Abstract
Post-weaning diarrhea caused by enterotoxigenic Escherichia coli F18 (E. coli F18) causes significant economic losses for pig producers. N6-methyladenosine (m6A) is a highly abundant epitranscriptomic marker that has been found to be involved in regulating the resistance of host cells to pathogenic infection, but its potential role in E. coli F18-exposed intestinal porcine epithelial cells (IPEC-J2) remains undetermined. Here, we demonstrated that m6A and its regulators modulate E. coli F18 susceptibility. Briefly, we revealed that the Wilms’ tumor 1-associating protein (WTAP) expressions were markedly elevated in IPEC-J2 cells upon E. coli F18 exposure. WTAP are required for the regulation of E. coli F18 adhesion in IPEC-J2 cells. Additionally, WTAP knockdown significantly suppressed m6A level at N-acetyllactosaminide beta-1,6-N-acetylglucosaminyl-transferase (GCNT2) 3′UTR, resulting in the enhancement of TH N6-methyladenosine RNA binding protein 2 (YTHDF2)-mediated GCNT2 mRNA stability. Subsequently, the altered GCNT2 expressions could inhibit the glycosphingolipid biosynthesis, thus improving resistance to E. coli F18 infection in IPEC-J2. Collectively, our analyses highlighted the mechanism behind the m6A-mediated management of E. coli F18 susceptibility, which will aid in the development of novel approaches that protect against bacterial diarrhea in piglets.
1. Introduction
Piglets’ bacterial diarrhea is one of the most common intestinal inflammatory diseases, which becomes the biggest contributor to financial loss to pig farmers everywhere. Enterotoxigenic Escherichia coli F18 (ETEC F18) is a major pathogen that induces post-weaning piglet diarrhea, and it strongly interacts with the intestinal porcine epithelial cell (IPEC-J2) using pili [1]. F18 fimbriae are commonly found in E. coli extracted from weaned piglets, and they can be categorized into two major antigenic variants, F18ab and F18ac. F18ab is closely related to the Stx2e-producing strains that cause edema disease (ED). However, many F18ab isolates (10 of 11 isolates, 90.9%) are also found in ETEC. F18ac is associated with enterotoxigenic bacteria that encodes STa and STb, which causes post-weaning diarrhea (PWD) [2]. It is well reported that the E. coli F18 pathogenicity is based on the levels of corresponding receptors that express on the brush border of the piglet IPEC-J2, resulting in the production of LPS-enterotoxin and causing diarrhea [3]. Thus, resistance against the E. coli F18 invasion depends on the intestinal immunity and F18-receptor content in pigs. Recently, several studies analyzed the association between immune-related genes (CD14, TLR5) [4,5], or fucosetransferase genes (FUT2, FUT3) [6,7] and E. coli F18 susceptibility in piglets. Nevertheless, the mechanism behind piglets’ resistance to E. coli F18 invasion is not fully understood. Therefore, it is necessary to carry out a comprehensive and in-depth investigation into this process.
Emerging evidence revealed that chemical-based RNA modifications modulate gene expression. The N6-methyladenosine (m6A) methylation is relatively common among mammalian mRNAs, and it is strongly conserved in eukaryotes from yeast to humans [8,9]. Several reports demonstrated that the m6A transcript or non-coding RNA modification can regulate RNA fate and activity. Generally, m6A is deposited on RNAs via the m6A methyltransferase complex (METTL3/METTL14/WTAP), reversed using demethylases (FTO/ALKBH5), and identified by the m6A interacting proteins (YTHDF1-3/YTHDC1,2) [10]. Molecular studies revealed that this epigenetic alteration modulates RNA stability, translation, alternative splicing, and nuclear export [11,12,13,14]. Thus, m6A are intricately related to numerous physiological processes, namely, tissue development, tumorigenesis and cell division [15]. Although numerous investigations have examined the association between m6A modifications and physical traits in humans, great progress has been made in the study of m6A regulating pig characters, including fat deposition [16], lipid metabolism [17], stem cell differentiation [18], liver development [19], etc. Nonetheless, litter studies have not yet characterized the association between m6A modifications and piglets’ bacterial diarrhea, underscoring a novel direction for the ongoing epigenetic studies on economically important livestock.
In general, the intestinal porcine epithelial cell (IPEC-J2) is an efficient cell model for the examination of the underlying mechanism behind the host anti-E. coli F18 pathogen [7,20]. In addition, multiple studies have revealed that the IPEC-J2 exposure to E. coli triggers oxidative damage and inflammatory responses, which results in enhancing apoptotic cell death and diminishing functionality [21,22,23]. Nevertheless, the explicit biological role of m6A regulators in conferring resistance against E. coli F18-based infection in IPEC-J2 cells remains undetermined. Herein, we demonstrated a crucial role of m6A and its regulators in affecting the susceptibility of IPEC-J2 cells to E. coli F18 exposure. We revealed that m6A regulator (WTAP) depletion significantly enhanced the adhesive capacity of E. coli F18-fimbriae to IPEC-J2 cells. Moreover, we showed that WTAP regulated target expression by modulating mRNA stability using an m6A-YTHDF2-based system. In short, we highlighted the mechanism behind the m6A-mediated regulation of resistance to E. coli F18-based infection in IPEC-J2 cells and will provide valuable targets that induce resistance to bacterial diarrhea in pigs.
2. Results
2.1. Upregulated WTAP Expressions in IPEC-J2 Cells after E. coli F18 Exposure
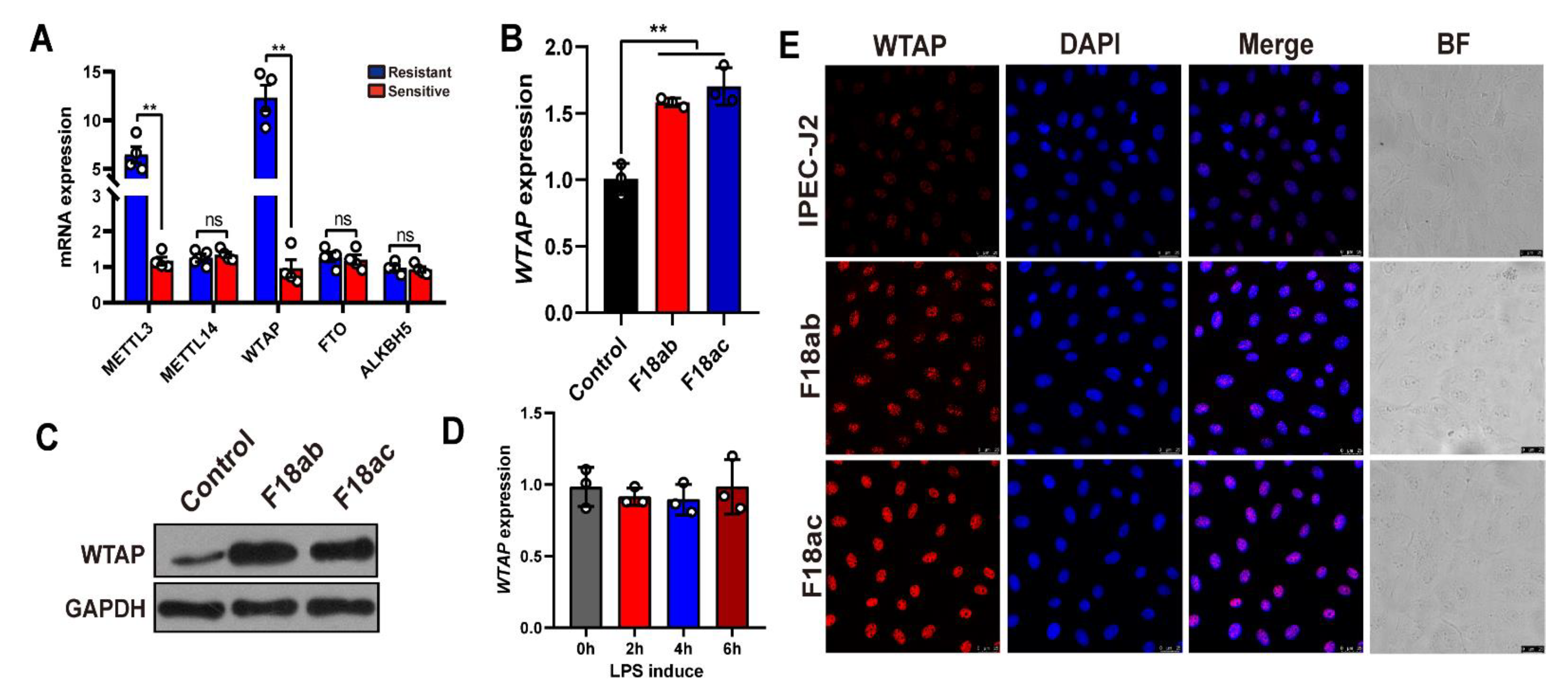
To preliminarily examine the role of m6A methyl markers in resistance to E. coli F18 infection in piglets, we detected the levels of three m6A writers (METTL3, METTL14, and WTAP) and two m6A erasers (ALKBH5 and FTO) in the duodenum of E. coli F18-sensitive and -resistant piglets. Based on our qPCR analysis (Figure 1A), the METTL3 and WTAP expressions were markedly elevated in the resistant versus sensitive piglets (p < 0.01). We next assessed the levels of WTAP expressions in IPEC-J2 cells following E. coli F18 exposure. We revealed that both WTAP transcript and protein levels were considerably high in the F18ab/ac-treated cells (Figure 1B,C, p < 0.01). The lipopolysaccharide (LPS) layer in E. coli stabilizes the outer membrane of the bacteria [24]. Studies revealed that the IPEC-J2-exposed LPS triggers cell immune responses [5,25]. Our findings demonstrated that the WTAP expression showed no significant change in IPEC-J2 cells following a LPS induction (Figure 1D, p > 0.05). These results indicated that WTAP did not have a potential role in modulating E. coli F18-induced immune response but may be involved in other aspects of regulation. Moreover, immunofluorescence analysis revealed that the F18ab/ac-bacteria stimulation enhanced WTAP levels in the IPEC-J2 cells (Figure 1E).
