3.1. Challenges of Using Rye Breeding Material for Mapping
A big challenge is the naturally cross-pollination character of rye and inbred lines can only be produced due to a self-fertility gene. Each self-pollination, requires isolation bags and results in only a few grams of seed per bag. Moreover, inbred lines are characterized by inbreeding depression that is characterized by loss of vitality. The production of double haploid lines has not been successful yet in elite rye breeding material. This all represents a big difference to self-pollinating species, where inbred lines can be produced with low workload and in almost unlimited population sizes and seed quantities.
This project was based on a private-public partnership and thus material from the respective company could only be tested on company-owned sites and stations of the scientific partners. This limited the comparison of absolute values of genotype means across different material. However, as the purpose of this study was to identify new resistance loci within each population, the calculation of means across environments for each population separately suffices. To further reduce the error for mapping, the genotypes from each single population were always grown together in randomized experiments at all locations.
Special attention was paid to heterozygous genotypes that were in rather early inbreeding generation. To conduct replicated experiments in different environments, we tested genotypes in single rows (plots). Plants of a heterozygous genotype with a single dominant gene grown in a single plot (DNA sampling from seed bulk), were segregating within the plot and as an average score per plot was given, a fully dominant gene (1:3 segregation within the plot) could never reach the same resistance level of the (homozygously) resistant parent. This must be considered when interpreting effects of heterozygous genotypes. We discussed this already in detail in our previous study [
16] and tried to adjust the marker coding for QTL mapping there. In this study, we used another approach by fitting an additional dominance effect for each marker. This allowed a more flexible estimate for any kind of dominance effects. An additional consequence of this within plot segregation of heterozygous genotypes was the potential of additional errors, with just slightly different allele ratios caused by the limited number of plants per plot. Actually, we observed very high standard errors of our QTL effect estimates (
Table 4 and
Table 9). However, we considered this experimental side effect of less importance than the need for multi-environmental field trials. This segregation problem of course also affected the seedling test in similar fashion and the dominance effect estimated across single plants from the same genotype was not as high as would had been expected for a full dominance effect on single plant basis.
Another experimental challenge was the choice of appropriate population size. Grains for multi-environmental field trials were limited due to aforementioned reasons and to broaden a breeder’s portfolio of resistance sources, it was reasonable to test several populations with small population sizes. Further, minimum population size could also be calculated from statistical theory, e.g., in Falconer and Mackay [
28] a formula from Sokal and Rohlfs [
29] is used:
. According to this formula, the size of each marker allele group depends on the smallest relevant difference
δ between marker classes, its standard deviation
σW, and the quantiles of the normal distribution
z for the acceptable error rate of false positives (
α) and false negatives (
β). If both,
α and
β, are set to 0.05,
δ to 10, and based on standard error estimates between 1.7 and 6.7 from previous studies [
16], that would be equal to
σW between 10 and 40, n would be between 26 and 400. Thus, our chosen population sizes of about 90 (
n = 45) was within this rage, but it becomes clear that
σW is the crucial parameter for planning of experiments and the low heritability for SR in some populations resulted in very large standard errors (
Table 4). The formula used to calculate the sample size was based on differences detected by a
t-test. A thorough review on approaches to determine sample sizes can be found in [
30].
Additionally, we considered it as a proof of methodology that loci across populations were found at similar chromosomal positions and loci, such as
Pgs3.1, QTL-SR9/QTL-SR11, and QTL-SR8/QTL-SR10, were close to or overlapping loci found previously (
Pgs3, QTL-SR1, QTL-SR3 in [
16,
17]).
3.2. Resistance to Stem Rust
Because SR does not yet occur regularly at all locations, plots were artificially inoculated. This, however, was no guarantee for high SR severity and in some environments, severities were very low. This resulted in low repeatability estimates and data from the respective environments was dropped resulting in less test environments for analysis across environments (
Table S1). We attributed the low SR severity to unfavorable weather conditions, especially in 2020 when the spring and summer was wet and cold. In 2017 and 2018, we performed similar experiments for SR resistance with other breeding material in almost the same locations and could achieve generally higher infection levels and higher repeatabilities for the single locations [
16]. The modelling of fixed marker-environment interaction (instead of random) in the mapping procedure should further help to additionally track those environmental differences as significant marker-environment effects in QTL mapping and prevent that the actual marker effect would be lost in a large random marker-environment interaction.
