Effects of Indole-3-Lactic Acid, a Metabolite of Tryptophan, on IL-4 and IL-13-Induced Human Skin-Equivalent Atopic Dermatitis Models
Abstract
1. Introduction
2. Results and Discussion
2.1. MTT Assay for Cytotoxicity Test
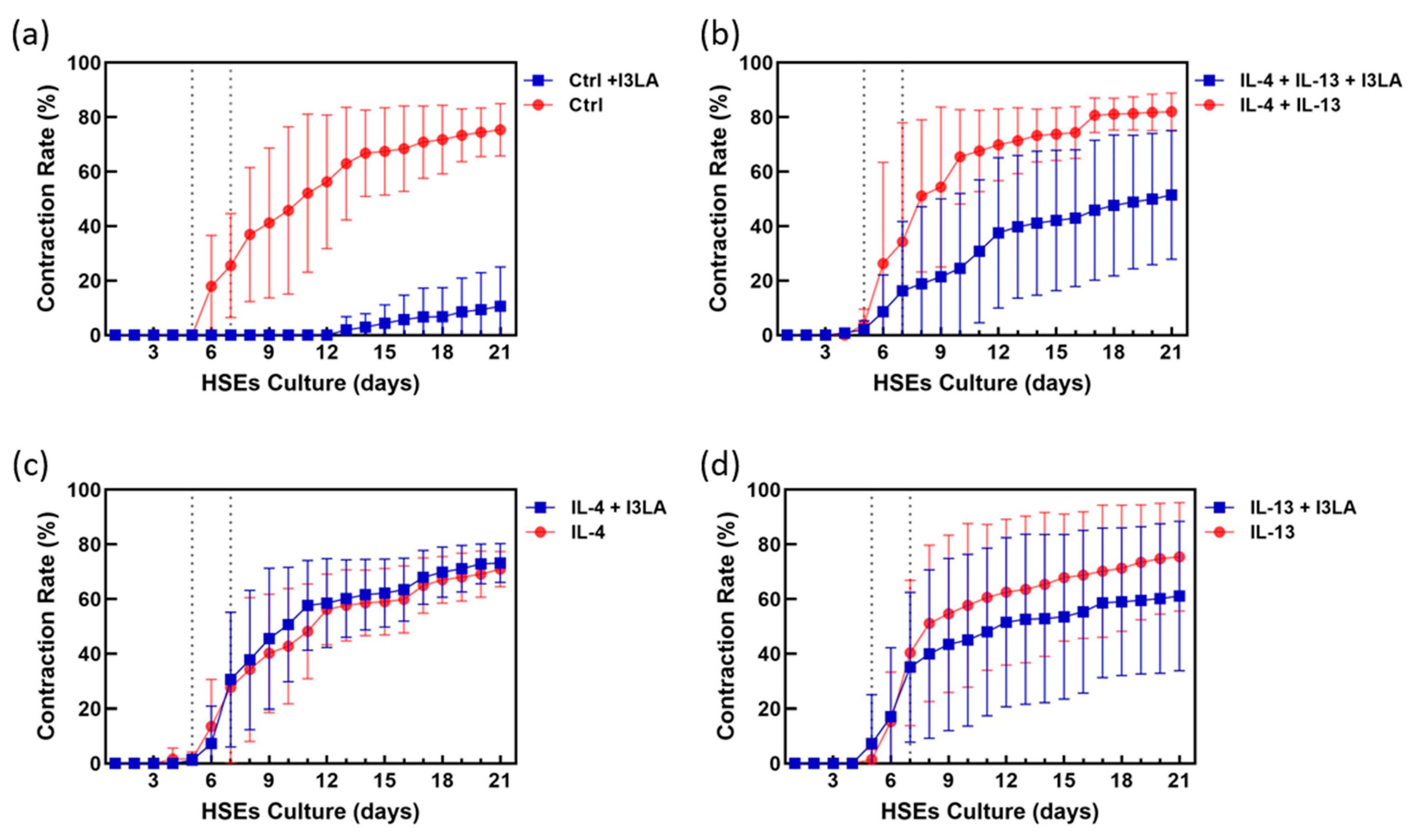
2.2. Inhibitory Effect of Indole-3-Lactic Acid on Contraction
2.3. Inhibitory Effect of Indole-3-Lactic Acid on Contraction
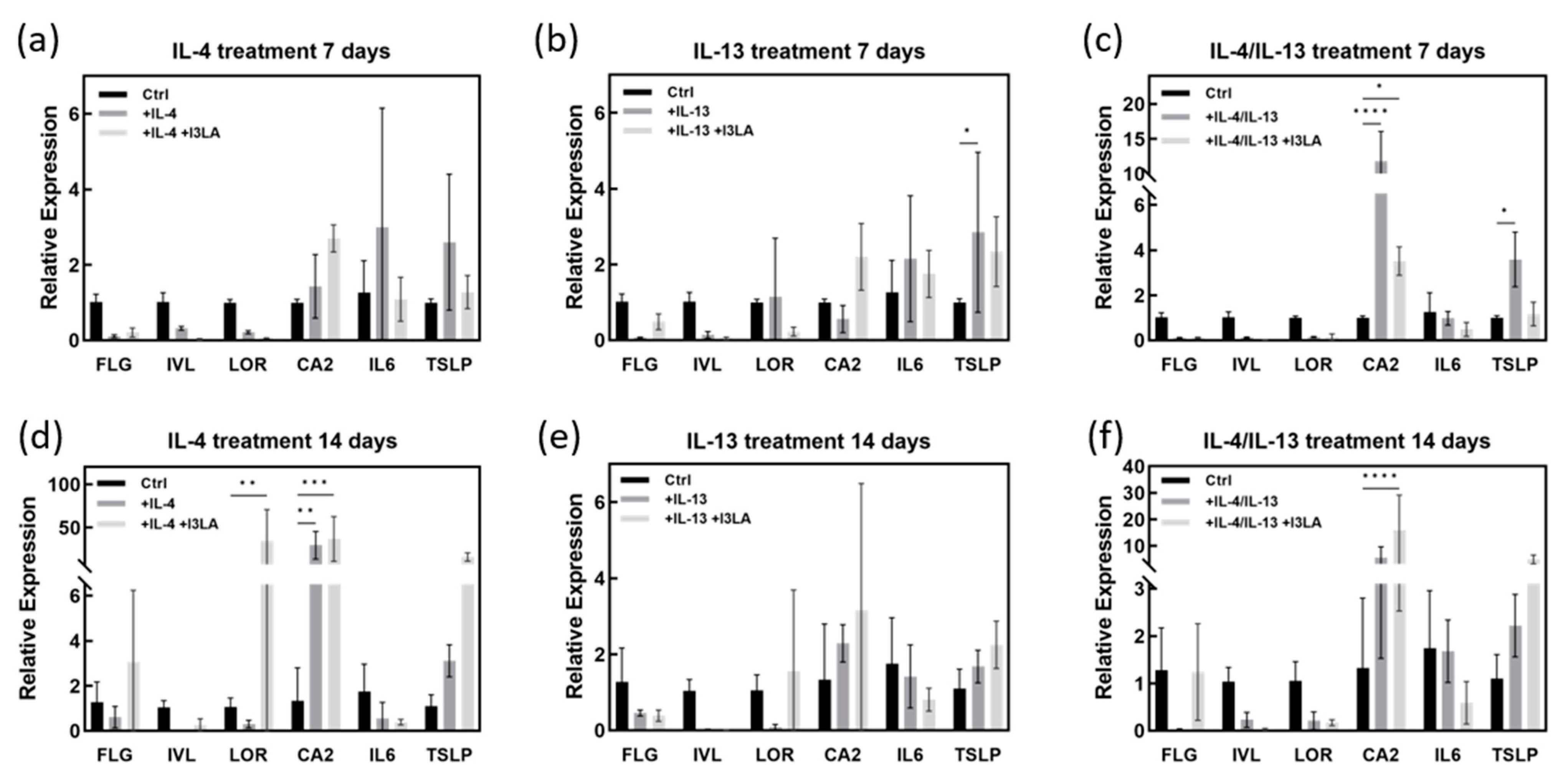
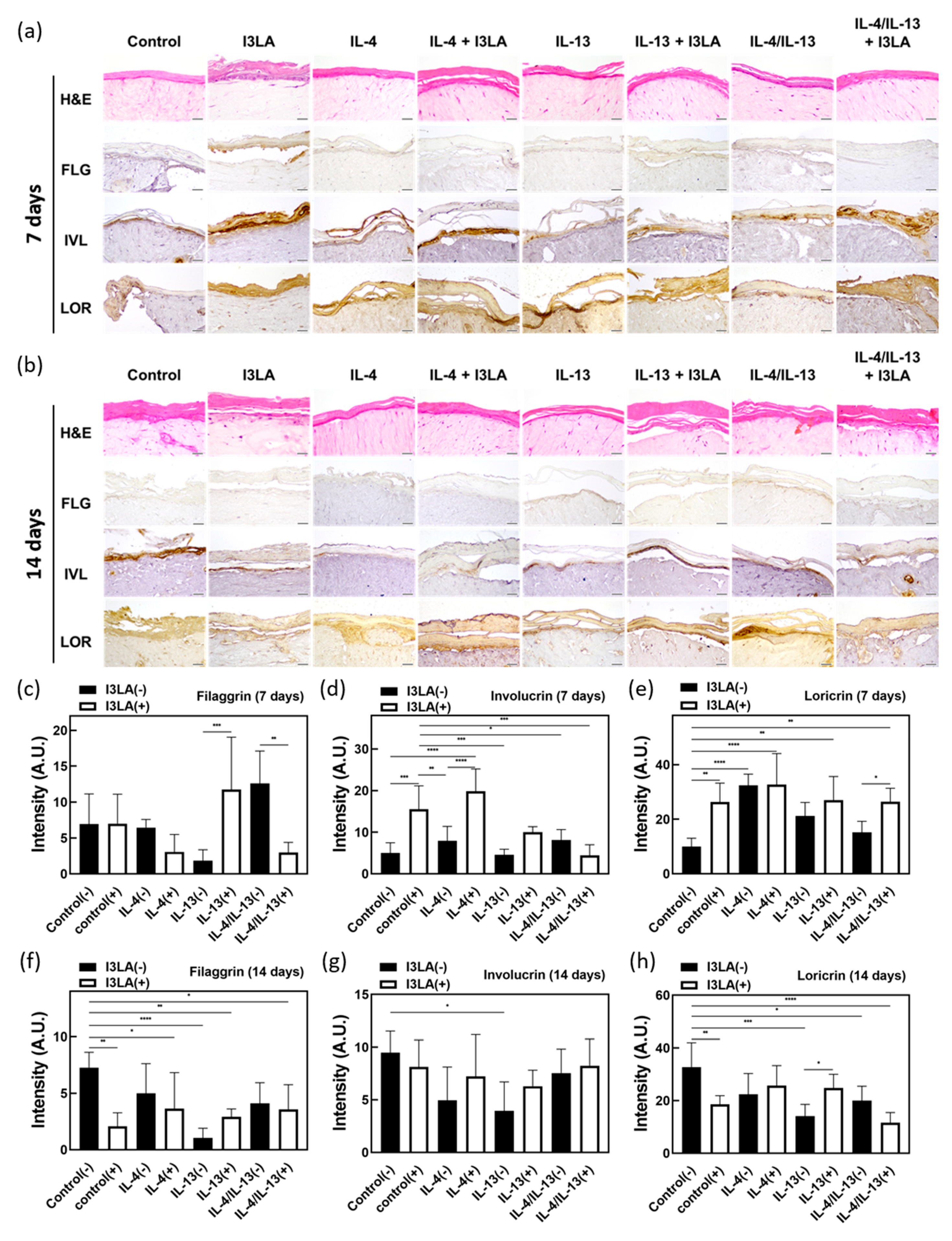
2.4. Skin Barrier Recovery Effect by I3LA
3. Materials and Methods
3.1. Cell Culture
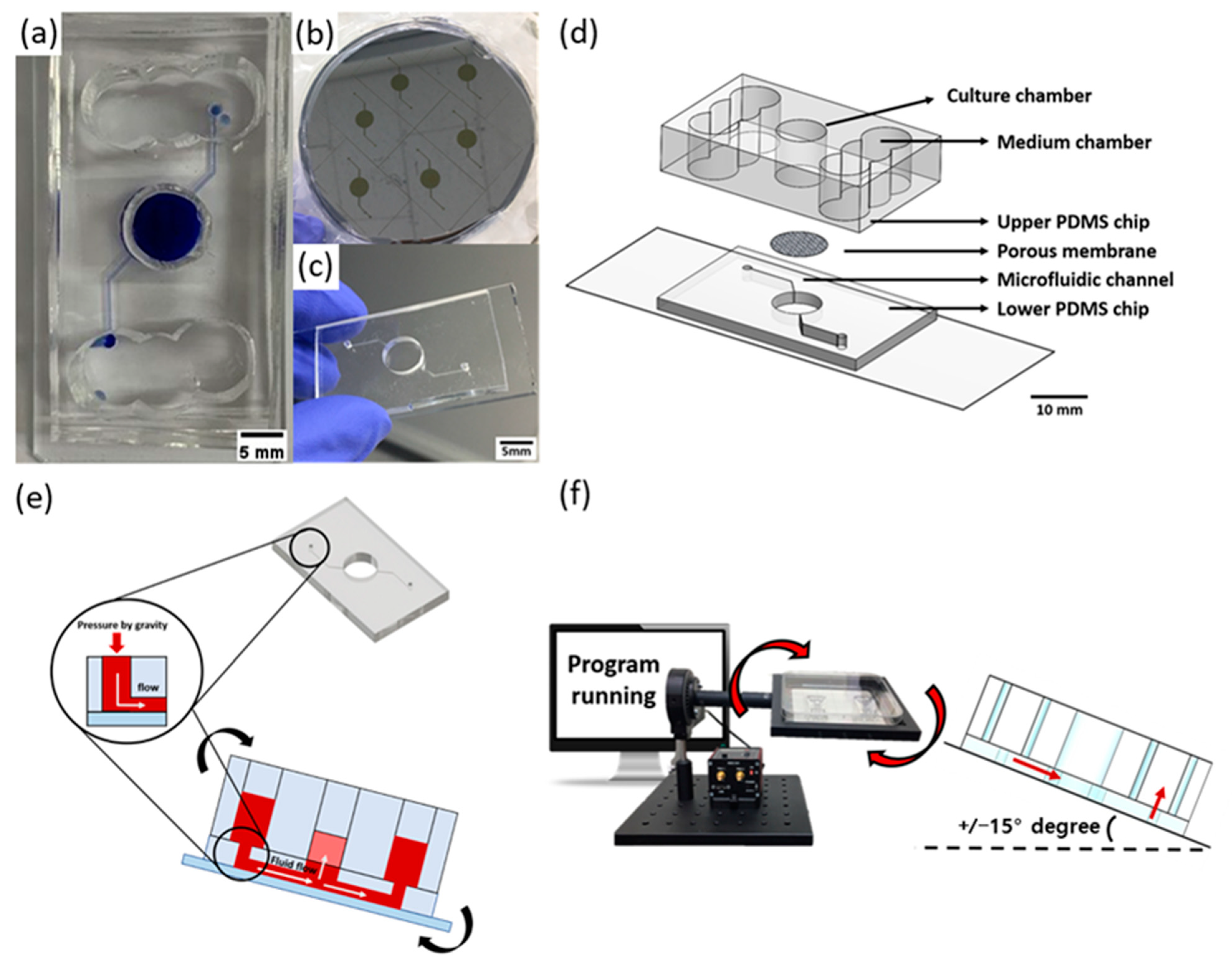
3.2. Surface Functionalization Using Sulfo-SANPAH
3.3. Soft Lithography
3.4. Gravity Flow System for Pumpless Microfluidic Chip
3.5. MTT Assay
3.6. Fabrication of AD-HSEs
3.7. H&E and IHC Staining
3.8. Measurement of HSE Contraction
3.9. Quantitative Real-Time PCR (qPCR)
3.10. Scanning Electron Miscroscopy Images
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Thomsen, S.F. Atopic dermatitis: Natural history, diagnosis, and treatment. Int. Sch. Res. Not. 2014, 2014, 354250. [Google Scholar] [CrossRef] [PubMed]
- De Vuyst, E.; Salmon, M.; Evrard, C.; Lambert de Rouvroit, C.; Poumay, Y. Atopic dermatitis studies through in vitro models. Front. Med. 2017, 4, 119. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.Y.; Soter, N.A. Cellular and immunologic mechanisms in atopic dermatitis. J. Am. Acad. Dermatol. 2001, 44, S1–S12. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, H.; Sung, G.Y. An Interleukin-4 and Interleukin-13 Induced Atopic Dermatitis Human Skin Equivalent Model by a Skin-On-A-Chip. Int. J. Mol. Sci. 2022, 23, 2116. [Google Scholar] [CrossRef] [PubMed]