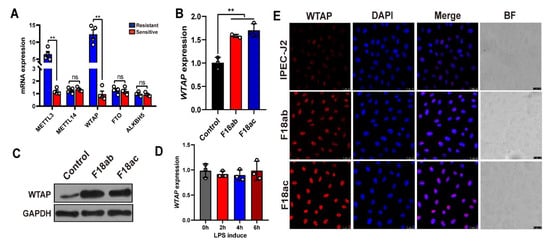
Figure 1.
Expression analysis of m6A methyl markers in IPEC-J2 cells upon E. coli F18 exposure. (A) qPCR validation of METTL3, WTAP, METTL14, FTO, ALKBH5 expression in duodenum tissues between F18-resistant (n = 4) and -sensitive piglets (n = 4). (B,C) Expression changes of WTAP in F18ab/ac-stimulated IPEC-J2 cells by using qPCR and Western blotting analysis. (D) Expression changes of WTAP in LPS-induced IPEC-J2 cells. IPEC-J2 cells were treated with 0.1 μg/mL LPS for 0, 2, 4, and 6 h. (E) Immunofluorescence analysis of WTAP in IPEC-J2 upon E. coli F18ab/ac exposure. Scale bar, 25 μm. All data are presented as the mean ± SD, ns p > 0.05, ** p < 0.01.
2.2. WTAP Is Required for the E. coli F18-Based Adhesion Regulation in IPEC-J2 Cells
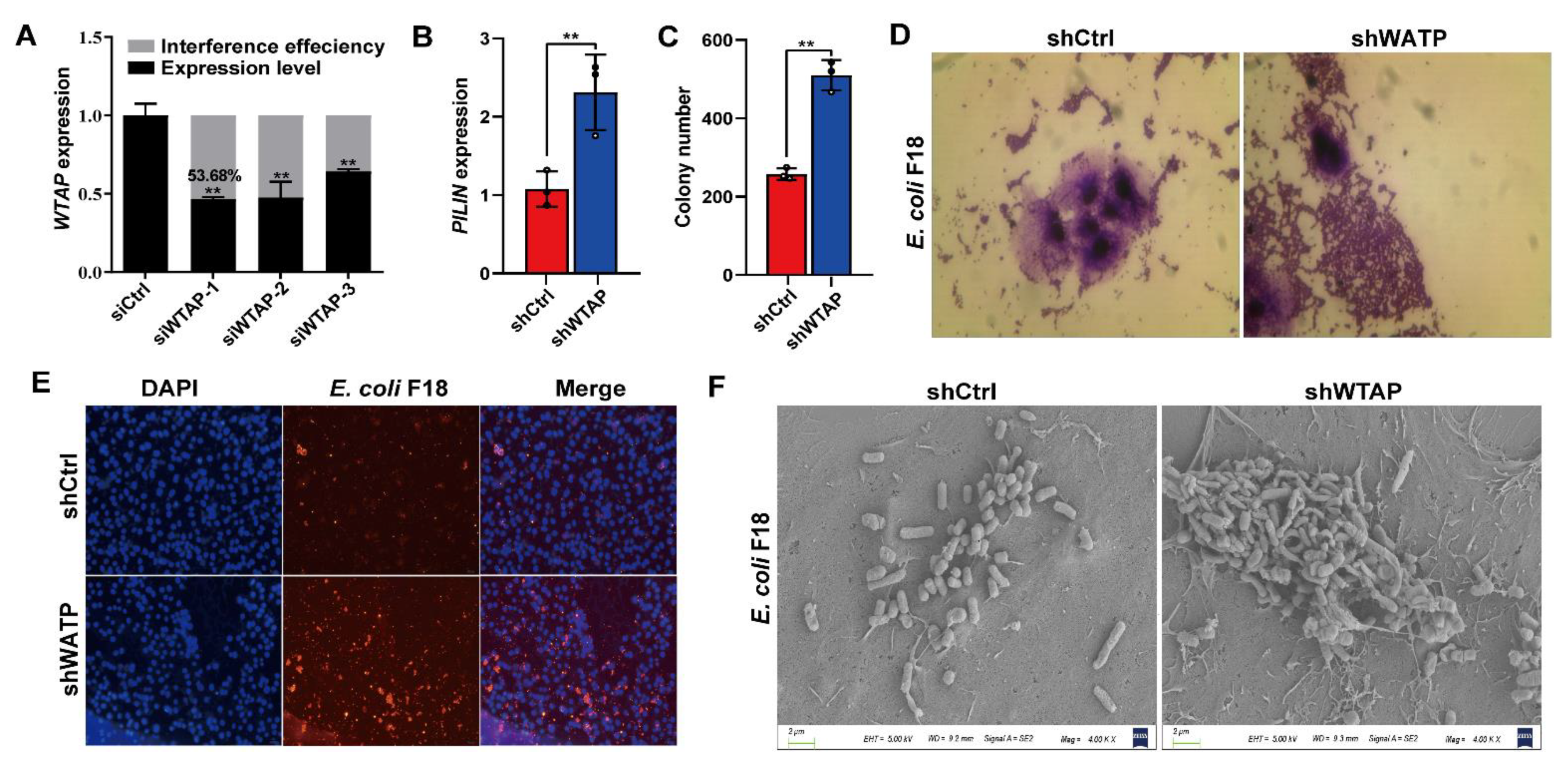
To elucidate the mechanism behind the WTAP-mediated regulation of E. coli F18 susceptibility, we assessed how WTAP levels modulated the adhesive capacity of F18ac-expressing fimbriae to IPEC-J2 cells. First, we generated an overexpression vector and designed three siRNA vectors for the WTAP gene. The optimal interference efficiency was 53.68% in IPEC-J2 cells with siWTAP vectors (Figure 2A) as evidenced by qPCR verification. F18-fimbriae protein (PILIN) expression detection (Figure 2B) and colony counting (Figure 2C) revealed a significantly higher quantity of bacteria adhering to the IPEC-J2 cells in the siWTAP group (p < 0.01). In addition, we also assessed the adhesive capacity of E. coli F18 to IPEC-J2 cells using gram staining (Figure 2D), indirect immunofluorescence (Figure 2E) and scanning electron microscope (Figure 2F). Consistently, the results revealed that the WTAP knockdown markedly increased the adhesion level of E. coli F18 to IPEC-J2 cells. Thus, our findings highlighted the significance of WTAP in regulating E. coli F18 invasion and confirmed that WTAP deficiency markedly enhanced E. coli F18 adhesion to IPEC-J2 cells.
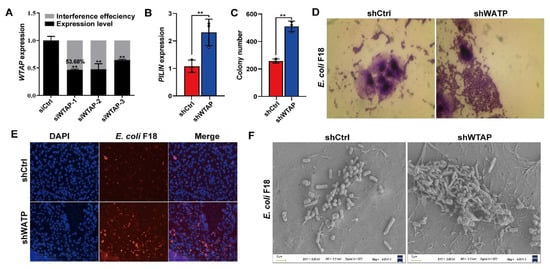
Figure 2.
WTAP is required for affecting E. coli F18 adhesion in IPEC-J2 cells. (A) Knockdown efficiency of WTAP gene in IPEC-J2 cells was determined using qRT-PCR. (B) Expression detection of E. coi F18 fimbriae gene (PILIN) via relative quantification in WTAP-silenced IPEC-J2 cells. (C) Colony number of E. coi F18 fimbria adhering to IPEC-J2 cells were evaluated in treatment group. (D) Gram staining assay, an optical microscope (400×, scale bar = 100 μm) was used to observe the IPEC-J2 cells. (E) Indirect immunofluorescence assay, blue fluorescence indicates nuclear staining via DAPI; red fluorescence indicates staining with the anti-E. coli antibody. Cells were observed under a fluorescence microscope (100×, scale bar = 1 mm). (F) Scanning electron microscopy (SEM) assay, cells were observed under a scanning electron microscope (2000×). All data are presented as the mean ± SD, ** p < 0.01.
