Reaction of Corroles with Sarcosine and Paraformaldehyde: A New Facet of Corrole Chemistry
Abstract
1. Introduction
2. Results and Discussion
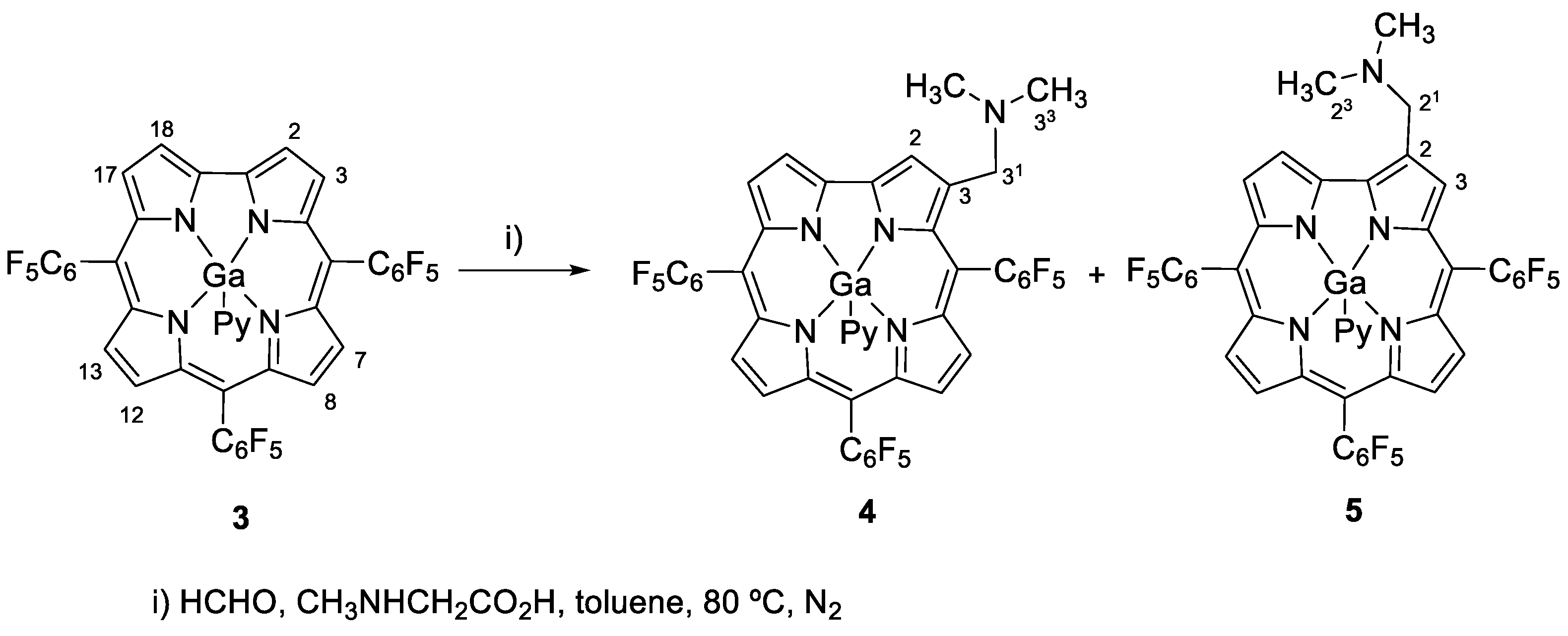
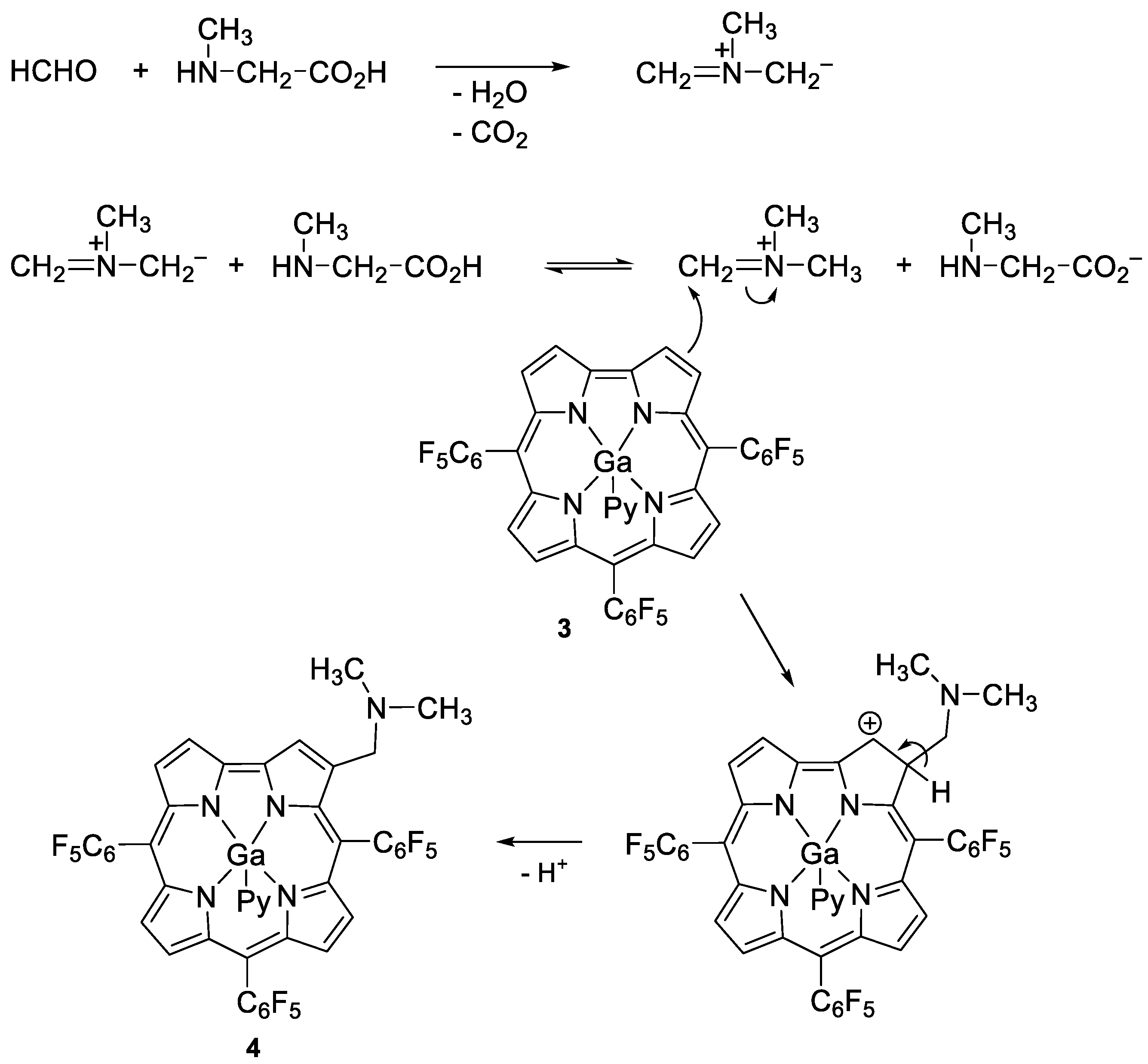
2.1. Reactions Involving 5,10,15-Tris(Pentafluorophenyl)Corrolatogallium(III)
2.2. Reactions Involving 5,10,15-Tris(Pentafluorophenyl)Corrole
3. Materials and Methods
3.1. Materials
3.2. Techniques
3.3. Theoretical Calculations for Model Structures
3.4. Experimental Procedure
3.4.1. General Procedure for the Synthesis of Compounds 4 and 5
3.4.2. General Experimental Procedure for Compound 9
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barata, J.F.B.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Tomé, A.C.; Cavaleiro, J.A.S. Strategies for Corrole Functionalization. Chem. Rev. 2017, 117, 3192–3253. [Google Scholar] [CrossRef] [PubMed]
- Barata, J.F.B.; Santos, C.I.M.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Cavaleiro, J.A.S. Functionalization of Corroles. In Synthesis and Modifications of Porphyrinoids. Topics in Heterocyclic Chemistry; Paolesse, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; Volume 33, pp. 79–141. [Google Scholar] [CrossRef]
- Kumar, A.; Yadav, P.; Majdoub, M.; Saltsman, I.; Fridman, N.; Kumar, S.; Kumar, A.; Mahammed, A.; Gross, Z. Corroles: The Hitherto Elusive Parent Macrocycle and its Metal Complexes. Angew. Chem. Int. Ed. 2021, 60, 25097–25103. [Google Scholar] [CrossRef]
- Mahammed, A.; Gross, Z. Corroles as triplet photosensitizers. Coord. Chem. Rev. 2019, 379, 121–132. [Google Scholar] [CrossRef]
- Teo, R.D.; Hwang, J.Y.; Termini, J.; Gross, Z.; Gray, H.B. Fighting Cancer with Corroles. Chem. Rev. 2017, 117, 2711–2729. [Google Scholar] [CrossRef] [PubMed]
- Di Natale, C.; Gros, C.P.; Paolesse, R. Corroles at work: A small macrocycle for great applications. Chem. Soc. Rev. 2022, 51, 1277–1335. [Google Scholar] [CrossRef] [PubMed]
- Barata, J.F.B.; Zamarrón, A.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Tomé, A.C.; Cavaleiro, J.A.S.; Röder, B.; Juarranz, Á.; Sanz-Rodríguez, F. Photodynamic effects induced by meso-tris(pentafluorophenyl)corrole and its cyclodextrin conjugates on cytoskeletal components of HeLa cells. Eur. J. Med. Chem. 2015, 92, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.I.M.; Rodríguez-Pérez, L.; Gonçalves, G.; Pinto, S.N.; Melle-Franco, M.; Marques, P.A.A.P.; Faustino, M.A.F.; Herranz, M.Á.; Martin, N.; Neves, M.G.P.M.S.; et al. Novel hybrids based on graphene quantum dots covalently linked to glycol corroles for multiphoton bioimaging. Carbon N. Y. 2020, 166, 164–174. [Google Scholar] [CrossRef]
