Deciphering the Roles & Regulation of Estradiol Signaling during Female Mini-Puberty: Insights from Mouse Models
Abstract
:1. Introduction
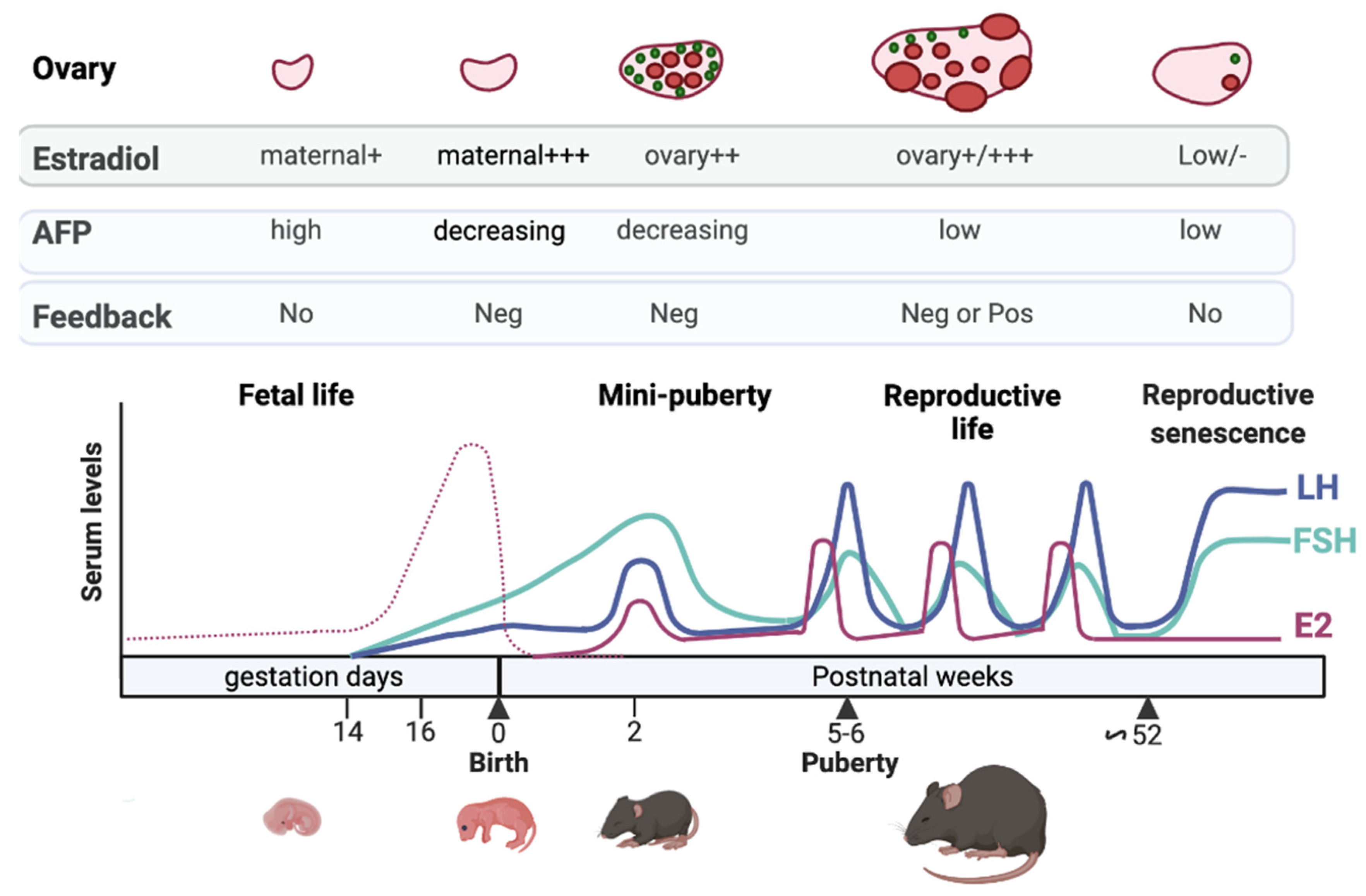
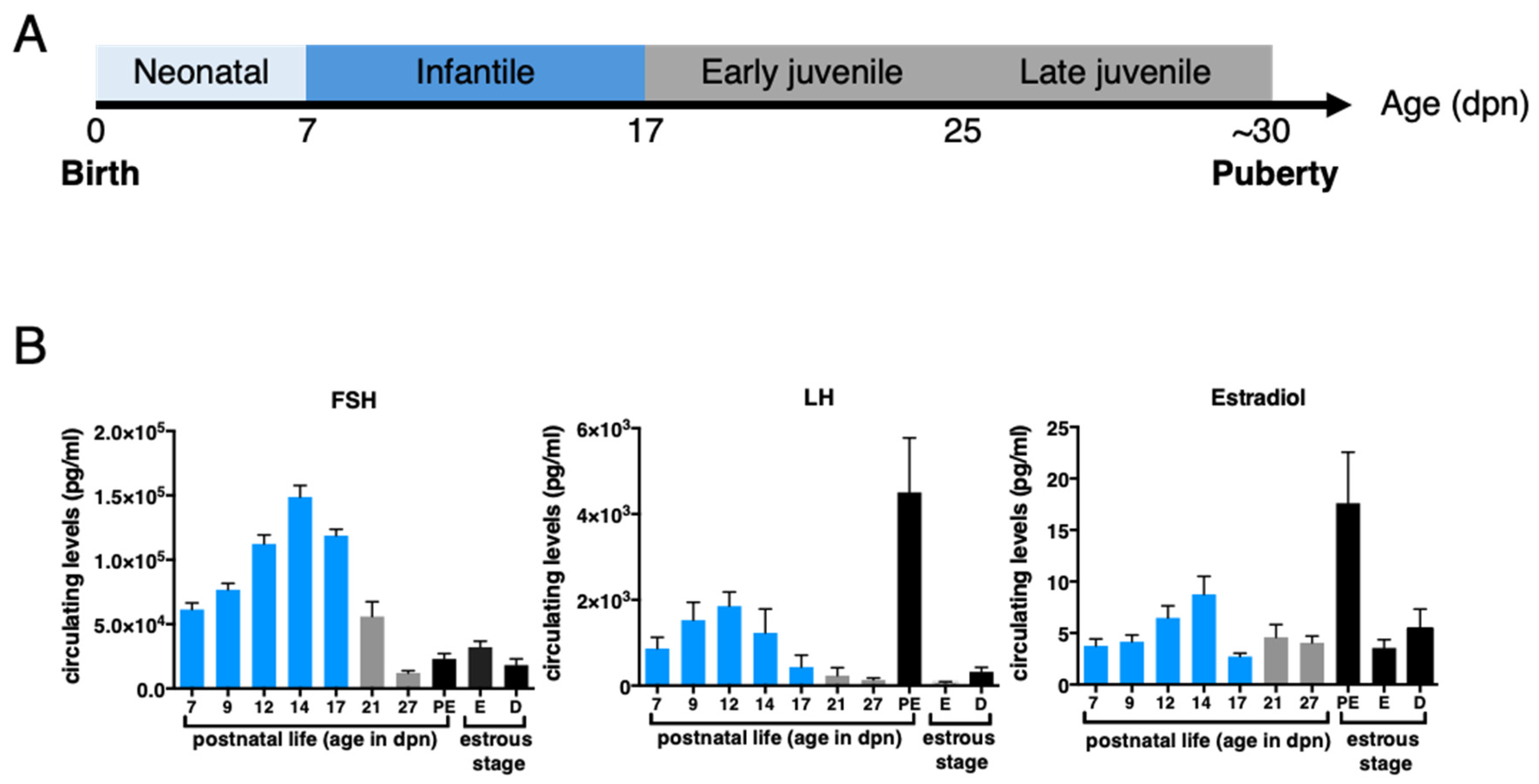
2. Defining HPO Axis Activation & Mini-Puberty Timing in Female Mammals
3. Possible Mechanisms Underlying Increasing Gonadotropin Levels during Fetal Life and Mini-Puberty
3.1. Lack of Sensitivity to Estrogen Negative Feedback during Fetal Life
3.2. Limited Negative Feedbacks Exerted by Estrogens and Inhibins at Mini-Puberty
4. The Ovary: From Its Differentiation to Its Endocrine Activation at Mini-Puberty
4.1. Follicle Formation
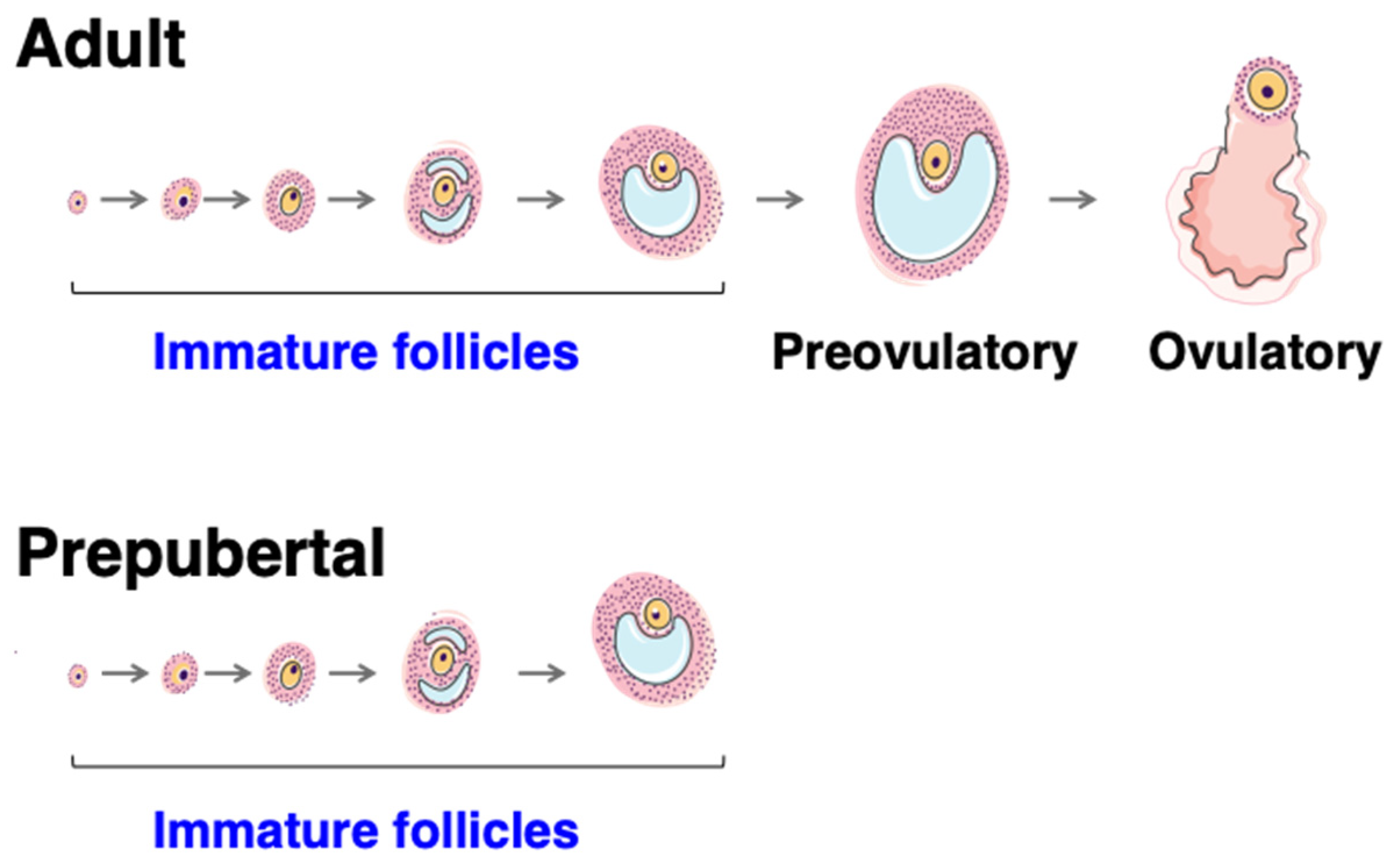
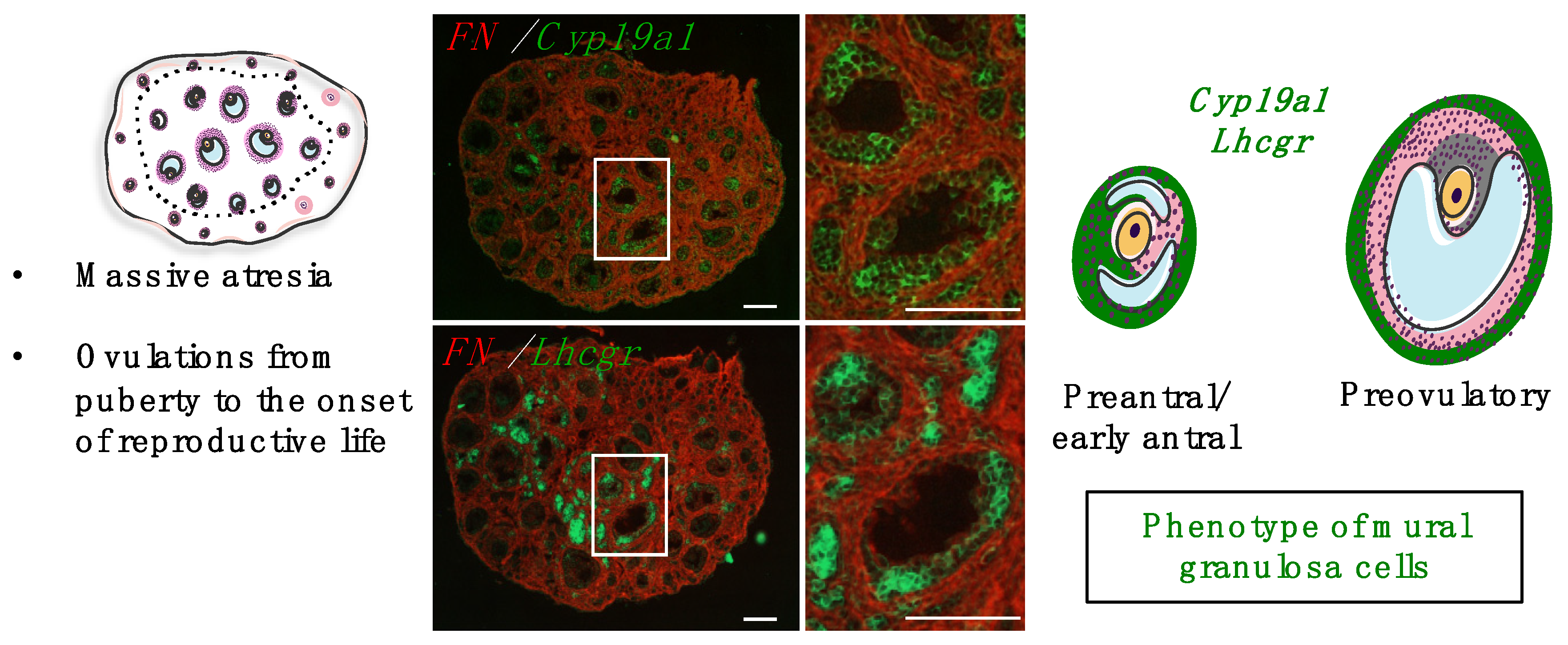
4.2. Roles and Dynamics of the First Follicular Waves
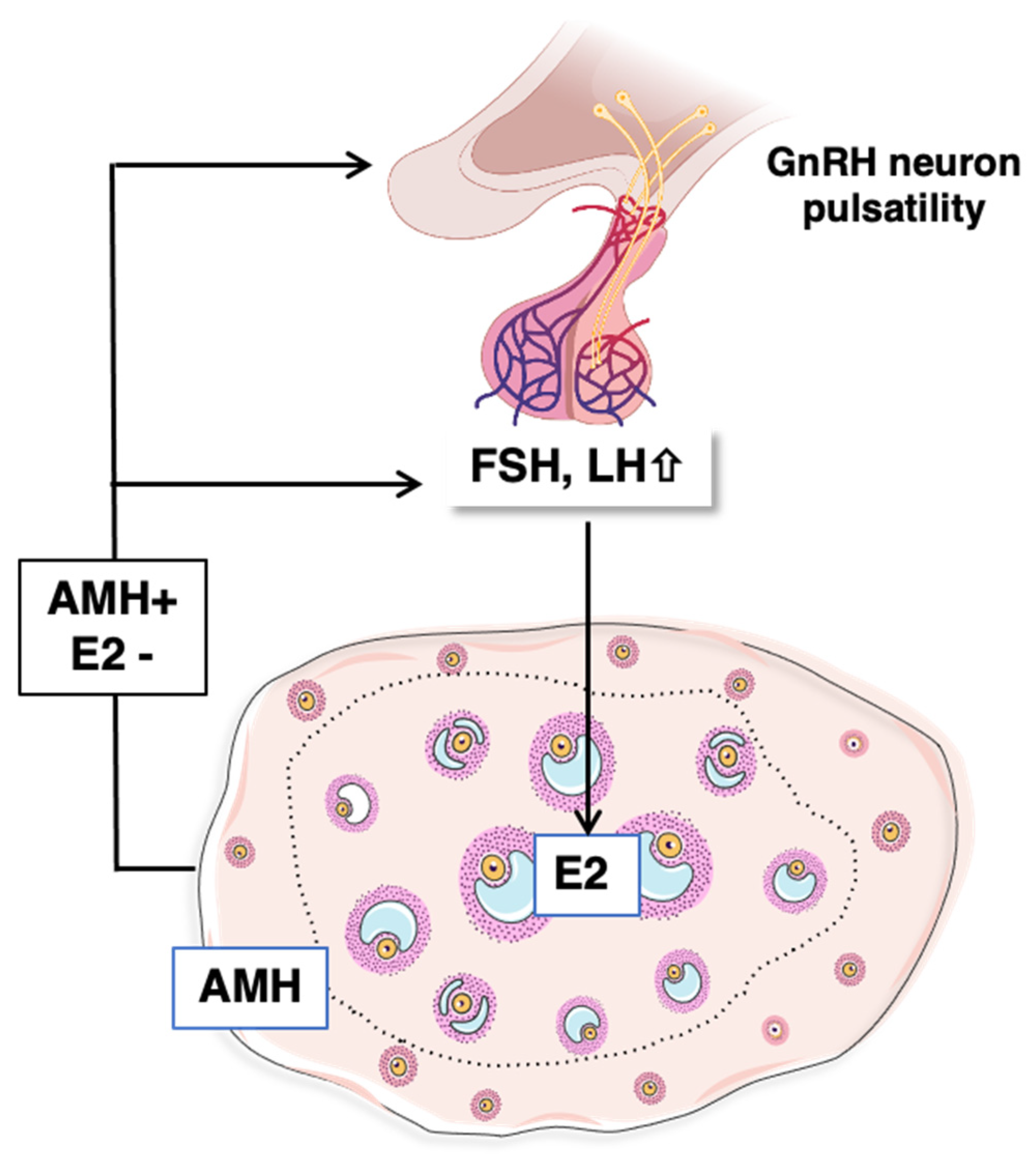
4.3. Mechanisms Controlling Intra-Ovarian Estradiol Production during Mini-Puberty
4.3.1. Regulation by Gonadotropins
4.3.2. Contribution of AMH Signaling
4.3.3. Effects of Exposure to Endocrine Disruptor Chemicals (EDCs) on Mini-Pubertal Estradiol Production
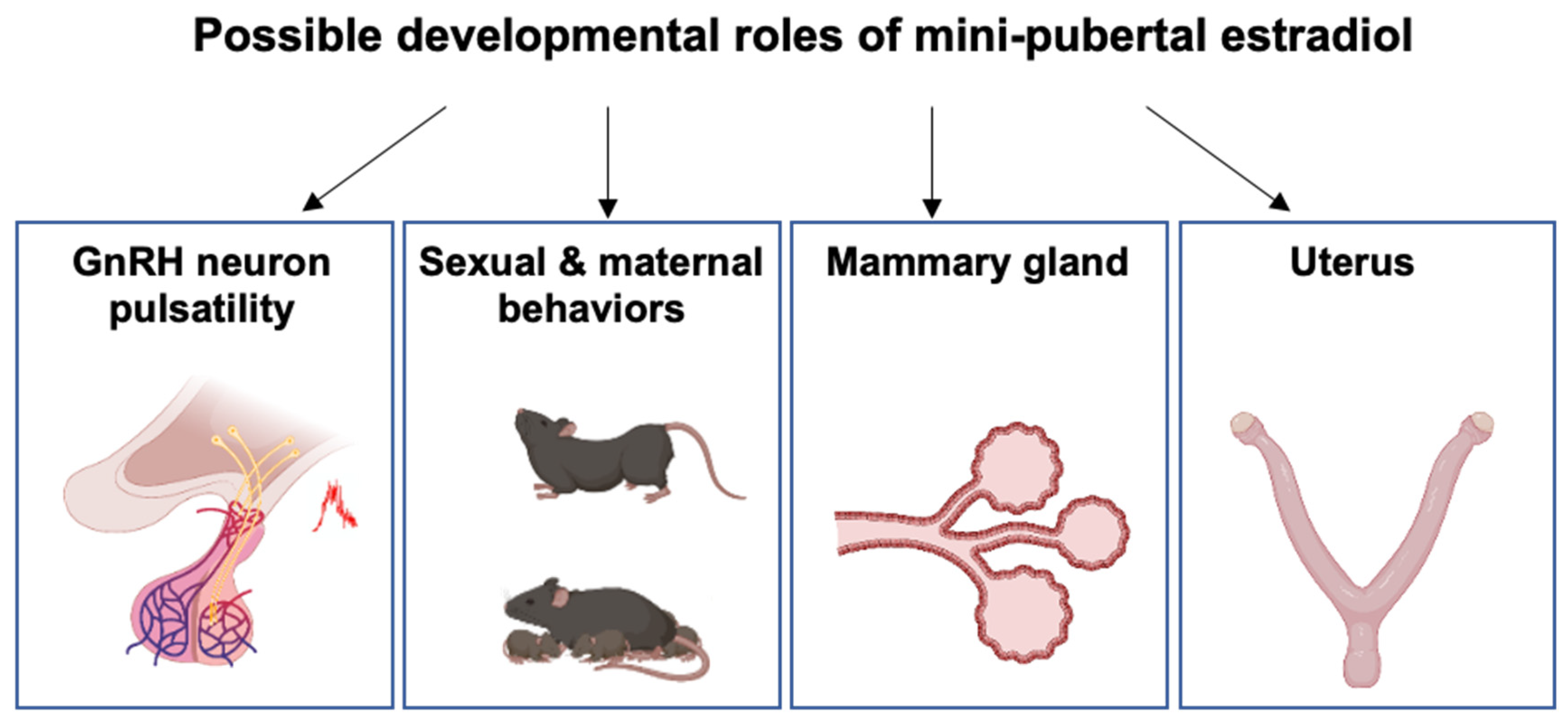
5. Putative Physiological Roles of Estradiol during Mini-Puberty
5.1. Developmental Organization of the HPO Axis and Female Behavior
5.2. Mammary Gland Development
5.3. Uterus Development
6. Concluding Remarks, Perspectives and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Hadziselimovic, F.; Zivkovic, D.; Bica, D.T.G.; Emmons, L.R. The importance of mini-puberty for fertility in cryptorchidism. J. Urol. 2005, 174, 1536–1539, discussion 1538–1539. [Google Scholar] [CrossRef] [PubMed]
- Johannsen, T.H.; Main, K.M.; Ljubicic, M.L.; Jensen, T.K.; Andersen, H.R.; Andersen, M.S.; Petersen, J.H.; Andersson, A.-M.; Juul, A. Sex Differences in Reproductive Hormones during Mini-Puberty in Infants with Normal and Disordered Sex Development. J. Clin. Endocrinol. Metab. 2018, 103, 3028–3037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rey, R.A. Mini-puberty and true puberty: Differences in testicular function. Ann. Endocrinol. 2014, 75, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Phoenix, C.H.; Goy, R.W.; Gerall, A.A.; Young, W.C. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology 1959, 65, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Tofovic, S.P.; Jackson, E.K. Estradiol Metabolism: Crossroads in Pulmonary Arterial Hypertension. Int. J. Mol. Sci. 2019, 21, 116. [Google Scholar] [CrossRef] [Green Version]
- Lanciotti, L.; Cofini, M.; Leonardi, A.; Penta, L.; Esposito, S. Up-To-Date Review about Minipuberty and Overview on Hypothalamic-Pituitary-Gonadal Axis Activation in Fetal and Neonatal Life. Front. Endocrinol. 2018, 9, 410. [Google Scholar] [CrossRef] [Green Version]
- Becker, M.; Hesse, V. Minipuberty: Why Does it Happen? Horm. Res. Paediatr. 2020, 93, 76–84. [Google Scholar] [CrossRef]
- Bizzarri, C.; Cappa, M. Ontogeny of Hypothalamus-Pituitary Gonadal Axis and Minipuberty: An Ongoing Debate? Front. Endocrinol. 2020, 11, 187. [Google Scholar] [CrossRef]
- Kermath, B.A.; Gore, A.C. Neuroendocrine control of the transition to reproductive senescence: Lessons learned from the female rodent model. Neuroendocrinology 2012, 96, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Oduwole, O.O.; Huhtaniemi, I.T.; Misrahi, M. The Roles of Luteinizing Hormone, Follicle-Stimulating Hormone and Testosterone in Spermatogenesis and Folliculogenesis Revisited. Int. J. Mol. Sci. 2021, 22, 12735. [Google Scholar] [CrossRef]
- Parkening, T.A.; Collins, T.J.; Smith, E.R. Plasma and pituitary concentrations of luteinizing hormone, follicle-stimulating hormone and prolactin in aged, ovariectomized CD-1 and C57BL/6 mice. Exp. Gerontol. 1982, 17, 437–443. [Google Scholar] [CrossRef]
- Pointis, G.; Latreille, M.T.; Cedard, L. Gonado-pituitary relationships in the fetal mouse at various times during sexual differentiation. J. Endocrinol. 1980, 86, 483–488. [Google Scholar] [CrossRef]
- Stiff, M.E.; Bronson, F.H.; Stetson, M.H. Plasma gonadotropins in prenatal and prepubertal female mice: Disorganization of pubertal cycles in the absence of a male. Endocrinology 1974, 94, 492–496. [Google Scholar] [CrossRef]
- Kreisman, M.J.; Song, C.I.; Yip, K.; Natale, B.V.; Natale, D.R.; Breen, K.M. Androgens Mediate Sex-Dependent Gonadotropin Expression during Late Prenatal Development in the Mouse. Endocrinology 2017, 158, 2884–2894. [Google Scholar] [CrossRef]
