Comprehensive Analysis of StSRO Gene Family and Its Expression in Response to Different Abiotic Stresses in Potato
Abstract
1. Introduction
2. Results
2.1. Identification of SRO Genes in Potato
2.2. Gene Structure and Conserved Motifs of SRO Proteins in Potato
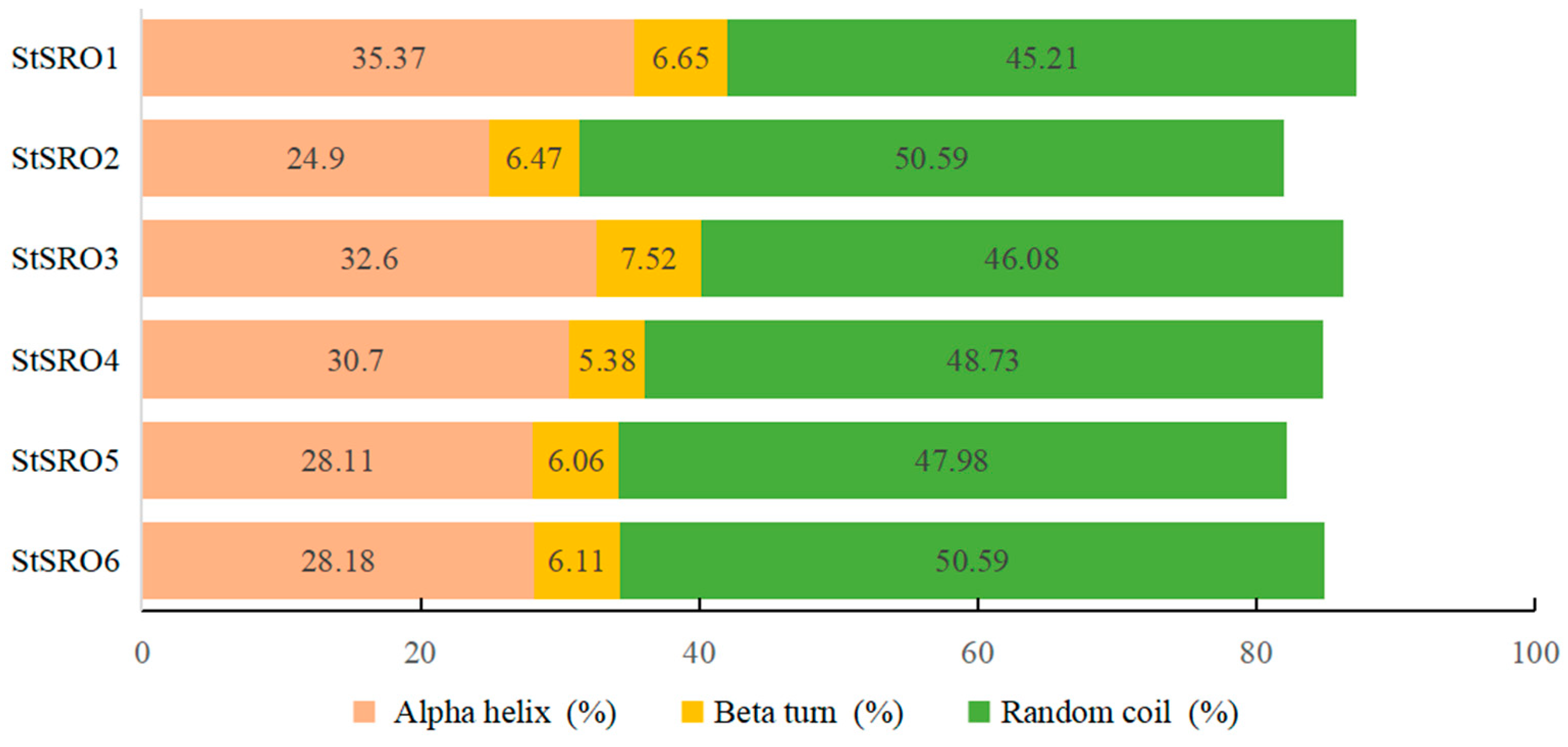
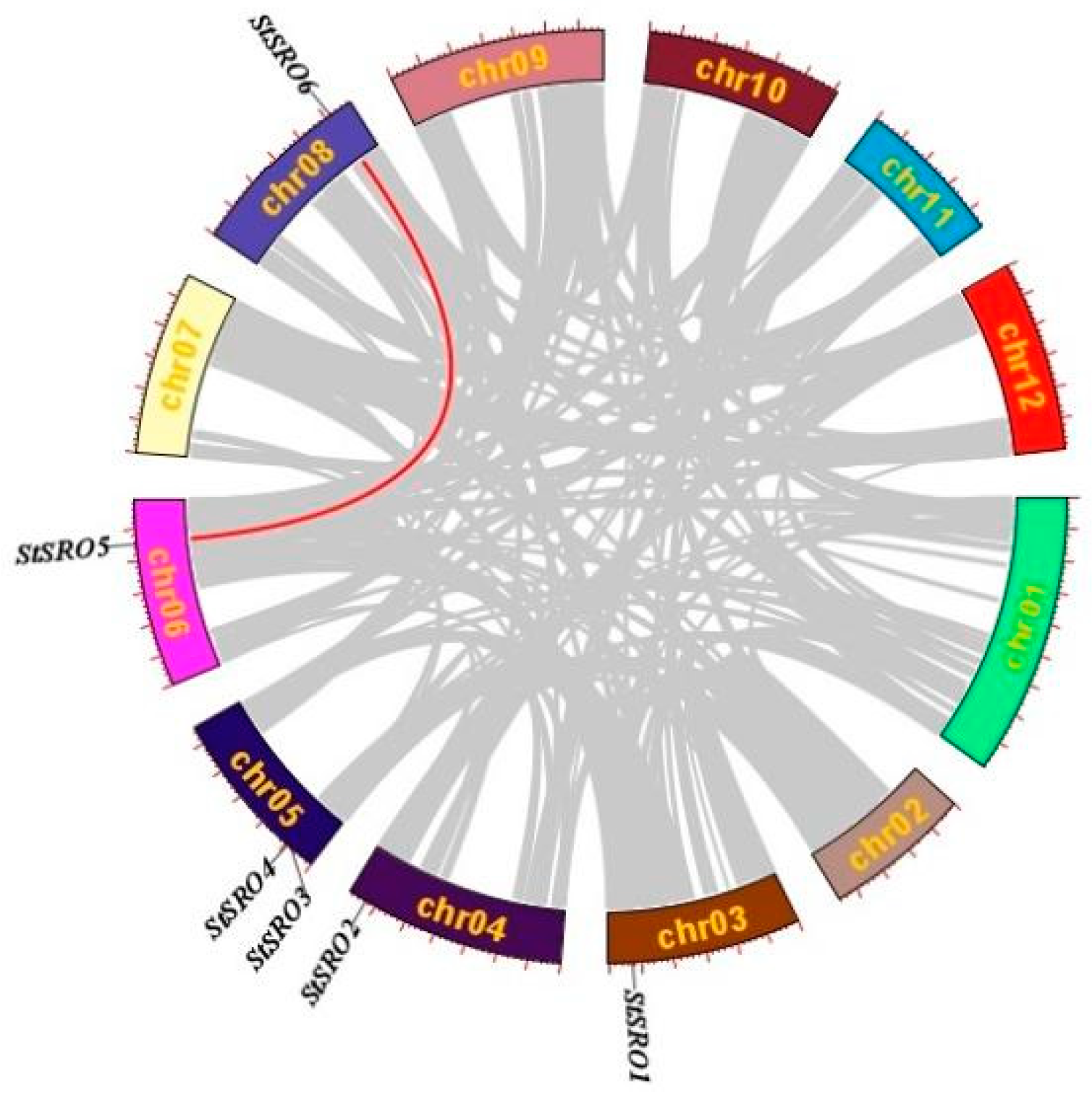
2.3. Chromosomal Mapping and Secondary Structure Analysis of StSRO Genes in Potato
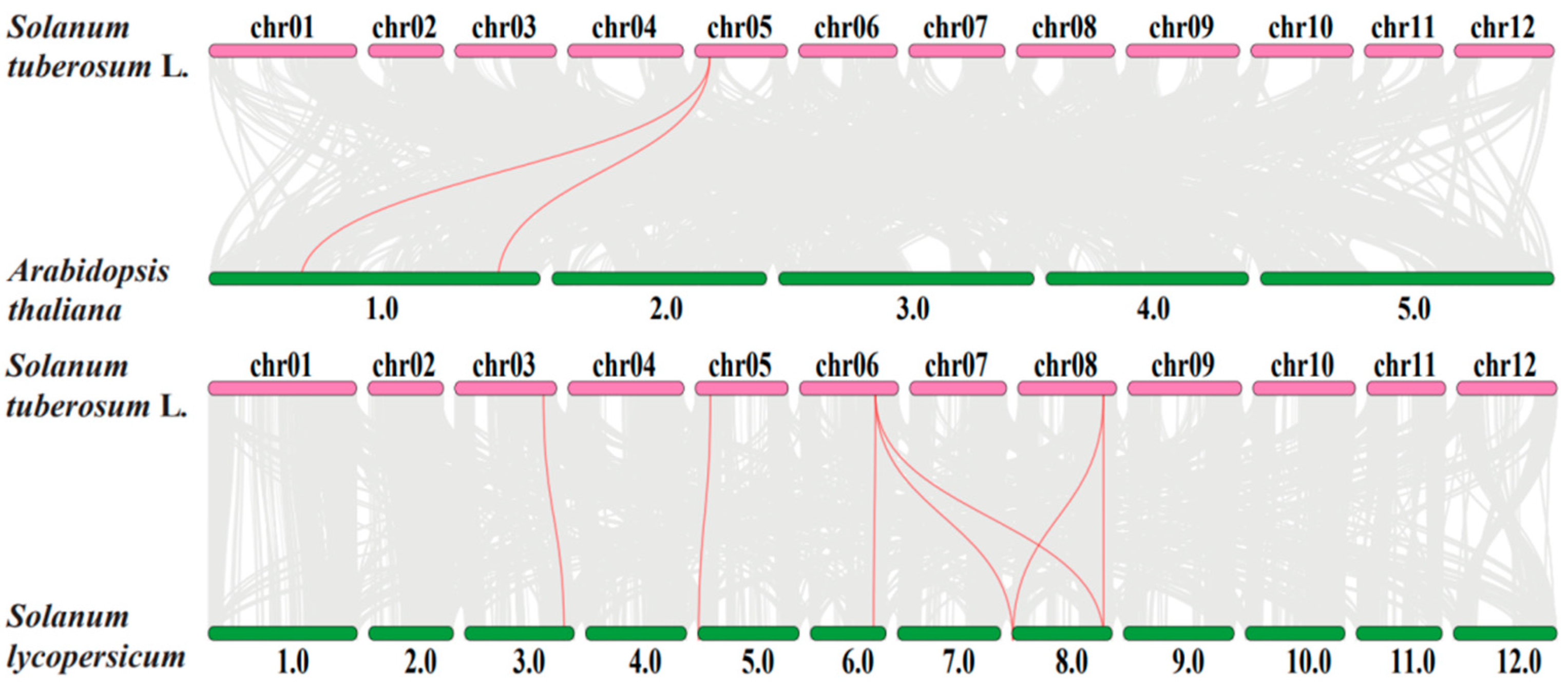
2.4. Synteny Analysis of SRO Gene Family
2.5. Phylogenetic Tree Analysis of SRO Genes
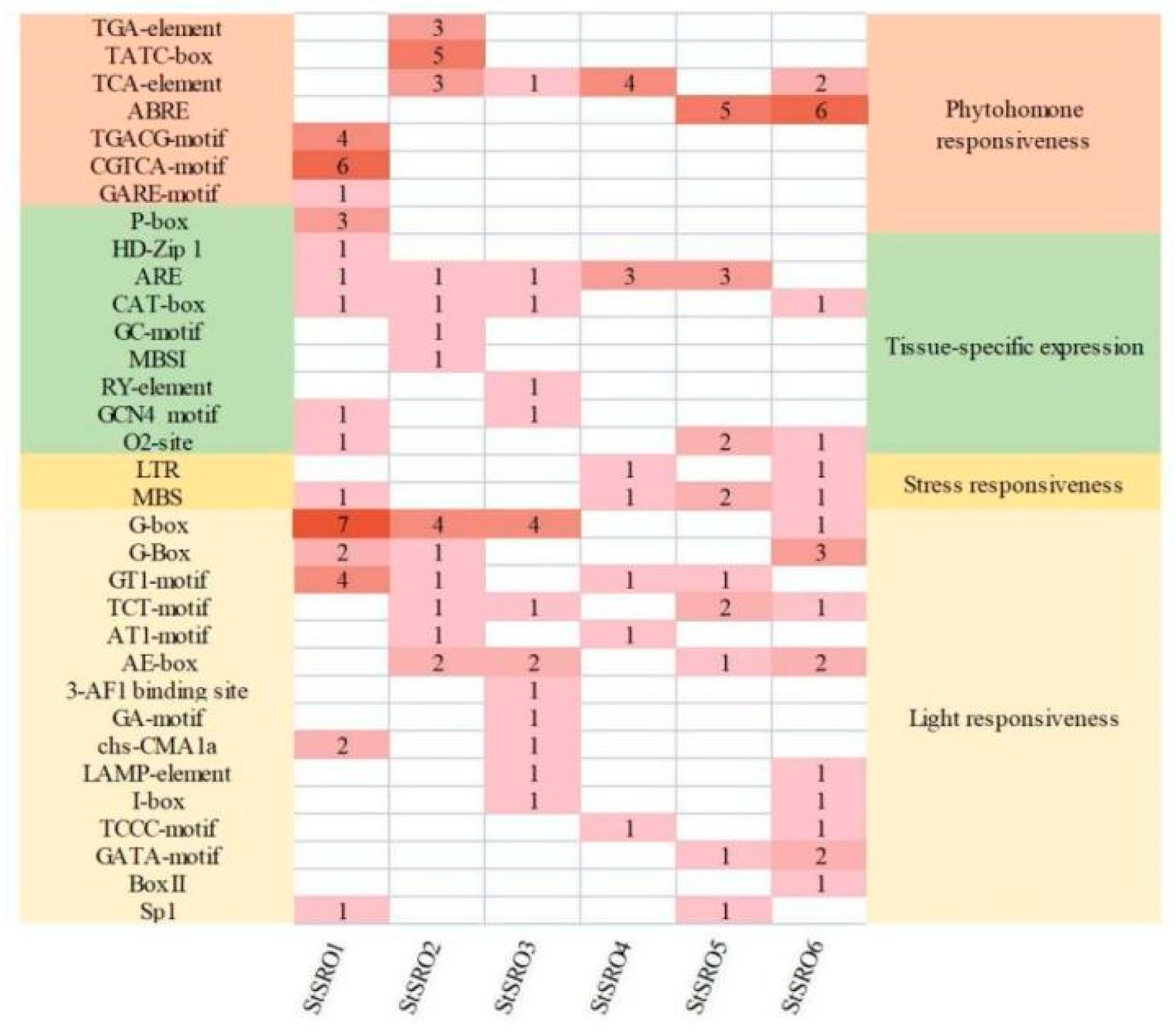
2.6. Analysis of Cis-Acting Elements of SRO Gene Family in Potato
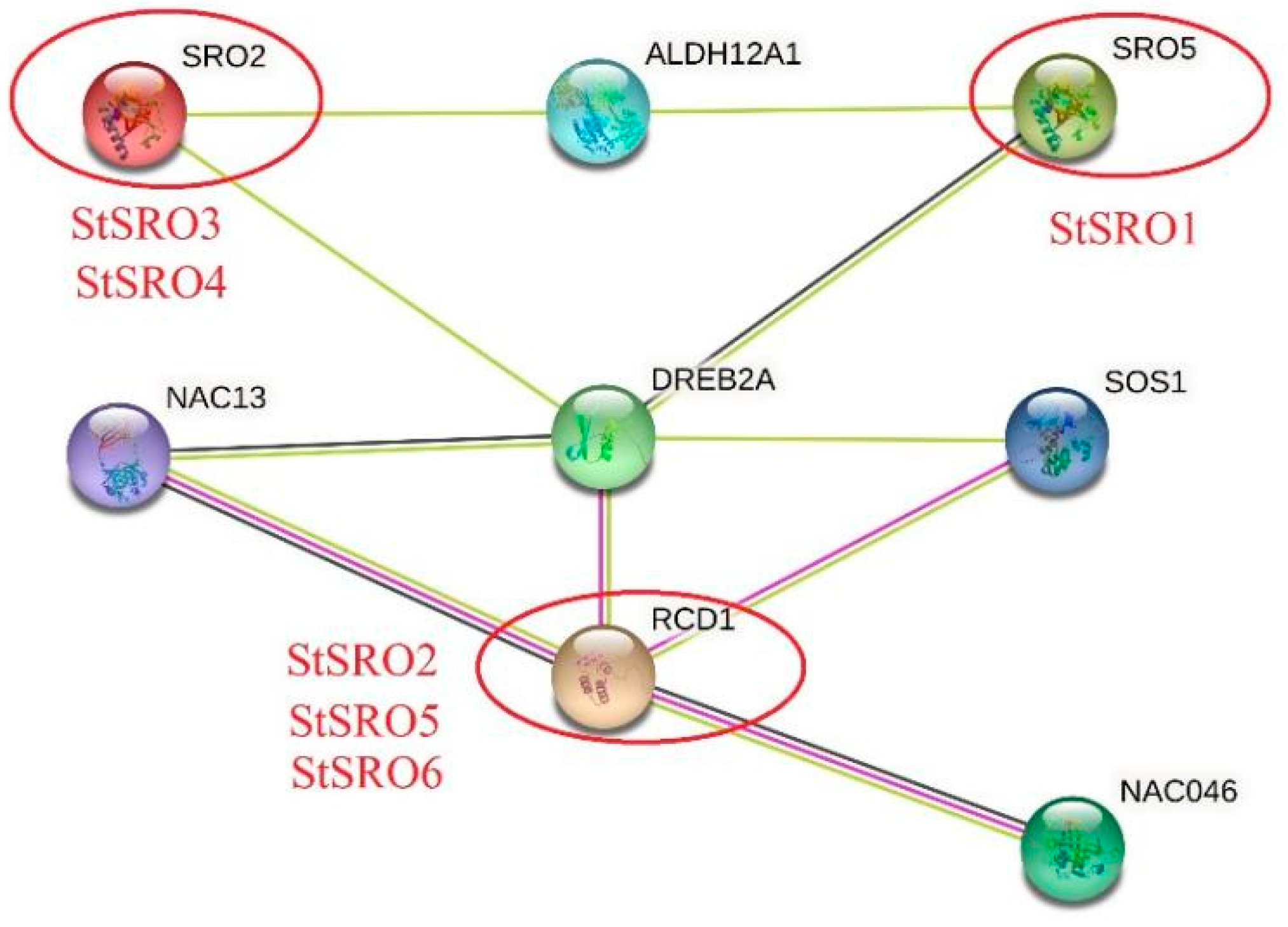
2.7. Construction of SRO Protein Interaction Network and Functional Annotation in Potato
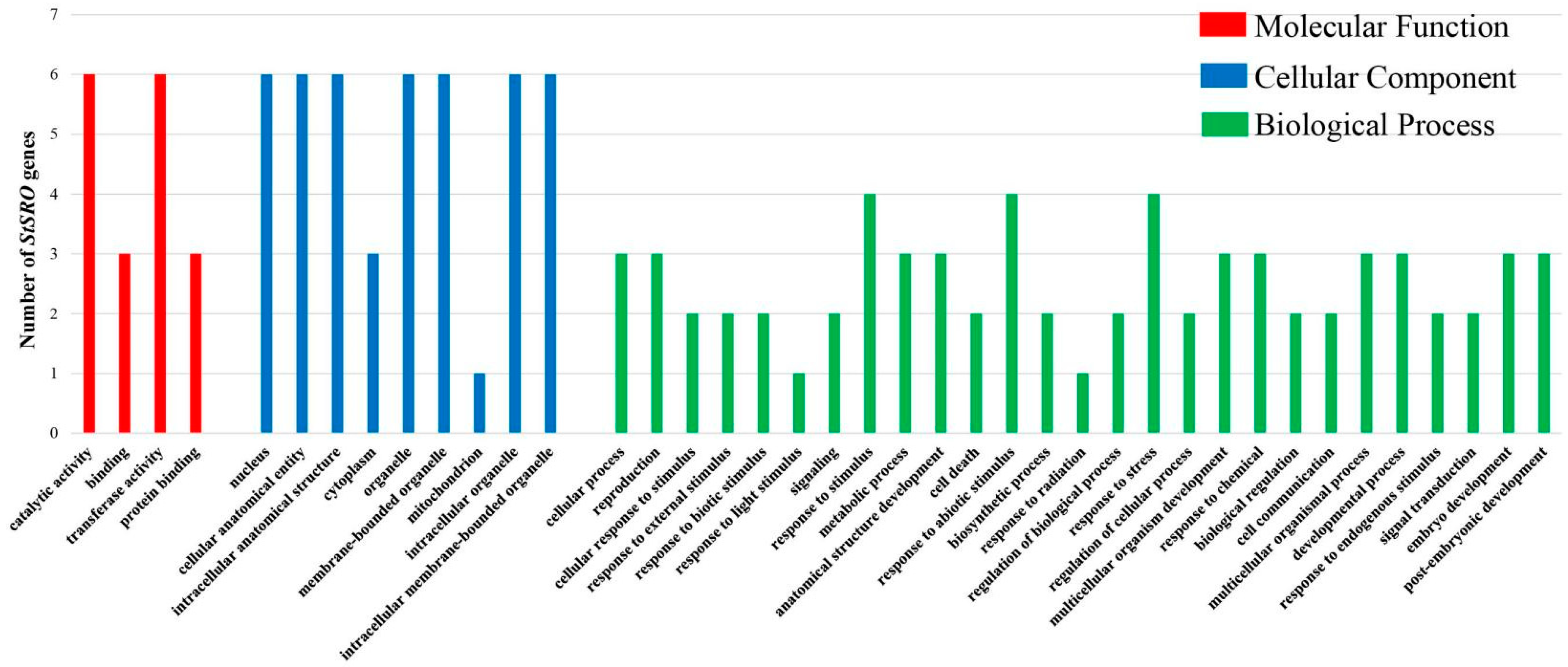
2.8. Functional Annotation of SRO Genes in Potato
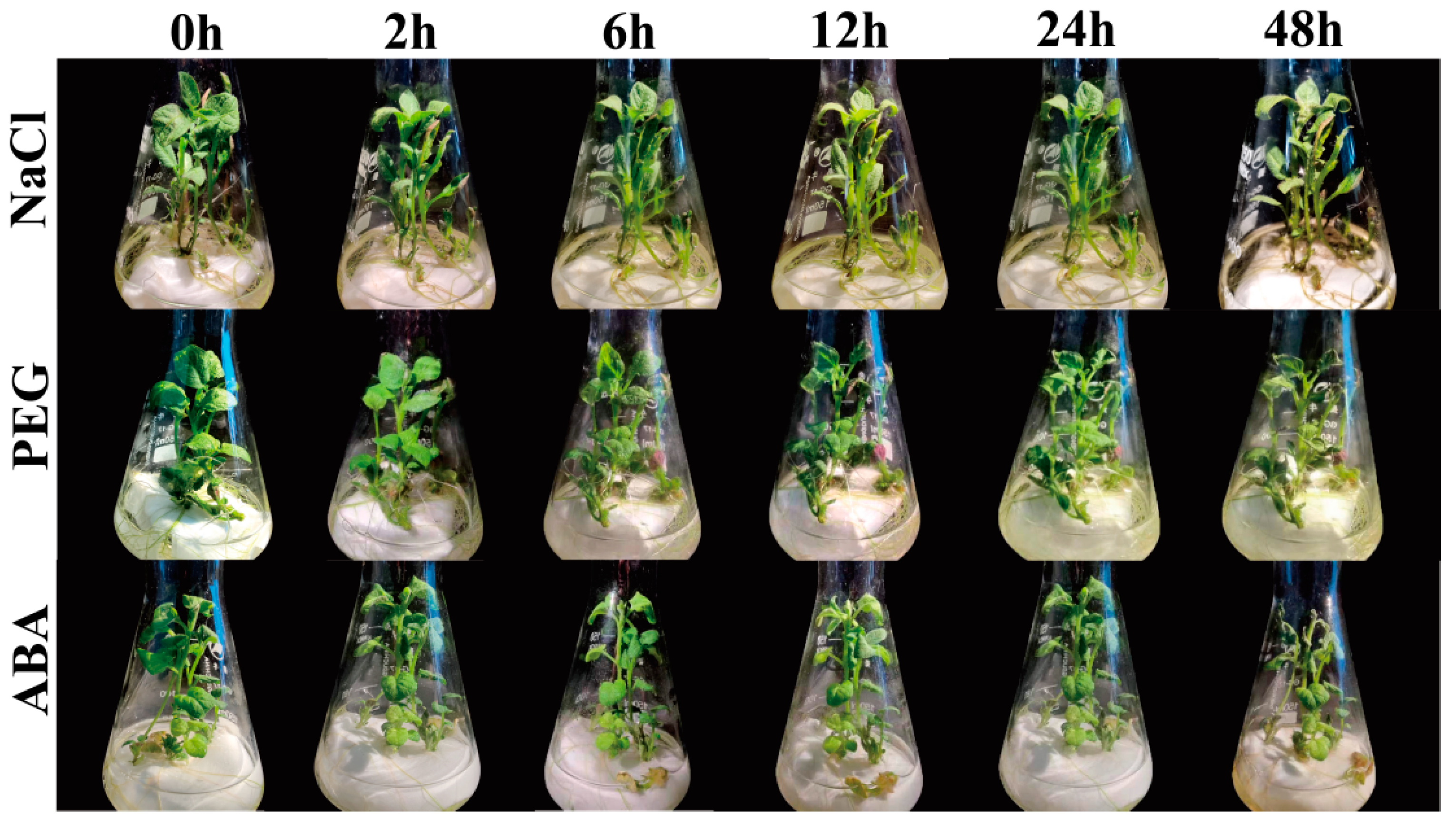
2.9. Effects of Abiotic Stresses and Hormone Treatments on Potato Plantlets In Vitro
2.10. Analysis of SRO Gene Expression in Different Tissues and Treatments in DM Potato
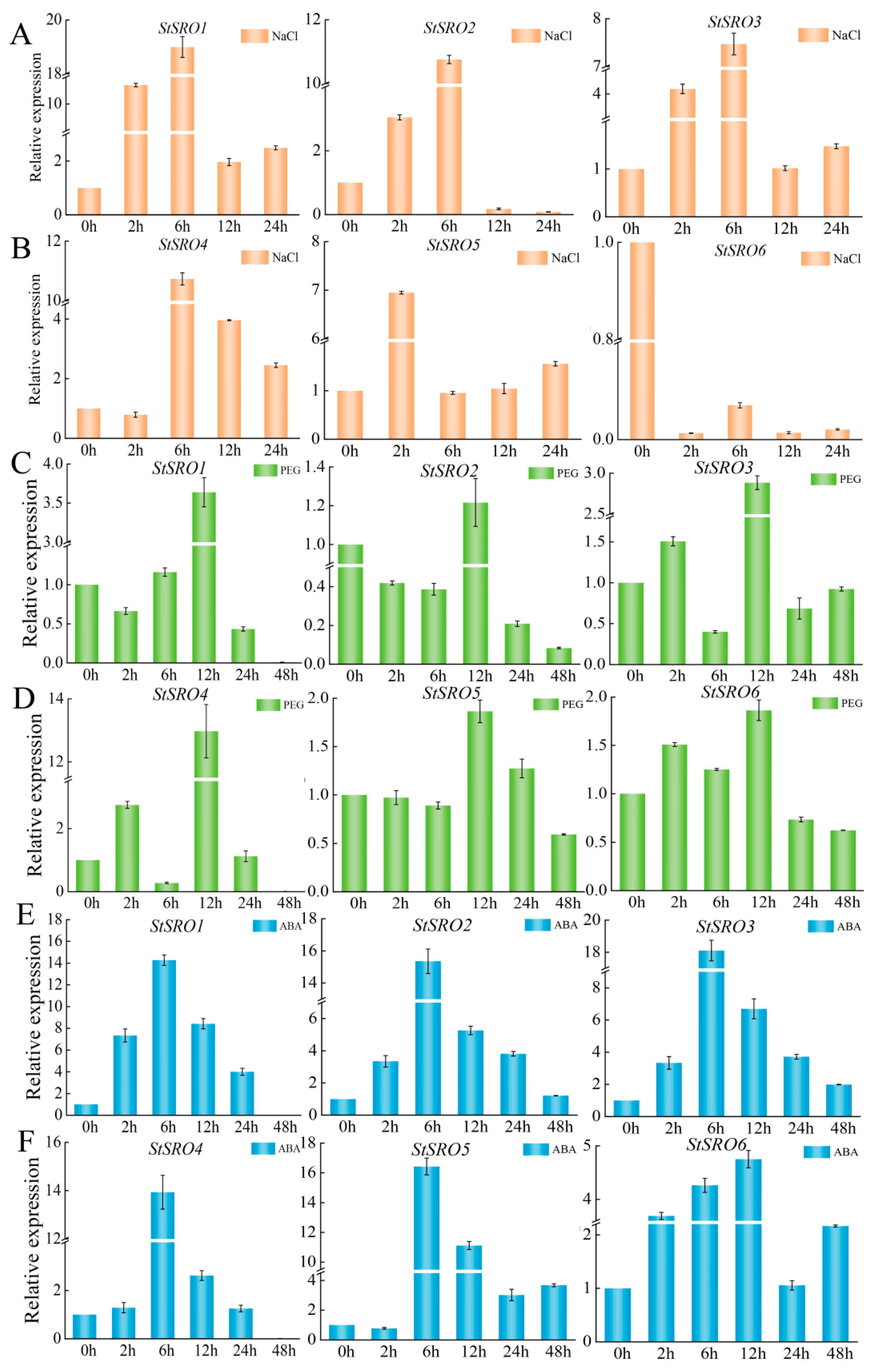
2.11. Expression Levels of StSRO Genes under NaCl, PEG and ABA Treatments
2.12. Transcriptome-Based Expression Profiling of StSRO Genes in Potato Cultivar “Zihuabai” under Salt Stress
3. Discussion
4. Materials and Methods
4.1. Identification and Sequence Analysis of SRO Gene Family Members in Potato
4.2. Gene Structure and Conserved Motifs of SRO Proteins in Potato
4.3. Chromosomal Mapping and Secondary Structure Analysis of SRO Genes in Potato
4.4. Synteny Analysis of SRO Gene Family
4.5. Phylogenetic Tree Analysis of SRO Genes in Potato
4.6. Analysis of Cis-Acting Elements of SRO Gene Family in Potato
4.7. Construction of SRO Protein Interaction Network in Potato
4.8. Gene Ontology (GO) Analysis of SRO Genes in Potato
4.9. Analysis of Potato SRO Gene Expression in Different Tissues and Different Treatments in DM Potato
4.10. RNA Isolation and qRT-PCR
4.11. Plant Materials, Growth Conditions and Treatments
4.12. RNA-Seq Data Analysis
4.13. Differential Expression Genes Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, W.; Geng, Y.; Liu, Y.; Chen, S.; Cao, S.; Li, W.; Chen, H.; Ma, D.; Yin, J. Genome-wide identification and characterization of SRO gene family in wheat: Molecular evolution and expression profiles during different stresses. Plant Physiol. Biochem. 2020, 154, 590–611. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.N.; Zhu, C.; Jiang, J.; Zhang, H.; Zhu, J.K.; Duan, C.G. Epigenetic regulation in plant abiotic stress responses. J. Integr. Plant Biol. 2020, 62, 563–580. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Xiong, L.; Shi, H.; Yang, S.; Herrera-Estrella, L.R.; Xu, G.; Chao, D.Y.; Li, J.; Wang, P.Y.; Qin, F.; et al. Plant abiotic stress response and nutrient use efficiency. Sci. China Life Sci. 2020, 63, 635–674. [Google Scholar] [CrossRef] [PubMed]