- Araújo, A.R.L.; Tomé, A.C.; Santos, C.I.M.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Simões, M.M.Q.; Moura, N.M.M.; Abu-Orabi, S.T.; Cavaleiro, J.A.S. Azides and Porphyrinoids: Synthetic Approaches and Applications. Part 1—Azides, Porphyrins and Corroles. Molecules 2020, 25, 1662. [Google Scholar] [CrossRef]
- Alberto, M.E.; De Simone, B.C.; Liuzzi, S.; Marino, T.; Russo, N.; Toscano, M. Iodine substituted phosphorus corrole complexes as possible photosensitizers in photodynamic therapy: Insights from theory. J. Comput. Chem. 2020, 41, 1395–1401. [Google Scholar] [CrossRef]
- Thomassen, I.K.; McCormick-McPherson, L.J.; Borisov, S.M.; Ghosh, A. Iridium Corroles Exhibit Weak Near-Infrared Phosphorescence but Efficiently Sensitize Singlet Oxygen Formation. Sci. Rep. 2020, 10, 7551. [Google Scholar] [CrossRef]
- Lacerda, P.S.S.; Bartolomeu, M.; Gomes, A.T.P.C.; Duarte, A.S.; Almeida, A.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Barata, J.F.B. Can Corrole Dimers Be Good Photosensitizers to Kill Bacteria? Microorganisms 2022, 10, 1167. [Google Scholar] [CrossRef] [PubMed]
- Preuß, A.; Saltsman, I.; Mahammed, A.; Pfitzner, M.; Goldberg, I.; Gross, Z.; Röder, B. Photodynamic inactivation of mold fungi spores by newly developed charged corroles. J. Photochem. Photobiol. B Biol. 2014, 133, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Cardote, T.A.F.; Barata, J.F.B.; Amador, C.; Alves, E.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S.; Cunha, Â.; Almeida, A.; Faustino, M.A.F. Evaluation of meso-substituted cationic corroles as potential antibacterial agents. An. Acad. Bras. Ciênc. 2018, 90, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.-G.; Sun, Y.-M.; Liu, Z.-Y.; Liao, Y.-H.; Zeng, L.; Ye, Y.; Liu, H.-Y. Halogenated Gallium Corroles:DNA Interaction and Photodynamic Antitumor Activity. Inorg. Chem. 2021, 60, 2234–2245. [Google Scholar] [CrossRef]
- Sun, Y.-M.; Jiang, X.; Liu, Z.-Y.; Liu, L.-G.; Liao, Y.-H.; Zeng, L.; Ye, Y.; Liu, H.-Y. Hydroxy-corrole and its gallium(III) complex as new photosensitizer for photodynamic therapy against breast carcinoma. Eur. J. Med. Chem. 2020, 208, 112794. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, H.-J.; Huang, H.; Wan, B.; Wu, S.; Liu, H.-Y.; Zhang, H.-T. The photocytotoxicity effect of cationic sulfonated corrole towards lung cancer cells: In vitro and in vivo study. Lasers Med. Sci. 2019, 34, 1353–1363. [Google Scholar] [CrossRef]
