Immune Tolerance of Embryo Implantation and Pregnancy: The Role of Human Decidual Stromal Cell- and Embryonic-Derived Extracellular Vesicles
Abstract
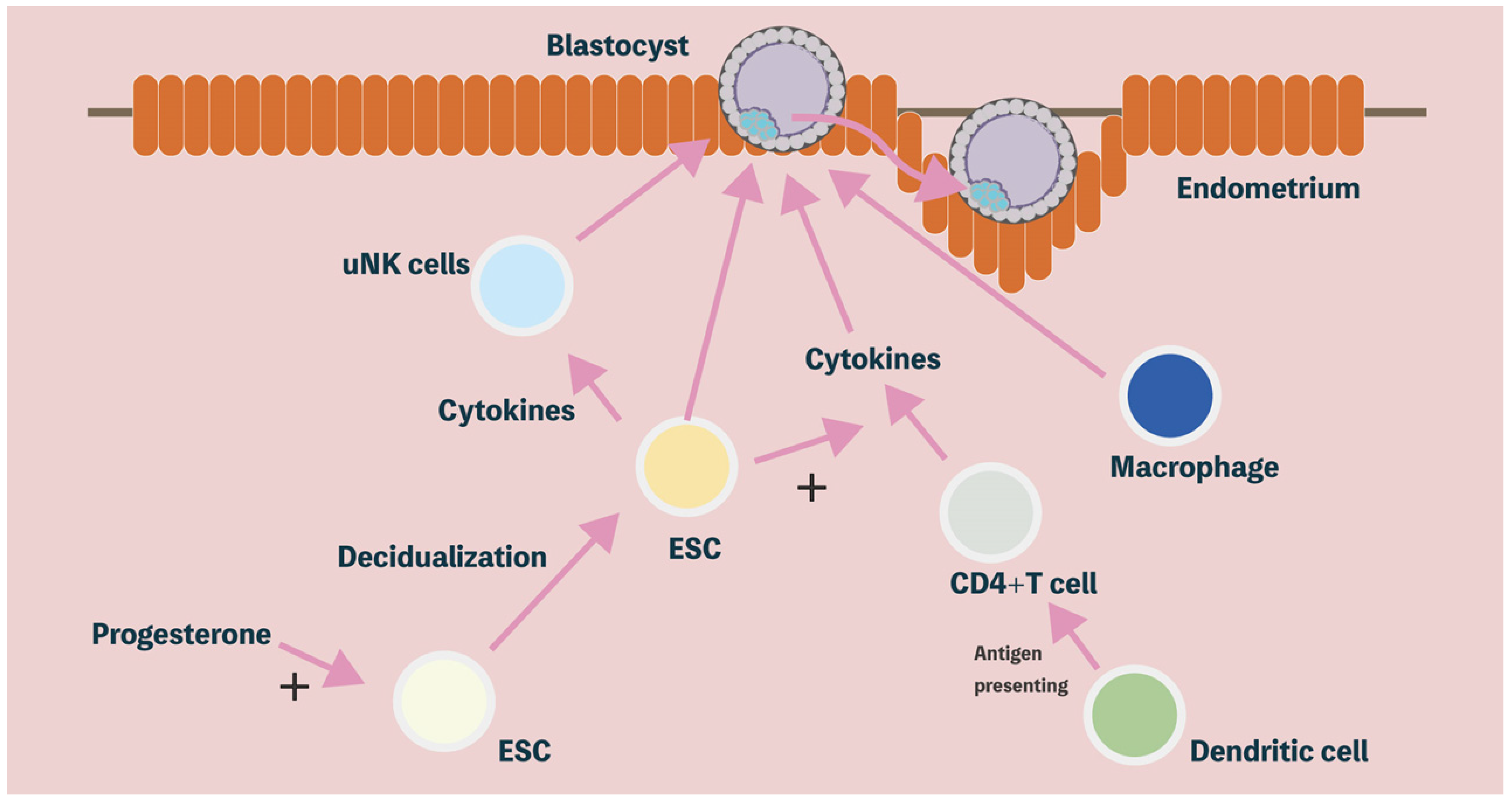
:1. Introduction
2. Decidualization of the Human Endometrium
2.1. Morphological Differentiation in the Human Endometrium
2.2. Functional Differentiation in Human Endometrium
3. Immune Modulatory Properties of Decidual Stromal Cells
3.1. Uterine Natural Killer (uNK) Cells
3.2. Cytokines
3.3. T Cells
3.4. Macrophages
3.5. Other Immune Cells
| Immune Cells and Cytokines | Effect and Mechanism | Present in Uterus * | Role in Human Decidua | |
|---|---|---|---|---|
| Cytokines [74,75,76] | Immunoregulatory | ++ | Modulating receptive decidualization, maternal tolerance to embryo invasion | |
| Uterine natural killer cells [46,47,48] | Cytokine secretion, cytotoxicity | +++ | Angiogenesis, tissue remodeling, regulation of trophoblast invasion | |
| T cells | CD 4+ T cells [92,93,94,95] | Cytokine secretion, recruit effector memory T cells | ++ | Promote trophoblast invasion, vascular and tissue remodeling |
| CD 4+ Treg cells [96] | Immune regulation | + | Promote decidual vascular and tissue remodeling | |
| CD 8+ T cells [107,108] | Cytotoxicity, cytokine secretion | ++ | Immune regulation | |
| Macrophages [110] | Phagocytosis, antigen-presenting cells (APCs), cytokine secretion | +++ | Pathogen clearance, trophoblast invasion, tissue and vascular remodeling | |
| Other immune cells | Dendritic cell (DCs) [117] | Antigen-presenting cells (APCs) | + | T cell induction, immune tolerance |
| B cells [129,130] | Antibody production, cytokine secretion | + | Immune regulation | |
4. Identification of Decidual Stromal Cell-Associated Extracellular Vesicles
5. The Content of Extracellular Vesicles
5.1. microRNA
5.2. Protein
5.3. Lipids
6. Physiological and Immunological Functions of Extracellular Vesicles
6.1. The Immune Modulatory Effects of Extracellular Vesicles
6.2. Communication between the Embryo and the Maternal Immune System through Extracellular Vesicles
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gellersen, B.; Reimann, K.; Samalecos, A.; Aupers, S.; Bamberger, A.M. Invasiveness of human endometrial stromal cells is promoted by decidualization and by trophoblast-derived signals. Hum. Reprod. 2010, 25, 862–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blois, S.M.; Kammerer, U.; Soto, C.A.; Tometten, M.C.; Shaikly, V.; Barrientos, G.; Jurd, R.; Rukavina, D.; Thomson, A.W.; Klapp, B.F.; et al. Dendritic cells: Key to fetal tolerance? Biol. Reprod. 2007, 77, 590–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kliman, H.J.; Frankfurter, D. Clinical approach to recurrent implantation failure: Evidence-based evaluation of the endometrium. Fertil. Steril. 2019, 111, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.; Salamonsen, L.A.; Winship, A.; Menkhorst, E.; Nie, G.; Gargett, C.E.; Dimitriadis, E. Fertile ground: Human endometrial programming and lessons in health and disease. Nat. Rev. Endocrinol. 2016, 12, 654–667. [Google Scholar] [CrossRef]
- Ashary, N.; Tiwari, A.; Modi, D. Embryo Implantation: War in Times of Love. Endocrinology 2018, 159, 1188–1198. [Google Scholar] [CrossRef] [Green Version]
- Greening, D.W.; Nguyen, H.P.; Evans, J.; Simpson, R.J.; Salamonsen, L.A. Modulating the endometrial epithelial proteome and secretome in preparation for pregnancy: The role of ovarian steroid and pregnancy hormones. J. Proteom. 2016, 144, 99–112. [Google Scholar] [CrossRef]
- Wu, H.M.; Lo, T.C.; Tsai, C.L.; Chen, L.H.; Huang, H.Y.; Wang, H.S.; Yu, J. Extracellular Vesicle-Associated MicroRNA-138-5p Regulates Embryo Implantation and Early Pregnancy by Adjusting GPR124. Pharmaceutics 2022, 14, 1172. [Google Scholar] [CrossRef]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [Green Version]
- Vyas, N.; Dhawan, J. Exosomes: Mobile platforms for targeted and synergistic signaling across cell boundaries. Cell. Mol. Life Sci. 2017, 74, 1567–1576. [Google Scholar] [CrossRef]
- Pollet, H.; Conrard, L.; Cloos, A.S.; Tyteca, D. Plasma Membrane Lipid Domains as Platforms for Vesicle Biogenesis and Shedding? Biomolecules 2018, 8, 94. [Google Scholar] [CrossRef]
- Simon, C.; Greening, D.W.; Bolumar, D.; Balaguer, N.; Salamonsen, L.A.; Vilella, F. Extracellular Vesicles in Human Reproduction in Health and Disease. Endocr. Rev. 2018, 39, 292–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurian, N.K.; Modi, D. Extracellular vesicle mediated embryo-endometrial cross talk during implantation and in pregnancy. J. Assist. Reprod. Genet. 2019, 36, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.; Sun, X.; Dey, S.K. Mechanisms of implantation: Strategies for successful pregnancy. Nat. Med. 2012, 18, 1754–1767. [Google Scholar] [CrossRef]
- Fox, C.; Morin, S.; Jeong, J.W.; Scott, R.T., Jr.; Lessey, B.A. Local and systemic factors and implantation: What is the evidence? Fertil. Steril. 2016, 105, 873–884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawkins, S.M.; Matzuk, M.M. The menstrual cycle: Basic biology. Ann. N. Y. Acad. Sci. 2008, 1135, 10–18. [Google Scholar] [CrossRef]
- Brosens, J.J.; Pijnenborg, R.; Brosens, I.A. The myometrial junctional zone spiral arteries in normal and abnormal pregnancies: A review of the literature. Am. J. Obstet. Gynecol. 2002, 187, 1416–1423. [Google Scholar] [CrossRef]
- Gellersen, B.; Brosens, J.J. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr. Rev. 2014, 35, 851–905. [Google Scholar] [CrossRef] [Green Version]
- Murata, H.; Tanaka, S.; Tsuzuki-Nakao, T.; Kido, T.; Kakita-Kobayashi, M.; Kida, N.; Hisamatsu, Y.; Tsubokura, H.; Hashimoto, Y.; Kitada, M.; et al. The transcription factor HAND2 up-regulates transcription of the IL15 gene in human endometrial stromal cells. J. Biol. Chem. 2020, 295, 9596–9605. [Google Scholar] [CrossRef]