- Veyssière, G.; Berger, M.; Jean-Faucher, C.; De Turckheim, M.; Jean, C. Pituitary and plasma levels of luteinizing hormone and follicle-stimulating hormone in male and female rabbit fetuses. J. Endocrinol. 1982, 92, 381–387. [Google Scholar] [CrossRef]
- Takagi, S.; Yoshida, T.; Tsubata, K.; Ozaki, H.; Fujii, T.K.; Nomura, Y.; Sawada, M. Sex differences in fetal gonadotropins and androgens. J. Steroid Biochem. 1977, 8, 609–620. [Google Scholar] [CrossRef]
- Reyes, F.I.; Boroditsky, R.S.; Winter, J.S.; Faiman, C. Studies on human sexual development. II. Fetal and maternal serum gonadotropin and sex steroid concentrations. J. Clin. Endocrinol. Metab. 1974, 38, 612–617. [Google Scholar] [CrossRef]
- Ellinwood, W.E.; Resko, J.A. Sex differences in biologically active and immunoreactive gonadotropins in the fetal circulation of rhesus monkeys. Endocrinology 1980, 107, 902–907. [Google Scholar] [CrossRef]
- Salfi, V.; Ventura, T.; Caraceni, D. Follicles development in the foetal human ovary. Experientia 1979, 35, 543–544. [Google Scholar] [CrossRef]
- Ellinwood, W.E.; McClellan, M.C.; Brenner, R.M.; Resko, J.A. Estradiol synthesis by fetal monkey ovaries correlates with antral follicle formation. Biol. Reprod. 1983, 28, 505–516. [Google Scholar] [CrossRef]
- Pezzi, V.; Mathis, J.M.; Rainey, W.E.; Carr, B.R. Profiling transcript levels for steroidogenic enzymes in fetal tissues. J. Steroid Biochem. Mol. Biol. 2003, 87, 181–189. [Google Scholar] [CrossRef]
- Sokka, T.; Huhtaniemi, I. Ontogeny of gonadotrophin receptors and gonadotrophin-stimulated cyclic AMP production in the neonatal rat ovary. J. Endocrinol. 1990, 127, 297–303. [Google Scholar] [CrossRef]
- Weniger, J.P.; Zeis, A.; Chouraqui, J. Estrogen production by fetal and infantile rat ovaries. Reprod. Nutr. Dev. 1993, 33, 129–136. [Google Scholar] [CrossRef] [Green Version]
- O’Shaughnessy, P.J.; McLelland, D.; McBride, M.W. Regulation of luteinizing hormone-receptor and follicle-stimulating hormone-receptor messenger ribonucleic acid levels during development in the neonatal mouse ovary. Biol. Reprod. 1997, 57, 602–608. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, S.L.; Grumbach, M.M. The ontogenesis of human foetal hormones. II. Luteinizing hormone (LH) and follicle stimulating hormone (FSH). Acta Endocrinol. 1976, 81, 808–829. [Google Scholar] [CrossRef]
- Beck-Peccoz, P.; Padmanabhan, V.; Baggiani, A.M.; Cortelazzi, D.; Buscaglia, M.; Medri, G.; Marconi, A.M.; Pardi, G.; Beitins, I.Z. Maturation of hypothalamic-pituitary-gonadal function in normal human fetuses: Circulating levels of gonadotropins, their common alpha-subunit and free testosterone, and discrepancy between immunological and biological activities of circulating follicle-stimulating hormone. J. Clin. Endocrinol. Metab. 1991, 73, 525–532. [Google Scholar] [CrossRef]
- Döhler, K.D.; Wuttke, W. Changes with age in levels of serum gonadotropins, prolactin and gonadal steroids in prepubertal male and female rats. Endocrinology 1975, 97, 898–907. [Google Scholar] [CrossRef] [PubMed]
- Chellakooty, M.; Schmidt, I.M.; Haavisto, A.M.; Boisen, K.A.; Damgaard, I.N.; Mau, C.; Petersen, J.H.; Juul, A.; Skakkebaek, N.E.; Main, K.M. Inhibin A, inhibin B, follicle-stimulating hormone, luteinizing hormone, estradiol, and sex hormone-binding globulin levels in 473 healthy infant girls. J. Clin. Endocrinol. Metab. 2003, 88, 3515–3520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- François, C.M.; Petit, F.; Giton, F.; Gougeon, A.; Ravel, C.; Magre, S.; Cohen-Tannoudji, J.; Guigon, C.J. A novel action of follicle-stimulating hormone in the ovary promotes estradiol production without inducing excessive follicular growth before puberty. Sci. Rep. 2017, 7, 46222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herath, C.B.; Yamashita, M.; Watanabe, G.; Jin, W.; Tangtrongsup, S.; Kojima, A.; Groome, N.P.; Suzuki, A.K.; Taya, K. Regulation of follicle-stimulating hormone secretion by estradiol and dimeric inhibins in the infantile female rat. Biol. Reprod. 2001, 65, 1623–1633. [Google Scholar] [CrossRef]
- Frederiksen, H.; Johannsen, T.H.; Andersen, S.E.; Albrethsen, J.; Landersoe, S.K.; Petersen, J.H.; Andersen, A.N.; Vestergaard, E.T.; Schorring, M.E.; Linneberg, A.; et al. Sex-specific Estrogen Levels and Reference Intervals from Infancy to Late Adulthood Determined by LC-MS/MS. J. Clin. Endocrinol. Metab. 2020, 105, dgz196. [Google Scholar] [CrossRef]
- Burger, H.G.; Yamada, Y.; Bangah, M.L.; McCloud, P.I.; Warne, G.L. Serum gonadotropin, sex steroid, and immunoreactive inhibin levels in the first two years of life. J. Clin. Endocrinol. Metab. 1991, 72, 682–686. [Google Scholar] [CrossRef]
