Abstract
Cholesterol synthesis occurs in almost all cells, but mainly in hepatocytes in the liver. Cholesterol is garnering increasing attention for its central role in various metabolic diseases. In addition, cholesterol is one of the most essential elements for cells as both a structural source and a player participating in various metabolic pathways. Accurate regulation of cholesterol is necessary for the proper metabolism of fats in the body. Disturbances in cholesterol homeostasis have been linked to various metabolic diseases, such as hyperlipidemia and non-alcoholic fatty liver disease (NAFLD). For many years, the use of synthetic chemical drugs has been effective against many health conditions. Furthermore, from ancient to modern times, various plant-based drugs have been considered local medicines, playing important roles in human health. Phytochemicals are bioactive natural compounds that are derived from medicinal plants, fruit, vegetables, roots, leaves, and flowers and are used to treat a variety of diseases. They include flavonoids, carotenoids, polyphenols, polysaccharides, vitamins, and more. Many of these compounds have been proven to have antioxidant, anti-inflammatory, antiobesity and antihypercholesteremic activity. The multifaceted role of phytochemicals may provide health benefits to humans with regard to the treatment and control of cholesterol metabolism and the diseases associated with this disorder, such as NAFLD. In recent years, global environmental climate change, the COVID-19 pandemic, the current war in Europe, and other conflicts have threatened food security and human nutrition worldwide. This further emphasizes the urgent need for sustainable sources of functional phytochemicals to be included in the food industry and dietary habits. This review summarizes the latest findings on selected phytochemicals from sustainable sources—algae and edible mushrooms—that affect the synthesis and metabolism of cholesterol and improve or prevent NAFLD.
Keywords:
phytochemicals; active compounds; algae; mushrooms; fungi; cholesterol; inflammation; NAFLD; NASH; sustainable 1. Introduction
Due to its role in different metabolic diseases, interest in cholesterol is growing. Cholesterol is essential for cells, both as a structural element and as a participant in various metabolic pathways. Accurate regulation of cholesterol is important for normal lipid metabolism in the body. Disruptions of cholesterol homeostasis have been linked to various metabolic diseases, such as type 2 diabetes, atherosclerosis, heart disease, hypercholesterolemia, and even liver diseases, such as non-alcoholic fatty liver disease (NAFLD) []. NAFLD is a broad term for a variety of liver diseases caused by Western diets based on high fat and high sugar. The main characteristic of NAFLD is a high accumulation of fat in the liver, which can lead to non-alcoholic steatohepatitis (NASH), a severe form of fatty liver disease that involves inflammation of the liver and may progress to liver failure due to cirrhosis and fibrosis []. The overall prevalence of NAFLD worldwide has been recently estimated at 32.4%, increasing significantly over time from 25.5% in 2005 to 37.8% in 2016 []. The therapeutic status of NAFLD today includes several treatments, such as antibiotics, Farnesoid X receptor (FXR) agonists, and probiotics, none of which provide a full response. Furthermore, many clinical trials in recent years have failed to provide the desired results. Therefore, NAFLD remains a disease with no satisfactory therapeutic response [].
Animal cells synthesize cholesterol from acetyl coenzyme A (acetyl-CoA) via more than 20 enzymatic subreactions []. In addition, cells receive cholesterol from the circulating blood in the form of mainly low-density lipoprotein (LDL) containing apolipoprotein B. LDL particles enter the cells via the LDL receptor (LDLR) located on the cell surface and are hydrolyzed to free cholesterol in the cell lysosome []. Cholesterol concentration in the cells is tightly regulated by various mechanisms related to transcriptional and post-transformation levels []. Several genes and transcription factors have been linked to the synthesis and regulation of cholesterol, one of the most prominent being the sterol regulatory element-binding protein (SREBP) family. SREBP transcription factors are encoded by SREBF1 and SREBF2. SREBP-2 activates genes involved in cholesterol synthesis, such as those encoding 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR), LDLR and mevalonate kinase (MVK), among several others (Table 1). In addition, several microRNAs (miRNAs) are also involved in the synthesis and regulation of cholesterol, including miR-122, miR-33, and miR-370 []. These miRNAs regulate the expression of a number of genes responsible for the control of cholesterol levels, such as HMGCR, LDLR, MVK, sterol cleavage-activating protein (SCAP), the phosphoribulokinase (PRKA) family, ATP-binding cassette subfamily A member 1 (ABCA1) and subfamily G member 1 (ABCG1), and cytochrome P450 family 7 subfamily A member 1 (CYP7A1) []. Changes in these miRNAs have been reported to contribute to the development or worsening of NAFLD—one of the most common diseases in the western world—caused by the activation of HMGCR. Understanding the relationship between cholesterol metabolism and NAFLD may have preventive or therapeutic benefits [].
Many studies have demonstrated the diverse effects of phytochemicals on metabolic diseases, cholesterol intake, and dyslipidemia []. Phytochemicals are a rich source of bioactive compounds and have anti-inflammatory, antioxidant, and immunomodulatory properties. In addition, different phytochemicals have been shown to have a positive effect on LDL levels and to help prevent the oxidation of lipids in the blood [].
Worldwide changes in recent years are affecting global supply chains, food security, and nutritional intake. Factors such as climate change, the COVID-19 pandemic, and the current war in Europe are raising the need for phytochemicals from sustainable sources. Algae and edible mushrooms are well-known for their rapid growth while requiring fewer natural resources than higher plants [,]. Mushrooms play beneficial roles in improving soil health and increasing agricultural efficiency; they also play a beneficial role in biodiversity and the health of ecosystems and humans alike and have enormous potential for creating food with minimal waste of resources []. Algae are also considered a prominent and highly sustainable source of food ingredients. Microalgae have photoautotrophic capacity and yield more vegetation than other, more conventional crops; in addition, they do not need land for cultivation, and they contribute significantly to CO2 absorption []. Thus, including sustainable sources such as algae and mushrooms in man’s diet has great potential to sustain the global food system.
This review will focus on the effects of alga- and edible mushroom-based phytochemicals on cholesterol metabolism and its relationship to NAFLD development.
2. Cholesterol Metabolism, Biosynthesis, and Related Genes
About 20–25% of the cholesterol produced by the body daily is produced in the liver; other cholesterol-producing tissues are the adrenal glands, intestine, and reproductive organs []. Cholesterol is an important lipid molecule that serves many biological functions. It is essential for the formation of steroid hormones and bile salts and for the transfer of lipids in the blood. In addition, it is involved in maintaining the membrane’s permeability and fluidity and in the modulation of transmembrane signaling pathways [].
In the blood, there are several types of cholesterol-carrying lipoproteins. These are, from least to most dense: chylomicrons, very low-density lipoproteins (VLDLs), LDLs, intermediate-density lipoproteins (IDLs), and high-density lipoproteins (HDLs) []; the more fat and less protein contained by the lipoprotein, the lower its density. Cholesterol appears in these lipoprotein particles as either free cholesterol or cholesterol-ester, to which a fatty acid is attached []. For cholesterol transfer to a cell, the lipoprotein particles attach to receptors on the target cell surface through apo-lipoproteins, used as ligands. Chylomicrons, the cholesterol-carrying particles with the lowest density, carry fats that have been absorbed in the intestine to the muscle tissue, as well as to other tissues that require fatty acids for energy production or fat storage []. Cholesterol that is not consumed by the muscle or other tissues remains in the remnants of the cholesterol-rich chylomicrons; these are absorbed by the liver. The liver produces VLDL particles which contain excess triglycerides and cholesterol that are not needed by the liver for the synthesis of bile acids []. During their passage through the blood, IDL particles (average density lipoproteins) are formed, which contain a higher percentage of cholesterol []. Some of the IDL particles can be absorbed by LDL receptors on the surface of liver cells, whereas the others continue to lose triglyceride molecules in the blood until they become LDL particles with the highest cholesterol concentration []. In addition to LDL, HDL is the good cholesterol that transports cholesterol from peripheral tissues to the liver; the liver removes it from the body. Higher HDL levels are linked to a lower chance of heart disease and stroke []. The HDL receptor facilitates reverse cholesterol transport. It enables the delivery of lipids to and from HDL lipoproteins by mediating the transfer of cholesterol from the plasma membrane to the HDL lipoprotein. Therefore, LDL particles are the main carriers of cholesterol in the blood, with each particle containing approximately 1500 cholesterol-ester molecules []. The LDL envelope contains only one molecule of apolipoprotein B100, which is recognized by LDLRs in the peripheral tissues. The LDL–LDLR complex is introduced into the cell through endocytosis to form an endosome particle, which later fuses with a lysosome; the cholesterol-ester molecules are hydrolyzed in the lysosome by the enzyme lysosomal acid lipase []. The free cholesterol is released inside the cell and can be used for biosynthesis of the cell membrane; if it is not needed, it can undergo re-esterification for storage inside the cell until it is needed [].
Cholesterol biosynthesis involves enzymes located in the cytoplasm, microsomes (endoplasmic reticulum [ER]), and peroxisomes. The process starts with an acetyl-CoA molecule and usually occurs in the liver cell’s ER []. The first step in cholesterol synthesis produces the intermediate mevalonate, whose synthesis is a committed and rate-limiting step in cholesterol formation. In this reaction, two molecules of acetyl-CoA condense to form acetoacetyl-CoA; catalyzed by 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) synthase, this molecule condenses with a third acetyl-CoA molecule to form HMG-CoA. The latter is then reduced to mevalonate by NADPH, catalyzed by HMGCR []. This is the main regulatory point in cholesterol synthesis that reduces HMG-CoA to mevalonate. Mevalonate then goes through a series of reactions, ultimately yielding cholesterol [] (Figure 1). The synthesis of HMGCR is controlled by SREBPs, a family of integral ER proteins that regulate the transcription of various genes involved in the cellular uptake of cholesterol and its metabolism. After HMGCR’s synthesis, the SREBPs form complexes with SCAP. The insulin-induced genes (INSIGs), which are related to SCAP, are also involved in the SREBP process. Dissociation of INSIGs from this complex allows its transfer from the ER to the Golgi apparatus []. The SREBP–SCAP–INSIG complex is the central regulator of cholesterol levels through the following process. Once the cell’s cholesterol level is high, the binding of cholesterol to SCAP stabilizes the SREBP–SCAP–INSIG complex, and this blocks movement to the Golgi, which causes a decrease in nuclear SREBP and suppresses transcription of the relevant genes, which causes a delay in cholesterol synthesis []. Once the level of cholesterol in the cell is depleted, INSIG1 dissociates from the SCAP–SREBP complex, and the latter is transported to the Golgi apparatus []. An excessive increase in cholesterol level is associated with a higher risk of developing heart disease, stroke, and peripheral vascular disease. High cholesterol is also linked to diabetes, high blood pressure, liver diseases, and metabolic diseases associated with oxidative and ER stress []. In addition to the transcriptional regulation of genes involved in cholesterol metabolism, miRNAs serve as gene regulators, mainly after their transcription []. The miRNAs are small non-coding RNA molecules of about 22 nucleotides in length that affect the post-transcriptional expression of genes by modulating the stability of their mRNA or its translation into a protein []. The involvement of miRNAs in cholesterol metabolism and their role in regulating gene expression have only just begun to be investigated []. Studies by Kathryn et al. showed the involvement of several miRNAs—including miR-122, miR-33, and miR-370—in cholesterol metabolism through the regulation of expression of several genes involved in the control of cholesterol levels such as HMGCR, LDLR, MVK, SCAP, the PRKAs family, ABCA1, ABCG1 and CYP7A1 [], as well as a potential role in the development of liver diseases. Several miRNAs have been shown to participate in the development of NAFLD, such as miR-34a, miR-122, miR-21, and miR-33 [].
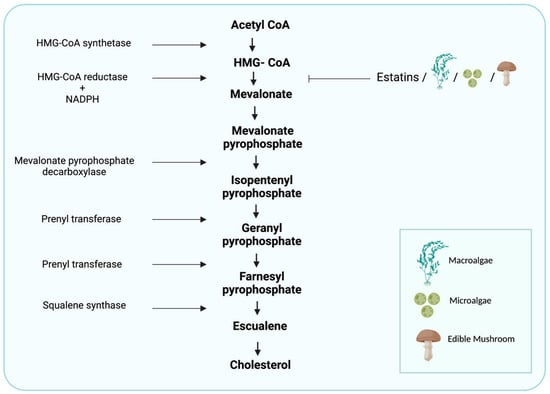
Figure 1.
Cholesterol-synthesis pathway. The diagram includes the most relevant enzymes, the mediators of synthesis, and the point at which statins and algal- and edible mushroom-based phytochemicals interfere with the metabolism of HMG-CoA. (Created with BioRender.com).
Cholesterol synthesis occurs in most cells but mainly in hepatocytes and enterocytes. There are several mechanisms related to cholesterol absorption in these latter cells. More than 30 chemical reactions are needed for the biosynthesis of cholesterol, which occurs in the ER. The first two chemical reactions are reversible reactions and are catalyzed by the enzymes thiolase and HMG-CoA synthase, leading to the condensation of two acetate molecules to form acetoacetyl-CoA []; this then condenses with a third acetate molecule to form HMG-CoA. Another reaction then occurs, catalyzed by the enzyme HMGCR—a transmembrane protein of the ER that reduces HMG-CoA to mevalonate [,]. HMGCR is a key player in cholesterol synthesis. The transcription of this enzyme is responsible for limiting the rate of cholesterol synthesis and is in equilibrium with SREBP-2, which means that its expression is controlled by the concentration of cholesterol in the cells [].
SREBP-2 is a regulatory protein involved in the third feedback mechanism of cholesterol biosynthesis regulation. This protein is the central regulator of genes involved in the synthesis of cholesterol [,]. Located in the ER membrane, SREBP-2 is a double-helix transmembrane protein with two domains: an amino-terminal domain and a carboxy-terminal domain facing the cytosol []. The amino terminus includes an active domain that is transported to the nucleus and binds to the sterol regulatory element (SRE) responsible for the activation and translation of genes related to cholesterol synthesis. SREBP is located on the ER membrane connected to two other receptors: SCAP and INSIG, which together form a complex. This complex can only be transported upon dissociation of INSIG1, which is degraded [] by ubiquitin; the resultant SREBP-2–SCAP complex is inserted into a vesicle called coat protein II (COPII), coated by the proteins Sec23, Sec24, and Sar1 complex [,]. COPII is then moved to the Golgi apparatus and is degraded by site-1 protease (S1P) and site-2 protease (S2P). This releases the amino-terminal active domain of SREBP-2 and SCAP []. SREBP-2 then moves to the nucleus and binds to the SRE, inducing the transcription of genes involved in cholesterol synthesis, such as HMGCR, HMG-CoA synthase, and MVK, as well as LDLR, which is responsible for cholesterol uptake [] (Figure 2).

Figure 2.
Regulation of cholesterol biosynthesis. SREBP-2 is the main regulator of cholesterol biosynthesis and is responsible for increases in blood cholesterol; it is attached to the ER membrane through its interaction with SCAP and INSIG1. When cholesterol levels decrease, INSIG1 is degraded, and the remaining SREBP-2–SCAP complex is inserted into the COPII vesicle in a process driven by GTPase and with the help of a protein complex consisting of Sar1 and Sec23–25. The SREBP-2–SCAP complex is transported to the Golgi by the COPII vesicle, where it is degraded by proteases S1P and S2P. This causes the release of the active domain of SREBP-2, which is transferred to the nucleus and binds to the SRE, resulting in the transcription of genes involved in cholesterol synthesis: HMGCR, HMG-CoA synthase, and MVK, as well as LDLR, which is responsible for cholesterol uptake. (Created with BioRender.com (accessed on 1 September 2022)).
3. Cholesterol Metabolism and Pathophysiology of NAFLD
NAFLD is the most common liver disease worldwide, with a prevalence of 20–30% in the general population of western countries. In NAFLD, excess fat accumulates in the liver (to more than 5% of the liver’s weight) of individuals who drink little or no alcohol []. The most common form of NAFLD is non-alcoholic fatty liver (NAFL), where the fat accumulates in the liver cells; although this condition is not normal, it probably does not damage the liver in and of itself, and there is no inflammation []. However, 10–20% of NAFL patients will progress to a more severe state of the disease termed NASH, in which fat accumulation activates inflammatory processes. NASH can lead to severe scarring of the liver and cirrhosis. Cirrhosis occurs when the liver is significantly damaged and can lead to liver cancer []. Recent studies have shown that a disruption in the balance of cholesterol metabolism in the liver causes the accumulation of lipids and, consequently, liver toxicity and NAFLD. Free cholesterol may accumulate in NAFLD due to its upregulation in the input pathways or a decrease in its elimination. Studies have shown that NAFL/NASH patients have increased expression of HMGCR [,].