2.3. GCNT2 3′UTR Mediates WTAP-m6A Regulation
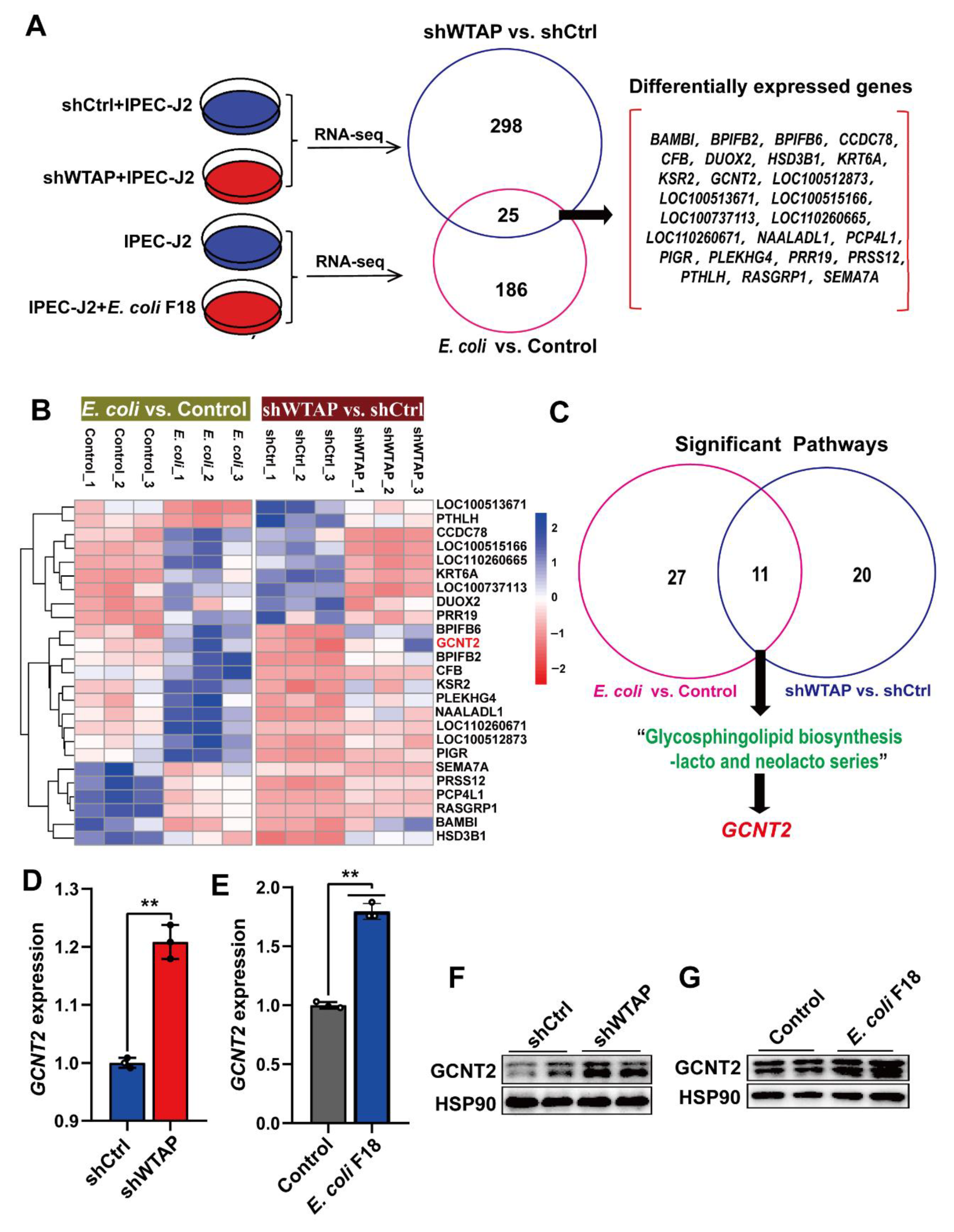
To understand the underlying mechanism by which WTAP regulates E. coli F18 susceptibility in IPEC-J2 cells, we employed a comparative transcriptome sequencing to highlight the transcriptional differences following WTAP knockdown or E. coli F18 infection. Overall, we identified 323 differentially expressed genes (DEGs) between the shWTAP and shCtrl groups (Figure S1), and 211 DEGs between the E. coli F18-treated and control samples (Figure S2). Interestingly, 25 common DEGs were screened in both the shWTAP vs. shCtrl and E. coli vs. control groups (Figure 3A,B). To further assess the potential biological impact of DEGs in WTAP knockdown or E. coli-treated cells, KEGG pathway enrichment analyses were performed. Our analysis revealed that the DEGs transcripts from the shWTAP vs. shCtrl and E. coli vs. control groups were involved in 11 common signaling pathways (Table S3), including a glycosphingolipid biosynthesis-lacto and neolacto series (Figure 3C), which is likely linked to the E. coli F18 receptor formation [26,27]. Among the 25 common DEGs, GCNT2 serves as a critical molecule participating in the glycosphingolipid biosynthesis pathway. Based on our qRT-PCR data (Figure 3D,E), the GCNT2 levels were markedly upregulated in both WTAP knockdown and E. coli F18-treated cells (p < 0.01). Similarly, our Western blot results revealed (Figure 3F,G) that the GCNT2 protein levels were also significantly enhanced in these treated cells. Thus, our findings preliminarily identified GCNT2 as a downstream target of WTAP by using transcriptome sequencing.
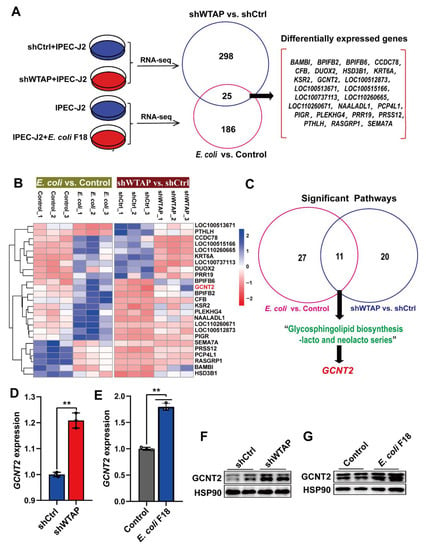
Figure 3.
Transcriptome sequencing identified GCNT2 as a WTAP downstream target. (A) Venny analysis of differentially expressed genes (DEGs) in IPEC-J2 from shWTAP and shCtrl group, E. coli and Control group by comparative transcriptome sequencing. (B) Hierarchical clustering analysis of common DEGs between shWTAP vs. shCtrl and E. coli vs. Control. (C) Venny analysis of significant pathways that DEGs were involved in. (D) qPCR analysis of GCNT2 expression in IPEC-J2 cells transfected with control and shWTAP vector. (E) qPCR analysis of the expression change of GCNT2 in IPEC-J2 cells after E. coli F18 infection. (F,G) Western blotting analysis of GACN2 expression in IPEC-J2 cells after WTAP knockdown or E. coli F18 infection. All data are presented as the mean ± SD, ** p < 0.01.
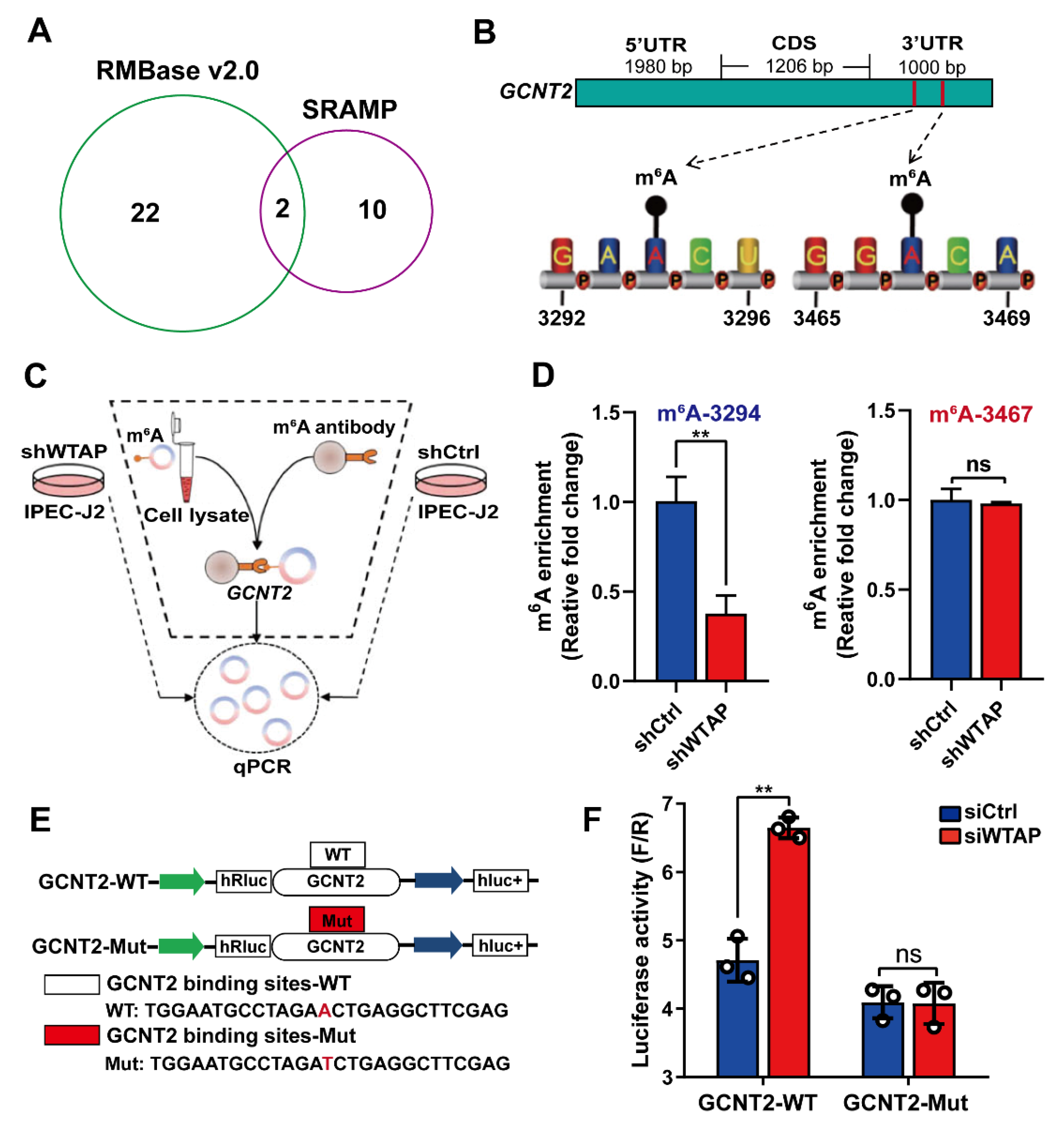
To further explore whether m6A modification are essential for the WTAP-based regulation of GCNT2 expression, we employed the SRAMP [28] and RMBase v2.0 [29] to predict m6A sites. We identified two m6A sites (3294, 3467) in the 3′UTR of the GCNT2 gene (Figure 4A,B). Importantly, using the m6A-specific immunoprecipitation assay (Figure 4C), we revealed a significant downregulation of m6A levels in the 3′UTR region (3294) of GCNT2 in the WTAP knockdown IPEC-J2 cells, relative to mock-vehicle controls (p < 0.01). In contrast, the m6A level at the 3′UTR site (3467) displayed no significant change in the WTAP-silenced cells (Figure 4D, p > 0.05). We also conducted GCNT2-DLR and mutagenesis assays in the WTAP knockdown cells (Figure 4E). Based on our results, WTAP knockdown markedly enhanced bioluminescence of reporter carrying the GCNT2 3′UTR-WT fragment, compared to controls (Figure 4F). Taken together, our results confirmed that the GCNT2 transcript was a functionally important substrate of WTAP.