- Winter, J.S.; Faiman, C.; Hobson, W.C.; Prasad, A.V.; Reyes, F.I. Pituitary-gonadal relations in infancy. I. Patterns of serum gonadotropin concentrations from birth to four years of age in man and chimpanzee. J. Clin. Endocrinol. Metab. 1975, 40, 545–551. [Google Scholar] [CrossRef]
- Penny, R.; Olambiwonnu, N.O.; Frasier, S.D. Serum gonadotropin concentrations during the first four years of life. J. Clin. Endocrinol. Metab. 1974, 38, 320–321. [Google Scholar] [CrossRef]
- Schmidt, H.; Schwarz, H.P. Serum concentrations of LH and FSH in the healthy newborn. Eur. J. Endocrinol. 2000, 143, 213–215. [Google Scholar] [CrossRef] [Green Version]
- Bidlingmaier, F.; Strom, T.M.; Dörr, H.G.; Eisenmenger, W.; Knorr, D. Estrone and estradiol concentrations in human ovaries, testes, and adrenals during the first two years of life. J. Clin. Endocrinol. Metab. 1987, 65, 862–867. [Google Scholar] [CrossRef] [Green Version]
- Dullaart, J.; Kent, J.; Ryle, M. Serum gonadotrophin concentrations in infantile female mice. J. Reprod. Fertil. 1975, 43, 189–192. [Google Scholar] [CrossRef] [Green Version]
- Michael, S.D.; Kaplan, S.B.; Macmillan, B.T. Peripheral plasma concentrations of LH, FSH, prolactin and GH from birth to puberty in male and female mice. J. Reprod. Fertil. 1980, 59, 217–222. [Google Scholar] [CrossRef] [Green Version]
- Torres-Rovira, L.; Succu, S.; Pasciu, V.; Manca, M.E.; Gonzalez-Bulnes, A.; Leoni, G.G.; Pennino, M.G.; Spezzigu, A.; Gallus, M.; Dattena, M.; et al. Postnatal pituitary and follicular activation: A revisited hypothesis in a sheep model. Reprod. Camb. Engl. 2016, 151, 215–225. [Google Scholar] [CrossRef] [Green Version]
- Rawlings, N.C.; Evans, A.C.O.; Honaramooz, A.; Bartlewski, P.M. Antral follicle growth and endocrine changes in prepubertal cattle, sheep and goats. Anim. Reprod. Sci. 2003, 78, 259–270. [Google Scholar] [CrossRef]
- Fuller, G.B.; Faiman, C.; Winter, J.S.; Reyes, F.I.; Hobson, W.C. Sex-dependent gonadotropin concentrations in infant chimpanzees and rhesus monkeys. Proc. Soc. Exp. Biol. Med. 1982, 169, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Aubert, M.L.; Begeot, M.; Winiger, B.P.; Morel, G.; Sizonenko, P.C.; Dubois, P.M. Ontogeny of hypothalamic luteinizing hormone-releasing hormone (GnRH) and pituitary GnRH receptors in fetal and neonatal rats. Endocrinology 1985, 116, 1565–1576. [Google Scholar] [CrossRef] [PubMed]
- Bugnon, C.; Bloch, B.; Lenys, D.; Fellmann, D. Ultrastructural study of the LH-RH containing neurons in the human fetus. Brain Res. 1977, 137, 175–180. [Google Scholar] [CrossRef]
- Paulin, C.; Dubois, M.P.; Barry, J.; Dubois, P.M. Immunofluorescence study of LH-RH producing cells in the human fetal hypothalamus. Cell Tissue Res. 1977, 182, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Groom, G.V.; Boyns, A.R. Effect of hypothalamic releasing factors and steroids on release of gonadotrophins by organ cultures of human foetal pituitaries. J. Endocrinol. 1973, 59, 511–522. [Google Scholar] [CrossRef]
- Bakker, J.; De Mees, C.; Douhard, Q.; Balthazart, J.; Gabant, P.; Szpirer, J.; Szpirer, C. Alpha-fetoprotein protects the developing female mouse brain from masculinization and defeminization by estrogens. Nat. Neurosci. 2006, 9, 220–226. [Google Scholar] [CrossRef]
- Kuiri-Hänninen, T.; Kallio, S.; Seuri, R.; Tyrväinen, E.; Liakka, A.; Tapanainen, J.; Sankilampi, U.; Dunkel, L. Postnatal developmental changes in the pituitary-ovarian axis in preterm and term infant girls. J. Clin. Endocrinol. Metab. 2011, 96, 3432–3439. [Google Scholar] [CrossRef] [Green Version]
- Dulka, E.A.; Moenter, S.M. Prepubertal Development of Gonadotropin-Releasing Hormone Neuron Activity Is Altered by Sex, Age, and Prenatal Androgen Exposure. Endocrinology 2017, 158, 3943–3953. [Google Scholar] [CrossRef] [Green Version]
- Prévot, V. Puberty in Mice and Rats. In Knobil and Neill’s Physiology of Reproduction, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1395–1439. [Google Scholar]
- Garrel, G.; Racine, C.; L’Hôte, D.; Denoyelle, C.; Guigon, C.J.; di Clemente, N.; Cohen-Tannoudji, J. Anti-Müllerian hormone: A new actor of sexual dimorphism in pituitary gonadotrope activity before puberty. Sci. Rep. 2016, 6, 23790. [Google Scholar] [CrossRef] [Green Version]