- Einrem, R.F.; Alemayehu, A.B.; Borisov, S.M.; Ghosh, A.; Gederaas, O.A. Amphiphilic Rhenium-Oxo Corroles as a New Class of Sensitizers for Photodynamic Therapy. ACS Omega 2020, 5, 10596–10601. [Google Scholar] [CrossRef]
- Sun, Y.-M.; Akram, W.; Cheng, F.; Liu, Z.-Y.; Liao, Y.-H.; Ye, Y.; Liu, H.-Y. DNA interaction and photodynamic antitumor activity of transition metal mono-hydroxyl corrole. Bioorg. Chem. 2019, 90, 103085. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, H.-J.; Huang, H.; Wang, H.-H.; Wu, S.; Liu, H.-Y.; Zhang, H.-T. The photodynamic activity and toxicity evaluation of 5,10,15-tris(ethoxylcarbonyl)corrole phosphorus(V) in vivo and in vitro. Eur. J. Med. Chem. 2019, 163, 779–786. [Google Scholar] [CrossRef]
- Wang, J.-M.; Li, Y.; Yuan, H.-Q.; Wu, D.-H.; Ying, X.; Shi, L.; Zhang, H.-T.; Liu, H.-Y. Methyl Benzoate Gallium(III) corrole complexes: DNA-binding, Photocleavage Activity, Cytotoxicity on Tumor Cells. Appl. Organomet. Chem. 2017, 31, e3571. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H.-H.; Yu, H.-J.; Xiong, Y.-Z.; Zhang, H.-T.; Ji, L.-N.; Liu, H.-Y. Synthesis, characterization and in vitro and in vivo photodynamic activities of a gallium(III) tris(ethoxycarbonyl)corrole. Dalton Trans. 2017, 46, 9481–9490. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Huang, L.; Wang, H.; Liu, Y.; Kandhadi, J.; Wang, H.; Ji, L.; Liu, H. Photodynamic Therapy Activities of 10-(4-Formylphenyl)-5,15-bis(pentafluorophenyl)corrole and Its Gallium Complex. Chin. J. Chem. 2017, 35, 86–92. [Google Scholar] [CrossRef]
- Alemayehu, A.B.; Day, N.U.; Mani, T.; Rudine, A.B.; Thomas, K.E.; Gederaas, O.A.; Vinogradov, S.A.; Wamser, C.C.; Ghosh, A. Gold Tris(carboxyphenyl)corroles as Multifunctional Materials: Room Temperature Near-IR Phosphorescence and Applications to Photodynamic Therapy and Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2016, 8, 18935–18942. [Google Scholar] [CrossRef] [PubMed]
- Jaworska, E.; Caroleo, F.; Di Natale, C.; Maksymiuk, K.; Paolesse, R.; Michalska, A. Si-corrole-based fluoride fluorometric turn-on sensor. J. Porphyr. Phthalocyanines 2020, 24, 929–937. [Google Scholar] [CrossRef]
- Cai, F.; Xia, F.; Guo, Y.; Zhu, W.; Fu, B.; Liang, X.; Wang, S.; Cai, Z.; Xu, H. “Off–on–off” type of selectively pH-sensing 8-hydroxyquinoline-substituted galliumIII) corrole. New J. Chem. 2019, 43, 18012–18017. [Google Scholar] [CrossRef]