- Jaspers, P.; Overmyer, K.; Wrzaczek, M.; Vainonen, J.P.; Blomster, T.; Salojärvi, J.; Reddy, R.A.; Kangasjärvi, J. The RST and PARP-like domain containing SRO protein family: Analysis of protein structure, function and conservation in land plants. BMC Genom. 2010, 11, 170. [Google Scholar] [CrossRef]
- Xiong, Y.; Fang, J.; Jiang, X.; Wang, T.; Liu, K.; Peng, H.; Zhang, X.; Zhang, A. Genome-wide analysis of multiple organellar RNA editing factor (MORF) family in Kiwifruit (Actinidia chinensis) reveals its roles in chloroplast RNA editing and pathogens stress. Plants 2022, 11, 146. [Google Scholar] [CrossRef]
- Du, W.; Yang, J.; Li, Q.; Su, Q.; Yi, D.; Pang, Y. Genome-wide identification and characterization of growth regulatory factor family genes in Medicago. Int. J. Mol. Sci. 2022, 23, 6905. [Google Scholar] [CrossRef]
- Wang, S.; Duan, Z.; Yan, Q.; Wu, F.; Zhou, P.; Zhang, J. Genome-wide identification of the GRAS family genes in Melilotus albus and expression analysis under various tissues and abiotic stresses. Int. J. Mol. Sci. 2022, 23, 7403. [Google Scholar] [CrossRef]
- Teotia, S.; Lamb, R.S. The paralogous genes RADICAL-INDUCED CELL DEATH1 and SIMILAR TO RCD ONE1 have partially redundant functions during Arabidopsis development. Plant Physiol. 2009, 151, 180–198. [Google Scholar] [CrossRef]
- Liu, A.; Wei, M.; Zhou, Y.; Li, D.; Zhou, R.; Zhang, Y.; Zhang, X.; Wang, L.; You, J. Comprehensive analysis of SRO gene family in Sesamum indicum (L.) reveals its association with abiotic stress responses. Int. J. Mol. Sci. 2021, 22, 13048. [Google Scholar] [CrossRef]
- Sharma, S.; Kaur, C.; Singla-Pareek, S.L.; Sopory, S.K. OsSRO1a interacts with RNA binding domain-containing protein (OsRBD1) and functions in abiotic stress tolerance in Yeast. Front. Plant Sci. 2016, 7, 62. [Google Scholar] [CrossRef]
- Li, B.Z.; Zhao, X.; Zhao, X.L.; Peng, L. Structure and function analysis of Arabidopsis thaliana SRO protein family. Hereditas 2013, 35, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Zong, W.; Du, H.; Hu, H.; Xiong, L. A special member of the rice SRO family, OsSRO1c, mediates responses to multiple abiotic stresses through interaction with various transcription factors. Plant Mol. Biol. 2014, 84, 693–705. [Google Scholar] [CrossRef]
- Li, H.; Li, R.; Qu, F.; Yao, J.; Hao, Y.; Wang, X.; You, C. Identification of the SRO gene family in apples (Malus×domestica) with a functional characterization of MdRCD1. Tree Genet. Genomes 2017, 13, 94. [Google Scholar] [CrossRef]
- Yuan, B.; Chen, M.; Li, S. Isolation and identification of Ipomoea cairica (L.) Sweet gene IcSRO1 encoding a SIMILAR TO RCD-ONE protein, which improves salt and drought tolerance in transgenic Arabidopsis. Int. J. Mol. Sci. 2020, 21, 1017. [Google Scholar] [CrossRef] [PubMed]