- Hau, J.; Svendsen, P.; Teisner, B.; Pederson, G.T.; Kristiansen, B. Correlation between fetal weight and maternal serum levels of murine alpha-fetoprotein and quantitation of four molecular forms. Biol. Reprod. 1981, 24, 683–689. [Google Scholar] [CrossRef]
- Belayew, A.; Tilghman, S.M. Genetic analysis of alpha-fetoprotein synthesis in mice. Mol. Cell. Biol. 1982, 2, 1427–1435. [Google Scholar] [CrossRef]
- Ojeda, S.R.; Ramirez, V.D. Short-term steroid treatment on plasma LH and FSH in castrated rats from birth to puberty. Neuroendocrinology 1973, 13, 100–114. [Google Scholar] [CrossRef]
- Meijs-Roelofs, H.M.; Kramer, P. Maturation of the inhibitory feedback action of oestrogen on follicle-stimulating hormone secretion in the immature female rat: A role for alpha-foetoprotein. J. Endocrinol. 1979, 81, 199–208. [Google Scholar] [CrossRef]
- Plant, T.M. A striking sex difference in the gonadotropin response to gonadectomy during infantile development in the rhesus monkey (Macaca mulatta). Endocrinology 1986, 119, 539–545. [Google Scholar] [CrossRef]
- Kuiri-Hänninen, T.; Dunkel, L.; Sankilampi, U. Sexual dimorphism in postnatal gonadotrophin levels in infancy reflects diverse maturation of the ovarian and testicular hormone synthesis. Clin. Endocrinol. 2018, 89, 85–92. [Google Scholar] [CrossRef] [Green Version]
- Sawyer, H.R.; Smith, P.; Heath, D.A.; Juengel, J.L.; Wakefield, S.J.; McNatty, K.P. Formation of ovarian follicles during fetal development in sheep. Biol. Reprod. 2002, 66, 1134–1150. [Google Scholar] [CrossRef] [Green Version]
- Hirshfield, A.N.; DeSanti, A.M. Patterns of ovarian cell proliferation in rats during the embryonic period and the first three weeks postpartum. Biol. Reprod. 1995, 53, 1208–1221. [Google Scholar] [CrossRef] [Green Version]
- Mazaud, S.; Guyot, R.; Guigon, C.J.; Coudouel, N.; Le Magueresse-Battistoni, B.; Magre, S. Basal membrane remodeling during follicle histogenesis in the rat ovary: Contribution of proteinases of the MMP and PA families. Dev. Biol. 2005, 277, 403–416. [Google Scholar] [CrossRef] [Green Version]
- Mork, L.; Maatouk, D.M.; McMahon, J.A.; Guo, J.J.; Zhang, P.; McMahon, A.P.; Capel, B. Temporal differences in granulosa cell specification in the ovary reflect distinct follicle fates in mice. Biol. Reprod. 2012, 86, 37. [Google Scholar] [CrossRef]
- Harikae, K.; Miura, K.; Shinomura, M.; Matoba, S.; Hiramatsu, R.; Tsunekawa, N.; Kanai-Azuma, M.; Kurohmaru, M.; Morohashi, K.-I.; Kanai, Y. Heterogeneity in sexual bipotentiality and plasticity of granulosa cells in developing mouse ovaries. J. Cell Sci. 2013, 126, 2834–2844. [Google Scholar] [CrossRef]
- Hartshorne, G.M.; Lyrakou, S.; Hamoda, H.; Oloto, E.; Ghafari, F. Oogenesis and cell death in human prenatal ovaries: What are the criteria for oocyte selection? Mol. Hum. Reprod. 2009, 15, 805–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGee, E.A.; Hsueh, A.J. Initial and cyclic recruitment of ovarian follicles. Endocr. Rev. 2000, 21, 200–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guigon, C.J.; Mazaud, S.; Forest, M.G.; Brailly-Tabard, S.; Coudouel, N.; Magre, S. Unaltered development of the initial follicular waves and normal pubertal onset in female rats after neonatal deletion of the follicular reserve. Endocrinology 2003, 144, 3651–3662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, W.; Zhang, H.; Gorre, N.; Risal, S.; Shen, Y.; Liu, K. Two classes of ovarian primordial follicles exhibit distinct developmental dynamics and physiological functions. Hum. Mol. Genet. 2014, 23, 920–928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazaud, S.; Guigon, C.J.; Lozach, A.; Coudouel, N.; Forest, M.G.; Coffigny, H.; Magre, S. Establishment of the reproductive function and transient fertility of female rats lacking primordial follicle stock after fetal gamma-irradiation. Endocrinology 2002, 143, 4775–4787. [Google Scholar] [CrossRef] [Green Version]
- White, S.S.; Ojeda, S.R. Changes in ovarian luteinizing hormone and follicle-stimulating hormone receptor content and in gonadotropin-induced ornithine decarboxylase activity during prepubertal and pubertal development of the female rat. Endocrinology 1981, 109, 152–161. [Google Scholar] [CrossRef]
- Camp, T.A.; Rahal, J.O.; Mayo, K.E. Cellular localization and hormonal regulation of follicle-stimulating hormone and luteinizing hormone receptor messenger RNAs in the rat ovary. Mol. Endocrinol. 1991, 5, 1405–1417. [Google Scholar] [CrossRef] [Green Version]
- Fortune, J.E.; Eppig, J.J. Effects of gonadotropins on steroid secretion by infantile and juvenile mouse ovaries in vitro. Endocrinology 1979, 105, 760–768. [Google Scholar] [CrossRef]
