receptors in postnatal skeletal muscle of male mice does not alter muscle mass and response to pathological injury. Endocrinology 2013, 154, 3776–3783. [Google Scholar] [CrossRef][Green Version]
- Pryce, B.R.; Al-Zahrani, K.N.; Dufresne, S.; Belkina, N.; Labreche, C.; Patino-Lopez, G.; Frenette, J.; Shaw, S.; Sabourin, L.A. Deletion of the Ste20-like kinase SLK in skeletal muscle results in a progressive myopathy and muscle weakness. Skelet. Muscle 2017, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Redelsperger, F.; Raddi, N.; Bacquin, A.; Vernochet, C.; Mariot, V.; Gache, V.; Blanchard-Gutton, N.; Charrin, S.; Tiret, L.; Dumonceaux, J.; et al. Genetic Evidence That Captured Retroviral Envelope syncytins Contribute to Myoblast Fusion and Muscle Sexual Dimorphism in Mice. PLoS Genet. 2016, 12, e1006289. [Google Scholar] [CrossRef] [PubMed]
- Kaspar, P.; Ilencikova, K.; Zikova, M.; Horvath, O.; Cermak, V.; Bartunek, P.; Strnad, H. c-Myb inhibits myoblast fusion. PLoS ONE 2013, 8, e76742. [Google Scholar] [CrossRef] [PubMed]
- Griffin, D.A.; Johnson, R.W.; Whitlock, J.M.; Pozsgai, E.R.; Heller, K.N.; Grose, W.E.; Arnold, W.D.; Sahenk, Z.; Hartzell, H.C.; Rodino-Klapac, L.R. Defective membrane fusion and repair in Anoctamin5-deficient muscular dystrophy. Hum. Mol. Genet. 2016, 25, 1900–1911. [Google Scholar] [CrossRef] [PubMed]
- Trapani, L.; Segatto, M.; La Rosa, P.; Fanelli, F.; Moreno, S.; Marino, M.; Pallottini, V. 3-hydroxy 3-methylglutaryl coenzyme A reductase inhibition impairs muscle regeneration. J. Cell. Biochem. 2012, 113, 2057–2063. [Google Scholar] [CrossRef] [PubMed]
- Hamoud, N.; Tran, V.; Aimi, T.; Kakegawa, W.; Lahaie, S.; Thibault, M.P.; Pelletier, A.; Wong, G.W.; Kim, I.S.; Kania, A.; et al. Spatiotemporal regulation of the GPCR activity of BAI3 by C1qL4 and Stabilin-2 controls myoblast fusion. Nat. Commun. 2018, 9, 4470. [Google Scholar] [CrossRef] [PubMed]
- Tamilarasan, K.P.; Temmel, H.; Das, S.K.; Al Zoughbi, W.; Schauer, S.; Vesely, P.W.; Hoefler, G. Skeletal muscle damage and impaired regeneration due to LPL-mediated lipotoxicity. Cell Death Dis. 2012, 3, e354. [Google Scholar] [CrossRef]
- Yoshida, N.; Endo, J.; Kinouchi, K.; Kitakata, H.; Moriyama, H.; Kataoka, M.; Yamamoto, T.; Shirakawa, K.; Morimoto, S.; Nishiyama, A.; et al. (Pro)renin receptor accelerates development of sarcopenia via activation of Wnt/YAP signaling axis. Aging Cell 2019, 18, e12991. [Google Scholar] [CrossRef] [PubMed]
- Youm, T.H.; Woo, S.H.; Kwon, E.S.; Park, S.S. NADPH Oxidase 4 Contributes to Myoblast Fusion and Skeletal Muscle Regeneration. Oxid. Med. Cell. Longev. 2019, 2019, 3585390. [Google Scholar] [CrossRef]
- Teng, S.; Stegner, D.; Chen, Q.; Hongu, T.; Hasegawa, H.; Chen, L.; Kanaho, Y.; Nieswandt, B.; Frohman, M.A.; Huang, P. Phospholipase D1 facilitates second-phase myoblast fusion and skeletal muscle regeneration. Mol. Biol. Cell 2015, 26, 506–517. [Google Scholar] [CrossRef]
- Krause, M.P.; Moradi, J.; Coleman, S.K.; D’Souza, D.M.; Liu, C.; Kronenberg, M.S.; Rowe, D.W.; Hawke, T.J.; Hadjiargyrou, M. A novel GFP reporter mouse reveals Mustn1 expression in adult regenerating skeletal muscle, activated satellite cells and differentiating myoblasts. Acta Physiol. 2013, 208, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Kurosaka, M.; Ogura, Y.; Funabashi, T.; Akema, T. Involvement of Transient Receptor Potential Cation Channel Vanilloid 1 (TRPV1) in Myoblast Fusion. J. Cell. Physiol. 2016, 231, 2275–2285. [Google Scholar] [CrossRef] [PubMed]
- Singhal, N.; Martin, P.T. A role for Galgt1 in skeletal muscle regeneration. Skelet. Muscle 2015, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Yalvac, M.E.; Amornvit, J.; Braganza, C.; Chen, L.; Hussain, S.A.; Shontz, K.M.; Montgomery, C.L.; Flanigan, K.M.; Lewis, S.; Sahenk, Z. Impaired regeneration in calpain-3 null muscle is associated with perturbations in mTORC1 signaling and defective mitochondrial biogenesis. Skelet. Muscle 2017, 7, 27. [Google Scholar] [CrossRef]
- Ogawa, R.; Ma, Y.; Yamaguchi, M.; Ito, T.; Watanabe, Y.; Ohtani, T.; Murakami, S.; Uchida, S.; De Gaspari, P.; Uezumi, A.; et al. Doublecortin marks a new population of transiently amplifying muscle progenitor cells and is required for myofiber maturation during skeletal muscle regeneration. Development 2015, 142, 810. [Google Scholar] [CrossRef]
- Ohno, Y.; Ando, K.; Ito, T.; Suda, Y.; Matsui, Y.; Oyama, A.; Kaneko, H.; Yokoyama, S.; Egawa, T.; Goto, K. Lactate Stimulates a Potential for Hypertrophy and Regeneration of Mouse Skeletal Muscle. Nutrients 2019, 11, 869. [Google Scholar] [CrossRef]
- Hoshino, S.; Sakamoto, K.; Vassilopoulos, S.; Camus, S.M.; Griffin, C.A.; Esk, C.; Torres, J.A.; Ohkoshi, N.; Ishii, A.; Tamaoka, A.; et al. The CHC22 clathrin-GLUT4 transport pathway contributes to skeletal muscle regeneration. PLoS ONE 2013, 8, e77787. [Google Scholar] [CrossRef]
- Agbulut, O.; Li, Z.; Perie, S.; Ludosky, M.A.; Paulin, D.; Cartaud, J.; Butler-Browne, G. Lack of desmin results in abortive muscle regeneration and modifications in synaptic structure. Cell Motil. Cytoskelet. 2001, 49, 51–66. [Google Scholar] [CrossRef]
- Cicchillitti, L.; Di Stefano, V.; Isaia, E.; Crimaldi, L.; Fasanaro, P.; Ambrosino, V.; Antonini, A.; Capogrossi, M.C.; Gaetano, C.; Piaggio, G.; et al. Hypoxia-inducible factor 1-alpha induces miR-210 in normoxic differentiating myoblasts. J. Biol. Chem. 2012, 287, 44761–44771. [Google Scholar] [CrossRef]
- Piccioni, A.; Gaetani, E.; Neri, V.; Gatto, I.; Palladino, M.; Silver, M.; Smith, R.C.; Giarretta, I.; Pola, E.; Hlatky, L.; et al. Sonic hedgehog therapy in a mouse model of age-associated impairment of skeletal muscle regeneration. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 245–252. [Google Scholar] [CrossRef]
- Liu, N.; Garry, G.A.; Li, S.; Bezprozvannaya, S.; Sanchez-Ortiz, E.; Chen, B.; Shelton, J.M.; Jaichander, P.; Bassel-Duby, R.; Olson, E.N. A Twist2-dependent progenitor cell contributes to adult skeletal muscle. Nat. Cell Biol. 2017, 19, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Shaikh, S.; Baig, M.H.; Park, S.Y.; Lim, J.H.; Ahmad, S.S.; Ali, S.; Ahmad, K.; Choi, I. MIF1 and MIF2 Myostatin Peptide Inhibitors as Potent Muscle Mass Regulators. Int. J. Mol. Sci. 2022, 23, 4222. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Kay, D.I.; Rudra, R.T.; Chen, B.M.; Hsu, N.; Izumiya, Y.; Martinez, L.; Spencer, M.J.; Walsh, K.; Grinnell, A.D.; et al. Myogenic Akt signaling attenuates muscular degeneration, promotes myofiber regeneration and improves muscle function in dystrophin-deficient mdx mice. Hum. Mol. Genet. 2011, 20, 1324–1338. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Nie, M.; Liu, J.; Hu, X.; Ma, L.; Deng, Z.L.; Wang, D.Z. Trbp Is Required for Differentiation of Myoblasts and Normal Regeneration of Skeletal Muscle. PLoS ONE 2016, 11, e0155349. [Google Scholar] [CrossRef]
- Murray, I.R.; Gonzalez, Z.N.; Baily, J.; Dobie, R.; Wallace, R.J.; Mackinnon, A.C.; Smith, J.R.; Greenhalgh, S.N.; Thompson, A.I.; Conroy, K.P.; et al. alphav integrins on mesenchymal cells regulate skeletal and cardiac muscle fibrosis. Nat. Commun. 2017, 8, 1118. [Google Scholar] [CrossRef]
- Zhao, L.; Son, J.S.; Wang, B.; Tian, Q.; Chen, Y.; Liu, X.; de Avila, J.M.; Zhu, M.J.; Du, M. Retinoic acid signalling in fibro/adipogenic progenitors robustly enhances muscle regeneration. EBioMedicine 2020, 60, 103020. [Google Scholar] [CrossRef]
- Zanotti, S.; Gibertini, S.; Blasevich, F.; Bragato, C.; Ruggieri, A.; Saredi, S.; Fabbri, M.; Bernasconi, P.; Maggi, L.; Mantegazza, R.; et al. Exosomes and exosomal miRNAs from muscle-derived fibroblasts promote skeletal muscle fibrosis. Matrix Biol. 2018, 74, 77–100. [Google Scholar] [CrossRef]
- Vumbaca, S.; Giuliani, G.; Fiorentini, V.; Tortolici, F.; Cerquone Perpetuini, A.; Riccio, F.; Sennato, S.; Gargioli, C.; Fuoco, C.; Castagnoli, L.; et al. Characterization of the Skeletal Muscle Secretome Reveals a Role for Extracellular Vesicles and IL1alpha/IL1beta in Restricting Fibro/Adipogenic Progenitor Adipogenesis. Biomolecules 2021, 11, 1171. [Google Scholar] [CrossRef]
- Stepien, D.M.; Hwang, C.; Marini, S.; Pagani, C.A.; Sorkin, M.; Visser, N.D.; Huber, A.K.; Edwards, N.J.; Loder, S.J.; Vasquez, K.; et al. Tuning Macrophage Phenotype to Mitigate Skeletal Muscle Fibrosis. J. Immunol. 2020, 204, 2203–2215. [Google Scholar] [CrossRef]
- Burks, T.N.; Andres-Mateos, E.; Marx, R.; Mejias, R.; Van Erp, C.; Simmers, J.L.; Walston, J.D.; Ward, C.W.; Cohn, R.D. Losartan restores skeletal muscle remodeling and protects against disuse atrophy in sarcopenia. Sci. Transl. Med. 2011, 3, 82ra37. [Google Scholar] [CrossRef]