- Paolesse, R.; Nardis, S.; Monti, D.; Stefanelli, M.; Di Natale, C. Porphyrinoids for Chemical Sensor Applications. Chem. Rev. 2017, 117, 2517–2583. [Google Scholar] [CrossRef]
- Fischer, S.; Vestfrid, J.; Mahammed, A.; Herrmann-Westendorf, F.; Schulz, M.; Müller, J.; Kiesewetter, O.; Dietzek, B.; Gross, Z.; Presselt, M. Photometric Detection of Nitric Oxide Using a Dissolved Iron(III) Corrole as a Sensitizer. ChemPlusChem 2016, 81, 594–603. [Google Scholar] [CrossRef]
- Santos, C.I.M.; Oliveira, E.; Barata, J.F.B.; Faustino, M.A.F.; Cavaleiro, J.A.S.; Neves, M.G.P.M.S.; Lodeiro, C. New gallium(III) corrole complexes as colorimetric probes for toxic cyanide anion. Inorganica Chim. Acta 2014, 417, 148–154. [Google Scholar] [CrossRef]
- Santos, C.I.M.; Oliveira, E.; Barata, J.F.B.; Faustino, M.A.F.; Cavaleiro, J.A.S.; Neves, M.G.P.M.S.; Lodeiro, C. Corroles as anion chemosensors: Exploiting their fluorescence behaviour from solution to solid-supported devices. J. Mater. Chem. 2012, 22, 13811–13819. [Google Scholar] [CrossRef]
- Berna, B.B.; Platzer, B.; Wolf, M.; Lavarda, G.; Nardis, S.; Galloni, P.; Torres, T.; Guldi, D.M.; Paolesse, R. Panchromatic Light Harvesting and Stabilizing Charge-Separated States in Corrole–Phthalocyanine Conjugates through Coordinating a Subphthalocyanine. Chem. Eur. J. 2020, 26, 13451–13461. [Google Scholar] [CrossRef]
- Mahammed, A.; Chen, K.; Vestfrid, J.; Zhao, J.; Gross, Z. Phosphorus corrole complexes: From property tuning to applications in photocatalysis and triplet–triplet annihilation upconversion. Chem. Sci. 2019, 10, 7091–7103. [Google Scholar] [CrossRef] [PubMed]
- Golf, H.R.A.; Oltmanns, A.M.; Trieu, D.H.; Reissig, H.-U.; Wiehe, A. Synthesis of Functionalized BODIPYs, BODIPY-Corrole, and BODIPY-Porphyrin Arrays with 1,2,3-Triazole Linkers Using the 4-Azido(tetrafluorophenyl)-BODIPY Building Block. Eur. J. Org. Chem. 2015, 2015, 4224–4237. [Google Scholar] [CrossRef]
- Dedić, D.; Dorniak, A.; Rinner, U.; Schöfberger, W. Recent Progress in (Photo-)-Electrochemical Conversion of CO2 With Metal Porphyrinoid-Systems. Front. Chem. 2021, 9, 685619. [Google Scholar] [CrossRef] [PubMed]
- Timelthaler, D.; Schöfberger, W.; Topf, C. Selective and Additive-Free Hydrogenation of Nitroarenes Mediated by a DMSO-Tagged Molecular Cobalt Corrole Catalyst. Eur. J. Org. Chem. 2021, 2021, 2114–2120. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, W.; Zhu, W.; Liang, X. meso -Expanded Co(III)corroles through Suzuki-Miyaura couplings: Synthesis and tunable electrocatalytic hydrogen evolutions and oxygen reductions. J. Porphyr. Phthalocyanines 2021, 25, 273–281. [Google Scholar] [CrossRef]