- Balla, A.; Danilovich, N.; Yang, Y.; Sairam, M.R. Dynamics of ovarian development in the FORKO immature mouse: Structural and functional implications for ovarian reserve. Biol. Reprod. 2003, 69, 1281–1293. [Google Scholar] [CrossRef]
- Halpin, D.M.; Jones, A.; Fink, G.; Charlton, H.M. Postnatal ovarian follicle development in hypogonadal (hpg) and normal mice and associated changes in the hypothalamic-pituitary ovarian axis. J. Reprod. Fertil. 1986, 77, 287–296. [Google Scholar] [CrossRef]
- Richards, J.S.; Fitzpatrick, S.L.; Clemens, J.W.; Morris, J.K.; Alliston, T.; Sirois, J. Ovarian cell differentiation: A cascade of multiple hormones, cellular signals, and regulated genes. Recent Prog. Horm. Res. 1995, 50, 223–254. [Google Scholar] [CrossRef]
- Hardy, K.; Fenwick, M.; Mora, J.; Laird, M.; Thomson, K.; Franks, S. Onset and Heterogeneity of Responsiveness to FSH in Mouse Preantral Follicles in Culture. Endocrinology 2017, 158, 134–147. [Google Scholar] [CrossRef] [Green Version]
- di Clemente, N.; Racine, C.; Pierre, A.; Taieb, J. Anti-Müllerian Hormone in Female Reproduction. Endocr. Rev. 2021, 42, 753–782. [Google Scholar] [CrossRef]
- Baarends, W.M.; Uilenbroek, J.T.; Kramer, P.; Hoogerbrugge, J.W.; van Leeuwen, E.C.; Themmen, A.P.; Grootegoed, J.A. Anti-müllerian hormone and anti-müllerian hormone type II receptor messenger ribonucleic acid expression in rat ovaries during postnatal development, the estrous cycle, and gonadotropin-induced follicle growth. Endocrinology 1995, 136, 4951–4962. [Google Scholar] [CrossRef]
- Devillers, M.M.; Petit, F.; Cluzet, V.; François, C.M.; Giton, F.; Garrel, G.; Cohen-Tannoudji, J.; Guigon, C.J. FSH inhibits AMH to support ovarian estradiol synthesis in infantile mice. J. Endocrinol. 2019, 240, 215–228. [Google Scholar] [CrossRef]
- Durlinger, A.L.; Gruijters, M.J.; Kramer, P.; Karels, B.; Kumar, T.R.; Matzuk, M.M.; Rose, U.M.; de Jong, F.H.; Uilenbroek, J.T.; Grootegoed, J.A.; et al. Anti-Müllerian hormone attenuates the effects of FSH on follicle development in the mouse ovary. Endocrinology 2001, 142, 4891–4899. [Google Scholar] [CrossRef]
- Roy, S.; Gandra, D.; Seger, C.; Biswas, A.; Kushnir, V.A.; Gleicher, N.; Kumar, T.R.; Sen, A. Oocyte-Derived Factors (GDF9 and BMP15) and FSH Regulate AMH Expression Via Modulation of H3K27AC in Granulosa Cells. Endocrinology 2018, 159, 3433–3445. [Google Scholar] [CrossRef] [Green Version]
- Cimino, I.; Casoni, F.; Liu, X.; Messina, A.; Parkash, J.; Jamin, S.P.; Catteau-Jonard, S.; Collier, F.; Baroncini, M.; Dewailly, D.; et al. Novel role for anti-Müllerian hormone in the regulation of GnRH neuron excitability and hormone secretion. Nat. Commun. 2016, 7, 10055. [Google Scholar] [CrossRef] [Green Version]
- Lucaccioni, L.; Trevisani, V.; Boncompagni, A.; Marrozzini, L.; Berardi, A.; Iughetti, L. Minipuberty: Looking Back to Understand Moving Forward. Front. Pediatr. 2020, 8, 612235. [Google Scholar] [CrossRef]
- Berger, K.; Eskenazi, B.; Kogut, K.; Parra, K.; Lustig, R.H.; Greenspan, L.C.; Holland, N.; Calafat, A.M.; Ye, X.; Harley, K.G. Association of Prenatal Urinary Concentrations of Phthalates and Bisphenol A and Pubertal Timing in Boys and Girls. Environ. Health Perspect. 2018, 126, 97004. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Ding, G.; Tian, Y.; Zhou, Z.; Wang, X.; Shen, L.; Huang, H. Association between bisphenol a exposure and idiopathic central precocious puberty (ICPP) among school-aged girls in Shanghai, China. Environ. Int. 2018, 115, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Busbee, P.B.; Rouse, M.; Nagarkatti, M.; Nagarkatti, P.S. Use of natural AhR ligands as potential therapeutic modalities against inflammatory disorders. Nutr. Rev. 2013, 71, 353–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baba, T.; Mimura, J.; Nakamura, N.; Harada, N.; Yamamoto, M.; Morohashi, K.-I.; Fujii-Kuriyama, Y. Intrinsic function of the aryl hydrocarbon (dioxin) receptor as a key factor in female reproduction. Mol. Cell. Biol. 2005, 25, 10040–10051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandez-Ochoa, I.; Barnett-Ringgold, K.R.; Dehlinger, S.L.; Gupta, R.K.; Leslie, T.C.; Roby, K.F.; Flaws, J.A. The ability of the aryl hydrocarbon receptor to regulate ovarian follicle growth and estradiol biosynthesis in mice depends on stage of sexual maturity. Biol. Reprod. 2010, 83, 698–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larigot, L.; Juricek, L.; Dairou, J.; Coumoul, X. AhR signaling pathways and regulatory functions. Biochim. Open 2018, 7, 1–9. [Google Scholar] [CrossRef]