- Vainonen, J.P.; Jaspers, P.; Wrzaczek, M.; Lamminmäki, A.; Reddy, R.A.; Vaahtera, L.; Brosché, M.; Kangasjärvi, J. RCD1-DREB2A interaction in leaf senescence and stress responses in Arabidopsis thaliana. Biochem. J. 2012, 442, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Jaspers, P.; Blomster, T.; Brosché, M.; Salojärvi, J.; Ahlfors, R.; Vainonen, J.P.; Reddy, R.A.; Immink, R.; Angenent, G.; Turck, F.; et al. Unequally redundant RCD1 and SRO1 mediate stress and developmental responses and interact with transcription factors. Plant J. 2009, 60, 268–279. [Google Scholar] [CrossRef]
- Carmel-Harel, O.; Stearman, R.; Gasch, A.P.; Botstein, D.; Brown, P.O.; Storz, G. Role of thioredoxin reductase in the Yap1p-dependent response to oxidative stress in Saccharomyces cerevisiae. Mol. Microbiol. 2001, 39, 595–605. [Google Scholar] [CrossRef]
- Fernandes, L.; Rodrigues-Pousada, C.; Struhl, K. Yap, a novel family of eight bZIP proteins in Saccharomyces cerevisiae with distinct biological functions. Mol. Cell. Biol. 1997, 17, 6982–6993. [Google Scholar] [CrossRef]
- Belles-Boix, E.; Babiychuk, E.; Van Montagu, M.; Inzé, D.; Kushnir, S. CEO1, a new protein from Arabidopsis thaliana, protects yeast against oxidative damage. FEBS Lett. 2000, 482, 19–24. [Google Scholar] [CrossRef]
- Ahlfors, R.; Lång, S.; Overmyer, K.; Jaspers, P.; Brosché, M.; Tauriainen, A.; Kollist, H.; Tuominen, H.; Belles-Boix, E.; Piippo, M.; et al. Arabidopsis RADICAL-INDUCED CELL DEATH1 belongs to the WWE protein-protein interaction domain protein family and modulates abscisic acid, ethylene, and methyl jasmonate responses. Plant Cell 2004, 16, 1925–1937. [Google Scholar] [CrossRef]
- Lu, C.; Li, Y.; Sun, D.; Niu, X.; Chang, Q. Genome-wide identification and expression analysis of GATA gene family in Foxtail Mille. Acta Agric. Boreali-Occident. Sin. 2021, 30, 883–893. [Google Scholar] [CrossRef]
- Spooner, D.M.; McLean, K.; Ramsay, G.; Waugh, R.; Bryan, G.J. A single domestication for potato based onmultilocus amplified fragment length polymorphism genotyping. Proc. Natl. Acad. Sci. USA 2005, 102, 14694–14699. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xie, H.; He, M.; Su, W.; Yang, Y.; Wang, J.; Ye, G.; Zhou, Y. Genome-wide identification and expression analysis of the StSWEET family genes in potato (Solanum tuberosum L.). Genes Genom. 2020, 42, 135–153. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Ma, Z.; Chen, H.; Liu, M. MYB gene family in potato (Solanum tuberosum L.): Genome-wide identification of hormone-responsive reveals their potential functions in growth and development. Int. J. Mol. Sci. 2019, 20, 4847. [Google Scholar] [CrossRef]
- Pham, G.M.; Hamilton, J.P.; Wood, J.C.; Burke, J.T.; Zhao, H.; Vaillancourt, B.; Ou, S.; Jiang, J.; Buell, C.R. Construction of a chromosome-scale long-read reference genome assembly for potato. Gigascience 2020, 9, giaa100. [Google Scholar] [CrossRef]
- Hardigan, M.A.; Crisovan, E.; Hamilton, J.P.; Kim, J.; Laimbeer, P.; Leisner, C.P.; Manrique-Carpintero, N.C.; Newton, L.; Pham, G.M.; Vaillancourt, B.; et al. Genome reduction uncovers a large dispensable genome and adaptive role for copy number variation in asexually propagated Solanum tuberosum. Plant Cell 2016, 28, 388–405. [Google Scholar] [CrossRef]
