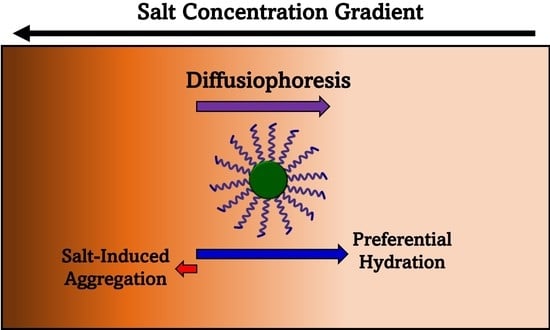
Diffusiophoresis of a Nonionic Micelle in Salt Gradients; Roles of Preferential Hydration and Salt-Induced Surfactant Aggregation
Abstract
:1. Introduction
2. Theoretical Background
3. Results and Discussion
3.1. Phase Diagram
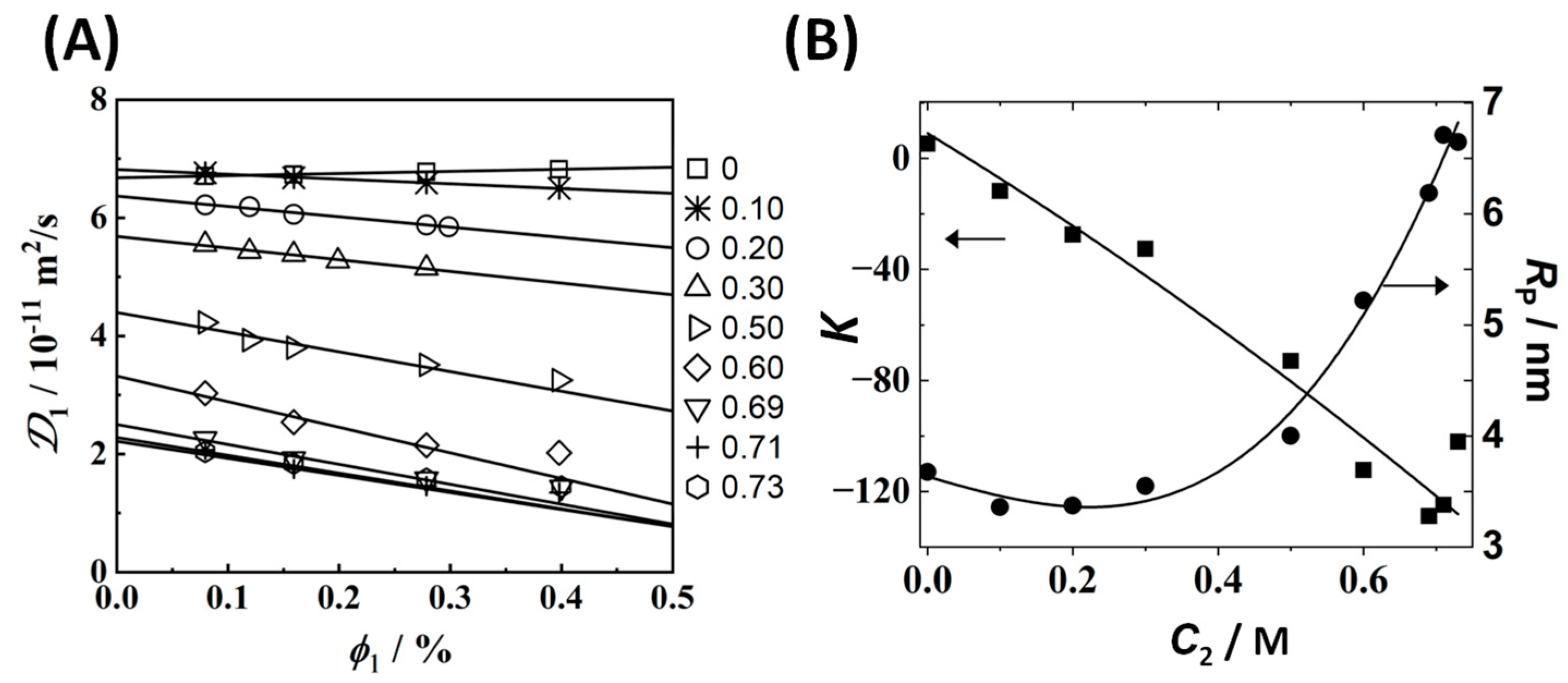
3.2. Multicomponent Diffusion Coefficients
3.3. DLS Diffusion Coefficients
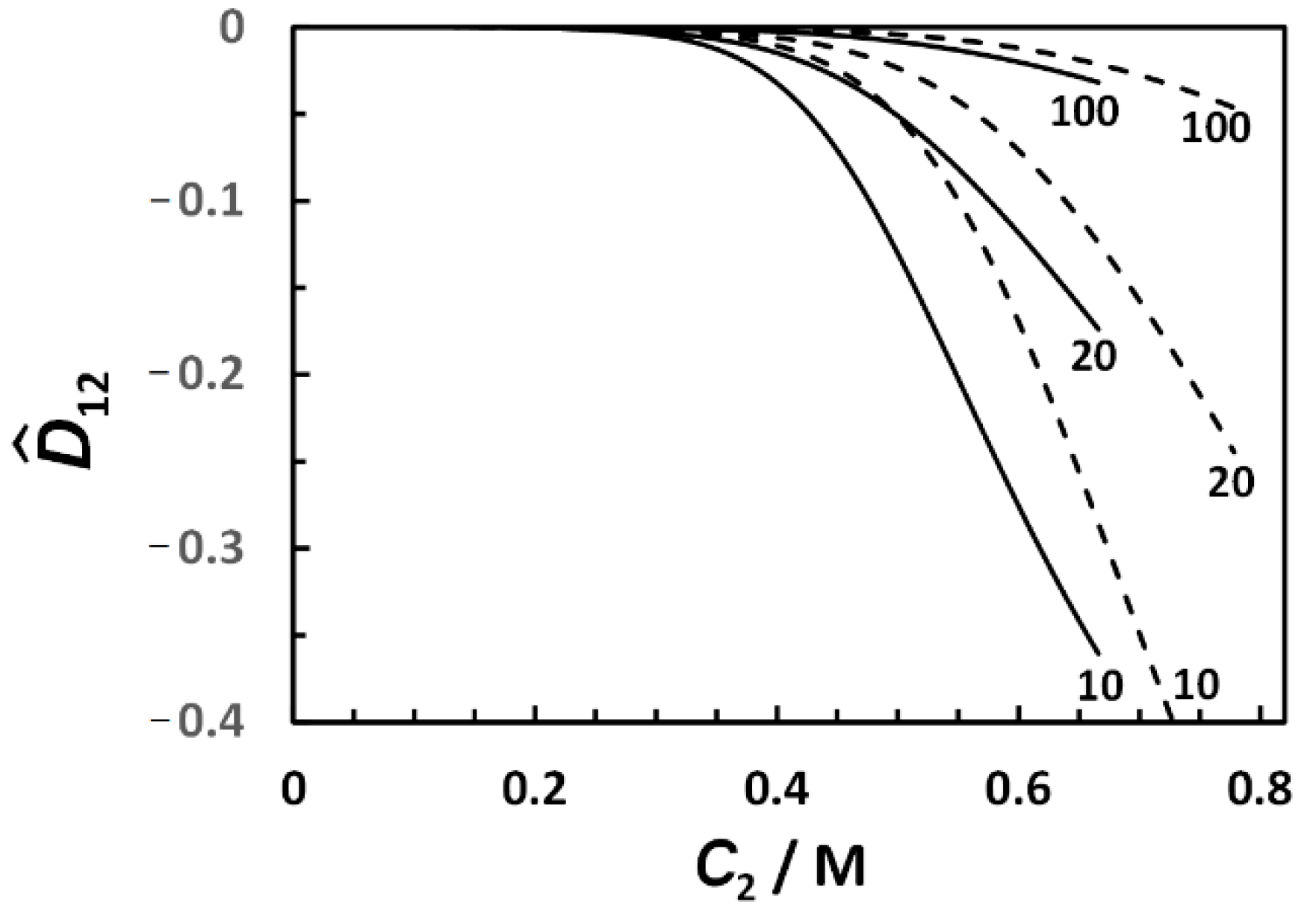
3.4. Micelle Diffusiophoresis and Salt Osmotic Diffusion
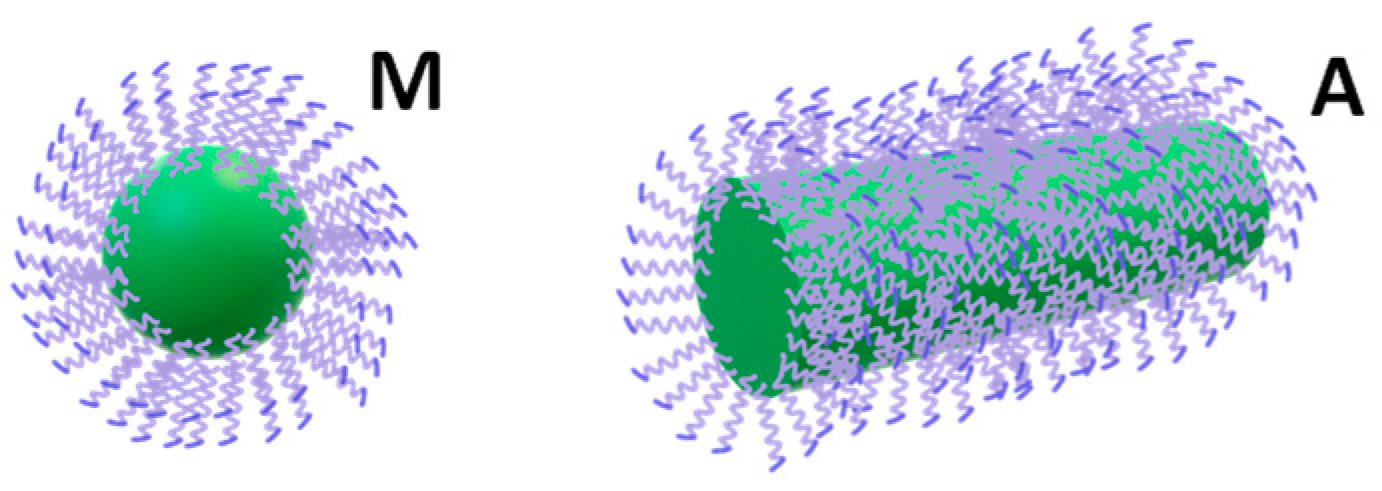
3.5. Role of Salt-Induced Surfactant Aggregation
4. Experimental Section
4.1. Materials
4.2. Rayleigh Interferometry
4.3. Dynamic Light Scattering (DLS)
4.4. Cloud Point Measurements
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Grier, D.G. A revolution in optical manipulation. Nature 2003, 424, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Ha, D.; Seo, S.; Lee, K.; Kim, T. Dynamic transport control of colloidal particles by repeatable active switching of solute gradients. ACS Nano 2019, 13, 12939–12948. [Google Scholar] [CrossRef] [PubMed]
- Frimmel, F.H.; von der Kammer, F.; Flemming, H.C. Colloidal Transport in Porous Media; Springer: Heidelberg, Germany, 2007. [Google Scholar]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Lee, J.; Yoon, H.; Shin, S. Microfluidic investigation of salinity-induced oil recovery in porous media during chemical flooding. Energy Fuels 2021, 35, 4885–4892. [Google Scholar] [CrossRef]
- Shin, S. Diffusiophoretic separation of colloids in microfluidic flows. Phys. Fluids 2020, 32, 15. [Google Scholar] [CrossRef]
- Dey, K.K.; Das, S.; Poyton, M.F.; Sengupta, S.; Butler, P.J.; Cremer, P.S.; Sen, A. Chemotactic separation of enzymes. ACS Nano 2014, 8, 11941–11949. [Google Scholar] [CrossRef] [Green Version]
- Abecassis, B.; Cottin-Bizonne, C.; Ybert, C.; Ajdari, A.; Bocquet, L. Boosting migration of large particles by solute contrasts. Nat. Mater. 2008, 7, 785–789. [Google Scholar] [CrossRef]
- Shin, S.; Shardt, O.; Warren, P.B.; Stone, H.A. Membraneless water filtration using co2. Nat. Commun. 2017, 8, 15181. [Google Scholar] [CrossRef] [Green Version]
- Guha, R.; Shang, X.; Zydney, A.L.; Velegol, D.; Kumar, M. Diffusiophoresis contributes significantly to colloidal fouling in low salinity reverse osmosis systems. J. Membr. Sci. 2015, 479, 67–76. [Google Scholar] [CrossRef]