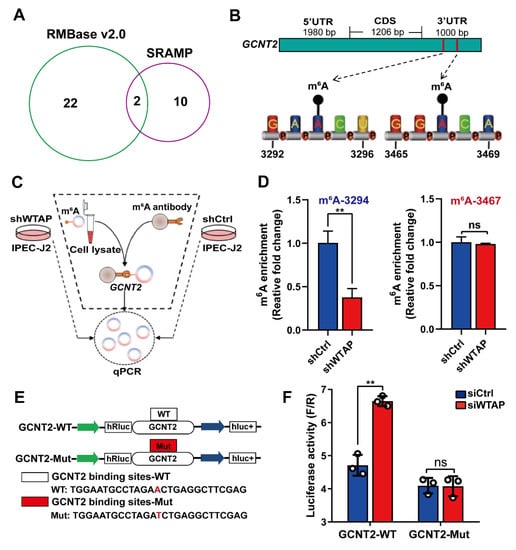
Figure 4.
WTAP regulates GCNT2 expression via m6A methylation. (A) Predicted m6A sites in GCNT2 from overlapping results of SRAMP and RMBase v2.0 software. (B) Schematic diagram of predicted m6A sites in the 3′UTR of GCNT2 gene. (C) Flow diagram of m6A-specific immunoprecipitation (MeRIP) assays. (D) MeRIP assays for m6A-modified GCNT2 in shWTAP and shCtrl cell lines. (E) Construction of luciferase vector with GCNT2 wild-type 3′UTR (WT) or mutant-type 3′UTR (Mut). (F) Relative activity of the wild-type or mutant GCNT2 3′UTR firefly luciferase reporter in IPEC-J2 cells transfected with control or WTAP siRNAs. All data are presented as the mean ± SD, ** p < 0.01, ns p > 0.05.
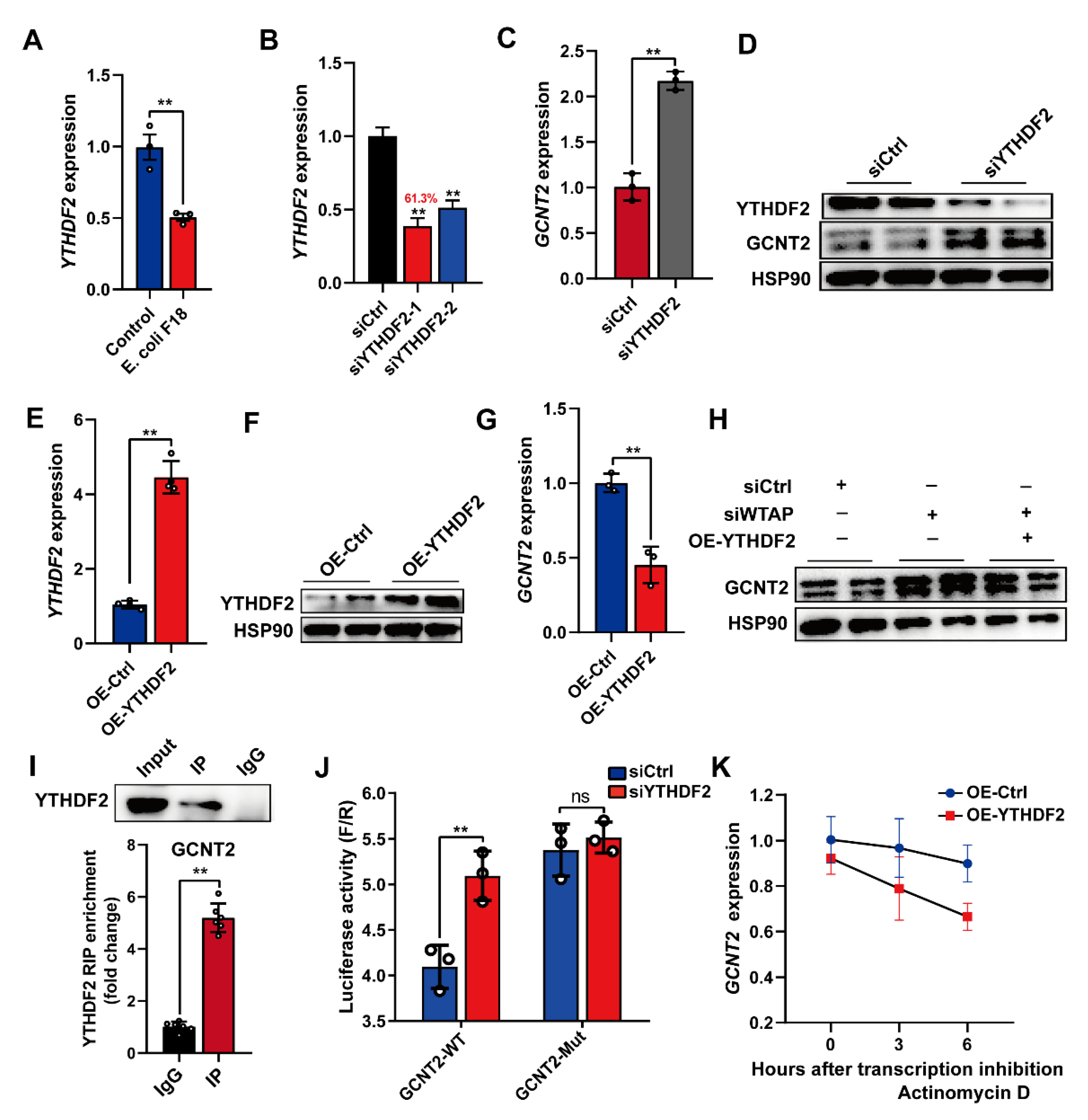
2.4. WTAP Reduces GCNT2 mRNA Stability through an m6A-YTHDF2-Regulated Manner
Furthermore, we examined the m6A-mediated regulation of GCNT2. Based on published reports, the m6A “reader” protein-like YTHDF2 employs m6A modification to mediate degradation of m6A-rich transcripts [11]. This suggests that the YTHDF2 protein may recognize and interact with the m6A-rich GCNT2 transcript. To examine this hypothesis, we detected the mRNA levels of YTHDF2 in E. coli F18-treated IPEC-J2 cells, and its expression demonstrated significant downregulation in IPEC-J2 cells upon E. coli F18 exposure (Figure 5A, p < 0.01). Subsequently, we established YTHDF2-silenced IPEC-J2 cells with a knockdown efficiency of 61.3% (Figure 5B). Upon YTHDF2 knockdown, the GCNT2 mRNA and protein expressions were markedly elevated in IPEC-J2 cells (Figure 5C,D, p < 0.01). We next assessed the overexpression efficiency of YTHDF2 in IPEC-J2 cells incorporated with the pcDNA3.1-YTHDF2, using qPCR and Western blot analyses (Figure 5E,F). YTHDF2 overexpression markedly reduced GCNT2 transcript levels in IPEC-J2 cells (Figure 5G). Interestingly, the WTAP knockdown-mediated regulation of GCNT2 protein expression was reversed in cells with YTHDF2 overexpression (Figure 5H), thus indicating that YTHDF2 modulates GCNT2 expression. Likewise, RIP-qPCR analysis confirmed that GCNT2 acts as a target of YTHDF2 (Figure 5I). Furthermore, YTHDF2 overexpression markedly enhanced bioluminescence of luciferase reporters carrying the GCNT2 3′UTR-WT fragment (Figure 5J, p < 0.01). To further elucidate the importance of mRNA stability in the YTHDF2-mediated regulation of GCNT2 expression, we evaluated expression alterations of GCNT2 mRNA in the transcriptional inhibitor (actinomycin D) treated IPEC-J2 cells with carrying the control or YTHDF2 overexpression vector. We clearly demonstrated that YTHDF2 overexpression obviously diminished the half-life of GCNT2 mRNAs (Figure 5K). Given these data, it was obvious that YTHDF2 was critical in modulating the WTAP-mediated action on GCNT2 expression by affecting mRNA stability.
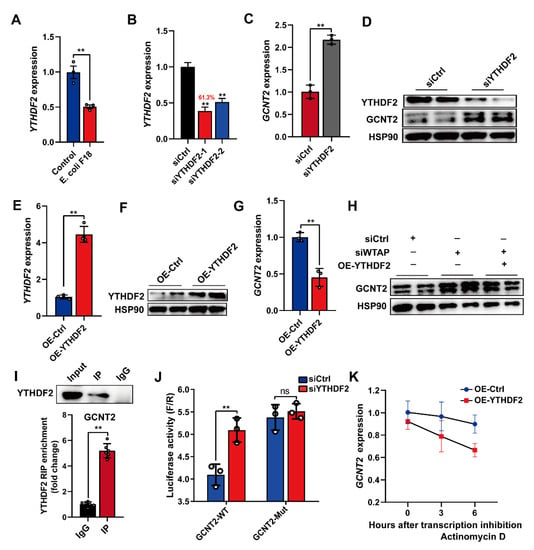
Figure 5.
Silencing of WTAP enhanced GCNT2 mRNA stability via YTHDF2-dependent mechanism. (A) qPCR detection of YTHDF2 expression in IPEC-J2 cells upon E. coli F18 exposure. (B) Knockdown efficiency of YTHDF2 gene in IPEC-J2 was assessed using qPCR. (C,D) qPCR and Western blot analysis of GCNT2 expression in control and YTHDF2-knockdown IPEC-J2 cells. (E,F) Overexpression efficiency of YTHDF2 gene in IPEC-J2 cells was determined using qPCR and Western blotting. (G) qPCR analysis of GCNT2 expression in control and YTHDF2-overexpressed IPEC-J2 cells. (H) Western blot analysis of GCNT2 expression in control, WTAP-knockdown, siWTAP + YTHDF2-overexpressed IPEC-J2 cells. (I) RIP assays of YTHDF2 association with IKBKG. Nuclear lysates of IPEC-J2 cells were immunoprecipitated with control mouse IgG or anti-histone YTHDF2 antibody, and the enrichment of GCNT2 was analyzed by using qPCR. (J) Relative luciferase activity of wild-type (WT) or mutant-type (Mut) GCNT2-3′UTR luciferase reporter in IPEC-J2 transfected with control or YTHDF2 siRNAs. (K) mRNA stability analysis of GCNT2 mRNA in control, YTHDF2-overexpressed IPEC-J2 treated with actinomycin D for 3 and 6 h. All data are presented as the mean ± SD, ** p < 0.01, ns p > 0.05.