- Ma, W.; Han, A.; Nai, G.; Li, Y.; Li, Y.; Mao, J. Identification and expression analysis of NF-Y nuclear factor family in Grape. Acta Agric. Boreali-Occident. Sin. 2021, 30, 870–882. [Google Scholar] [CrossRef]
- Li, N.; Xu, R.; Wang, B.; Wang, J.; Huang, S.; Yu, Q.; Gao, J. Genome-wide identification and evolutionary analysis of the SRO gene family in tomato. Front. Genet. 2021, 12, 753638. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, N.; Zhu, X.; Ma, R.; Liu, S.; Wang, X.; Yang, J.; Si, H. Genome-wide analysis of NF-Y genes in potato and functional identification of StNF-YC9 in drought tolerance. Front. Plant Sci. 2021, 12, 749688. [Google Scholar] [CrossRef]
- Athanasios, A.; Charalampos, V.; Vasileios, T.; Ashraf, G.M. Protein-protein interaction (PPI) network: Recent advances in drug discovery. Curr. Drug Metab. 2017, 18, 5–10. [Google Scholar] [CrossRef]
- Wei, H.; Movahedi, A.; Liu, G.; Li, Y.; Liu, S.; Yu, C.; Chen, Y.; Zhong, F.; Zhang, J. Comprehensive analysis of carotenoid cleavage dioxygenases gene family and its expression in response to abiotic stress in poplar. Int. J. Mol. Sci. 2022, 23, 1418. [Google Scholar] [CrossRef] [PubMed]
- Bader, G.D.; Hogue, C.W. An automated method for finding molecular complexes in large protein interaction networks. BMC Bioinform. 2003, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, L.; Zhou, C.; Zhuang, J.; Wang, L.; Sun, Y.; Sun, C. Protein-protein interaction networks and different clustering analysis in Burkitt’s lymphoma. Hematology 2018, 23, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Guo, X.; Liu, J.; Zhou, F.; Liu, W.; Wu, J.; Zhang, H.; Cao, H.; Su, H.; Wen, R. Genome-wide identification, characterization, and expression analysis of the NAC transcription factor in Chenopodium quinoa. Genes 2019, 10, 500. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, Y.; Zhu, J.; Ma, W.; Li, Z.; Bi, Z.; Sun, C.; Bai, J.; Zhang, J.; Liu, Y. Genome-wide identification and analysis of the NF-Y gene family in Potato (Solanum tuberosum L.). Front. Genet. 2021, 12, 739989. [Google Scholar] [CrossRef]
- Wang, B.; Zhu, X.; Wei, X. Genome-wide identification, structural analysis, and expression profiles of the BZR gene family in tomato. J. Plant Biochem. Biotechnol. 2021, 31, 739–750. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, B.; Wang, X.; Zhang, C.; Wei, X. Genome-wide identification, characterization and expression analysis of the LIM transcription factor family in quinoa. Physiol. Mol. Biol. Plants 2021, 27, 787–800. [Google Scholar] [CrossRef]
- Zhou, X.; Ma, Y.; Liu, Z. Gansu Provincial Key Laboratory of Aridland Crop Science/College of Life Science and Technology, Gansu Agricultural University, Lanzhou, Gansu, China. Manuscript in preparation. 2022. [Google Scholar]