- Sear, R.P.; Warren, P.B. Diffusiophoresis in nonadsorbing polymer solutions: The asakura-oosawa model and stratification in drying films. Phys. Rev. E 2017, 96, 11. [Google Scholar] [CrossRef]
- Larson, R.G. Transport and deposition patterns in drying sessile droplets. Aiche J. 2014, 60, 1538–1571. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Du, D.J.; Pu, W.F.; Zhang, J.; Fan, X.B. Enhanced oil recovery potential of alkyl alcohol polyoxyethylene ether sulfonate surfactants in high-temperature and high-salinity reservoirs. Energy Fuels 2018, 32, 12128–12140. [Google Scholar] [CrossRef]
- Sinz, D.K.N.; Hanyak, M.; Darhuber, A.A. Self-induced surfactant transport along discontinuous liquid-liquid interfaces. J. Phys. Chem. Lett. 2013, 4, 1039–1043. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Doan, V.S.; Feng, J. Osmotic delivery and release of lipid-encapsulated molecules via sequential solution exchange. Phys. Rev. Appl. 2019, 12, 8. [Google Scholar] [CrossRef]
- Wesselingh, J.A. Controlling diffusion. J. Control. Release 1993, 24, 47–60. [Google Scholar] [CrossRef]
- Zhang, H.X.; Annunziata, O. Modulation of drug transport properties by multicomponent diffusion in surfactant aqueous solutions. Langmuir 2008, 24, 10680–10687. [Google Scholar] [CrossRef]
- Shin, S.; Warren, P.B.; Stone, H.A. Cleaning by surfactant gradients: Particulate removal from porous materials and the significance of rinsing in laundry detergency. Phys. Rev. Appl. 2018, 9, 6. [Google Scholar] [CrossRef] [Green Version]
- Annunziata, O.; Buzatu, D.; Albright, J.G. Protein diffusiophoresis and salt osmotic diffusion in aqueous solutions. J. Phys. Chem. B 2012, 116, 12694–12705. [Google Scholar] [CrossRef]
- Fahim, A.; Annunziata, O. Amplification of salt-induced protein diffusiophoresis by varying salt from potassium to sodium to magnesium chloride in water. Langmuir 2020, 36, 2635–2643. [Google Scholar] [CrossRef]
- McAfee, M.S.; Zhang, H.; Annunziata, O. Amplification of salt-induced polymer diffusiophoresis by increasing salting-out strength. Langmuir 2014, 30, 12210–12219. [Google Scholar] [CrossRef]
- Velegol, D.; Garg, A.; Guha, R.; Kar, A.; Kumar, M. Origins of concentration gradients for diffusiophoresis. Soft Matter 2016, 12, 4686–4703. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.L. Colloid transport by interfacial forces. Annu. Rev. Fluid Mech. 1989, 21, 61–99. [Google Scholar] [CrossRef]
