Overexpression of a Senescence-Related Gene CpSRG1 from Wintersweet (Chimonanthus praecox) Promoted Growth and Flowering, and Delayed Senescence in Transgenic Arabidopsis
Abstract
1. Introduction
2. Results
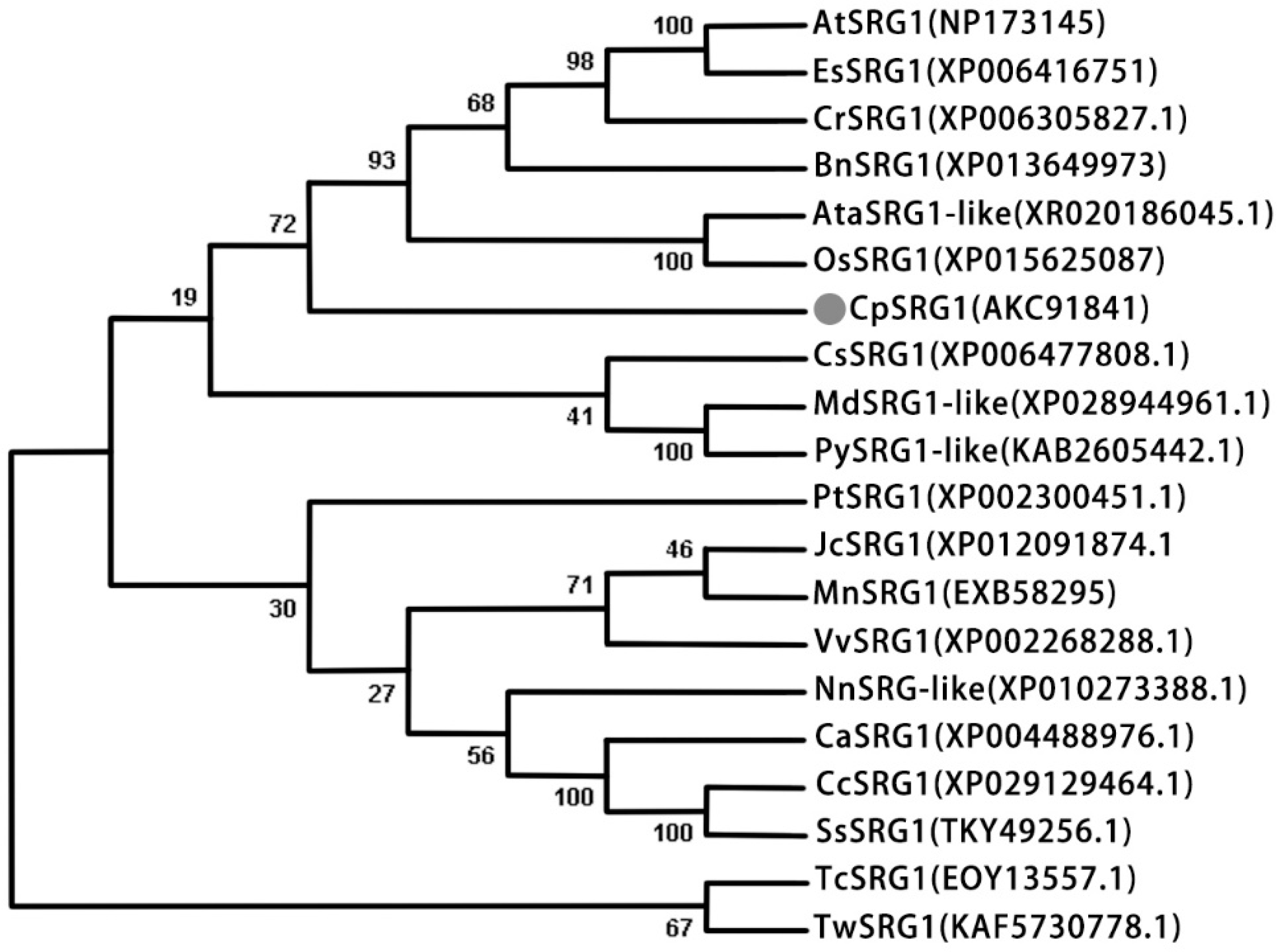
2.1. Isolation and Characterization of CpSRG1 from Wintersweet
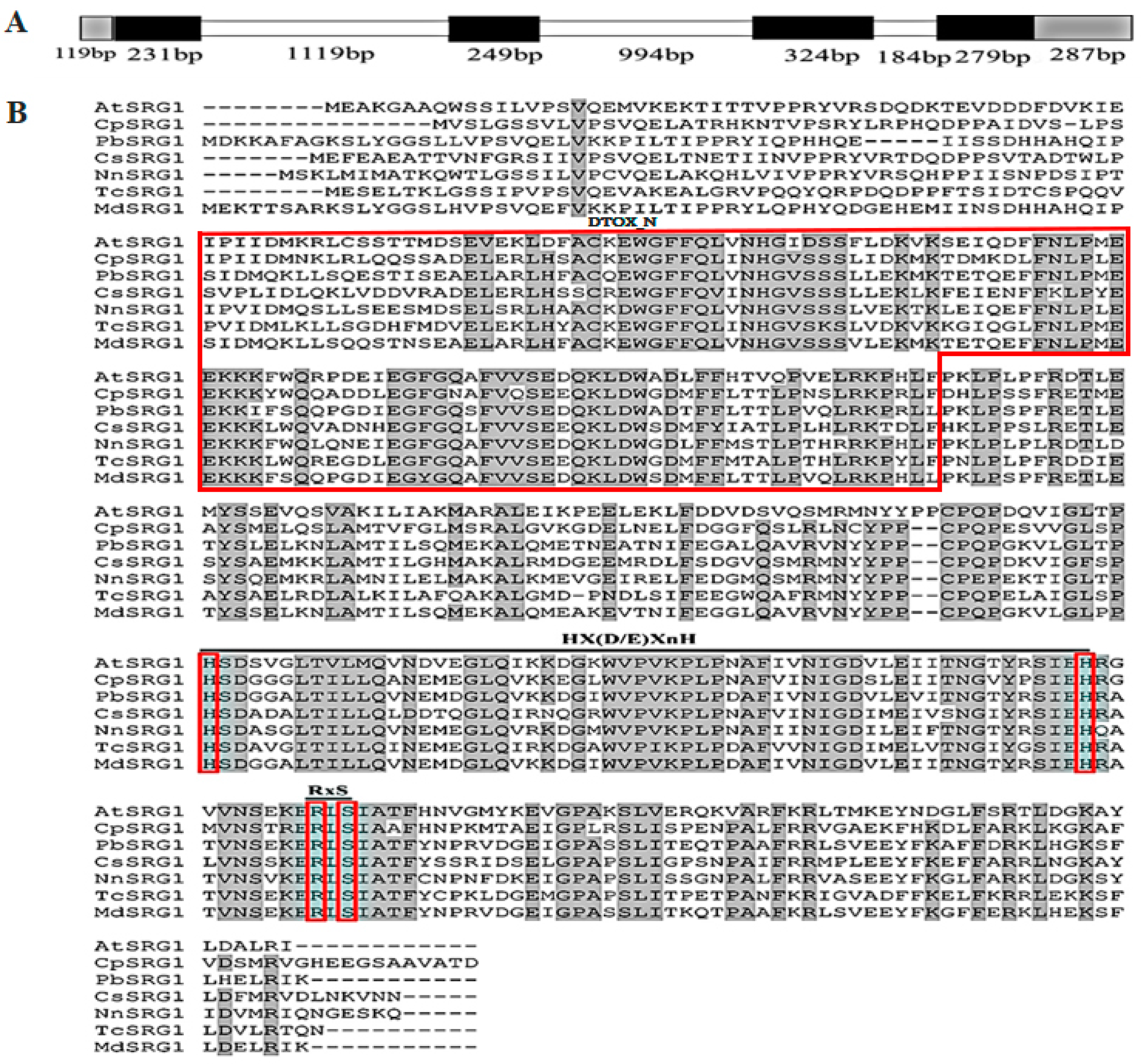
2.2. The Expression Pattern of CpSRG1
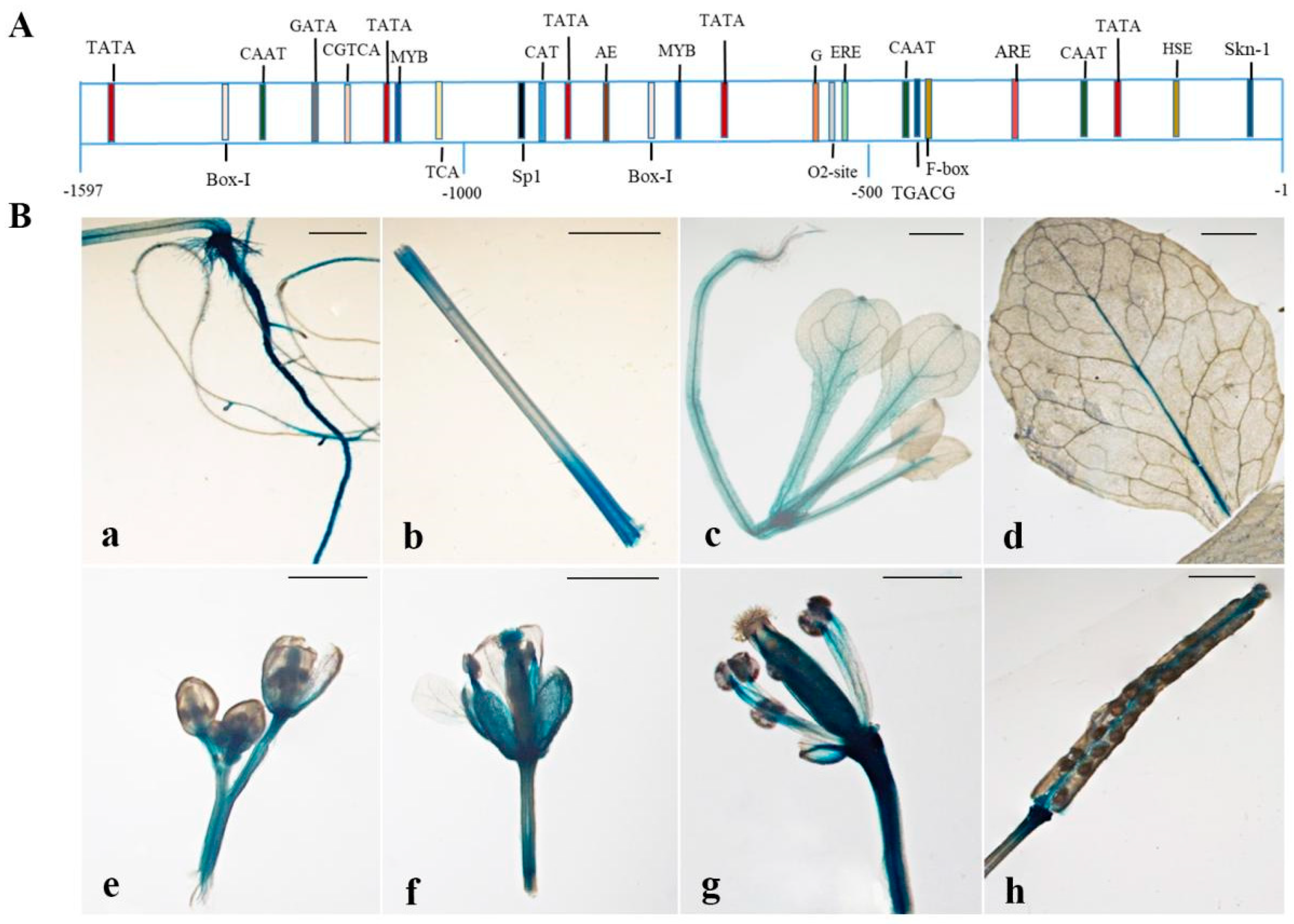
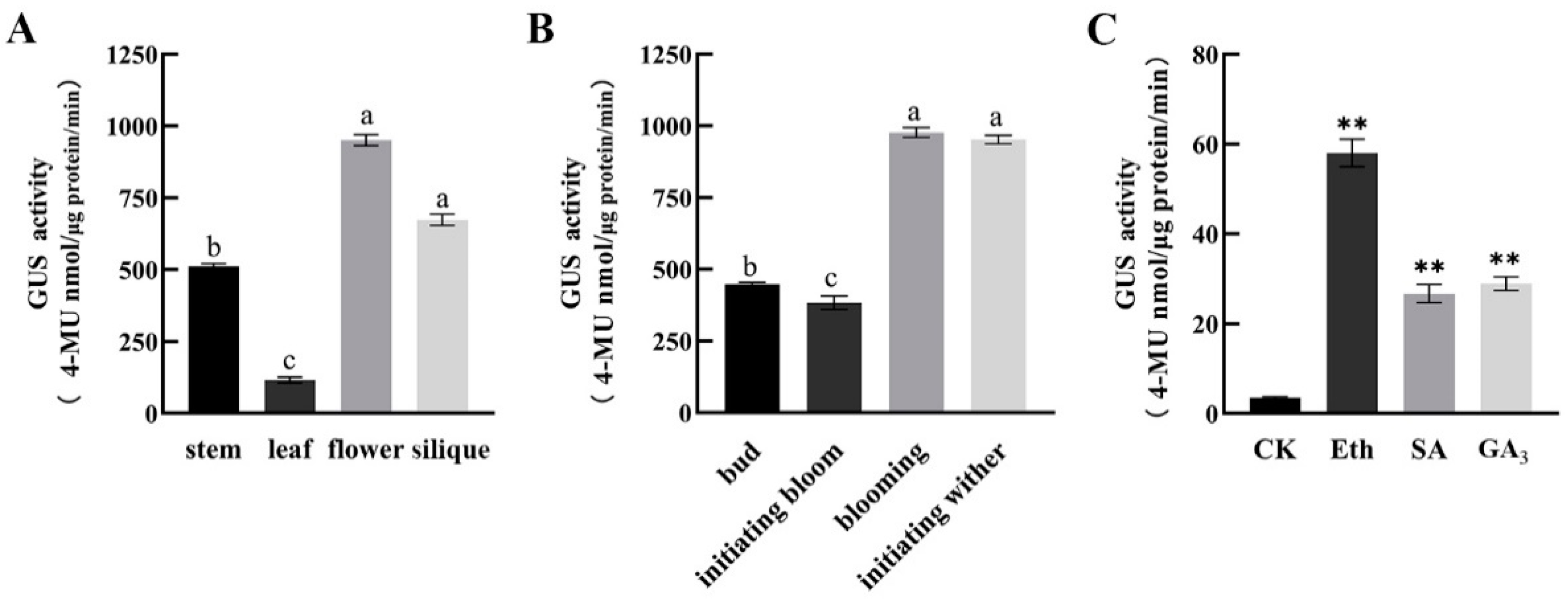
2.3. The GUS Activities of CpSRG1
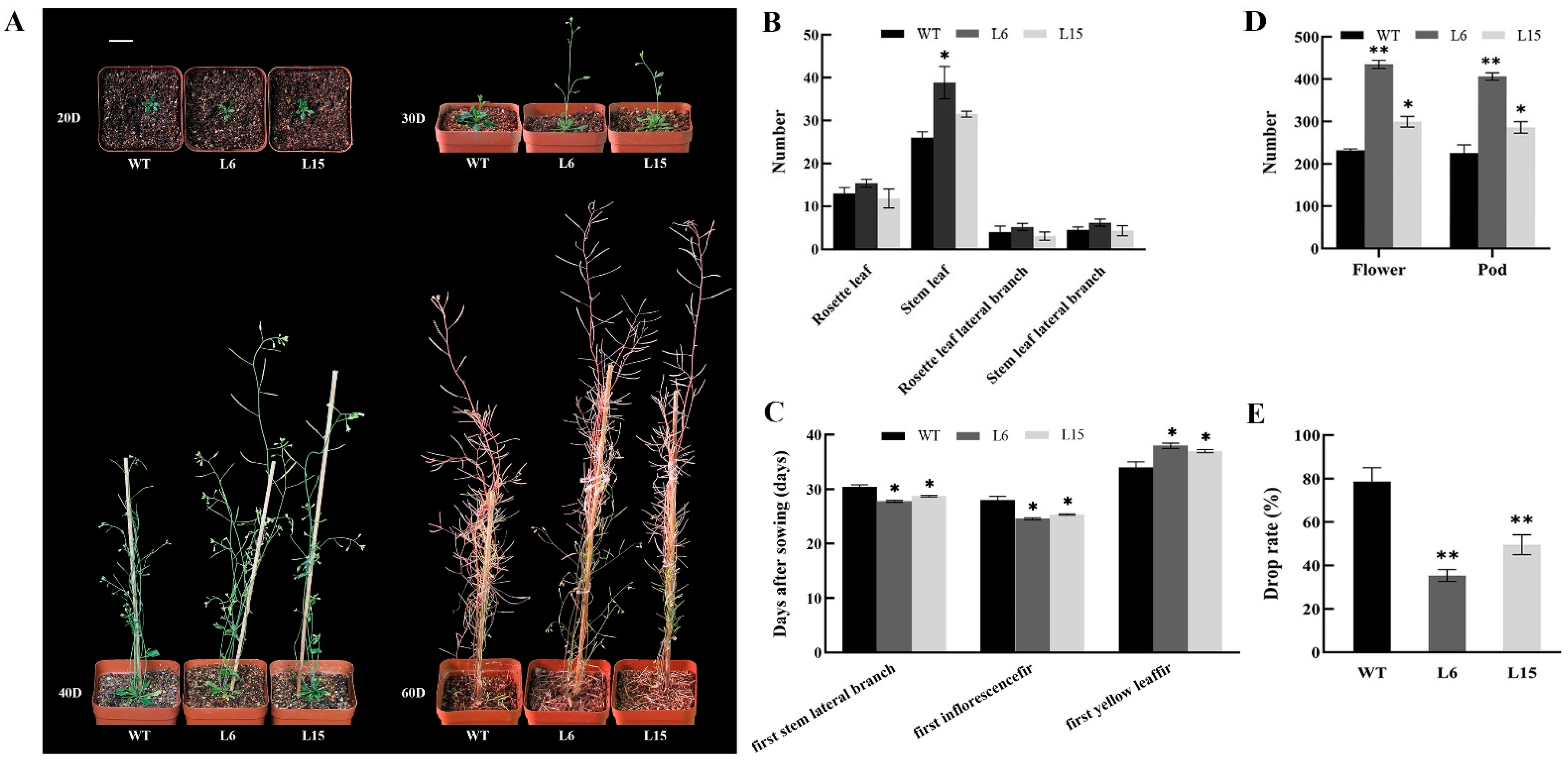
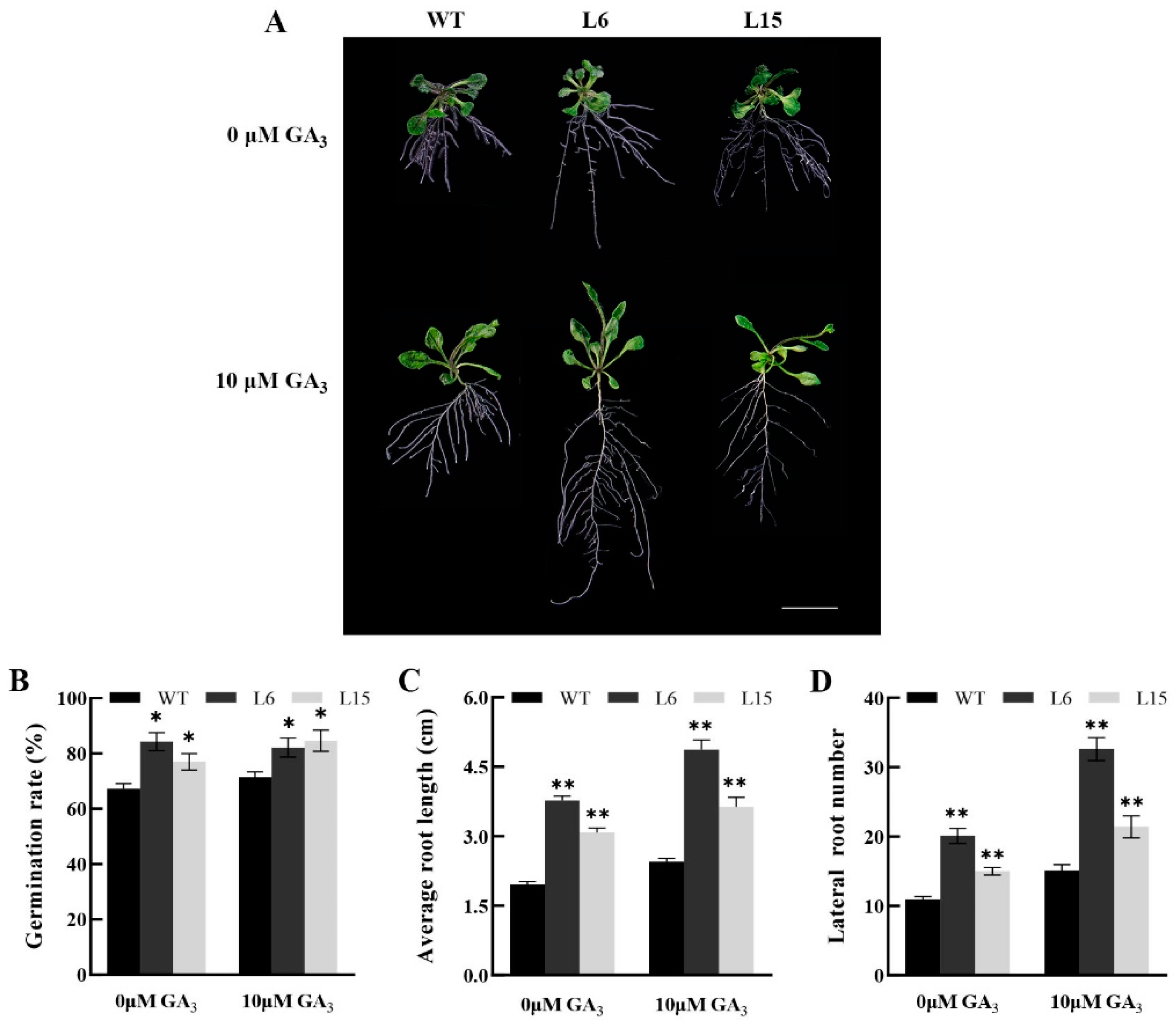
2.4. Overexpression of CpSRG1 Promoted Growth and Flowering, and Delayed Senescence in Arabidopsis
2.5. Overexpression of CpSRG1 Improved the Utilization of Nitrogen in Arabidopsis
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Isolation of CpSRG1 and Its Promoter
4.3. Plasmid Constructs
4.4. Plant Treatment
4.5. Quantitative Real Time-PCR Analysis
4.6. Arabidopsis Transformation
4.7. GUS Histochemical and GUS Activity Assays
4.8. Phenotypic Analysis
4.9. Analysis of Chlorophyll and Flavonoids Content
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shahri, W.; Tahir, I. Flower Senescence-Strategies and Some Associated Events. Bot. Rev. 2011, 77, 152–184. [Google Scholar] [CrossRef]
- Yamada, T.; Ichimura, K.; Kanekatsu, M.; van Doorn, W.G. Gene expression in opening and senescing petals of morning glory (Ipomoea nil) flowers. Plant Cell Rep. 2007, 26, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Masclaux, C.; Valadier, M.H.; Brugiere, N.; Morot-Gaudry, J.F.; Hirel, B. Characterization of the sink/source transition in tobacco (Nicotiana tabacum L.) shoots in relation to nitrogen management and leaf senescence. Planta 2000, 211, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Hortensteiner, S.; Feller, U. Nitrogen metabolism and remobilization during senescence. J. Exp. Bot. 2002, 53, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Rogers, H.J. From models to ornamentals: How is flower senescence regulated? Plant Mol. Biol. 2013, 82, 563–574. [Google Scholar] [CrossRef]