- Bosnakovski, D.; Oyler, D.; Mitanoska, A.; Douglas, M.; Ener, E.T.; Shams, A.S.; Kyba, M. Persistent Fibroadipogenic Progenitor Expansion Following Transient DUX4 Expression Provokes a Profibrotic State in a Mouse Model for FSHD. Int. J. Mol. Sci. 2022, 23, 1983. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, S.; Cheng, X.W.; Inoue, A.; Hu, L.; Piao, L.; Yu, C.; Goto, H.; Xu, W.; Zhao, G.; Lei, Y.; et al. Cathepsin K activity controls cardiotoxin-induced skeletal muscle repair in mice. J. Cachexia Sarcopenia Muscle 2018, 9, 160–175. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Lee, Y.S.; Zimmers, T.A.; Soleimani, A.; Matzuk, M.M.; Tsuchida, K.; Cohn, R.D.; Barton, E.R. Regulation of muscle mass by follistatin and activins. Mol. Endocrinol. 2010, 24, 1998–2008. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, F.; Zhang, Y.; Mondragon-Gonzalez, R.; Harvey, J.; Perlingeiro, R.C.R. Treatment with rGDF11 does not improve the dystrophic muscle pathology of mdx mice. Skelet. Muscle 2016, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Mignemi, N.A.; Yuasa, M.; Baker, C.E.; Moore, S.N.; Ihejirika, R.C.; Oelsner, W.K.; Wallace, C.S.; Yoshii, T.; Okawa, A.; Revenko, A.S.; et al. Plasmin Prevents Dystrophic Calcification After Muscle Injury. J. Bone Miner. Res. 2017, 32, 294–308. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lee, S.J. Regulation of GDF-11 and myostatin activity by GASP-1 and GASP-2. Proc. Natl. Acad. Sci. USA 2013, 110, E3713–E3722. [Google Scholar] [CrossRef]
- Zhao, Y.; Urganus, A.L.; Spevak, L.; Shrestha, S.; Doty, S.B.; Boskey, A.L.; Pachman, L.M. Characterization of dystrophic calcification induced in mice by cardiotoxin. Calcif. Tissue Int. 2009, 85, 267–275. [Google Scholar] [CrossRef]
- Lounev, V.Y.; Ramachandran, R.; Wosczyna, M.N.; Yamamoto, M.; Maidment, A.D.; Shore, E.M.; Glaser, D.L.; Goldhamer, D.J.; Kaplan, F.S. Identification of progenitor cells that contribute to heterotopic skeletogenesis. J. Bone Joint Surg. Am. 2009, 91, 652–663. [Google Scholar] [CrossRef]
- Drouin, G.; Couture, V.; Lauzon, M.A.; Balg, F.; Faucheux, N.; Grenier, G. Muscle injury-induced hypoxia alters the proliferation and differentiation potentials of muscle resident stromal cells. Skelet. Muscle 2019, 9, 18. [Google Scholar] [CrossRef]
- Arsic, N.; Zacchigna, S.; Zentilin, L.; Ramirez-Correa, G.; Pattarini, L.; Salvi, A.; Sinagra, G.; Giacca, M. Vascular endothelial growth factor stimulates skeletal muscle regeneration in vivo. Mol. Ther. 2004, 10, 844–854. [Google Scholar] [CrossRef]
- Sinha-Hikim, I.; Braga, M.; Shen, R.; Sinha Hikim, A.P. Involvement of c-Jun NH2-terminal kinase and nitric oxide-mediated mitochondria-dependent intrinsic pathway signaling in cardiotoxin-induced muscle cell death: Role of testosterone. Apoptosis 2007, 12, 1965–1978. [Google Scholar] [CrossRef] [PubMed]
- Min, K.; Lawan, A.; Bennett, A.M. Loss of MKP-5 promotes myofiber survival by activating STAT3/Bcl-2 signaling during regenerative myogenesis. Skelet. Muscle 2017, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Tjondrokoesoemo, A.; Schips, T.; Kanisicak, O.; Sargent, M.A.; Molkentin, J.D. Genetic overexpression of Serpina3n attenuates muscular dystrophy in mice. Hum. Mol. Genet. 2016, 25, 1192–1202. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.N.; Hawley, G.D.; Smith, E.N.; Mignemi, N.A.; Ihejirika, R.C.; Yuasa, M.; Cates, J.M.; Liu, X.; Schoenecker, J.G. Validation of a Radiography-Based Quantification Designed to Longitudinally Monitor Soft Tissue Calcification in Skeletal Muscle. PLoS ONE 2016, 11, e0159624. [Google Scholar] [CrossRef] [PubMed]
- Ieronimakis, N.; Hays, A.; Reyes, M. Bone marrow-derived cells do not engraft into skeletal muscle microvasculature but promote angiogenesis after acute injury. Exp. Hematol. 2012, 40, 238–249.e233. [Google Scholar] [CrossRef][Green Version]
- Bellamy, L.M.; Johnston, A.P.; De Lisio, M.; Parise, G. Skeletal muscle-endothelial cell cross talk through angiotensin II. Am. J. Physiol. Cell Physiol. 2010, 299, C1402–C1408. [Google Scholar] [CrossRef]
- Mellows, B.; Mitchell, R.; Antonioli, M.; Kretz, O.; Chambers, D.; Zeuner, M.T.; Denecke, B.; Musante, L.; Ramachandra, D.L.; Debacq-Chainiaux, F.; et al. Protein and Molecular Characterization of a Clinically Compliant Amniotic Fluid Stem Cell-Derived Extracellular Vesicle Fraction Capable of Accelerating Muscle Regeneration Through Enhancement of Angiogenesis. Stem Cells Dev. 2017, 26, 1316–1333. [Google Scholar] [CrossRef]
- Hosaka, Y.; Yokota, T.; Miyagoe-Suzuki, Y.; Yuasa, K.; Imamura, M.; Matsuda, R.; Ikemoto, T.; Kameya, S.; Takeda, S. Alpha1-syntrophin-deficient skeletal muscle exhibits hypertrophy and aberrant formation of neuromuscular junctions during regeneration. J. Cell Biol. 2002, 158, 1097–1107. [Google Scholar] [CrossRef]
- Daneshvar, N.; Tatsumi, R.; Peeler, J.; Anderson, J.E. Premature satellite cell activation before injury accelerates myogenesis and disrupts neuromuscular junction maturation in regenerating muscle. Am. J. Physiol. Cell Physiol. 2020, 319, C116–C128. [Google Scholar] [CrossRef]
- Sawano, S.; Suzuki, T.; Do, M.K.; Ohtsubo, H.; Mizunoya, W.; Ikeuchi, Y.; Tatsumi, R. Supplementary immunocytochemistry of hepatocyte growth factor production in activated macrophages early in muscle regeneration. Anim. Sci. J. 2014, 85, 994–1000. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Shono, J.; Suzuki, T.; Sawano, S.; Anderson, J.E.; Do, M.K.; Ohtsubo, H.; Mizunoya, W.; Sato, Y.; Nakamura, M.; et al. Implication of anti-inflammatory macrophages in regenerative moto-neuritogenesis: Promotion of myoblast migration and neural chemorepellent semaphorin 3A expression in injured muscle. Int. J. Biochem. Cell Biol. 2014, 54, 272–285. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Cha, H.N.; Jo, H.J.; Song, I.H.; Baek, S.H.; Dan, J.M.; Kim, Y.W.; Kim, J.Y.; Lee, I.K.; Seo, J.S.; et al. TLR2 deficiency attenuates skeletal muscle atrophy in mice. Biochem. Biophys. Res. Commun. 2015, 459, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Oikawa, S.; Lee, M.; Akimoto, T. Conditional Deletion of Dicer in Adult Mice Impairs Skeletal Muscle Regeneration. Int. J. Mol. Sci. 2019, 20, 5686. [Google Scholar] [CrossRef] [PubMed]
- Hiramuki, Y.; Sato, T.; Furuta, Y.; Surani, M.A.; Sehara-Fujisawa, A. Mest but Not MiR-335 Affects Skeletal Muscle Growth and Regeneration. PLoS ONE 2015, 10, e0130436. [Google Scholar] [CrossRef]
- Norton, C.R.; Chen, Y.; Han, X.H.; Bradley, C.K.; Krebs, L.T.; Yoon, J.K.; Gridley, T. Absence of a major role for the snai1 and snai3 genes in regulating skeletal muscle regeneration in mice. PLoS Curr. 2013, 5. [Google Scholar] [CrossRef]
- Call, J.A.; Wilson, R.J.; Laker, R.C.; Zhang, M.; Kundu, M.; Yan, Z. Ulk1-mediated autophagy plays an essential role in mitochondrial remodeling and functional regeneration of skeletal muscle. Am. J. Physiol. Cell Physiol. 2017, 312, C724–C732. [Google Scholar] [CrossRef]
- Chaturvedi, N.; Ahmad, K.; Yadav, B.S.; Lee, E.J.; Sonkar, S.C.; Marina, N.; Choi, I. Understanding Calcium-Dependent Conformational Changes in S100A1 Protein: A Combination of Molecular Dynamics and Gene Expression Study in Skeletal Muscle. Cells 2020, 9, 181. [Google Scholar] [CrossRef]
- Parks, C.A.; Pak, K.; Pinal-Fernandez, I.; Huang, W.; Derfoul, A.; Mammen, A.L. Trim33 (Tif1gamma) is not required for skeletal muscle development or regeneration but suppresses cholecystokinin expression. Sci. Rep. 2019, 9, 18507. [Google Scholar] [CrossRef]
- Goetsch, S.C.; Martin, C.M.; Embree, L.J.; Garry, D.J. Myogenic progenitor cells express filamin C in developing and regenerating skeletal muscle. Stem Cells Dev. 2005, 14, 181–187. [Google Scholar] [CrossRef]
- Wardrop, K.E.; Dominov, J.A. Proinflammatory signals and the loss of lymphatic vessel hyaluronan receptor-1 (LYVE-1) in the early pathogenesis of laminin alpha2-deficient skeletal muscle. J. Histochem. Cytochem. 2011, 59, 167–179. [Google Scholar] [CrossRef]
- Merkulova, T.; Dehaupas, M.; Nevers, M.C.; Creminon, C.; Alameddine, H.; Keller, A. Differential modulation of alpha, beta and gamma enolase isoforms in regenerating mouse skeletal muscle. Eur. J. Biochem. 2000, 267, 3735–3743. [Google Scholar] [CrossRef] [PubMed]
- Yuasa, K.; Hagiwara, Y.; Ando, M.; Nakamura, A.; Takeda, S.; Hijikata, T. MicroRNA-206 is highly expressed in newly formed muscle fibers: Implications regarding potential for muscle regeneration and maturation in muscular dystrophy. Cell Struct. Funct. 2008, 33, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Casciola-Rosen, L.; Hall, J.C.; Mammen, A.L.; Christopher-Stine, L.; Rosen, A. Isolated elevation of aldolase in the serum of myositis patients: A potential biomarker of damaged early regenerating muscle cells. Clin. Exp. Rheumatol. 2012, 30, 548–553. [Google Scholar]