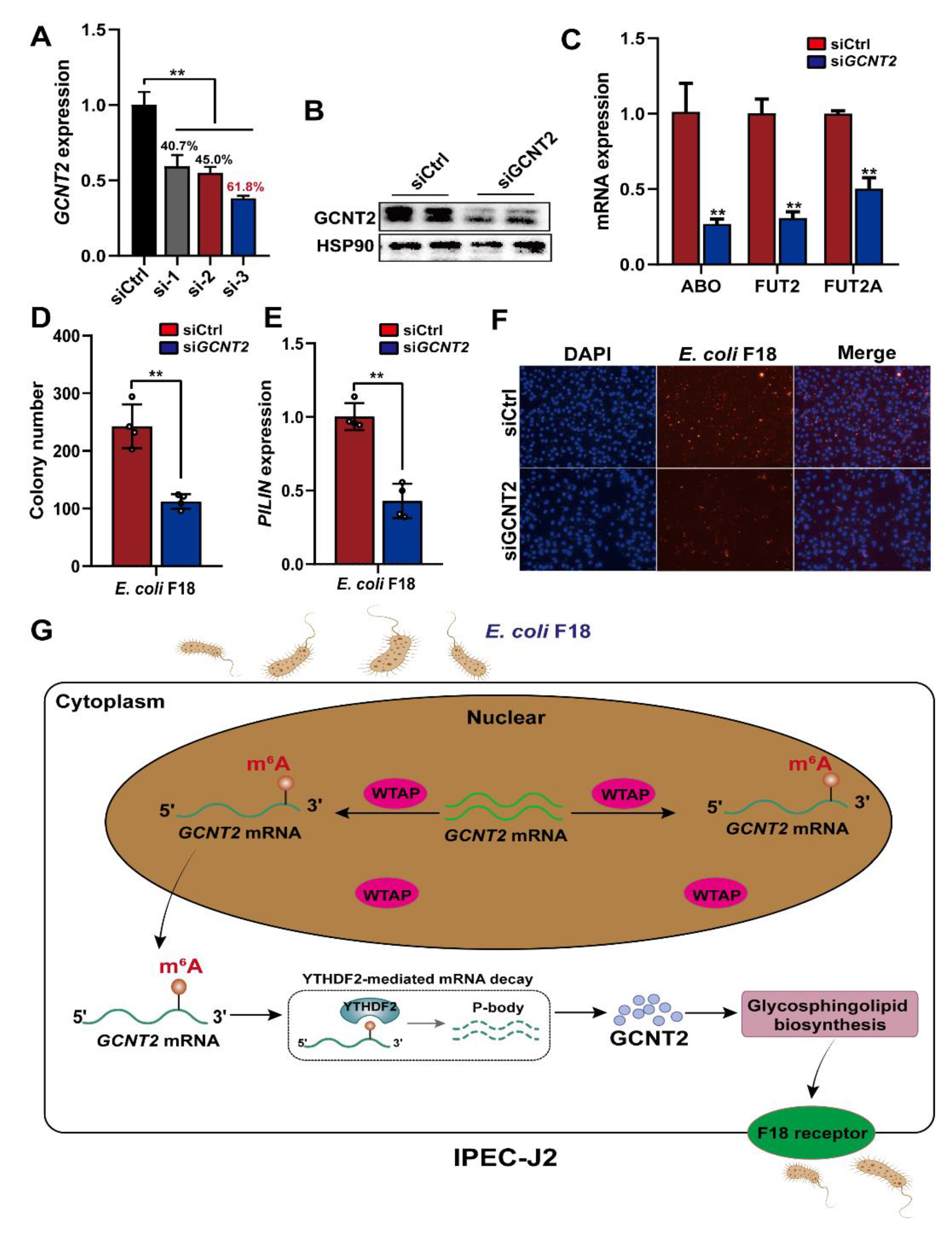
2.5. WTAP Potentially Regulates E. coli F18 Receptor Formation by Inhibiting the GCNT2-Mediated Glycosphingolipid Biosynthesis
To further elucidate the GCNT2-mediated regulation of E. coli F18 susceptibility in IPEC-J2 cells, we confirmed that GCNT2 was a target of WTAP and it participated in the glycosphingolipid biosynthesis pathway (Figure 3C). Studies revealed that the glycosphingolipid biosynthesis was closely associated with F18 receptor formation [26,27]. Thus, we speculated that WTAP potentially regulates E. coli F18 receptor formation via the GCNT2-mediated glycosphingolipid biosynthesis. Thus, we constructed three siRNA vectors of GCNT2 in IPEC-J2 cells, with an optimal knockdown efficiency of 61.8% (Figure 6A). Our Western blot analysis revealed that the GCNT2 protein expression was obviously decreased in the IPEC-J2 cells after GCNT2 knockdown (Figure 6B). Moreover, GCNT2 knockdown significantly reduced the expression of glycosphingolipid biosynthesis-related genes, including fucosyltransferase 2 (FUT2), galactoside 2-alpha-L-fucosyltransferase 2 (FUT2A), alpha 1-3-N-acetylgalactosaminyltransferase, and alpha 1-3-galactosyltransferase (ABO) (Figure 6C). Moreover, GCNT2 knockdown considerably diminished the adhesive capacity of E. coli F18 to IPEC-J2 cells, as assessed by colony counting, PILIN expression, and indirect immunofluorescence assay (Figure 6D–F). Taken together, our findings indicated that GCNT2 served a crucial function in controlling E. coli F18 adhesion via the regulation of glycosphingolipid biosynthesis.
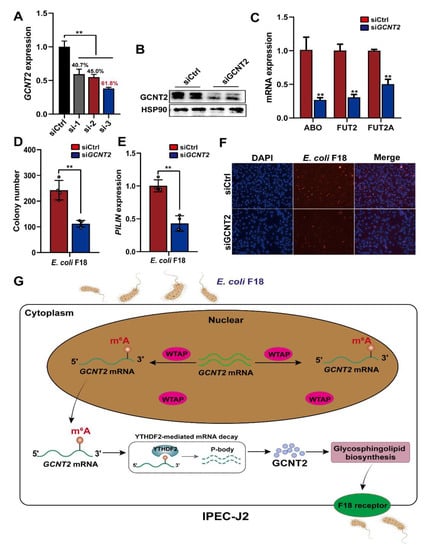
Figure 6.
WTAP affects the formation of E. coli F18 receptor by inhibiting glycosphingolipid biosynthesis pathway. (A,B) Knockdown efficiency of IKBKG gene in IPEC-J2 was assessed using qPCR and Western blot analysis. (C) Expression detection of glycosphingolipid biosynthesis-related genes in IPEC-J2 cells with or without GCNT2 knockdown. (D–F) Effect of GCNT2 knockdown on the ability of E. coi F18 fimbria to adhere to IPEC-J2 cells was analyzed by colony counting, PILIN expression detection, indirect immunofluorescence assay. (G) A working model summarizing the mechanism of m6A modification and its modulators in regulating E. coli F18 susceptibility in IPEC-J2 cells. m6A methyltransferase WTAP increases the m6A levels of GCNT2 mRNA, leading to WTAP attenuating the YTHDF2-dependent mRNA stability of GCNT2, WTAP thereby restraining the formation of E. coli F18 receptor by inhibiting GCNT2-mediated glycosphingolipid biosynthesis, resulting in the resistance of IPEC-J2 to E. coli F18 infection. All data are presented as the mean ± SD, ** p < 0.01.
3. Discussion
To successfully utilize IPEC-J2 cells in simulating the phenomenon of E. coli F18 adhesion in vitro, it is crucial to enhance our comprehension of the underlying mechanisms behind the m6A-mediated regulation of IPEC-J2 cells following E. coli F18 exposure. Herein, we identified an important mechanism whereby m6A modification regulates E. coli F18 susceptibility by modulating the NF-κB and glycosphingolipid biosynthesis signaling via the WTAP-m6A-based and YTHDF2-dependent post-transcriptional regulations (Figure 6G). Briefly, WTAP enhances the m6A status in GCNT2 transcripts, which attenuates the WTAP-induced YTHDF2-dependent mRNA stability of GCNT2, thereby restraining the WTAP-based synthesis of E. coli F18 receptor by inhibiting the GCNT2-mediated glycosphingolipid biosynthesis. Collectively, these activities enhance resistance to E. coli F18 infection.
Functionally, m6A modification control numerous biological processes, such as metabolism, differentiation, and disease pathology [30]. Prior investigations demonstrated m6A as a critical contributor to host resistance of multiple pathogen/microbial infections [31,32,33,34]. METTL3 is a methyltransferase that accelerates m6A synthesis, which regulates viral replication and E. coli infection. Hao et al. (2019) reported that the host m6A complex METTL3 binds to viral proteins, and controls EV71 replication [35]. Zong et al. (2021) reported that the enterotoxigenic E. coli infection induces enteric defensin expression via the FOXO6-METTL3-m6A-GPR161 signaling axis [36]. WTAP is critical for m6A deposition and aids in the METTL3-METTL14 heterodimer heterodimer localizing to transcription sites [37,38]. Recent studies documented that the WTAP-mediated m6A modification critically regulates viral infection [39,40]. However, the potential contribution of WTAP regulating bacterial infection and its underlying mechanisms remain largely unknown. Our findings demonstrated that WTAP depletion enhanced the adhesive capacity of E. coli F18 fimbria to IPEC-J2 cells. In this study, we found that WTAP serves critical roles in the formation of E. coli F18 receptor via regulating GCNT2-based glycosphingolipid biosynthesis. In IPEC-J2 cells upon E. coli exposure, the E. coli F18 adhesion caused the upregulated expression of WTAP gene and activated the synthesis of F18 receptor. Given this evidence, we identified that the m6A methyl marker (WTAP) strongly modulated resistance to E. coli F18 infection.