- Prieve, D.C. Migration of a colloidal particle in a gradient of electrolyte concentration. Adv. Colloid Interface Sci. 1982, 16, 321–335. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Wong, T.I.; Liu, X.; Zhou, X.; Liedberg, B. Electrofocusing-enhanced localized surface plasmon resonance biosensors. Nanoscale 2015, 7, 17244–17248. [Google Scholar] [CrossRef]
- Prieve, D.C.; Malone, S.M.; Khair, A.S.; Stout, R.F.; Kanj, M.Y. Diffusiophoresis of charged colloidal particles in the limit of very high salinity. Proc. Natl. Acad. Sci. USA 2019, 116, 18257–18262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kar, A.; Guha, R.; Dani, N.; Velegol, D.; Kumar, M. Particle deposition on microporous membranes can be enhanced or reduced by salt gradients. Langmuir 2014, 30, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Kar, A.; Chiang, T.Y.; Rivera, I.O.; Sen, A.; Velegol, D. Enhanced transport into and out of dead-end pores. ACS Nano 2015, 9, 746–753. [Google Scholar] [CrossRef]
- Leaist, D.G. The role of supporting electrolytes in protein diffusion. J. Phys. Chem. 1989, 93, 474–479. [Google Scholar] [CrossRef]
- Gosting, L.J. Measurement and interpretation of diffusion coefficients of proteins. Adv. Protein Chem. 1956, 11, s0065–s3233. [Google Scholar]
- Albright, J.G.; Annunziata, O.; Miller, D.G.; Paduano, L.; Pearlstein, A.J. Precision measurements of binary and multicomponent diffusion coefficients in protein solutions relevant to crystal growth: Lysozyme chloride in water and aqueous NaCl at ph 4.5 and 25 degrees c-perpendicular to. J. Am. Chem. Soc. 1999, 121, 3256–3266. [Google Scholar] [CrossRef]
- McAfee, M.S.; Annunziata, O. Effect of particle size on salt-induced diffusiophoresis compared to brownian mobility. Langmuir 2014, 30, 4916–4923. [Google Scholar] [CrossRef] [PubMed]
- McAfee, M.S.; Annunziata, O. Effects of salting-in interactions on macromolecule diffusiophoresis and salt osmotic diffusion. Langmuir 2015, 31, 1353–1361. [Google Scholar] [CrossRef] [PubMed]
- Timasheff, S.N. Protein-solvent preferential interactions, protein hydration, and the modulation of biochemical reactions by solvent components. Proc. Natl. Acad. Sci. USA 2002, 99, 9721–9726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arakawa, T.; Timasheff, S.N. Preferential interactions of proteins with salts in concentrated solutions. Biochemistry 1982, 21, 6545–6552. [Google Scholar] [CrossRef] [PubMed]