Increasing evidence indicates that a change in miRNAs results in the constant activation of HMGCoAr. Studies have shown that several miRNAs are associated with the development of liver diseases and NAFLD [], including miR-34a, miR-122, miR-21, and miR-33, which have a regulatory role in hepatic functions []. miR-122 is the first miRNA to be described in humans for its effect on the liver; it is highly expressed in the liver, representing about 70% of the total miRNA pool in that organ. miR-122 modulates the inactive form of SREBP-2, and a significant decrease in miR-122 has been found in patients with NASH, potentially explaining the increase in SREBP-2 []. In NAFL/NASH patients, there is an increase in SREBP-2 expression; its overexpression is not found in obese patients or patients with other liver diseases, such as hepatitis C. SREBP-2 may be activated in NAFL/NASH by an additional molecular pathway that is responsible for the increase in hepatic free cholesterol levels, but it is independent of the pathway related to changes in cellular cholesterol []. miR-34a has an important role in NAFLD development: it is involved in the dysregulation of cholesterol metabolism because it causes the suppression of sirtuin 1 (encoded by SIRT1), a molecule that is responsible for the regulation of AMP kinase (AMPK) activity, which regulates HMGCR phosphorylation [,]. Increasing evidence suggests miR-34a silencing is a novel therapeutic approach for the treatment of NAFLD. miR-33 has increased expression in the liver of NAFLD patients []. It regulates the metabolism of lipids and insulin-signaling pathways. Its overexpression in NAFLD patients greatly suppresses the expression of ABCA1, promotes cellular cholesterol efflux, and mediates the efflux of cholesterol to lipid-poor apolipoproteins (apoA-I). ABCA1 repression decreases cholesterol efflux to ApoA-I, which has a major role in regulating reverse cholesterol transport []. miR-21 expression level is also increased in NAFL/NASH patients. miR-21 induces hepatic lipid accumulation through interaction with several factors, such as SREBP-1, HMGCR, and fatty acid binding protein 7 (FABP7) [] (Figure 3).
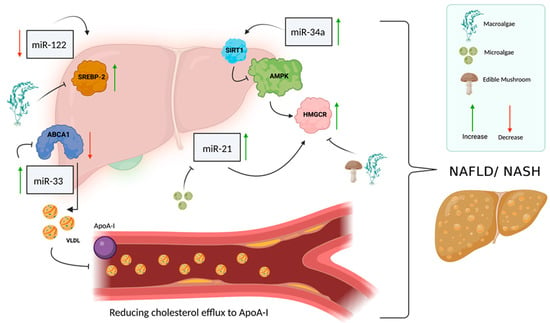
Figure 3.
miRNAs involved in cholesterol metabolism and NAFLD. Several miRNAs are involved in cholesterol metabolism and NAFLD, such as miR-122, miR-34a, miR-33 and miR-21. miR-122 is responsible for the regulation of SREBP-2 in its inactive form and is normally expressed at a high level in the liver, but in NAFLD patients, there seems to be a decrease in miR-122 expression level, which causes an increase in the transcription factor SREBP-2. miR-34a has a key role in the development of NAFLD through its involvement in the dysregulation of cholesterol metabolism. miR-34a is responsible for the suppression of sirtuin 1 (encoded by SIRT1), which in turn is responsible for regulating AMP kinase (AMPK) activity, which regulates HMGCR phosphorylation. When miR-34a levels increase, SIRT1 suppresses AMPK, which causes a decrease in HMGCR levels. As a result of this series of actions, the regulation of cholesterol metabolism is impaired, and there is an increase in cholesterol accumulation in the liver, which contributes to the development of NAFLD. miR-33 is overexpressed in the liver of NAFLD patients and functions as a regulator of lipid and insulin metabolism; when it is overexpressed, it suppresses ABCA1 expression. Suppression of ABCA1 results in a reduction in cholesterol efflux to ApoA-I, which is a key player in the regulation of reverse cholesterol transport. High levels of miR-21 also characterize NAFLD/NASH patients; a high level of miR-21 causes the accumulation of fats in the liver and leads to an increase in HMGCR expression and impaired regulation of cholesterol metabolism. (Created with BioRender.com (accessed on 1 September 2022)).
The liver plays a central role in cholesterol homeostasis. In NAFL/NASH patients, in addition to the increase in cholesterol synthesis due to the activation of HMGCR, there are changes in the pathways involved in cholesterol elimination. A decrease in the expressions of CYP7A1 (responsible for cholesterol catabolism and synthesis of bile acids) and ABCG5/G8 (responsible for cholesterol secretion into bile) has been reported []. Therefore, it is clear to us that in NAFL/NASH patients, there is a basic increase in the synthesis and accumulation of free cholesterol in the liver and a decrease in cholesterol elimination.
4. Western Diet and Relation to Cholesterol Metabolism and NAFLD
Obesity is an increasing health concern worldwide that is influenced by the western diet and lack of physical activity. High cholesterol content in the western diet and lifestyle plays a central role in the development of many metabolic diseases, such as type 2 diabetes, cardiovascular diseases, and NAFLD.
The western diet was originally attributed to the United States, but it has migrated to many countries in the world, including Asian countries [,]. This diet was developed with the aim of getting the population to regularly consume certain food products by adding taste-stimulating and addictive ingredients such as sugar, salt, and processed fats, mostly soy and canola, in very large quantities []. The western diet also includes high consumption of red meat, processed meat, packaged food, fried food, high-fat dairy products, eggs, processed grains, potatoes, corn and high-fructose corn syrup, high-sugar drinks, and food additives (as classified by E numbers, such as titanium dioxide) []. This diet also minimizes and/or eliminates the consumption of plant-based foods, meaning less dietary fiber and fewer vitamins, minerals, and antioxidants [].
In recent years, the relationship between nutrition and metabolic diseases has been well established. Today, it is known that consumption of a high-fat diet (HFD) that raises blood cholesterol (LDL) levels contributes to the development of fatty liver diseases such as NAFLD []. Moreover, a recent study has shown that a toxic combination of cholesterol and dietary fat affects components of the immune system in the liver, such as macrophages. By using an in vivo model, that study presented the cascade of events occurring in the immune system in the liver, which ultimately contributed to the development of local inflammation and scarring of the liver, similar to that which occurs in non-alcoholic steatohepatitis (NASH). Mice were fed an HFD, and this dietary composition had synergistic harmful effects on the genes that regulate inflammation and scarring of the liver [].
5. Effect of Cholesterol-Synthesis Inhibitors on NAFLD
Today, statins are useful drugs for the inhibition of cholesterol synthesis. They include lovastatin, simvastatin, pravastatin, fluvastatin, atorvastatin, and cerivastatin. These six drugs inhibit the enzyme HMGCR, resulting in decreased synthesis of cholesterol in human hepatocytes []; on the other hand, they cause increased transcription of LDLR and the absorption of LDL cholesterol (LDL-c) in the liver []. Statins are indeed effective for most people, but they are also associated with muscle pain, mental fogginess in some individuals, sleep problems, digestive problems, a decrease in blood platelet count, and in rare cases, they can cause liver damage []. Studies have shown that statin therapy seems safe for use in patients with NAFLD. However, it is not yet known whether they should be used for the specific treatment of NAFLD.


Table 1.
Transcription factor, enzyme, receptors, and their associated genes involved in cholesterol metabolism and NAFLD.
Table 1.
Transcription factor, enzyme, receptors, and their associated genes involved in cholesterol metabolism and NAFLD.
| Transcription Factor/Enzyme/Receptor | Related Gene | Cholesterol Metabolism | NAFL/NASH | References |
|---|---|---|---|---|
| SREBPs (SREBP-2) | SREBF1/SREBF2 | Transcription factors activate genes that are involved in the synthesis of cholesterol, such as HMGCR, LDLR and MVK | Increases gene expression levels. NAFLD progression and deterioration | [] [] |
| HMG-CoA | HMGCR | Converts HMG-CoA to mevalonate, which is an enzyme that limits cholesterol biosynthesis | Increases gene expression levels. NAFLD progression and deterioration | [] [] |
| LDLR | LDLR | Receptor; binds to LDL particles, which serve as the main carrier of cholesterol in the blood | Decrease gene expression levels. NAFLD progression and deterioration | [] [] |
| MVK | MVK | Catalyzes the phosphorylation of mevalonate to mevalonate 5 phosphate, a key step in cholesterol biosynthesis | Increases gene expression levels. NAFLD progression and deterioration | [] [] [] |
| SCAP | SCAP | Part of the SREBP–SCAP complex that causes translocation of SREBP from the ER to the Golgi apparatus, resulting in the regulation of cholesterol levels | No data. NAFLD progression and deterioration | [] |
| AMPK | α—PRKAA1, PRKAA2 β—PRKAB1, PRKAB2 γ—PRKAG1, PRKAG2, PRKAG3 | A kinase-type enzyme that plays a role in cells’ energy homeostasis, causes the oxidation of fatty acids in the liver, inhibits cholesterol and triglyceride, consists of several subunits | Decreases gene expression levels. NAFLD progression and deterioration | [,] |
| ABCA1 | ABCA1 | Transporter, cholesterol-efflux mediator for apolipoprotein (apoA-I), maintains cholesterol homeostasis in the body | Decreases gene expression levels. NAFLD progression and deterioration | [] [,] |
| ABCG1 | ABCG1 | Transporter, excess cholesterol flows from the cells to HDL particles, resulting in reverse cholesterol transport, and considered the only factor that removes cholesterol from the body | Decreases gene expression levels. NAFLD progression and deterioration | [] [] |
| CYP7A1 | CYP7A1 | A receptor that accelerates the initial step in cholesterol catabolism and bile acid synthesis | Increases gene expression levels. NAFLD progression and deterioration | [] [] |
6. Phytochemical Effects on Cholesterol Metabolism and NAFLD
Phytochemicals are secondary metabolites and bioactive compounds primarily derived from fruit, vegetables, cereals, and other natural sources. Beyond their use in basic foods as nutrients, they may offer health benefits by reducing cholesterol metabolism and the risk of chronic diseases such as NAFLD due to their anti-inflammatory and immunomodulatory [] properties. Several phytochemicals exert their beneficial effects by lowering circulating cholesterol levels or preventing lipid oxidation. Laka et al. showed that phytochemicals inhibit the expression of enzymes and transcription factors associated with cholesterol metabolism, resulting in lower liver cholesterol levels. Moreover, phytochemicals decrease the level of LDL, suggesting the prevention of cholesterol accumulation. The major classes of phytochemicals include vitamins, polysaccharides, polyphenols, terpenoids, steroids, alkaloids, tannins, saponins, and thiols [] (Figure 4).

Figure 4.
Scheme of alga- and edible mushroom-based phytochemicals. Phytochemicals are secondary metabolites and bioactive compounds naturally found in plants and organisms such as microalgae, macroalgae and edible mushrooms. Among phytochemicals, there are carotenoids, sterols, polysaccharides, and phenols that have biological activity that affects human health. Many phytochemicals act as antioxidants; others succeed in suppressing harmful processes in the body’s cells, preventing the proliferation of cancer cells, regulating lipid levels and having anti-inflammatory activity. (Created with BioRender.com (accessed on 1 September 2022)).
6.1. Clinical Significance of Phytochemicals in Therapeutic Approaches to NAFLD
NAFLD is characterized by the accumulation of excess lipids in the liver tissue, leading to inflammatory and oxidative damage []. There are a number of clinical trials exploring phytochemicals as a therapeutic approach for NAFLD. One such study evaluated a curcumin-based polyphenol of nutritional origin for its effect on lipid properties and its anti-inflammatory and antioxidant activity []. The effectiveness and safety of supplementation with encapsulated curcumin-based polyphenol (phytosomal form) were assessed in 102 NAFLD patients in different stages of the disease (1–3, classified according to liver ultrasound examination). The patients received 1000 mg per day of curcumin in phytosomal form for 8 weeks. The curcumin-based polyphenol was found to be associated with a decrease in body mass index, an improvement in liver ultrasound findings showing a decrease in hepatic fat levels, and a reduction in serum aspartate aminotransferase and alanine aminotransferase levels. Curcumin-based polyphenol appeared to improve liver fat levels, and its use appeared to be safe and well-tolerated during the trial []. In another randomized controlled clinical trial in which children with NAFLD (classified by biopsy) participated, the addition of 2000 IU per day of vitamin D for 6 months was associated with significant improvement in liver steatosis and lobular inflammation, as determined by liver biopsy taken after treatment []. Another active clinical trial illustrating the potential of phytochemicals for the treatment of NAFLD bears mentioning: a randomized, double-blind, placebo-controlled, parallel-group clinical trial examining the potential of vitamin E and docosahexaenoic acid ethyl ester (DHA-EE) administration for NAFLD patients is still in progress [,].
6.2. Vitamins
6.2.1. Vitamin E
The most significant lipid-soluble antioxidant is vitamin E. It effectively prevents many disease processes linked to oxidative stress []. Due to its solubility, bile salts and pancreatic secretion are required for intestinal absorption []. When vitamin E enters the intestine, it forms micelles that are passively diffused and partially mediated by cholesterol membrane transporters through the membrane []. Some of the vitamin E moves to HDL, and the rest remains in the chylomicron [] remnants. In the liver, these remnants bind to α-tocopherol, lipoproteins, and triglycerides. They form VLDL, which is then transported to the plasma. VLDL is separated into VLDL, HDL, and LDL [], and therefore, vitamin E may act as an inhibitor of cholesterol biosynthesis. Sato et al. investigated the clinical effect of vitamin E on NAFLD patients and showed significant improvement in their liver function. The vitamin E supplement decreased liver fat by 0.54 U/L []. As the number of patients with hepatic steatosis rises, vitamin E supplementation may be an adequate treatment opportunity for preventing the deterioration of NAFLD [].
6.2.2. Vitamin B12
Vitamin B12 is an essential cofactor in important methylation processes related to DNA and cell metabolism []. Studies have also demonstrated that a low level of vitamin B12 is related to a high total cholesterol level together with an increased ratio of LDL to HDL in the serum, causing the biosynthesis of cholesterol and homocysteine in adipocytes. Moreover, a low level of vitamin B12 increases the expression of SREBPs and genes responsible for the biosynthesis of cholesterol and leads to a reduction in the levels of AdoMet to AdoHcy and hypomethylation of the regulatory regions of SREBF1 and LDLR [].
6.3. Polyphenols
Polyphenols have been described as plant secondary metabolites. They are usually found in foods such as cereals, fruit, vegetables, wine, coffee, tea, and many other derived foods. Their most salient health benefits are associated with their antioxidant and anti-inflammatory properties. The advantages of polyphenols as a prebiotic substrate are attributed to the intestinal microbiota’s ability to metabolize phenolic compounds [], such as pomegranate peel polyphenols, their main component punicalagin, and its metabolite pomegranate ellagic acid. These polyphenols were tested in human hepatocyte cell line L-02; all of them reduced lipids and enhanced cholesterol metabolism, with ellagic acid being the most effective, followed by peel polyphenols. The molecular mechanism may be via activation of the target gene peroxisome proliferator-activated receptor gamma (PPARγ) and then upregulation of the expression of the downstream genes ABCA1 and CYP7A1 via a PPARγ ABCA1/CYP7A1 cell-signaling pathway, leading to cholesterol metabolism in liver cells. In addition, pomegranate peel polyphenols promote cholesterol metabolism in liver cells, so they could be used in a drug regimen or as a functional food-based material and may be a more appropriate prevention and treatment option for diseases related to excessive cholesterol accumulation [].
6.4. Flavonoids
Flavonoids are a group of natural substances with variable phenolic structures, including flavonols, flavanols, flavanones, flavones, and isoflavones. They are found in fruit, vegetables, grain, bark, roots, stems, flowers, tea and wine and are well known for their beneficial effects on health [].
6.4.1. Flavonols
Flavonols are widely distributed in plants and in large amounts in fruit and vegetables. Aside from their antioxidant effects, they interfere with many biochemical signaling pathways []. Quercetin is a flavonol found in capers. Chambers et al. showed that in rats, quercetin increases Cyp7a1 in the liver and liver X receptor α (LXRα) mRNA and protein, along with the increased secretion of bile acid (BA) and hepatic BAs. Quercetin also increased the expression of ABCG1 mRNA and protein, suggesting its involvement in the regulation of hepatic cholesterol efflux. Another flavonoid, kaempferol, increased hepatic CYP7A1, fecal cholesterol, and BAs, possibly via its binding to LXRα [].
6.4.2. Flavanols
Flavanols (also termed flavan-3-ols) are a family of bioactive compounds found in cocoa, red wine, green tea, red grapes, berries, and apples. These potent antioxidants scavenge free radicals. They may exhibit other activities as well, including modulation of intracellular signaling, effects on membrane fluidity, and regulation of cytokine release or action []. The catechin flavonoids have been found to increase CYP7A1 mRNA levels, with epicatechin gallate showing the most marked induction. Conversely, epigallocatechin gallate is a known activator of Farnesoid X receptor signaling, which suppresses CYP7A1 expression. Human hepatocytes treated with epigallocatechin gallate in the absence of serum showed a reduction in CYP7A1 gene expression [].
6.4.3. Flavanones, Flavones, and Isoflavones
The flavone naringin occurs naturally in citrus fruit []. It has been shown to induce LDLR and CYP7A1 expression in liver cells through nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and extracellular signal-regulated kinase (ERK) signaling pathways, as well as PPARy. The isoflavone puerarin (from arrowroot) has been shown to increase hepatic CYP7A1 expression and suppress hepatic cholesterol. In mice and rats, isoflavone-rich extracts increased hepatic Cyp7a1 expression and BA secretion, respectively. Soy milk and fermented soy milk, both of which contain isoflavones, bioactive proteins and peptides, reduced liver cholesterol and triglyceride levels and increased hepatic Cyp7a1 expression in rats [].