It is well reported that m6A modification is primarily enforced by particular m6A-interacting proteins called m6A readers [15]. Given this scenario, some signaling pathways may be simultaneously modulated by m6A and its multiple m6A readers. Wu et al. (2019) demonstrated that m6A modulates the pluripotency of porcine pluripotent stem cells by influencing the SOCS3/JAK2/STAT3 axis in a YTHDF1/YTHDF2-regulated fashion [18]. Yao et al. (2019) reported that METTL3 suppresses BMSC adipogenic differentiation via modulation of the JAK1/STAT5/C/EBPβ axis in an m6A-YTHDF2-regulated fashion [41]. YTHDF2 recognizes and destabilizes m6A-rich transcripts [11], but YTHDF1 and YTHDF3 modulate transcript translation and protein synthesis [12,42]. Because m6A and the YTH domain family are ubiquitous among eukaryotes and they strongly regulate multiple biological processes, we proposed that the m6A interacting proteins serve discrete functions in the m6A-mediated regulation of E. coli susceptibility. In this study, we demonstrated that WTAP attenuated GCNT2 mRNA stability and inhibited glycosphingolipid biosynthesis in a m6A-YTHDF2-dependent manner. Given this evidence, our research revealed crucial roles of YTHDF2 in E. coli F18 susceptibility-related gene regulation in IPEC-J2 cells. Du et al. (2016) reported that m6A reader protein YTHDF2 recruits the CCR4-NOT deadenylase complex by directly interacting with the superfamily homology (SH) domain of CNOT1, the scaffolding subunit of the complex, to initiate the deadenylation and decay of m6A-containing mRNAs [43]. In this study, the mechanism of YTHDF2 interacting with GCNT2 should still be further explored. WTAP is a Wilms’ tumor 1 (WT1) related protein that is necessary for numerous physiological processes, such as G2/M transition and pre-mRNA splicing [44,45,46]. Moreover, multiple reports highlighted the essential role of WTAP in the progression of cancers [47]. However, the m6A-based physiological function in IPEC-J2 is not yet determined. Here, we reported that WTAP affected the YTHDF2-based mRNA stability of GCNT2 in E. coli F18-exposed IPEC-J2 cells. It was reported that the mRNA stability of cyclin A2 and CDK2 regulated by WTAP contributed to the variation of mitotic cycle transition [46,48]. Taken together, our findings for the first time revealed the WTAP-involved m6A mechanism in IPEC-J2 cells following E. coli F18 exposure.
4. Materials and Methods
4.1. Reagents
The following antibodies were employed in immunoprecipitation (IP), indirect immunofluorescence assay (IFA) and Western blot assays: YTHDF2 (ab220163, rabbit, 1:30), WTAP (ab195380, rabbit, 1:1000), E. coli (ab137967, rabbit, 1:200) and GAPDH (ab9485, rabbit, 1:2500), HSP90 (ab59459, mouse, 1:500) were acquired from abcam (Shanghai, China). GCNT2 (Q06430, rabbit, 1:1000, Abcepta Biotech Ltd. Co., Suzhou, China); m6A antibody (56593, rabbit, 1:1000 Cell Signaling Technology, Boston, MA, USA); IgG (Q6005, rabbit, 1:1000 Dia-An Biotech, Wuhan, China).
4.2. Ethics Statement
All experimental protocols received ethical approval from the Yangzhou University (Pig: SYXK (Su) 2012-0029) and abided by the ethical standards of the People’s Republic of China. Our research did not warrant any other permission or approval.
4.3. Experimental Animals
Eight Meishan piglets (Kunshan Conservation Ltd., Suzhou, China) were used for these experiments. In prior studies, we obtained four E. coli F18-resistant and four E. coli F18-sensitive piglets by challenging with the E. coli F18 strain, and identifying via assays, such as bacterial counting, histopathological, and in vitro adherence assays of IPEC-J2 cells [5]. All piglet sacrifices were performed humanely via an intravenous administration of pentobarbital sodium, prior to the collection of duodenal tissues and storage in liquid nitrogen for additional use.
4.4. Cell Culture and E. coli F18 Exposure
IPEC-J2 cells were grown in complete culture medium (Dulbecco’s modified Eagle’s medium: F12 medium = 1:1, 10% fetal calf serum; Gibco BRL, Life Technologies, New York, NY, USA), and seeded into 12-well plates at 5.0 × 105 cells per well, prior to a 24 h incubation in a humid chamber at 37 °C and 5% CO2. E. coli F18ab {107/86 (O139:K12:H1)} and E. coli F18ac {2134P (O157:H19)} fimbriae standard strains were obtained from the Institute of Microbiology, University of Pennsylvania. E. coli F18 strains were introduced to the LB culture medium, and maintained on a rocking platform for 12 h at 200 r/min. Next, 1 × 109 CFU of E. coli bacteria were introduced to a monolayer of about 5 × 105 IPEC-J2 cells in each well of a 12-well culture plate (Corning, NY, USA) for 3 h at 37 °C. To obtain the two forms of E. coli, the cultures were centrifuged for 5 min at 4000 rpm, followed by supernatant filtration (pore size, 0.22 μm), bacterial resuspension, and three wash cycles.
4.5. In Vitro Adherence Assays
Adherence assays of E. coli F18ac-fimbriae to IPEC-J2 cells in vitro were carried out as described previously [49]. To evaluate the adhesive capacity of E. coli, a relative quantification technique [50], along with colony counting, scanning electron microscopy (SEM), indirect immunofluorescence and gram staining were employed as previously reported [4].
4.6. qPCR Analysis
Trizol (Invitrogen, Shanghai, China) was used to extract total RNAs from duodenal tissues or IPEC-J2 cells. Next, the reverse transcription of RNA was conducted using a Superscript First-Strand Synthesis system (Promega, Madison, WI, USA). SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA) was employed to conduct qPCR analysis. All qRT-PCR assay primers are summarized in Table S1.
4.7. Western Blotting
Total protein isolation was performed with an NE-PER kit (Nuclear and Cytoplasmic Extraction Reagents) (Thermo Fisher Scientific, New York, NY, USA). A bicinchoninic acid (BCA, Nanjing Keygen Biotech, Nanjing, China) kit was employed to normalize protein levels. Proteins were transferred to PVDF membranes and immunoblotted with antibodies against WTAP, GCNT2 and YTHDF2. Horseradish peroxidase-labeled antibody (HRP, 1:5000) was employed as secondary antibody, and GAPDH and HSP90 protein were the endogenous controls.
4.8. Indirect Immunofluorescence (IFA)
IPEC-J2 cells (E. coli F18 exposure vs. control) were carefully rinsed with PBS, prior to fixation in 4% paraformaldehyde, and treatment with Triton X-100. Subsequently, the cells were treated with goat-blocking serum for 1 h, followed by incubation with WTAP antibody (1:500), and secondary antibody IgG-HRP (1:3000). Next, they were treated with DAPI (1:1000), and thrice rinsed with PBS before mounting on slides with a fade-resistant fluorescent mounting medium, prior to analysis via confocal microscopy.
4.9. Transcriptome Sequencing
To conduct transcript sequencing, the total RNA was extracted from normal (Control, n = 3), E. coli F18-exposed (E. coli, n = 3), negative control (shCtrl, n = 3), and WTAP knockdown (shWTAP, n = 3) IPEC-J2 cells. All RNA samples were converted to double-stranded cDNA, which were then sequenced on the Illumina Hiseq2500 platform by Oebiotech Corporation (Shanghai, China). The transcript sequencing data were then uploaded to the NCBI SRA repository under the BioProject IDs: PRJNA826808, PRJNA826988.
4.10. RNA Knockdown and Overexpression
In case of WTAP, GCNT2, YTHDF2, small interfering RNA (siRNA) vector and negative control (siCtrl) (Table S2) were chemically synthesized by Ribobio (Guangzhou, China), and incorporated into cultured IPEC-J2 cells, along with Lipofectamine 2000 from Invitrogen (Waltham, MA, USA). Overexpressing vector (pGLV5-YTHDF2) and a negative control (pGLV5-NC) were synthesized by GenePharma (Shanghai, China), and incorporated into cultured IPEC-J2 cells.
4.11. Methylated RNA Immunoprecipitation (MeRIP)-qPCR
MeRIP assay was employed based on a previously published protocol [51]. In brief, following total RNAs extraction from IPEC-J2 cells, the fragmented RNAs were treated with magnetic Dynabeads associated with anti-m6A antibody to detect transcripts with m6A. The beads were then treated with Proteinase K, and RNA was isolated for verification via qPCR. The primer sequences that amplified the m6A peak region are the following: GCNT2-m6A-3294, F: CAAGCCTGTGTTGACTGTTTCTTGT; R: TCACTCTGATTTAGGGTTCTTTCTC. GCNT2-m6A-3467, F: TGTGAAATGTTTGTCTGGCACA; R: AGAATGCTTCCTCTAGCACTGT
4.12. Dual-luciferase Reporter (DLR) Assays
Wild-type (WT) or mutant-type (Mut) GCNT2-3′UTR (chr7: 7419012-7419243) were ligated downstream of the pmirGLO Dual-Luciferase vector. To conduct DLR assay, cells were seeded in 24-well plates and co-incorporated with wild-type (GCNT2-WT) or mutant-type (GCNT2-Mut) and siCtrl (or siWTAP, or siYTHDF2). Then, the firefly and Renilla bioluminescence were recorded using DLR Gene Assay Kit (Beyotime, Shanghai, China). Data normalization was performed via computation of the ratio between firefly and Renilla luciferase activities.