- Patel, S.; Zhou, C.; Rattan, S.; Flaws, J.A. Effects of Endocrine-Disrupting Chemicals on the Ovary. Biol. Reprod. 2015, 93, 20. [Google Scholar] [CrossRef]
- Magre, S.; Rebourcet, D.; Ishaq, M.; Wargnier, R.; Debard, C.; Meugnier, E.; Vidal, H.; Cohen-Tannoudji, J.; Le Magueresse-Battistoni, B. Gender differences in transcriptional signature of developing rat testes and ovaries following embryonic exposure to 2,3,7,8-TCDD. PLoS ONE 2012, 7, e40306. [Google Scholar] [CrossRef] [Green Version]
- Devillers, M.M.; Petit, F.; Giton, F.; François, C.M.; Juricek, L.; Coumoul, X.; Magre, S.; Cohen-Tannoudji, J.; Guigon, C.J. Age-dependent vulnerability of the ovary to AhR-mediated TCDD action before puberty: Evidence from mouse models. Chemosphere 2020, 258, 127361. [Google Scholar] [CrossRef]
- Franceschini, I.; Desroziers, E. Development and Aging of the Kisspeptin-GPR54 System in the Mammalian Brain: What are the Impacts on Female Reproductive Function? Front. Endocrinol. 2013, 4, 22. [Google Scholar] [CrossRef] [Green Version]
- Messager, S.; Chatzidaki, E.E.; Ma, D.; Hendrick, A.G.; Zahn, D.; Dixon, J.; Thresher, R.R.; Malinge, I.; Lomet, D.; Carlton, M.B.L.; et al. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc. Natl. Acad. Sci. USA 2005, 102, 1761–1766. [Google Scholar] [CrossRef]
- Clarkson, J.; Herbison, A.E. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology 2006, 147, 5817–5825. [Google Scholar] [CrossRef] [Green Version]
- Mhaouty-Kodja, S.; Naulé, L.; Capela, D. Sexual Behavior: From Hormonal Regulation to Endocrine Disruption. Neuroendocrinology 2018, 107, 400–416. [Google Scholar] [CrossRef]
- Bakker, J.; Honda, S.-I.; Harada, N.; Balthazart, J. The aromatase knock-out mouse provides new evidence that estradiol is required during development in the female for the expression of sociosexual behaviors in adulthood. J. Neurosci. Off. J. Soc. Neurosci. 2002, 22, 9104–9112. [Google Scholar] [CrossRef] [Green Version]
- Bakker, J.; Pierman, S.; González-Martínez, D. Effects of aromatase mutation (ArKO) on the sexual differentiation of kisspeptin neuronal numbers and their activation by same versus opposite sex urinary pheromones. Horm. Behav. 2010, 57, 390–395. [Google Scholar] [CrossRef]
- Brock, O.; Baum, M.J.; Bakker, J. The development of female sexual behavior requires prepubertal estradiol. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 5574–5578. [Google Scholar] [CrossRef] [Green Version]
- Brock, O.; Bakker, J. The two kisspeptin neuronal populations are differentially organized and activated by estradiol in mice. Endocrinology 2013, 154, 2739–2749. [Google Scholar] [CrossRef] [Green Version]
- Clarkson, J. Effects of estradiol on kisspeptin neurons during puberty. Front. Neuroendocrinol. 2013, 34, 120–131. [Google Scholar] [CrossRef]
- Cao, J.; Patisaul, H.B. Sexually dimorphic expression of hypothalamic estrogen receptors α and β and Kiss1 in neonatal male and female rats. J. Comp. Neurol. 2011, 519, 2954–2977. [Google Scholar] [CrossRef] [Green Version]
- Brock, O.; De Mees, C.; Bakker, J. Hypothalamic expression of oestrogen receptor α and androgen receptor is sex-, age- and region-dependent in mice. J. Neuroendocrinol. 2015, 27, 264–276. [Google Scholar] [CrossRef]
- Ikeda, Y.; Kato-Inui, T.; Tagami, A.; Maekawa, M. Expression of progesterone receptor, estrogen receptors α and β, and kisspeptin in the hypothalamus during perinatal development of gonad-lacking steroidogenic factor-1 knockout mice. Brain Res. 2019, 1712, 167–179. [Google Scholar] [CrossRef]
- Naulé, L.; Robert, V.; Parmentier, C.; Martini, M.; Keller, M.; Cohen-Solal, M.; Hardin-Pouzet, H.; Grange-Messent, V.; Franceschini, I.; Mhaouty-Kodja, S. Delayed pubertal onset and prepubertal Kiss1 expression in female mice lacking central oestrogen receptor beta. Hum. Mol. Genet. 2015, 24, 7326–7338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayer, C.; Acosta-Martinez, M.; Dubois, S.L.; Wolfe, A.; Radovick, S.; Boehm, U.; Levine, J.E. Timing and completion of puberty in female mice depend on estrogen receptor alpha-signaling in kisspeptin neurons. Proc. Natl. Acad. Sci. USA 2010, 107, 22693–22698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trouillet, A.-C.; Ducroq, S.; Naulé, L.; Capela, D.; Parmentier, C.; Radovick, S.; Hardin-Pouzet, H.; Mhaouty-Kodja, S. Deletion of neural estrogen receptor alpha induces sex differential effects on reproductive behavior in mice. Commun. Biol. 2022, 5, 383. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, K. In vitro analysis of the hormonal basis for the sexual dimorphism in the embryonic development of the mouse mammary gland. J. Embryol. Exp. Morphol. 1971, 25, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Imagawa, W.; Bandyopadhyay, G.K.; Nandi, S. Regulation of mammary epithelial cell growth in mice and rats. Endocr. Rev. 1990, 11, 494–523. [Google Scholar] [CrossRef]