6.5. Anthocyanins
Anthocyanins suppress lipid accumulation in adipocytes as a result of a broad inhibition of the transcription factors that regulate lipogenesis []. One of the most common anthocyanidins (aglycons of anthocyanins) is cyanidin, a compound that is naturally abundant in fruit and vegetables []. It affects all three PPAR subtypes, showing the greatest affinity for PPARα []. Cyanidin-3-O-β-glucoside (C3G) activates LXR-ATP-binding cassette transporter-dependent cholesterol efflux and phosphorylation of cellular AMPK in human hepatoma (HepG2) cells []. C3G was shown to decrease the expression of adipogenic transcription factors involved in hepatic lipid metabolism, including SREBP-1c, CCAAT enhancer-binding protein α (C/EBPα), and PPARγ, in both HepG2 cells and the liver of supplemented mice []. The effect of anthocyanins on suppressing SREBP-1c and C/EBPα gene expression has also been verified in other studies [,,].
6.6. Iridoids
Iridoids are a type of monoterpenoid found in a wide variety of plants, typically as glycosides. Gentiopicroside, a subgroup of seco-iridoid compounds, ameliorated dyslipidemia and improved nerve blood flow by regulating the PPARγ/AMPK/acetyl-CoA carboxylase (ACC) signaling pathway in a rat model. Moreover, in both acute and chronic alcohol-induced mouse hepatosteatosis (the latter stage of alcoholic liver disease), gentiopicroside decreased SREBP-1 upregulation and PPARα downregulation via the activation of AMPK. A decrease in SREBP-1 expression was observed in a nonalcoholic fatty liver disease model [].
6.7. Palmitoylethanolamide
Palmitoylethanolamide regulates LXR activity, which causes activation of PPARα by PPARγ, in addition to the downstream ABCA1. In contrast, it does not affect retinoid X receptor α. Palmitoylethanolamide promoted cholesterol removal by increasing fecal bile acid and upregulating the two pathways LXR/PPAR–ABCA1 [].
6.8. Alkaloids
Alkaloids are important secondary metabolites with therapeutic properties. They can prevent the onset of various degenerative diseases via free-radical scavenging or binding with the oxidative reaction catalyst [].
6.8.1. Nuciferine
Nuciferine is an active aporphine alkaloid that has a significant effect on reducing intracellular triglyceride levels and improving lipid metabolism in 3T3-L1 adipocytes []. Nuciferine prevented hepatic steatosis in golden hamsters fed with HFD []. Supplementation with this compound significantly alleviated serum and hepatic lipid levels and enhanced expression levels of PPARα and carnitine palmitoyl transferase 1 (CPT1), which contributed to increased fatty acid oxidation. In addition, nuciferine treatment significantly reversed the increase in hepatic levels of proteins SREBP-1c and fatty acid synthase (encoded by FASN) caused by the HFD, indicating that nuciferine can suppress the development of hepatic steatosis induced by an HFD [,].
6.8.2. Betaine
Betaine notably enhances hepatic steatosis in C57BL/6J mice through AMPK activation and SREBP-1c downregulation []. It also suppressed triglyceride accumulation in the liver by reducing methylation of PPARα, thus increasing its expression []. Similar results were found in rats fed an HFD, where betaine enhanced hepatic lipid export and fatty acid oxidation by increasing the expression of PPARα and CPT1 []. In addition to this, betaine has shown a promotion in the absorption of fatty acids through increasing the expression of genes involved in fatty acid transport, such as cluster of differentiation (FAT/CD36)/fatty acid translocase, fatty acid transport protein (FATP1) and FABP3 in the muscle tissue of finishing pigs [].
6.9. Omega-3 Fatty Acids
The polyunsaturated omega-3 fatty acids regulate transcription factors associated with lipid metabolism in the liver, resulting in higher oxidation of fatty acids and the regulation of downstream proinflammatory genes []. Omega-3 fatty acids have also been shown to reduce circulating triglycerides by reducing the hepatic secretion of VLDL cholesterol or increasing chylomicron metabolism. Chan et al. showed that fish oil supplementation lowers triglycerides (−18%), VLDL apolipoprotein B (−20%) and hepatic excretion of VLDL apolipoprotein B (−29%) compared to supplementation with placebo []. This effect resulted in a 35% reduction in the synthesis of triglycerides and an increase in the mitochondrial oxidation of fatty acids [].
7. Effects of Plant-Based Active Compounds on Cholesterol Metabolism and NAFLD
7.1. Microalgae
Microalgae are a diverse class of eukaryotic and prokaryotic unicellular organisms that are predominantly autotrophic. Only a few species have been thoroughly explored: Dunaliella, Chlorella, Isochrysis, Nannochloropsis, Chlamydomonas, Haematococcus, and Spirulina, although the number of microalgal species has been estimated at around 200,000 (according to AlgaeBase, www.algaebase.org (accessed on 1 September 2022)). Due to their well-balanced chemical components, microalgae are one of the most viable sources of nutrients and bioactive substances for new food products that can be utilized to improve the nutritional content of foods. In fact, adding microalgal biomass to food products is an intriguing way to add biologically active supplements to the diet, such as vitamins A, B1, B2, B6, B12, C, and E, proteins with essential amino acids, polysaccharides, monounsaturated and polyunsaturated fatty acids (MUFAs and PUFAs, respectively), nucleic acids, minerals such as iodine, potassium, iron, magnesium, and calcium [,], and several bioactive compounds such as dietary fiber, polyphenols, carotenoids, phycobiliproteins, and polysaccharides [] (Figure 4). The complement of bioactive compounds differs among species growing in different environments—temperature, light conditions, pH, CO2 level, nutrients, and salts []. Due to their antioxidant, antimicrobial, anti-inflammatory, anticarcinogenic, antidiabetic, antihypertensive, antihyperlipidemic, and antiobesity activities, these molecules have potential health applications [,]. Microalgae are applied in the cosmetic, food, and pharmaceutical industries and are considered to be therapeutic agents for a number of medical conditions due to their ability to produce a variety of primary and secondary metabolites []. One of the many health benefits of microalgae is balancing cholesterol metabolism by regulating several genes that also affect liver function, which plays a key role in cholesterol homeostasis [].
7.1.1. Microalgal Phytochemicals Affecting Cholesterol Metabolism in the Liver and NAFLD
The major phytochemicals—important secondary metabolites—are alkaloids, flavonoids, terpenoids, carotenoids, glycosides, phenols, and sterols []. Phytochemicals are essential to several processes, serving as inhibitors of oxidation, cancer, viruses, bacteria, and enzymes. There are about 4000 phytochemicals of both terrestrial and marine origin that have established health benefits, with marine phytochemicals having higher potential biological activity than terrestrial ones []. With their high concentration of phenols and terpenes, microalgae are a significant source of phytochemicals and nutritional supplements [].
Carotenoids are lipid-soluble pigments that are primarily responsible for living organisms’ distinctive purple, red, orange, and yellow colors []. They are divided into carotenes (lycopene and β-carotene) and xanthophylls (lutein, zeaxanthin and astaxanthin) [].
Microalgal Astaxanthin
Yoshida et al. investigated the administration of astaxanthin on serum HDL cholesterol (HDL-c) and found several mechanisms, including reverse cholesterol transport from peripheral tissues to the liver, where astaxanthin can be used to treat impaired lipid metabolism and improve the serum lipid profile in humans, including an increase in HDL-c and significant negative risk for several diseases []. In patients with NAFLD and NASH, miR-21 was more highly expressed than the other miRNAs [], and its increase was in direct response to fast food and, therefore, to increases in the hepatic level of unsaturated fatty acids []. Shatoor et al. investigated the effect of astaxanthin on NAFLD in relation to miR-21. In rats fed an HFD, they examined whether astaxanthin can improve hepatic steatosis by modifying the nuclear factor erythroid-2-related factor 2(Nrf2)/miR-21 axis []. Further studies proved that Nrf2—a fundamental antioxidant transcription factor [] and a dominant inhibitor of miR-21 []—was elevated and activated, mediating the hypolipidemic and antisteatotic actions of astaxanthin [].
Astaxanthin was also investigated as a preventive of NAFLD through its decrease in SREBP1/2 expression, which inhibits oxidative stress and inflammation, and through activation of PPARα, which significantly promotes fatty acid oxidation and suppresses the production of triglycerides. SREBP1/2 are the primary transcriptional factors promoting the production of triglycerides and cholesterol []. Shatoor observed a significant reduction in serum levels of triglycerides (38%), cholesterol (33%), and LDL (32%), and hepatic levels of triglycerides (13%) in rats fed a standard diet with astaxanthin treatment compared to those fed a standard diet alone (p < 0.05). In contrast, the HFD + astaxanthin group significantly outperformed the HFD group in terms of liver weight (107% improvement), serum triglycerides (91.3%), cholesterol (106%), and LDL-c (60%) levels, as well as hepatic triglycerides (88%), cholesterol (75%), and free fatty acid (84%) levels (p < 0.0001). The percentages of improvement were calculated as follows: (levels for HFD + astaxanthin − levels for HFD]/[levels for HFD − levels for standard diet). Shatoor et al.’s results suggest that astaxanthin reduces hepatic lipid production via an Nrf2-dependent increase in hepatic free fatty acid oxidation and suppression of cholesterol and triglyceride synthesis. When Shatoor et al. investigated the changes in the expression of miR-21 and lipogenic genes, they observed a high positive association between the mRNA levels of hepatic miR-21 and SERBP-1/2 (r2 = 0.886 and r2 = 0.8682, respectively). These findings show that astaxanthin reduces Srebp-1 and Srebp-2 expression while increasing PPAR expression in the livers of HFD-fed rats. These results imply that astaxanthin may inhibit miR-21 in an Nrf2-dependent manner to prevent the initiation and progression of NAFLD in rats fed an HFD diet [] (Figure 3).
Microalgal Fucoxanthin
Antioxidant, anticancer, anti-inflammatory, and antiobesity benefits are only a few of the health-promoting properties of fucoxanthin []. Fucoxanthin significantly influences the enzymes that control cholesterol levels and effectively lowers triglyceride levels in the liver and adipose tissue []. Fucoxanthin also impacts the expression of genes involved in lipid metabolism, including those for fatty acid synthase, stearoyl-CoA desaturase-1 (SCD1), C/EBP, PPAR, and SREBP []. Koo et al. evaluated body and organ weight following supplementation with Phaeodatylum extract (PE), known as a potential source of fucoxanthin, and found uncoupling protein 1 (UCP1) upregulation in brown adipose tissue and PPAR downregulation, indicating the mechanism by which PE regulates lipid metabolism []. Those authors also determined that PE controls lipid metabolism via PPAR and UCP1 and, as a result, has an antiobesity effect. Koo et al. also explored the effect of PE on serum lipid parameters to evaluate its effect on lipid metabolism and liver damage. PE treatment resulted in a dose-dependent reduction in LDL levels and could also reduce lipid metabolism dysfunction and liver damage. Livers of the HFD-fed group had more lipid droplets than those of the normal diet group, but PE treatment reduced lipid droplet number and size. Triglyceride level was also reduced in the PE-treated group. These results show that PE affects hepatic steatosis mainly by reducing excessive fat accumulation [,]. Mayer et al. examined whether lipids and carotenoids from the microalga Phaeodactylum tricornutum can prevent the cell characteristics of NAFLD. The human hepatocarcinoma cell line HepG2, frequently used to research lipid metabolism and metabolic abnormalities in the liver, including those related to NAFLD [], served as the cell model. Cells were treated with 250 mM palmitate for 24 h—a procedure typically applied to promote lipotoxicity and hepatic steatosis in HepG2 cells—to induce NAFLD [,]. They showed that carotenoid extract (CE) inhibits the increase in total cholesterol, cholesterol-ester, and triglycerides and reduces the lipid droplet accumulation induced by palmitate. The major bioactive component of CE, fucoxanthin, may control the expression of lipid metabolism-related enzymes such as ACCα, fatty acid synthase, diacylglycerol O-acyltransferase 1, and SCD1; additionally, it could decrease the mRNA levels of acyl-CoA:cholesterol acyltransferase 1/sterol O-acyltransferase 1 (ACAT1/SOAT1) in HepG2 cells [,]. Finally, CE elevated CPT1A’s mRNA level, suggesting that fucoxanthin functions by promoting the beta-oxidation pathway to reduce lipid droplet accumulation and production. Furthermore, Mayer et al. explain that CE appears to have modifying effects on lipid metabolism by increasing the mRNA levels of CPT1A, a gene associated with fatty acid oxidation, and decreasing the mRNA levels of lipogenesis genes, indicating that fucoxanthin CE may serve as a powerful anti-NAFLD therapy.
Microalgal Polysaccharides
Microalgal polysaccharides may constitute another category of natural lipid-regulating compounds, particularly considering the non-digestible polysaccharides from a variety of food sources that have been recognized for their lipid-regulating properties []. Xylose, glucose, and galactose are the major sugars in the heteropolymeric cell wall polysaccharides []. The combination of glucuronic acid and sulfate groups gives these molecules a negative charge []. Dvir et al. used Porphyridium sp. Polysaccharides to investigate their therapeutic effect on the lipid metabolism of hypercholesterolemic rats fed diets rich in cholesterol []. The results were similar to those in other studies investigating the effect of microalgae on lipid metabolism [,,,] and showed a decrease in the accumulation of hepatic cholesterol, plasma triglycerides, and VLDL cholesterol. In another study, animals fed soluble fibers to reduce cholesterol were shown to have higher levels of HMGCR, which is essential for endogenous cholesterol formation []. Compared to control animals, hypercholesterolemic rats fed microalgal polysaccharides at levels of 5% or 10% had increased levels of HMGCR.
Wan et al. analyzed hepatic mRNA and protein levels of genes associated with the AMPK pathway, an important factor in regulating glycolipid metabolism, to examine the mechanism governing microalgal polysaccharides’ effects on the hypolipidemic activity in diet-induced rats []. After treatment with microalgal polysaccharides, hepatic steatosis and lipid droplets decreased, along with liver triglycerides, total cholesterol and body weight []. Therefore, microalgal polysaccharides could serve as a good source for improving lipid metabolism by regulating triglyceride and LDL-c metabolism. It was also observed that in comparison to the HFD group, microalgal polysaccharide significantly increased AMPK mRNA expression, which in turn reduced HMG-CoA and SREBP-1 levels and inhibited the synthesis of fatty acids [] (Figure 3).
Gou et al. examined the potential effects on the lipid metabolism of polysaccharides isolated from Chlorella pyrenoidosa (CPC) and Spirulina platensis (SPC) in mice fed an HFD with a reference compound of β-glucan []. A significant role of the ligand-dependent transcription factor PPAR was identified in the late phase of adipocyte development []. Through the activation of PPAR expression, the production of an endogenous PPAR ligand, and the regulation of the expression of several crucial genes for lipid biosynthesis, the transcription factor SREBP-1c plays an essential role in the development of adipocytes []. Gou et al. used Western blot analysis to show that the hepatic PPAR and Srebp-1c levels in HFD control mice were significantly higher than those in low-fat diet control animals, indicating that HFD-fed mice had higher levels of adipogenesis. Oral administration of β-glucan, CPS, and SPS significantly inhibited the HFD-induced rise in both adipogenic transcription factors, supporting their contribution to lowering lipogenesis in mice fed the HFD [] and therefore characterizing them as a potential source for improving lipid metabolism. Fucoidan, a low-molecular-weight polysaccharide found in algae, reduced hepatic accumulation of triglycerides and cholesterol, as well as total cholesterol, in mice. In addition, this polysaccharide elevated the level of the anti-inflammatory adiponectin, an adipose hormone associated with inflammation, while down-regulating various proinflammatory cytokines and transcription factors. Fucoidan treatment also resulted in the activation of hepatic AMPK signaling. According to the findings of Zheng et al., fucoidan polysaccharide prevents NAFLD in mice by inducing the AMPK signaling system [].
Microalgal Phytosterols
Phytosterols are secondary metabolites of cholesterol-like substances found naturally in plants. They reduce plasma LDL-c and cholesterol absorption []. As secondary metabolites of steryl glycosides, steroid glycosides are a class of major natural compounds with nutritional and pharmaceutical value []. Steryl glycosides are a group of sterol metabolites in which a sugar compound is bound to the hydroxyl group of the sterol. In plants, steryl-D-mono glucopyranoside is the most common steryl glycoside []. Steryl glycosides have significant biological activities, such as controlling lipid metabolism, developmental processes, and host defenses against pathogens []. A great source of steryl glycosides is microalgae. Recently, it was revealed that the marine microalga Pavlova viridis has a 5.234 mg/kg dry weight of steryl glycosides. Microalgae can produce a more diverse range of sterols than higher plants []. However, there is considerable variation in steryl glycoside composition across various microalgae []. In general, phytosterols are considered to lower cholesterol levels by competitively inhibiting cholesterol absorption and modifying the genes in enterocytes and hepatocytes that are involved in cholesterol metabolism []. According to a recent study, dietary phytosterols increase the expression of the hepatic HMGCR in response to the low hepatic cholesterol levels brought on by the phytosterol-induced reduction in intestinal cholesterol levels []. Feng et al. examined, by quantitative PCR, the expression of genes involved in lipid and bile acid metabolism to understand the mechanisms governing phytosterol’s reduction of hepatic lipid buildup and bile acid pool size. Their results showed that HMGCR mRNA expression in the liver was significantly reduced in a high-fat western diet, but phytosterols stopped the reduction. PPAR expression also showed a significant increase upon phytosterol supplementation. Feng et al. also reported that long-term phytosterol consumption inhibits NAFLD development via cholesterol reduction in the liver []. In addition, phytosterols have been shown to be novel treatment compounds for hepatic fibrosis, LDL concentration reduction, improving insulin resistance, down-regulating systemic inflammation, and increasing circulation of endothelial progenitor cells in patients with NAFLD [,].