- Fang, J.-J.; Lan, J.; Yang, G.; Yuan, G.-Q.; Liu, H.-Y.; Si, L.-P. Synthesis of cobalt A2B triaryl corroles bearing aldehyde and amide pyridyl groups and their performance in electrocatalytic hydrogen evolution. New J. Chem. 2021, 45, 5127–5136. [Google Scholar] [CrossRef]
- Rana, A.; Lee, Y.-M.; Li, X.; Cao, R.; Fukuzumi, S.; Nam, W. Highly Efficient Catalytic Two-Electron Two-Proton Reduction of Dioxygen to Hydrogen Peroxide with a Cobalt Corrole Complex. ACS Catal. 2021, 11, 3073–3083. [Google Scholar] [CrossRef]
- Reddy, S.N.; Krishnamurthy, C.B.; Grinberg, I. First-Principles Study of the Ligand Substituent Effect on ORR Catalysis by Metallocorroles. J. Phys. Chem. C 2020, 124, 11275–11283. [Google Scholar] [CrossRef]
- Sinha, W.; Mahammed, A.; Fridman, N.; Gross, Z. Water Oxidation Catalysis by Mono- and Binuclear Iron Corroles. ACS Catal. 2020, 10, 3764–3772. [Google Scholar] [CrossRef]
- Chen, Y.; Fan, Q.-H.; Hossain, M.S.; Zhan, S.-Z.; Liu, H.-Y.; Si, L.-P. Electrocatalytic Hydrogen Evolution of Cobalt and Free-base Triaryl Corrole Bearing Hydroxyethyl Amino Groups. Eur. J. Inorg. Chem. 2020, 2020, 491–498. [Google Scholar] [CrossRef]
- Zhang, W.; Lai, W.; Cao, R. Energy-Related Small Molecule Activation Reactions: Oxygen Reduction and Hydrogen and Oxygen Evolution Reactions Catalyzed by Porphyrin- and Corrole-Based Systems. Chem. Rev. 2017, 117, 3717–3797. [Google Scholar] [CrossRef] [PubMed]
- Nardis, S.; Mandoj, F.; Stefanelli, M.; Paolesse, R. Metal complexes of corrole. Coord. Chem. Rev. 2019, 388, 360–405. [Google Scholar] [CrossRef]
- Sample, H.C.; Senge, M.O. Nucleophilic Aromatic Substitution (SNAr) and Related Reactions of Porphyrinoids: Mechanistic and Regiochemical Aspects. Eur. J. Org. Chem. 2021, 2021, 7–42. [Google Scholar] [CrossRef] [PubMed]
- Ooi, S.; Ueta, K.; Tanaka, T.; Osuka, A. Singly, Doubly, and Triply Linked Corrole Oligomers: Synthesis, Structures, and Linking Position Dependent Properties. ChemPlusChem 2019, 84, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Ueta, K.; Osuka, A. Development of the Peripheral Functionalization Chemistry of meso -Free Corroles. Chem. Eur. J. 2021, 27, 15605–15615. [Google Scholar] [CrossRef]
- Cavaleiro, J.A.S.; Tomé, A.C.; Neves, M.G.P.M.S. meso -Tetraarylporphyrin Derivatives: New Synthetic Methodologies. In Handbook of Porphyrin Science; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; World Scientific: Singapore, 2010; Volume 2, Chapter 9; pp. 193–294. [Google Scholar]
