Influence of the Substituent’s Size in the Phosphinate Group on the Conformational Possibilities of Ferrocenylbisphosphinic Acids in the Design of Coordination Polymers and Metal–Organic Frameworks
Abstract
:1. Introduction
2. Results and Discussion
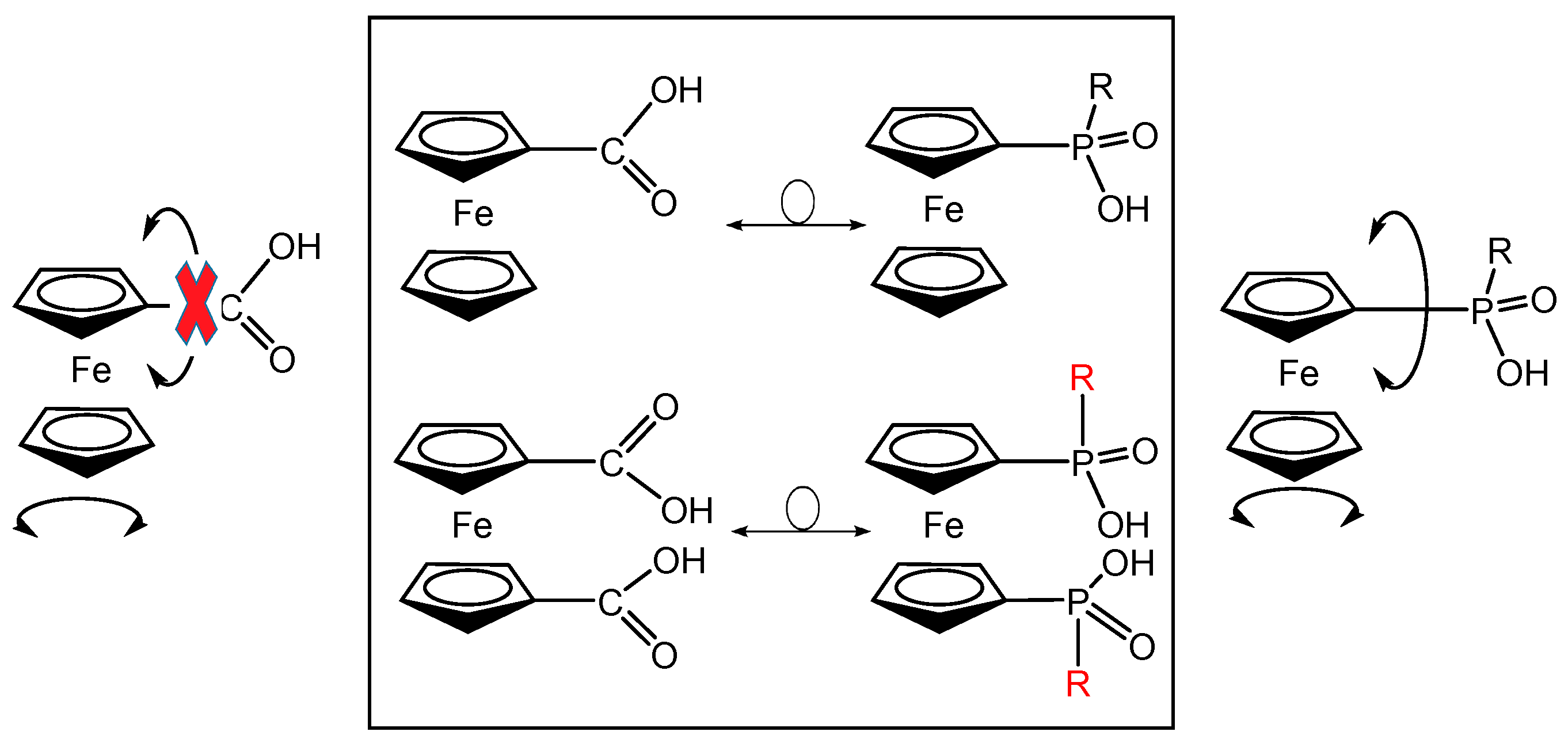
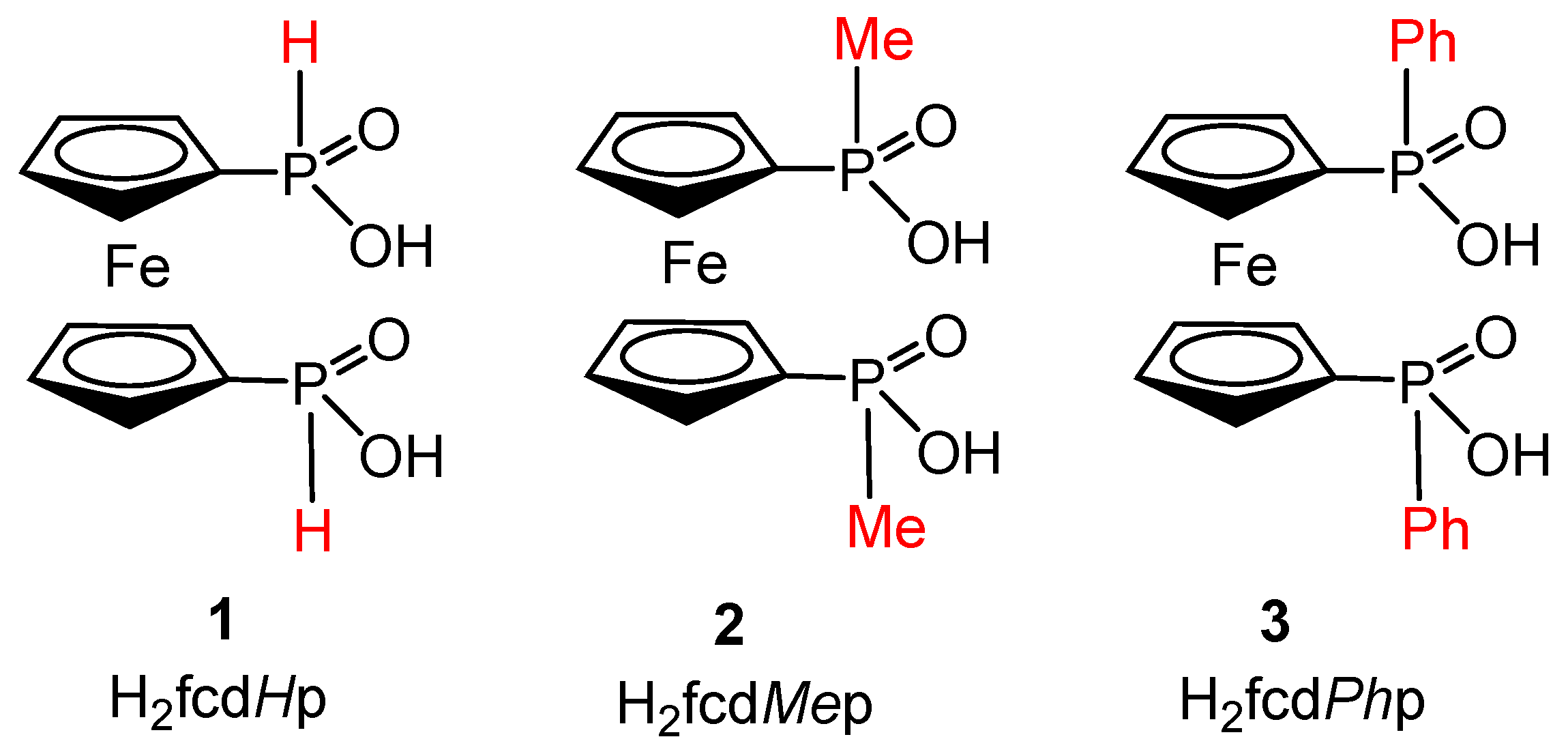
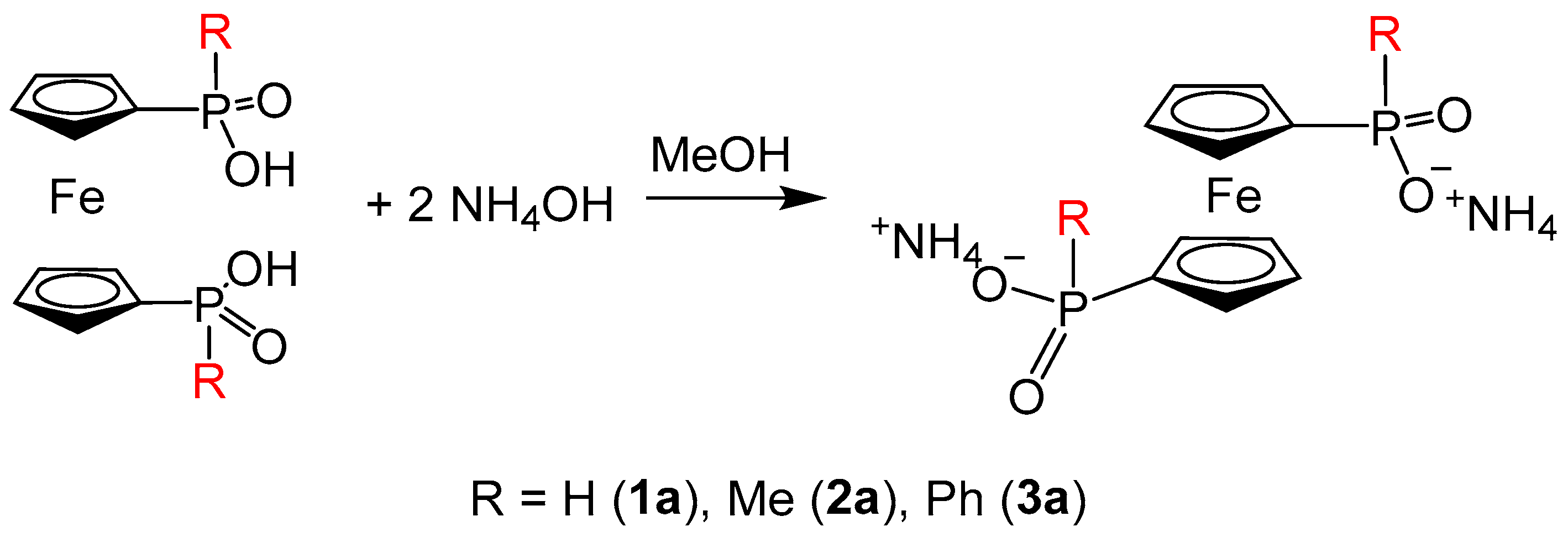
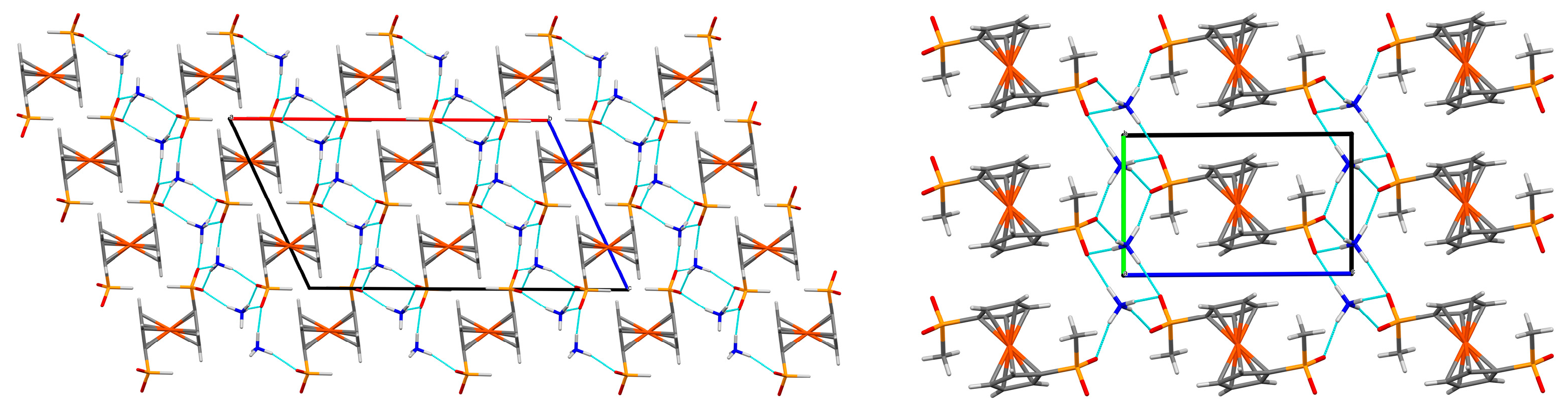
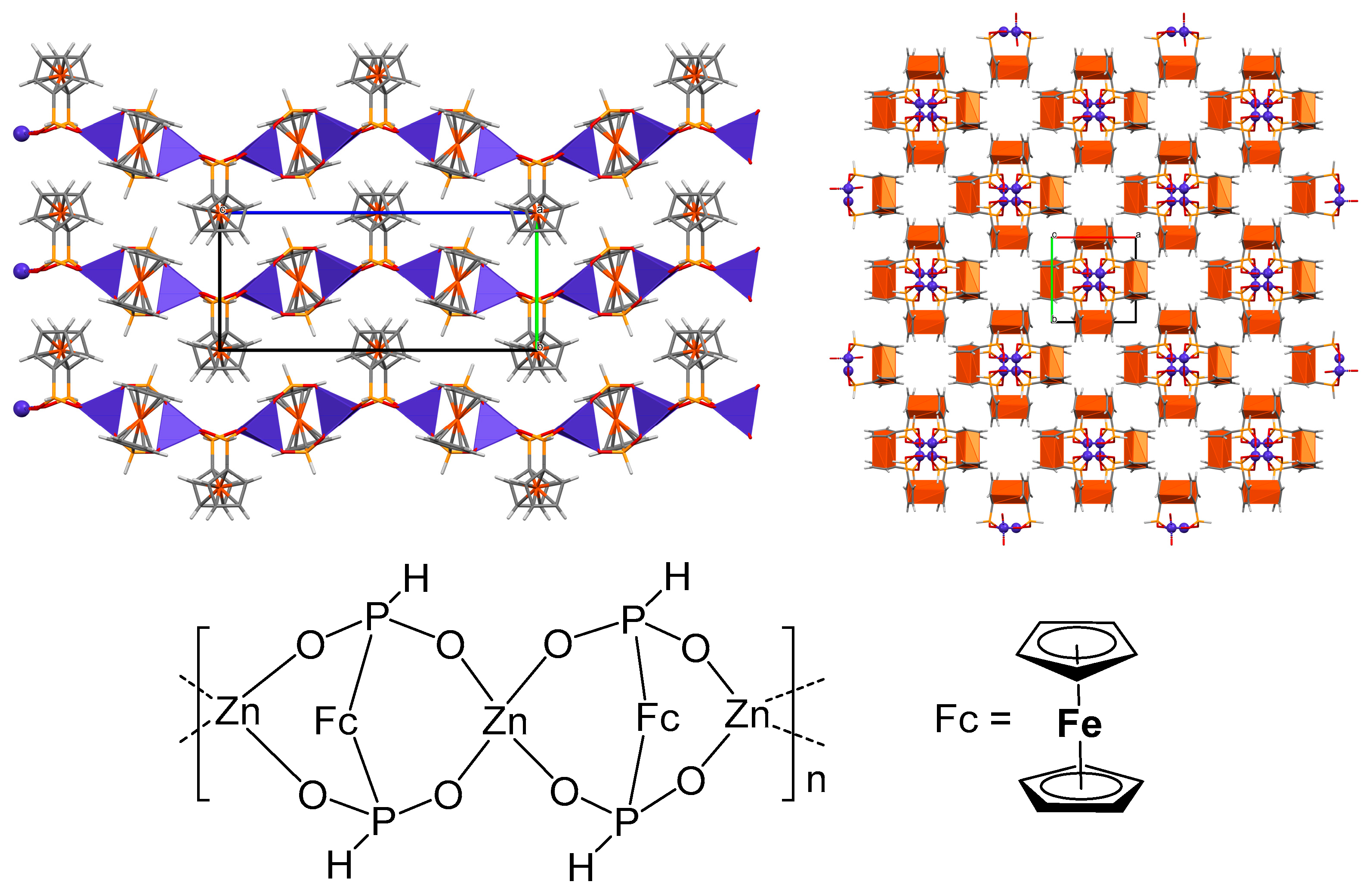
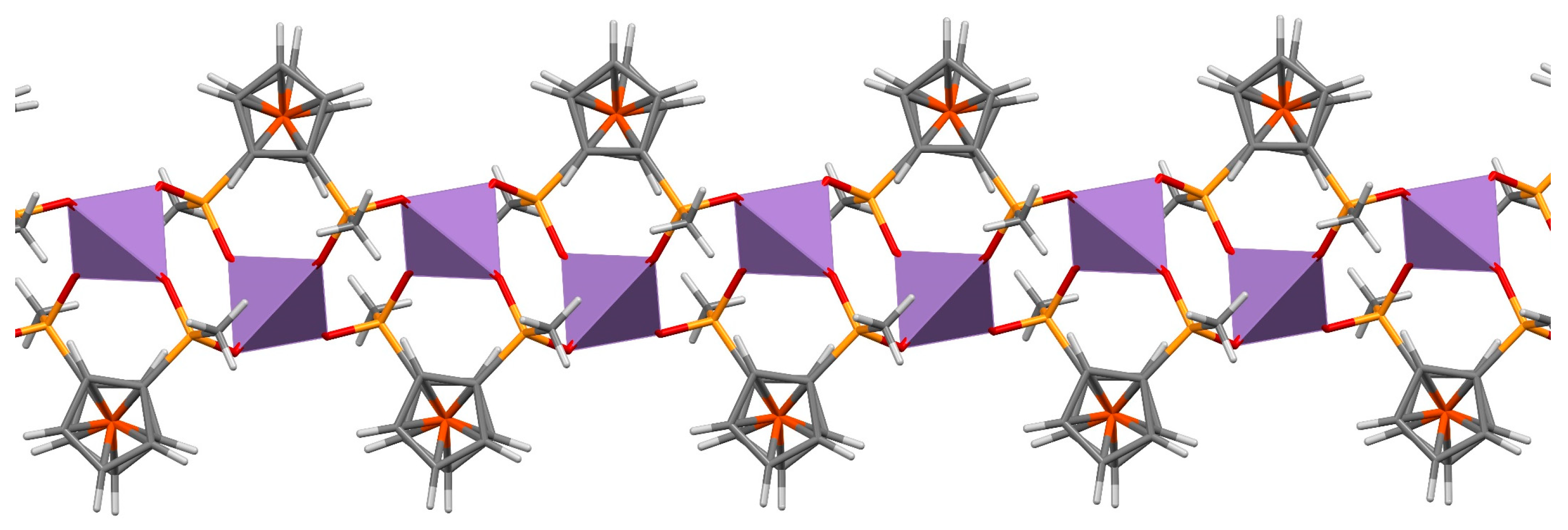
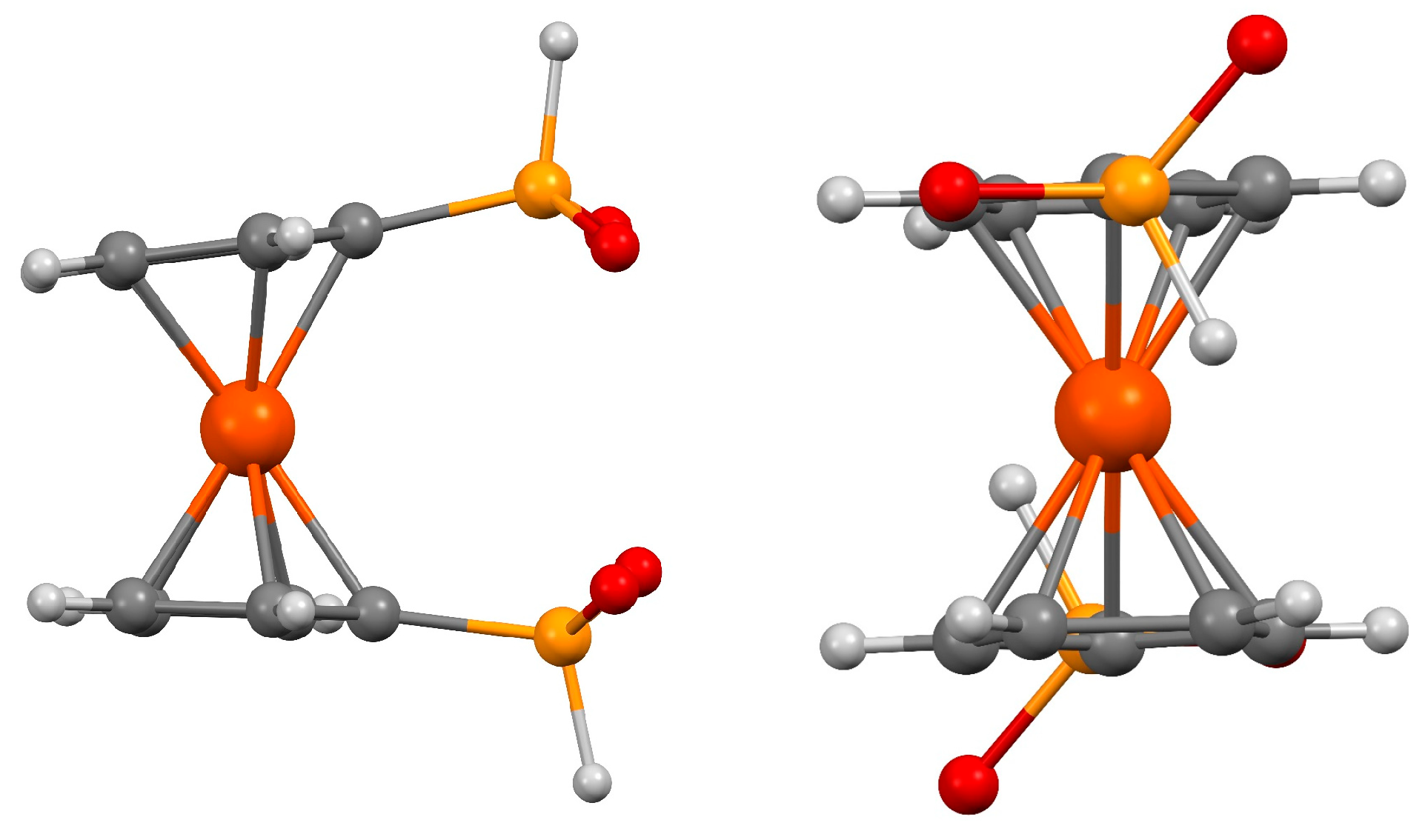
2.1. Supramolecular Architecture of Diammonium Ferrocene-1,1′-diyldi(R-phosphinate)
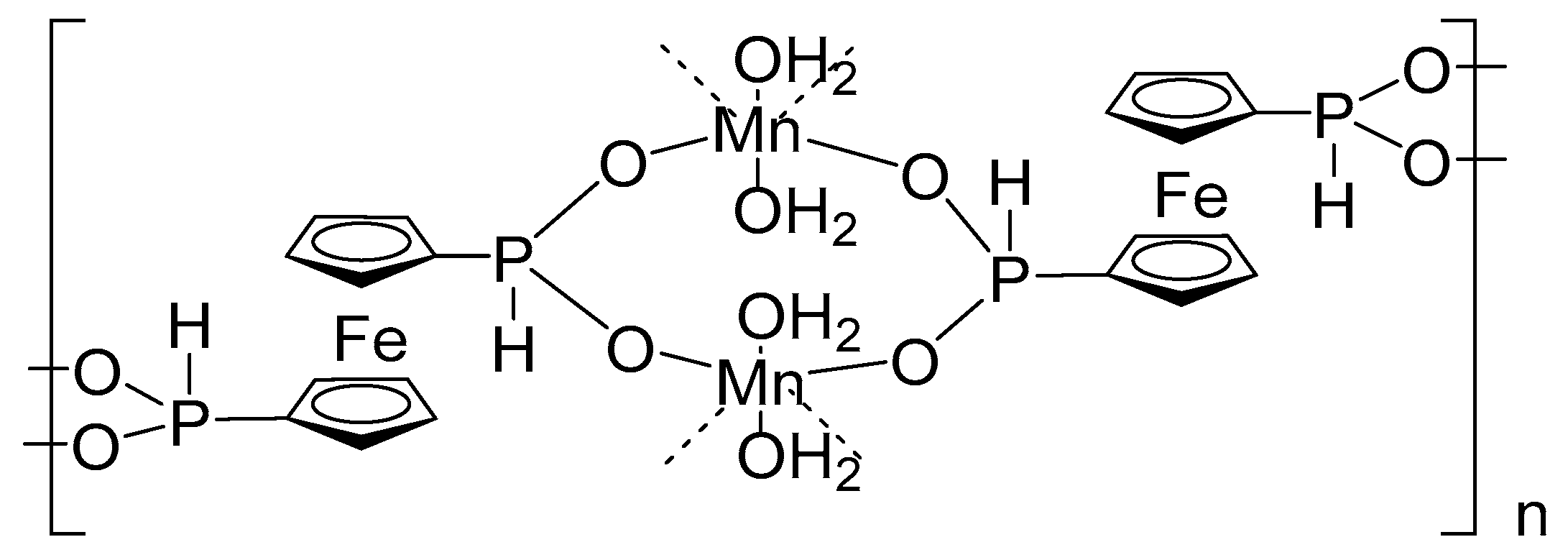
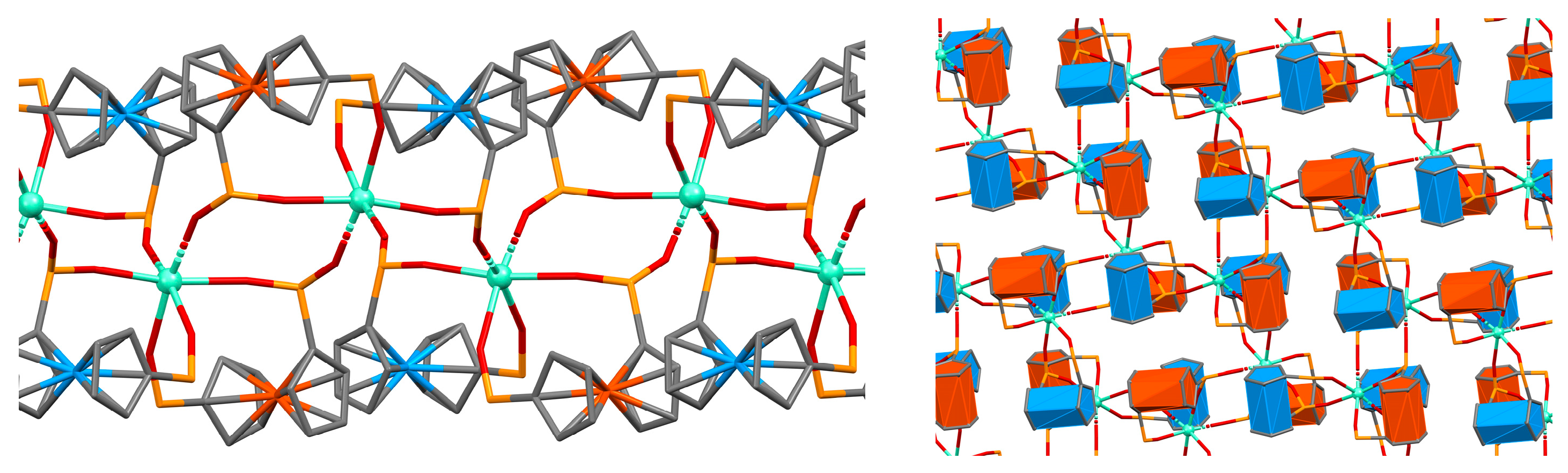
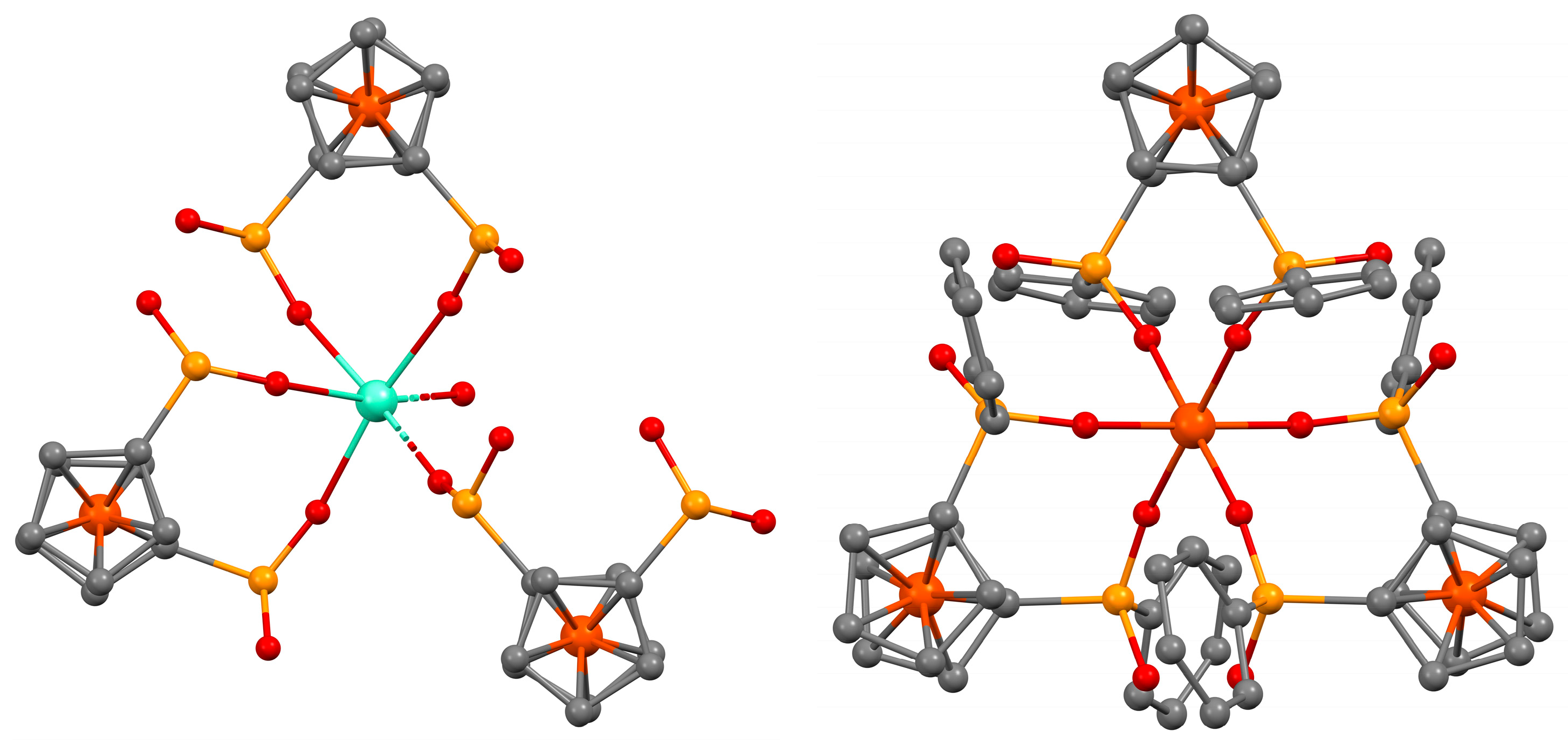
2.2. Crystal Structures and Control of Coordination Mode with M2+ and M3+
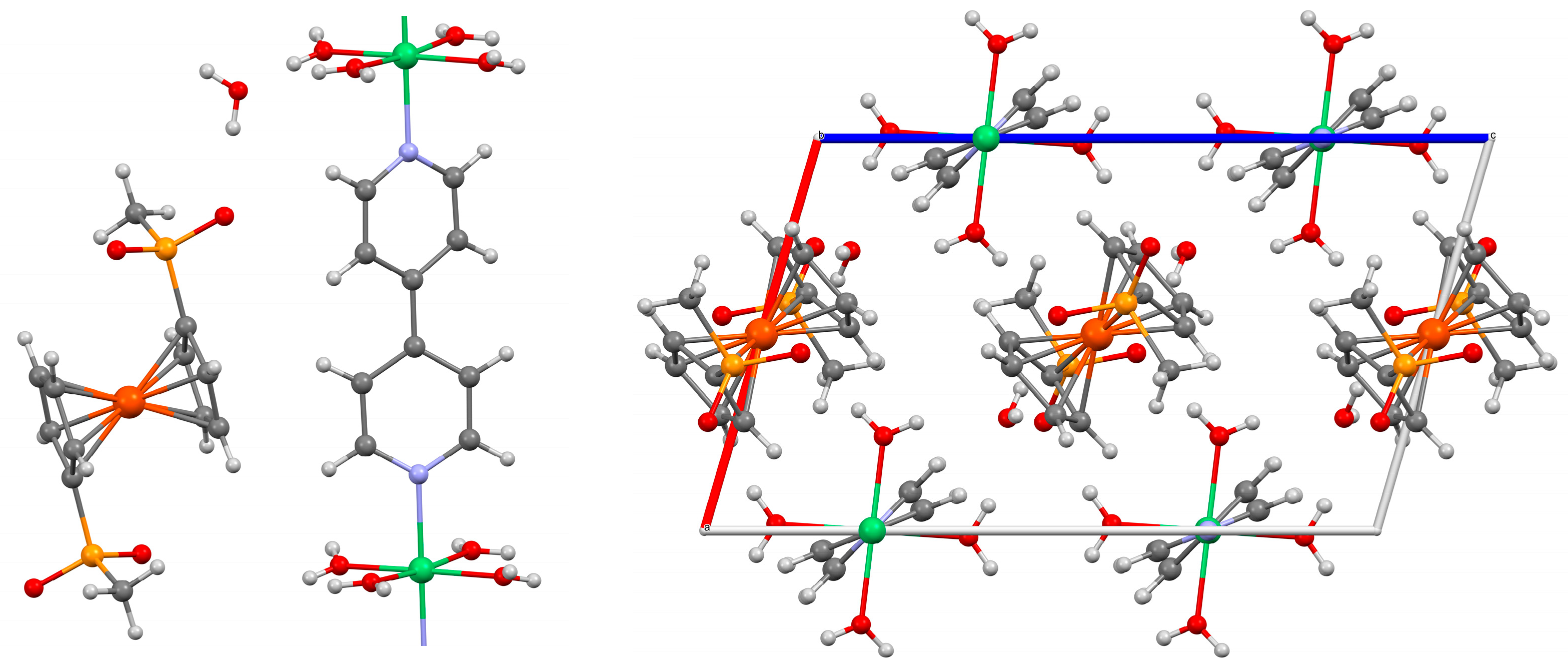
2.3. Role of 4,4′-Bipyridine in the Solvothermal Synthesis of MOFs Based on Ferrocenyl-R-phosphinates
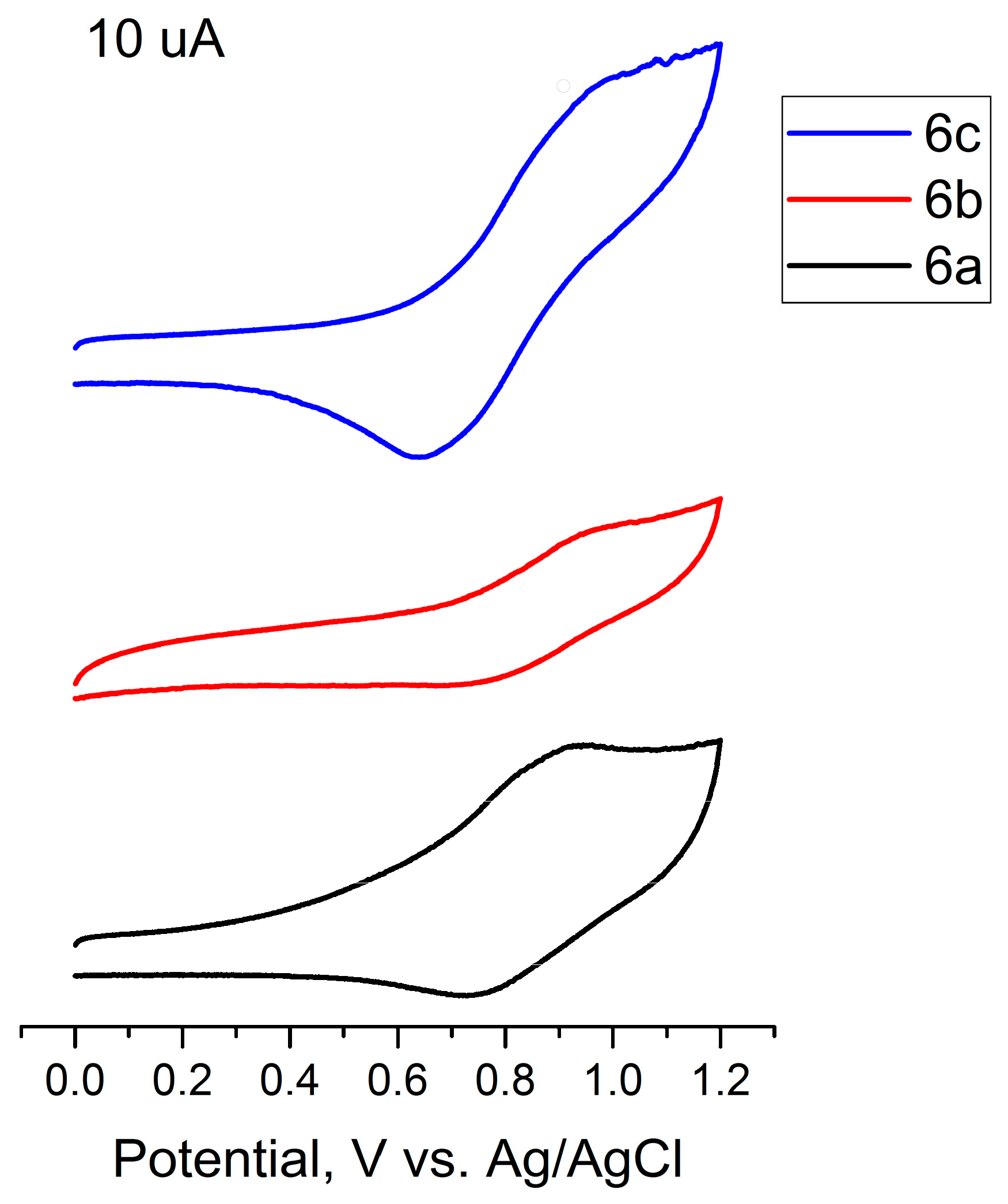
2.4. Physico-Chemical Properties
3. Materials and Methods
3.1. General
3.2. X-ray Diffraction Analysis
3.3. Synthesis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, W.; Liang, T.; Lin, Y.; Wu, W.; Li, S. In Silico Screening of Metal-Organic Frameworks for Formaldehyde Capture with and without Humidity by Molecular Simulation. Int. J. Mol. Sci. 2022, 23, 13672. [Google Scholar] [CrossRef] [PubMed]
- Timofeeva, M.N.; Panchenko, V.N.; Jhung, S.H. Insights into the Structure–Property–Activity Relationship of Zeolitic Imidazolate Frameworks for Acid–Base Catalysis. Int. J. Mol. Sci. 2023, 24, 4370. [Google Scholar] [CrossRef]
- Ajibade, P.A.; Oloyede, S.O. Synthesis of Metal–Organic Frameworks Quantum Dots Composites as Sensors for Endocrine-Disrupting Chemicals. Int. J. Mol. Sci. 2022, 23, 7980. [Google Scholar] [CrossRef]
- Saura-Sanmartin, A. Photoresponsive Metal-Organic Frameworks as Adjustable Scaffolds in Reticular Chemistry. Int. J. Mol. Sci. 2022, 23, 7121. [Google Scholar] [CrossRef] [PubMed]
- Khrizanforov, M. Editorial of Special Issue “Synthesis and Molecular Applications of Metal-Organic Frameworks (MOFs)”. Int. J. Mol. Sci. 2023, 24, 7857. [Google Scholar] [CrossRef]
- Horcajada, P.; Gref, R.; Baati, T.; Allan, P.K.; Maurin, G.; Couvreur, P.; Férey, G.; Morris, R.E.; Serre, C. Metal-Organic Frameworks in Biomedicine. Chem. Rev. 2012, 112, 1232–1268. [Google Scholar] [CrossRef]
- Gao, X.; Yan, W.-H.; Hu, B.-Y.; Huang, Y.-X.; Zheng, S.-M. Porous Metal–Organic Frameworks for Light Hydrocarbon Separation. Molecules 2023, 28, 6337. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-N.; Yin, S.-H.; Li, X.; Wang, Y.-Y.; Zhang, K.; Li, J.-A. Research and Application of Metal–Organic Framework in Surface Modification of Biomaterials—A Review. Metals 2023, 13, 1511. [Google Scholar] [CrossRef]
