In Silico Screening of Metal-Organic Frameworks for Formaldehyde Capture with and without Humidity by Molecular Simulation
Abstract
1. Introduction
2. Results and Discussion
2.1. HCHO Capture Performance without Humidity
2.2. HCHO Capture Performance with Humidity
3. Materials and Methods
3.1. MOFs Database
3.2. Grand Canonical Monte Carlo
3.3. Force Field
3.4. The Descriptor of MOF Characteristic
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kim, W.J.; Terada, N.; Nomura, T.; Takahashi, R.; Lee, S.D.; Park, J.H.; Konno, A. Effect of formaldehyde on the expression of adhesion molecules in nasal microvascular endothelial cells: The role of formaldehyde in the pathogenesis of sick building syndrome. Clin. Exp. Allergy 2002, 32, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Wood, R.W.; Coleman, J.B. Behavioral evaluation of the irritant properties of formaldehyde. Toxicol. Appl. Pharmacol. 1995, 130, 67–72. [Google Scholar] [CrossRef]
- Gu, Z.-Y.; Wang, G.; Yan, X.-P. MOF-5 Metal-Organic Framework as Sorbent for in-Field Sampling and Preconcentration in Combination with Thermal Desorption GC/MS for Determination of Atmospheric Formaldehyde. Anal. Chem. 2010, 82, 1365–1370. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Xu, P.; Wang, H.; Zeng, G.; Huang, D.; Chen, M.; Lai, C.; Zhang, C.; Wan, J.; Xue, W. Strategies to improve metal organic frameworks photocatalyst’s performance for degradation of organic pollutants. Coord. Chem. Rev. 2018, 376, 449–466. [Google Scholar] [CrossRef]
- Szulejko, J.E.; Kim, K.-H.; Parise, J. Seeking the most powerful and practical real-world sorbents for gaseous benzene as a representative volatile organic compound based on performance metrics. Sep. Purif. Technol. 2019, 212, 980–985. [Google Scholar] [CrossRef]
- Lai, C.; Wang, Z.; Qin, L.; Fu, Y.; Li, B.; Zhang, M.; Liu, S.; Li, L.; Yi, H.; Liu, X.; et al. Metal-organic frameworks as burgeoning materials for the capture and sensing of indoor VOCs and radon gases. Coord. Chem. Rev. 2021, 427, 213565. [Google Scholar] [CrossRef]