- Morris, K.; Mackerness, S.A.H.; Page, T.; John, C.F.; Murphy, A.M.; Carr, J.P.; Buchanan-Wollaston, V. Salicylic acid has a role in regulating gene expression during leaf senescence. Plant J. 2000, 23, 677–685. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Xu, Y.C.; Li, W.L.; Yang, L.; Yue, X.; Zhang, X.S.; Zhao, X.Y. Transcriptional analyses of natural leaf senescence in maize. PLoS ONE 2014, 9, e115617. [Google Scholar] [CrossRef]
- Guo, Y.; Cai, Z.; Gan, S. Transcriptome of Arabidopsis leaf senescence. Plant Cell Environ. 2004, 27, 521–549. [Google Scholar] [CrossRef]
- Shahri, W.; Tahir, I. Flower senescence: Some molecular aspects. Planta 2014, 239, 277–297. [Google Scholar] [CrossRef]
- Obregón, P.; Martín, R.; Sanz, A.; Castresana, C. Activation of defence-related genes during senescence: A correlation between gene expression and cellular damage. Plant Mol. Biol. 2001, 46, 67–77. [Google Scholar] [CrossRef]
- Ding, Q.; Wang, F.; Xue, J.; Yang, X.; Fan, J.; Chen, H.; Li, Y.; Wu, H. Identification and Expression Analysis of Hormone Biosynthetic and Metabolism Genes in the 2OGD Family for Identifying Genes That May Be Involved in Tomato Fruit Ripening. Int. J. Mol. Sci. 2020, 21, 5344. [Google Scholar] [CrossRef] [PubMed]
- Hedden, P.; Thomas, S.G. Gibberellin biosynthesis and its regulation. Biochem. J. 2012, 444, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Halitschke, R.; Yin, C.; Liu, C.J.; Gan, S.S. Salicylic acid 3-hydroxylase regulates Arabidopsis leaf longevity by mediating salicylic acid catabolism. Proc. Natl. Acad. Sci. USA 2013, 110, 14807–14812. [Google Scholar] [CrossRef] [PubMed]
- Hammond, J.P.; Bennett, M.J.; Bowen, H.C.; Broadley, M.R.; Eastwood, D.C.; May, S.T.; Rahn, C.; Swarup, R.; Woolaway, K.E.; White, P.J. Changes in gene expression in Arabidopsis shoots during phosphate starvation and the potential for developing smart plants. Plant Physiol. 2003, 132, 578–596. [Google Scholar] [CrossRef] [PubMed]