- Budnikova, Y.H. Recent advances in metal–organic frameworks for electrocatalytic hydrogen evolution and overall water splitting reactions. Dalton Trans. 2020, 49, 12483–12502. [Google Scholar] [CrossRef]
- Zhang, W.; Ye, G.; Liao, D.; Chen, X.; Lu, C.; Nezamzadeh-Ejhieh, A.; Khan, M.S.; Liu, J.; Pan, Y.; Dai, Z. Recent Advances of Silver-Based Coordination Polymers on Antibacterial Applications. Molecules 2022, 27, 7166. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Li, B.; Guo, X.; Zhang, J.; Zhu, G. Activation of Ferrocene-Based Coordination Compounds for Oxygen Evolution and the Application in Zinc-air Battery. J. Alloys Compd. 2023, 967, 171665. [Google Scholar] [CrossRef]
- Khrizanforov, M.; Zagidullin, A.; Shekurov, R.; Akhmatkhanova, F.; Bezkishko, I.; Ermolaev, V.; Miluykov, V. Inorganic and Organometallic Polymers as Energy Storage Materials and Enhancing Their Efficiency. Comments Inorg. Chem. 2023. [Google Scholar] [CrossRef]
- Mousa, A.O.; Chuang, C.-H.; Kuo, S.-W.; Mohamed, M.G. Strategic Design and Synthesis of Ferrocene Linked Porous Organic Frameworks toward Tunable CO2 Capture and Energy Storage. Int. J. Mol. Sci. 2023, 24, 12371. [Google Scholar] [CrossRef]
- Li, C.; Zhang, C.; Xie, J.; Wang, K.; Li, J.; Zhang, Q. Ferrocene-based metal-organic framework as a promising cathode in lithium-ion battery. Chem. Eng. J. 2021, 404, 126463. [Google Scholar] [CrossRef]
- Huang, Z.; Yu, H.; Wang, L.; Liu, X.; Lin, T.; Haq, F.; Vatsadze, S.Z.; Lemenovskiy, D.A. Ferrocene-contained metal organic frameworks: From synthesis to applications. Coord. Chem. Rev. 2021, 430, 213737. [Google Scholar] [CrossRef]
- Horikoshi, R.; Mochida, T. Ferrocene-Containing Coordination Polymers: Ligand Design and Assembled Structures. Eur. J. Inorg. Chem. 2010, 2010, 5355–5371. [Google Scholar] [CrossRef]
- Chandrasekhar, V.; Thirumoorthi, R. Coordination polymers containing ferrocene backbone. Synthesis, structure and electrochemistry. Dalton Trans. 2010, 39, 2684–2691. [Google Scholar] [CrossRef]
- Hu, M.-L.; Abbasi-Azad, M.; Habibi, B.; Rouhani, F.; Moghanni-Bavil-Olyaei, H.; Liu, K.-G.; Morsali, A. Electrochemical Applications of Ferrocene-Based Coordination Polymers. ChemPlusChem 2020, 85, 2397–2418. [Google Scholar] [CrossRef]
- Meng, X.; Li, G.; Hou, H.; Han, H.; Fan, Y.; Zhu, Y.; Du, C. A series of novel metal-ferrocenedicarboxylate coordination polymers: Crystal structures, magnetic and luminescence properties. J. Organomet. Chem. 2003, 679, 153–161. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, Y.; Liang, F.; Zhou, Z. Synthesis, crystal structure, and magnetic properties of two manganese (II) polymers bearing ferrocenecarboxylato ligands. Eur. J. Inorg. Chem. 2007, 14, 2040–2045. [Google Scholar] [CrossRef]