- Hoffjan, S.; Stemmler, S. On the role of the epidermal differentiation complex in ichthyosis vulgaris, atopic dermatitis and psoriasis. Br. J. Dermatol. 2007, 157, 441–449. [Google Scholar] [CrossRef]
- Furue, M.; Tsuji, G.; Mitoma, C.; Nakahara, T.; Chiba, T.; Morino-Koga, S.; Uchi, H. Gene regulation of filaggrin and other skin barrier proteins via aryl hydrocarbon receptor. J. Dermatol. Sci. 2015, 80, 83–88. [Google Scholar] [CrossRef]
- Howell, M.D.; Kim, B.E.; Gao, P.; Grant, A.V.; Boguniewicz, M.; DeBenedetto, A.; Schneider, L.; Beck, L.A.; Barnes, K.C.; Leung, D.Y. Cytokine modulation of atopic dermatitis filaggrin skin expression. J. Allergy Clin. Immunol. 2009, 124, R7–R12. [Google Scholar] [CrossRef]
- Takei, K.; Mitoma, C.; Hashimoto-Hachiya, A.; Uchi, H.; Takahara, M.; Tsuji, G.; Kido-Nakahara, M.; Nakahara, T.; Furue, M. Antioxidant soybean tar G lyteer rescues T-helper-mediated downregulation of filaggrin expression via aryl hydrocarbon receptor. J. Dermatol. 2015, 42, 171–180. [Google Scholar] [CrossRef]
- Kamsteeg, M.; Bergers, M.; de Boer, R.; Zeeuwen, P.L.; Hato, S.V.; Schalkwijk, J.; Tjabringa, G.S. Type 2 helper T-cell cytokines induce morphologic and molecular characteristics of atopic dermatitis in human skin equivalent. Am. J. Pathol. 2011, 178, 2091–2099. [Google Scholar] [CrossRef]
- Ziegler, S.F.; Artis, D. Sensing the outside world: TSLP regulates barrier immunity. Nat. Immunol. 2010, 11, 289–293. [Google Scholar] [CrossRef]