- Rizhsky, L.; Liang, H.; Shuman, J.; Shulaev, V.; Davletova, S.; Mittler, R. When defense pathways collide. The response of Arabidopsis to a combination of drought and heat stress. Plant Physiol. 2004, 134, 1683–1696. [Google Scholar] [CrossRef]
- Pourtau, N.; Jennings, R.; Pelzer, E.; Pallas, J.; Wingler, A. Effect of sugar-induced senescence on gene expression and implications for the regulation of senescence in Arabidopsis. Planta 2006, 224, 556–568. [Google Scholar] [CrossRef]
- Barah, P.; Winge, P.; Kusnierczyk, A.; Tran, D.H.; Bones, A.M. Molecular signatures in Arabidopsis thaliana in response to insect attack and bacterial infection. PLoS ONE 2013, 8, e58987. [Google Scholar] [CrossRef]
- Wu, K.; Tian, L.; Hollingworth, J.; Brown, D.C.W.; Miki, B. Functional Analysis of Tomato Pti4 in Arabidopsis. Plant Physiol. 2002, 128, 30–37. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheetamun, R.; Dhugga, K.S.; Rafalski, J.A.; Tingey, S.V.; Shirley, N.J.; Taylor, J.; Hayes, K.; Beatty, M.; Bacic, A.; et al. Spatial gradients in cell wall composition and transcriptional profiles along elongating maize internodes. BMC Plant Biol. 2014, 14, 27. [Google Scholar] [CrossRef]
- Rios, G.; Naranjo, M.A.; Rodrigo, M.J.; Alos, E.; Zacarias, L.; Cercos, M.; Talon, M. Identification of a GCC transcription factor responding to fruit colour change events in citrus through the transcriptomic analyses of two mutants. BMC Plant Biol. 2010, 10, 276. [Google Scholar] [CrossRef]
- Zhang, X.; Ju, H.W.; Chung, M.S.; Huang, P.; Ahn, S.J.; Kim, C.S. The R-R-type MYB-like transcription factor, AtMYBL, is involved in promoting leaf senescence and modulates an abiotic stress response in Arabidopsis. Plant Cell Physiol. 2011, 52, 138–148. [Google Scholar] [CrossRef]