- Hirai, K.; Uehara, H.; Kitagawa, S.; Furukawa, S. Redox reaction in two-dimensional porous coordination polymers based on ferrocenedicarboxylates. Dalton Trans. 2012, 41, 3924–3927. [Google Scholar] [CrossRef]
- Mi, L.; Hou, H.; Song, Z.; Han, H.; Xu, H.; Fan, Y.; Ng, S.W. Rational Construction of Porous Polymeric Cadmium Ferrocene-1,1′-disulfonates for Transition Metal Ion Exchange and Sorption. Cryst. Growth Des. 2007, 7, 2553–2561. [Google Scholar] [CrossRef]
- Ospina-Castro, M.L.; Ávila, E.E.; Briceño, A.; Reiber, A.; Pacheco-Londoño, L.C.; Galan-Freyle, N.J. Self-assembly and supramolecular isomerism in 1D metal–organometallic networks based on transition-metal assemblies from 1,1′-ferrocene-dicarboxylic acid and ancillary nitrogen heterocycle ligands. CrystEngComm 2021, 23, 8198–8208. [Google Scholar] [CrossRef]
- Miao, Q.; Rouhani, F.; Moghanni-Bavil-Olyaei, H.; Liu, K.-G.; Gao, X.-M.; Li, J.-Z.; Hu, X.-D.; Jin, Z.-M.; Hu, M.-L.; Morsali, A. Comparative Study of the Supercapacitive Performance of Three Ferrocene-Based Structures: Targeted Design of a Conductive Ferrocene-Functionalized Coordination Polymer as a Supercapacitor Electrode. Chem. Eur. J. 2020, 26, 9518–9526. [Google Scholar] [CrossRef] [PubMed]
- Rajak, R.; Saraf, M.; Verma, S.K.; Kumar, R.; Mobin, S.M. Dy(III)-Based Metal–Organic Framework as a Fluorescent Probe for Highly Selective Detection of Picric Acid in Aqueous Medium. Inorg. Chem. 2019, 58, 16065–16074. [Google Scholar] [CrossRef]
- Benecke, J.; Grape, E.S.; Fuß, A.; Wöhlbrandt, S.; Engesser, T.A.; Inge, A.K.; Stock, N.; Reinsch, H. Polymorphous indium metal–organic frameworks based on a ferrocene linker: Redox activity, porosity, and structural diversity. Inorg. Chem. 2020, 59, 9969–9978. [Google Scholar] [CrossRef]
- Gao, R.; Chen, S.-M.; Wang, F.; Zhang, J. Single-Crystal Syntheses and Properties of Indium–Organic Frameworks Based on 1,1′-Ferrocenedicarboxylic Acid. Inorg. Chem. 2021, 60, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Schull, T.L.; Knight, D.A. Organometallic phosphonic acids: Synthesis and coordination chemistry. Coord. Chem. Rev. 2005, 249, 1269–1282. [Google Scholar] [CrossRef]
- Egger, M.; Koehne, I.; Wickenhauser, D.; Schlemmer, W.; Spirk, S.; Pietschnig, R. Electrochemistry and Stability of 1, 1′-Ferrocene-Bisphosphonates. ACS Omega 2023, 8, 10899–10905. [Google Scholar] [CrossRef]