- Dou, H.; Long, D.; Rao, X.; Zhang, Y.; Qin, Y.; Pan, F.; Wu, K. Photocatalytic Degradation Kinetics of Gaseous Formaldehyde Flow Using TiO2 Nanowires. ACS Sustain. Chem. Eng. 2019, 7, 4456–4465. [Google Scholar] [CrossRef]
- Yi, H.; Yan, M.; Huang, D.; Zeng, G.; Lai, C.; Li, M.; Huo, X.; Qin, L.; Liu, S.; Liu, X.; et al. Synergistic effect of artificial enzyme and 2D nano-structured Bi2WO6 for eco-friendly and efficient biomimetic photocatalysis. Appl. Catal. B Environ. 2019, 250, 52–62. [Google Scholar] [CrossRef]
- Yan, S.; Su, Y.; Deng, D.; Hu, J.; Lv, Y. Formaldehyde sensing based on high photoluminescence and strong oxidizing degradation of NH2-Fe(III)-nMOFs. Sens. Actuators B Chem. 2021, 333, 129140. [Google Scholar] [CrossRef]
- Zhang, S.; Zhuo, Y.; Ezugwu, C.I.; Wang, C.-c.; Li, C.; Liu, S. Synergetic Molecular Oxygen Activation and Catalytic Oxidation of Formaldehyde over Defective MIL-88B(Fe) Nanorods at Room Temperature. Environ. Sci. Technol. 2021, 55, 8341–8350. [Google Scholar] [CrossRef]
- Hu, S.-C.; Chen, Y.-C.; Lin, X.-Z.; Shiue, A.; Huang, P.-H.; Chen, Y.-C.; Chang, S.-M.; Tseng, C.-H.; Zhou, B. Characterization and adsorption capacity of potassium permanganate used to modify activated carbon filter media for indoor formaldehyde removal. Environ. Sci. Pollut. Res. 2018, 25, 28525–28545. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Deng, X.; Cai, C.; Shi, Z.; Liang, H.; Li, S.; Qiao, Z. Machine learning and high-throughput computational screening of hydrophobic metal–organic frameworks for capture of formaldehyde from air. Green Energy Environ. 2021, 6, 759–770. [Google Scholar] [CrossRef]
- Bellat, J.P.; Bezverkhyy, I.; Weber, G.; Royer, S.; Averlant, R.; Giraudon, J.M.; Lamonier, J.F. Capture of formaldehyde by adsorption on nanoporous materials. J. Hazard. Mater. 2015, 300, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Miyawaki, J.; Shiratori, N.; Yoon, S.-H.; Jang, J. Toward an effective adsorbent for polar pollutants: Formaldehyde adsorption by activated carbon. J. Hazard. Mater. 2013, 260, 82–88. [Google Scholar] [CrossRef]