- Dunn, C.L.; Kelly, R.W.; Critchley, H.O. Decidualization of the human endometrial stromal cell: An enigmatic transformation. Reprod. Biomed. Online 2003, 7, 151–161. [Google Scholar] [CrossRef]
- Ihnatovych, I.; Livak, M.; Reed, J.; de Lanerolle, P.; Strakova, Z. Manipulating actin dynamics affects human in vitro decidualization. Biol. Reprod. 2009, 81, 222–230. [Google Scholar] [CrossRef]
- Irwin, J.C.; Utian, W.H.; Eckert, R.L. Sex steroids and growth factors differentially regulate the growth and differentiation of cultured human endometrial stromal cells. Endocrinology 1991, 129, 2385–2392. [Google Scholar] [CrossRef] [PubMed]
- Tabanelli, S.; Tang, B.; Gurpide, E. In vitro decidualization of human endometrial stromal cells. J. Steroid Biochem. Mol. Biol. 1992, 42, 337–344. [Google Scholar] [CrossRef]
- Sawai, K.; Matsuzaki, N.; Okada, T.; Shimoya, K.; Koyama, M.; Azuma, C.; Saji, F.; Murata, Y. Human decidual cell biosynthesis of leukemia inhibitory factor: Regulation by decidual cytokines and steroid hormones. Biol. Reprod. 1997, 56, 1274–1280. [Google Scholar] [CrossRef] [PubMed]
- Kastner, P.; Krust, A.; Turcotte, B.; Stropp, U.; Tora, L.; Gronemeyer, H.; Chambon, P. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J. 1990, 9, 1603–1614. [Google Scholar] [CrossRef]
- Mesiano, S.; Wang, Y.; Norwitz, E.R. Progesterone receptors in the human pregnancy uterus: Do they hold the key to birth timing? Reprod. Sci. 2011, 18, 6–19. [Google Scholar] [CrossRef]
- Goldman, S.; Weiss, A.; Almalah, I.; Shalev, E. Progesterone receptor expression in human decidua and fetal membranes before and after contractions: Possible mechanism for functional progesterone withdrawal. Mol. Hum. Reprod. 2005, 11, 269–277. [Google Scholar] [CrossRef] [Green Version]
- Tang, M.; Mazella, J.; Gao, J.; Tseng, L. Progesterone receptor activates its promoter activity in human endometrial stromal cells. Mol. Cell. Endocrinol. 2002, 192, 45–53. [Google Scholar] [CrossRef]
- Patel, B.; Elguero, S.; Thakore, S.; Dahoud, W.; Bedaiwy, M.; Mesiano, S. Role of nuclear progesterone receptor isoforms in uterine pathophysiology. Hum. Reprod. Update 2015, 21, 155–173. [Google Scholar] [CrossRef] [Green Version]
- Stefanoska, I.; Krivokuca, M.J.; Vasilijic, S.; Cujic, D.; Vicovac, L. Prolactin stimulates cell migration and invasion by human trophoblast in vitro. Placenta 2013, 34, 775–783. [Google Scholar] [CrossRef]
- Matsumoto, H.; Sakai, K.; Iwashita, M. Insulin-like growth factor binding protein-1 induces decidualization of human endometrial stromal cells via alpha5beta1 integrin. Mol. Hum. Reprod. 2008, 14, 485–489. [Google Scholar] [CrossRef]
- Kim, J.J.; Buzzio, O.L.; Li, S.; Lu, Z. Role of FOXO1A in the regulation of insulin-like growth factor-binding protein-1 in human endometrial cells: Interaction with progesterone receptor. Biol. Reprod. 2005, 73, 833–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tseng, L.; Tang, M.; Wang, Z.; Mazella, J. Progesterone receptor (hPR) upregulates the fibronectin promoter activity in human decidual fibroblasts. DNA Cell Biol. 2003, 22, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Marwood, M.; Visser, K.; Salamonsen, L.A.; Dimitriadis, E. Interleukin-11 and leukemia inhibitory factor regulate the adhesion of endometrial epithelial cells: Implications in fertility regulation. Endocrinology 2009, 150, 2915–2923. [Google Scholar] [CrossRef] [Green Version]
- Suman, P.; Shembekar, N.; Gupta, S.K. Leukemia inhibitory factor increases the invasiveness of trophoblastic cells through integrated increase in the expression of adhesion molecules and pappalysin 1 with a concomitant decrease in the expression of tissue inhibitor of matrix metalloproteinases. Fertil. Steril. 2013, 99, 533–542. [Google Scholar] [CrossRef]
- Evans, J.; Catalano, R.D.; Brown, P.; Sherwin, R.; Critchley, H.O.; Fazleabas, A.T.; Jabbour, H.N. Prokineticin 1 mediates fetal-maternal dialogue regulating endometrial leukemia inhibitory factor. FASEB J. 2009, 23, 2165–2175. [Google Scholar] [CrossRef] [PubMed]
- Suman, P.; Poehlmann, T.G.; Prakash, G.J.; Markert, U.R.; Gupta, S.K. Interleukin-11 increases invasiveness of JEG-3 choriocarcinoma cells by modulating STAT3 expression. J. Reprod. Immunol. 2009, 82, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Suano, A.; Hamilton, A.B.; Betz, A.G. Gimme shelter: The immune system during pregnancy. Immunol. Rev. 2011, 241, 20–38. [Google Scholar] [CrossRef]
- Saito, S.; Nakashima, A.; Shima, T.; Ito, M. Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am. J. Reprod. Immunol. 2010, 63, 601–610. [Google Scholar] [CrossRef]
- Oliver, C.; Montes, M.J.; Galindo, J.A.; Ruiz, C.; Olivares, E.G. Human decidual stromal cells express alpha-smooth muscle actin and show ultrastructural similarities with myofibroblasts. Hum. Reprod. 1999, 14, 1599–1605. [Google Scholar] [CrossRef] [Green Version]
- Macias, M.I.; Grande, J.; Moreno, A.; Dominguez, I.; Bornstein, R.; Flores, A.I. Isolation and characterization of true mesenchymal stem cells derived from human term decidua capable of multilineage differentiation into all 3 embryonic layers. Am. J. Obstet. Gynecol. 2010, 203, 495.e9–495.e23. [Google Scholar] [CrossRef]
- Erkers, T.; Nava, S.; Yosef, J.; Ringden, O.; Kaipe, H. Decidual stromal cells promote regulatory T cells and suppress alloreactivity in a cell contact-dependent manner. Stem Cells Dev. 2013, 22, 2596–2605. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Morschauser, A.; Zhang, X.; Lu, X.; Gleason, J.; He, S.; Chen, H.J.; Jankovic, V.; Ye, Q.; Labazzo, K.; et al. Human placenta-derived adherent cells induce tolerogenic immune responses. Clin. Transl. Immunol. 2014, 3, e14. [Google Scholar] [CrossRef] [PubMed]
- Kesting, M.R.; Wolff, K.D.; Hohlweg-Majert, B.; Steinstraesser, L. The role of allogenic amniotic membrane in burn treatment. J. Burn Care Res. 2008, 29, 907–916. [Google Scholar] [CrossRef]