- Jungwirth, P.; Cremer, P.S. Beyond hofmeister. Nat. Chem. 2014, 6, 261–263. [Google Scholar] [CrossRef]
- Annunziata, O.; Fahim, A. A unified description of macroion diffusiophoresis, salt osmotic diffusion and collective diffusion coefficient. Int. J. Heat Mass Transf. 2020, 163, 11. [Google Scholar] [CrossRef]
- Lightfoot, E.N.; Cussler, E.L.; Rettig, R.L. Applicability of the stefan-maxwell equations to multicomponent diffusion in liquids. AIChE J. 1962, 8, 708–710. [Google Scholar] [CrossRef]
- Krishna, R. Diffusing uphill with james clerk maxwell and josef stefan. Chem. Eng. Sci. 2019, 195, 851–880. [Google Scholar] [CrossRef]
- Lechlitner, L.R.; Annunziata, O. Macromolecule diffusiophoresis induced by concentration gradients of aqueous osmolytes. Langmuir 2018, 34, 9525–9531. [Google Scholar] [CrossRef]
- Cruz Barrios, E.; Krause, T.C.; Annunziata, O. Salt-induced diffusiophoresis of a nonionic micelle: Roles of salting out and proximity to surfactant cloud point. J. Mol. Liq. 2022, 359, 10. [Google Scholar] [CrossRef]
- Leaist, D.G. Diffusion of ionic micelles in salt-solutions–sodium dodecyl-sulfate + sodium-chloride + water. J. Colloid Interface Sci. 1986, 111, 240–249. [Google Scholar] [CrossRef]
- Chelazzi, D.; Giorgi, R.; Baglioni, P. Microemulsions, micelles, and functional gels: How colloids and soft matter preserve works of art. Angew. Chem. Int. Edit. 2018, 57, 7296–7303. [Google Scholar] [CrossRef] [PubMed]
- Patist, A.; Kanicky, J.R.; Shukla, P.K.; Shah, D.O. Importance of micellar kinetics in relation to technological processes. J. Colloid Interface Sci. 2002, 245, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, A.; Shahzad, S.; Munir, A.; Nadagouda, M.N.; Khan, G.S.; Shams, D.F.; Dionysiou, D.D.; Rana, U.A. Micelles as soil and water decontamination agents. Chem. Rev. 2016, 116, 6042–6074. [Google Scholar] [CrossRef]
- La Sorella, G.; Strukul, G.; Scarso, A. Recent advances in catalysis in micellar media. Green Chem. 2015, 17, 644–683. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, E.S.; Yang, J.H.; Cao, Z.Q. Strategies to improve micelle stability for drug delivery. Nano Res. 2018, 11, 4985–4998. [Google Scholar] [CrossRef]