- Horký, F.; Císařová, I.; Schulz, J.; Štěpnička, P. Synthesis and structural characterisation of 1’-(diphenylphosphino) ferrocene-1-phosphonic acid, its ammonium salts and Pd (II) complexes. J. Organomet. Chem. 2019, 891, 44–53. [Google Scholar] [CrossRef]
- Kloda, M.; Ondrušová, S.; Lang, K.; Demel, J. Phosphinic acids as building units in materials chemistry. Coord. Chem. Rev. 2021, 433, 213748. [Google Scholar] [CrossRef]
- Edmundson, R.S. Properties and Reactions of Phosphonic and Phosphinic Acids and Their Derivatives; John Wiley and Sons, Ltd.: Hoboken, NJ, USA, 2009. [Google Scholar] [CrossRef]
- Tranchemontagne, D.J.; Mendoza-Cortés, J.L.; O’keeffe, M.; Yaghi, O.M. Secondary building units, nets and bonding in the chemistry of metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1257–1283. [Google Scholar] [CrossRef] [PubMed]
- Carson, I.; Healy, M.R.; Doidge, E.D.; Love, J.B.; Morrison, C.A.; Tasker, P.A. Metal-binding motifs of alkyl and aryl phosphinates; versatile mono and polynucleating ligands. Coord. Chem. Rev. 2017, 335, 150–171. [Google Scholar] [CrossRef]
- Gholivand, K.; Fallah, N.; Valmoozi, A.A.E.; Gholami, A.; Dusek, M.; Eigner, V.; Pooyan, M.; Mohammadpanah, F. Synthesis and structural characterization of phosphinate coordination polymers with tin(IV) and copper(II). J. Mol. Struct. 2020, 1202, 127369. [Google Scholar] [CrossRef]
- Rohlíček, J.; Bůžek, D.; Brázda, P.; Kobera, L.; Hynek, J.; Brus, J.; Lang, K.; Demel, J. Novel Cerium Bisphosphinate Coordination Polymer and Unconventional Metal–Organic Framework. Crystals 2019, 9, 303. [Google Scholar] [CrossRef]
- Shearan, S.J.; Stock, N.; Emmerling, F.; Demel, J.; Wright, P.A.; Demadis, K.D.; Vassaki, M.; Costantino, F.; Vivani, R.; Sallard, S.; et al. New Directions in Metal Phosphonate and Phosphinate Chemistry. Crystals 2019, 9, 270. [Google Scholar] [CrossRef]
- Hynek, J.; Brázda, P.; Rohlíček, J.; Londesborough, M.G.; Demel, J. Phosphinic acid based linkers: Building blocks in metal–organic framework chemistry. Angew. Chem. 2018, 130, 5110–5113. [Google Scholar] [CrossRef]
- Ondrušová, S.; Kloda, M.; Rohlíček, J.; Taddei, M.; Zaręba, J.K.; Demel, J. Exploring the Isoreticular Continuum between Phosphonate-and Phosphinate-Based Metal–Organic Frameworks. Inorg. Chem. 2022, 61, 18990–18997. [Google Scholar] [CrossRef]
- Kloda, M.; Plechacek, T.; Ondrušová, S.; Brázda, P.; Chalupský, P.; Rohlicek, J.; Demel, J.; Hynek, J. Phosphinate MOFs Formed from Tetratopic Ligands as Proton-Conductive Materials. Inorg. Chem. 2022, 61, 7506–7512. [Google Scholar] [CrossRef]
- Buzek, D.; Ondrusova, S.; Hynek, J.; Kovar, P.; Lang, K.; Rohlicek, J.; Demel, J. Robust Aluminum and Iron Phosphinate Metal–Organic Frameworks for Efficient Removal of Bisphenol A. Inorg. Chem. 2020, 59, 5538–5545. [Google Scholar] [CrossRef]