- Mammen, A.L.; Mahoney, J.A.; St Germain, A.; Badders, N.; Taylor, J.P.; Rosen, A.; Spinette, S. A novel conserved isoform of the ubiquitin ligase UFD2a/UBE4B is expressed exclusively in mature striated muscle cells. PLoS ONE 2011, 6, e28861. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Tsukamoto, Y.; Hijiya, N.; Higuchi, Y.; Yano, S.; Yokoyama, S.; Kumamoto, T.; Moriyama, M. Induction of GNE in myofibers after muscle injury. Pathobiology 2010, 77, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Do, M.K.; Suzuki, T.; Ohtsubo, H.; Mizunoya, W.; Nakamura, M.; Furuse, M.; Ikeuchi, Y.; Tatsumi, R. Satellite cells produce neural chemorepellent semaphorin 3A upon muscle injury. Anim. Sci. J. 2013, 84, 185–189. [Google Scholar] [CrossRef]
- Garry, D.J.; Meeson, A.; Elterman, J.; Zhao, Y.; Yang, P.; Bassel-Duby, R.; Williams, R.S. Myogenic stem cell function is impaired in mice lacking the forkhead/winged helix protein MNF. Proc. Natl. Acad. Sci. USA 2000, 97, 5416–5421. [Google Scholar] [CrossRef]
- Kemp, M.W.; Edwards, B.; Burgess, M.; Clarke, W.T.; Nicholson, G.; Parry, D.A.; Davies, K.E. Syncoilin isoform organization and differential expression in murine striated muscle. J. Struct. Biol. 2009, 165, 196–203. [Google Scholar] [CrossRef]
- Miura, P.; Thompson, J.; Chakkalakal, J.V.; Holcik, M.; Jasmin, B.J. The utrophin A 5’-untranslated region confers internal ribosome entry site-mediated translational control during regeneration of skeletal muscle fibers. J. Biol. Chem. 2005, 280, 32997–33005. [Google Scholar] [CrossRef]
- Wang, Q.; Qiu, X.; Liu, T.; Ahn, C.; Horowitz, J.F.; Lin, J.D. The hepatokine TSK maintains myofiber integrity and exercise endurance and contributes to muscle regeneration. JCI Insight 2022, 7, e154746. [Google Scholar] [CrossRef]
- McCullagh, K.J.; Edwards, B.; Kemp, M.W.; Giles, L.C.; Burgess, M.; Davies, K.E. Analysis of skeletal muscle function in the C57BL6/SV129 syncoilin knockout mouse. Mamm. Genome 2008, 19, 339–351. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Demonbreun, A.R.; Lapidos, K.A.; Heretis, K.; Levin, S.; Dale, R.; Pytel, P.; Svensson, E.C.; McNally, E.M. Myoferlin regulation by NFAT in muscle injury, regeneration and repair. J. Cell Sci. 2010, 123, 2413–2422. [Google Scholar] [CrossRef] [PubMed]
- Maeda, Y.; Yonemochi, Y.; Nakajyo, Y.; Hidaka, H.; Ikeda, T.; Ando, Y. CXCL12 and osteopontin from bone marrow-derived mesenchymal stromal cells improve muscle regeneration. Sci. Rep. 2017, 7, 3305. [Google Scholar] [CrossRef] [PubMed]
- Darabi, R.; Gehlbach, K.; Bachoo, R.M.; Kamath, S.; Osawa, M.; Kamm, K.E.; Kyba, M.; Perlingeiro, R.C. Functional skeletal muscle regeneration from differentiating embryonic stem cells. Nat. Med. 2008, 14, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Di Rocco, A.; Uchibe, K.; Larmour, C.; Berger, R.; Liu, M.; Barton, E.R.; Iwamoto, M. Selective Retinoic Acid Receptor gamma Agonists Promote Repair of Injured Skeletal Muscle in Mouse. Am. J. Pathol. 2015, 185, 2495–2504. [Google Scholar] [CrossRef]
- Bryer, S.C.; Koh, T.J. The urokinase-type plasminogen activator receptor is not required for skeletal muscle inflammation or regeneration. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R1152–R1158. [Google Scholar] [CrossRef]
- Bryan, B.A.; Mitchell, D.C.; Zhao, L.; Ma, W.; Stafford, L.J.; Teng, B.B.; Liu, M. Modulation of muscle regeneration, myogenesis, and adipogenesis by the Rho family guanine nucleotide exchange factor GEFT. Mol. Cell. Biol. 2005, 25, 11089–11101. [Google Scholar] [CrossRef]
- Mathes, A.L.; Lafyatis, R. Role for Toll-like receptor 3 in muscle regeneration after cardiotoxin injury. Muscle Nerve 2011, 43, 733–740. [Google Scholar] [CrossRef]
- Wu, G.; Sher, R.B.; Cox, G.A.; Vance, D.E. Differential expression of choline kinase isoforms in skeletal muscle explains the phenotypic variability in the rostrocaudal muscular dystrophy mouse. Biochim. Biophys. Acta 2010, 1801, 446–454. [Google Scholar] [CrossRef]
- Fujita, R.; Kawano, F.; Ohira, T.; Nakai, N.; Shibaguchi, T.; Nishimoto, N.; Ohira, Y. Anti-interleukin-6 receptor antibody (MR16-1) promotes muscle regeneration via modulation of gene expressions in infiltrated macrophages. Biochim. Biophys. Acta 2014, 1840, 3170–3180. [Google Scholar] [CrossRef]
- Wu, G.; Sher, R.B.; Cox, G.A.; Vance, D.E. Understanding the muscular dystrophy caused by deletion of choline kinase beta in mice. Biochim. Biophys. Acta 2009, 1791, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Wada, E.; Kato, M.; Yamashita, K.; Kokuba, H.; Liang, W.C.; Bonne, G.; Hayashi, Y.K. Deficiency of emerin contributes differently to the pathogenesis of skeletal and cardiac muscles in LmnaH222P/H222P mutant mice. PLoS ONE 2019, 14, e0221512. [Google Scholar] [CrossRef] [PubMed]
- Mofarrahi, M.; McClung, J.M.; Kontos, C.D.; Davis, E.C.; Tappuni, B.; Moroz, N.; Pickett, A.E.; Huck, L.; Harel, S.; Danialou, G.; et al. Angiopoietin-1 enhances skeletal muscle regeneration in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R576–R589. [Google Scholar] [CrossRef]
- Gattazzo, F.; Molon, S.; Morbidoni, V.; Braghetta, P.; Blaauw, B.; Urciuolo, A.; Bonaldo, P. Cyclosporin A Promotes in vivo Myogenic Response in Collagen VI-Deficient Myopathic Mice. Front. Aging Neurosci. 2014, 6, 244. [Google Scholar] [CrossRef] [PubMed]
- Laziz, I.; Armand, A.S.; Pariset, C.; Lecolle, S.; Della Gaspera, B.; Charbonnier, F.; Chanoine, C. Sprouty gene expression is regulated by nerve and FGF6 during regeneration of mouse muscles. Growth Factors 2007, 25, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Armand, A.S.; Della Gaspera, B.; Launay, T.; Charbonnier, F.; Gallien, C.L.; Chanoine, C. Expression and neural control of follistatin versus myostatin genes during regeneration of mouse soleus. Dev. Dyn. 2003, 227, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Gao, H.L.; Shimokawa, T.; Nabeka, H.; Hamada, F.; Araki, H.; Cao, Y.M.; Kobayashi, N.; Matsuda, S. Prosaposin expression in the regenerated muscles of mdx and cardiotoxin-treated mice. Histol. Histopathol. 2013, 28, 875–892. [Google Scholar] [CrossRef]
- Cizkova, D.; Vavrova, J.; Micuda, S.; Filip, S.; Brcakova, E.; Bruckova, L.; Mokry, J. Role of transplanted bone marrow cells in response to skeletal muscle injury. Folia Biol. 2011, 57, 232–241. [Google Scholar]
- Wernig, G.; Janzen, V.; Schafer, R.; Zweyer, M.; Knauf, U.; Hoegemeier, O.; Mundegar, R.R.; Garbe, S.; Stier, S.; Franz, T.; et al. The vast majority of bone-marrow-derived cells integrated into mdx muscle fibers are silent despite long-term engraftment. Proc. Natl. Acad. Sci. USA 2005, 102, 11852–11857. [Google Scholar] [CrossRef]
- Jung, J.E.; Song, M.J.; Shin, S.; Choi, Y.J.; Kim, K.H.; Chung, C.J. Local myogenic pulp-derived cell injection enhances craniofacial muscle regeneration in vivo. Orthod. Craniofac. Res. 2017, 20, 35–43. [Google Scholar] [CrossRef]
- de la Garza-Rodea, A.S.; van der Velde, I.; Boersma, H.; Goncalves, M.A.; van Bekkum, D.W.; de Vries, A.A.; Knaan-Shanzer, S. Long-term contribution of human bone marrow mesenchymal stromal cells to skeletal muscle regeneration in mice. Cell Transplant 2011, 20, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Mori, J.; Ishihara, Y.; Matsuo, K.; Nakajima, H.; Terada, N.; Kosaka, K.; Kizaki, Z.; Sugimoto, T. Hematopoietic contribution to skeletal muscle regeneration in acid alpha-glucosidase knockout mice. J. Histochem. Cytochem. 2008, 56, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, J.; Dumont, N.; Lebel, C.; Quenneville, S.P.; Cote, C.H.; Frenette, J.; Tremblay, J.P. Dystrophin expression following the transplantation of normal muscle precursor cells protects mdx muscle from contraction-induced damage. Cell Transplant 2010, 19, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Gang, E.J.; Darabi, R.; Bosnakovski, D.; Xu, Z.; Kamm, K.E.; Kyba, M.; Perlingeiro, R.C. Engraftment of mesenchymal stem cells into dystrophin-deficient mice is not accompanied by functional recovery. Exp. Cell Res. 2009, 315, 2624–2636. [Google Scholar] [CrossRef] [PubMed]