- Mangiapia, G.; D’Errico, G.; Simeone, L.; Irace, C.; Radulescu, A.; Di Pascale, A.; Colonna, A.; Montesarchio, D.; Paduano, L. Ruthenium-based complex nanocarriers for cancer therapy. Biomaterials 2012, 33, 3770–3782. [Google Scholar] [CrossRef]
- Shin, S.; Um, E.; Sabass, B.; Ault, J.T.; Rahimi, M.; Warren, P.B.; Stone, H.A. Size-dependent control of colloid transport via solute gradients in dead-end channels. Proc. Natl. Acad. Sci. USA 2016, 113, 257–261. [Google Scholar] [CrossRef] [Green Version]
- Rard, J.A.; Miller, D.G. Isopiestic determination of the osmotic coefficients of aqueous Na2SO4, MgSO4, and Na2SO4-MgSO4 at 25-degrees-c. J. Chem. Eng. Data 1981, 26, 33–38. [Google Scholar] [CrossRef]
- Rard, J.A.; Miller, D.G. Mutual diffusion-coefficients of na2so4-h2o and mgso4-h2o at 25 c from rayleigh interferometry. J. Solut. Chem. 1979, 8, 755–766. [Google Scholar] [CrossRef]
- Wu, J.Z.; Liu, F.H.; Chen, G.; Wu, X.; Ma, D.S.; Liu, Q.J.; Xu, S.J.; Huang, S.Z.; Chen, T.; Zhang, W.; et al. Effect of ionic strength on the interfacial forces between oil/brine/rock interfaces: A chemical force microscopy study. Energy Fuels 2016, 30, 273–280. [Google Scholar] [CrossRef]
- Strand, S.; Austad, T.; Puntervold, T.; Hognesen, E.J.; Olsen, M.; Barstad, S.M.F. “Smart water” for oil recovery from fractured limestone: A preliminary study. Energy Fuels 2008, 22, 3126–3133. [Google Scholar] [CrossRef] [Green Version]
- Dharaiya, N.; Aswal, V.K.; Bahadur, P. Characterization of triton x-100 and its oligomer (tyloxapol) micelles vis-à-vis solubilization of bisphenol a by spectral and scattering techniques. Colloids Surf. A Physicochem. Eng. Asp. 2015, 470, 230–239. [Google Scholar] [CrossRef]
- Regev, O.; Zana, R. Aggregation behavior of tyloxapol, a nonionic surfactant oligomer, in aqueous solution. J. Colloid Interface Sci. 1999, 210, 8–17. [Google Scholar] [CrossRef]
- Schott, H. Comparing the surface chemical properties and the effect of salts on the cloud point of a conventional nonionic surfactant, octoxynol 9 (triton x-100), and of its oligomer, tyloxapol (triton wr-1339). J. Colloid Interface Sci. 1998, 205, 496–502. [Google Scholar] [CrossRef]
- Barrios, E.C.; Annunziata, O. Determination of critical micelle concentration from the diffusion-driven dilution of micellar aqueous mixtures. Langmuir 2021, 37, 2855–2862. [Google Scholar] [CrossRef]