- Liu, Y.-J. Thymic stromal lymphopoietin: Master switch for allergic inflammation. J. Exp. Med. 2006, 203, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Soumelis, V.; Reche, P.A.; Kanzler, H.; Yuan, W.; Edward, G.; Homey, B.; Gilliet, M.; Ho, S.; Antonenko, S.; Lauerma, A. Human epithelial cells trigger dendritic cell–mediated allergic inflammation by producing TSLP. Nat. Immunol. 2002, 3, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Castillo, J.M.; Hener, P.; Jiang, H.; Li, M. TSLP produced by keratinocytes promotes allergen sensitization through skin and thereby triggers atopic march in mice. J. Investig. Dermatol. 2013, 133, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Hengge, U.R.; Ruzicka, T.; Schwartz, R.A.; Cork, M.J. Adverse effects of topical glucocorticosteroids. J. Am. Acad. Dermatol. 2006, 54, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Coondoo, A.; Phiske, M.; Verma, S.; Lahiri, K. Side-effects of topical steroids: A long overdue revisit. Indian Dermatol. Online J. 2014, 5, 416. [Google Scholar] [CrossRef]
- Fisher, D.A. Adverse effects of topical corticosteroid use. West. J. Med. 1995, 162, 123. [Google Scholar]
- Abraham, A.; Roga, G. Topical steroid-damaged skin. Indian J. Dermatol. 2014, 59, 456. [Google Scholar] [CrossRef]
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef]
- Levy, M.; Blacher, E.; Elinav, E. Microbiome, metabolites and host immunity. Curr. Opin. Microbiol. 2017, 35, 8–15. [Google Scholar] [CrossRef]