- Silva, A.M.G.; de Oliveira, K.T.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Tomé, A.C.; Silva, A.M.S.; Cavaleiro, J.A.S.; Brandão, P.; Felix, V. Chemical Transformations of Mono- and Bis(buta-1,3-dien-1-yl)porphyrins: A New Synthetic Approach to Mono- and Dibenzoporphyrins. Eur. J. Org. Chem. 2008, 2008, 704–712. [Google Scholar] [CrossRef]
- Moura, N.M.M.; Monteiro, C.J.P.; Tomé, A.C.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S. Synthesis of chlorins and bacteriochlorins from cycloaddition reactions with porphyrins. Arkivoc 2022, 2022, 54–98. [Google Scholar] [CrossRef]
- Barata, J.F.B.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S. Recent advances in the functionalization of meso -triarylcorroles via cycloaddition reactions. J. Porphyr. Phthalocyanines 2009, 13, 415–418. [Google Scholar] [CrossRef]
- Vale, L.S.H.P.; Barata, J.F.B.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Tomé, A.C.; Silva, A.M.S.; Paz, F.A.A.; Cavaleiro, J.A.S. Novel quinone-fused corroles. Tetrahedron Lett. 2007, 48, 8904–8908. [Google Scholar] [CrossRef]
- Vale, L.S.H.P.; Barata, J.F.B.; Santos, C.I.M.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Tomé, A.C.; Silva, A.M.S.; Paz, F.A.A.; Cavaleiro, J.A.S. Corroles in 1,3-dipolar cycloaddition reactions. J. Porphyr. Phthalocyanines 2009, 13, 358–368. [Google Scholar] [CrossRef]
- Hata, H.; Kamimura, Y.; Shinokubo, H.; Osuka, A. Synthesis of Pyrrolidine-Fused [34]- and [36] Octaphyrins via 1,3-Dipolar Cycloaddition. Org. Lett. 2006, 8, 1169–1172. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Nakamura, Y.; Aratani, N.; Osuka, A. Regioselective [3+4] cycloaddition of an azomethine ylide to meso-meso, β–β, β′–β′ triply linked diporphyrins. Tetrahedron Lett. 2008, 49, 3308–3311. [Google Scholar] [CrossRef]
- Ramos, C.I.V.; Santana-Marques, M.G.; Ferrer-Correia, A.J.; Barata, J.F.B.; Tomé, A.C.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S.; Abreu, P.E.; Pereira, M.M.; Pais, A.A.C.C. Differentiation of aminomethyl corrole isomers by mass spectrometry. J. Mass Spectrom. 2012, 47, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.M.G.; Tomé, A.C.; Neves, M.G.P.M.S.; Silva, A.M.S.; Cavaleiro, J.A.S. 1,3-Dipolar Cycloaddition Reactions of Porphyrins with Azomethine Ylides. J. Org. Chem. 2005, 70, 2306–2314. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, A.F.R.; Snarskis, G.; Zurauskas, J.; Guieu, S.; Paz, F.A.A.; Tomé, A.C. Site-Selective Modification of a Porpholactone—Selective Synthesis of 12,13- and 17,18-Dihydroporpholactones. Molecules 2020, 25, 2642. [Google Scholar] [CrossRef]