- Molina-Bolívar, J.A.; Aguiar, J.; Ruiz, C.C. Growth and hydration of triton x-100 micelles in monovalent alkali salts: A light scattering study. J. Phys. Chem. B 2002, 106, 870–877. [Google Scholar] [CrossRef]
- Vergara, A.; Capuano, F.; Paduano, L.; Sartorio, R. Lysozyme mutual diffusion in solutions crowded by poly(ethylene glycol). Macromolecules 2006, 39, 4500–4506. [Google Scholar] [CrossRef]
- Ribeiro, A.C.F.; Gomes, J.C.S.; Santos, C.I.A.V.; Lobo, V.M.M.; Esteso, M.A.; Leaist, D.G. Ternary mutual diffusion coefficients of aqueous nicl2+ nacl and nicl2+ hcl solutions at 298.15 k. J. Chem. Eng. Data 2011, 56, 4696–4699. [Google Scholar] [CrossRef]
- Alexander, N.P.; Phillips, R.J.; Dungan, S.R. Multicomponent diffusion in aqueous solutions of nonionic micelles and decane. Langmuir 2019, 35, 13595–13606. [Google Scholar] [CrossRef]
- Miller, D.G.; Vitagliano, V.; Sartorio, R. Some comments on multicomponent diffusion–negative main term diffusion-coefficients, 2nd law constraints, solvent choices, and reference frame transformations. J. Phys. Chem. 1986, 90, 1509–1519. [Google Scholar] [CrossRef]
- Zhang, H.X.; Annunziata, O. Macromolecular hydration compared with preferential hydration and their role on macromolecule-osmolyte coupled diffusion. Phys. Chem. Chem. Phys. 2009, 11, 8923–8932. [Google Scholar] [CrossRef] [PubMed]
- Pusey, P.N.; Tough, R.J.A. Particle Interactions. In Dynamic Light Scattering. Applications of Photon Correlation Spectroscopy; Plenum Press: New York, NY, USA, 1985. [Google Scholar]
- Rard, J.A.; Clegg, S.L.; Palmer, D.A. Isopiestic determination of the osmotic coefficients of Na2SO4(aq) at 25 and 50c, and representation with ion-interaction (pitzer) and mole fraction thermodynamic models. J. Solut. Chem. 2000, 29, 1–49. [Google Scholar] [CrossRef]
- Anderson, C.F.; Courtenay, E.S.; Record, M.T. Thermodynamic expressions relating different types of preferential interaction coefficients in solutions containing two solute components. J. Phys. Chem. B 2002, 106, 418–433. [Google Scholar] [CrossRef]
- Weckstrom, K.; Papageorgiou, A.C. Lower consolute boundaries of the nonionic surfactant c8e5 in aqueous alkali halide solutions: An approach to reproduce the effects of alkali halides on the cloud-point temperature. J. Colloid Interface Sci. 2007, 310, 151–162. [Google Scholar] [CrossRef] [PubMed]