Microalgal Phenolics
The largest class of phytochemicals are polyphenols, many of which were discovered in foods derived from plants []. These plant-derived secondary metabolites classified as phenolic compounds share an aromatic ring with one or more hydroxyl groups []. Plant sources have been identified with several phenolic substances, such as simple phenols, flavonoids, lignins and lignans, tannins, xanthones, and coumarins []. Safafar et al. investigated the total phenolic content in several different microalgal species using the Folin–Ciocalteu method and revealed a significant difference among them, which may depend on their growth conditions and oxidative stress. The highest concentration was found in Desmodesmus with 7.72 mg/g []. As a defense strategy against oxidative stress brought on by excess reactive oxygen species, polyphenols are essential compounds that support and enhance the activities of antioxidant vitamins and enzymes. Therefore, polyphenol-rich diets have been associated with a number of health advantages against, for example, cancer, inflammation, cardiovascular diseases [,] and liver injuries [].
Several signaling pathways, including those that increase fatty acid oxidation by upregulating PPAR, decrease lipogenesis by downregulating SREBP-1c and activating AMPK and increase antioxidant defense through the Nrf2 pathway. Polyphenols may protect against hepatocyte injury caused by NAFLD. Various polyphenols are considered to act through AMPK regulation, especially with c-Jun N-terminal kinase (JNK) and p38 MAPK, which have been suggested as viable pathways for active therapy for NAFLD []. SREBP-1c has also been recognized as a target in NAFLD therapy. Its increase in the liver promotes fatty liver, and studies have shown that phenols may inhibit SREBP-1c by downregulating its protein and gene expression [].
7.2. Macroalgae
Macroalgae, commonly known as seaweed, are large, macroscopic algae that resemble grass. Due to their size, macroalgae have the ability to eliminate harmful toxins due to their large surface area, which allows biological absorption of toxic compounds from the environment. In addition, they have essential ingredients with health benefits, such as proteins, polysaccharides and omega-3 fatty acids [] (Figure 4). Macroalgae are classified into brown algae, green algae and red algae. Red and brown algae are considered marine algae, whereas green algae may also be found in freshwater, such as lakes and rivers []. Macroalgae may contain large amounts of tocopherols, a group of compounds that have vitamin E activity and are powerful antioxidants [], as well as additional antioxidants such as vitamins A and C. Moreover, macroalgae contain a number of protective pigments and secondary metabolites []. These macroalgal compounds have been shown in recent studies to have anti-inflammatory, antioxidant, anticarcinogenic, antidiabetic, anti-Alzheimer, and antimicrobial activity, as well as antihyperlipidemic and antiobesity effects []; they play a significant role in health promotion.
7.2.1. Macroalgal Phytochemicals Affecting Cholesterol Metabolism in the Liver and NAFLD
Cholesterol is an essential factor for cell homeostasis. Cholesterol homeostasis is a tightly regulated process, and any imbalance can result in hypercholesterolemia, including high levels of total cholesterol, LDL-c, or triglycerides, and even a decrease in HDL-c [].
In vivo studies have reported the hypocholesterolemic effect of different macroalgal species [,,,]. In most studies, algae rich in phytochemical extracts were used as a dietary supplement, resulting in decreased total cholesterol, triglyceride and LDL-c levels and increased HDL-c levels. Those effects on blood lipid levels might be explained by the modification of cholesterol biosynthesis due to a modulatory effect on the high-affinity receptor of lipoprotein metabolism. In addition, macroalgae have the reported ability to absorb cholesterol and excrete it from the body through the feces. A recent in vivo study of rats fed a 20% HFD and an additional 2.5% of extracts from the macroalgae Ecklonia arborea or Silvetia compressa showed different results for the two algae with respect to cholesterol levels and liver histology []. S. compressa caused an 18% decrease in total cholesterol, whereas E. arborea increased cholesterol levels by 5.8% compared to the control rats. In addition, the group fed the S. compressa extract had lower levels of LDL-c. Liver histology showed a reduction in steatosis in the group fed S. compressa vs. E. arborea extract. This suggests S. compressa as a resource for the reduction of serum cholesterol and liver tissue damage [].
Macroalgal Polysaccharides
Polysaccharides derived from plant foods are major components of the human diet and serve as major sources of energy in all diets []. NAFLD is caused by the accumulation of fat in the liver and is associated with a disorder in cholesterol metabolism []. Previous studies have shown that polysaccharides from the alga Enteromorpha prolifera lower cholesterol levels in diabetic rats []. A study based on those results examined the possible effect of E. prolifera polysaccharides on the development of NAFLD and its underlying mechanisms. These polysaccharides, supplemented at 200 mg per kg body weight, significantly reduced liver weight and also decreased mRNA and protein expression of HMGCR in the liver []. They also suppressed the regulatory element of sterol-binding protein 2—a key transcription factor in cholesterol metabolism and regulator of HMGCR expression. In light of these results, E. prolifera polysaccharides may provide a functional food that can prevent NAFLD []. Moreover, microalgal polysaccharides can reduce blood lipid levels and total cholesterol and simultaneously increase HDL-c levels [,]. In addition, they reduce the absorption of cholesterol in the body by binding the diet-supplied cholesterol compounds and excreting them from the body through the feces []. Cholesterol absorption by polysaccharides is affected by the ellagic acid found in large quantities in macroalgae. Sodium alginate in seaweed turns into free ellagic acid, and in this process, it is transformed into a gel that prevents absorption in the small intestine and drives the excretion of cholesterol. In addition, in aged hypercholesterolemia-induced rats that received HFD for 12 weeks together with an aquatic extract of Ulva fasciata polysaccharides, the extract showed antihypercholesterolemic properties. Thus, it could be used as a natural lipid regulator []. Another study examining the effect of polysaccharides from U. fasciata showed significant decreases in total cholesterol, triglycerides and total lipids compared to the untreated rats [].
Macroalgal Fucoxanthin
Seaweed’s antioxidant capacity is known to be due in part to carotenoids. Fucoxanthin [] is the most abundant carotenoid in macroalgae. Its hypolipidemic effect has been described in various studies, where it lowered the levels of cholesterol and triglycerides in the liver and therefore affected both the absorption of cholesterol in the liver and by inhibiting HMGCR and its synthesis [,] (Figure 4). Fucoxanthin also causes the absorption of cholesterol and its excretion through the feces by reducing the mRNA levels of ACAT and increasing those of lecithin cholesterol acyltransferase; these two enzymes are responsible for catalyzing the esterification of free cholesterol to cholesteryl ester [].
Macroalgal Phenolics
The main phenolic compounds in macroalgae, and in particular red macroalgae, are phlorotannins, which make up as much as 25% of the algae’s dry weight. Phlorotannin activity and its role in hypolipidemic activity have not yet been completely described, but it is now known that these compounds have an effect on the biosynthesis and absorption of cholesterol []. A phenolic-rich extract from Fucus vesiculosus and Ecklonia cava was able to inhibit cholesterol absorption, although the mode of action has yet to be clarified [,]. Moreover, other studies have shown that E. cava can inhibit cholesterol esterase, which limits the absorption of dietary cholesterol []. Similarly, phenolic compounds isolated from plants or fruit showed the ability to inhibit NPC1-like intracellular cholesterol transporter 1 (NPC1L1), a transporter that takes part in cholesterol absorption, similar to the HMGCR inhibitor (statin) [,]. Ezetimibe Na et al. isolated new anti-lipid compounds from the edible brown macroalga Ecklonia stolonifera. They showed that an ethanolic extract enriched with phenolic phlorotannin affected lipid levels in the blood of rats with hyperlipidemia induced by HFD. The results showed reductions in triglycerides, total cholesterol, and LDL-c levels, as well as a significant increase in HDL-c levels.
7.3. Edible Mushrooms
In addition to their aromatic taste and culinary properties, edible mushrooms are a rich source of bioactive compounds. Mushrooms have recently become attractive as a functional food and for their potential impact on human health. Many cultures over the years have consumed mushrooms as both food and medicine. Today, mushrooms are consumed as food due to their low-fat content and high nutritional value, as they contain proteins, polysaccharides, polyphenols, fibers, minerals and vitamins [,,] (Figure 4). The bioactive compounds found in mushrooms, in particular edible mushrooms, have anti-inflammatory, anticancer, antioxidant and anticholesterolemic properties [,].
7.3.1. Fungal-Based Phytochemicals Affecting Cholesterol Metabolism in the Liver and NAFLD
Studies have revealed a hypocholesterolemic effect of Pleurotus ostreatus on rats fed an HFD and those with a hereditary cholesterol disorder []. The decrease in cholesterol levels was also demonstrated by a decrease in LDL levels, in addition to the elimination of HMG-CoA reductase activity [] and a reduction in total cholesterol and triglycerides in the plasma [,]. Moreover, an interesting study on the Mexican mushroom Ganoderma lucidum provided evidence for its hypocholesterolemic effects in C57BL/6 mice. In that study, a significant reduction was observed in the expression of lipogenic genes, such as HMRC, FASN, SREBP-1C and ACACA, and of genes responsible for the reverse transport of cholesterol, such as ABCG5 and ABCG8, as well as increased expression of LDLR in the liver [].
Previous studies have shown that vitamin D deficiency is linked to NAFLD, and both conditions are considered risk factors for metabolic diseases []. In addition, there is evidence linking vitamin D, NAFLD, and increased cholesterol levels [].
C57BL/6 mice fed an HFD together with Lentinula edodes mushroom extract or with vitamin D-enriched mushroom extract for 25 weeks showed significant attenuation of total body fat accumulation rate, decreasing liver fat content, a significant decrease in serum triglycerides and LDL-c, and an increase in the HDL/LDL ratio. These results indicate that the consumption of mushrooms enriched with vitamin D may serve to treat or prevent the development of NAFLD [].
Fungal Polysaccharides
Polysaccharides are the most common carbohydrates in foods, with a wide variety of physiological functions according to their composition and molecular weight [].
Pleurotus ostreatus, also called the oyster mushroom, is considered one of the most consumed mushrooms in the world, along with the white button mushroom (Agaricus bisporus). A particularly notable advantage of this mushroom is the fact that it grows quickly compared to other mushrooms, and in addition, it contains many bioactive compounds, including β-glucans—a polysaccharide derived from D-glucose that is linked by glycosidic bonds, which has been shown to decrease serum LDL-c. P. ostreatus contains twice as much β-glucan as A. bisporus []. P. ostreatus is also known for its potent ability to produce lovastatin, a pharmaceutical therapy agent which helps lower cholesterol levels by inhibiting HMG-Co-A reductase. Therefore, consumption of a diet rich in P. ostreatus results in lower triglyceride and cholesterol levels. In addition, polysaccharides from L. edodes showed a decrease in the expression level of vascular cell adhesion molecule 1 (VCAM-1) mRNA of the thoracic aorta endothelial cells in rats, with increased antioxidant enzyme activity and a significant decrease in total cholesterol, triglycerides, and lipoprotein-cholesterol levels together with inhibition of damage caused by oxidative stress in the blood due to an HFD []. Li et al. examined the impact of polysaccharides found in the edible fungus Grifola frondosa on potential regulatory mechanisms controlling lipid and cholesterol metabolism in rats []. The production of 7-α-hydroxycholesterol, which is crucial for the maintenance of cholesterol levels, is catalyzed by CYP7A1, a critical rate-limiting enzyme in the synthesis of bile acids []. Livers of rats supplemented with G. frondosa had significantly higher levels of hepatic CYP7A1 mRNA, which may have enhanced the production of bile acids and decreased hepatic triglycerides [].
Previous research has shown that Ganoderma amboinense polysaccharide (GAP) can protect the liver. In a recently published study in which mice fed an HFD were given GAP for 8 weeks, GAP effectively prevented NAFLD development and reduced body weight, liver weight, and blood lipid levels. It appears that GAP promotes the transport of fat in the liver by regulating the content of phosphatidylcholine in the blood and regulates several metabolic pathways that protect the mitochondrial function responsible for the rapid catabolism of liver cell fats. According to these results, it appears that GAP may help prevent and treat NAFLD [].
Fungal Phenolics
Phenols are a large family of organic compounds that are found in plants and fungi. Polyphenols are widely used as antioxidants and have a beneficial effect on human health. Their regular consumption is associated with reductions in several metabolic diseases, including cardiovascular disease, obesity, liver disease, and insulin resistance []. Recent studies have reported that a diet rich in polyphenols may play an important role in preventing high cholesterol levels. One example of polyphenols are flavonoids, which improve glucose and lipid metabolism in the liver []. Suillus luteus and Boletus badius are edible mushrooms that are rich in polyphenols []. Both polyphenols and dietary fiber from mushrooms can reduce cholesterol and bile acids produced by the liver. Bile acids are secreted into the small intestine to facilitate digestion and absorption of dietary fats. They are reabsorbed by enterocytes and transported back to the liver through the enterohepatic circulation. When polyphenols and dietary fibers mix with bile acids in the enterohepatic circulation, the bile acids are excreted together with cholesterol through the feces. This results in reduced cholesterol levels and has a positive effect on the blood lipid profile [].
Fungal Flavonoids
Flavonoids are found naturally in many plants, as well as in edible mushrooms. Flavonoids are known to have antioxidant activity and a positive effect on metabolic diseases such as type 2 diabetes and inflammatory, heart, and liver diseases []. Increased consumption of flavonoids may reduce the risk of many chronic health conditions. Ganoderma lucidum is a white Basidiomycete root mushroom that has been eaten in the Far East for many years and is believed to improve health and enhance longevity []. G. lucidum contains high amounts of flavonoids, such as quercetin and kaempferol []. According to Jung et al. [], a fraction isolated from G. lucidum improved non-alcoholic steatosis and the associated complex disorders by inducing energy-metabolizing enzymes. In an in vivo experiment, G. lucidum helped reduce the weight of the epididymal and perirenal adipose tissue and reduced serum cholesterol and LDL levels in mice fed an HFD. In addition, G. lucidum reduced AMPK and ACC phosphorylation in HFD-fed mouse liver and caused increased phosphorylation of AMPK and ACC in HepG2 and 3T3-L1 cells. Therefore, G. lucidum appears to participate in energy metabolism regulation and lipid accumulation directly in adipose tissue and in the liver. Therefore, G. lucidum may serve as a treatment or preventive measure for NAFLD or other metabolic disorders [].
Fungal Sterols
Ergosterol is a sterol found in fungal cell membranes; it performs many of the same functions as cholesterol in animal cells or phytosterols in plants. Because many fungi cannot survive without ergosterol, its synthesis-related enzymes have emerged as crucial drug-discovery targets. Ergosterol is a provitamin form of vitamin D2, which is used in human nutrition []. Das et al. investigated fat-soluble components extracted from Agaricus bisporus (button mushroom) and their biological activities on fat-related metabolic processes and gene expression. The results revealed that treatment with ergosterol at 34.96 mg/g of extract in an HFD group could reduce hepatic cholesterol accumulation, as well as liver triglyceride levels by 26%. The ergosterol extract significantly reduced HMG-CoA and SREBP-2 expression, and the expression of LDLR, which is responsible for hepatic cholesterol clearance, was greatly enhanced. Therefore, these findings may contribute to the use of ergosterols as therapeutic agents for NAFLD and other liver diseases [].
8. Conclusions and Future Directions
Many studies support the claim that metabolic diseases can be prevented by adopting healthy lifestyle habits and reducing risk factors that can lead to an increase in LDL-c levels. Consumption of phytochemicals and biological compounds from plant sources results in better control of blood lipid levels.
Our review examines the positive effects of sustainable sources such as algal- and fungal-based phytochemicals on managing hyperlipidemia and preventing the development of NAFLD, as well as their related effects on the various pathways and genes involved in the development of these conditions.
Phytochemicals regulate lipid metabolism and have multi-target involvement in absorption, transport, elimination, and cholesterol biosynthesis. HMGCR, MVK, LDLR and CYP7A1 remain the key molecules involved in these processes.
In addition, following these processes, transcription factors such as SREBPs take an active part in helping phytochemicals lower lipid levels. The effects of phytochemicals seem promising and emphasize the need for and importance of their use as potential treatments. The algal and mushroom-based phytochemicals described in this review are diverse, including polysaccharides, phenols, sterols, and flavonoids, and are considered safe and well-tolerated in most cases. Phytochemicals can reduce LDL-c levels and prevent NAFLD by regulating various metabolic pathways. In addition, in this review, we described the involvement of a number of miRNAs—miR-122, miR-33, and miR-370—in cholesterol metabolism and the development of NAFLD. Various phytochemicals also seem to have an effect on these components, which can lead to a reduction in blood lipid levels. Dietary fibers, such as the β-glucans found in edible mushrooms and algae, are known to inhibit cholesterol absorption in the intestines, prevent the absorption of bile acids, and affect cholesterol synthesis in the liver to decrease LDL-c levels.