- Shi, B.; Bangayan, N.J.; Curd, E.; Taylor, P.A.; Gallo, R.L.; Leung, D.Y.; Li, H. The skin microbiome is different in pediatric versus adult atopic dermatitis. J. Allergy Clin. Immunol. 2016, 138, 1233–1236. [Google Scholar] [CrossRef]
- Kennedy, E.A.; Connolly, J.; Hourihane, J.O.B.; Fallon, P.G.; McLean, W.I.; Murray, D.; Jo, J.-H.; Segre, J.A.; Kong, H.H.; Irvine, A.D. Skin microbiome before development of atopic dermatitis: Early colonization with commensal staphylococci at 2 months is associated with a lower risk of atopic dermatitis at 1 year. J. Allergy Clin. Immunol. 2017, 139, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.J.; Marsland, B.J.; Bunyavanich, S.; O’Mahony, L.; Leung, D.Y.; Muraro, A.; Fleisher, T.A. The microbiome in allergic disease: Current understanding and future opportunities—2017 PRACTALL document of the American Academy of Allergy, Asthma & Immunology and the European Academy of Allergy and Clinical Immunology. J. Allergy Clin. Immunol. 2017, 139, 1099–1110. [Google Scholar] [PubMed]
- Chng, K.R.; Tay, A.S.L.; Li, C.; Ng, A.H.Q.; Wang, J.; Suri, B.K.; Matta, S.A.; McGovern, N.; Janela, B.; Wong, X.F.C.C. Whole metagenome profiling reveals skin microbiome-dependent susceptibility to atopic dermatitis flare. Nat. Microbiol. 2016, 1, 16106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.S.; Davies, S.S. Microbial metabolism of dietary components to bioactive metabolites: Opportunities for new therapeutic interventions. Genome Med. 2016, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, W. Indole inhibits bacterial quorum sensing signal transmission by interfering with quorum sensing regulator folding. Microbiology 2013, 159 Pt 12, 2616–2625. [Google Scholar] [CrossRef]