- Baerenfaller, K.; Massonnet, C.; Walsh, S.; Baginsky, S.; Buhlmann, P.; Hennig, L.; Hirsch-Hoffmann, M.; Howell, K.A.; Kahlau, S.; Radziejwoski, A.; et al. Systems-based analysis of Arabidopsis leaf growth reveals adaptation to water deficit. Mol. Syst. Biol. 2012, 8, 606. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.G.; Zhang, Q.; Wang, L.G.; Duan, K.; Pan, A.H.; Tang, X.M.; Sui, S.Z.; Li, M.Y. The AGL6-like Gene CpAGL6, a Potential Regulator of Floral Time and Organ Identity in Wintersweet (Chimonanthus praecox). J. Plant Growth Regul. 2011, 30, 343–352. [Google Scholar] [CrossRef]
- Huang, R.; Liu, D.; Huang, M.; Ma, J.; Li, Z.; Li, M.; Sui, S. CpWRKY71, a WRKY Transcription Factor Gene of Wintersweet (Chimonanthus praecox), Promotes Flowering and Leaf Senescence in Arabidopsis. Int. J. Mol. Sci. 2019, 20, 5325. [Google Scholar] [CrossRef] [PubMed]
- Farrow, S.C.; Facchini, P.J. Functional diversity of 2-oxoglutarate/Fe(II)-dependent dioxygenases in plant metabolism. Front. Plant Sci. 2014, 5, 524. [Google Scholar] [CrossRef]
- Kawai, Y.; Ono, E.; Mizutani, M. Evolution and diversity of the 2-oxoglutarate-dependent dioxygenase superfamily in plants. Plant J. 2014, 78, 328–343. [Google Scholar] [CrossRef]
- Duan, H.C.; Wei, L.H.; Zhang, C.; Wang, Y.; Chen, L.; Lu, Z.; Chen, P.R.; He, C.; Jia, G. ALKBH10B Is an RNA N 6-Methyladenosine Demethylase Affecting Arabidopsis Floral Transition. Plant Cell 2017, 29, 2995–3011. [Google Scholar] [CrossRef]
- Martinez-Perez, M.; Aparicio, F.; Lopez-Gresa, M.P.; Belles, J.M.; Sanchez-Navarro, J.A.; Pallas, V. Arabidopsis m(6)A demethylase activity modulates viral infection of a plant virus and the m(6)A abundance in its genomic RNAs. Proc. Natl. Acad. Sci. USA 2017, 114, 10755–10760. [Google Scholar] [CrossRef]
- Velasquez, S.M.; Ricardi, M.M.; Poulsen, C.P.; Oikawa, A.; Dilokpimol, A.; Halim, A.; Mangano, S.; Denita Juarez, S.P.; Marzol, E.; Salgado Salter, J.D.; et al. Complex regulation of prolyl-4-hydroxylases impacts root hair expansion. Mol. Plant 2015, 8, 734–746. [Google Scholar] [CrossRef]