GCNT2-3’UTR with WT m6A sites:
CGGCATCTGTATCTATGGAAACGGAGACTTAAAGTGGCTGATGAATTCATCAAGCCTCTTTGCTAACAAGTTTGAGCTCAGTACCTACCCTCTTACCGTGGAATGCCTAGAACTGAGGCTTCGAGAAAGAACCCTAAATCAGAGTGAAATTGAAATACAGCCCAGCTGGTATTTTTGATTGGCTGCCACTCACAGGTGAAGGGAAATCACAGCTGGGAAGGAAAACCTTTCT
GCNT2-3’UTR with Mut m6A sites:
CGGCATCTGTATCTATGGAAACGGAGACTTAAAGTGGCTGATGAATTCATCAAGCCTCTTTGCTAACAAGTTTGAGCTCAGTACCTACCCTCTTACCGTGGAATGCCTAGATCTGAGGCTTCGAGAAAGAACCCTAAATCAGAGTGAAATTGAAATACAGCCCAGCTGGTATTTTTGATTGGCTGCCACTCACAGGTGAAGGGAAATCACAGCTGGGAAGGAAAACCTTTCT
4.13. RNA Immunoprecipitation (RIP)
The RIP assay was performed in IPEC-J2 cells according to the RNA Immunoprecipitation Kit (24T, Geneseed Biotech Co., Ltd., Guangzhou, China) following the manufacturer’s instructions. YTHDF2 antibody or rabbit IgG (as control) were used for the RIP assay. Both input and co-RIPs were extracted via Trizol (Thermo Fisher Scientific, New York, NY, USA) and assessed via qPCR analysis.
4.14. mRNA Stability Analysis
To assess GCNT2 mRNA stability, the OE-Ctrl and OE-YTHDF2 IPEC-J2 cells were exposed to 10 μg/mL actinomycin D (MedChemExpress, Shanghai, China) for 0, 3 h and 6 h. Following cell harvest, total RNA was isolated for reverse transcription and qPCR analysis of GCNT2 transcript.
4.15. Statistical Analysis
Data analyses were performed via Student’s t test (two-tailed) using the SPSS v.20 (IBM Corp, Armonk, NY, USA) and GraphPad Prism 6.0 software (GraphPad Inc., La Jolla, CA, USA), and are presented as mean ± SD. * p < 0.05 and ** p < 0.01 were set as the significance thresholds.
5. Conclusions
In conclusion, we identified the m6A methyltransferase WTAP as a critical regulator of E. coli F18 susceptibility in IPEC-J2 cells. This is a novel study that suggests that m6A regulates E. coli F18 invasion by targeting the GCNT2/glycosphingolipid biosynthesis in a YTHDF2-dependent fashion. Our findings offer a novel insight into the underlying mechanism of m6A modification and its regulators in IPEC-J2 cell biology. However, the in vivo data should be extended to strengthen our conclusion, such as the WTAP knockout mice models. A more comprehensive understanding of RNA modifications can enable us to establish small-molecule inhibitors or gene therapy approaches that target proteins to control gene expression or protein translation. Our research will provide theoretical guidance that will help resolve the challenge of combating bacterial diarrhea in piglets.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms232113602/s1.
Author Contributions
Conceived and designed the experiments: W.B. and S.W.; Performed the experiments: Z.W., Y.W., T.L. and L.Y.; Analyzed the data: Z.W.; Contributed reagents/materials/analysis tools: J.J.; Contributed to the writing of the manuscript: Z.W. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Open competition mechanism to select the best candidates Foundation for breeding industry prosperity of Jiangsu Province, China (JBGS [2021]098), China Postdoctoral Science Foundation (2021M702764), College Students’ Innovation and Entrepreneurship Training Program of Yangzhou University (X20220649) and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank Oebiotech Corporation (Shanghai, China) for Illumina sequencing.
Conflicts of Interest
The authors declare that they have no competing interest.
Abbreviations
| m6A | N6-methyladenosine |
| PWD | post-weaning diarrhea |
| E. coli | Escherichia coli |
| IPEC-J2 | intestinal porcine epithelial cell |
| IFA | indirect immunofluorescence assay |
| MeRIP | methylated RNA immunoprecipitation |
| SEM | scanning electron microscopy |
| UTR | untranslated regions |
| DGEs | differential expression genes |
| YTHDF | N6-methyladenosine RNA binding protein 2 |
| WTAP | Wilms’ tumor 1-associating protein |
| GCNT2 | N-acetyllactosaminide beta-1,6-N-acetylglucosaminyl-transferase |
References
- Luppi, A.; Gibellini, M.; Gin, T.; Vangroenweghe, F.; Vandenbroucke, V.; Bauerfeind, R.; Bonilauri, P.; Labarque, G.; Hidalgo, Á. Prevalence of virulence factors in enterotoxigenic Escherichia coli isolated from pigs with post-weaning diarrhoea in Europe. Porcine Health Manag. 2016, 2, 20. [Google Scholar] [CrossRef] [PubMed]
- Do, K.H.; Byun, J.W.; Lee, W.K. Prevalence of O-serogroups, virulence genes, and F18 antigenic variants in Escherichia coli isolated from weaned piglets with diarrhea in Korea during 2008–2016. J. Vet. Sci. 2019, 20, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Boldin, B. Persistence and spread of gastro-intestinal infections: The case of enterotoxigenic Escherichia coli in piglets. Bull. Math. Biol. 2008, 70, 2077–2101. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Yang, L.; Jin, J.; Wang, H.; Wu, S.; Bao, W. Regulation and molecular mechanism of TLR5 on resistance to Escherichia coli F18 in weaned piglets. Animals 2019, 9, 735. [Google Scholar] [CrossRef]
- Wu, Z.C.; Liu, Y.; Dong, W.H.; Zhu, G.Q.; Wu, S.L.; Bao, W.B. CD14 in the TLRs signaling pathway is associated with the resistance to E. coli F18 in Chinese domestic weaned piglets. Sci. Rep. 2016, 6, 24611. [Google Scholar] [CrossRef]
- Wu, Z.; Feng, H.; Cao, Y.; Huang, Y.; Dai, C.; Wu, S.; Bao, W. New Insight into the molecular mechanism of the FUT2 regulating Escherichia coli F18 resistance in weaned piglets. Int. J. Mol. Sci. 2018, 19, 3301. [Google Scholar] [CrossRef]
- Wu, Z.; Shi, D.; Jin, J.; Fan, H.; Bao, W.; Wu, S. DNA methylation of pig FUT3 promoter alters mRNA expression to regulate E. coli F18 susceptibility. Genes 2021, 12, 1586. [Google Scholar] [CrossRef]
- Dominissini, D.; Moshitch-Moshkovitz, S.; Schwartz, S.; Salmon-Divon, M.; Ungar, L.; Osenberg, S.; Cesarkas, K.; Jacob-Hirsch, J.; Amariglio, N.; Kupiec, M.; et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 2012, 485, 201–206. [Google Scholar] [CrossRef]
- Wu, R.F.; Jiang, D.H.; Wang, Y.Z.; Wang, X.X. N(6)-methyladenosine (m(6)A) methylation in mRNA with A dynamic andreversible epigenetic modification. Mol. Biotechnol. 2016, 58, 450–459. [Google Scholar] [CrossRef]
- Yang, Y.; Hsu, P.J.; Chen, Y.S.; Yang, Y.G. Dynamic transcriptomic m6A decoration: Writers, erasers, readers and functions in RNA metabolism. Cell Res. 2018, 28, 616–624. [Google Scholar] [CrossRef]
- Wang, X.; Lu, Z.; Gomez, A.; Hon, G.C.; Yue, Y.; Han, D.; Fu, Y.; Parisien, M.; Dai, Q.; Jia, G.; et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature 2014, 505, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, B.S.; Roundtree, I.A.; Lu, Z.; Han, D.; Ma, H.; Weng, H.; Chen, K.; Shi, H.; He, C. N(6)-methyladenosine modulates messenger RNA translation efficiency. Cell 2015, 161, 1388–1399. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yang, Y.; Sun, B.F.; Shi, Y.; Yang, X.; Xiao, W.; Hao, Y.-J.; Ping, X.-L.; Chen, Y.-S.; Wang, W.-J.; et al. FTO-dependent demethylation of N6-methyladenosine regulates mRNA splicing and is required for adipogenesis. Cell Res. 2014, 24, 1403–1419. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Adhikari, S.; Dahal, U.; Chen, Y.S.; Hao, Y.J.; Sun, B.F.; Sun, H.-Y.; Li, A.; Ping, X.-L.; Lai, W.-Y.; et al. Nuclear m(6)A Reader YTHDC1 Regulates mRNA Splicing. Mol. Cell 2016, 61, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Dominissini, D.; Rechavi, G.; He, C. Gene expression regulation mediated through reversible m(6)A RNA methylation. Nat. Rev. Genet. 2014, 15, 293–306. [Google Scholar] [CrossRef]
- Heng, J.; Tian, M.; Zhang, W.; Chen, F.; Guan, W.; Zhang, S. Maternal heat stress regulates the early fat deposition partly through modification of m6A RNA methylation in neonatal piglets. Cell Stress Chaperones 2019, 24, 635–645. [Google Scholar] [CrossRef]
- Lu, N.; Li, X.; Yu, J.; Li, Y.; Wang, C.; Zhang, L.; Wang, T.; Zhong, X. Curcumin Attenuates Lipopolysaccharide-Induced Hepatic Lipid Metabolism Disorder by Modification of m6A RNA Methylation in Piglets. Lipids 2018, 53, 53–63. [Google Scholar] [CrossRef]
- Wu, R.F.; Liu, Y.H.; Zhao, Y.L.; Bi, Z.; Yao, Y.X.; Liu, Q.; Wang, F.; Wang, Y.; Wang, X. m6A methylation controls pluripotency of porcine induced pluripotent stem cells by targeting SOCS3/JAK2/STAT3 pathway in a YTHDF1/YTHDF2-orchestrated manner. Cell Death Dis. 2019, 10, 171. [Google Scholar] [CrossRef]