- Xu, Z.; Yu, J.; Xiao, W. Microemulsion-Assisted Preparation of a Mesoporous Ferrihydrite/SiO2 Composite for the Efficient Removal of Formaldehyde from Air. Chem. -A Eur. J. 2013, 19, 9592–9598. [Google Scholar] [CrossRef]
- Xu, Z.; Yu, J.; Liu, G.; Cheng, B.; Zhou, P.; Li, X. Microemulsion-assisted synthesis of hierarchical porous Ni(OH)2/SiO2 composites toward efficient removal of formaldehyde in air. Dalton Trans. 2013, 42, 10190–10197. [Google Scholar] [CrossRef]
- Xu, Z.; Yu, J.; Low, J.; Jaroniec, M. Microemulsion-Assisted Synthesis of Mesoporous Aluminum Oxyhydroxide Nanoflakes for Efficient Removal of Gaseous Formaldehyde. ACS Appl. Mater. Interfaces 2014, 6, 2111–2117. [Google Scholar] [CrossRef]
- Ewlad-Ahmed, A.M.; Morris, M.A.; Patwardhan, S.V.; Gibson, L.T. Removal of Formaldehyde from Air Using Functionalized Silica Supports. Environ. Sci. Technol. 2012, 46, 13354–13360. [Google Scholar] [CrossRef]
- Wen, Q.; Li, C.; Cai, Z.; Zhang, W.; Gao, H.; Chen, L.; Zeng, G.; Shu, X.; Zhao, Y. Study on activated carbon derived from sewage sludge for adsorption of gaseous formaldehyde. Bioresour. Technol. 2011, 102, 942–947. [Google Scholar] [CrossRef]
- Laszlo, K. Characterization and adsorption properties of polymer-based microporous carbons with different surface chemistry. Microporous Mesoporous Mater. 2005, 80, 205–211. [Google Scholar] [CrossRef]
- Ferey, G. Hybrid porous solids: Past, present, future. Chem. Soc. Rev. 2008, 37, 191–214. [Google Scholar] [CrossRef] [PubMed]
- Bobbitt, N.S.; Mendonca, M.L.; Howarth, A.J.; Islamoglu, T.; Hupp, J.T.; Farha, O.K.; Snurr, R.Q. Metal-organic frameworks for the removal of toxic industrial chemicals and chemical warfare agents. Chem. Soc. Rev. 2017, 46, 3357–3385. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.-X.; Yang, H.; Zhang, J. Zeolitic imidazolate framework as formaldehyde gas sensor. Inorg. Chem. 2014, 53, 5411–5413. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, Z.; Zhou, J.; Fang, H.; He, X.; Jena, P.; Zeng, J.-B.; Wang, W.-N. Simultaneous Detection and Removal of Formaldehyde at Room Temperature: Janus Au@ZnO@ZIF-8 Nanoparticles. Nano-Micro Lett. 2017, 10, 4. [Google Scholar] [CrossRef]