Unexpectedly, we found only one single ASR gene for SR in six populations. In wheat, most SR resistance genes are ASR genes and to the best of our knowledge already
Sr gene number 62 has been described [
31], that was like many other before derived from introgressions of other cereals or grass species. Reasons why, in contrast to this study on rye, only a few QTLs for APR for SR in wheat were known, may be that in wheat studies most of the testing was done in the seedling stage or that the cross-pollinating character of rye generally results in a larger resistance gene diversity and during evolution, domestication and breeding of rye, APR QTLs accumulated as consequence of higher plant fitness. Overall, we found 10 SR-QTLs with effect sizes ranging from 4 to 17 percentage points SR reduction. Three pairs of SR-QTLs were mapped at similar chromosomal regions (QTL-SR9/QTL-SR11, QTL-SR7/QTL-SR13, QTL-SR8/QTL-SR10). Interestingly, and a bit unfortunately for breeders, some QTLs for SR resistance, e.g., QTL-SR4 or QTL-SR9/QTL-SR11, were located in centromeric regions that were generally characterized by low recombination frequency [
32]. Similar results have been observed for barley [
33]. There, most of those race-nonspecific genes were even active against several pathogens making those genes more appealing for breeders. In our material, no QTL could be proven to be active against SR and LR simultaneously.
To the best of our knowledge, no other marker-linked resistance loci for SR resistance in rye has been published, except in our previous studies [
16,
17]. QTLs and genes with overlapping positions or close by previously identified loci were
Pgs3.1 close to
Pgs3, QTL-SR9/QTL-SR11 close to QTL-SR1 as well as QTL-SR8/QTL-SR10 and QTL-SR3. However, the same marker position is no proof for having the same gene. Moreover, our definition of two QTLs in different populations being the same QTL if a common marker could be used to detect it is only a further argument for it and no final proof. Common markers were most probably not detecting the causal mutation in the respective gene. Even though no rye-SR genes were found, material and markers were overlapping with other studies. The population Gator, which was the resistance donor of
Pgs3.1, has been studied by Tan et al. [
13,
14] and they also found a single dominant gene (
SrC) that could be the same as
Pgs3.1. A wheat-SR gene,
Sr59, which is located on a rye translocation in wheat, was associated with three markers from a rye SNP chip [
34,
35]. One of them (c20194_115) could be referenced on the linkage map of Bauer et al. [
27] at 175.2 cM, which is close to
Pgs3.1. The population Elbon, which was the resistance donor of P4, was studied by Tan et al. [
13,
14], too. However, for this population, the results did not agree well with ours. When studying the response to f. sp.
secalis, they found genes in the seedling stage that were not active in the adult-plant stage, and when studying the response to f. sp.
tritici, they found two dominant genes active in seedling and adult plant stage.
By identifying field resistance loci in the rye genome, a seedling test was necessary to distinguish ASR from APR. However, only Pgs3.1 from P5 was segregating in both, seedling and adult-plant stage. This could be shown by correlating adjusted means from seedling and field test, but also by mapping the same resistance locus based on both tests. The difference between susceptible and resistant genotypes was already observed when parents of this population were tested in a pre-test for seedling resistance. Accordingly, among parents from all populations, the parents of P5 had the largest IT differences.
As smaller differences between parents from other populations (P3, P6) could also be observed in the seedling test (
Table 5), the question concerned how this difference in resistance was related to field resistance and whether responsible gene loci could be found. P3 was studied in detail, because its parents had different ITs in the seedling stage and high genetic variance was also found when analyzing the offspring. Based on a correlation of 0.59 between adjusted means from field and seedling experiments, it was concluded that some genes/QTL must be different between seedling and adult-plant stage, but also some common genes must be given. The two clusters observed in the seedling stage (
Figure 5 and
Figure 6) were also significantly different based on field data. Surprisingly, no resistance locus could be found in this population (
Figure 3 and
Figure 7). We repeated the seedling test for all genotypes with a detached leaf-segment test but, again, no significant marker–trait association was found.