- Kano, K.; Horiuchi, K.; Yoshida, Y.; Hayasaka, T.; Kabara, M.; Tomita, Y.; Tatsukawa, T.; Matsuo, R.; Sawada, J.; Nakagawa, N.; et al. EphA7(+) perivascular cells as myogenic and angiogenic precursors improving skeletal muscle regeneration in a muscular dystrophic mouse model. Stem Cell Res. 2020, 47, 101914. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, X.; Sun, Z.; Chen, B.; Han, Q.; Li, J.; Zhao, R.C. Flk-1+ adipose-derived mesenchymal stem cells differentiate into skeletal muscle satellite cells and ameliorate muscular dystrophy in mdx mice. Stem Cells Dev. 2007, 16, 695–706. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, S.; Zhou, J.; Chen, B.; Shang, Y.; Gao, T.; Wang, X.; Xie, H.; Chen, F. Clone-derived human AF-amniotic fluid stem cells are capable of skeletal myogenic differentiation in vitro and in vivo. J. Tissue Eng. Regen. Med. 2012, 6, 598–613. [Google Scholar] [CrossRef]
- Kim, J.A.; Shon, Y.H.; Lim, J.O.; Yoo, J.J.; Shin, H.I.; Park, E.K. MYOD mediates skeletal myogenic differentiation of human amniotic fluid stem cells and regeneration of muscle injury. Stem Cell Res. Ther. 2013, 4, 147. [Google Scholar] [CrossRef]
- Drapeau, C.; Antarr, D.; Ma, H.; Yang, Z.; Tang, L.; Hoffman, R.M.; Schaeffer, D.J. Mobilization of bone marrow stem cells with StemEnhance improves muscle regeneration in cardiotoxin-induced muscle injury. Cell Cycle 2010, 9, 1819–1823. [Google Scholar] [CrossRef][Green Version]
- Xuan, W.; Khan, M.; Ashraf, M. Pluripotent stem cell-induced skeletal muscle progenitor cells with givinostat promote myoangiogenesis and restore dystrophin in injured Duchenne dystrophic muscle. Stem Cell Res. Ther. 2021, 12, 131. [Google Scholar] [CrossRef]
- Naldaiz-Gastesi, N.; Goicoechea, M.; Aragon, I.M.; Perez-Lopez, V.; Fuertes-Alvarez, S.; Herrera-Imbroda, B.; Lopez de Munain, A.; de Luna-Diaz, R.; Baptista, P.M.; Fernandez, M.A.; et al. Isolation and characterization of myogenic precursor cells from human cremaster muscle. Sci. Rep. 2019, 9, 3454. [Google Scholar] [CrossRef] [PubMed]
- Abedi, M.; Foster, B.M.; Wood, K.D.; Colvin, G.A.; McLean, S.D.; Johnson, K.W.; Greer, D.A. Haematopoietic stem cells participate in muscle regeneration. Br. J. Haematol. 2007, 138, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.; Suk, S.; Shih, Y.R.; Seo, T.; Du, B.; Xie, Y.; Li, Z.; Varghese, S. WNT3A promotes myogenesis of human embryonic stem cells and enhances in vivo engraftment. Sci. Rep. 2014, 4, 5916. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, M.; Franzin, C.; Bertin, E.; Urbani, L.; Blaauw, B.; Repele, A.; Taschin, E.; Cenedese, A.; Zanon, G.F.; Andre-Schmutz, I.; et al. Amniotic fluid stem cells restore the muscle cell niche in a HSA-Cre, Smn(F7/F7) mouse model. Stem Cells 2012, 30, 1675–1684. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Chen, M.; Lee, C.H.; Yoon, R.; Lal, S.; Mao, J.J. Clones of ectopic stem cells in the regeneration of muscle defects in vivo. PLoS ONE 2010, 5, e13547. [Google Scholar] [CrossRef]
- Bossolasco, P.; Corti, S.; Strazzer, S.; Borsotti, C.; Del Bo, R.; Fortunato, F.; Salani, S.; Quirici, N.; Bertolini, F.; Gobbi, A.; et al. Skeletal muscle differentiation potential of human adult bone marrow cells. Exp. Cell Res. 2004, 295, 66–78. [Google Scholar] [CrossRef]
- Fukada, S.; Miyagoe-Suzuki, Y.; Tsukihara, H.; Yuasa, K.; Higuchi, S.; Ono, S.; Tsujikawa, K.; Takeda, S.; Yamamoto, H. Muscle regeneration by reconstitution with bone marrow or fetal liver cells from green fluorescent protein-gene transgenic mice. J. Cell Sci. 2002, 115, 1285–1293. [Google Scholar] [CrossRef]
- Luth, E.S.; Jun, S.J.; Wessen, M.K.; Liadaki, K.; Gussoni, E.; Kunkel, L.M. Bone marrow side population cells are enriched for progenitors capable of myogenic differentiation. J. Cell Sci. 2008, 121, 1426–1434. [Google Scholar] [CrossRef][Green Version]
- Zheng, J.K.; Wang, Y.; Karandikar, A.; Wang, Q.; Gai, H.; Liu, A.L.; Peng, C.; Sheng, H.Z. Skeletal myogenesis by human embryonic stem cells. Cell Res. 2006, 16, 713–722. [Google Scholar] [CrossRef]
- Meeson, A.P.; Hawke, T.J.; Graham, S.; Jiang, N.; Elterman, J.; Hutcheson, K.; Dimaio, J.M.; Gallardo, T.D.; Garry, D.J. Cellular and molecular regulation of skeletal muscle side population cells. Stem Cells 2004, 22, 1305–1320. [Google Scholar] [CrossRef]
- Kowalski, K.; Archacki, R.; Archacka, K.; Streminska, W.; Paciorek, A.; Golabek, M.; Ciemerych, M.A.; Brzoska, E. Stromal derived factor-1 and granulocyte-colony stimulating factor treatment improves regeneration of Pax7-/- mice skeletal muscles. J. Cachexia Sarcopenia Muscle 2016, 7, 483–496. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.; Mellows, B.; Sheard, J.; Antonioli, M.; Kretz, O.; Chambers, D.; Zeuner, M.T.; Tomkins, J.E.; Denecke, B.; Musante, L.; et al. Secretome of adipose-derived mesenchymal stem cells promotes skeletal muscle regeneration through synergistic action of extracellular vesicle cargo and soluble proteins. Stem Cell Res. Ther. 2019, 10, 116. [Google Scholar] [CrossRef] [PubMed]
- Tobin, S.W.; Alibhai, F.J.; Wlodarek, L.; Yeganeh, A.; Millar, S.; Wu, J.; Li, S.H.; Weisel, R.D.; Li, R.K. Delineating the relationship between immune system aging and myogenesis in muscle repair. Aging Cell 2021, 20, e13312. [Google Scholar] [CrossRef] [PubMed]

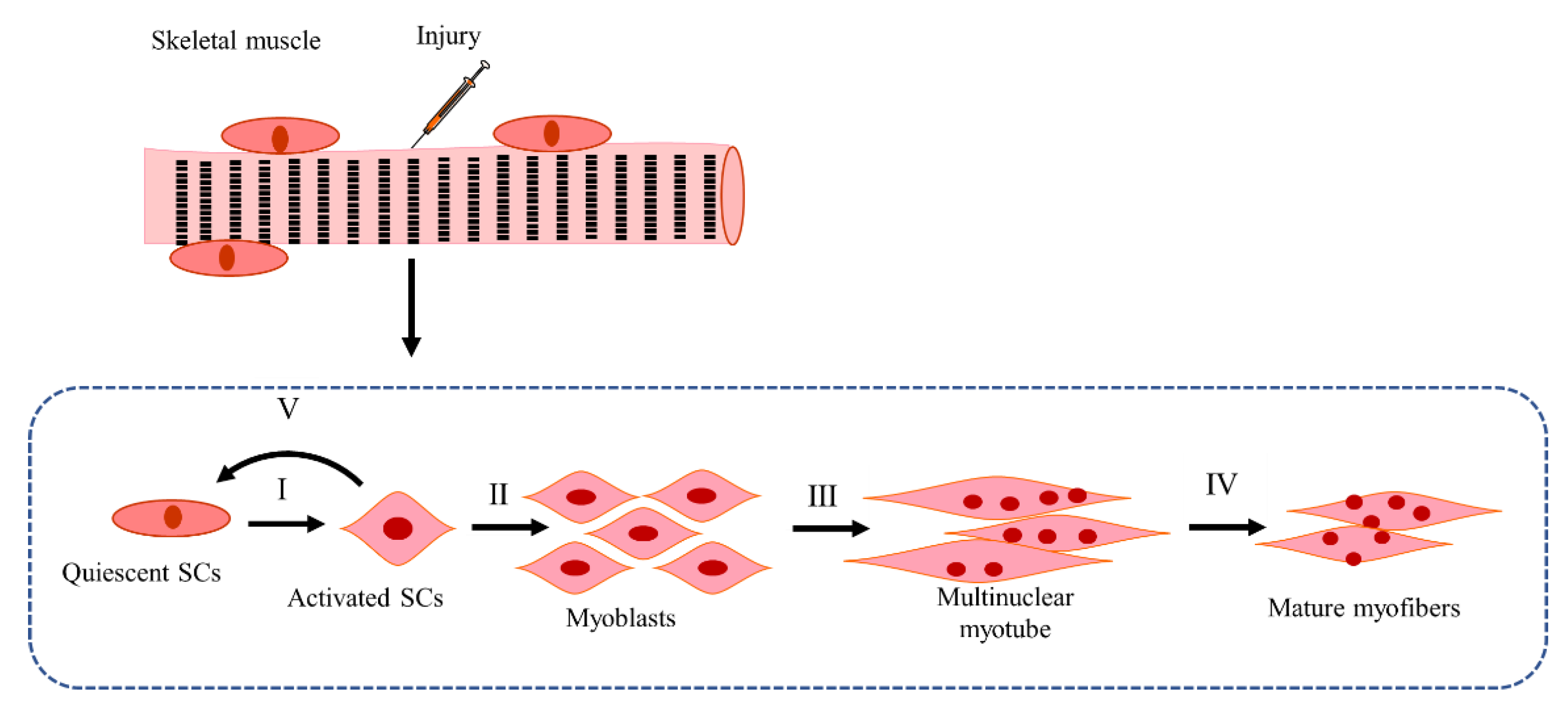
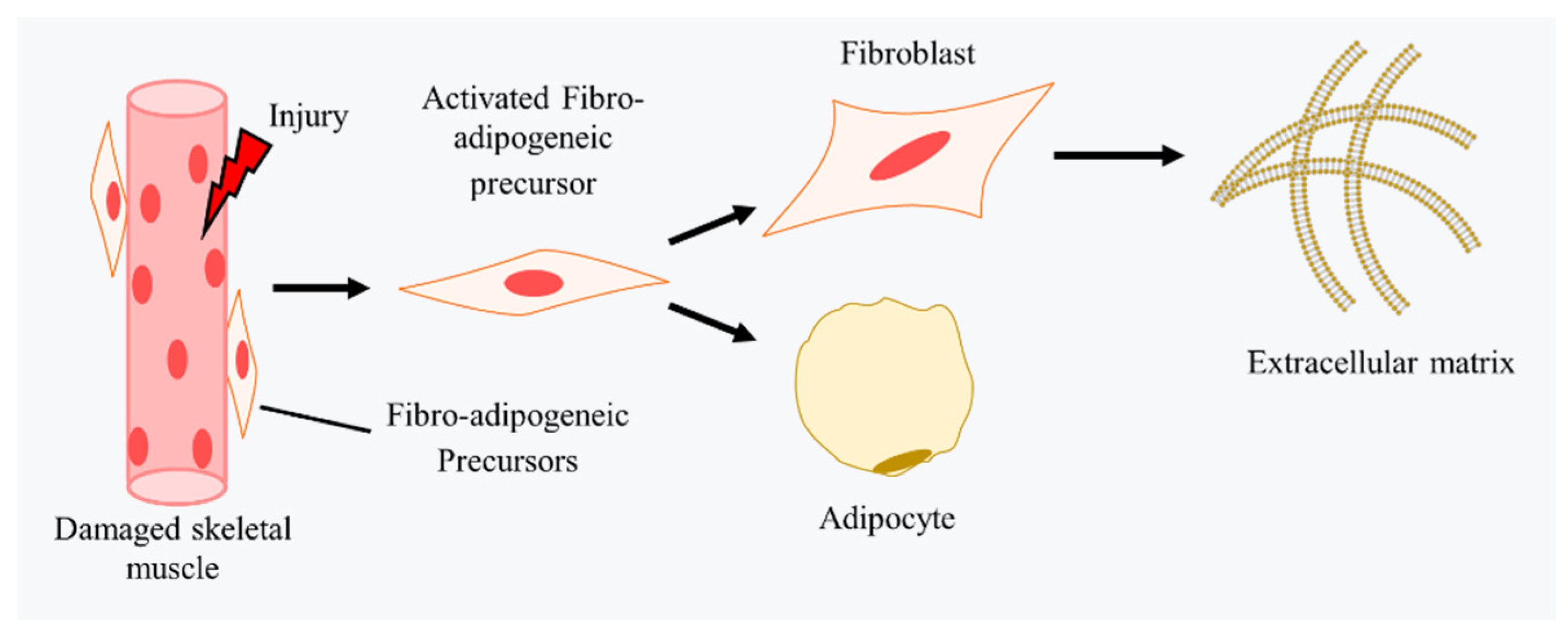
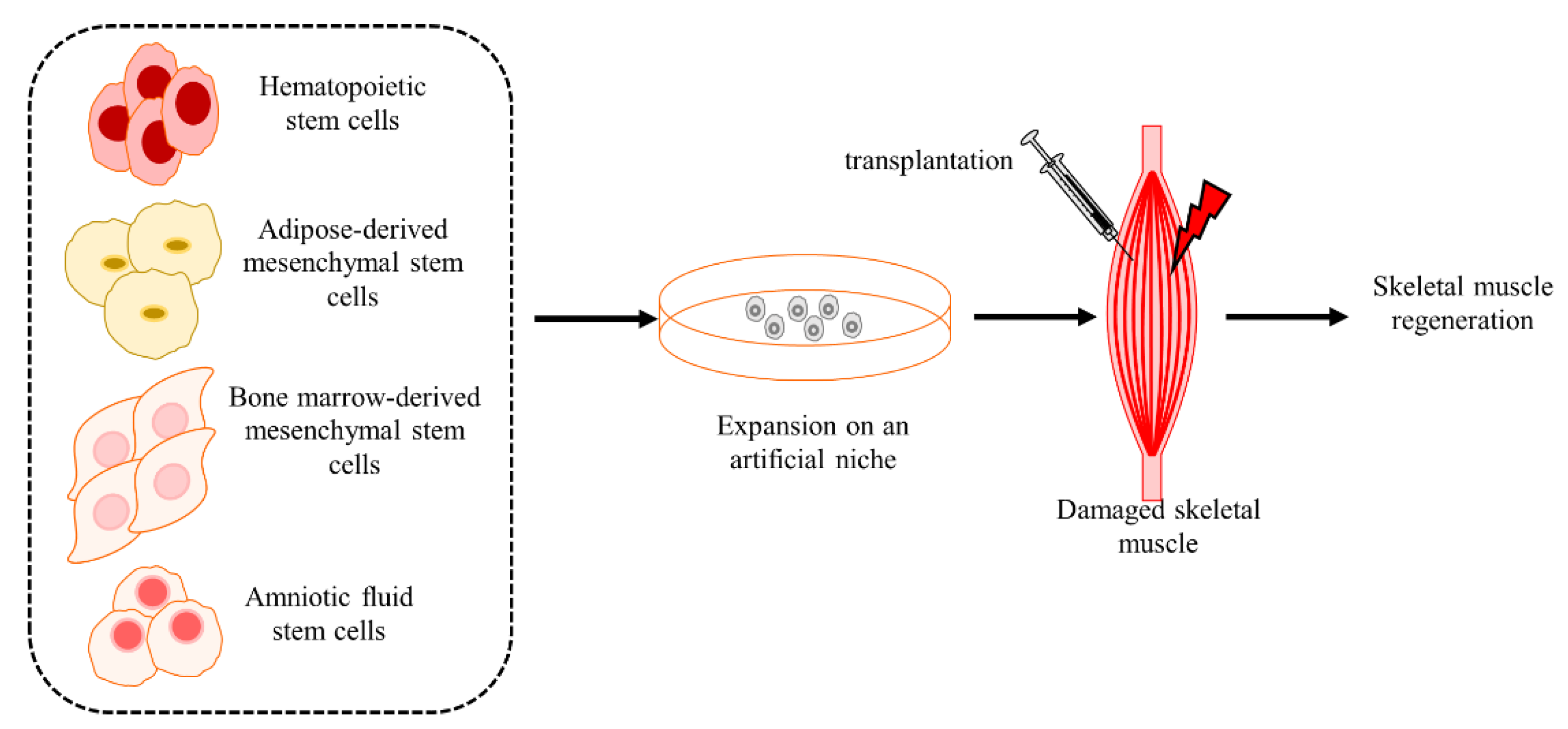
| Author, Year | Injury Portions | Mouse Models | Targets | Regeneration (Impair/Improve) | Ref. |
|---|---|---|---|---|---|
| Mouisel E, 2010 | Right tibialis anterior | 5 weeks,5, 12 and 18–24 months mdx mice | Different ages | Impair | [28] |
| Fearing CM, 2016 | Right hind limb anterior and post compartments | Male and female C57BL/6J mice; young:4-6 months, middle:12–19 months, old 25–30 months and very old:32–33 months | Ages and sex | Impair; —— | [40] |
| Takahashi Y, 2021 | Left tibialis anterior | 22 weeks C57BL/6-WT, C57BL/6-Akita, KK/Ta-WT, and KK/Ta-Akita male mice | Diabetes | Impair | [20] |
| Vignaud A, 2007 | Right tibialis anterior | 3–4 months STZ-treated Swiss and Akita male mice | Diabetes | Impair | [21] |
| Chaiyasing R, 2021 | Right tibialis anterior | 12 weeks ovariectomized and normal C5BL/6JJcl female mice | Estrogen | Impair | [41] |
| Rebalka IA, 2017 | Tibialis anterior | 10–12W STZ-treated and normal male C57BL/6J mice | Fluvastatin | Impair | [42] |
| Nguyen MH, 2011 | Extensor digitorum longus | 14–16 weeks leptin-deficient, leptin receptor-mutant mice and a group of C57BL/6 mice fed a high-fat diet | ob/ob and db/db | Impair | [22] |
| D’Souza DM, 2015 | Left gastrocnemius-plantaris, tibialis anterior, quadriceps | 16 weeks male C57BL/6J mice fed a high-fat diet | Diet-induced obesity | Impair | [24] |
| Jinno N, 2014 | Right gastrocnemius | 8 weeks male C57BL/6 mice: a marginally zinc-deficient diet-fed group, a zinc-adequate diet-fed group and a zinc-high diet-fed group | Zinc | Impair | [36] |
| Matsuba Y, 2009 | Soleus muscle | gravitational unloading | Gravitational unloading | Impair | [32] |
| Jeong J, 2013 | Tibialis anterior and gastrocnemius | 2–4 months C57BL/6 male mice treated with STZ, 20–24 months C57BL/6 male mice and C57BL/6J-Ins2Akita mice | Diabetes | Impair | [43] |
| Inaba S, 2018 | Tibialis anterior | cancer cachexia | Cancer cachexia | Impair | [26] |
| McHale MJ, 2012 | Right hind limb anterior and posterior compartment | 4–6 months ovariectomized, castrated and normal male and female C57BL/6J mice | Sex hormones | —— | [44] |
| Patsalos A, 2017 | Tibialis anterior | 2–6 months BoyJ, C57BL/6J male mice with or without irradiation and Pax7 Cre-Rosa26 DTA mice | Irradiation | Impair | [29] |
| Ikeda Y, 2019 | Gastrocnemius | 8 weeks male C57BL/6J mice with or without iron overload | Iron | Impair | [45] |
| Attia M, 2017 | Right tibialis anterior | 10 weeks myasthenia gravis and normal female C57BL/6J mice | Myasthenia gravis | Impair | [46] |
| Saliu TP, 2022 | Left tibialis anterior and gastrocnemius | 9 weeks normal and NAFLD male CD-1 mice | NAFLD | Impair | [47] |
| Rahman FA, 2020 | Left tibialis anterior | Young (3 months) and old (18 months) male C57BL/6J mice | Aging | Impair | [48] |
| Kohno S, 2012 | Soleus | C57BL/6J mice with or without tail suspension | Unloading | Impair | [38] |
| Kimoloi S, 2022 | Tibialis anterior | Both male and female K320Eskm and K320Emsc transgenic mice | Mitochondrial DNA alterations | Impair | [39] |
| Paiva-Oliveira EL, 2017 | Right gastrocnemius | Isogenic 6–8 weeks C3H/HeJ (TLR4 defective), C3H/HeN(TLR4 WT), C57BL/6 (WT) and TLR-4 knockout male mice | Different strains | —— | [49] |
| Yoshioka K, 2021 | Tibialis anterior and masseter | C57BL/6J and mdx mice | Position specificity | —— | [50] |
| Joanisse S, 2016 | Tibialis anterior | 24 months old male C57BL/6J mice with or without exercise and 8 weeks young male mice | Exercise and age | Improve; impair | [33] |