- Yoo, M.-J.; Lee, M.-H.; Szulejko, J.E.; Vikrant, K.; Kim, K.-H. A quantitation method for gaseous formaldehyde based on gas chromatography with metal–organic framework cold-trap sorbent as an effective alternative for HPLC-based standard protocol. Microchem. J. 2021, 160, 105624. [Google Scholar] [CrossRef]
- Wang, L.; Liang, X.-Y.; Chang, Z.-Y.; Ding, L.-S.; Zhang, S.; Li, B.-J. Effective Formaldehyde Capture by Green Cyclodextrin-Based Metal-Organic Framework. ACS Appl. Mater. Interfaces 2018, 10, 42–46. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, W.; Jiang, D.; Zhang, L.; Zheng, Y. Diamine-appended metal-organic frameworks: Enhanced formaldehyde-vapor adsorption capacity, superior recyclability and water resistibility. Dalton. Trans. 2016, 45, 11306–11311. [Google Scholar] [CrossRef]
- Tran, T.Y.; Younis, S.A.; Heynderickx, P.M.; Kim, K.-H. Validation of two contrasting capturing mechanisms for gaseous formaldehyde between two different types of strong metal-organic framework adsorbents. J. Hazard. Mater. 2022, 424, 127459. [Google Scholar] [CrossRef]
- Boyd, P.G.; Chidambaram, A.; Garcia-Diez, E.; Ireland, C.P.; Daff, T.D.; Bounds, R.; Gladysiak, A.; Schouwink, P.; Moosavi, S.M.; Maroto-Valer, M.M.; et al. Data-driven design of metal-organic frameworks for wet flue gas CO2 capture. Nature 2019, 576, 253–256. [Google Scholar] [CrossRef]
- Bobbitt, N.S.; Snurr, R.Q. Molecular modelling and machine learning for high-throughput screening of metal-organic frameworks for hydrogen storage. Mol. Simul. 2019, 45, 1069–1081. [Google Scholar] [CrossRef]
- Budhathoki, S.; Ajayi, O.; Steckel, J.A.; Wilmer, C.E. High-throughput computational prediction of the cost of carbon capture using mixed matrix membranes. Energy Environ. Sci. 2019, 12, 1255–1264. [Google Scholar] [CrossRef]
- Qiao, Z.; Xu, Q.; Jiang, J. Computational screening of hydrophobic metal–organic frameworks for the separation of H2S and CO2 from natural gas. J. Mater. Chem. A 2018, 6, 18898–18905. [Google Scholar] [CrossRef]