- Guo, Y.; Wang, P.; Chen, D.; Zheng, Y.; Chen, X.; Naixing, Y.E. Genome-wide identification and expression analysis of SRO gene family in Camellia sinensis. J. Tea Sci. 2019, 39, 392–402. [Google Scholar] [CrossRef]
- Qiao, Y.; Gao, X.; Liu, Z.; Wu, Y.; Hu, L.; Yu, J. Genome-wide identification and analysis of SRO gene family in Chinese Cabbage (Brassica rapa L.). Plants 2020, 9, 1235. [Google Scholar] [CrossRef]
- Zhao, Q.; Ma, H.; Jia, L.; Chen, S.; Chen, H. Genome-wide identification and expression analysis of SRO genes family in maize. Sci. Agric. Sin. 2018, 51, 3009–3019. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, M.; Singer, S.D.; Fei, Z.; Wang, H.; Wang, X. Genome-wide identification and analysis of the TIFY gene family in grape. PLoS ONE 2012, 7, e44465. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.C.; Purugganan, M.D. The early stages of duplicate gene evolution. Proc. Natl. Acad. Sci. USA 2003, 100, 15682–15687. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.; Landherr, L.L.; Frohlich, M.W.; Leebens-Mack, J.; Ma, H.; DePamphilis, C.W. Patterns of gene duplication in the plant SKP1 gene family in angiosperms: Evidence for multiple mechanisms of rapid gene birth. Plant J. 2007, 50, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Luo, B.; Wang, Y.; Li, J.; Hu, Z.; Xie, Q.; Wu, T.; Chen, G. Genome-wide identification, classification and expression analysis of m6A gene family in Solanum lycopersicum. Int. J. Mol. Sci. 2022, 23, 4522. [Google Scholar] [CrossRef] [PubMed]
- Mnaimneh, S.; Davierwala, A.P.; Haynes, J.; Moffat, J.; Peng, W.T.; Zhang, W.; Yang, X.; Pootoolal, J.; Chua, G.; Lopez, A.; et al. Exploration of essential gene functions via titratable promoter alleles. Cell 2004, 118, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, P.; Cheng, B.; Zhang, Y.; Shen, Y.; Wang, X.; Zhang, Q.; Lou, Q.; Zhang, S.; Wang, B.; et al. Identification of TALE transcription factor family and expression patterns related to fruit chloroplast development in tomato (Solanum lycopersicum L.). Int. J. Mol. Sci. 2022, 23, 4507. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, D.; Hu, H.; Li, W.; Hu, Y.; Xie, J.; Huang, S.; Wang, W. Genome-wide characterization of a SRO gene family involved in response to biotic and abiotic stresses in banana (Musa spp.). BMC Plant Biol. 2019, 19, 211. [Google Scholar] [CrossRef]
- Liang, W.; Ma, X.; Wan, P.; Liu, L. Plant salt-tolerance mechanism: A review. Biochem. Biophys. Res. Commun. 2018, 495, 286–291. [Google Scholar] [CrossRef]
- Wang, Q.; Guan, C.; Wang, P.; Ma, Q.; Bao, A.; Zhang, J.; Wang, S.M. The effect of Athkt1;1 or AtSOS1 mutation on the expressions of Na+ or K+ transporter genes and ion homeostasis in Arabidopsis thaliana under salt stress. Int. J. Mol. Sci. 2019, 20, 1085. [Google Scholar] [CrossRef]
- Lyu, Y.; Yang, W.; Zhao, L.; Yao, J.; Chen, W.; Li, Y.; Zhang, Y.S. Genome-wide identification and expression analysis of SRO genes family in Gossypium hirsutum L. Acta Agron. Sin. 2017, 43, 10. [Google Scholar] [CrossRef]