- Moodley, Y.; Ilancheran, S.; Samuel, C.; Vaghjiani, V.; Atienza, D.; Williams, E.D.; Jenkin, G.; Wallace, E.; Trounson, A.; Manuelpillai, U. Human amnion epithelial cell transplantation abrogates lung fibrosis and augments repair. Am. J. Respir. Crit. Care Med. 2010, 182, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Carrega, P.; Ferlazzo, G. Natural killer cell distribution and trafficking in human tissues. Front. Immunol. 2012, 3, 347. [Google Scholar] [CrossRef] [Green Version]
- Hanna, J.; Goldman-Wohl, D.; Hamani, Y.; Avraham, I.; Greenfield, C.; Natanson-Yaron, S.; Prus, D.; Cohen-Daniel, L.; Arnon, T.I.; Manaster, I.; et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat. Med. 2006, 12, 1065–1074. [Google Scholar] [CrossRef]
- Hanna, J.; Wald, O.; Goldman-Wohl, D.; Prus, D.; Markel, G.; Gazit, R.; Katz, G.; Haimov-Kochman, R.; Fujii, N.; Yagel, S.; et al. CXCL12 expression by invasive trophoblasts induces the specific migration of CD16− human natural killer cells. Blood 2003, 102, 1569–1577. [Google Scholar] [CrossRef]
- Vacca, P.; Vitale, C.; Montaldo, E.; Conte, R.; Cantoni, C.; Fulcheri, E.; Darretta, V.; Moretta, L.; Mingari, M.C. CD34+ hematopoietic precursors are present in human decidua and differentiate into natural killer cells upon interaction with stromal cells. Proc. Natl. Acad. Sci. USA 2011, 108, 2402–2407. [Google Scholar] [CrossRef] [Green Version]
- Carlino, C.; Rippo, M.R.; Lazzarini, R.; Monsurro, V.; Morrone, S.; Angelini, S.; Trotta, E.; Stabile, H.; Bastianelli, C.; Albertini, M.C.; et al. Differential microRNA expression between decidual and peripheral blood natural killer cells in early pregnancy. Hum. Reprod. 2018, 33, 2184–2195. [Google Scholar] [CrossRef]
- Faas, M.M.; de Vos, P. Uterine NK cells and macrophages in pregnancy. Placenta 2017, 56, 44–52. [Google Scholar] [CrossRef]
- He, Y.; Tian, Z. NK cell education via nonclassical MHC and non-MHC ligands. Cell. Mol. Immunol. 2017, 14, 321–330. [Google Scholar] [CrossRef] [Green Version]
- Jabrane-Ferrat, N. Features of Human Decidual NK Cells in Healthy Pregnancy and During Viral Infection. Front. Immunol. 2019, 10, 1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallace, A.E.; Fraser, R.; Cartwright, J.E. Extravillous trophoblast and decidual natural killer cells: A remodelling partnership. Hum. Reprod. Update 2012, 18, 458–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimitriadis, E.; Menkhorst, E.; Saito, S.; Kutteh, W.H.; Brosens, J.J. Recurrent pregnancy loss. Nat. Rev. Dis. Prim. 2020, 6, 98. [Google Scholar] [CrossRef] [PubMed]
- Lucas, E.S.; Vrljicak, P.; Muter, J.; Diniz-da-Costa, M.M.; Brighton, P.J.; Kong, C.S.; Lipecki, J.; Fishwick, K.J.; Odendaal, J.; Ewington, L.J.; et al. Recurrent pregnancy loss is associated with a pro-senescent decidual response during the peri-implantation window. Commun. Biol. 2020, 3, 37. [Google Scholar] [CrossRef] [Green Version]
- Moretta, L.; Pietra, G.; Montaldo, E.; Vacca, P.; Pende, D.; Falco, M.; Del Zotto, G.; Locatelli, F.; Moretta, A.; Mingari, M.C. Human NK cells: From surface receptors to the therapy of leukemias and solid tumors. Front. Immunol. 2014, 5, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vento-Tormo, R.; Efremova, M.; Botting, R.A.; Turco, M.Y.; Vento-Tormo, M.; Meyer, K.B.; Park, J.E.; Stephenson, E.; Polanski, K.; Goncalves, A.; et al. Single-cell reconstruction of the early maternal-fetal interface in humans. Nature 2018, 563, 347–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parham, P.; Moffett, A. Variable NK cell receptors and their MHC class I ligands in immunity, reproduction and human evolution. Nat. Rev. Immunol. 2013, 13, 133–144. [Google Scholar] [CrossRef] [Green Version]
- Moffett, A.; Colucci, F. Co-evolution of NK receptors and HLA ligands in humans is driven by reproduction. Immunol. Rev. 2015, 267, 283–297. [Google Scholar] [CrossRef] [Green Version]
- Moffett, A.; Chazara, O.; Colucci, F.; Johnson, M.H. Variation of maternal KIR and fetal HLA-C genes in reproductive failure: Too early for clinical intervention. Reprod. Biomed. Online 2016, 33, 763–769. [Google Scholar] [CrossRef]
- Chazara, O.; Xiong, S.; Moffett, A. Maternal KIR and fetal HLA-C: A fine balance. J. Leukoc. Biol. 2011, 90, 703–716. [Google Scholar] [CrossRef] [PubMed]
- Alecsandru, D.; Garrido, N.; Vicario, J.L.; Barrio, A.; Aparicio, P.; Requena, A.; Garcia-Velasco, J.A. Maternal KIR haplotype influences live birth rate after double embryo transfer in IVF cycles in patients with recurrent miscarriages and implantation failure. Hum. Reprod. 2014, 29, 2637–2643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menkhorst, E.M.; Van Sinderen, M.L.; Rainczuk, K.; Cuman, C.; Winship, A.; Dimitriadis, E. Invasive trophoblast promote stromal fibroblast decidualization via Profilin 1 and ALOX5. Sci. Rep. 2017, 7, 8690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.M.; Chen, L.H.; Schally, A.V.; Huang, H.Y.; Soong, Y.K.; Leung, P.C.K.; Wang, H.S. Impact of growth hormone-releasing hormone antagonist on decidual stromal cell growth and apoptosis in vitrodagger. Biol. Reprod. 2022, 106, 145–154. [Google Scholar] [CrossRef]
- Sternberg, A.K.; Buck, V.U.; Classen-Linke, I.; Leube, R.E. How Mechanical Forces Change the Human Endometrium during the Menstrual Cycle in Preparation for Embryo Implantation. Cells 2021, 10, 2008. [Google Scholar] [CrossRef]
- Ng, S.W.; Norwitz, G.A.; Pavlicev, M.; Tilburgs, T.; Simon, C.; Norwitz, E.R. Endometrial Decidualization: The Primary Driver of Pregnancy Health. Int. J. Mol. Sci. 2020, 21, 4092. [Google Scholar] [CrossRef]
- Yang, F.; Zheng, Q.; Jin, L. Dynamic Function and Composition Changes of Immune Cells During Normal and Pathological Pregnancy at the Maternal-Fetal Interface. Front. Immunol. 2019, 10, 2317. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Hao, S.; Chen, X.; Zhao, H.; Du, L.; Ren, H.; Wang, C.; Mao, H. Human placental trophoblast cells contribute to maternal-fetal tolerance through expressing IL-35 and mediating iTR35 conversion. Nat. Commun. 2019, 10, 4601. [Google Scholar] [CrossRef] [Green Version]