- Lei, B.; Li, W.; Wei, Z.; Liu, X.; Li, S. Formaldehyde Adsorption Performance of Selected Metal-Organic Frameworks from High-throughput Computational Screening. Acta Chim. Sin. 2018, 76, 303–310. [Google Scholar]
- Daubert, T.E. Physical and Thermodynamic Properties of Pure Chemicals: Data Compilation; Design Institute for Physacal Property Data (DIPPR): New York, NY, USA, 1989. [Google Scholar]
- Wilmer, C.E.; Leaf, M.; Lee, C.Y.; Farha, O.K.; Hauser, B.G.; Hupp, J.T.; Snurr, R.Q. Large-scale Screening of Hypothetical Metal-organic Frameworks. Nat. Chem. 2012, 4, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, D.; Simon, C.M.; Plonka, A.M.; Motkuri, R.K.; Liu, J.; Chen, X.; Smit, B.; Parise, J.B.; Haranczyk, M.; Thallapally, P.K. Metal-organic framework with optimally selective xenon adsorption and separation. Nat. Commun. 2016, 7, ncomms11831. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.C.; Burhan, M.; Shahzad, M.W.; Ismail, A.B. A Universal Isotherm Model to Capture Adsorption Uptake and Energy Distribution of Porous Heterogeneous Surface. Sci. Rep. 2017, 7, 10634. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chung, Y.G.; Snurr, R.Q. High-Throughput Screening of Metal-Organic Frameworks for CO2 Capture in the Presence of Water. Langmuir 2016, 32, 10368–10376. [Google Scholar] [CrossRef]
- Chung, Y.G.; Camp, J.; Haranczyk, M.; Sikora, B.J.; Bury, W.; Krungleviciute, V.; Yildirim, T.; Farha, O.K.; Sholl, D.S.; Snurr, R.Q. Computation-Ready, Experimental Metal-Organic Frameworks: A Tool To Enable High-Throughput Screening of Nanoporous Crystals. Chem. Mater. 2014, 26, 6185–6192. [Google Scholar] [CrossRef]
- Manz, T.A.; Sholl, D.S. Improved Atoms-in-Molecule Charge Partitioning Functional for Simultaneously Reproducing the Electrostatic Potential and Chemical States in Periodic and Nonperiodic Materials. J. Chem. Theory Comput. 2012, 8, 2844–2867. [Google Scholar] [CrossRef]
- Nazarian, D.; Camp, J.S.; Sholl, D.S. A Comprehensive Set of High-Quality Point Charges for Simulations of Metal–Organic Frameworks. Chem. Mater. 2016, 28, 785–793. [Google Scholar] [CrossRef]