- He, S.; Wang, H.; Liu, R.; He, M.N.; Che, T.D.; Jin, L.; Deng, L.; Tian, S.; Li, Y.; Lu, H.; et al. mRNA N6-methyladenosine methylation of postnatal liver development in pig. PLoS ONE 2017, 12, e0173421. [Google Scholar] [CrossRef]
- Zong, Q.; Jing, P.; Sun, S.; Wang, H.; Wu, S.; Bao, W. Effects of HSP27 gene expression on the resistance to Escherichia coli infection in piglets. Gene 2021, 773, 145415. [Google Scholar] [CrossRef]
- Dou, C.; Shang, Z.; Qiao, J.; Wang, Y.; Li, H. Clostridium butyricum Protects IPEC-J2 Cells from ETEC K88-Induced Oxidative Damage by Activating the Nrf2/ARE Signaling Pathway. Oxid. Med. Cell Longev. 2021, 2021, 4464002. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Fu, Q.; Su, G.; Chen, D.; Yu, B.; Luo, Y.; Zheng, P.; Mao, X.; Huang, Z.; Yu, J.; et al. Protective effect of Bombyx mori gloverin on intestinal epithelial cells exposure to enterotoxigenic E. coli. Braz. J. Microbiol. 2021, 52, 1235–1245. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Chen, J.; Yang, J.; He, Q.; Luo, B.; Lu, Y.; Zou, T.; Wang, Z.; You, J. Seaweed polysaccharide mitigates intestinal barrier dysfunction induced by enterotoxigenic Escherichia coli through NF-κB pathway suppression in porcine intestinal epithelial cells. J. Anim. Physiol. Anim. Nutr. 2021, 105, 1063–1074. [Google Scholar] [CrossRef] [PubMed]
- Nikaido, H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. R. 2003, 67, 593–656. [Google Scholar] [CrossRef]
- Zhu, Z.; Xueying, L.; Chunlin, L.; Wen, X.; Rongrong, Z.; Jing, H.; Meilan, J.; Yuwei, X.; Zili, W. Effect of berberine on LPS-induced expression of NF-κB/MAPK signalling pathway and related inflammatory cytokines in porcine intestinal epithelial cells. Innate Immun. 2020, 26, 627–634. [Google Scholar] [CrossRef]
- Coddens, A.; Diswall, M.; Ångström, J.; Breimer, M.E.; Goddeeris, B.; Cox, E.; Teneberg, S. Recognition of blood group ABH type 1 determinants by the FedF adhesin of F18-fimbriated Escherichia coli. J. Biol. Chem. 2009, 284, 9713–9726. [Google Scholar] [CrossRef]
- Moonens, K.; Bouckaert, J.; Coddens, A.; Tran, T.; Panjikar, S.; De Kerpel, M.; Cox, E.; Remaut, H.; De Greve, H. Structural insight in histo-blood group binding by the F18 fimbrial adhesin FedF. Mol. Microbiol. 2012, 86, 82–95. [Google Scholar] [CrossRef]
- Zhou, Y.; Zeng, P.; Li, Y.H.; Zhang, Z.; Cui, Q. SRAMP: Prediction of mammalian N6-methyladenosine (m6A) sites based on sequence-derived features. Nucleic Acids Res. 2016, 44, e91. [Google Scholar] [CrossRef]
- Xuan, J.-J.; Sun, W.-J.; Ling-Ling, Z.; Zhou, K.-R.; Liu, S.; Zheng, L.-L.; Qu, L.-H.; Yang, J.-H. RMBase v2.0: Deciphering the map of RNA modifications from epitranscriptome sequencing data. Nucleic Acids Res. 2018, 46, D327–D334. [Google Scholar] [CrossRef]
- Mi, S.; Shi, Y.; Dari, G.; Yu, Y. Function of m6A and its regulation of domesticated animals’ complex traits. J. Anim. Sci. 2022, 100, skac034. [Google Scholar] [CrossRef]
- Li, N.; Hui, H.; Bray, B.; Gonzalez, G.M.; Zeller, M.; Anderson, K.G.; Knight, R.; Smith, D.; Wang, Y.; Carlin, A.F.; et al. METTL3 regulates viral m6A RNA modification and host cell innate immune responses during SARS-CoV-2 infection. Cell Rep. 2021, 35, 109091. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yang, Q.; Zhang, J.; Gao, X.; Luo, R.; Xie, K.; Wang, W.; Li, J.; Huang, X.; Yan, Z.; et al. N6-Methyladenosine Methylation Analysis of Long Noncoding RNAs and mRNAs in IPEC-J2 Cells Treated with Clostridium perfringens beta2 Toxin. Front. Immunol. 2021, 12, 769204. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Lin, C.; Zhu, Y.; Xu, H.; Yin, Y.; Wang, C.; Tang, X.; Song, T.; Guo, A.; Chen, Y.; et al. Transcriptome Profiling of m6A mRNA modification in bovine mammary epithelial cells treated with Escherichia coli. Int. J. Mol. Sci. 2021, 22, 6254. [Google Scholar] [CrossRef]
- Brocard, M.; Ruggieri, A.; Locker, N. m6A RNA methylation, a new hallmark in virus-host interactions. J. Gen. Virol. 2017, 98, 2207–2214. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.J.; Hao, S.J.; Chen, H.H.; Chen, Z.; Zhang, Y.F.; Wang, J.; Wang, H.; Zhang, B.; Qiu, J.; Deng, F.; et al. N6-methyladenosine modification and METTL3 modulate enterovirus 71 replication. Nucleic Acids Res. 2019, 47, 362–374. [Google Scholar] [CrossRef]
- Zong, X.; Wang, H.; Xiao, X.; Zhang, Y.; Hu, Y.; Wang, F.; Wang, Y.; Lu, Z. Enterotoxigenic Escherichia coli infection promotes enteric defensin expression via FOXO6-METTL3-m6A-GPR161 signaling axis. RNA Biol. 2021, 18, 576–586. [Google Scholar] [CrossRef]
- Ping, X.-L.; Sun, B.-F.; Wang, L.; Xiao, W.; Yang, X.; Wang, W.-J.; Adhikari, S.; Shi, Y.; Lv, Y.; Chen, Y.-S.; et al. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res. 2014, 24, 177–189. [Google Scholar] [CrossRef]
- Śledź, P.; Jinek, M. Structural insights into the molecular mechanism of the m6A writer complex. eLife 2016, 5, e18434. [Google Scholar] [CrossRef]
- Xiao, H.; Zhang, Y.; Sun, L.; Zhao, Z.; Liu, W.; Luo, B. EBV downregulates the m6A “writer” WTAP in EBV-associated gastric carcinoma. Virus Res. 2021, 304, 198510. [Google Scholar] [CrossRef]
- Ge, Y.; Ling, T.; Wang, Y.; Jia, X.; Xie, X.; Chen, R.; Chen, S.; Yuan, S.; Xu, A. Degradation of WTAP blocks antiviral responses by reducing the m6 A levels of IRF3 and IFNAR1 mRNA. EMBO Rep. 2021, 22, e52101. [Google Scholar] [CrossRef]
- Yao, Y.; Bi, Z.; Wu, R.; Zhao, Y.; Liu, Y.; Liu, Q.; Wang, Y.; Wang, X. METTL3 inhibits BMSC adipogenic differentiation by targeting the JAK1/STAT5/C/EBPβ pathway via an m6A-YTHDF2-dependent manner. FASEB J. 2019, 33, 7529–7544. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Wang, X.; Lu, Z.; Zhao, B.S.; Ma, H.; Hsu, P.J.; Liu, C.; He, C. YTHDF3 facilitates translation and decay of N6-methyladenosine-modifified RNA. Cell Res. 2017, 27, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Zhao, Y.; He, J.; Zhang, Y.; Xi, H.; Liu, M.; Ma, J.; Wu, L. YTHDF2 destabilizes m(6)A-containing RNA through direct recruitment of the CCR4-NOT deadenylase complex. Nat. Commun. 2016, 7, 12626. [Google Scholar] [CrossRef] [PubMed]
- Little, N.A.; Hastie, N.D.; Davies, R.C. Identifification of WTAP, a novel Wilms’ tumour 1-associating protein. Hum. Mol. Genet. 2000, 9, 2231–2239. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, K.; Kawamura, T.; Iwanari, H.; Ohashi, R.; Naito, M.; Kodama, T.; Hamakubo, T. Identification of Wilms’ tumor 1-associating protein complex and its role in alternative splicing and the cell cycle. J. Biol. Chem. 2013, 288, 33292–33302. [Google Scholar] [CrossRef]
- Horiuchi, K.; Umetani, M.; Minami, T.; Okayama, H.; Takada, S.; Yamamoto, M.; Aburatani, H.; Reid, P.C.; Housman, D.E.; Hamakubo, T.; et al. Wilms’ tumor 1-associating protein regulates G2/M transition through stabilization of cyclin A2 mRNA. Proc. Natl. Acad. Sci. USA 2006, 103, 17278–17283. [Google Scholar] [CrossRef]
- Xie, W.; Wei, L.; Guo, J.; Guo, H.; Song, X.; Sheng, X. Physiological functions of Wilms’ tumor 1-associating protein and its role in tumourigenesis. J. Cell Biochem. 2019. [Google Scholar] [CrossRef]
- Tang, J.; Wang, F.; Cheng, G.; Si, S.; Sun, X.; Han, J.; Yu, H.; Zhang, W.; Lv, Q.; Wei, J.-F.; et al. Wilms’ tumor 1-associating protein promotes renal cell carcinoma proliferation by regulating CDK2 mRNA stability. J. Exp. Clin. Cancer Res. 2018, 37, 40. [Google Scholar] [CrossRef]
- Xia, P.; Song, Y.; Zou, Y.; Yang, Y.; Zhu, G. F4+ enterotoxigenic Escherichia coli (ETEC) adhesion mediated by the major fimbrial subunit FaeG. J. Basic Microbiol. 2015, 55, 1118–1124. [Google Scholar] [CrossRef]
- Dai, C.H.; Gan, L.N.; Bao, W.B.; Wu, S.L.; Qin, W.U.; Zi, C.; Zhu, G.Q. Use of fluorescence quantitative polymerase chain reaction (PCR) for the detection of Escherichia coli adhesion to pig intestinal epithelial cells. Pol. J. Vet. Sci. 2016, 19, 619–625. [Google Scholar] [CrossRef]
- Dominissini, D.; Moshitch-Moshkovitz, S.; Salmon-Divon, M.; Amariglio, N.; Rechavi, G. Transcriptome-wide mapping of N6-methyladenosine by m6A-seq based on immunocapturing and massively parallel sequencing. Nat. Protoc. 2013, 8, 176–189. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).