- Krzymowski, T.; Stefanczyk-Krzymowska, S. Advances in understanding the physiological mechanism of maternal immune tolerance to the embryo. Reprod. Biol. 2012, 12, 265–270. [Google Scholar] [CrossRef]
- Bert, S.; Ward, E.J.; Nadkarni, S. Neutrophils in pregnancy: New insights into innate and adaptive immune regulation. Immunology 2021, 164, 665–676. [Google Scholar] [CrossRef]
- PrabhuDas, M.; Bonney, E.; Caron, K.; Dey, S.; Erlebacher, A.; Fazleabas, A.; Fisher, S.; Golos, T.; Matzuk, M.; McCune, J.M.; et al. Immune mechanisms at the maternal-fetal interface: Perspectives and challenges. Nat. Immunol. 2015, 16, 328–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitazawa, J.; Kimura, F.; Nakamura, A.; Morimune, A.; Takahashi, A.; Takashima, A.; Amano, T.; Tsuji, S.; Kaku, S.; Kasahara, K.; et al. Endometrial Immunity for Embryo Implantation and Pregnancy Establishment. Tohoku J. Exp. Med. 2020, 250, 49–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nancy, P.; Erlebacher, A. T cell behavior at the maternal-fetal interface. Int. J. Dev. Biol. 2014, 58, 189–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- von Rango, U. Fetal tolerance in human pregnancy—A crucial balance between acceptance and limitation of trophoblast invasion. Immunol. Lett. 2008, 115, 21–32. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, M.; Lee, S.K. Role of endometrial immune cells in implantation. Clin. Exp. Reprod. Med. 2011, 38, 119–125. [Google Scholar] [CrossRef] [Green Version]
- Jena, M.K.; Nayak, N.; Chen, K.; Nayak, N.R. Role of Macrophages in Pregnancy and Related Complications. Arch. Immunol. Ther. Exp. 2019, 67, 295–309. [Google Scholar] [CrossRef]
- Harrity, C.; Shkrobot, L.; Walsh, D.; Marron, K. ART implantation failure and miscarriage in patients with elevated intracellular cytokine ratios: Response to immune support therapy. Fertil. Res. Pract. 2018, 4, 7. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Sung, N.; Gilman-Sachs, A.; Kwak-Kim, J. T Helper (Th) Cell Profiles in Pregnancy and Recurrent Pregnancy Losses: Th1/Th2/Th9/Th17/Th22/Tfh Cells. Front. Immunol. 2020, 11, 2025. [Google Scholar] [CrossRef]
- Mor, G.; Cardenas, I.; Abrahams, V.; Guller, S. Inflammation and pregnancy: The role of the immune system at the implantation site. Ann. N. Y. Acad. Sci. 2011, 1221, 80–87. [Google Scholar] [CrossRef] [Green Version]
- Romero, R.; Espinoza, J.; Goncalves, L.F.; Kusanovic, J.P.; Friel, L.A.; Nien, J.K. Inflammation in preterm and term labour and delivery. Semin. Fetal Neonatal Med. 2006, 11, 317–326. [Google Scholar] [CrossRef]
- Meazza, R.; Azzarone, B.; Orengo, A.M.; Ferrini, S. Role of common-gamma chain cytokines in NK cell development and function: Perspectives for immunotherapy. J. Biomed. Biotechnol. 2011, 2011, 861920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okada, S.; Okada, H.; Sanezumi, M.; Nakajima, T.; Yasuda, K.; Kanzaki, H. Expression of interleukin-15 in human endometrium and decidua. Mol. Hum. Reprod. 2000, 6, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Okada, H.; Nakajima, T.; Yasuda, K.; Kanzaki, H. Interleukin-1 inhibits interleukin-15 production by progesterone during in vitro decidualization in human. J. Reprod. Immunol. 2004, 61, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Gimeno, P.; Horcajadas, J.A.; Martinez-Conejero, J.A.; Esteban, F.J.; Alama, P.; Pellicer, A.; Simon, C. A genomic diagnostic tool for human endometrial receptivity based on the transcriptomic signature. Fertil. Steril. 2011, 95, 50–60, 60 e51-15. [Google Scholar] [CrossRef] [PubMed]
- Perez-Sepulveda, A.; Torres, M.J.; Khoury, M.; Illanes, S.E. Innate immune system and preeclampsia. Front. Immunol. 2014, 5, 244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sykes, L.; MacIntyre, D.A.; Yap, X.J.; Teoh, T.G.; Bennett, P.R. The Th1:th2 dichotomy of pregnancy and preterm labour. Mediat. Inflamm. 2012, 2012, 967629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lissauer, D.; Kilby, M.D.; Moss, P. Maternal effector T cells within decidua: The adaptive immune response to pregnancy? Placenta 2017, 60, 140–144. [Google Scholar] [CrossRef] [Green Version]
- Bartmann, C.; Segerer, S.E.; Rieger, L.; Kapp, M.; Sutterlin, M.; Kammerer, U. Quantification of the predominant immune cell populations in decidua throughout human pregnancy. Am. J. Reprod. Immunol. 2014, 71, 109–119. [Google Scholar] [CrossRef]
- Monteiro, C.; Kasahara, T.M.; Castro, J.R.; Sacramento, P.M.; Hygino, J.; Centuriao, N.; Cassano, T.; Lopes, L.M.F.; Leite, S.; Silva, V.G.; et al. Pregnancy favors the expansion of circulating functional follicular helper T Cells. J. Reprod. Immunol. 2017, 121, 1–10. [Google Scholar] [CrossRef]
- Kieffer, T.E.; Faas, M.M.; Scherjon, S.A.; Prins, J.R. Pregnancy persistently affects memory T cell populations. J. Reprod. Immunol. 2017, 119, 1–8. [Google Scholar] [CrossRef]
- Rowe, J.H.; Ertelt, J.M.; Xin, L.; Way, S.S. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature 2012, 490, 102–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powell, R.M.; Lissauer, D.; Tamblyn, J.; Beggs, A.; Cox, P.; Moss, P.; Kilby, M.D. Decidual T Cells Exhibit a Highly Differentiated Phenotype and Demonstrate Potential Fetal Specificity and a Strong Transcriptional Response to IFN. J. Immunol. 2017, 199, 3406–3417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, W.; Liu, X.; Liu, Z.; Zheng, Y.; Yu, T.; Fu, S.; Li, X.; Zhang, J.; Zhang, S.; Ma, X.; et al. Deep Surveying of the Transcriptional and Alternative Splicing Signatures for Decidual CD8(+) T Cells at the First Trimester of Human Healthy Pregnancy. Front. Immunol. 2018, 9, 937. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.X.; Jin, L.P.; Xu, B.; Liang, S.S.; Li, D.J. Decidual stromal cells recruit Th17 cells into decidua to promote proliferation and invasion of human trophoblast cells by secreting IL-17. Cell. Mol. Immunol. 