Results pointing in the similar direction were reported by Tan et al. [
13] who discovered genes based on phenotypic segregation causing phenotypic differences in the seedling stage but not at the adult-plant stage. Johnson [
36] studied environmental factors affecting the infection level in SR seedling tests of wheat and discovered for some isolates and genotypes a highly environment-dependent resistance reaction, which was termed X-type reaction, and with similar definition, this X-type reaction could also be found in the work of Stakman [
37]. In our experiments, an X-type would be expressed as differences between plants of a single genotype. As we fitted a statistical model to analyze our data, the presence of X-types would be expressed by low genetic variance and high variances of experimental factors instead, but this was not observed (
Table 6).
3.3. Resistance to Leaf Rust
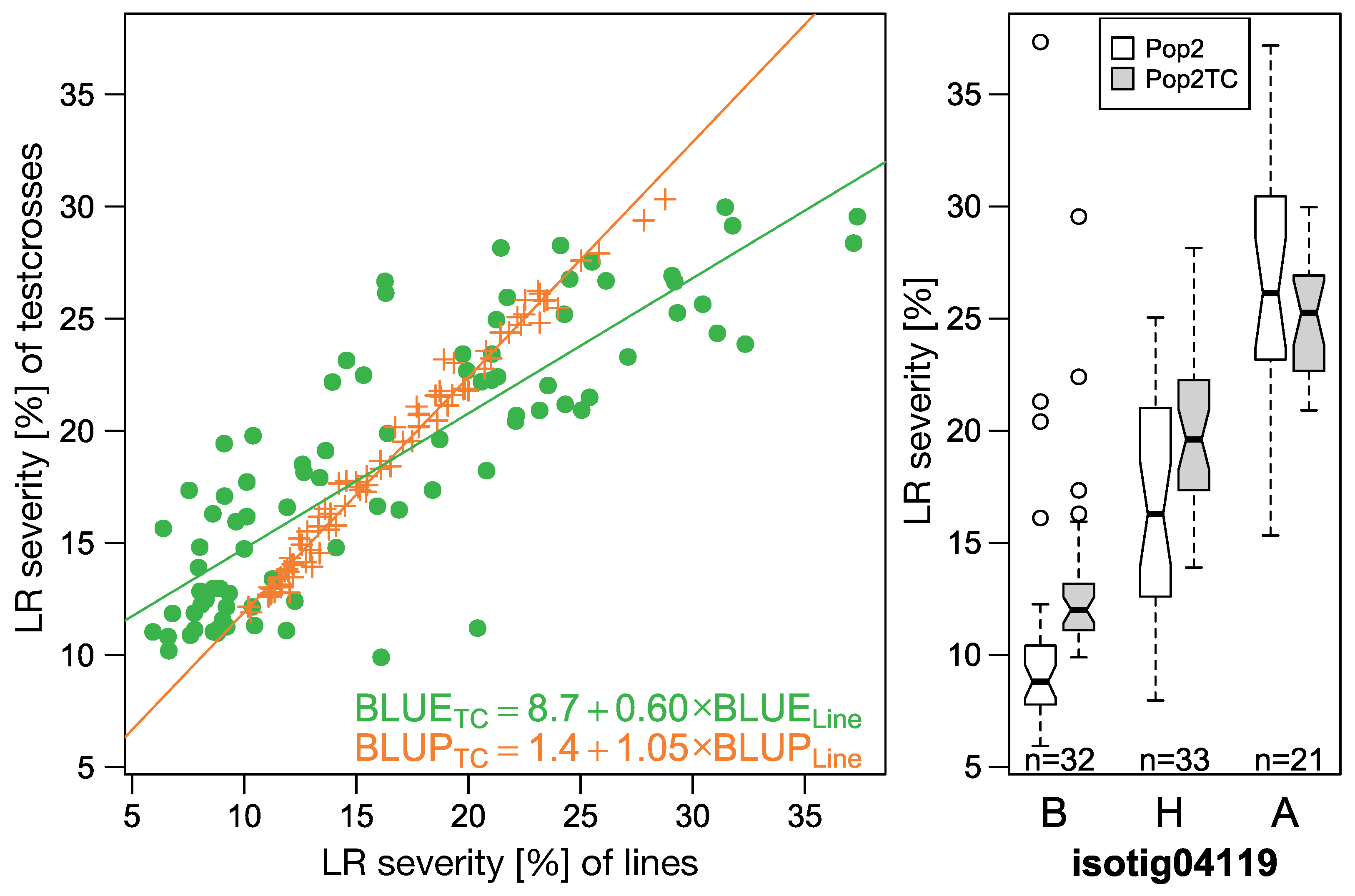
LR infections are occurring naturally in all environments. LR is much more adapted to the climate in Central Europe and in contrast to SR the urediniospores can overwinter on the crop and thus easily boost the disease as soon as the conditions are favorable. However, the consequence of natural inoculum is that there may be different pathotypes of the fungus at different locations. Again, the mapping statistics was adjusted for such cases by modelling a fixed effect for marker-environment interaction. One LR resistance gene,
Pr7, was active in all locations and did not show significant marker-environment interaction (
Figure 10,
Table 7). The other identified resistance gene,
Pr8, was only segregating in one environment and should be again tested in more environments.
For LR resistance in rye, to the best of our knowledge only ten chromosome-associated and thereof six marker-associated genes have been reported [
18,
23]. No marker-linked genes were reported on chromosome 3R and thus the LR resistances detected in P2 and P2TC was assigned with the tentative name
Pr7. On chromosome 1R, several LR resistances have been found [
25,
26,
38]. However, it is difficult to compare the previously found genes with ours. According to chromosomal location, the most reasonable genes would be
Pr3,
Pr4, or
Pr5 [
25], which were all located between the two markers
Xscm1 [
39] and
Xps162 [