Today, there is a therapeutic gap for patients suffering from hypercholesteremia: 70% of patients do not respond well to statins. In addition, there is no suitable therapeutic approach for NAFLD, which is currently considered the leading chronic liver disease in the western world. The relationship between phytochemicals and these diseases may offer a new direction, with a therapeutic advantage for those suffering from these disorders or as a potential supportive treatment through dietary inclusion.
Plant foods are viewed in a positive light due to their health benefits. It is worth noting that in light of the global challenges facing the world food system, edible mushrooms and algae—which are considered sustainable sources—might be important sources of functional phytochemicals promoting health and wellbeing and enabling food security worldwide. In contrast, there is an issue with microalgae in that only a few species have been approved for human consumption (GRAS): Arthrospira maxima, Arthrospira platensis, Chlamydomonas reinhardtii, Chlorella protothecoides, Dunaliella bardawil, Haematococcus pluvialis and Prototheca moriformis. Further studies supporting the importance of additional microalgae-based phytochemicals for cholesterol metabolism and NAFLD could convince the regulators to approve new algae for human consumption.
Despite this limitation, the consumption of algal- and edible mushroom-based phytochemicals is an effective strategy for managing cholesterol metabolism and NAFLD, and these could potentially be used as functional ingredients for the food industry.
Author Contributions
Conceptualization, D.A., Y.E. and N.P.; writing—original draft preparation, Y.E., N.P., H.K., N.S. and R.T.; writing—review and editing, D.A.; visualization, Y.E. and H.K.; supervision, D.A.; funding acquisition, D.A. All authors have read and agreed to the published version of the manuscript.
Funding
This review was funded by the European Union’s HORIZON 2020 RIA programme grant number [101000501].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Glass, C.K.; Witztum, J.L. Atherosclerosis: The Road Ahead. Cell 2001, 104, 503–516. [Google Scholar] [CrossRef]
- Vancells Lujan, P.; Viñas Esmel, E.; Sacanella Meseguer, E. Overview of Non-Alcoholic Fatty Liver Disease (NAFLD) and the Role of Sugary Food Consumption and Other Dietary Components in Its Development. Nutrients 2021, 13, 1442. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Chang, Y.; Ryu, S.; Kim, Y.; Cho, Y.K.; Sung, E.; Kim, H.N.; Ahn, J.; Jung, H.S.; Yun, K.E.; et al. Low Levels of Alcohol Consumption, Obesity, and Development of Fatty Liver With and Without Evidence of Advanced Fibrosis. Hepatology 2020, 71, 861–873. [Google Scholar] [CrossRef] [PubMed]
- Smeuninx, B.; Boslem, E.; Febbraio, M.A. Current and Future Treatments in the Fight Against Non-Alcoholic Fatty Liver Disease. Cancers 2020, 12, 1714. [Google Scholar] [CrossRef] [PubMed]
- Dawidowicz, E.A. Dynamics of membrane lipid metabolism and turnover. Ann. Rev. Biochem. 1987, 56, 43–61. [Google Scholar] [CrossRef]
- Brown, M.S.; Goldstein, J.L. Receptor-mediated control of cholesterol metabolism. Science 1976, 191, 150–154. [Google Scholar] [CrossRef]
- Brown, M.S.; Goldstein, J.L. The SREBP Pathway: Regulation of Cholesterol Metabolism by Proteolysis of a Membrane-Bound Transcription Factor. Cell 1997, 89, 331–340. [Google Scholar] [CrossRef]
- Iliopoulos, D.; Drosatos, K.; Hiyama, Y.; Goldberg, I.J.; Zannis, V.I. MicroRNA-370 controls the expression of microRNA-122 and Cpt1alpha and affects lipid metabolism. J. Lipid Res. 2010, 51, 1513–1523. [Google Scholar] [CrossRef]
- Moore, K.J.; Rayner, K.J.; Suárez, Y.; Fernández-Hernando, C. microRNAs and cholesterol metabolism. Trends Endocrinol. Metab. 2010, 21, 699–706. [Google Scholar] [CrossRef]
- Thakur, M.; Singh, K.; Khedkar, R. Phytochemicals: Extraction Process, Safety Assessment, Toxicological Evaluations, And Regulatory Issues. In Functional and Preservative Properties of Phytochemicals; Academic Press: Cambridge, MA, USA, 2020; pp. 341–361. [Google Scholar]
- Laka, K.; Makgoo, L.; Mbita, Z. Cholesterol-Lowering Phytochemicals: Targeting the Mevalonate Pathway for Anticancer Interventions. Front. Genet. 2022, 13, 841639. [Google Scholar] [CrossRef]
- Eating Sustainably—Easier than you Think? European Climate Pact. Available online: https://climate-pact.europa.eu/news/eating-sustainably-easier-you-think-2022-07-28_en (accessed on 1 September 2022).
- Why Sustainable Food Systems are Needed in a Post-COVID World. UNEP. 2020. Available online: http://www.unep.org/news-and-stories/story/why-sustainable-food-systems-are-needed-post-covid-world (accessed on 1 September 2022).
- Good, K. Sustainable Innovation: How Fungi-Based Proteins Are Changing the Game. Plant Based Foods Assoc. 2022. Available online: https://www.plantbasedfoods.org/sustainable-innovation-how-fungi-based-proteins-are-changing-the-game/ (accessed on 1 September 2022).
- Kusmayadi, A.; Leong, Y.K.; Yen, H.-W.; Huang, C.-Y.; Chang, J.-S. Microalgae as sustainable food and feed sources for animals and humans—Biotechnological and environmental aspects. Chemosphere 2021, 271, 129800. [Google Scholar] [CrossRef]
- Espenshade, P.J. SREBPs: Sterol-regulated transcription factors. J. Cell Sci. 2006, 119, 973–976. [Google Scholar] [CrossRef]
- Afonso, M.S.; Machado, R.M.; Lavrador, M.S.; Quintao, E.C.R.; Moore, K.J.; Lottenberg, A.M. Molecular Pathways Underlying Cholesterol Homeostasis. Nutrients 2018, 10, 760. [Google Scholar] [CrossRef]
- Wang, H.H.; Garruti, G.; Liu, M.; Portincasa, P.; Wang, D.Q.-H. Cholesterol and Lipoprotein Metabolism and Atherosclerosis: Recent Advances in Reverse Cholesterol Transport. Ann. Hepatol. 2017, 16 (Suppl. S1), S27–S42. [Google Scholar] [CrossRef]
- Sacks, F.M.; Lichtenstein, A.H.; Wu, J.H.; Appel, L.J.; Creager, M.A.; Kris-Etherton, P.M.; Miller, M.; Rimm, E.B.; Rudel, L.L.; Robinson, J.G.; et al. Dietary Fats and Cardiovascular Disease: A Presidential Advisory From the American Heart Association. Circulation 2017, 136, e1–e23. [Google Scholar] [CrossRef]
- Di Ciaula, A.; Garruti, G.; Baccetto, R.L.; Molina-Molina, E.; Bonfrate, L.; Portincasa, P.; Wang, D.Q. Bile acid physiology. Ann. Hepatol. 2017, 16, s4–s14. [Google Scholar] [CrossRef]
- Cox, R.A.; García-Palmieri, M.R. Cholesterol, Triglycerides, and Associated Lipoproteins. In Clinical Methods: The History, Physical, and Laboratory Examinations; Walker, H.K., Hall, W.D., Hurst, J.W., Eds.; Butterworths: Boston, MA, USA, 2011. [Google Scholar]
- Lloyd-Jones, D.M.; Goff, D.C.; Stone, N.J. Treatment of Blood Cholesterol to Reduce Risk for Atherosclerotic Cardiovascular Disease. Ann. Intern. Med. 2016, 164, 136. [Google Scholar] [CrossRef]
- Javitt, N.B. Bile acid synthesis from cholesterol: Regulatory and auxiliary pathways. FASEB J. 1994, 8, 1308–1311. [Google Scholar] [CrossRef]
- Sekhar, M.S.; Marupuru, S.; Reddy, B.S.; Kurian, S.J.; Rao, M. Physiological role of cholesterol in human body. In Dietary Sugar, Salt and Fat in Human Health; Academic Press: Cambridge, MA, USA, 2020; pp. 453–481. [Google Scholar]
- Kellner-Weibel, G.; de la Llera-Moya, M. Update on HDL receptors and cellular cholesterol transport. Curr. Atheroscler. Rep. 2011, 13, 233–241. [Google Scholar] [CrossRef]
- Mc Auley, M.T. Modeling cholesterol metabolism and atherosclerosis. WIREs Mech. Dis. 2022, 14, e1546. [Google Scholar] [CrossRef]
- Griffiths, W.J.; Wang, Y. Cholesterol metabolism: From lipidomics to immunology. J. Lipid Res. 2022, 63, 100165. [Google Scholar] [CrossRef] [PubMed]
- Craig, M.; Yarrarapu, S.N.S.; Dimri, M. Biochemistry, Cholesterol. In StatPearls; StatPearls Publishing: Tampa, FL, USA, 2021. [Google Scholar]
- Radhakrishnan, A.; Ikeda, Y.; Kwon, H.J.; Brown, M.S.; Goldstein, J.L. Sterol-regulated transport of SREBPs from endoplasmic reticulum to Golgi: Oxysterols block transport by binding to Insig. Proc. Natl. Acad. Sci. USA 2007, 104, 6511–6518. [Google Scholar] [CrossRef] [PubMed]
- Sozen, E.; Ozer, N.K.; Dominiczak, M.H.; Beastall, G.; Wallace, A.M. Biosynthesis of cholesterol and steroids. In Medical Biochemistry E-Book; Elsevier: Amsterdam, The Netherlands, 2014; p. 200. [Google Scholar]
- Impact of high cholesterol and endoplasmic reticulum stress on metabolic diseases: An updated mini-review. Redox Biol. 2017, 12, 456–461. [CrossRef] [PubMed]
- Citrin, K.M.; Fernández-Hernando, C.; Suárez, Y. MicroRNA regulation of cholesterol metabolism. Ann. N. Y. Acad. Sci. 2021, 1495, 55–77. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Yang, Y.-L.; Wang, P.-W.; Wang, F.-S.; Huang, Y.-H. The Emerging Role of MicroRNAs in NAFLD: Highlight of MicroRNA-29a in Modulating Oxidative Stress, Inflammation, and Beyond. Cells 2020, 9, 1041. [Google Scholar] [CrossRef]
- Alphonse, P.A.S.; Jones, P.J.H. Revisiting Human Cholesterol Synthesis and Absorption: The Reciprocity Paradigm and its Key Regulators. Lipids 2016, 51, 519–536. [Google Scholar] [CrossRef]
- Vaklavas, C.; Chatzizisis, Y.S.; Ziakas, A.; Zamboulis, C.; Giannoglou, G.D. Molecular basis of statin-associated myopathy. Atherosclerosis 2009, 202, 18–28. [Google Scholar] [CrossRef]
- Brown, M.S.; Goldstein, J.L. Cholesterol feedback: From Schoenheimer’s bottle to Scap’s MELADL. J. Lipid Res. 2009, 50, S15–S27. [Google Scholar] [CrossRef]
- Adams, C.M.; Reitz, J.; De Brabander, J.K.; Feramisco, J.D.; Li, L.; Brown, M.S.; Goldstein, J.L. Cholesterol and 25-hydroxycholesterol inhibit activation of SREBPs by different mechanisms, both involving SCAP and Insigs. J. Biol. Chem. 2004, 279, 52772–52780. [Google Scholar] [CrossRef]
- Brown, M.S.; Radhakrishnan, A.; Goldstein, J.L. Retrospective on Cholesterol Homeostasis: The Central Role of Scap. Annu. Rev. Biochem. 2018, 87, 783–807. [Google Scholar] [CrossRef]
- Gong, Y.; Lee, J.N.; Lee, P.C.; Goldstein, J.L.; Brown, M.S.; Ye, J. Sterol-regulated ubiquitination and degradation of Insig-1 creates a convergent mechanism for feedback control of cholesterol synthesis and uptake. Cell Metab. 2006, 3, 15–24. [Google Scholar] [CrossRef]
- Sato, K. COPII coat assembly and selective export from the endoplasmic reticulum. J. Biochem. 2004, 136, 755–760. [Google Scholar] [CrossRef]
- Brown, A.J.; Sun, L.; Feramisco, J.D.; Brown, M.S.; Goldstein, J.L. Cholesterol addition to ER membranes alters conformation of SCAP, the SREBP escort protein that regulates cholesterol metabolism. Mol. Cell 2002, 10, 237–245. [Google Scholar] [CrossRef]
- Bellentani, S.; Scaglioni, F.; Marino, M.; Bedogni, G. Epidemiology of Non-Alcoholic Fatty Liver Disease. DDI 2010, 28, 155–161. [Google Scholar] [CrossRef]
- Bellentani, S. The epidemiology of non-alcoholic fatty liver disease. Liver Int. 2017, 37 (Suppl. S1), 81–84. [Google Scholar] [CrossRef]
- Spiers, J.; Brindley, J.H.; Li, W.; Alazawi, W. What’s new in non-alcoholic fatty liver disease? Frontline Gastroenterol. 2022, 13, e102–e108. [Google Scholar] [CrossRef]
- Kuperman, Y.; Weiss, M.; Dine, J.; Staikin, K.; Golani, O.; Ramot, A.; Nahum, T.; Kühne, C.; Shemesh, Y.; Wurst, W.; et al. CRFR1 in AgRP Neurons Modulates Sympathetic Nervous System Activity to Adapt to Cold Stress and Fasting. Cell Metab. 2016, 23, 1185–1199. [Google Scholar] [CrossRef]
- Malhotra, P.; Gill, R.K.; Saksena, S.; Alrefai, W.A. Disturbances in Cholesterol Homeostasis and Non-alcoholic Fatty Liver Diseases. Front. Med. 2020, 7, 467. [Google Scholar] [CrossRef]
- McDaniel, K.; Herrera, L.; Zhou, T.; Francis, H.; Han, Y.; Levine, P.; Lin, E.; Glaser, S.; Alpini, G.; Meng, F. The functional role of microRNAs in alcoholic liver injury. J. Cell. Mol. Med. 2014, 18, 197–207. [Google Scholar] [CrossRef]
- Cheung, O.; Puri, P.; Eicken, C.; Contos, M.J.; Mirshahi, F.; Maher, J.W.; Kellum, J.M.; Min, H.; Luketic, V.A.; Sanyal, A.J. Nonalcoholic steatohepatitis is associated with altered hepatic MicroRNA expression. Hepatology 2008, 48, 1810–1820. [Google Scholar] [CrossRef] [PubMed]
- Van Rooyen, D.M.; Farrell, G.C. SREBP-2: A link between insulin resistance, hepatic cholesterol, and inflammation in NASH. J. Gastroenterol. Hepatol. 2011, 26, 789–792. [Google Scholar] [CrossRef] [PubMed]
- Min, H.-K.; Kapoor, A.; Fuchs, M.; Mirshahi, F.; Zhou, H.; Maher, J.; Kellum, J.; Warnick, R.; Contos, M.J.; Sanyal, A.J. Increased hepatic synthesis and dysregulation of cholesterol metabolism is associated with the severity of nonalcoholic fatty liver disease. Cell Metab. 2012, 15, 665–674. [Google Scholar] [CrossRef]
- Gori, M.; Arciello, M.; Balsano, C. MicroRNAs in nonalcoholic fatty liver disease: Novel biomarkers and prognostic tools during the transition from steatosis to hepatocarcinoma. Biomed. Res. Int. 2014, 2014, 741465. [Google Scholar] [CrossRef] [PubMed]
- Lendvai, G.; Jármay, K.; Karácsony, G.; Halász, T.; Kovalszky, I.; Baghy, K.; Wittmann, T.; Schaff, Z.; Kiss, A. Elevated miR-33a and miR-224 in steatotic chronic hepatitis C liver biopsies. World J. Gastroenterol. 2014, 20, 15343–15350. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, Z.; Kusumanchi, P.; Han, S.; Liangpunsakul, S. Critical Role of microRNA-21 in the Pathogenesis of Liver Diseases. Front. Med. 2020, 7, 7. [Google Scholar] [CrossRef]
- Koh, Y.-C.; Lin, Y.C.; Lee, P.S.; Lu, T.J.; Lin, K.Y.; Pan, M.H. A multi-targeting strategy to ameliorate high-fat-diet- and fructose-induced (western diet-induced) non-alcoholic fatty liver disease (NAFLD) with supplementation of a mixture of legume ethanol extracts. Food Funct. 2020, 11, 7545–7560. [Google Scholar] [CrossRef]