- Zhen, J.; Zhao, P.; Li, Y.; Cai, Y.; Yu, W.; Wang, W.; Zhao, L.; Wang, H.; Huang, G.; Xu, A. The Multiomics Analyses of Gut Microbiota, Urine Metabolome and Plasma Proteome Revealed Significant Changes in Allergy Featured with Indole Derivatives of Tryptophan. J. Asthma Allergy 2022, 15, 117. [Google Scholar] [CrossRef]
- Scott, S.A.; Fu, J.; Chang, P.V. Microbial tryptophan metabolites regulate gut barrier function via the aryl hydrocarbon receptor. Proc. Natl. Acad. Sci. USA 2020, 117, 19376–19387. [Google Scholar] [CrossRef]
- Park, S.L.; Justiniano, R.; Williams, J.D.; Cabello, C.M.; Qiao, S.; Wondrak, G.T. The tryptophan-derived endogenous aryl hydrocarbon receptor ligand 6-formylindolo [3, 2-b] carbazole is a nanomolar UVA photosensitizer in epidermal keratinocytes. J. Investig. Dermatol. 2015, 135, 1649–1658. [Google Scholar] [CrossRef]
- Yu, J.; Luo, Y.; Zhu, Z.; Zhou, Y.; Sun, L.; Gao, J.; Sun, J.; Wang, G.; Yao, X.; Li, W. A tryptophan metabolite of the skin microbiota attenuates inflammation in patients with atopic dermatitis through the aryl hydrocarbon receptor. J. Allergy Clin. Immunol. 2019, 143, 2108–2119.e12. [Google Scholar] [CrossRef]
- Bao, L.; Zhang, H.; Chan, L.S. The involvement of the JAK-STAT signaling pathway in chronic inflammatory skin disease atopic dermatitis. Jak-Stat 2013, 2, e24137. [Google Scholar] [CrossRef]