2014, 11, 253–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, S.; Nakabayashi, Y.; Nakashima, A.; Shima, T.; Yoshino, O. A new era in reproductive medicine: Consequences of third-party oocyte donation for maternal and fetal health. Semin. Immunopathol. 2016, 38, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Ticconi, C.; Pietropolli, A.; Di Simone, N.; Piccione, E.; Fazleabas, A. Endometrial Immune Dysfunction in Recurrent Pregnancy Loss. Int. J. Mol. Sci. 2019, 20, 5332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bettelli, E.; Carrier, Y.; Gao, W.; Korn, T.; Strom, T.B.; Oukka, M.; Weiner, H.L.; Kuchroo, V.K. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 2006, 441, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Samstein, R.M.; Josefowicz, S.Z.; Arvey, A.; Treuting, P.M.; Rudensky, A.Y. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell 2012, 150, 29–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mold, J.E.; Venkatasubrahmanyam, S.; Burt, T.D.; Michaelsson, J.; Rivera, J.M.; Galkina, S.A.; Weinberg, K.; Stoddart, C.A.; McCune, J.M. Fetal and adult hematopoietic stem cells give rise to distinct T cell lineages in humans. Science 2010, 330, 1695–1699. [Google Scholar] [CrossRef] [Green Version]
- Guerin, L.R.; Prins, J.R.; Robertson, S.A. Regulatory T-cells and immune tolerance in pregnancy: A new target for infertility treatment? Hum. Reprod. Update 2009, 15, 517–535. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.X.; Kang, X.M.; Zhao, A.M. Regulation of CD4(+)FOXP3(+) T cells by CCL20/CCR6 axis in early unexplained recurrent miscarriage patients. Genet. Mol. Res. 2015, 14, 9145–9154. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Zhang, N.; Lin, J.; Wang, C.; Pan, X.; Chen, L.; Li, D.; Wang, L. Distinct pattern of Th17/Treg cells in pregnant women with a history of unexplained recurrent spontaneous abortion. Biosci. Trends 2018, 12, 157–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuda, S.; Zhang, X.; Hamana, H.; Shima, T.; Ushijima, A.; Tsuda, K.; Muraguchi, A.; Kishi, H.; Saito, S. Clonally Expanded Decidual Effector Regulatory T Cells Increase in Late Gestation of Normal Pregnancy, but Not in Preeclampsia, in Humans. Front. Immunol. 2018, 9, 1934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nadkarni, S.; Smith, J.; Sferruzzi-Perri, A.N.; Ledwozyw, A.; Kishore, M.; Haas, R.; Mauro, C.; Williams, D.J.; Farsky, S.H.; Marelli-Berg, F.M.; et al. Neutrophils induce proangiogenic T cells with a regulatory phenotype in pregnancy. Proc. Natl. Acad. Sci. USA 2016, 113, E8415–E8424. [Google Scholar] [CrossRef] [Green Version]
- van der Zwan, A.; Bi, K.; Norwitz, E.R.; Crespo, A.C.; Claas, F.H.J.; Strominger, J.L.; Tilburgs, T. Mixed signature of activation and dysfunction allows human decidual CD8(+) T cells to provide both tolerance and immunity. Proc. Natl. Acad. Sci. USA 2018, 115, 385–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Zwan, A.; van der Meer-Prins, E.M.W.; van Miert, P.; van den Heuvel, H.; Anholts, J.D.H.; Roelen, D.L.; Claas, F.H.J.; Heidt, S. Cross-Reactivity of Virus-Specific CD8+ T Cells Against Allogeneic HLA-C: Possible Implications for Pregnancy Outcome. Front. Immunol. 2018, 9, 2880. [Google Scholar] [CrossRef] [PubMed]
- Tilburgs, T.; Scherjon, S.A.; Roelen, D.L.; Claas, F.H. Decidual CD8+CD28- T cells express CD103 but not perforin. Hum. Immunol. 2009, 70, 96–100. [Google Scholar] [CrossRef]
- Wang, S.C.; Li, Y.H.; Piao, H.L.; Hong, X.W.; Zhang, D.; Xu, Y.Y.; Tao, Y.; Wang, Y.; Yuan, M.M.; Li, D.J.; et al. PD-1 and Tim-3 pathways are associated with regulatory CD8+ T-cell function in decidua and maintenance of normal pregnancy. Cell Death Dis. 2015, 6, e1738. [Google Scholar] [CrossRef] [Green Version]
- Solano, M.E.; Kowal, M.K.; O’Rourke, G.E.; Horst, A.K.; Modest, K.; Plosch, T.; Barikbin, R.; Remus, C.C.; Berger, R.G.; Jago, C.; et al. Progesterone and HMOX-1 promote fetal growth by CD8+ T cell modulation. J. Clin. Investig. 2015, 125, 1726–1738. [Google Scholar] [CrossRef] [Green Version]
- Ander, S.E.; Diamond, M.S.; Coyne, C.B. Immune responses at the maternal-fetal interface. Sci. Immunol. 2019, 4, 73–82. [Google Scholar] [CrossRef]
- Vishnyakova, P.; Elchaninov, A.; Fatkhudinov, T.; Sukhikh, G. Role of the Monocyte-Macrophage System in Normal Pregnancy and Preeclampsia. Int. J. Mol. Sci. 2019, 20, 3695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, Y.; Xu, X.H.; Jin, L. Macrophage Polarization in Physiological and Pathological Pregnancy. Front. Immunol. 2019, 10, 792. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Wang, S.; Du, M. Functional regulation of decidual macrophages during pregnancy. J. Reprod. Immunol. 2021, 143, 103264. [Google Scholar] [CrossRef] [PubMed]
- Parasar, P.; Guru, N.; Nayak, N.R. Contribution of macrophages to fetomaternal immunological tolerance. Hum. Immunol. 2021, 82, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Palma, A.; Jarrah, A.S.; Tieri, P.; Cesareni, G.; Castiglione, F. Gene Regulatory Network Modeling of Macrophage Differentiation Corroborates the Continuum Hypothesis of Polarization States. Front. Physiol. 2018, 9, 1659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jantsch, J.; Schultze, J.L.; Kurts, C. Immunophysiology: Macrophages as key regulators of homeostasis in various organs. Pflug. Arch. 2017, 469, 363–364. [Google Scholar] [CrossRef] [Green Version]
- Faas, M.M.; De Vos, P. Innate immune cells in the placental bed in healthy pregnancy and preeclampsia. Placenta 2018, 69, 125–133. [Google Scholar] [CrossRef]
- Jiang, X.; Du, M.R.; Li, M.; Wang, H. Three macrophage subsets are identified in the uterus during early human pregnancy. Cell. Mol. Immunol. 2018, 15, 1027–1037. [Google Scholar] [CrossRef] [Green Version]