- Popkin, B.M.; Adair, L.S.; Ng, S.W. Global nutrition transition and the pandemic of obesity in developing countries. Nutr. Rev. 2012, 70, 3–21. [Google Scholar] [CrossRef]
- Drake, I.; Sonestedt, E.; Ericson, U.; Wallström, P.; Orho-Melander, M. A Western dietary pattern is prospectively associated with cardio-metabolic traits and incidence of the metabolic syndrome. Br. J. Nutr. 2018, 119, 1168–1176. [Google Scholar] [CrossRef]
- Ha, B. The Power of Plants: Is a Whole-Foods, Plant-Based Diet the Answer to Health, Health Care, and Physician Wellness? Perm. J. 2019, 23, 19-003. [Google Scholar] [CrossRef]
- Ichimura, M.; Kawase, M.; Masuzumi, M.; Sakaki, M.; Nagata, Y.; Tanaka, K.; Suruga, K.; Tamaru, S.; Kato, S.; Tsuneyama, K.; et al. High-fat and high-cholesterol diet rapidly induces non-alcoholic steatohepatitis with advanced fibrosis in Sprague-Dawley rats. Hepatol. Res. 2015, 45, 458–469. [Google Scholar] [CrossRef]
- Rao, A.; Kosters, A.; Mells, J.E.; Zhang, W.; Setchell, K.D.; Amanso, A.M.; Wynn, G.M.; Xu, T.; Keller, B.T.; Yin, H.; et al. Inhibition of ileal bile acid uptake protects against nonalcoholic fatty liver disease in high-fat diet-fed mice. Sci. Transl. Med. 2016, 8, 357ra122. [Google Scholar] [CrossRef]
- Feingold, K.R. Cholesterol-Lowering Drugs; Endotext: South Dartmouth, MA, USA, 2021. [Google Scholar]
- Yu, Q.; Chen, Y.; Xu, C.-B. Statins and New-Onset Diabetes Mellitus: LDL Receptor May Provide a Key Link. Front. Pharmacol. 2017, 8, 372. [Google Scholar] [CrossRef]
- Ratchford, E.V.; Martin, S.S. Statins. Vasc. Med. 2017, 22, 442–445. [Google Scholar] [CrossRef]
- Kim, J.Y.; Garcia-Carbonell, R.; Yamachika, S.; Zhao, P.; Dhar, D.; Loomba, R.; Kaufman, R.J.; Saltiel, A.R.; Karin, M. ER Stress Drives Lipogenesis and Steatohepatitis via Caspase-2 Activation of S1P. Cell 2018, 175, 133–145.e15. [Google Scholar] [CrossRef]
- Lu, X.-Y.; Lu, X.Y.; Shi, X.J.; Hu, A.; Wang, J.Q.; Ding, Y.; Jiang, W.; Sun, M.; Zhao, X.; Luo, J.; et al. Feeding induces cholesterol biosynthesis via the mTORC1-USP20-HMGCR axis. Nature 2020, 588, 479–484. [Google Scholar] [CrossRef]
- Ye, Z.-J.; Go, G.W.; Singh, R.; Liu, W.; Keramati, A.R.; Mani, A. LRP6 protein regulates low density lipoprotein (LDL) receptor-mediated LDL uptake. J. Biol. Chem. 2012, 287, 1335–1344. [Google Scholar] [CrossRef]
- Simon, A. Cholesterol metabolism and immunity. N. Engl. J. Med. 2014, 371, 1933–1935. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, J.-H.; Im, S.-S. The cellular function of SCAP in metabolic signaling. Exp. Mol. Med. 2020, 52, 724–729. [Google Scholar] [CrossRef]
- Jeon, S.-M. Regulation and function of AMPK in physiology and diseases. Exp. Mol. Med. 2016, 48, e245. [Google Scholar] [CrossRef]
- Lee, J.Y.; Karwatsky, J.; Ma, L.; Zha, X. ABCA1 increases extracellular ATP to mediate cholesterol efflux to ApoA-I. Am. J. Physiol. Cell Physiol. 2011, 301, C886–C894. [Google Scholar] [CrossRef] [PubMed]
- Sag, D.; Cekic, C.; Wu, R.; Linden, J.; Hedrick, C.C. The cholesterol transporter ABCG1 links cholesterol homeostasis and tumour immunity. Nat. Commun. 2015, 6, 6354. [Google Scholar] [CrossRef] [PubMed]
- Pullinger, C.R.; Eng, C.; Salen, G.; Shefer, S.; Batta, A.K.; Erickson, S.K.; Verhagen, A.; Rivera, C.R.; Mulvihill, S.J.; Malloy, M.J.; et al. Human cholesterol 7alpha-hydroxylase (CYP7A1) deficiency has a hypercholesterolemic phenotype. J. Clin. Investig. 2002, 110, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Shanley, P.; Luz, L. The Impacts of Forest Degradation on Medicinal Plant Use and Implications for Health Care in Eastern Amazonia. BioScience 2003, 53, 573–584. [Google Scholar] [CrossRef]
- Williamson, R.M.; Price, J.F.; Glancy, S.; Perry, E.; Nee, L.D.; Hayes, P.C.; Frier, B.M.; Van Look, L.A.; Johnston, G.I.; Reynolds, R.M.; et al. Prevalence of and risk factors for hepatic steatosis and nonalcoholic Fatty liver disease in people with type 2 diabetes: The Edinburgh Type 2 Diabetes Study. Diabetes Care 2011, 34, 1139–1144. [Google Scholar] [CrossRef]
- Panahi, Y.; Badeli, R.; Karami, G.-R.; Sahebkar, A. Investigation of the efficacy of adjunctive therapy with bioavailability-boosted curcuminoids in major depressive disorder. Phytother. Res. 2015, 29, 17–21. [Google Scholar] [CrossRef]
- Panahi, Y.; Kianpour, P.; Mohtashami, R.; Jafari, R.; Simental-Mendía, L.E.; Sahebkar, A. Efficacy and Safety of Phytosomal Curcumin in Non-Alcoholic Fatty Liver Disease: A Randomized Controlled Trial. Drug Res. 2017, 67, 244–251. [Google Scholar] [CrossRef]
- El Amrousy, D.; Abdelhai, D.; Shawky, D. Vitamin D and nonalcoholic fatty liver disease in children: A randomized controlled clinical trial. Eur. J. Pediatr. 2022, 181, 579–586. [Google Scholar] [CrossRef]
- Vitamin E and DHA-EE on NAFLD—Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Clinical Trial (PUVENAFLD)—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04198805 (accessed on 1 September 2022).
- Vitamin E and DHA-EE on NAFLD—Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Clinical Trial (PUVENAFLD). Available online: https://www.smartpatients.com/trials/NCT04198805 (accessed on 1 September 2022).
- Violi, F.; Nocella, C.; Loffredo, L.; Carnevale, R.; Pignatelli, P. Interventional study with vitamin E in cardiovascular disease and meta-analysis. Free Radic. Biol. Med. 2022, 178, 26–41. [Google Scholar] [CrossRef]
- Gallo-Torres, H.E. Obligatory role of bile for the intestinal absorption of vitamin E. Lipids 1970, 5, 379–384. [Google Scholar] [CrossRef]
- Reboul, E.; Klein, A.; Bietrix, F.; Gleize, B.; Malezet-Desmoulins, C.; Schneider, M.; Margotat, A.; Lagrost, L.; Collet, X.; Borel, P. Scavenger Receptor Class B Type I (SR-BI) Is Involved in Vitamin E Transport across the Enterocyte*. J. Biol. Chem. 2006, 281, 4739–4745. [Google Scholar] [CrossRef]
- Reboul, E. Vitamin E intestinal absorption: Regulation of membrane transport across the enterocyte. IUBMB Life 2019, 71, 416–423. [Google Scholar] [CrossRef]
- Lemaire-Ewing, S.; Desrumaux, C.; Néel, D.; Lagrost, L. Vitamin E transport, membrane incorporation and cell metabolism: Is α-tocopherol in lipid rafts an oar in the lifeboat? Mol. Nutr. Food Res. 2010, 54, 631–640. [Google Scholar] [CrossRef]
- Sato, K.; Gosho, M.; Yamamoto, T.; Kobayashi, Y.; Ishii, N.; Ohashi, T.; Nakade, Y.; Ito, K.; Fukuzawa, Y.; Yoneda, M. Vitamin E has a beneficial effect on nonalcoholic fatty liver disease: A meta-analysis of randomized controlled trials. Nutrition 2015, 31, 923–930. [Google Scholar] [CrossRef]
- Presa, N.; Clugston, R.D.; Lingrell, S.; Kelly, S.E.; Merrill, A.H., Jr.; Jana, S.; Kassiri, Z.; Gómez-Muñoz, A.; Vance, D.E.; Jacobs, R.L.; et al. Vitamin E alleviates non-alcoholic fatty liver disease in phosphatidylethanolamine N-methyltransferase deficient mice. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2019, 1865, 14–25. [Google Scholar] [CrossRef]
- Hunt, A.; Harrington, D.; Robinson, S. Vitamin B12 deficiency. BMJ 2014, 349, g5226. [Google Scholar] [CrossRef]
- Adaikalakoteswari, A.; Finer, S.; Voyias, P.D.; McCharty, C.M.; Vatish, M.; Moore, J.; Smart-Halajko, M.; Bawazeer, N.; Al-Daghri, N.M.; McThernan, P.G.; et al. Vitamin B12 insufficiency induces cholesterol biosynthesis by limiting s-adenosylmethionine and modulating the methylation of SREBF1 and LDLR genes. Clin. Epigenet. 2015, 7, 14. [Google Scholar] [CrossRef]
- Plamada, D.; Vodnar, D.C. Polyphenols-Gut Microbiota Interrelationship: A Transition to a New Generation of Prebiotics. Nutrients 2021, 14, 137. [Google Scholar] [CrossRef]
- Lv, O.; Wang, L.; Li, J.; Ma, Q.; Zhao, W. Effects of pomegranate peel polyphenols on lipid accumulation and cholesterol metabolic transformation in L-02 human hepatic cells via the PPARγ-ABCA1/CYP7A1 pathway. Food Funct. 2016, 7, 4976–4983. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Perez-Vizcaino, F.; Duarte, J. Flavonols and cardiovascular disease. Mol. Aspects Med. 2010, 31, 478–494. [Google Scholar] [CrossRef] [PubMed]
- Chambers, K.F.; Day, P.E.; Aboufarrag, H.T.; Kroon, P.A. Polyphenol Effects on Cholesterol Metabolism via Bile Acid Biosynthesis, CYP7A1: A Review. Nutrients 2019, 11, 2588. [Google Scholar] [CrossRef] [PubMed]
- Hackman, R.M.; Polagruto, J.A.; Zhu, Q.Y.; Sun, B.; Fujii, H.; Keen, C.L. Flavanols: Digestion, absorption and bioactivity. Phytochem. Rev. 2007, 7, 195–208. [Google Scholar] [CrossRef]
- Alam, M.A.; Subhan, N.; Rahman, M.M.; Uddin, S.J.; Reza, H.M.; Sarker, S.D. Effect of citrus flavonoids, naringin and naringenin, on metabolic syndrome and their mechanisms of action. Adv. Nutr. 2014, 5, 404–417. [Google Scholar] [CrossRef] [PubMed]
- Danielewski, M.; Matuszewska, A.; Szeląg, A.; Sozański, T. The Impact of Anthocyanins and Iridoids on Transcription Factors Crucial for Lipid and Cholesterol Homeostasis. Int. J. Mol. Sci. 2021, 22, 6074. [Google Scholar] [CrossRef] [PubMed]
- Danielewski, M.; Matuszewska, A.; Nowak, B.; Kucharska, A.Z.; Sozański, T. The Effects of Natural Iridoids and Anthocyanins on Selected Parameters of Liver and Cardiovascular System Functions. Oxid. Med. Cell. Longev. 2020, 2020, 2735790. [Google Scholar] [CrossRef]
- Jia, Y.; Kim, J.Y.; Jun, H.J.; Kim, S.J.; Lee, J.H.; Hoang, M.H.; Kim, H.S.; Chang, H.I.; Hwang, K.Y.; Um, S.J.; et al. Cyanidin is an agonistic ligand for peroxisome proliferator-activated receptor-alpha reducing hepatic lipid. Biochim. Biophys. Acta 2013, 1831, 698–708. [Google Scholar] [CrossRef]
- Fu, Y.; Wei, Z.; Zhou, E.; Zhang, N.; Yang, Z. Cyanidin-3-O-β-glucoside inhibits lipopolysaccharide-induced inflammatory response in mouse mastitis model. J. Lipid Res. 2014, 55, 1111–1119. [Google Scholar] [CrossRef]
- Park, M.; Yoo, J.-H.; Lee, Y.-S.; Lee, H.-J. Lonicera caerulea Extract Attenuates Non-Alcoholic Fatty Liver Disease in Free Fatty Acid-Induced HepG2 Hepatocytes and in High Fat Diet-Fed Mice. Nutrients 2019, 11, 494. [Google Scholar] [CrossRef]
- Khan, M.I.; Shin, J.H.; Shin, T.S.; Kim, M.Y.; Cho, N.J.; Kim, J.D. Anthocyanins from Cornus kousa ethanolic extract attenuate obesity in association with anti-angiogenic activities in 3T3-L1 cells by down-regulating adipogeneses and lipogenesis. PLoS ONE 2018, 13, e0208556. [Google Scholar] [CrossRef]
- Park, S.; Kang, S.; Jeong, D.Y.; Jeong, S.Y.; Park, J.J.; Yun, H.S. Cyanidin and malvidin in aqueous extracts of black carrots fermented with Aspergillus oryzae prevent the impairment of energy, lipid and glucose metabolism in estrogen-deficient rats by AMPK activation. Genes Nutr. 2015, 10, 1–14. [Google Scholar] [CrossRef]
- de Sousaa, A.R.; de Castro Moreira, M.E.; Toledo, R.C.L.; dos Anjos Benjamin, L.; Queiroz, V.A.V.; Veloso, M.P.; de Souza Reis, K.; Martino, H.S.D. Extruded sorghum (Sorghum bicolor L.) reduces metabolic risk of hepatic steatosis in obese rats consuming a high fat diet. Food Res. Int. 2018, 112, 48–55. [Google Scholar] [CrossRef]
- Liu, R.; de Castro Moreira, M.E.; Toledo, R.C.L.; dos Anjos Benjamin, L.; Queiroz, V.A.V.; Veloso, M.P.; de Souza Reis, K.; Martino, H.S.D. Effects of ellagic acid-rich extract of pomegranates peel on regulation of cholesterol metabolism and its molecular mechanism in hamsters. Food Funct. 2014, 6, 780–787. [Google Scholar] [CrossRef]
- Roy, A. A Review on the Alkaloids an Important Therapeutic Compound from Plants. Int. J. Plant Biotechnol. 2017, 3, 1–9. [Google Scholar]
- Cheng, C.; Li, Z.; Zhao, X.; Liao, C.; Quan, J.; Bode, A.M.; Cao, Y.; Luo, X. Natural alkaloid and polyphenol compounds targeting lipid metabolism: Treatment implications in metabolic diseases. Eur. J. Pharmacol. 2020, 870, 172922. [Google Scholar] [CrossRef]
- Zhang, C.; Deng, J.; Liu, D.; Tuo, X.; Xiao, L.; Lai, B.; Yao, Q.; Liu, J.; Yang, H.; Wang, N. Nuciferine ameliorates hepatic steatosis in high-fat diet/streptozocin-induced diabetic mice through a PPARα/PPARγ coactivator-1α pathway. Br. J. Pharmacol. 2018, 175, 4218–4228. [Google Scholar] [CrossRef]
- Guo, F.; Yang, X.; Li, X.; Feng, R.; Guan, C.; Wang, Y.; Li, Y.; Sun, C. Nuciferine Prevents Hepatic Steatosis and Injury Induced by a High-Fat Diet in Hamsters. PLoS ONE 2013, 8, e63770. [Google Scholar] [CrossRef]
- Xu, G.; Huang, K.; Zhou, J. Hepatic AMP Kinase as a Potential Target for Treating Nonalcoholic Fatty Liver Disease: Evidence from Studies of Natural Products. Curr. Med. Chem. 2018, 25, 889–907. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Tan, Y.; Wei, J.; Chang, Y.; Jin, T.; Zhu, H. Betaine supplement alleviates hepatic triglyceride accumulation of apolipoprotein E deficient mice via reducing methylation of peroxisomal proliferator-activated receptor alpha promoter. Lipids Health Dis. 2013, 12, 1–9. [Google Scholar] [CrossRef]
- Xu, L.; Huang, D.; Hu, Q.; Wu, J.; Wang, Y.; Feng, J. Betaine alleviates hepatic lipid accumulation via enhancing hepatic lipid export and fatty acid oxidation in rats fed with a high-fat diet. Br. J. Nutr. 2015, 113, 1835–1843. [Google Scholar] [CrossRef]
- Pacifico, L.; Giansanti, S.; Gallozzi, A.; Chiesa, C. Long chain omega-3 polyunsaturated fatty acids in pediatric metabolic syndrome. Mini Rev. Med. Chem. 2014, 14, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.C.; Watts, G.F.; Mori, T.A.; Barrett, P.H.R.; Redgrave, T.G.; Beilin, L.J. Randomized controlled trial of the effect of n–3 fatty acid supplementation on the metabolism of apolipoprotein B-100 and chylomicron remnants in men with visceral obesity. Am. J. Clin. Nutr. 2003, 77, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Perla, F.M.; Prelati, M.; Lavorato, M.; Visicchio, D.; Anania, C. The Role of Lipid and Lipoprotein Metabolism in Non-Alcoholic Fatty Liver Disease. Children 2017, 4, 46. [Google Scholar] [CrossRef] [PubMed]