- Huang, W.; Cho, K.Y.; Meng, D.; Walker, W.A. The impact of indole-3-lactic acid on immature intestinal innate immunity and development: A transcriptomic analysis. Sci. Rep. 2021, 11, 8088. [Google Scholar] [CrossRef] [PubMed]
- Song, H.J.; Lim, H.Y.; Chun, W.; Choi, K.C.; Lee, T.-Y.; Sung, J.H.; Sung, G.Y. Development of 3D skin-equivalent in a pump-less microfluidic chip. J. Ind. Eng. Chem. 2018, 60, 355–359. [Google Scholar] [CrossRef]
- Jeon, H.M.; Kim, K.; Choi, K.C.; Sung, G.Y. Side-effect test of sorafenib using 3-D skin equivalent based on microfluidic skin-on-a-chip. J. Ind. Eng. Chem. 2020, 82, 71–80. [Google Scholar] [CrossRef]
- Jeong, S.; Kim, J.; Jeon, H.M.; Kim, K.; Sung, G.Y. Development of an Aged Full-Thickness Skin Model Using Flexible Skin-on-a-Chip Subjected to Mechanical Stimulus Reflecting the Circadian Rhythm. Int. J. Mol. Sci. 2021, 22, 12788. [Google Scholar] [CrossRef]
- OECD. Test No. 439: In Vitro Skin Irritation—Reconstructed Human Epidermis Test Method; OECD Publishing: Paris, Franch, 2013. [Google Scholar]
- Hänel, K.H.; Cornelissen, C.; Lüscher, B.; Baron, J.M. Cytokines and the skin barrier. Int. J. Mol. Sci. 2013, 14, 6720–6745. [Google Scholar] [CrossRef]
- Kim, B.E.; Leung, D.Y.; Boguniewicz, M.; Howell, M.D. Loricrin and involucrin expression is down-regulated by Th2 cytokines through STAT-6. Clin. Immunol. 2008, 126, 332–337. [Google Scholar] [CrossRef]
- van den Bogaard, E.H.; Bergboer, J.G.; Vonk-Bergers, M.; van Vlijmen-Willems, I.M.; Hato, S.V.; van der Valk, P.G.; Schröder, J.M.; Joosten, I.; Zeeuwen, P.L.; Schalkwijk, J. Coal tar induces AHR-dependent skin barrier repair in atopic dermatitis. J. Clin. Investig. 2013, 123, 917–927. [Google Scholar] [CrossRef]