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.H.; He, M.; Wang, Y.; Liao, A.H. Modulators of the Balance between M1 and M2 Macrophages during Pregnancy. Front. Immunol. 2017, 8, 120. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, L.; Hu, X.; Ji, J.; Mor, G.; Liao, A. The role of the PD-1/PD-L1 axis in macrophage differentiation and function during pregnancy. Hum. Reprod. 2019, 34, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, K.C.; Jena, M.K.; Pradhan, B.S.; Nayak, N.; Das, S.; Hsu, C.D.; Wheeler, D.S.; Chen, K.; Nayak, N.R. VEGF may contribute to macrophage recruitment and M2 polarization in the decidua. PLoS ONE 2018, 13, e0191040. [Google Scholar] [CrossRef] [PubMed]
- Hamelin-Morrissette, J.; Dallagi, A.; Girouard, J.; Ravelojaona, M.; Oufqir, Y.; Vaillancourt, C.; Van Themsche, C.; Carrier, C.; Reyes-Moreno, C. Leukemia inhibitory factor regulates the activation of inflammatory signals in macrophages and trophoblast cells. Mol. Immunol. 2020, 120, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.L.; Wang, C.J.; Lai, Z.Z.; Yang, S.L.; Zheng, Z.M.; Shi, J.W.; Li, M.Q.; Shao, J. Decidual stromal cells maintain decidual macrophage homeostasis by secreting IL-24 in early pregnancy. Am. J. Reprod. Immunol. 2020, 84, e13261. [Google Scholar] [CrossRef]
- Ding, J.; Yang, C.; Cheng, Y.; Wang, J.; Zhang, S.; Yan, S.; He, F.; Yin, T.; Yang, J. Trophoblast-derived IL-6 serves as an important factor for normal pregnancy by activating Stat3-mediated M2 macrophages polarization. Int. Immunopharmacol. 2021, 90, 106788. [Google Scholar] [CrossRef]
- Gamliel, M.; Goldman-Wohl, D.; Isaacson, B.; Gur, C.; Stein, N.; Yamin, R.; Berger, M.; Grunewald, M.; Keshet, E.; Rais, Y.; et al. Trained Memory of Human Uterine NK Cells Enhances Their Function in Subsequent Pregnancies. Immunity 2018, 48, 951–962.e5. [Google Scholar] [CrossRef] [Green Version]
- Collin, M.; Bigley, V. Human dendritic cell subsets: An update. Immunology 2018, 154, 3–20. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Wei, H.; Li, Y.; Huang, C.; Lian, R.; Xu, J.; Chen, L.; Zeng, Y. Downregulation of ILT4(+) dendritic cells in recurrent miscarriage and recurrent implantation failure. Am. J. Reprod. Immunol. 2018, 80, e12998. [Google Scholar] [CrossRef]
- Ziegler, K.B.; Muzzio, D.O.; Matzner, F.; Bommer, I.; Ventimiglia, M.S.; Malinowsky, K.; Ehrhardt, J.; Zygmunt, M.; Jensen, F. Human pregnancy is accompanied by modifications in B cell development and immunoglobulin profile. J. Reprod. Immunol. 2018, 129, 40–47. [Google Scholar] [CrossRef]
- Huang, B.; Faucette, A.N.; Pawlitz, M.D.; Pei, B.; Goyert, J.W.; Zhou, J.Z.; El-Hage, N.G.; Deng, J.; Lin, J.; Yao, F.; et al. Interleukin-33-induced expression of PIBF1 by decidual B cells protects against preterm labor. Nat. Med. 2017, 23, 128–135. [Google Scholar] [CrossRef] [Green Version]
- Szekeres-Bartho, J.; Polgar, B. PIBF: The double edged sword. Pregnancy and tumor. Am. J. Reprod. Immunol. 2010, 64, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Elfeky, O.; Longo, S.; Lai, A.; Rice, G.E.; Salomon, C. Influence of maternal BMI on the exosomal profile during gestation and their role on maternal systemic inflammation. Placenta 2017, 50, 60–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lotvall, J.; Hill, A.F.; Hochberg, F.; Buzas, E.I.; Di Vizio, D.; Gardiner, C.; Gho, Y.S.; Kurochkin, I.V.; Mathivanan, S.; Quesenberry, P.; et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: A position statement from the International Society for Extracellular Vesicles. J. Extracell. Vesicles 2014, 3, 26913. [Google Scholar] [CrossRef] [PubMed]
- Tong, M.; Kleffmann, T.; Pradhan, S.; Johansson, C.L.; DeSousa, J.; Stone, P.R.; James, J.L.; Chen, Q.; Chamley, L.W. Proteomic characterization of macro-, micro- and nano-extracellular vesicles derived from the same first trimester placenta: Relevance for feto-maternal communication. Hum. Reprod. 2016, 31, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Sarker, S.; Scholz-Romero, K.; Perez, A.; Illanes, S.E.; Mitchell, M.D.; Rice, G.E.; Salomon, C. Placenta-derived exosomes continuously increase in maternal circulation over the first trimester of pregnancy. J. Transl. Med. 2014, 12, 204. [Google Scholar] [CrossRef] [Green Version]
- Boriachek, K.; Masud, M.K.; Palma, C.; Phan, H.P.; Yamauchi, Y.; Hossain, M.S.A.; Nguyen, N.T.; Salomon, C.; Shiddiky, M.J.A. Avoiding Pre-Isolation Step in Exosome Analysis: Direct Isolation and Sensitive Detection of Exosomes Using Gold-Loaded Nanoporous Ferric Oxide Nanozymes. Anal. Chem. 2019, 91, 3827–3834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koh, Y.Q.; Peiris, H.N.; Vaswani, K.; Reed, S.; Rice, G.E.; Salomon, C.; Mitchell, M.D. Characterization of exosomal release in bovine endometrial intercaruncular stromal cells. Reprod. Biol. Endocrinol. 2016, 14, 78. [Google Scholar] [CrossRef] [Green Version]
- Harp, D.; Driss, A.; Mehrabi, S.; Chowdhury, I.; Xu, W.; Liu, D.; Garcia-Barrio, M.; Taylor, R.N.; Gold, B.; Jefferson, S.; et al. Exosomes derived from endometriotic stromal cells have enhanced angiogenic effects in vitro. Cell Tissue Res. 2016, 365, 187–196. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Chen, X.; Chang, Q.X.; Hua, R.; Wei, Y.X.; Huang, L.P.; Liao, Y.X.; Yue, X.J.; Hu, H.Y.; Sun, F.; et al. Decidual small extracellular vesicles induce trophoblast invasion by upregulating N-cadherin. Reproduction 2020, 159, 171–180. [Google Scholar] [CrossRef]
- Shah, R.; Patel, T.; Freedman, J.E. Circulating Extracellular Vesicles in Human Disease. N. Engl. J. Med. 2018, 379, 958–966. [Google Scholar] [CrossRef]
- Bjorge, I.M.; Kim, S.Y.; Mano, J.F.; Kalionis, B.; Chrzanowski, W. Extracellular vesicles, exosomes and shedding vesicles in regenerative medicine—A new paradigm for tissue repair. Biomater. Sci. 2017, 6, 60–78. [Google Scholar] [CrossRef] [PubMed]