- Zhang, C.; Seidel, D. Nontraditional Reactions of Azomethine Ylides: Decarboxylative Three-Component Couplings of α-Amino Acids. J. Am. Chem. Soc. 2010, 132, 1798–1799. [Google Scholar] [CrossRef]
- Stewart, J.J.P. MOPAC2016. Stewart Comput. Chem., Colorado Springs, CO, USA. 2016. Available online: http//OpenMOPAC.net (accessed on 30 June 2022).
- Silva, A.M.G.; Tomé, A.C.; Neves, M.G.P.M.S.; Silva, A.M.S.; Cavaleiro, J.A.S. meso-Tetraarylporphyrins as dipolarophiles in 1,3-dipolar cycloaddition reactions. Chem. Commun. 1999, 1767–1768. [Google Scholar] [CrossRef]
- Yadav, P.; Sankar, M. Spectroscopic and theoretical studies of anionic corroles derived from phosphoryl and carbomethoxyphenyl substituted corroles. Chem. Phys. Lett. 2017, 677, 107–113. [Google Scholar] [CrossRef]
- Iglesias, B.A.; Barata, J.F.B.; Domingues, M.R.M.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S. The gas-phase fragmentation behavior of protonated meso-trans-A2B-corroles studied by ESI–MS/MS: The influence of the meso-10-aryl substituent. Int. J. Mass Spectrom. 2014, 363, 1–7. [Google Scholar] [CrossRef]
- Lau, K.S.F.; Sadilek, M.; Gouterman, M.; Khalil, G.E.; Brückner, C. Observation of phenyl-fused porphyrinoids during the ESI mass spectrometric analysis of meso-pentafluorophenyl-substituted porphyrin and corrole. J. Am. Soc. Mass Spectrom. 2006, 17, 1306–1314. [Google Scholar] [CrossRef][Green Version]
- Iglesias, B.A.; Barata, J.F.B.; Ramos, C.I.V.; Santana-Marques, M.G.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S. Adventures in corrole features by electrospray ionization mass spectrometry studies. RSC Adv. 2014, 4, 16824–16838. [Google Scholar] [CrossRef]
- Domingues, M.R.M.; Domingues, P.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S. Recent developments in the structural characterization of substituted meso-tetraarylporphyrins by electrospray tandem mass spectrometry. J. Porphyr. Phthalocyanines 2009, 13, 524–527. [Google Scholar] [CrossRef]
- Stewart, J.J.P. Optimization of parameters for semiempirical methods V: Modification of NDDO approximations and application to 70 elements. J. Mol. Model. 2007, 13, 1173–1213. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.J.P. Optimization of parameters for semiempirical methods VI: More modifications to the NDDO approximations and re-optimization of parameters. J. Mol. Model. 2013, 19, 1–32. [Google Scholar] [CrossRef]