40]. The BLAST of the respective sequences resulted in alignments at 137,570,945bp and 629,653,045bp in the Lo7 reference genome [
32], respectively, and our best marker for the LR gene on 1R aligned at 713,072,950bp, outside the defined interval. Interestingly, the associated marker in Rakoczy-Trojanowska et al. [
38],
3363612 (pers. commun. Rakoczy-Trojanowska), mapped on chromosome 2R (not on 1R) in the Lo7 reference genome. The marker AX-99805135 associated with leaf-rust resistance in Vendelbo et al. [
26] was aligned at 625.54 MB in the Lo7 reference genome and was thus close to ours. For this, however, also no previously mentioned gene denomination was proposed, and thus we assigned the LR resistance found in P6 to a new locus
Pr8.
3.4. Breeding Rust-Resistant Hybrid Rye
Hybrid cultivars are usually based on three- or four-way hybrids with different genetical composition (CMS vs. restorer pool). This would allow breeding strategies with different resistances in both breeding pools and stacking of dominant genes in the final hybrid. We have shown for
Pr7 and
Pgs3.1 that the resistances for LR and SR act dominantly. Similar results as well as additional genes have been reported elsewhere [
17,
18] and combinations of all could be used as stacked resistance genes. The use of the detected APR QTLs would require more breeding effort, because effect sizes were smaller (approximately by factor 2–3,
Figure 4) and thus several QTLs must be added up to result in a cultivar with high resistance level. In our material, the combination of a single or two QTLs already resulted in very resistant genotypes (
Figure 4). Unfortunately for breeding, the APR resistance QTLs were not acting dominantly, so that in the case of rye hybrids, the QTLs must be introgressed into both pools to get the full effect. However, it is not known which level of resistance would be necessary to prevent the cultivars from yield losses caused by SR infection and small effect genes could already slow down rust infections until harvest, which is in Central Europe generally a bit earlier than the harvest of winter wheat.
Our study shows that there is a large potential to further optimize rye cultivars in terms of resistance. If our results presented here could be transferred into diagnostic marker sequences in future studies, breeders would be well equipped and not restricted to intensive field testing anymore.