| Nagata K, 2013 | Tibialis anterior | 8 weeks C57BL/6J mice with ultrasound exposure | Ultrasound | Improve | [51] |
| Morioka S, 2008 | Soleus | 10 weeks male C57BL/6J mice with or without functional overloading | Functional overloading | Improve | [37] |
| Fujiya H, 2015 | Left tibialis anterior | 7 weeks male C57BL/6J mice with or without microcurrent electrical neuromuscular stimulation (MENS) | MENS | Improve | [35] |
| Author, Year | Injury Portions | Target Molecule/ Drug | Target Process | Expression | Effects (Positive/Negative) | Regeneration (Impair/Improve) | Ref. |
|---|---|---|---|---|---|---|---|
| Shi D, 2018 | Tibialis anterior | CaMKIV | Infiltration of macrophages | Up/Down | Positive/Negative | Impair/improve | [57] |
| Neves Jde C, 2015 | Right tibialis anterior | Neuraminidase-1 | Inflammatory response; myofiber maturation | Down | Positive; Negative | Impair | [58] |
| Liao ZH, 2019 | Tibialis anterior | Estrogen signaling | Inflammation infiltration; conversion of macrophages from M1 to M2 | Down | Positive; Negative | Impair | [59] |
| Kohno S, 2011 | Tibialis anterior | Cbl-b | Cytotoxic T-cell infiltration | Down | Positive | Impair | [60] |
| Wang H, 2014 | Right tibialis anterior and hindlimb posterior compartment | Monocyte/ macrophage | Monocyte and macrophages recruitment; conversion of macrophages from M1 to M2 | —— | Positive; Negative | Impair | [61] |
| Park CY, 2010 | Gastrocnemius and soleus | skNAC | Inflammation infiltration and myonecrosis | Down | Positive | Impair | [62] |
| Shi H, 2010 | Right tibialis anterior | MKP-1 | Inflammation; myoblast proliferation; differentiation | Down | Positive; Negative; Positive | Impair | [63] |
| Hu J, 2019 | Tibialis anterior | CaM signaling | Inflammatory response | Up | Positive | Improve | [64] |
| Manoharan P, 2019 | Gastrocnemius | Klf2 | Inflammatory response | Down | Positive | Improve | [65] |
| Gao Y, 2012 | Unilateral tibialis anterior | STAT1 | Inflammatory response | Down | Positive | Improve | [66] |
| Kozakowska M, 2018 | Gastrocnemius | Hmox1 | Inflammation and SC proliferation | Down | Positive | Improve | [67] |
| Koh, 2005 | Extensor digitorum longus | PAI-1 | Macrophage and SC migration | Down | Positive | Improve | [2] |
| Zhang, 2020 | Tibialis anterior and gastrocnemius | IFN-γ/CXCL10/ CXCR3 | Macrophages and myoblast proliferation | —— | Positive | Improve | [68] |
| Yaden BC, 2014 | Right Gastrocnemius | Activin A | Macrophage infiltration | Up | Positive | Improve | [69] |
| Mothe-Satney I, 2017 | Left Tibialis Anterior | PPARβ | Macrophage recruitment | Up | Positive | Improve | [70] |
| Tanaka Y, 2019 | Tibialis Anterior | APN | Elimination of the necrotic fibers | Up | Positive | Improve | [71] |
| Dinulovic I, 2016 | Tibialis Anterior | PGC-1α | Conversion of macrophages from M1 to M2 | Up/Down | Positive; Negative | Improve/Impair | [56] |
| Sugihara H, 2018 | Tibialis Anterior | PGRN | Prolonged Persistence of M2 Macrophages | Down | Positive | Improve | [72] |
| Lo Sicco, 2017 | Tibialis Anterior | Extracellular vesicles released by human adipose derived-MSCs | Conversion of macrophages from M1 to M2 | Up | Positive | Improve | [73] |
| Yang M, 2022 | tibialis anterior | Balenine | Phagocytosis ability of macrophages | —— | Positive | improve | [74] |
| Cardoso ES, 2016 | Gastrocnemius | Thymol | Inflammatory response | —— | Negative | Improve | [75] |
| Wang ZG, 2021 | Gastrocnemius | Conversion of n-6 to n-3 PUFAs | Inflammatory response; SC activation | Up | Negative; Positive | Improve | [76] |
| Chaweewannakorn C, 2018 | Unilaterally tibialis anterior | IL-1a/β | Inflammatory response | Down | Negative | Impair | [77] |
| Senf SM, 2013 | Tibialis anterior | Hsp70 | Inflammatory response | Down | Negative | Impair | [78] |
| Mojumdar K, 2016 | Tibialis anterior | TLR2 | Macrophage accumulation; elimination of the necrotic fibers | Down | Negative; Negative | Impair | [79] |
| Varga T, 2013 | Tibialis anterior | NUR77 | Macrophage development | Down | —— | Impair | [80] |
| Ochoa O, 2007 | Right anterior and posterior compartment | CCR2 | Macrophage recruitment and angiogenesis and VEGF production | Down | Negative | Impair | [81] |
| Zhang C, 2013 | Tibialis anterior and gastrocnemius | IL-6/STAT3 | Infiltration of macrophages and myoblast proliferation | Down | Negative | Impair | [82] |
| Zhang J, 2014 | Tibialis anterior | CD8 | Macrophage recruitment | Down | Negative | Impair | [83] |
| Martinez CO, 2010 | Right tibialis anterior and hindlimb posterior compartment | CCR-2/MCP-1 | Macrophage recruitment | Down | Negative | Impair | [84] |
| Krause MP, 2013 | Left tibialis anterior and gastrocnemius-plantaris-soleus | Diabetes | Macrophages infiltration | —— | —— | Impair | [23] |
| Cheng M, 2008 | Extensor digitorum longus and tibialis anterior | IFN-γ | Macrophages infiltration; myoblast proliferation | Down | Negative | Impair | [85] |
| Zhang, 2017 | Tibialis anterior and gastrocnemius | C3a | Monocyte/macrophage infiltration | Down | Negative | Impair | [86] |
| Sun D, 2009 | Right hind limb anterior and posterior compartment | CCR2 | Recruitment of macrophages and neutrophils | Down | Negative | Impair | [87] |
| Nishimura D, 2015 | Tibialis anterior | ADAM8 | Elimination of the necrotic fibers | Down | Negative | Impair | [88] |
| AI-Zaeed N, 2021 | Tibialis anterior | TAM kinase receptor Mer | Elimination of the necrotic fibers and conversion of macrophage from M1 to m2s | Down | Negative | Impair | [89] |
| Zhang J, 2019 | Tibialis anterior | SRB1 | Elimination of the necrotic fibers and conversion of macrophage from M1 to M2 | Down | Negative | Impair | [90] |
| Jin R M, 2018 | One or more hindlimb muscles | Preexisting inflammatory environment | Conversion of macrophage from M1 to M2 | —— | Negative | Impair | [91] |
| Bronisz-Budzyńska I, 2020 | Gastrocnemius | Nrf2 | Inflammatory response | Down | Positive | No effects | [92] |
| Tarban N, 2022 | Tibialis anterior | Retinol saturase | Phagocytosis ability of macrophages | Down | Negative | No effects | [93] |
| Dalle S, 2020 | Tibialis anterior | Ibuprofen | Inflammatory response | —— | Negative | No effects | [94] |
| Shen W, 2008 | Gastrocnemius | Macrophage, TFG-β1 and COX-2 | Inflammatory response | —— | —— | —— | [95] |
| Rousseau AS, 2021 | Left tibialis anterior | PPARβ/δ | T cell dynamic | Down | —— | —— | [96] |
| Author, Year | Injury Portions | Target Molecule/ Drug | Target Process | Expression | Effects (Positive/Negative) | Regeneration (Impair/Improve) | Ref. |
|---|---|---|---|---|---|---|---|
| Schaaf G J, 2018 | Quadriceps femoris and gastrocnemius | Acid alpha glucosidase | SC activation | Down | Negative | Impair | [115] |
| Serra C, 2013 | Left tibialis anterior | Testosterone | SC activation | Up | Positive | Improve | [116] |
| Liu Q, 2021 | Left tibialis anterior | Salvador | SC activation and angiogenesis | Down | Positive | Improve | [117] |
| Zeng L, 2010 | Tibialis anterior or gastrocnemius | Insl6 | SC activation and proliferation | Up/Down | Positive/Negative | Improve/impair | [108] |
| Shelar SB, 2016 | Tibialis anterior | Nrf2 | SC activation and proliferation | Down | Negative | Impair | [113] |
| Rebalka IA, 2018 | Left tibialis anterior or left gastrocnemius | Lcn2 | SC activation and fibrosis | Down | Negative | Impair | [114] |
| Zeng P, 2016 | Right tibialis anterior | Mir-378/IGF1R | SC activation and differentiation | Up | Negative | Impair | [118] |
| Nissar AA, 2011 | Tibialis anterior | Xin | SC activation | Down | Negative | Impair | [111] |
| Lagalice L, 2018 | Tibialis anterior | Acid alpha glucosidase | SC activation | Down | Negative | Impair | [119] |
| Mizbani A, 2016 | Tibialis anterior | Mirna-501 | SC activation | Down | Negative | Impair | [120] |
| Fiore PF, 2020 | Tibialis anterior | Pkcθ | SC self-renewal | Down | Positive | Improve | [121] |
| Fortier M, 2013 | Tibialis anterior | S1pr3 | SC proliferation | Down | Positive | Improve | [122] |
| Cai S, 2020 | Tibialis anterior | Mll1/myf5 | SC proliferation | Up | Positive | Improve | [123] |
| Sincennes MC, 2021 | Tibialis anterior | Pax7 acetylation | SC pool | Down | Positive | Improve | [124] |
| Naito T, 2009 | Tibialis anterior | G-csf | SC number | Up | Positive | Improve | [125] |
| Ohno Y, 2016 | Left soleus | Mstn | SC number | Down | Positive | Improve | [31] |
| Price FD, 2014 | Tibialis anterior | Jak/stat | SC number | Down | Positive | Improve | [126] |
| Hillege MMG, 2022 | Tibialis anterior | TGF-β signaling | SC number | Down | Positive | Improve | [112] |