- McAfee, M.S.; Annunziata, O. Cross-diffusion in a colloid-polymer aqueous system. Fluid Phase Equilib. 2013, 356, 46–55. [Google Scholar] [CrossRef]
- Korosi, A.; Fabuss, B.M. Viscosities of binary aqueous solutions of sodium chloride, potassium chloride, sodium sulfate, and magnesium sulfate at concentrations and temperatures of interest in desalination processes. J. Chem. Eng. Data 2002, 13, 548–552. [Google Scholar] [CrossRef]
- Corti, M.; Degiorgio, V. Micellar properties and critical fluctuations in aqueous solutions of nonionic amphiphiles. J. Phys. Chem. 1981, 85, 1442–1445. [Google Scholar] [CrossRef]
- Annunziata, O.; Buzatu, D.; Albright, J.G. Effect of lysozyme proteins on the mutual-diffusion coefficient of sodium chloride in water. J. Chem. Eng. Data 2011, 56, 4849–4852. [Google Scholar] [CrossRef]
- Onsager, L. Reciprocal relations in irreversible processes. I. Phys. Rev. 1931, 37, 405–426. [Google Scholar] [CrossRef]
- Record, M.T.; Anderson, C.F. Interpretation of preferential interaction coefficients of nonelectrolytes and of electrolyte ions in terms of a two-domain model. Biophys. J. 1995, 68, 786–794. [Google Scholar] [CrossRef]
- Robson, R.J.; Dennis, E.A. The size, shape, and hydration of nonionic surfactant micelles. Triton x-100. J. Phys. Chem. 1976, 81, 1075–1078. [Google Scholar] [CrossRef]
- Perrin, F. Mouvement brownien d’un ellipsoide (ii). Rotation libre et dépolarisation des fluorescences. Translation et diffusion de molécules ellipsoidales. J. Phys. Radium 1936, 7, 1–11. [Google Scholar] [CrossRef]
- Neurath, H. The apparent shape of protein molecules. J. Am. Chem. Soc. 2002, 61, 1841–1844. [Google Scholar] [CrossRef]
- Miller, D.G.; Albright, J.G. Optical Methods. In Measurement of the Transport Properties of Fluids: Experimental Thermodynamics; Wakeham, W.A., Nagashima, A., Sengers, J.V., Eds.; Blackwell Scientific Publications: Oxford, UK, 1991; Volume 3, pp. 272–294. [Google Scholar]
- Annunziata, O.; Buzatu, D.; Albright, J.G. Protein diffusion coefficients determined by macroscopic-gradient rayleigh interferometry and dynamic light scattering. Langmuir 2005, 21, 12085–12089. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.G. A method for obtaining multicomponent diffusion coefficients directly from rayleigh and gouy fringe position data. J. Phys. Chem. 1988, 92, 4222–4226. [Google Scholar] [CrossRef]