- Sun, F.; Chen, Q.; Chen, Q.; Jiang, M.; Zeng, Q.; Qu, Y. Genome-wide identification and expression analysis of the 2OG-Fe(II) oxygenase gene family in upland cotton (Gossypium hirsutum L.). Physiol. Mol. Biol. Plants 2021, 27, 1969–1977. [Google Scholar] [CrossRef] [PubMed]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.; Tosatto, S.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The protein families database in 2021. Nucleic Acids Res. 2021, 49, D412–D419. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Li, S.; Yang, J.; Luo, H.; Zhang, N.; Si, H. Identification and expressional analysis of StCDPKs gene family in potato. J. Gansu Agric. Univ. 2020, 55, 11. [Google Scholar] [CrossRef]
- Wilkins, M.R.; Gasteiger, E.; Bairoch, A.; Sanchez, J.C.; Williams, K.L.; Appel, R.D.; Hochstrasser, D.F. Protein identification and analysis tools in the ExPASy server. In 2-D Proteome Analysis Protocols; Link, A.J., Ed.; Methods in Molecular Biology; Humana: Totowa, NJ, USA, 1999; Volume 112, pp. 531–552. [Google Scholar] [CrossRef]
- Horton, P.; Park, K.J.; Obayashi, T.; Fujita, N.; Harada, H.; Adams-Collier, C.J.; Nakai, K. WoLF PSORT: Protein localization predictor. Nucleic Acids Res. 2007, 35, W585–W587. [Google Scholar] [CrossRef]
- Lei, P.; Wei, X.; Gao, R.; Huo, F.; Nie, X.; Tong, W.; Song, W. Genome-wide identification of PYL gene family in wheat: Evolution, expression and 3D structure analysis. Genomics 2021, 113, 854–866. [Google Scholar] [CrossRef]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef]
- Zhang, P.; Witters, N.A.; Duhamel, G.E. Recovery from colonic infection elicits serum IgG antibodies to specific Serpulina pilosicoli outer membrane antigens (SPOMA). Adv. Exp. Med. Biol. 1999, 473, 191–197. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; Debarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef]
- Hall, B.G. Building phylogenetic trees from molecular data with MEGA. Mol. Biol. Evol. 2013, 30, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.; Sun, X.; Cao, L.; Zhang, S.; Yu, J.; Xu, X.; Xu, Z.; Liu, G.; Qu, C. Genome-wide identification of FRK genes in Populus trichocarpa and their expression under different nitrogen treatments. Physiol. Mol. Biol. Plants 2021, 27, 1919–1931. [Google Scholar] [CrossRef] [PubMed]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Bagchi, A.; Banerjee, A.; Chakrabortty, S. Rindler Physics on the String Worldsheet. Phys. Rev. Lett. 2021, 126, 31601. [Google Scholar] [CrossRef] [PubMed]
- Cantalapiedra, C.P.; Hernández-Plaza, A.; Letunic, I.; Bork, P.; Huerta-Cepas, J. eggNOG-mapper v2: Functional Annotation, Orthology Assignments, and Domain Prediction at the Metagenomic Scale. Mol. Biol. Evol. 2021, 38, 5825–5829. [Google Scholar] [CrossRef]
- Seifert, E. OriginPro 9.1: Scientific data analysis and graphing software—Software review. J. Chem. Inf. Model. 2014, 54, 1552. [Google Scholar] [CrossRef]