- Costantino, F.; Gentili, P.L.; Guerri, A.; Ienco, A.; Midollini, S.; Oberhauser, W. Structural similarities in 1D coordination polymers of alkaline earth diphosphinates. Inorg. Chim. Acta 2012, 391, 150–157. [Google Scholar] [CrossRef]
- Bataille, T.; Costantino, F.; Ienco, A.; Guerri, A.; Marmottini, F.; Midollini, S. A snapshot of a coordination polymerself-assembly process: The crystallization of a metastable 3D network followed by the spontaneous transformation in water to a 2D pseudopolymorphic phase. Chem. Commun. 2008, 47, 6381–6383. [Google Scholar] [CrossRef] [PubMed]
- Costantino, F.; Midollini, S.; Orlandini, A. Cobalt(II) and nickel(II) coordination polymers constructed from P,P′-diphenylmethylenediphosphinic acid (H2pcp) and 4,4′-bipyridine (bipy): Structural isomerism in [Co(pcp)(bipy)0.5(H2O)2]. Inorg. Chim. Acta 2008, 361, 327–334. [Google Scholar] [CrossRef]
- Costantino, F.; Ienco, A.; Midollini, S. Different Structural Networks Determined by Variation of the Ligand Skeleton in Copper(II) Diphosphinate Coordination Polymers. Cryst. Growth Des. 2010, 10, 7–10. [Google Scholar] [CrossRef]
- Shekurov, R.; Miluykov, V.; Islamov, D.; Krivolapov, D.; Kataeva, O.; Gerasimova, T.; Katsyuba, S.; Nasybullina, G.; Yanilkin, V.; Sinyashin, O. Synthesis and structure of ferrocenylphosphinic acids. J. Organomet. Chem. 2014, 766, 40–48. [Google Scholar] [CrossRef]
- Shekurov, R.P.; Tufatullin, A.I.; Milyukov, V.A.; Kataeva, O.N.; Sinyashin, O.G. Supramolecular architecture of diammonium ferrocene-1,1′-diyldiphosphinates. Russ. Chem. Bull. 2014, 63, 178–181. [Google Scholar] [CrossRef]
- Shekurov, R.; Khrizanforov, M.; Ivshin, K.; Miluykov, V.; Budnikova, Y.; Kataeva, O. Supramolecular architecture of diammonium ferrocene-1,1′-diyldi(methylphosphinate). J. Organomet. Chem. 2019, 904, 121004. [Google Scholar] [CrossRef]
- Li, Z.; Lu, Y.C. Material Design of Aqueous Redox Flow Batteries: Fundamental Challenges and Mitigation Strategies. Adv. Mater. 2020, 32, 2002132. [Google Scholar] [CrossRef]
- Schrage, B.R.; Frkonja-Kuczin, A.; Zhang, B.; Hobbs, M.S.; Chen, W.Y.; Boika, A.; Ziegler, C.J. Pyridinium ferrocene sulfonate salts: Highly soluble materials for electrochemical applications. J. Organomet. Chem. 2023, 991, 122695. [Google Scholar] [CrossRef]
- Shekurov, R.; Khrizanforova, V.; Gilmanova, L.; Khrizanforov, M.; Miluykov, V.; Kataeva, O.; Yamaleeva, Z.; Burganov, T.; Gerasimova, T.; Khamatgalimov, A.; et al. Zn and Co Redox Active Coordination Polymers Based on Ferrocene-containing Diphosphinate Ligand as Efficient Electrocatalysts for Hydrogen Evolution Reaction. Dalton Trans. 2019, 48, 3601–3609. [Google Scholar] [CrossRef]