| Angione AR, 2011 | Tibialis anterior and gastrocnemius | Pparδ | SC number and proliferation | Down | Negative | Impair | [127] |
| Nishizawa S, 2013 | Left soleus | Hsf1 | SC number and proinflammatory response | Down | Negative | Impair | [128] |
| Ahrens HE, 2018 | Right tibialis anterior | Klotho | SC number and function | Down | Negative | Impair | [129] |
| Sakamoto K, 2019 | Forearm muscle | R3hdml | SC number | down | Negative | Impair | [130] |
| Bye-A-Jee H, 2018 | Tibialis anterior | ZFP36L1 and ZFP36L2 | SC number | Down | Negative | Impair | [131] |
| Tonami K, 2013 | Left tibialis anterior | Capn6 | Myoblast differentiation | Down | Positive | Improve | [132] |
| Accornero F, 2014 | Tibialis anterior | TGF-β | SC number and activity; decreased degeneration | Down | Positive; Positive | Improve | [133] |
| Van Ry PM, 2014 | Tibialis anterior | Laminin-111 | SC pool; fibrosis | Up | Positive; Negative | Improve | [134] |
| Hosoyama T, 2011 | Tibialis anterior | Rb1 | SC pool; differentiation | Down | Positive; Negative | Impair | [135] |
| Rion N, 2019 | Unilaterally tibialis anterior | mTORC2 | SC pool replenishment | Down | Negative | Impair | [136] |
| Yoshida T, 2013 | Unilateral gastrocnemius | Angiotensin II | SC pool and proliferation | Up | Negative | Impair | [137] |
| Armand AS, 2011 | Soleus or EDL | AIF | SC pool | Down | Negative | Impair | [138] |
| Milanesi A, 2017 | Right tibialis anterior or quadriceps femoris | Thyroid hormone receptor alpha | SC pool | Down | Negative | Impair | [139] |
| Castets P, 2011 | Unilateral tibialis anterior and soleus muscles | SelN | SC pool | Down | Negative | Impair | [140] |
| Fujimaki S, 2018 | Right tibialis anterior | Notch1/Notch2 | SC pool and proliferation; myoblast differentiation; fibrosis | Down | Negative; Positive; Positive | Impair | [141] |
| Johnston AP, 2011 | Tibialis anterior | Ang II | SC number; myoblast differentiation | Down | Positive; Negative | Impair | [142] |
| Jaafar M N, 2016 | Right tibialis anterior | Primary cilium | SC self-renewal | —— | —— | —— | [143] |
| Leung C, 2020 | Tibialis anterior/Extensor digitorum longus | Lgr5 | SC replenish and myofiber formation | —— | Positive | Improve | [144] |
| Buono R, 2012 | Tibialis anterior and quadriceps | NO signaling | SC self-renewal and proliferation | Down | Negative | Impair | [145] |
| Urciuolo A, 2014 | Tibialis anterior | Collagen VI | SC self-renewal | Down | Negative | Impair | [146] |
| Alexeev V, 2014 | Left gastrocnemius muscle | Adipose-derived stem cells | Migration of SCs | —— | Positive | Improve | [147] |
| Brien P, 2013 | Tibialis anterior/extensor digitorum longus muscle group | P38α | Myoblast proliferation; differentiation | Down | Positive; Negative | Impair | [148] |
| Hawke TJ, 2003 | Tibialis anterior | p21 | Myoblast proliferation; differentiation | Down | Positive; Negative | Impair | [149] |
| Cortez-Toledo O, 2017 | Tibialis anterior | Nur77 | Myoblast proliferation | Down | Negative | No effects | [150] |
| Alves, 2019 | Rectus femoral muscle | Kinin-B2 receptor | Myoblast proliferation; differentiation | Down | Positive; Negative | Impair | [4] |
| Wu, 2014 | Right tibialis anterior | Duxbl | Myoblast proliferation and differentiation | Up | Positive; Negative | Impair | [151] |
| Chen Y, 2012 | Tibialis anterior | miR-351 | Myoblast proliferation and differentiation | Up/Down | Positive/Negative | Improve/impair | [152] |
| Tseng C, 2019 | Gastrocnemius | Sod1/Cat | Myoblast proliferation and differentiation | Up | Positive | Improve | [153] |
| Jia Y, 2012 | Gastrocnemius | EPO | Proliferation and survival of the SCs | —— | Positive | Improve | [154] |
| Shibasaki H, 2019 | Tibialis anterior | miR-188 | Myoblast fusion | Up/Down | Positive/Negative | Improve/impair | [155] |
| Hawke TJ, 2007 | Tibialis anterior | Xin | Myoblast proliferation and migration | Down | Positive | Improve | [109] |
| Meng, 2014 | Tibialis anterior | RNF13 | Myoblast proliferation and differentiation | Up | Positive | Improve | [104] |
| Lee EJ, 2021 | Left gastrocnemius | Glycyrrhiza uralensis-extracted compounds | Myoblast proliferation and differentiation | —— | Positive | Improve | [156] |
| Galimov A, 2016 | Tibialis anterior | FGF2 | Myoblast proliferation | Up | Positive | Improve | [107] |
| Armand, 2003 | Soleus | FGF6 | Myoblast proliferation | Down/Up | Negative/Positive | Impair/improve | [157] |
| Shi, 2010 | Tibialis anterior and gastrocnemius | Tceal7 | Myoblast proliferation; differentiation | Up | Negative; Positive | —— | [1] |
| Pessemesse L, 2019 | Right tibialis anterior | p43 | Myoblast proliferation | Down/Up | Negative/Positive | Impair/improve | [158] |
| Ye, 2016 | Gastrocnemius | CaMKK2 | Myoblast proliferation and differentiation | Up/Down | Negative/Positive | Impair/ Improve | [159] |
| Minetti GC, 2014 | Tibialis anterior | Gαi2 | Myoblast proliferation, differentiation and fusion | Down | Negative | Impair | [160] |
| Zhang CC, 2021 | Tibialis anterior | Cyp4a14 | Myoblast proliferation and differentiation and inflammatory response | Down | Negative | Impair | [161] |
| Ding, 2021 | Tibialis anterior | Tfr1 | Myoblast proliferation and differentiation | Down | Negative | Impair | [162] |
| Naito M, 2016 | Tibialis anterior | Dnmt3a | Myoblast proliferation | Down | Negative | Impair | [163] |
| Bae, 2020 | Tibialis anterior | Cdon | Myoblast proliferation and senescence | Down | Negative | Impair | [164] |
| Rooney JE, 2009 | Left tibialis anterior | α7 integrin | Myoblast proliferation and differentiation | Down | Negative | Impair | [165] |
| Katsushi, 2020 | Tibialis anterior | Bach 1 | Myoblast proliferation and differentiation | Down | Negative | Impair | [166] |
| Yamashita, 2016 | Gastrocnemius | FOXO1 | Myoblast proliferation | Up | Negative | Impair | [167] |
| Al-Sajee D, 2015 | Left tibialis anterior, gastrocnemius/ Plantaris/soleus, quadriceps muscles | Xin | Myoblast proliferation | Down | Negative | Impair | [110] |
| Girgenrath, 2006 | Tibialis anterior | Fn14 | Myoblast proliferation | Down | Negative | Impair | [168] |
| Martinet C, 2016 | Tibialis anterior | H19 | Myoblast proliferation | Down | Negative | Impair | [169] |
| Yahiaoui, 2008 | Tibialis anterior | MCP-1 | Myoblast proliferation | Up | Negative | Impair | [170] |
| Kursaka, 2017 | One leg of tibialis anterior | Egr3 | Myoblast proliferation | Down | Negative | Impair | [171] |
| Ochiai N, 2016 | Tibialis anterior | fad24 | Myoblast proliferation | Down | Negative | Impair | [172] |
| Zanou, 2012 | Tibialis anterior and extensor digitorium longus | Trpc1 | Myoblast migration and differentiation | Down | Negative | Impair | [173] |
| Yablonka-Reuveni Z, 2015 | Unilateral tibialis anterior | FGFR1 | Myoblast proliferation | Down | Negative | No effects | [174] |
| Ohtsubo, 2017 | Gastrocnemius | APOBEC2 | Myoblast differentiation and fusion | Down | Positive | Improve | [8] |
| He, 2019 | Extensor digitorum longus | Nicotine | Myoblast differentiation | —— | Positive | Improve | [175] |
| Wu, 2007 | Tibialis anterior | Sema4C | Myoblast differentiation | Up/Down | Positive/Negative | Improve/impair | [176] |
| Liu, 2012 | Tibialis anterior | miR-206 | Myoblast differentiation | Up | Positive | Improve | [177] |
| Song, 2018 | Tibialis anterior | Linc-smad7 | Myoblast differentiation | Up | Positive | Improve | [178] |
| Gatta L, 2017 | Right tibialis anterior | Trimetazidine | Myoblast differentiation | —— | Positive | Improve | [179] |
| Gagan, 2011 | Tibialis anterior | miR-378 | Myoblast differentiation | Up | positive | Improve | [180] |
| Mikami T, 2012 | Tibialis anterior | Chondroitin sulfate | Myoblast differentiation | Down | Positive | Improve | [181] |
| Lee KP, 2015 | Hindlimb muscle | miR-431 | Myoblast differentiation | Up | positive | Improve | [27] |
| Storbeck CJ, 2013 | Tibialis anterior | SLK | Myoblast differentiation | Down | Positive | Improve | [182] |
| Lei, 2013 | One leg of tibialis anterior | MMP-13 | Myoblast migration | Down/Up | Negative/Positive | Impair/improve | [183] |
| Das, 2017 | Tibialis anterior | ACL | Myoblast differentiation | Down/Up | Negative/Positive | Impair/improve | [184] |
| Yoshida T, 2014 | Gastrocnemius | AT2R | Myoblast differentiation | Down/Up | Negative/Positive | Impair/improve | [185] |
| Huang Y, 2016 | Right tibialis anterior | Ccndbp1 | Myoblast differentiation | Down/Up | Negative/Positive | Impair/ Improve | [186] |
| Chen SE, 2007 | Soleus | TNF-α | Myoblast differentiation | Down/Up | Negative/Positive | Impair/ Improve | [187] |