- Sahoo, M.R.; Devi, T.R.; Dasgupta, M.; Nongdam, P.; Prakash, N. Reactive oxygen species scavenging mechanisms associated with polyethylene glycol mediated osmotic stress tolerance in Chinese potato. Sci. Rep. 2020, 10, 5404. [Google Scholar] [CrossRef]
- Tang, X.; Ghimire, S.; Liu, W.; Fu, X.; Zhang, H.; Sun, F.; Zhang, N.; Si, H. Genome-wide identification of U-box genes and protein ubiquitination under PEG-induced drought stress in potato. Physiol. Plant. 2022, 174, e13475. [Google Scholar] [CrossRef]





| Gene Name | Gene ID (DM v6.1) | PARP | RST | WWE | Transcript ID (DM v6.1) | Transcript ID (DM v4.03/4.04) |
|---|---|---|---|---|---|---|
| StSRO1 | Soltu.DM.03G028360 | + | + | - | Soltu.DM.03G028360.1 | PGSC0003DMT400063250 |
| Soltu.DM.03G028360.2 | PGSC0003DMT400063249 | |||||
| Soltu.DM.03G028360.3 | PGSC0003DMT400063247 | |||||
| StSRO2 | Soltu.DM.04G031810 | + | + | - | Soltu.DM.04G031810.1 | PGSC0003DMT400012724 |
| StSRO3 | Soltu.DM.05G008850 | + | + | - | Soltu.DM.05G008850.1 | PGSC0003DMT400035039 |
| StSRO4 | Soltu.DM.05G008950 | + | + | - | Soltu.DM.05G008950.1 | PGSC0003DMT400035057 |
| Soltu.DM.05G008950.2 | ||||||
| Soltu.DM.05G008950.3 | PGSC0003DMT400035056 | |||||
| StSRO5 | Soltu.DM.06G018860 | + | + | - | Soltu.DM.06G018860.1 | PGSC0003DMT400043197 |
| Soltu.DM.06G018860.2 | PGSC0003DMT400043196 | |||||
| StSRO6 | Soltu.DM.08G022220 | + | + | + | Soltu.DM.08G022220.1 | PGSC0003DMT400038368 |
| Gene Name | Chr. | Protein Length (AA) | MW (kD) | pI | GRAVY | II | AI | SL |
|---|---|---|---|---|---|---|---|---|
| StSRO1 | 3 | 376 | 41,505.63 | 8.52 | −0.446 | 49.19 | 68.46 | N, C, CP, P |
| StSRO2 | 4 | 510 | 57,088.39 | 5.97 | −0.234 | 38.53 | 87.82 | CP, N, C, V, CY |
| StSRO3 | 5 | 319 | 33,719.99 | 9.16 | −0.291 | 52.78 | 86.49 | C, N, CP, E, CY |
| StSRO4 | 5 | 316 | 35,377.56 | 7.64 | −0.260 | 47.20 | 88.20 | C, N, E, V |
| StSRO5 | 6 | 594 | 67,469.25 | 6.55 | −0.414 | 43.15 | 86.13 | N, C, V |
| StSRO6 | 8 | 589 | 66,542.67 | 6.63 | −0.431 | 46.06 | 81.19 | N, C, CP, V |
| Duplicated Genes | Ka | Ks | Ka/Ks |
|---|---|---|---|
| StSRO5/StSRO6 | 0.26898 | 0.82258 | 0.32699 |
| Class | GO Term | Annotation | StSRO Genes |
|---|---|---|---|
| MF | GO:0003824 | catalytic activity | StSRO1, StSRO2, StSRO3, StSRO4, StSRO5, StSRO6 |
| GO:0005488 | binding | StSRO1, StSRO5, StSRO6 | |
| GO:0016740 | transferase activity | StSRO1, StSRO2, StSRO3 StSRO4, StSRO5, StSRO6 | |
| GO:0005515 | protein binding | StSRO1, StSRO5, StSRO6 | |
| CC | GO:0005634 | nucleus | StSRO1, StSRO2, StSRO3, StSRO4, StSRO5, StSRO6 |
| GO:0110165 | cellular anatomical entity | StSRO1, StSRO2, StSRO3, StSRO4, StSRO5, StSRO6 | |
| GO:0005622 | intracellular anatomical structure | StSRO1, StSRO2, StSRO3, StSRO4, StSRO5, StSRO6 | |
| GO:0005737 | cytoplasm | StSRO1, StSRO5, StSRO6 | |
| GO:0043226 | organelle | StSRO1, StSRO2, StSRO3, StSRO4, StSRO5, StSRO6 | |
| GO:0043227 | membrane-bounded organelle | StSRO1, StSRO2, StSRO3, StSRO4, StSRO5, StSRO6 | |
| GO:0005739 | mitochondrion | StSRO1 | |