- Argueso, C.T.; Hansen, M.; Kieber, J.J. Regulation of Ethylene Biosynthesis. J. Plant Growth Regul. 2007, 26, 92–105. [Google Scholar] [CrossRef]
- Caarls, L.; Elberse, J.; Awwanah, M.; Ludwig, N.R.; de Vries, M.; Zeilmaker, T.; Van Wees, S.C.M.; Schuurink, R.C.; Van den Ackerveken, G. Arabidopsis JASMONATE-INDUCED OXYGENASES down-regulate plant immunity by hydroxylation and inactivation of the hormone jasmonic acid. Proc. Natl. Acad. Sci. USA 2017, 114, 6388–6393. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhang, Y.; Liu, X.; Zhang, X.; Liu, S.; Yu, X.; Ren, Y.; Zheng, X.; Zhou, K.; Jiang, L.; et al. A role for a dioxygenase in auxin metabolism and reproductive development in rice. Dev. Cell 2013, 27, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Brewer, P.B.; Yoneyama, K.; Filardo, F.; Meyers, E.; Scaffidi, A.; Frickey, T.; Akiyama, K.; Seto, Y.; Dun, E.A.; Cremer, J.E.; et al. LATERAL BRANCHING OXIDOREDUCTASE acts in the final stages of strigolactone biosynthesis in Arabidopsis. Proc. Natl. Acad. Sci. USA 2016, 113, 6301–6306. [Google Scholar] [CrossRef]
- Liu Ailing, C.A.U. Cloning and expression analysis of PacFLS in sweet cherry (Prunus avium L.). J. China Agric. Univ. 2013, 18, 56–63. [Google Scholar]
- Wang, H.; Zou, Z.; Ming, G. Gene Cloning and Cold-induced Expression Analysis of the Gene Encoding Gibberellin 20 Oxidase from Jatropha curcas. Biotechnol. Bull. 2015, 31, 140–148. [Google Scholar]
- Kang, H.G.; Jun, S.H.; Kim, J.; Kawaide, H.; Kamiya, Y.; An, G. Cloning and Molecular Analyses of a Gibberellin 20-Oxidase Gene Expressed Specifically in Developing Seeds of Watermelon1. Plant Physiol. 1999, 121, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, Y.; Li, Y.; Li, W. Function Prediction and Expression Analysis of GmDAO1 Gene in Soybean. Genom. Appl. Biol. 2017, 36, 2480–2485. [Google Scholar]
- Huang, C.H.; Gao, Y.H.; Zhu, Y.Q.; Tong, Z.K.; Xiao-Feng, A.J. Cloning and Expression Analysis of Flavanone 3-hydroxylase Gene LrF3H from Lycoris radiata. Acta Hortic. Sin. 2013, 40, 960–970. [Google Scholar]