- Matos, J.; Cardoso, C.; Bandarra, N.M.; Afonso, C. Microalgae as healthy ingredients for functional food: A review. Food Funct. 2017, 8, 2672–2685. [Google Scholar] [CrossRef]
- Batista, A.P.; Niccolai, A.; Bursic, I.; Sousa, I.; Raymundo, A.; Rodolfi, L.; Biondi, N.; Tredici, M.R. Microalgae as Functional Ingredients in Savory Food Products: Application to Wheat Crackers. Foods 2019, 8, 611. [Google Scholar] [CrossRef]
- Ramos-Romero, S.; Torrella, J.R.; Pagès, T.; Viscor, G.; Torres, J.L. Edible Microalgae and Their Bioactive Compounds in the Prevention and Treatment of Metabolic Alterations. Nutrients 2021, 13, 563. [Google Scholar] [CrossRef]
- Mimouni, V.; Ulmann, L.; Pasquet, V.; Mathieu, M.; Picot, L.; Bougaran, G.; Cadoret, J.P.; Morant-Manceau, A.; Schoefs, B. The Potential of Microalgae for the Production of Bioactive Molecules of Pharmaceutical Interest. Curr. Pharm. Biotechnol. 2012, 13, 2733–2750. [Google Scholar] [CrossRef]
- Barkia, I.; Saari, N.; Manning, S.R. Microalgae for High-Value Products Towards Human Health and Nutrition. Mar. Drugs 2019, 17, 304. [Google Scholar] [CrossRef]
- Ampofo, J.; Lord, A. Microalgae: Bioactive Composition, Health Benefits, Safety and Prospects as Potential High-Value Ingredients for the Functional Food Industry. Foods 2022, 11, 1744. [Google Scholar] [CrossRef]
- Januário, A.P.; Félix, R.; Félix, C.; Reboleira, J.; Valentão, P.; Lemos, M.F. Red Seaweed-Derived Compounds as a Potential New Approach for Acne Vulgaris Care. Pharmaceutics 2021, 13, 1930. [Google Scholar] [CrossRef]
- Shibata, S.; Hayakawa, K.; Egashira, Y.; Sanada, H. Hypocholesterolemic Mechanism of Chlorella: Chlorella and Its Indigestible Fraction Enhance Hepatic Cholesterol Catabolism through Up-Regulation of Cholesterol 7α-Hydroxylase in Rats. Biosci. Biotechnol. Biochem. 2007, 71, 916–925. [Google Scholar] [CrossRef]
- Singh, P.; Singh, M.; Singh, R.K.; Prasad, S.M. Physiology of Salt Stress in Plants: Perception, Signalling, Omics and Tolerance Mechanism; John Wiley Sons: New York, NY, USA, 2021. [Google Scholar]
- Holst, B.; Williamson, G. Nutrients and phytochemicals: From bioavailability to bioefficacy beyond antioxidants. Curr. Opin. Biotechnol. 2008, 19, 73–82. [Google Scholar] [CrossRef]
- Mandotra, S.K.; Upadhyay, A.K.; Ahluwalia, A.S. Algae: Multifarious Applications for a Sustainable World; Springer Nature: Singapore, 2020. [Google Scholar]
- Hu, J.; Nagarajan, D.; Zhang, Q.; Chang, J.S.; Lee, D.J. Heterotrophic cultivation of microalgae for pigment production: A review. Biotechnol. Adv. 2018, 36, 54–67. [Google Scholar] [CrossRef]
- Yoshida, H.; Yanai, H.; Ito, K.; Tomono, Y.; Koikeda, T.; Tsukahara, H.; Tada, N. Administration of natural astaxanthin increases serum HDL-cholesterol and adiponectin in subjects with mild hyperlipidemia. Atherosclerosis 2010, 209, 520–523. [Google Scholar] [CrossRef]
- Shatoor, A.S.; Al Humayed, S.; Almohiy, H.M. Astaxanthin attenuates hepatic steatosis in high-fat diet-fed rats by suppressing microRNA-21 via transactivation of nuclear factor erythroid 2-related factor 2. J. Physiol. Biochem. 2021, 78, 151–168. [Google Scholar] [CrossRef]
- Yang, Y.; Pham, T.X.; Wegner, C.J.; Kim, B.; Ku, C.S.; Park, Y.K.; Lee, J.Y. Astaxanthin lowers plasma TAG concentrations and increases hepatic antioxidant gene expression in diet-induced obesity mice. Br. J. Nutr. 2014, 112, 1797–1804. [Google Scholar] [CrossRef]
- Macedo, R.C.; Bolin, A.P.; Marin, D.P.; Otton, R. Astaxanthin addition improves human neutrophils function: In vitro study. Eur. J. Nutr. 2010, 49, 447–457. [Google Scholar] [CrossRef]
- Yang, Y.; Seo, J.M.; Nguyen, A.; Pham, T.X.; Park, H.J.; Park, Y.; Kim, B.; Bruno, R.S.; Lee, J. Astaxanthin-rich extract from the green alga Haematococcus pluvialis lowers plasma lipid concentrations and enhances antioxidant defense in apolipoprotein E knockout mice. J. Nutr. 2011, 141, 1611–1617. [Google Scholar] [CrossRef]
- Lee, S.H.; Min, K.H.; Han, J.S.; Lee, D.H.; Park, D.B.; Jung, W.K.; Park, P.J.; Jeon, B.T.; Kim, S.K.; Jeon, Y.J. Effects of brown alga, Ecklonia cava on glucose and lipid metabolism in C57BL/KsJ-db/db mice, a model of type 2 diabetes mellitus. Food Chem. Toxicol. 2012, 50, 575–582. [Google Scholar] [CrossRef]
- Beppu, F.; Hosokawa, M.; Niwano, Y.; Miyashita, K. Effects of dietary fucoxanthin on cholesterol metabolism in diabetic/obese KK-A y mice. Lipids Health Dis. 2012, 11, 1–8. [Google Scholar] [CrossRef]
- Tong, L. Acetyl-coenzyme A carboxylase: Crucial metabolic enzyme and attractive target for drug discovery. Cell. Mol. Life Sci. 2005, 62, 1784–1803. [Google Scholar] [CrossRef] [PubMed]
- Koo, S.Y.; Hwang, J.H.; Yang, S.H.; Um, J.I.; Hong, K.W.; Kang, K.; Pan, C.H.; Hwang, K.T.; Kim, S.M. Anti-Obesity Effect of Standardized Extract of Microalga Phaeodactylum tricornutum Containing Fucoxanthin. Mar. Drugs 2019, 17, 311. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Takahashi, N.; Kato, S.; Egawa, K.; Ebisu, S.; Moriyama, T.; Fushiki, T.; Kawada, T. Phytol directly activates peroxisome proliferator-activated receptor α (PPARα) and regulates gene expression involved in lipid metabolism in PPARα-expressing HepG2 hepatocytes. Biochem. Biophys. Res. Commun. 2005, 337, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Mayer, C.; Côme, M.; Blanckaert, V.; Chini Zittelli, G.; Faraloni, C.; Nazih, H.; Ouguerram, K.; Mimouni, V.; Chénais, B. Effect of Carotenoids from Phaeodactylum tricornutum on Palmitate-Treated HepG2 Cells. Molecules 2020, 25, 2845. [Google Scholar] [CrossRef] [PubMed]
- Youn, Y.; Kim, Y.-S. Inhibitory effects of Citrus unshiu pericarpium extracts on palmitate-induced lipotoxicity in HepG2 cells. Food Sci. Biotechnol. 2016, 25, 1709–1717. [Google Scholar] [CrossRef]
- Gammone, M.A.; D’Orazio, N. Anti-Obesity Activity of the Marine Carotenoid Fucoxanthin. Mar. Drugs 2015, 13, 2196–2214. [Google Scholar] [CrossRef]
- Eberlé, D.; Hegarty, B.; Bossard, P.; Ferré, P.; Foufelle, F. SREBP transcription factors: Master regulators of lipid homeostasis. Biochimie 2004, 86, 839–848. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, Q.; Fu, J.; Ren, R. Polysaccharides derived from natural sources regulate triglyceride and cholesterol metabolism: A review of the mechanisms. Food Funct. 2019, 5, 2330–2339. [Google Scholar] [CrossRef]
- Dvir, I.; Stark, A.H.; Chayoth, R.; Madar, Z.; Arad, S.M. Hypocholesterolemic effects of nutraceuticals produced from the red microalga Porphyridium sp. in rats. Nutrients 2009, 1, 156–167. [Google Scholar] [CrossRef]
- Ren, R.; Gong, J.; Zhao, Y.; Zhuang, X.; Ye, Y.; Huang, F.; Lin, W. Sulfated polysaccharide from Enteromorpha prolifera suppresses SREBP-1c and ACC expression to lower serum triglycerides in high-fat diet-induced hyperlipidaemic rats. J. Funct. Foods 2018, 40, 722–728. [Google Scholar] [CrossRef]
- Werman, M.J.; Sukenik, A.; Mokady, S. Effects of the marine unicellular alga Nannochloropsis sp. to reduce the plasma and liver cholesterol levels in male rats fed on diets with cholesterol. Biosci. Biotechnol. Biochem. 2003, 67, 2266–2268. [Google Scholar] [CrossRef]
- Ara, J.; Sultana, V.; Qasim, R.; Ahmad, V.U. Hypolipidaemic activity of seaweed from Karachi coast. Phytother. Res. 2002, 16, 479–483. [Google Scholar] [CrossRef]
- Amano, H.; Kakinuma, M.; Coury, D.A.; Ohno, H.; Hara, T. Effect of a seaweed mixture on serum lipid level and platelet aggregation in rats. Fish. Sci. 2005, 71, 1160–1166. [Google Scholar] [CrossRef]
- Pengzhan, Y.; Ning, L.; Xiguang, L.; Gefei, Z.; Quanbin, Z.; Pengcheng, L. Antihyperlipidemic effects of different molecular weight sulfated polysaccharides from Ulva pertusa (Chlorophyta). Pharmacol. Res. 2003, 48, 543–549. [Google Scholar] [CrossRef]
- Stark, A.; Nyska, A.; Madar, Z. Metabolic and Morphometric Changes in Small and Large Intestine in Rats Fed High-Fiber Diets. SAGE J. 1996, 24, 166–171. [Google Scholar] [CrossRef]
- Wan, X.-Z.; Ai, C.; Chen, Y.H.; Gao, X.X.; Zhong, R.T.; Liu, B.; Chen, X.H.; Zhao, C. Physicochemical Characterization of a Polysaccharide from Green Microalga Chlorella pyrenoidosa and Its Hypolipidemic Activity via Gut Microbiota Regulation in Rats. Agric. Food Chem. 2019, 68, 1186–1197. [Google Scholar] [CrossRef]
- Guo, W.; Zhu, S.; Li, S.; Feng, Y.; Wu, H.; Zeng, M. Microalgae polysaccharides ameliorates obesity in association with modulation of lipid metabolism and gut microbiota in high-fat-diet fed C57BL/6 mice. Int. J. Biol. Macromol. 2021, 182, 1371–1383. [Google Scholar] [CrossRef]
- Watanabe, M.; Takahashi, H.; Saeki, Y.; Ozaki, T.; Itoh, S.; Suzuki, M.; Mizushima, W.; Tanaka, K.; Hatakeyama, S. The E3 ubiquitin ligase TRIM23 regulates adipocyte differentiation via stabilization of the adipogenic activator PPARγ. Elife 2015, 4, e05615. [Google Scholar] [CrossRef]
- Payne, V.A.; Au, W.S.; Lowe, C.E.; Rahman, S.M.; Friedman, J.E.; O’Rahilly, S.; Rochford, J.J. C/EBP transcription factors regulate SREBP1c gene expression during adipogenesis. Biochem. J. 2009, 425, 215–223. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, T.; Wang, Z.; Xu, Y.; Zhang, Q.; Luo, D. Low molecular weight fucoidan attenuates liver injury via SIRT1/AMPK/PGC1α axis in db/db mice. Int. J. Biol. Macromol. 2018, 112, 929–936. [Google Scholar] [CrossRef]
- Ostlund, R.E., Jr.; Racette, S.B.; Stenson, W.F. Effects of trace components of dietary fat on cholesterol metabolism: Phytosterols, oxysterols, and squalene. Nutr. Rev. 2002, 60, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Ivanchina, N.V.; Kicha, A.A.; Stonik, V.A. Steroid glycosides from marine organisms. Steroids 2011, 76, 425–454. [Google Scholar] [CrossRef] [PubMed]
- Grille, S.; Zaslawski, A.; Thiele, S.; Plat, J.; Warnecke, D. The functions of steryl glycosides come to those who wait: Recent advances in plants, fungi, bacteria and animals. Prog. Lipid Res. 2010, 49, 262–288. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, M. Immunological functions of steryl glycosides. Arch. Immunol. Ther. Exp. 2012, 60, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Li, K.; Duan, X.; Hill, D.; Barrow, C.; Dunshea, F.; Martin, G.; Suleria, H. Bioactive compounds in microalgae and their potential health benefits. Food Biosci. 2022, 49, 101932. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, Y.; Ran, Y.; Lai, W.; Ran, Z.; Xu, J.; Zhou, C.; Yan, X. Characterization of steryl glycosides in marine microalgae by gas chromatography-triple quadrupole mass spectrometry (GC-QQQ-MS). J. Sci. Food Agric. 2018, 98, 1574–1583. [Google Scholar] [CrossRef]
- Feng, S.; Belwal, T.; Li, L.; Limwachiranon, J.; Liu, X.; Luo, Z. Phytosterols and their derivatives: Potential health-promoting uses against lipid metabolism and associated diseases, mechanism, and safety issues. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1243–1267. [Google Scholar] [CrossRef]
- Feng, S.; Dai, Z.; Liu, A.B.; Huang, J.; Narsipur, N.; Guo, G.; Kong, B.; Reuhl, K.; Lu, W.; Luo, Z.; et al. Intake of stigmasterol and β-sitosterol alters lipid metabolism and alleviates NAFLD in mice fed a high-fat western-style diet. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 1274–1284. [Google Scholar] [CrossRef]
- Chen, D.L.; Huang, P.H.; Chiang, C.H.; Leu, H.B.; Chen, J.W.; Lin, S.J. Phytosterols increase circulating endothelial progenitor cells and insulin-like growth factor-1 levels in patients with nonalcoholic fatty liver disease: A randomized crossover study. J. Funct. Foods 2015, 13, 148–157. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Russell, W.; Duthie, G. Plant secondary metabolites and gut health: The case for phenolic acids. Proc. Nutr. Soc. 2011, 70, 389–396. [Google Scholar] [CrossRef]
- Huang, W.-Y.; Cai, Y.-Z.; Zhang, Y. Natural phenolic compounds from medicinal herbs and dietary plants: Potential use for cancer prevention. Nutr. Cancer 2010, 62, 1–20. [Google Scholar] [CrossRef]
- Safafar, H.; Van Wagenen, J.; Møller, P.; Jacobsen, C. Carotenoids, Phenolic Compounds and Tocopherols Contribute to the Antioxidative Properties of Some Microalgae Species Grown on Industrial Wastewater. Mar. Drugs 2015, 13, 7339–7356. [Google Scholar] [CrossRef]
- Anantharaju, P.G.; Gowda, P.C.; Vimalambike, M.G.; Madhunapantula, S.V. An overview on the role of dietary phenolics for the treatment of cancers. Nutr. J. 2016, 15, 99. [Google Scholar] [CrossRef]
- Li, S.; Tan, H.Y.; Wang, N.; Cheung, F.; Hong, M.; Feng, Y. The Potential and Action Mechanism of Polyphenols in the Treatment of Liver Diseases. Oxid. Med. Cell. Longev. 2018, 2018, 8394818. [Google Scholar] [CrossRef]
- Wu, C.-H.; Lin, M.C.; Wang, H.C.; Yang, M.Y.; Jou, M.J.; Wang, C.J. Rutin inhibits oleic acid induced lipid accumulation via reducing lipogenesis and oxidative stress in hepatocarcinoma cells. J. Food Sci. 2011, 76, T65–T72. [Google Scholar] [CrossRef]
- Kraan, S. Mass-cultivation of carbohydrate rich macroalgae, a possible solution for sustainable biofuel production. Mitig. Adapt. Strateg. Glob. Change 2010, 18, 27–46. [Google Scholar] [CrossRef]
- Aguilar-Toalá, J.E.; Hall, F.G.; Urbizo-Reyes, U.; Liceaga, A.M. Sustainable, alternative sources of bioactive peptides. Biol. Act. Pept. 2021, 3, 427–453. [Google Scholar] [CrossRef]