| He, 2021 | Tibialis anterior | IRE1a | Myoblast differentiation and hypertrophy | Down/Up | Negative/ Positive | Impair/improve | [188] |
| Luca E, 2020 | Tibialis anterior | miRNA network | Myoblast differentiation | Down | Negative | Improve | [189] |
| Esteca MV, 2020 | Left tibialis anterior | Parkin | Myoblast differentiation | Down | Negative | Impair | [190] |
| Ishii A, 2001 | Tibialis anterior | Tensin | Myoblast differentiation and fusion | Down | Negative | Impair | [191] |
| Zhang M, 2020 | Tibialis anterior of one limb | Rbm24 | Myoblast differentiation | Down | Negative | Impair | [106] |
| Lee, 2015 | Tibialis anterior | miR-431 | Myoblast differentiation | Down | Negative | Impair | [192] |
| Fan, 2018 | Tibialis anterior | Hsp70 | Myoblast differentiation | Down | Negative | Impair | [16] |
| Cerquone, 2018 | Tibialis anterior | PAK1 | Myoblast differentiation | Down | Negative | Impair | [193] |
| Lee, 2020 | Tibialis anterior | PHF20 | Myoblast differentiation | Up | Negative | Impair | [194] |
| Hayashi, 2016 | Tibialis anterior | Klf5 | Myoblast differentiation | Down | Negative | Impair | [3] |
| Lee, 2015 | Tibialis anterior | AKAP6 | Myoblast differentiation | Down | Negative | Impair | [195] |
| Li, 2020 | Tibialis anterior | LRTM1 | Myoblast differentiation | Down | Negative | Impair | [196] |
| Lin, 2019 | Left gastrocnemius | MPM | Myoblast differentiation | Down | Negative | Impair | [197] |
| Harada, 2018 | Tibialis anterior | H3mm7 | Myoblast differentiation | Down | Negative | Impair | [198] |
| Tetsuaki, 2009 | Tibialis anterior | CT-1 | Myoblast differentiation | Up | Negative | Impair | [199] |
| Paul, 2012 | Tibialis anterior | COPR5 | Myoblast differentiation | Down | Negative | Impair | [200] |
| Faralli, 2011 | Tibialis anterior and gastrocnemius of one hind limb | Tshz3 | Myoblast differentiation | Up | Negative | Impair | [201] |
| Liu N, 2014 | Tibialis anterior | MEF2A, C and D | Myoblast differentiation | Down | Negative | Impair | [202] |
| Kielbasa OM, 2011 | Unilateral tibialis anterior | Myospryn | Myoblast differentiation | Up | Negative | Impair | [203] |
| Verpoorten S, 2020 | Tibialis anterior | CD36 | Myoblast differentiation | Down | Negative | Impair | [204] |
| Andrée B, 2002 | Right gastrocnemius and soleus | Pop | Myoblast differentiation | Down | Negative | Impair | [205] |
| Paolini A, 2018 | Tibialis anterior | Autophagy | Myoblast differentiation | Down | Negative | Impair | [206] |
| Clow C, 2010 | Tibialis anterior | BDNF | Myoblast differentiation | Down | Negative | Impair | [207] |
| Marshall JL, 2012 | Left quadriceps | SSPN | Myoblast differentiation | Down | Negative | Impair | [208] |
| Chen SE, 2005 | Soleus | TNF-α | Myoblast differentiation | Down | Negative | Impair | [209] |
| Ravel, 2014 | Tibialis anterior | Staufen1 | Myoblast differentiation | Up | Negative | Impair | [210] |
| Langsdorf A, 2007 | Tibialis anterior | Sulfs | Myoblast differentiation | Down | Negative | Impair | [211] |
| Liu H, 2011 | Tibialis anterior and soleus | β3-Integrin | Myoblast differentiation | Down | Negative | Impair | [212] |
| Watanabe S, 2007 | Right tibialis anterior | Lbx1 | Myoblast differentiation | Down | Negative | Impair | [213] |
| Fu D, 2015 | Tibialis anterior | Mdm2 | Myoblast differentiation | Down | Negative | Impair | [214] |
| Schroer, 2019 | Tibialis anterior | RGS12 | A switch from myoblast proliferation to differentiation | Down | Negative | Impair | [215] |
| Lim S, 2013 | Left tibialis anterior | CBR1 | Myoblast differentiation | Down | Negative | —— | [216] |
| Mammen AL, 2009 | Right tibialis anterior | Mi-2 | Myoblast differentiation | Up | Negative | —— | [217] |
| Wu Z, 2020 | Tibialis anterior | Andrographolide | Myoblast differentiation and fusion | —— | —— | Improve | [218] |
| Kurosaka, 2021 | Left tibialis anterior | STAT6 | Myoblast differentiation and fusion | Down | —— | Improve | [219] |
| Budai Z, 2021 | Tibialis anterior | TG2 | Myoblast fusion and conversion of macrophages from M1 to M2 | Down | Negative | Impair | [102] |
| Vijayakumar A, 2013 | Unilateral tibialis anterior | IGF-1R | Myoblast fusion | Down | Negative | Impair | [220] |
| Pryce BR, 2017 | Unilateral tibialis anterior | SLK | Myoblast fusion | Down | Negative | Impair | [221] |
| Redelsperger, 2016 | One tibialis anterior | Syncytin | Myoblast fusion | Down | Negative | Impair | [222] |
| Kaspar, 2013 | Tibialis anterior | 3′ untranslated region of c-Myb | Myoblast fusion | Down | Negative | Impair | [223] |
| Griffin, 2016 | Left tibialis anterior and gastrocnemius | ANO5 | Myoblast fusion | Down | Negative | Impair | [224] |
| Trapani L, 2012 | Right tibialis anterior | HMGR | Myoblast fusion | Down | Negative | Impair | [225] |
| Hamoud, 2018 | Tibialis anterior | BAI3 | Myoblast fusion | Down | Negative | Impair | [226] |
| Tamilarasan K P, 2012 | Gastrocnemius | Lipid accumulation | Myoblast fusion | Up | Negative | Impair | [227] |
| Yoshida N, 2019 | Tibialis anterior | (P)RR | Myoblast fusion | Up | Negative | Impair | [228] |
| Shibasaki H, 2019 | Tibialis anterior | miR-188 | Myoblast fusion | Up/Down | Positive/Negative | Improve/impair | [155] |
| Youm TH, 2019 | tibialis anterior | Nox4/ROS | Myoblast fusion | —— | Positive | Improve | [229] |
| Teng, 2015 | One tibialis anterior | PLD1 | Myoblast fusion | Up | Positive | Improve | [230] |
| Krause MP, 2013 | Left tibialis anterior | Mustn1 | Myoblast fusion | Up | Positive | Improve | [231] |
| Kurosaka, 2016 | Tibialis anterior | TRPV I | Myoblast fusion | Up | Positive | Improve | [232] |
| Singhal N, 2015 | Gastrocnemius, quadriceps, tibialis anterior | Galgt1 | Myoblast fusion; SC apoptosis | Down | Negative; Positive | Impair | [233] |
| Yalvac ME, 2017 | Left tibialis anterior and left gastrocnemius | Calpain-3 | Myoblast fusion; fibrosis | Down | Negative; Positive | Impair | [234] |
| Ogawa, 2015 | Tibialis anterior | Dcx | Myofiber maturation | Down | Negative | Impair | [235] |
| Ohno Y, 2019 | Right tibialis anterior | Lactate | Myotube formation | Up | Positive | Improve | [236] |
| Hoshino S, 2013 | Right tibialis anterior | CHC22 | Myofiber maturation | Up | Negative | Impair | [237] |
| Hu Z, 2010 | Unilateral tibialis anterior | PTEN | Myofiber maturation; fibrosis | Down | Positive | Improve | [25] |
| Agbulut O, 2001 | Gastrocnemius and soleus or tibialis anterior | Desmin | Myofiber maturation; neuromuscular junctions | Down | Negative | Impair | [238] |
| Cicchillitti, 2012 | Tibialis anterior | miR-210 | Myoblast differentiation | Down | —— | No effects | [239] |
| Piccioni A, 2014 | Tibialis anterior | Shh | Activated SCs | Up | —— | Improve | [240] |
| Ceco E, 2021 | Left tibialis anterior | Elevated CO2 exposure | Myoblast differentiation and fusion | —— | —— | Impair | [30] |
| Liu, 2017 | Tibialis anterior | Twist2 | Maintain SC state | —— | —— | —— | [241] |
| Author, Year | Injury Portions | Target Molecule/ Drug | Target Process | Expression | Effects (Positive/Negative) | Regeneration (Impair/Improve) | Ref. |
|---|---|---|---|---|---|---|---|
| Heredia JE, 2013 | Unilateral tibialis anterior | IL-4 | FAP proliferation | Down | Negative | Impair | [55] |
| Vumbaca S, 2021 | Tibialis anterior, quadriceps, and gastrocnemius | IL1a/IL1β and extracellular vesicles | FAP proliferation and differentiation | Up | Positive | —— | [248] |
| Zhao L, 2020 | Tibialis anterior | RA signaling | FAP proliferation | Up | positive | Improve | [246] |
| Zanotti S, 2018 | Tibialis anterior | Exosome miR-199a-5p/CAV1 | Fibrosis | Up | Positive | Impair | [247] |
| Horii N, 2018 | Tibialis anterior | C1q/Wnt and resistance training | Fibrosis | Up | positive | Impair | [34] |
| Murray, 2017 | Tibialis anterior | αV intergin | Fibrosis | Down | Negative | Improve | [245] |
| Burks, 2011 | Tibialis anterior | Losartam | Fibrosis | —— | Negative | Improve | [250] |
| Stepien DM, 2020 | Left tibialis anterior | TGF-β1 | Fibrosis | Down | Negative | Improve | [249] |
| Bosnakovski D, 2022 | Tibialis anterior | a prior DUX4 burst | Fibrosis | —— | Positive | —— | [251] |
| Ding, 2016 | Tibialis anterior | TAR RNA-binding protein (Trbp) | Fibrosis; myofiber formation | Down | Positive; Negative | Impair | [244] |
| Ogasawara S, 2018 | Left gastrocnemius | CatK | Fibrosis; inflammation and cell apoptosis | Down | Negative | Improve | [252] |
| Lee SJ, 2010 | Gastrocnemius | Follistatin | Fibrosis; myofiber maturation | Down | Negative | Impair | [253] |
| Rinaldi F, 2016 | Tibialis anterior | GDF11 | Collagen deposition | Up | Positive | No effects | [254] |