- Rappé, A.K.; Casewit, C.J.; Colwell, K.S.; Goddard, W.A., III; Skiff, W.M. UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations. J. Am. Chem. Soc. 1992, 114, 10024–10035. [Google Scholar] [CrossRef]
- Potoff, J.J.; Siepmann, J.I. Vapor-liquid equilibria of mixtures containing alkanes, carbon dioxide, and nitrogen. Aiche J. 2001, 47, 1676–1682. [Google Scholar] [CrossRef]
- Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Pedersen, L.G. A smooth particle mesh Ewald method. J. Chem. Phys. 1995, 103, 8577–8593. [Google Scholar] [CrossRef]
- Ghosh, P.; Kim, K.C.; Snurr, R.Q. Modeling Water and Ammonia Adsorption in Hydrophobic Metal–Organic Frameworks: Single Components and Mixtures. J. Phys. Chem. C 2014, 118, 1102–1110. [Google Scholar] [CrossRef]
- Hantal, G.; Jedlovszky, P.; Hoang, P.N.M.; Picaud, S. Calculation of the adsorption isotherm of formaldehyde on ice by grand canonical Monte Carlo simulation. J. Phys. Chem. C 2007, 111, 14170–14178. [Google Scholar] [CrossRef]




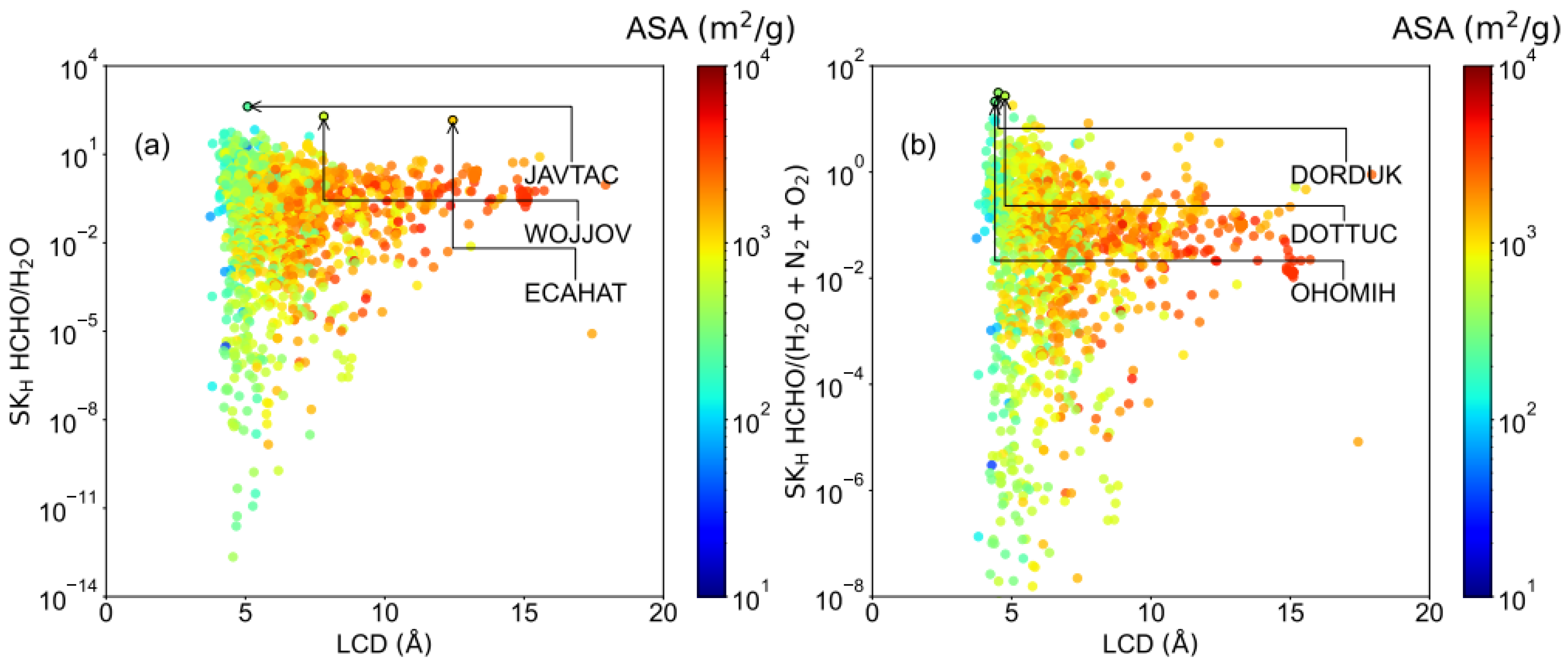

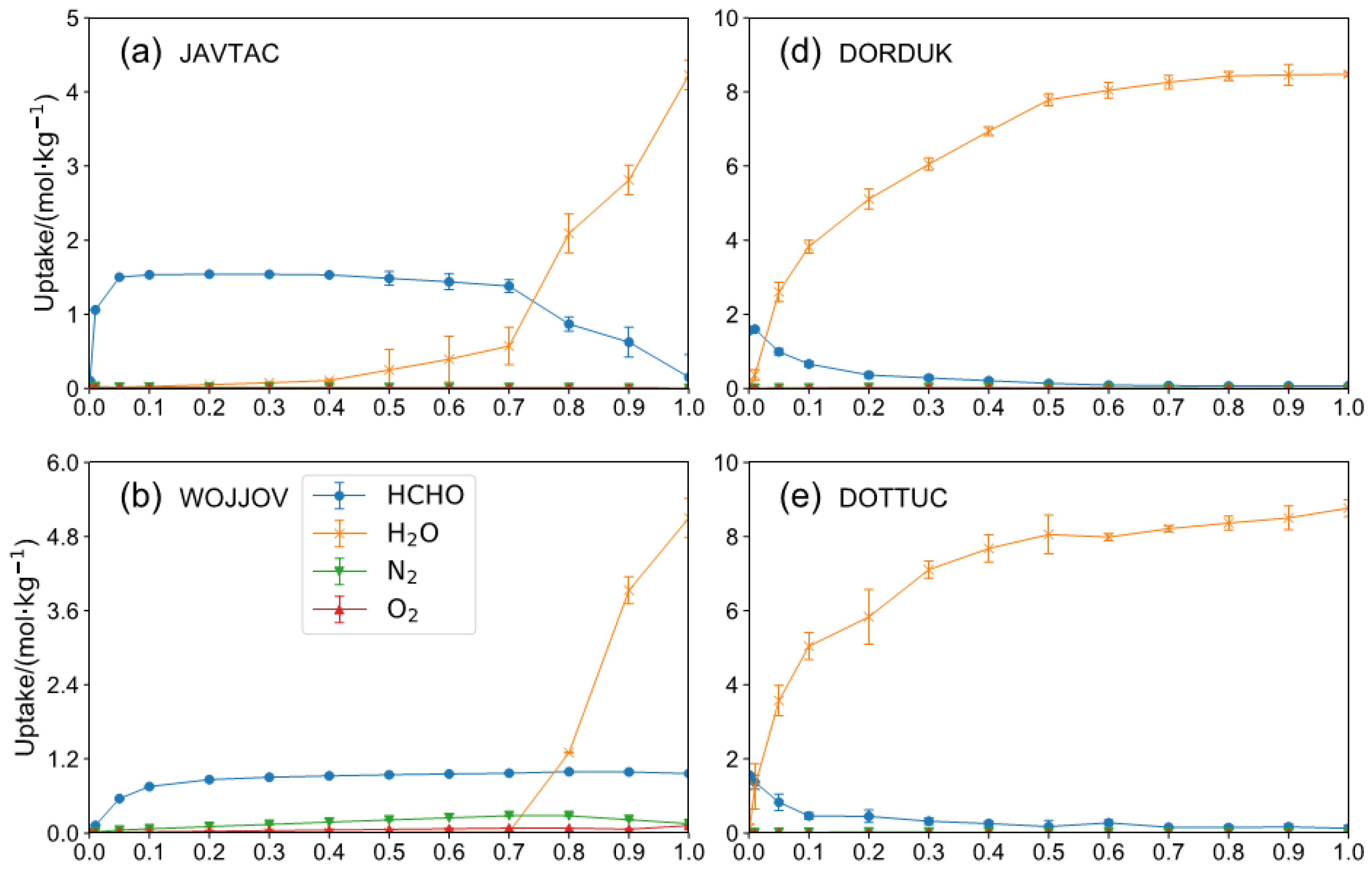
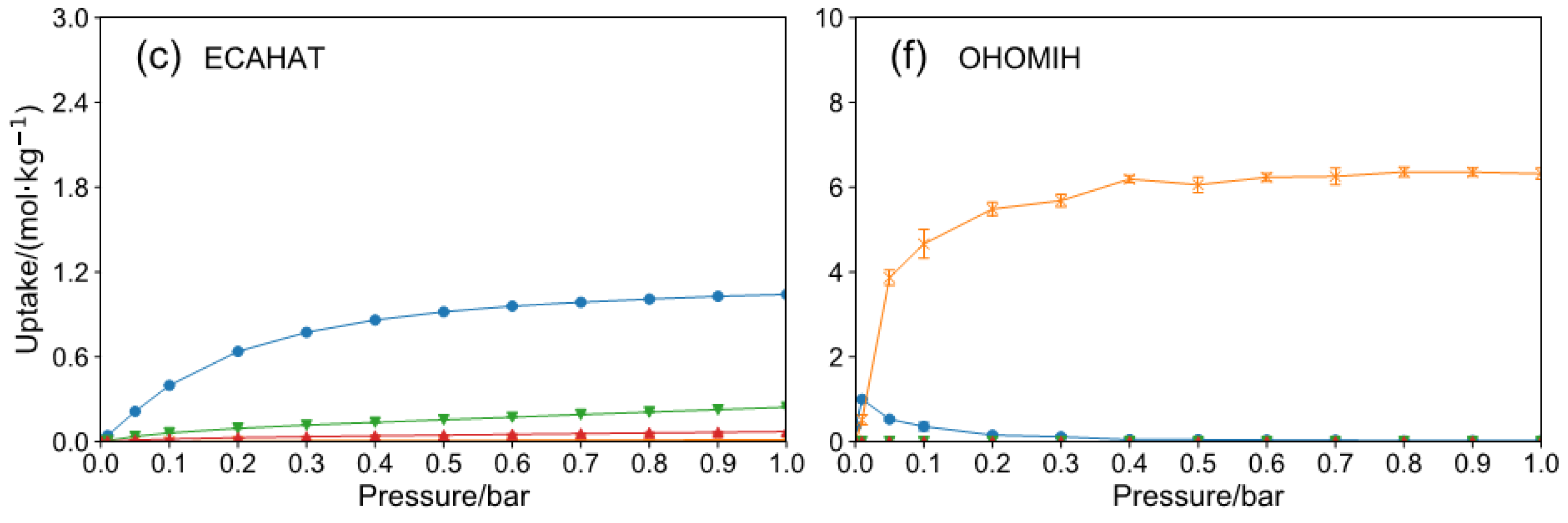
| REFCODE | LCD Å | MaxER | APC e | Aε kcal/mol | KH mol/(kg·Pa) | ΔW mol/kg | S |
|---|---|---|---|---|---|---|---|
| LAVSUY | 6.62 | 0.43 | 0.50 | 0.16 | 1.18 × 10−2 | 4.01 | 2722 |
| DUBWON | 5.20 | 0.38 | 0.48 | 0.14 | 4.19 × 10−2 | 3.98 | 8189 |
| PARMIG | 4.71 | 0.37 | 0.24 | 0.23 | 1.72 × 10−2 | 3.93 | 4044 |
| SEHTAB | 5.17 | 0.47 | 0.40 | 0.13 | 3.16 × 10−2 | 3.82 | 3157 |
| DEYJIC | 4.95 | 0.56 | 0.33 | 0.17 | 4.98 × 10−2 | 3.68 | 7689 |
| ADIQEL | 4.25 | 0.37 | 0.18 | 0.22 | 1.01 × 10−2 | 3.60 | 1453 |
| LIFWOO | 4.98 | 0.38 | 0.16 | 0.21 | 2.76 × 10−2 | 3.34 | 3486 |
| DEFKUU | 5.42 | 0.44 | 0.59 | 0.18 | 7.37 × 10−3 | 3.31 | 12,015 |
| NABMUA01 | 6.10 | 0.43 | 0.44 | 0.18 | 1.17 × 10−1 | 3.15 | 2180 |
| LOBHAM | 6.51 | 0.39 | 0.27 | 0.17 | 2.68 × 10−1 | 3.09 | 4160 |
| REFCODE | LCD Å | MPC e | MNC e | KH HCHO mol/(kg·Pa) | KH H2O mol/(kg·Pa) | KH N2 mol/(kg·Pa) | KH O2 mol/(kg·Pa) | SKH HCHO/H2O |
|---|---|---|---|---|---|---|---|---|