- Shekurov, R.; Khrizanforov, M.; Islamov, D.; Gerasimova, T.; Zagidullin, A.; Budnikova, Y.; Miluykov, V. Synthesis, crystal structure and electrochemical properties of poly(cadmium 1,1′-ferrocenediyl-bis(H-phosphinate)). J. Organomet. Chem. 2020, 914, 121233. [Google Scholar] [CrossRef]
- Khrizanforov, M.; Shekurov, R.; Miluykov, V.; Gilmanova, L.; Kataeva, O.; Yamaleeva, Z.; Gerasimova, T.; Ermolaev, V.; Gubaidullin, A.; Laskin, A.; et al. Excellent supercapacitor and sensor performance of robust cobalt phosphinate ferrocenyl organic framework materials achieved by intrinsic redox and structure properties. Dalton Trans. 2019, 48, 16986–16992. [Google Scholar] [CrossRef] [PubMed]
- Shekurov, R.; Miluykov, V.; Kataeva, O.; Krivolapov, D.; Sinyashin, O.; Gerasimova, T.; Katsyuba, S.; Kovalenko, V.; Krupskaya, Y.; Kataev, V.; et al. Reversible water-induced structural and magnetic transformations and selective water adsorption properties of poly (manganese 1,1′-ferrocenediyl-bis (H-phosphinate)). Cryst. Growth Des. 2016, 16, 5084–5090. [Google Scholar] [CrossRef]
- Khrizanforov, M.N.; Arkhipova, D.M.; Shekurov, R.P.; Gerasimova, T.P.; Ermolaev, V.V.; Islamov, D.R.; Miluykov, V.A.; Kataeva, O.N.; Khrizanforova, V.V.; Sinyashin, O.G.; et al. Novel paste electrodes based on phosphonium salt room temperature ionic liquids for studying the redox properties of insoluble compounds. J. Solid State Electrochem. 2015, 19, 2883–2890. [Google Scholar] [CrossRef]
- Khrizanforov, M.N.; Shekurov, R.P.; Ermolaev, V.V.; Arkhipova, D.M.; Miluykov, V.A.; Kataeva, O.N.; Khrizanforova, V.V.; Budnikova, Y.H. Novel phosphonium salt for paste electrode to study the redox properties of insoluble compounds. Phosphorus Sulfur Silicon Relat. Elem. 2016, 191, 1611–1612. [Google Scholar] [CrossRef]
- Shekurov, R.P.; Islamov, D.R.; Krivolapov, D.B.; Miluykov, V.A.; Kataeva, O.N.; Gerasimova, T.P.; Katsyuba, S.A.; Sinyashin, O.G. Synthesis and structure of the iron (iii) tris-chelate complex based on 1, 1´-ferrocenediylbis (phenylphosphinic acid). Russ. Chem. Bull. 2015, 64, 1819–1822. [Google Scholar] [CrossRef]
- Shekurov, R.P.; Gilmanova, L.H.; Miluykov, V.A. New porous Fe(III)-based ferrocene-containing diphosphinate. Phosphorus Sulfur Silicon Relat. Elem. 2019, 194, 1007–1009. [Google Scholar] [CrossRef]
- Khrizanforova, V.V.; Shekurov, R.P.; Nizameev, I.R.; Gerasimova, T.P.; Khrizanforov, M.N.; Bezkishko, I.A.; Miluykov, V.A.; Budnikova, Y.H. Aerogel based on nanoporous aluminium ferrocenyl diphosphinate metal-organic framework. Inorg. Chim. Acta 2021, 518, 120240. [Google Scholar] [CrossRef]