| Mignemi NA, 2017 | Posterior compartments of the lower extremities | Plasmin | Calcification | Down | Negative | Impair | [255] |
| Lee YS, 2013 | Right gastrocnemius | Gasp1 and/or Gasp2 | Calcified fibers and fibrosis | Down | Positive | Impair | [256] |
| Zhao Y, 2009 | Tibialis anterior | —— | Dystrophic calcification | —— | —— | —— | [257] |
| Lounev V Y, 2009 | Quadriceps | Tie2-expressing endothelial precursors | Heterotopic ossification | —— | —— | —— | [258] |
| Drouin G, 2019 | —— | Hypoxic state | Heterotopic ossification | —— | —— | —— | [259] |
| Arsic N, 2004 | Tibialis anterior | VEGF | Apoptosis | Up | Negative | improve | [260] |
| Sinha-Hikim I, 2007 | Gastrocnemius | JNK and iNOS signaling | Cell apoptosis | Down | Negative | improve | [261] |
| Min K, 2017 | Gastrocnemius/ Soleus | MKP-5 | myofiber apoptosis | Down | Negative | improve | [262] |
| Tjondrokoesoemo A, 2016 | Tibialis anterior | serpina3n | Stabilization of myofiber plasm membrane | Up | Positive | Improve | [263] |
| Author, Year | Injury Portions | Target Molecule/ Drug | Target Process | Expression | Effects (Positive/Negative) | Regeneration (Impair/Improve) | Ref. |
|---|---|---|---|---|---|---|---|
| Bellamy LM, 2010 | Unilateral tibialis anterior | Angiotensin II | Angiogenesis | Down | Negative | Impair | [266] |
| Ieronimakis N, 2012 | Tibialis anterior, quadriceps, gastrocnemius | Bone marrow-derived cells | Angiogenesis | —— | Positive | Improve | [265] |
| Mellows B, 2017 | Tibialis anterior | Extracellular vesicles-derived from amniotic fluid stem cell | Angiogenesis | —— | Positive | Improve | [267] |
| Hosaka Y, 2002 | Right tibialis anterior | α1-Syntrophin | Hypertrophy and neuromuscular junctions | Down | Positive; Negative | Improve | [268] |
| Daneshvar N, 2020 | Left tibialis anterior | Premature satellite cell activation | Maturation of neuromuscular junctions | Up | Negative | Improve | [269] |
| Kurosaka M, 2021 | Tibialis anterior | M2 macrophage | Motor innervation regeneration | —— | —— | Improve | [219] |
| Sawano S, 2014 | Tibialis anterior | M2 macrophage | Motor innervation regeneration | —— | —— | Improve | [270] |
| Randazzo D, 2019 | Unilaterally tibialis anterior | Tubb6 | Microtubule organization | Up | Negative | —— | [15] |
| Author, Year | Injury Portions | Target Molecule | Expression | Regeneration (Impair/Improve) | Ref. |
|---|---|---|---|---|---|
| Kim DS, 2015 | Left tibialis anterior | TLR2 | Down | Improve | [272] |
| Oikawa S, 2019 | Tibialis anterior | Dicer | Down | Impair | [273] |
| Hiramuki Y, 2015 | Tibialis anterior | Mest | Down | Impair | [274] |
| Norton CR, 2013 | Tibialis anterior | Snai1/Snai3 | Down | No effects | [275] |
| Call JA, 2017 | Left tibialis anterior and left flexor digitorum longus | Ulk1 | Down | —— | [276] |
| Chaturvedi N, 2020 | Left gastrocnemius | S100A1 | Down | —— | [277] |
| Parks CA, 2019 | Left tibialis anterior | Trim33 | Down | No effects | [278] |
| Goetsch SC, 2005 | Gastrocnemius | Filamin C | Up | —— | [279] |
| Wardrop KE, 2011 | Left tibialis anterior | LYVE-1 | Down | —— | [280] |
| Merkulova T, 2000 | Extensor digitorum longus and tibialis anterior | β enolase | Up | —— | [281] |
| Yuasa K, 2008 | Tibialis anterior | miR-206 | Up | —— | [282] |
| Casciola-Rosen L, 2012 | Right anterior tibilias | Aldolase A | Up | —— | [283] |
| Mammen AL, 2011 | Right tibialis anterior | UFD2a | Up | —— | [284] |
| Nakamura K, 2010 | Right gastrocnemius | GNE | Up | —— | [285] |
| Sato Y, 2013 | Left gastrocnemius | Sema3A | Up | —— | [286] |
| Garry, 2000 | Hind limbs | MNF | Down | Impair | [287] |
| Kemp MW, 2009 | Tibialis anterior | Syncoilin | Up | —— | [288] |
| Miura P, 2005 | Right tibialis anterior | Utrophin A | Up | —— | [289] |
| Wang Q, 2022 | Tibialis anterior | Tsukushi | Down | Impair | [290] |
| McCullagh KJ, 2008 | Unilateral tibialis anterior | Syncoilin | Down | No effects | [291] |
| Demonbreun AR, 2010 | Quadriceps or gastrocnemius/soleus | Ferlin | Up | —— | [292] |
| Maeda Y, 2017 | Right tibialis anterior | CXCL12 | Up | Improve | [293] |
| Darabi R, 2008 | Tibialis anterior | Pax3 | Up | Improve | [294] |
| Di Rocco A, 2015 | Tibialis anterior | RARγ | Down | Impair | [295] |
| Bryer SC, 2007 | Extensor digitorum longus and tibialis anterior | uPAR | —— | No effects | [296] |
| Bryan BA, 2005 | Tibialis anterior | GEFT | Up | Improve | [297] |
| Mathes AL, 2011 | Tibialis anterior | TLR-3 | Down | Impair | [298] |
| Wu G, 2010 | Forelimb leg muscle | Chkb | Down | No effects | [299] |
| Yan Z, 2003 | Tibialis anterior | E2f1 | Down | Impair | [5] |
| Fujita R, 2014 | Left tibialis anterior | IL-6R | Down | Improve | [300] |
| Wu G, 2009 | Gastrocnemius | Chkb | Down | Impair | [301] |
| Wada E, 2019 | Tibialis anterior | Emerin and lamin A/C | Down | Impair | [302] |
| Mofarrahi M, 2015 | Unilateral tibialis anterior | Ang-1 | Up | Improve | [303] |
| Gattazzo F, 2014 | Tibialis anterior | Cyclosporin A | Up | Improve | [304] |
| Kim MH, 2011 | Unilaterally tibialis anterior | Akt | Up | Improve | [243] |
| Laziz I, 2007 | Soleus | Spry | Down | —— | [305] |
| Armand AS, 2003 | Unilateral soleus | Follistatin and myostatin | Up/Down | Improve | [306] |
| Li C, 2013 | Right gastrocnemius | Prosaposin | Up | —— | [307] |
| Author, Year | Injury Portions | Target Molecule/Drug | Regeneration | Ref |
|---|---|---|---|---|
| Kano K, 2020 | Gastrocnemius | Capillary stem cells | Improve | [315] |
| Liu Y, 2007 | Unilateral tibialis anterior | Flk-1+ AD-MSCs | Improve | [316] |
| Kim JA, 2013 | Left tibialis anterior | hAFS cells transfected with MyoD | Improve | [318] |
| Xuan W, 2021 | Tibialis anterior | Pluripotent stem cells-induced skeletal muscle progenitor cells with givinostat | Improve | [320] |
| Naldaiz-Gastesi N, 2019 | Tibialis anterior | Human cremaster muscle-derived precursor cells | Improve | [321] |
| Mori J, 2008 | Tibialis anterior | CD45+: Sca-1+ hematopoietic stem cells | Improve | [312] |
| Abedi M, 2007 | Tibialis anterior | Hematopoietic stem cells | Improve | [322] |
| Hwang Y, 2014 | Tibialis anterior | Human embryonic stem cells | Improve | [323] |
| Piccoli M, 2012 | Tibialis anterior mice transplanted with bone marrow or amniotic fluid stem cells | Amniotic fluid stem cells | Improve | [324] |
| Yang R, 2010 | Right tibialis anterior | Clones of ectopic stem cells | Improve | [325] |
| Rousseau J, 2010 | EDL | Muscle precursor cells | Improve | [313] |
| Gang EJ, 2009 | Tibialis anterior | Mesenchymal stem cells | Improve | [314] |
| Jung JE, 2017 | Gastrocnemius and masseter | Pulp-derived cell | Improve | [310] |
| de la Garza-Rodea AS, 2011 | Tibialis anterior | BM-hMSCs | improve | [311] |
| Bossolasco, 2004 | Tibialis anterior | Human adult BM | Improve | [326] |
| Ma, 2012 | Tibialis anterior | Human AF-amniotic fluid stem cells | Improve | [317] |
| Fukada S, 2002 | Tibialis anterior | Bone marrow and fetal liver cells | Improve | [327] |
| Luth ES, 2008 | Tibialis anterior, quadriceps, and gastrocnemius | Bone marrow side population cells | Improve | [328] |
| Zheng JK, 2006 | Tibialis anterior | Human embryonic stem cells | Improve | [329] |
| Cížková D, 2011 | Right tibialis anterior | BMCs | Improve | [308] |
| Meeson AP, 2004 | Hindlimbs | Skeletal muscle side population | Improve | [330] |
| Drapeau C, 2010 | Right tibialis anterior | Mobilization of bone marrow stem cells | Improve | [319] |
| Kowalski K, 2016 | Gastrocnemius | Sdf-1 and granulocyte-colony stimulating factor | Improve | [331] |
| Mitchell R, 2019 | Right tibialis anterior | ADSC secretome | Improve | [332] |
| Tobin S, 2021 | Tibialis anterior; quadriceps; gastrocnemius | Young/aging macrophages | Improve/impair | [333] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Lu, J.; Liu, Y. Skeletal Muscle Regeneration in Cardiotoxin-Induced Muscle Injury Models. Int. J. Mol. Sci. 2022, 23, 13380. https://doi.org/10.3390/ijms232113380
Wang Y, Lu J, Liu Y. Skeletal Muscle Regeneration in Cardiotoxin-Induced Muscle Injury Models. International Journal of Molecular Sciences. 2022; 23(21):13380. https://doi.org/10.3390/ijms232113380
Chicago/Turabian StyleWang, Yanjie, Jianqiang Lu, and Yujian Liu. 2022. "Skeletal Muscle Regeneration in Cardiotoxin-Induced Muscle Injury Models" International Journal of Molecular Sciences 23, no. 21: 13380. https://doi.org/10.3390/ijms232113380
APA StyleWang, Y., Lu, J., & Liu, Y. (2022). Skeletal Muscle Regeneration in Cardiotoxin-Induced Muscle Injury Models. International Journal of Molecular Sciences, 23(21), 13380. https://doi.org/10.3390/ijms232113380