- Du, L.; Chen, K.; Liu, Y. Cloning of Flavonol Synthase Gene (FLS1) and Relativity Analysis of Its Expression with the Flower Color in Grape Hyacinth. J. Northwest For. Univ. 2017, 32, 106–113. [Google Scholar]
- Ma, Y.; Kang, J.; Wu, J.; Zhu, Y.; Wang, X. Identification of tapetum-specific genes by comparing global gene expression of four different male sterile lines in Brassica oleracea. Plant Mol. Biol. 2015, 87, 541–554. [Google Scholar] [CrossRef]
- Mateo-Bonmati, E.; Esteve-Bruna, D.; Juan-Vicente, L.; Nadi, R.; Candela, H.; Lozano, F.M.; Ponce, M.R.; Perez-Perez, J.M.; Micol, J.L. INCURVATA11 and CUPULIFORMIS2 Are Redundant Genes That Encode Epigenetic Machinery Components in Arabidopsis. Plant Cell 2018, 30, 1596–1616. [Google Scholar] [CrossRef]
- Yu, X.; Xu, Y.; Yan, S. Salicylic acid and ethylene coordinately promote leaf senescence. J. Integr. Plant Biol. 2021, 65, 823–827. [Google Scholar] [CrossRef]
- Lu, P.; Zhang, C.; Liu, J.; Liu, X.; Jiang, G.; Jiang, X.; Khan, M.A.; Wang, L.; Hong, B.; Gao, J. RhHB1 mediates the antagonism of gibberellins to ABA and ethylene during rose (Rosa hybrida) petal senescence. Plant J. 2014, 78, 578–590. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Wei, J.; Ma, Q.; Yu, D.; Li, J. Senescence of aerial parts is impeded by exogenous gibberellic acid in herbaceous perennial Paris polyphylla. J. Plant Physiol. 2009, 166, 819–830. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xiang, S.; Chen, Y.; Li, D.; Yu, D. Arabidopsis WRKY45 Interacts with the DELLA Protein RGL1 to Positively Regulate Age-Triggered Leaf Senescence. Mol. Plant 2017, 10, 1174–1189. [Google Scholar] [CrossRef]
- Xu, Y.M.; Xiao, X.M.; Zeng, Z.X.; Tan, X.L.; Liu, Z.L.; Chen, J.W.; Su, X.G.; Chen, J.Y. BrTCP7 Transcription Factor Is Associated with MeJA-Promoted Leaf Senescence by Activating the Expression of BrOPR3 and BrRCCR. Int. J. Mol. Sci. 2019, 20, 3963. [Google Scholar] [CrossRef] [PubMed]