- Haslam, S.Z. The ontogeny of mouse mammary gland responsiveness to ovarian steroid hormones. Endocrinology 1989, 125, 2766–2772. [Google Scholar] [CrossRef]
- Haslam, S.Z.; Nummy, K.A. The ontogeny and cellular distribution of estrogen receptors in normal mouse mammary gland. J. Steroid Biochem. Mol. Biol. 1992, 42, 589–595. [Google Scholar] [CrossRef]
- Feng, Y.; Manka, D.; Wagner, K.-U.; Khan, S.A. Estrogen receptor-alpha expression in the mammary epithelium is required for ductal and alveolar morphogenesis in mice. Proc. Natl. Acad. Sci. USA 2007, 104, 14718–14723. [Google Scholar] [CrossRef] [Green Version]
- Purup, S.; Sejrsen, K.; Foldager, J.; Akers, R.M. Effect of exogenous bovine growth hormone and ovariectomy on prepubertal mammary growth, serum hormones and acute in-vitro proliferative response of mammary explants from Holstein heifers. J. Endocrinol. 1993, 139, 19–26. [Google Scholar] [CrossRef]
- Dessauge, F.; Finot, L.; Wiart, S.; Aubry, J.M.; Ellis, S.E. Effects of ovariectomy in prepubertal goats. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2009, 60 (Suppl. 3), 127–133. [Google Scholar]
- Yart, L.; Lollivier, V.; Marnet, P.G.; Dessauge, F. Role of ovarian secretions in mammary gland development and function in ruminants. Anim. Int. J. Anim. Biosci. 2014, 8, 72–85. [Google Scholar] [CrossRef]
- Yart, L.; Finot, L.; Marnet, P.-G.; Dessauge, F. Suppression of ovarian secretions before puberty strongly affects mammogenesis in the goat. J. Dairy Res. 2012, 79, 157–167. [Google Scholar] [CrossRef]
- Capuco, A.V.; Ellis, S.; Wood, D.L.; Akers, R.M.; Garrett, W. Postnatal mammary ductal growth: Three-dimensional imaging of cell proliferation, effects of estrogen treatment, and expression of steroid receptors in prepubertal calves. Tissue Cell 2002, 34, 143–154. [Google Scholar] [CrossRef]
- Keeling, J.W.; Ozer, E.; King, G.; Walker, F. Oestrogen receptor alpha in female fetal, infant, and child mammary tissue. J. Pathol. 2000, 191, 449–451. [Google Scholar] [CrossRef]
- Kuiri-Hänninen, T.; Haanpää, M.; Turpeinen, U.; Hämäläinen, E.; Seuri, R.; Tyrväinen, E.; Sankilampi, U.; Dunkel, L. Postnatal ovarian activation has effects in estrogen target tissues in infant girls. J. Clin. Endocrinol. Metab. 2013, 98, 4709–4716. [Google Scholar] [CrossRef]
- Trotter, A.; Maier, L.; Kohn, T.; Böhm, W.; Pohlandt, F. Growth of the uterus and mammary glands and vaginal cytologic features in extremely premature infants with postnatal replacement of estradiol and progesterone. Am. J. Obstet. Gynecol. 2002, 186, 184–188. [Google Scholar] [CrossRef]
- Ogasawara, Y.; Okamoto, S.; Kitamura, Y.; Matsumoto, K. Proliferative pattern of uterine cells from birth to adulthood in intact, neonatally castrated, and/or adrenalectomized mice, assayed by incorporation of [125I] iododeoxyuridine. Endocrinology 1983, 113, 582–587. [Google Scholar] [CrossRef]
- Branham, W.S.; Sheehan, D.M. Ovarian and adrenal contributions to postnatal growth and differentiation of the rat uterus. Biol. Reprod. 1995, 53, 863–872. [Google Scholar] [CrossRef] [Green Version]
- Stewart, C.A.; Fisher, S.J.; Wang, Y.; Stewart, M.D.; Hewitt, S.C.; Rodriguez, K.F.; Korach, K.S.; Behringer, R.R. Uterine gland formation in mice is a continuous process, requiring the ovary after puberty, but not after parturition. Biol. Reprod. 2011, 85, 954–964. [Google Scholar] [CrossRef] [Green Version]
- Korach, K.S.; Horigome, T.; Tomooka, Y.; Yamashita, S.; Newbold, R.R.; McLachlan, J.A. Immunodetection of estrogen receptor in epithelial and stromal tissues of neonatal mouse uterus. Proc. Natl. Acad. Sci. USA 1988, 85, 3334–3337. [Google Scholar] [CrossRef] [Green Version]
- Cooke, P.S.; Buchanan, D.L.; Young, P.; Setiawan, T.; Brody, J.; Korach, K.S.; Taylor, J.; Lubahn, D.B.; Cunha, G.R. Stromal estrogen receptors mediate mitogenic effects of estradiol on uterine epithelium. Proc. Natl. Acad. Sci. USA 1997, 94, 6535–6540. [Google Scholar] [CrossRef] [PubMed]