- Pellerin, L.; Henry, J.; Hsu, C.-Y.; Balica, S.; Jean-Decoster, C.; Méchin, M.-C.; Hansmann, B.; Rodriguez, E.; Weindinger, S.; Schmitt, A.-M. Defects of filaggrin-like proteins in both lesional and nonlesional atopic skin. J. Allergy Clin. Immunol. 2013, 131, 1094–1102. [Google Scholar] [CrossRef]
- Mackenzie, I. Ordered structure of the epidermis. J. Investig. Dermatol. 1975, 65, 45–51. [Google Scholar] [CrossRef]
- Ali, S.M.; Yosipovitch, G. Skin pH: From basic science to basic skin care. Acta Derm. -Venereol. 2013, 93, 261–269. [Google Scholar] [CrossRef]
- Elias, P.M. Lipid abnormalities and lipid-based repair strategies in atopic dermatitis. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2014, 1841, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Jeon, H.M.; Choi, K.C.; Sung, G.Y. Testing the effectiveness of curcuma longa leaf extract on a skin equivalent using a pumpless skin-on-a-chip model. Int. J. Mol. Sci. 2020, 21, 3898. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, J.; Kim, H.; Sung, G.Y. Effect of α-Lipoic Acid on the Development of Human Skin Equivalents Using a Pumpless Skin-on-a-Chip Model. Int. J. Mol. Sci. 2021, 22, 2160. [Google Scholar] [CrossRef] [PubMed]


| Treated Cytokine Conditions | (−) I3LA | (+) I3LA |
|---|---|---|
| Control * | 0.00 | −71.27 |
| IL-4 | −10.88 | −8.64 |
| IL-13 | −6.38 | −20.75 |
| IL-4 & IL-13 | 0.14 | −30.37 |
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| GAPDH | 5′-CTCCTCTGACTTCAACAGCG-3′ | 5′-GCCAAATTCGTTGTCATACCAG-3′ |