- Maas, S.L.N.; Breakefield, X.O.; Weaver, A.M. Extracellular Vesicles: Unique Intercellular Delivery Vehicles. Trends Cell Biol. 2017, 27, 172–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robbins, P.D.; Dorronsoro, A.; Booker, C.N. Regulation of chronic inflammatory and immune processes by extracellular vesicles. J. Clin. Investig. 2016, 126, 1173–1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bidarimath, M.; Khalaj, K.; Wessels, J.M.; Tayade, C. MicroRNAs, immune cells and pregnancy. Cell. Mol. Immunol. 2014, 11, 538–547. [Google Scholar] [CrossRef] [Green Version]
- Altmae, S.; Martinez-Conejero, J.A.; Esteban, F.J.; Ruiz-Alonso, M.; Stavreus-Evers, A.; Horcajadas, J.A.; Salumets, A. MicroRNAs miR-30b, miR-30d, and miR-494 regulate human endometrial receptivity. Reprod. Sci. 2013, 20, 308–317. [Google Scholar] [CrossRef] [Green Version]
- Baltimore, D.; Boldin, M.P.; O’Connell, R.M.; Rao, D.S.; Taganov, K.D. MicroRNAs: New regulators of immune cell development and function. Nat. Immunol. 2008, 9, 839–845. [Google Scholar] [CrossRef]
- Taganov, K.D.; Boldin, M.P.; Baltimore, D. MicroRNAs and immunity: Tiny players in a big field. Immunity 2007, 26, 133–137. [Google Scholar] [CrossRef] [Green Version]
- Winger, E.E.; Reed, J.L.; Ji, X. First-trimester maternal cell microRNA is a superior pregnancy marker to immunological testing for predicting adverse pregnancy outcome. J. Reprod. Immunol. 2015, 110, 22–35. [Google Scholar] [CrossRef]
- Ng, Y.H.; Rome, S.; Jalabert, A.; Forterre, A.; Singh, H.; Hincks, C.L.; Salamonsen, L.A. Endometrial exosomes/microvesicles in the uterine microenvironment: A new paradigm for embryo-endometrial cross talk at implantation. PLoS ONE 2013, 8, e58502. [Google Scholar] [CrossRef] [Green Version]
- Vilella, F.; Moreno-Moya, J.M.; Balaguer, N.; Grasso, A.; Herrero, M.; Martinez, S.; Marcilla, A.; Simon, C. Hsa-miR-30d, secreted by the human endometrium, is taken up by the pre-implantation embryo and might modify its transcriptome. Development 2015, 142, 3210–3221. [Google Scholar] [CrossRef]
- Burns, G.; Brooks, K.; Wildung, M.; Navakanitworakul, R.; Christenson, L.K.; Spencer, T.E. Extracellular vesicles in luminal fluid of the ovine uterus. PLoS ONE 2014, 9, e90913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Racicot, K.; Schmitt, A.; Ott, T. The myxovirus-resistance protein, MX1, is a component of exosomes secreted by uterine epithelial cells. Am. J. Reprod. Immunol. 2012, 67, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Greening, D.W.; Nguyen, H.P.; Elgass, K.; Simpson, R.J.; Salamonsen, L.A. Human Endometrial Exosomes Contain Hormone-Specific Cargo Modulating Trophoblast Adhesive Capacity: Insights into Endometrial-Embryo Interactions. Biol. Reprod. 2016, 94, 38. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.; Rai, A.; Nguyen, H.P.T.; Poh, Q.H.; Elglass, K.; Simpson, R.J.; Salamonsen, L.A.; Greening, D.W. Human Endometrial Extracellular Vesicles Functionally Prepare Human Trophectoderm Model for Implantation: Understanding Bidirectional Maternal-Embryo Communication. Proteomics 2019, 19, e1800423. [Google Scholar] [CrossRef]
- Record, M.; Silvente-Poirot, S.; Poirot, M.; Wakelam, M.J.O. Extracellular vesicles: Lipids as key components of their biogenesis and functions. J. Lipid. Res. 2018, 59, 1316–1324. [Google Scholar] [CrossRef] [Green Version]
- O’Neil, E.V.; Burns, G.W.; Ferreira, C.R.; Spencer, T.E. Characterization and regulation of extracellular vesicles in the lumen of the ovine uterusdagger. Biol. Reprod. 2020, 102, 1020–1032. [Google Scholar] [CrossRef]
- Edwin, S.S.; Mitchell, M.D.; Silver, R.M.; Branch, D.W.; Dudley, D.J. Ceramide stimulates prostaglandin production by human amnion and decidual cells. J. Soc. Gynecol. Investig. 1997, 4, 274–278. [Google Scholar] [CrossRef]
- Fonseca, B.M.; Correia-da-Silva, G.; Teixeira, N.A. The endocannabinoid anandamide induces apoptosis of rat decidual cells through a mechanism involving ceramide synthesis and p38 MAPK activation. Apoptosis 2013, 18, 1526–1535. [Google Scholar] [CrossRef]
- Brunnert, D.; Sztachelska, M.; Bornkessel, F.; Treder, N.; Wolczynski, S.; Goyal, P.; Zygmunt, M. Lysophosphatidic acid and sphingosine 1-phosphate metabolic pathways and their receptors are differentially regulated during decidualization of human endometrial stromal cells. Mol. Hum. Reprod. 2014, 20, 1016–1025. [Google Scholar] [CrossRef]
- Dunlap, K.A.; Kwak, H.I.; Burghardt, R.C.; Bazer, F.W.; Magness, R.R.; Johnson, G.A.; Bayless, K.J. The sphingosine 1-phosphate (S1P) signaling pathway is regulated during pregnancy in sheep. Biol. Reprod. 2010, 82, 876–887. [Google Scholar] [CrossRef]
- Fox, C.; Eichelberger, K. Maternal microbiome and pregnancy outcomes. Fertil. Steril. 2015, 104, 1358–1363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, B.; Li, X.; Sun, R.; Tong, X.; Ling, B.; Tian, Z.; Wei, H. Natural killer cells promote immune tolerance by regulating inflammatory TH17 cells at the human maternal-fetal interface. Proc. Natl. Acad. Sci. USA 2013, 110, E231–E240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, J.M.; Bell, M.J. If we know so much about preeclampsia, why haven’t we cured the disease? J. Reprod. Immunol. 2013, 99, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Billingham, R.E.; Brent, L.; Medawar, P.B. Actively acquired tolerance of foreign cells. Nature 1953, 172, 603–606. [Google Scholar] [CrossRef]
- Stenqvist, A.C.; Nagaeva, O.; Baranov, V.; Mincheva-Nilsson, L. Exosomes secreted by human placenta carry functional Fas ligand and TRAIL molecules and convey apoptosis in activated immune cells, suggesting exosome-mediated immune privilege of the fetus. J. Immunol. 2013, 191, 5515–5523. [Google Scholar] [CrossRef] [Green Version]
- Djurisic, S.; Hviid, T.V. HLA Class Ib Molecules and Immune Cells in Pregnancy and Preeclampsia. Front. Immunol. 2014, 5, 652. [Google Scholar] [CrossRef] [Green Version]