- Klotz, D.M.; Hewitt, S.C.; Ciana, P.; Raviscioni, M.; Lindzey, J.K.; Foley, J.; Maggi, A.; DiAugustine, R.P.; Korach, K.S. Requirement of Estrogen Receptor-α in Insulin-like Growth Factor-1 (IGF-1)-induced Uterine Responses and in Vivo Evidence for IGF-1/Estrogen Receptor Cross-talk. J. Biol. Chem. 2002, 277, 8531–8537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lubahn, D.B.; Moyer, J.S.; Golding, T.S.; Couse, J.F.; Korach, K.S.; Smithies, O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc. Natl. Acad. Sci. USA 1993, 90, 11162–11166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curtis, S.W.; Clark, J.; Myers, P.; Korach, K.S. Disruption of estrogen signaling does not prevent progesterone action in the estrogen receptor alpha knockout mouse uterus. Proc. Natl. Acad. Sci. USA 1999, 96, 3646–3651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nanjappa, M.K.; Medrano, T.I.; March, A.G.; Cooke, P.S. Neonatal uterine and vaginal cell proliferation and adenogenesis are independent of estrogen receptor 1 (ESR1) in the mouse. Biol. Reprod. 2015, 92, 78. [Google Scholar] [CrossRef]
- Carpenter, K.D.; Gray, C.A.; Bryan, T.M.; Welsh, T.H.; Spencer, T.E. Estrogen and antiestrogen effects on neonatal ovine uterine development. Biol. Reprod. 2003, 69, 708–717. [Google Scholar] [CrossRef] [Green Version]
- Carpenter, K.D.; Hayashi, K.; Spencer, T.E. Ovarian regulation of endometrial gland morphogenesis and activin-follistatin system in the neonatal ovine uterus. Biol. Reprod. 2003, 69, 851–860. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, K.; O’Connell, A.R.; Juengel, J.L.; McNatty, K.P.; Davis, G.H.; Bazer, F.W.; Spencer, T.E. Postnatal uterine development in Inverdale ewe lambs. Reprod. Camb. Engl. 2008, 135, 357–365. [Google Scholar] [CrossRef] [Green Version]
- Brandenberger, A.W.; Tee, M.K.; Lee, J.Y.; Chao, V.; Jaffe, R.B. Tissue distribution of estrogen receptors alpha (ER-alpha) and beta (ER-beta) mRNA in the midgestational human fetus. J. Clin. Endocrinol. Metab. 1997, 82, 3509–3512. [Google Scholar] [CrossRef] [Green Version]
- WHO (World Health Organization). State of the Science of Endocrine Disrupting Chemicals. 2012. Available online: http://www.who.int/ceh/publications/endocrine/en/ (accessed on 1 April 2022).
- Viguié, C.; Mhaouty-Kodja, S.; Habert, R.; Chevrier, C.; Michel, C.; Pasquier, E. Evidence-based adverse outcome pathway approach for the identification of BPA as en endocrine disruptor in relation to its effect on the estrous cycle. Mol. Cell. Endocrinol. 2018, 475, 10–28. [Google Scholar] [CrossRef]
| Species | Ages at Gonadotropin Surge | Ages at Estradiol Surge | Ages at Puberty |
|---|---|---|---|
| Mouse | FSH: 9–17 dpn; LH: 9–14 dpn [29] FSH, LH: 4–18 dpn [37] FSH: 11–16 dpn; LH: 11–14 dpn [38] | 12–14 dpn [29] | 28–45 dpn |
| Rat | FSH: 1–17 dpn; LH: 11–21 dpn [27] FSH: 10–20 dpn; LH: 10–25 dpn [30] | 9–19 dpn [27] 10–20 dpn [30] | 28–35 dpn |
| Sheep | FSH: 2–7 weeks [39] LH: ND | ND | 25–35 weeks |
| Cow | FSH, LH: 2–14 weeks [40] | ND | 6–20 months |
| Chimpanzee | FSH, LH: 1–4 months [33] FSH, LH: 0.1–5 months [41] | ND | 8–12 years |
| Rhesus Monkey | FSH: 0.5–4 months LH: ND | ND | 2.5–3 years |
| Human | FSH: 1–4 years, LH: 1–3 years [33] FSH, LH: 1 year [32] FSH, LH: 0.5–4 years [34] | 6 months [32] 2–3 months [28,31] | 8–14 years |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Devillers, M.M.; Mhaouty-Kodja, S.; Guigon, C.J. Deciphering the Roles & Regulation of Estradiol Signaling during Female Mini-Puberty: Insights from Mouse Models. Int. J. Mol. Sci. 2022, 23, 13695. https://doi.org/10.3390/ijms232213695
Devillers MM, Mhaouty-Kodja S, Guigon CJ. Deciphering the Roles & Regulation of Estradiol Signaling during Female Mini-Puberty: Insights from Mouse Models. International Journal of Molecular Sciences. 2022; 23(22):13695. https://doi.org/10.3390/ijms232213695
Chicago/Turabian StyleDevillers, Marie M., Sakina Mhaouty-Kodja, and Céline J. Guigon. 2022. "Deciphering the Roles & Regulation of Estradiol Signaling during Female Mini-Puberty: Insights from Mouse Models" International Journal of Molecular Sciences 23, no. 22: 13695. https://doi.org/10.3390/ijms232213695
APA StyleDevillers, M. M., Mhaouty-Kodja, S., & Guigon, C. J. (2022). Deciphering the Roles & Regulation of Estradiol Signaling during Female Mini-Puberty: Insights from Mouse Models. International Journal of Molecular Sciences, 23(22), 13695. https://doi.org/10.3390/ijms232213695