- Belghit, I.; Rasinger, J.D.; Heesch, S.; Biancarosa, I.; Liland, N.; Torstensen, B.; Waagbø, R.; Lock, E.J.; Bruckner, C.G. In-depth metabolic profiling of marine macroalgae confirms strong biochemical differences between brown, red and green algae. Algal Res. 2017, 26, 240–249. [Google Scholar] [CrossRef]
- Wan, A.H.L.; Davies, S.J.; Soler-Vila, A.; Fitzgerald, R.; Johnson, M.P. Macroalgae as a sustainable aquafeed ingredient. Rev. Aquac. 2018, 11, 458–492. [Google Scholar] [CrossRef]
- Roohinejad, S.; Koubaa, M.; Barba, F.J.; Saljoughian, S.; Amid, M.; Greiner, R. Application of seaweeds to develop new food products with enhanced shelf-life, quality and health-related beneficial properties. Food Res. Int. 2017, 99, 1066–1083. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Noda, H.; Amano, H.; Nishino, T.; Nishizawa, K. Study on Antihypertensive and Antihyperlipidemic Effects of Marine Algae. Fish. Sci. 1994, 60, 83–88. [Google Scholar] [CrossRef]
- Lin, H.-T.V.; Tsou, Y.-C.; Chen, Y.-T.; Lu, W.-J.; Hwang, P.-A. Effects of Low-Molecular-Weight Fucoidan and High Stability Fucoxanthin on Glucose Homeostasis, Lipid Metabolism, and Liver Function in a Mouse Model of Type II Diabetes. Mar. Drugs 2017, 15, 113. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, K.; Beppu, F.; Hosokawa, M.; Liu, X.; Wang, S. Nutraceutical characteristics of the brown seaweed carotenoid fucoxanthin. Arch. Biochem. Biophys. 2020, 686, 108364. [Google Scholar] [CrossRef]
- Olivares-Bañuelos, T.; Gutiérrez-Rodríguez, A.G.; Méndez-Bellido, R.; Tovar-Miranda, R.; Arroyo-Helguera, O.; Juárez-Portilla, C.; Meza-Menchaca, T.; Aguilar-Rosas, L.E.; Hernández-Kelly, L.C.; Ortega, A.; et al. Brown Seaweed’s Cytotoxic Activity against Brain Cancer Cell Lines. Molecules 2019, 24, 260. [Google Scholar] [CrossRef] [PubMed]
- Neto, R.T.; Marçal, C.; Queirós, A.S.; Abreu, H.; Silva, A.M.; Cardoso, S.M. Screening of Ulva rigida, Gracilaria sp., Fucus vesiculosus and Saccharina latissima as Functional Ingredients. Int. J. Mol. Sci. 2018, 19, 2987. [Google Scholar] [CrossRef]
- Laura, A.P.; de la Garza, A.R.M.; Elena, P.M.; Gutiérrez-Uribe, J.A.; Armando, T.C.; Cruz-Suárez, L.E.; Serna-Saldívar, S.O. Effects of Ecklonia arborea or Silvetia compressa algae intake on serum lipids and hepatic fat accumulation in Wistar rats fed hyperlipidic diets. Algal Res. 2020, 49, 101946. [Google Scholar] [CrossRef]
- Lovegrove, A.; Edwards, C.H.; De Noni, I.; Patel, H.; El, S.N.; Grassby, T.; Zielke, C.; Ulmius, M.; Nilsson, L.; Butterworth, P.J.; et al. Role of polysaccharides in food, digestion, and health. Crit. Rev. Food Sci. Nutr. 2017, 57, 237–253. [Google Scholar] [CrossRef]
- Ren, R.; Gong, J.; Zhao, Y.; Zhuang, X.; Ye, Y.; Lin, W. Sulfated polysaccharides from Enteromorpha prolifera suppress SREBP-2 and HMG-CoA reductase expression and attenuate non-alcoholic fatty liver disease induced by a high-fat diet. Food Funct. 2017, 8, 1899–1904. [Google Scholar] [CrossRef]
- Zha, X.Q.; Xiao, J.J.; Zhang, H.N.; Wang, J.H.; Pan, L.H.; Yang, X.F.; Luo, J.P. Polysaccharides in Laminaria japonica (LP): Extraction, physicochemical properties and their hypolipidemic activities in diet-induced mouse model of atherosclerosis. Food Chem. 2012, 134, 244–252. [Google Scholar] [CrossRef]
- Ben Gara, A.; Kolsi, R.B.A.; Chaaben, R.; Hammami, N.; Kammoun, M.; Patti, F.P.; El Feki, A.; Fki, L.; Belghith, H.; Belghith, K. Inhibition of key digestive enzymes related to hyperlipidemia and protection of liver-kidney functions by Cystoseira crinita sulphated polysaccharide in high-fat diet-fed rats. Biomed. Pharmacother. 2017, 85, 517–526. [Google Scholar] [CrossRef]
- Matanjun, P.; Mohamed, S.; Muhammad, K.; Mustapha, N.M. Comparison of cardiovascular protective effects of tropical seaweeds, Kappaphycus alvarezii, Caulerpa lentillifera, and Sargassum polycystum, on high-cholesterol/high-fat diet in rats. J. Med. Food 2010, 13, 792–800. [Google Scholar] [CrossRef]
- Rizk, M.Z.; Aly, H.F.; Matloub, A.A.; Fouad, G.I. The anti-hypercholesterolemic effect of ulvan polysaccharide extracted from the green alga Ulva fasciata on aged hypercholesterolemic rats. Asian J. Pharm. Clin. Res. 2016, 9, 165–176. [Google Scholar]
- Matloub, A.A.; AbouSamra, M.M.; Salama, A.H.; Rizk, M.Z.; Aly, H.F.; Fouad, G.I. Cubic liquid crystalline nanoparticles containing a polysaccharide with potent antihyperlipidaemic activity. Saudi. Pharm. J. 2018, 26, 224–231. [Google Scholar] [CrossRef]
- Ha, A.W.; Kim, W.K. The effect of fucoxanthin rich power on the lipid metabolism in rats with a high fat diet. Nutr. Res. Pract. 2013, 7, 287–293. [Google Scholar] [CrossRef]
- Woo, M.-N.; Jeon, S.M.; Kim, H.J.; Lee, M.K.; Shin, S.K.; Shin, Y.C.; Park, Y.B.; Choi, M.S. Fucoxanthin supplementation improves plasma and hepatic lipid metabolism and blood glucose concentration in high-fat fed C57BL/6N mice. Chem. Biol. Interact. 2010, 186, 316–322. [Google Scholar] [CrossRef]
- Yoon, N.Y.; Kim, H.R.; Chung, H.Y.; Choi, J.S. Anti-hyperlipidemic effect of an edible brown algae, Ecklonia stolonifera, and its constituents on poloxamer 407-induced hyperlipidemic and cholesterol-fed rats. Arch. Pharm. Res. 2008, 31, 1564–1571. [Google Scholar] [CrossRef]
- Shin, H.-C.; Kim, S.H.; Park, Y.; Lee, B.H.; Hwang, H.J. Effects of 12-week oral supplementation of Ecklonia cava polyphenols on anthropometric and blood lipid parameters in overweight Korean individuals: A double-blind randomized clinical trial. Phytother. Res. 2012, 26, 363–368. [Google Scholar] [CrossRef]
- André, R.; Guedes, L.; Melo, R.; Ascensão, L.; Pacheco, R.; Vaz, P.D.; Serralheiro, M.L. Effect of Food Preparations on In Vitro Bioactivities and Chemical Components of Fucus vesiculosus. Foods 2020, 9, 955. [Google Scholar] [CrossRef]
- Edible Seaweed and Microalgae—Regulatory Status in France and Europe—2019 Update. Ceva-algues.com. 2020. Available online: https://www.ceva-algues.com/en/document/edible-algae-regulatory-update/ (accessed on 1 September 2022).
- Arantes, A.A.; Falé, P.L.; Costa, L.C.; Pacheco, R.; Ascensão, L.; Serralheiro, M.L. Inhibition of HMG-CoA reductase activity and cholesterol permeation through Caco-2 cells by caffeoylquinic acids from Vernonia condensata leaves. Rev. Bras. Farmacogn. 2016, 26, 738–743. [Google Scholar] [CrossRef]
- Kobayashi, S. The Effect of Polyphenols on Hypercholesterolemia through Inhibiting the Transport and Expression of Niemann-Pick C1-Like 1. Int. J. Mol. Sci. 2019, 20, 4939. [Google Scholar] [CrossRef] [PubMed]
- Bano, Z.; Rajarathnam, S. Pleurotus mushrooms. Part II. Chemical composition, nutritional value, post-harvest physiology, preservation, and role as human food. Crit. Rev. Food Sci. Nutr. 1988, 27, 87–158. [Google Scholar] [CrossRef] [PubMed]
- Manzi, P.; Gambelli, L.; Marconi, S.; Vivanti, V.; Pizzoferrato, L. Nutrients in edible mushrooms: An inter-species comparative study. Food Chem. 1999, 65, 477–482. [Google Scholar] [CrossRef]
- Mattila, P.; Könkö, K.; Eurola, M.; Pihlava, J.M.; Astola, J.; Vahteristo, L.; Hietaniemi, V.; Kumpulainen, J.; Valtonen, M.; Piironen, V. Contents of vitamins, mineral elements, and some phenolic compounds in cultivated mushrooms. J. Agric. Food Chem. 2001, 49, 2343–2348. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Oinuma, T.; Niihashi, M.; Mitsumata, M.; Fujioka, T.; Hasegawa, K.; Nagaoka, H.; Itakura, H. Effects of Lentinus edodes mycelia on dietary-induced atherosclerotic involvement in rabbit aorta. J. Atheroscler. Thromb. 2002, 9, 149–156. [Google Scholar] [CrossRef]
- Mori, K.; Kobayashi, C.; Tomita, T.; Inatomi, S.; Ikeda, M. Antiatherosclerotic effect of the edible mushrooms Pleurotus eryngii (Eringi), Grifola frondosa (Maitake), and Hypsizygus marmoreus (Bunashimeji) in apolipoprotein E-deficient mice. Nutr. Res. 2008, 28, 335–342. [Google Scholar] [CrossRef]
- Bobek, P.; Ginter, E.; Jurcovicová, M.; Kuniak, L. Cholesterol-lowering effect of the mushroom Pleurotus ostreatus in hereditary hypercholesterolemic rats. Ann. Nutr. Metab. 1991, 35, 191–195. [Google Scholar] [CrossRef]
- Bobek, P.; Hromadová, M.; Ozdín, L. Oyster mushroom (Pleurotus ostreatus) reduces the activity of 3-hydroxy-3-methylglutaryl CoA reductase in rat liver microsomes. Experientia 1995, 51, 589–591. [Google Scholar] [CrossRef]
- Bobek, P.D.P.; Ondreička, D.I.R.; Klvanová, I.J.; Ozdín, P.D.Ĺ. Oyster mushroom (Pleurotus ostreatus) decreases serum and liver cholesterol and increases cholesterol 7α-hydroxylase activity and fecal excretion of neutral sterols and bile acids in hypercholesterolemic rats. Nutr. Res. 1994, 14, 1683–1688. [Google Scholar] [CrossRef]
- Bobek, P.; Ozdin, L.; Kuniak, L. Mechanism of hypocholesterolemic effect of oyster mushroom (Pleurotus ostreatus) in rats: Reduction of cholesterol absorption and increase of plasma cholesterol removal. Z. Ernahrungswiss. 1994, 33, 44–50. [Google Scholar] [CrossRef]
- Meneses, M.E.; Martínez-Carrera, D.; Torres, N.; Sánchez-Tapia, M.; Aguilar-López, M.; Morales, P.; Sobal, M.; Bernabé, T.; Escudero, H.; Granados-Portillo, O.; et al. Hypocholesterolemic Properties and Prebiotic Effects of Mexican Ganoderma lucidum in C57BL/6 Mice. PLoS ONE 2016, 11, e0159631. [Google Scholar] [CrossRef]
- Eliades, M.; Spyrou, E. Vitamin D: A new player in non-alcoholic fatty liver disease? World J. Gastroenterol. 2015, 21, 1718–1727. [Google Scholar] [CrossRef]
- Strange, R.C.; Shipman, K.E.; Ramachandran, S. Metabolic syndrome: A review of the role of vitamin D in mediating susceptibility and outcome. World J. Diabetes 2015, 6, 896–911. [Google Scholar] [CrossRef]
- Drori, A.; Rotnemer-Golinkin, D.; Avni, S.; Danay, O.; Levanon, D.; Tam, J.; Zolotarev, L.; Ilan, Y. Attenuating the rate of total body fat accumulation and alleviating liver damage by oral administration of vitamin D-enriched edible mushrooms in a diet-induced obesity murine model is mediated by an anti-inflammatory paradigm shift. BMC Gastroenterol. 2017, 17, 130. [Google Scholar] [CrossRef]
- Kozarski, M.; Klaus, A.; Jakovljevic, D.; Todorovic, N.; Vunduk, J.; Petrović, P.; Niksic, M.; Vrvic, M.M.; Van Griensven, L. Antioxidants of Edible Mushrooms. Molecules 2015, 20, 19489–19525. [Google Scholar] [CrossRef]
- Chutkan, R.; Fahey, G.; Wright, W.L.; McRorie, J. Viscous versus nonviscous soluble fiber supplements: Mechanisms and evidence for fiber-specific health benefits. J. Am. Acad. Nurse Pract. 2012, 24, 476–487. [Google Scholar] [CrossRef]
- Chen, X.; Zhong, H.Y.; Zeng, J.H.; Ge, J. The pharmacological effect of polysaccharides from Lentinus edodes on the oxidative status and expression of VCAM-1mRNA of thoracic aorta endothelial cell in high-fat-diet rats. Carbohydr. Polym. 2008, 74, 445–450. [Google Scholar]
- Li, L.; Guo, W.L.; Zhang, W.; Xu, J.X.; Qian, M.; Bai, W.D.; Zhang, Y.Y.; Rao, P.F.; Ni, L.; Lv, X.C. Grifola frondosa polysaccharides ameliorate lipid metabolic disorders and gut microbiota dysbiosis in high-fat diet fed rats. Food Funct. 2019, 10, 2560–2572. [Google Scholar] [CrossRef]
- Russell, D.W. The Enzymes, Regulation, and Genetics of Bile Acid Synthesis. Annu. Rev. Biochem. 2003, 72, 137–174. [Google Scholar] [CrossRef]
- Ren, F.; Chen, Q.; Meng, C.; Chen, H.; Zhou, Y.; Zhang, H.; Chen, W. Serum metabonomics revealed the mechanism of Ganoderma amboinense polysaccharides in preventing non-alcoholic fatty liver disease (NAFLD) induced by high-fat diet. J. Funct. Foods 2021, 82, 104496. [Google Scholar] [CrossRef]
- Brandão, Y.B.; de Oliveira, J.G.C.; Benachour, M. Phenolic wastewaters: Definition, sources and treatment processes. In Phenolic Compounds—Natural Sources, Importance and Applications; InTech: London, UK, 2017. [Google Scholar]
- Rodriguez-Ramiro, I.; Vauzour, D.; Minihane, A.M. Polyphenols and non-alcoholic fatty liver disease: Impact and mechanisms. Proc. Nutr. Soc. 2016, 75, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Radzki, W.; Sławińska, A.; Jabłońska-Ryś, E.; Gustaw, W. Antioxidant capacity and polyphenolic content of dried wild edible mushrooms from Poland. Int. J. Med. Mushrooms 2014, 16, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, F.; Chopra, H.; Baig, A.A.; Avula, S.K.; Kumari, S.; Mohanta, T.K.; Saravanan, M.; Mishra, A.K.; Sharma, N.; Mohanta, Y.K. Edible Mushrooms as Novel Myco-Therapeutics: Effects on Lipid Level, Obesity and BMI. J. Fungi. 2022, 8, 211. [Google Scholar] [CrossRef] [PubMed]
- Promden, W.; Viriyabancha, W.; Monthakantirat, O.; Umehara, K.; Noguchi, H.; De-Eknamkul, W. Correlation between the Potency of Flavonoids on Mushroom Tyrosinase Inhibitory Activity and Melanin Synthesis in Melanocytes. Molecules 2018, 23, 1403. [Google Scholar] [CrossRef]
- Ma, H.-T.; Hsieh, J.-F.; Chen, S.-T. Anti-diabetic effects of Ganoderma lucidum. Phytochemistry 2015, 114, 109–113. [Google Scholar] [CrossRef]
- Gąsecka, M.; Mleczek, M.; Siwulski, M.; Niedzielski, P.; Kozak, L. Phenolic and Flavonoid Content in Hericium Erinaceus, Ganoderma Lucidum, and Agrocybe Aegerita under Selenium Addition. Acta Aliment. 2016, 45, 300–308. [Google Scholar] [CrossRef]
- Jung, S.; Son, H.; Hwang, C.E.; Cho, K.M.; Park, S.W.; Kim, H.J. Ameliorates Non-Alcoholic Steatosis by Upregulating Energy Metabolizing Enzymes in the Liver. J. Clin. Med. Res. 2018, 7, 152. [Google Scholar]
- Weete, J.D.; Abril, M.; Blackwell, M. Phylogenetic distribution of fungal sterols. PLoS ONE 2010, 5, e10899. [Google Scholar] [CrossRef]
- Das, M.; Geetha, V.; Zarei, M.; Harohally, N.V.; Suresh Kumar, G. Modulation of obesity associated metabolic dysfunction by novel lipophilic fraction obtained from Agaricus bisporus. Life Sci. 2022, 305, 120779. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).