| GO:0043229 | intracellular organelle | StSRO1, StSRO2, StSRO3, StSRO4, StSRO5, StSRO6 | |
| GO:0043231 | intracellular membrane-bounded organelle | StSRO1, StSRO2, StSRO3, StSRO4, StSRO5, StSRO6 | |
| BP | GO:0009987 | cellular process | StSRO1, StSRO5, StSRO6 |
| GO:0000003 | reproduction | StSRO2, StSRO5, StSRO6 | |
| GO:0051716 | cellular response to stimulus | StSRO5, StSRO6 | |
| GO:0009605 | response to external stimulus | StSRO5, StSRO6 | |
| GO:0009607 | response to biotic stimulus | StSRO5, StSRO6 | |
| GO:0009416 | response to light stimulus | StSRO2 | |
| GO:0023052 | signaling | StSRO5, StSRO6 | |
| GO:0050896 | response to stimulus | StSRO1, StSRO2, StSRO5, StSRO6 | |
| GO:0008152 | metabolic process | StSRO1, StSRO5, StSRO6 | |
| GO:0048856 | anatomical structure development | StSRO2, StSRO5, StSRO6 | |
| GO:0008219 | cell death | StSRO5, StSRO6 | |
| GO:0009628 | response to abiotic stimulus | StSRO1, StSRO2, StSRO5, StSRO6 | |
| GO:0009058 | biosynthetic process | StSRO5, StSRO6 | |
| GO:0009314 | response to radiation | StSRO2 | |
| GO:0050789 | regulation of biological process | StSRO5, StSRO6 | |
| GO:0006950 | response to stress | StSRO1, StSRO2, StSRO5, StSRO6 | |
| GO:0050794 | regulation of cellular process | StSRO5, StSRO6 | |
| GO:0007275 | multicellular organism development | StSRO2, StSRO5, StSRO6 | |
| GO:0042221 | response to chemical | StSRO2, StSRO5, StSRO6 | |
| GO:0065007 | biological regulation | StSRO5, StSRO6 | |
| GO:0007154 | cell communication | StSRO5, StSRO6 | |
| GO:0032501 | multicellular organismal process | StSRO2, StSRO5, StSRO6 | |
| GO:0032502 | developmental process | StSRO2, StSRO5, StSRO6 | |
| GO:0009719 | response to endogenous stimulus | StSRO5, StSRO6 | |
| GO:0007165 | signal transduction | StSRO5, StSRO6 | |
| GO:0009790 | embryo development | StSRO2, StSRO5, StSRO6 | |
| GO:0009791 | post-embryonic development | StSRO2, StSRO5, StSRO6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Zhou, X.; Liu, Z.; Wu, B. Comprehensive Analysis of StSRO Gene Family and Its Expression in Response to Different Abiotic Stresses in Potato. Int. J. Mol. Sci. 2022, 23, 13518. https://doi.org/10.3390/ijms232113518
Ma Y, Zhou X, Liu Z, Wu B. Comprehensive Analysis of StSRO Gene Family and Its Expression in Response to Different Abiotic Stresses in Potato. International Journal of Molecular Sciences. 2022; 23(21):13518. https://doi.org/10.3390/ijms232113518
Chicago/Turabian StyleMa, Yanming, Xiangyan Zhou, Ziliang Liu, and Bing Wu. 2022. "Comprehensive Analysis of StSRO Gene Family and Its Expression in Response to Different Abiotic Stresses in Potato" International Journal of Molecular Sciences 23, no. 21: 13518. https://doi.org/10.3390/ijms232113518
APA StyleMa, Y., Zhou, X., Liu, Z., & Wu, B. (2022). Comprehensive Analysis of StSRO Gene Family and Its Expression in Response to Different Abiotic Stresses in Potato. International Journal of Molecular Sciences, 23(21), 13518. https://doi.org/10.3390/ijms232113518