- Sui, S.; Luo, J.; Ma, J.; Zhu, Q.; Lei, X.; Li, M. Generation and Analysis of Expressed Sequence Tags from Chimonanthus praecox (Wintersweet) Flowers for Discovering Stress-Responsive and Floral Development-Related Genes. Comp. Funct. Genom. 2012, 2012, 134596. [Google Scholar] [CrossRef]
- Liu, Y.G.; Chen, Y. High-efficiency thermal asymmetric interlaced PCR for amplification of unknown flanking sequences. Biotechniques 2007, 43, 649–650, 652, 654. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Meng, Y.; Liu, D.; Tang, H.; Lü, S.; Imtiaz, M.; Jiang, G.; Lü, P.; Ji, Y.; Gao, J.; et al. Responses of rose RhACS1 and RhACS2 promoters to abiotic stresses in transgenic Arabidopsis thaliana. Plant Cell Rep. 2015, 34, 795–804. [Google Scholar] [CrossRef]
- Tao, Y.; Wang, F.T.; Jia, D.M.; Li, J.T.; Zhang, Y.M.; Jia, C.G.; Wang, D.P.; Pan, H.Y. Cloning and Functional Analysis of the Promoter of a Stress-inducible Gene (ZmRXO1) in Maize. Plant Mol. Biol. Rep. 2015, 33, 200–208. [Google Scholar] [CrossRef]
- Mara, C.; Grigorova, B.; Liu, Z. Floral-dip transformation of Arabidopsis thaliana to examine pTSO2::beta-glucuronidase reporter gene expression. J. Vis. Exp. 2010, 11, 1952. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, R.A.; Kavanagh, T.A.; Bevan, M.W. GUS fusions: Beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987, 6, 3901–3907. [Google Scholar] [CrossRef] [PubMed]
- Niu, G.L.; Gou, W.; Han, X.L.; Qin, C.; Zhang, L.X.; Abomohra, A.; Ashraf, M. Cloning and Functional Analysis of Phosphoethanolamine Methyltransferase Promoter from Maize (Zea mays L.). Int. J. Mol. Sci. 2018, 19, 191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, L.; Yu, D. Transcription Factor WRKY75 Interacts with DELLA Proteins to Affect Flowering. Plant Physiol. 2018, 176, 790–803. [Google Scholar] [CrossRef]
- Wang, J.; Chen, P.; Chen, A.; Tian, J.; Muhammad, S.; Li, Y. Effects of drought stress on physiological and growth characteristics of the seedlings of three ornamental grasses. Pratacultural. Sci. 2019, 36, 1266–1274. [Google Scholar]