- Zhang, H.; Annunziata, O. Effect of macromolecular polydispersity on diffusion coefficients measured by rayleigh interferometry. J. Phys. Chem. B 2008, 112, 3633–3643. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Robinson, J.T.; Sun, X.M.; Dai, H.J. Pegylated nanographene oxide for delivery of water-insoluble cancer drugs. J. Am. Chem. Soc. 2008, 130, 10876–10877. [Google Scholar] [CrossRef] [Green Version]
- Kolate, A.; Baradia, D.; Patil, S.; Vhora, I.; Kore, G.; Misra, A. Peg–a versatile conjugating ligand for drugs and drug delivery systems. J. Control. Release 2014, 192, 67–81. [Google Scholar] [CrossRef]
- Dong, R.H.; Hao, J.C. Complex fluids of poly(oxyethylene) monoalkyl ether nonionic surfactants. Chem. Rev. 2010, 110, 4978–5022. [Google Scholar] [CrossRef]




| C2/M | D11a | D22 | D12/C1 | D21/D22 |
|---|---|---|---|---|
| 0 | 6.96 ± 0.01 b | |||
| 0.100 | 6.33 ± 0.02 | 58.5 ± 0.1 b | 59 ± 1 c | 1.18 ± 0.15 |
| 0.300 | 5.14 ± 0.03 | 48.9 ± 0.1 | 50 ± 1 | 3.09 ± 0.06 |
| 0.500 | 3.51 ± 0.02 | 44.2 ± 0.2 | 54 ± 3 | 5.57 ± 0.06 |
| 0.650 | 2.33 ± 0.01 | 42.2 ± 0.2 | 39 ± 4 | 7.08 ± 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz Barrios, E.; Penino, K.V.; Annunziata, O. Diffusiophoresis of a Nonionic Micelle in Salt Gradients; Roles of Preferential Hydration and Salt-Induced Surfactant Aggregation. Int. J. Mol. Sci. 2022, 23, 13710. https://doi.org/10.3390/ijms232213710
Cruz Barrios E, Penino KV, Annunziata O. Diffusiophoresis of a Nonionic Micelle in Salt Gradients; Roles of Preferential Hydration and Salt-Induced Surfactant Aggregation. International Journal of Molecular Sciences. 2022; 23(22):13710. https://doi.org/10.3390/ijms232213710
Chicago/Turabian StyleCruz Barrios, Eliandreina, Kyra V. Penino, and Onofrio Annunziata. 2022. "Diffusiophoresis of a Nonionic Micelle in Salt Gradients; Roles of Preferential Hydration and Salt-Induced Surfactant Aggregation" International Journal of Molecular Sciences 23, no. 22: 13710. https://doi.org/10.3390/ijms232213710
APA StyleCruz Barrios, E., Penino, K. V., & Annunziata, O. (2022). Diffusiophoresis of a Nonionic Micelle in Salt Gradients; Roles of Preferential Hydration and Salt-Induced Surfactant Aggregation. International Journal of Molecular Sciences, 23(22), 13710. https://doi.org/10.3390/ijms232213710