| FLG | 5′-GGAGTCACGTGGCAGTCCTCACA-3′ | 5′-GGTGTCTAAACCCGGATTCACC-3′ |
| IVL | 5′-CCGCAAATGAAACAGCCAACTCC-3′ | 5′-GGATTCCTCATGCTGTTCCCAG-3′ |
| LOR | 5′-GTCTGCGGAGGTGGTTCCTCT-3′ | 5′-TGCTGGGTCTGGTGGCAGATC-3′ |
| CA2 | 5′-AACAATGGTCATGCTTTCAACG-3′ | 5′-TGTCCATCAAGTGAACCCCAG-3′ |
| IL-6 | 5′-AGACAGCCACTCACCTCTTCAG-3′ | 5′-TTCTGCCAGTGCCTCTTTGCTG-3′ |
| TSLP | 5′-AGTGGGACCAAAAGTACCGAGTT-3′ | 5′-GGATTGAAGGTTAGGCTCTGG-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.; Kim, H.; Sung, G.Y. Effects of Indole-3-Lactic Acid, a Metabolite of Tryptophan, on IL-4 and IL-13-Induced Human Skin-Equivalent Atopic Dermatitis Models. Int. J. Mol. Sci. 2022, 23, 13520. https://doi.org/10.3390/ijms232113520
Kim K, Kim H, Sung GY. Effects of Indole-3-Lactic Acid, a Metabolite of Tryptophan, on IL-4 and IL-13-Induced Human Skin-Equivalent Atopic Dermatitis Models. International Journal of Molecular Sciences. 2022; 23(21):13520. https://doi.org/10.3390/ijms232113520
Chicago/Turabian StyleKim, Kyunghee, Hyeju Kim, and Gun Yong Sung. 2022. "Effects of Indole-3-Lactic Acid, a Metabolite of Tryptophan, on IL-4 and IL-13-Induced Human Skin-Equivalent Atopic Dermatitis Models" International Journal of Molecular Sciences 23, no. 21: 13520. https://doi.org/10.3390/ijms232113520
APA StyleKim, K., Kim, H., & Sung, G. Y. (2022). Effects of Indole-3-Lactic Acid, a Metabolite of Tryptophan, on IL-4 and IL-13-Induced Human Skin-Equivalent Atopic Dermatitis Models. International Journal of Molecular Sciences, 23(21), 13520. https://doi.org/10.3390/ijms232113520