- Shekurov, R.P.; Khrizanforov, M.N.; Zagidullin, A.A.; Zinnatullin, A.L.; Kholin, K.V.; Ivshin, K.A.; Gerasimova, T.P.; Sirazieva, A.R.; Kataeva, O.N.; Vagizov, F.G.; et al. The Phosphinate Group in the Formation of 2D Coordination Polymer with Sm(III) Nodes: X-ray Structural, Electrochemical and Mössbauer Study. Int. J. Mol. Sci. 2022, 23, 15569. [Google Scholar] [CrossRef]
- Shekurov, R.; Gilmanova, L.; Khrizanforov, M.; Strekalova, S.; Budnikova, Y.; Gerasimova, T.; Katsyuba, S.; Miluykov, V. Inhibitory property of poly(manganese 1,1′-ferrocenediyl-bis(H-phosphinate)). Phosphorus Sulfur Silicon Relat. Elem. 2019, 194, 459–462. [Google Scholar] [CrossRef]
- Khrizanforov, M.N.; Shekurov, R.P.; Gilmanova, L.H.; Gerasimova, T.P.; Miluykov, V.A.; Budnikova, Y.H. Electrochemical properties of poly ([Eu or Dy or Y] 1, 1′-ferrocenediyl-bis (H-phosphinates)). Phosphorus Sulfur Silicon Relat. Elem. 2019, 194, 1010–1012. [Google Scholar] [CrossRef]
- Khrizanforova, V.; Shekurov, R.; Miluykov, V.; Khrizanforov, M.; Bon, V.; Kaskel, S.; Gubaidullin, A.; Sinyashin, O.; Budnikova, Y. 3D Ni and Co Redox-Active Metal-Organic Frameworks Based on Ferrocenyl Diphosphinate and 4,4′-Bipyridine Ligands as Efficient Electrocatalysts for Hydrogen Evolution Reaction. Dalton Trans. 2020, 49, 2794–2802. [Google Scholar] [CrossRef] [PubMed]
- Maji, T.K.; Kitagawa, S. Chemistry of porous coordination polymers. Pure Appl. Chem. 2007, 79, 2155–2177. [Google Scholar] [CrossRef]
- Xanthopoulos, K.; Anagnostou, Z.; Chalkiadakis, S.; Choquesillo-Lazarte, D.; Mezei, G.; Zaręba, J.K.; Zoń, J.; Demadis, K.D. Platonic Relationships in Metal Phosphonate Chemistry: Ionic Metal Phosphonates. Crystals 2019, 9, 301. [Google Scholar] [CrossRef]
- Bruker. APEX3 Crystallography Software Suite; Area Detector Control and Integration Software, Version 5.x; Bruker AXS Inc.: Madison, WI, USA, 2016. [Google Scholar]
- Bruker. SAINT Crystallography Software Suite; Bruker AXS Inc.: Madison, WI, USA, 2016. [Google Scholar]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]







| Peculiarity | Metal Nodes | Application | Ref. |
|---|---|---|---|
| Aerogel | Al | The porous coordination polymers (PCPs) adsorb 1400 cm3/g of N2. The aerogel have a colloidal microstructure rather than a polymeric one, with a surface area of about 671 m2/g. | [59] |
| Aerogel | Fe | N2 is absorbed in the cavities, showing an isotherm typical of porous powders. The BET surface area is 617 m2/g with a significant pore volume. | [58] |
| 3D | Ni | The Ni-MOF excels in HER efficiency, needing just 350 mV for a 10 mA cm−2 current density. With a Tafel slope of 60 mV dec−1 and durability up to 10,000 cycles, it ranks among the best non-precious metal electrocatalysts | [63] |