- Routaboul, J.M.; Kerhoas, L.; Debeaujon, I.; Pourcel, L.; Caboche, M.; Einhorn, J.; Lepiniec, L. Flavonoid diversity and biosynthesis in seed of Arabidopsis thaliana. Planta 2006, 224, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Routaboul, J.M.; Dubos, C.; Beck, G.; Marquis, C.; Bidzinski, P.; Loudet, O.; Lepiniec, L. Metabolite profiling and quantitative genetics of natural variation for flavonoids in Arabidopsis. J. Exp. Bot. 2012, 63, 3749–3764. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Y.; Li, G.; Wang, X.; Huang, R.; Luo, J.; Li, M.; Liu, D.; Sui, S. Overexpression of a Senescence-Related Gene CpSRG1 from Wintersweet (Chimonanthus praecox) Promoted Growth and Flowering, and Delayed Senescence in Transgenic Arabidopsis. Int. J. Mol. Sci. 2022, 23, 13971. https://doi.org/10.3390/ijms232213971
Cao Y, Li G, Wang X, Huang R, Luo J, Li M, Liu D, Sui S. Overexpression of a Senescence-Related Gene CpSRG1 from Wintersweet (Chimonanthus praecox) Promoted Growth and Flowering, and Delayed Senescence in Transgenic Arabidopsis. International Journal of Molecular Sciences. 2022; 23(22):13971. https://doi.org/10.3390/ijms232213971
Chicago/Turabian StyleCao, Yinzhu, Guixiang Li, Xia Wang, Renwei Huang, Jianghui Luo, Mingyang Li, Daofeng Liu, and Shunzhao Sui. 2022. "Overexpression of a Senescence-Related Gene CpSRG1 from Wintersweet (Chimonanthus praecox) Promoted Growth and Flowering, and Delayed Senescence in Transgenic Arabidopsis" International Journal of Molecular Sciences 23, no. 22: 13971. https://doi.org/10.3390/ijms232213971
APA StyleCao, Y., Li, G., Wang, X., Huang, R., Luo, J., Li, M., Liu, D., & Sui, S. (2022). Overexpression of a Senescence-Related Gene CpSRG1 from Wintersweet (Chimonanthus praecox) Promoted Growth and Flowering, and Delayed Senescence in Transgenic Arabidopsis. International Journal of Molecular Sciences, 23(22), 13971. https://doi.org/10.3390/ijms232213971