- Holder, B.; Jones, T.; Shimizu, V.S.; Rice, T.F.; Donaldson, B.; Bouqueau, M.; Forbes, K.; Kampmann, B. Macrophage Exosomes Induce Placental Inflammatory Cytokines: A Novel Mode of Maternal-Placental Messaging. Traffic 2016, 17, 168–178. [Google Scholar] [CrossRef] [Green Version]
- Giacomini, E.; Alleva, E.; Fornelli, G.; Quartucci, A.; Privitera, L.; Vanni, V.S.; Vigano, P. Embryonic extracellular vesicles as informers to the immune cells at the maternal-fetal interface. Clin. Exp. Immunol. 2019, 198, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Benichou, G.; Wang, M.; Ahrens, K.; Madsen, J.C. Extracellular vesicles in allograft rejection and tolerance. Cell. Immunol. 2020, 349, 104063. [Google Scholar] [CrossRef]
- Ou, Q.; Dou, X.; Tang, J.; Wu, P.; Pan, D. Small extracellular vesicles derived from PD-L1-modified mesenchymal stem cell promote Tregs differentiation and prolong allograft survival. Cell Tissue Res. 2020, 389, 465–481. [Google Scholar] [CrossRef]
- Cho, K.; Kook, H.; Kang, S.; Lee, J. Study of immune-tolerized cell lines and extracellular vesicles inductive environment promoting continuous expression and secretion of HLA-G from semi-allograft immune tolerance during pregnancy. J. Extracell. Vesicles 2020, 9, 1795364. [Google Scholar] [CrossRef] [PubMed]
- Svensson-Arvelund, J.; Mehta, R.B.; Lindau, R.; Mirrasekhian, E.; Rodriguez-Martinez, H.; Berg, G.; Lash, G.E.; Jenmalm, M.C.; Ernerudh, J. The human fetal placenta promotes tolerance against the semiallogeneic fetus by inducing regulatory T cells and homeostatic M2 macrophages. J. Immunol. 2015, 194, 1534–1544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.H.; Aldo, P.; You, Y.; Ding, J.; Kaislasuo, J.; Petersen, J.F.; Lokkegaard, E.; Peng, G.; Paidas, M.J.; Simpson, S.; et al. Trophoblast-secreted soluble-PD-L1 modulates macrophage polarization and function. J. Leukoc. Biol. 2020, 108, 983–998. [Google Scholar] [CrossRef] [PubMed]
- Atay, S.; Gercel-Taylor, C.; Suttles, J.; Mor, G.; Taylor, D.D. Trophoblast-derived exosomes mediate monocyte recruitment and differentiation. Am. J. Reprod. Immunol. 2011, 65, 65–77. [Google Scholar] [CrossRef]
- Pallinger, E.; Bognar, Z.; Bogdan, A.; Csabai, T.; Abraham, H.; Szekeres-Bartho, J. PIBF+ extracellular vesicles from mouse embryos affect IL-10 production by CD8+ cells. Sci. Rep. 2018, 8, 4662. [Google Scholar] [CrossRef] [Green Version]
- Desrochers, L.M.; Bordeleau, F.; Reinhart-King, C.A.; Cerione, R.A.; Antonyak, M.A. Microvesicles provide a mechanism for intercellular communication by embryonic stem cells during embryo implantation. Nat. Commun. 2016, 7, 11958. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, K.; Kusama, K.; Ideta, A.; Kimura, K.; Hori, M.; Imakawa, K. Effects of miR-98 in intrauterine extracellular vesicles on maternal immune regulation during the peri-implantation period in cattle. Sci. Rep. 2019, 9, 20330. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, K.; Kusama, K.; Ideta, A.; Imakawa, K.; Hori, M. IFNT-independent effects of intrauterine extracellular vesicles (EVs) in cattle. Reproduction 2020, 159, 503–511. [Google Scholar] [CrossRef]
- Gurung, S.; Greening, D.W.; Catt, S.; Salamonsen, L.; Evans, J. Exosomes and soluble secretome from hormone-treated endometrial epithelial cells direct embryo implantation. Mol. Hum. Reprod. 2020, 26, 510–520. [Google Scholar] [CrossRef]
- Greening, D.W.; Xu, R.; Gopal, S.K.; Rai, A.; Simpson, R.J. Proteomic insights into extracellular vesicle biology—Defining exosomes and shed microvesicles. Expert Rev. Proteom. 2017, 14, 69–95. [Google Scholar] [CrossRef]
- Tan, Q.; Shi, S.; Liang, J.; Zhang, X.; Cao, D.; Wang, Z. MicroRNAs in Small Extracellular Vesicles Indicate Successful Embryo Implantation during Early Pregnancy. Cells 2020, 9, 645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giacomini, E.; Scotti, G.M.; Vanni, V.S.; Lazarevic, D.; Makieva, S.; Privitera, L.; Signorelli, S.; Cantone, L.; Bollati, V.; Murdica, V.; et al. Global transcriptomic changes occur in uterine fluid-derived extracellular vesicles during the endometrial window for embryo implantation. Hum. Reprod. 2021, 36, 2249–2274. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | ||||
|---|---|---|---|---|
| Contents | Origin | Size of EV | Member | Role |
| microRNA [132,144,145,146,147,148,149,150,151,152] | exosomes, microvesicles | 30–1500 nm | mcroRNA-494, microRNA-923, microRNA-30 family, microRNA-138-5p… | immune regulation, endometrial receptivity regulation |
| Protein [151,153,154] | exosomes, microvesicles | 30–1500 nm | 663 common proteins | regulate embryo apposition, adhesion, and implantation |
| Lipids [155,156,157,158,159,160] | exosomes, microvesicles | 30–1500 nm | eight classes | angiogenesis, decidual stromal cell apoptosis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.-M.; Chen, L.-H.; Hsu, L.-T.; Lai, C.-H. Immune Tolerance of Embryo Implantation and Pregnancy: The Role of Human Decidual Stromal Cell- and Embryonic-Derived Extracellular Vesicles. Int. J. Mol. Sci. 2022, 23, 13382. https://doi.org/10.3390/ijms232113382
Wu H-M, Chen L-H, Hsu L-T, Lai C-H. Immune Tolerance of Embryo Implantation and Pregnancy: The Role of Human Decidual Stromal Cell- and Embryonic-Derived Extracellular Vesicles. International Journal of Molecular Sciences. 2022; 23(21):13382. https://doi.org/10.3390/ijms232113382
Chicago/Turabian StyleWu, Hsien-Ming, Liang-Hsuan Chen, Le-Tien Hsu, and Chyong-Huey Lai. 2022. "Immune Tolerance of Embryo Implantation and Pregnancy: The Role of Human Decidual Stromal Cell- and Embryonic-Derived Extracellular Vesicles" International Journal of Molecular Sciences 23, no. 21: 13382. https://doi.org/10.3390/ijms232113382
APA StyleWu, H.-M., Chen, L.-H., Hsu, L.-T., & Lai, C.-H. (2022). Immune Tolerance of Embryo Implantation and Pregnancy: The Role of Human Decidual Stromal Cell- and Embryonic-Derived Extracellular Vesicles. International Journal of Molecular Sciences, 23(21), 13382. https://doi.org/10.3390/ijms232113382