| 3D | Co | The Co-MOF displays enhanced HER efficiency, necessitating an overpotential of just 400 mV to reach a current density of 10 mA cm−2. | |
| 2D | Mn | The 2D CP was investigated for its capability to inhibit the redox production of ●C6F13 from C6F13I. | [61] |
| 2D | Co | The electrode based on CP can detect upon 0.05% of water in solvents. The dehydrated form, [Co(Fc(PHOO)2)]n, used as a supercapacitor material, exhibits a high specific capacitance of 2517 F g−1 at 2 A g−1 and retains 90.1% of it after 1000 cycles. | [53] |
| 1D | Co Zn | These CPs excel as electrocatalysts for hydrogen formation, with the Co CP having a rapid turnover frequency of 300 s−1 in CH3CN. They require overpotentials of 820–840 mV for this rate. As HER catalysts in 0.5 M H2SO4, they display overpotentials between 220 and 450 mV and Tafel slopes of 110–120 mV dec−1, with notable durability. | [51] |
| Polymer | Epa, V | E1/2, V | ΔEpa-c, mV |
|---|---|---|---|
| Zn fcdHp 1D | 0.98 | 0.88 | 105 |
| Zn fcdMep 1D 6a | 0.93 | 0.83 | 188 |
| Co fcdHp 1D | 0.88 | 0.81 | 110 |
| Co fcdHp 2D | 0.62 | 0.57 | 120 |
| Co fcdMep 1D 6b | 0.98 | 0.83 | 219 |
| Mn fcdHp 2D | 0.93 | 0.89 | 80 |
| Mn fcdMep 1D 6c | 1.01 | 0.84 | 371 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shekurov, R.P.; Khrizanforov, M.N.; Bezkishko, I.A.; Ivshin, K.A.; Zagidullin, A.A.; Lazareva, A.A.; Kataeva, O.N.; Miluykov, V.A. Influence of the Substituent’s Size in the Phosphinate Group on the Conformational Possibilities of Ferrocenylbisphosphinic Acids in the Design of Coordination Polymers and Metal–Organic Frameworks. Int. J. Mol. Sci. 2023, 24, 14087. https://doi.org/10.3390/ijms241814087
Shekurov RP, Khrizanforov MN, Bezkishko IA, Ivshin KA, Zagidullin AA, Lazareva AA, Kataeva ON, Miluykov VA. Influence of the Substituent’s Size in the Phosphinate Group on the Conformational Possibilities of Ferrocenylbisphosphinic Acids in the Design of Coordination Polymers and Metal–Organic Frameworks. International Journal of Molecular Sciences. 2023; 24(18):14087. https://doi.org/10.3390/ijms241814087
Chicago/Turabian StyleShekurov, Ruslan P., Mikhail N. Khrizanforov, Ilya A. Bezkishko, Kamil A. Ivshin, Almaz A. Zagidullin, Anna A. Lazareva, Olga N. Kataeva, and Vasili A. Miluykov. 2023. "Influence of the Substituent’s Size in the Phosphinate Group on the Conformational Possibilities of Ferrocenylbisphosphinic Acids in the Design of Coordination Polymers and Metal–Organic Frameworks" International Journal of Molecular Sciences 24, no. 18: 14087. https://doi.org/10.3390/ijms241814087