| JAVTAC | 5.08 | 2.44 | −1.14 | 4.70 × 10−1 | 6.85 × 10−5 | 8.96 × 10−5 | 8.71 × 10−5 | 418.76 |
| WOJJOV | 7.81 | 1.72 | −0.68 | 5.16 × 10−2 | 1.62 × 10−5 | 2.29 × 10−5 | 2.32 × 10−5 | 194.11 |
| ECAHAT | 12.44 | 0.88 | −0.61 | 2.08 × 10−2 | 8.75 × 10−6 | 1.13 × 10−5 | 1.38 × 10−5 | 144.74 |
| PUQYAC | 5.33 | 1.03 | −0.62 | 2.10 × 10−3 | 1.88 × 10−6 | 8.87 × 10−6 | 9.35 × 10−6 | 68.04 |
| LIDZUV | 4.49 | 1.6 | −0.78 | 1.02 × 10−2 | 1.10 × 10−5 | 2.10 × 10−5 | 2.23 × 10−5 | 56.81 |
| ZERQOE | 4.24 | 1.59 | −0.78 | 4.56 × 10−3 | 5.39 × 10−6 | 1.50 × 10−5 | 1.93 × 10−5 | 51.59 |
| KAXQOR | 4.23 | 1.59 | −0.78 | 4.07 × 10−3 | 5.22 × 10−6 | 1.41 × 10−5 | 1.83 × 10−5 | 47.56 |
| IXISOX | 5.57 | 0.24 | −0.36 | 3.84 × 10−2 | 5.12 × 10−5 | 9.73 × 10−6 | 1.04 × 10−5 | 45.73 |
| PARMIG | 4.71 | 0.76 | −0.58 | 1.72 × 10−2 | 2.35 × 10−5 | 4.37 × 10−5 | 5.31 × 10−5 | 44.6 |
| GUPBEZ | 7.29 | 0.1 | −0.31 | 3.72 × 10−3 | 5.64 × 10−6 | 2.01 × 10−6 | 2.26 × 10−6 | 40.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Liang, T.; Lin, Y.; Wu, W.; Li, S. In Silico Screening of Metal-Organic Frameworks for Formaldehyde Capture with and without Humidity by Molecular Simulation. Int. J. Mol. Sci. 2022, 23, 13672. https://doi.org/10.3390/ijms232213672
Li W, Liang T, Lin Y, Wu W, Li S. In Silico Screening of Metal-Organic Frameworks for Formaldehyde Capture with and without Humidity by Molecular Simulation. International Journal of Molecular Sciences. 2022; 23(22):13672. https://doi.org/10.3390/ijms232213672
Chicago/Turabian StyleLi, Wei, Tiangui Liang, Yuanchuang Lin, Weixiong Wu, and Song Li. 2022. "In Silico Screening of Metal-Organic Frameworks for Formaldehyde Capture with and without Humidity by Molecular Simulation" International Journal of Molecular Sciences 23, no. 22: 13672. https://doi.org/10.3390/ijms232213672
APA StyleLi, W., Liang, T., Lin, Y., Wu, W., & Li, S. (2022). In Silico Screening of Metal-Organic Frameworks for Formaldehyde Capture with and without Humidity by Molecular Simulation. International Journal of Molecular Sciences, 23(22), 13672. https://doi.org/10.3390/ijms232213672