- Klamt, A.; Schüürmann, G. COSMO: A new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient. J. Chem. Soc. Perkin Trans. 2 1993, 799–805. [Google Scholar] [CrossRef]
- Available online: http://openmopac.net/manual/rsolv.html (accessed on 25 July 2022).
- Gryko, D.T.; Koszarna, B. Refined methods for the synthesis of meso-substituted A3- and trans-A2B-corroles. Org. Biomol. Chem. 2003, 1, 350–357. [Google Scholar] [CrossRef]
- Bendix, J.; Dmochowski, I.J.; Gray, H.B.; Mahammed, A.; Simkhovich, L.; Gross, Z. Structural, electrochemical, and photophysical properties of gallium(III) 5,10,15-tris(pentafluorophenyl)corrole. Angew. Chem. Int. Ed. 2000, 39, 4048–4051. [Google Scholar] [CrossRef]






| Compounds | Formation Enthalpy (kcal mol−1) (a) | Formation Enthalpy (kcal mol−1) (b) |
|---|---|---|
| 3 | −415.15 | −420.84 |
| 4 | −422.09 | −423.78 |
| 5 | −418.75 | −424.28 |
| 6 | −438.42 | −446.09 |
| 7 | −380.29 | −388.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barata, J.F.B.; Lacerda, P.S.S.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S.; Ramos, C.I.V.; Tomé, A.C.; Abreu, P.E.; Pais, A.A.C.C. Reaction of Corroles with Sarcosine and Paraformaldehyde: A New Facet of Corrole Chemistry. Int. J. Mol. Sci. 2022, 23, 13581. https://doi.org/10.3390/ijms232113581
Barata JFB, Lacerda PSS, Neves MGPMS, Cavaleiro JAS, Ramos CIV, Tomé AC, Abreu PE, Pais AACC. Reaction of Corroles with Sarcosine and Paraformaldehyde: A New Facet of Corrole Chemistry. International Journal of Molecular Sciences. 2022; 23(21):13581. https://doi.org/10.3390/ijms232113581
Chicago/Turabian StyleBarata, Joana F. B., Paula S. S. Lacerda, Maria Graça P. M. S. Neves, José A. S. Cavaleiro, Catarina I. V. Ramos, Augusto C. Tomé, Paulo E. Abreu, and Alberto A. C. C. Pais. 2022. "Reaction of Corroles with Sarcosine and Paraformaldehyde: A New Facet of Corrole Chemistry" International Journal of Molecular Sciences 23, no. 21: 13581. https://doi.org/10.3390/ijms232113581
APA StyleBarata, J. F. B., Lacerda, P. S. S., Neves, M. G. P. M. S., Cavaleiro, J. A. S., Ramos, C. I. V., Tomé, A. C., Abreu, P. E., & Pais, A. A. C. C. (2022). Reaction of Corroles with Sarcosine and Paraformaldehyde: A New Facet of Corrole Chemistry. International Journal of Molecular Sciences, 23(21), 13581. https://doi.org/10.3390/ijms232113581












