Heat Shock Transcription Factor 2 Is Significantly Involved in Neurodegenerative Diseases, Inflammatory Bowel Disease, Cancer, Male Infertility, and Fetal Alcohol Spectrum Disorder: The Novel Mechanisms of Several Severe Diseases
Abstract
1. Introduction
1.1. Classical HSFs, HSF1–4
1.2. The Importance of Non-Classical HSFs, HSF5, and HSFY, in Spermatogenesis and Fertility
2. HSF2 Research History in Early Period
2.1. HSF2 Is Activated by Various Stimuli but Not by Typical Heat Shock
2.2. HSF2 Is Involved in HSP70 and Other HSPs Induction
2.3. HSF2 Is Expressed in Embryos and during Their Development
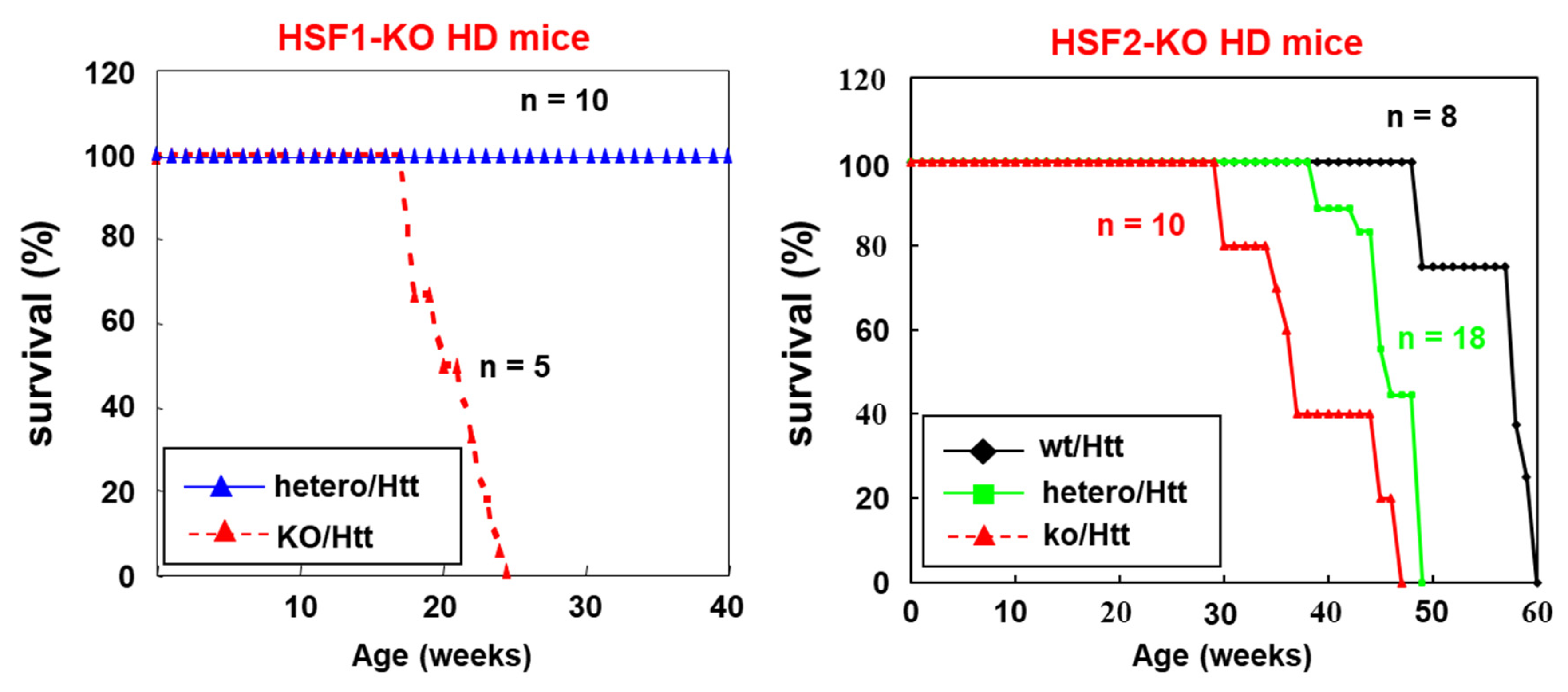
3. HSF2 Is Required for the Suppression of Polyglutamine Diseases Onset and Progression
4. HSF2 Has Essential Protective Roles in Ulcerative Colitis
5. HSF2 Affects Cancer Development and Progression
5.1. Involvement of HSF2 in Cancer Was Discovered Later Than HSF1
5.2. Discovery of the Relationship between HSF2 and Various Cancers
5.2.1. HSF2 Is Constitutively Active in Embryonic Carcinoma Cells
5.2.2. HSF2 in Hepatocellular Carcinoma
5.2.3. HSF2 in Head and Neck Squamous Cell Carcinoma
5.2.4. HSF2 in Breast Cancer
5.2.5. HSF2 in Lung Cancer
5.2.6. HSF2 in Thyroid Cancer
5.2.7. HSF2 in Esophageal Cancer
5.2.8. HSF2 in Prostate Cancer
5.3. Other HSF2-Mediated Novel Mechanisms in Cancer Cells and Possibility to Discover Therapeutic Target
| Type of Cancer | Report | Samples | Reference |
|---|---|---|---|
| Embryonic Carcinoma | HSF2 is constitutively active under 37 degrees condition | cell line | [83] |
| Hepatocellular Carcinoma | HSF2 is a novel target of Wnt/β-catenin/TCF signaling | cell line, xenograft | [88] |
| Hepatocellular Carcinoma | HSF2 regulates aerobic glycolysis through the suppression of FBP1 | cell line | [90] |
| Hepatocellular Carcinoma | HSF2 regulates transcription of DNAJC24 | patients | [94] |
| Head and Neck Squamous Cell Carcinoma | Anti-HSF2 antibodies are detected | patients | [95] |
| Breast Cancer | HSF2 regulates transcription of miR-183/-96/-182 cluster | patients and cell lines | [96] |
| Breast Cancer | HSF2 regulates transcription of ALG3 | patients | [97] |
| Lung Cancer | Expression of HSF2 is significantly upregulated the expression of HSPs | cell line | [57] |
| Thyroid Carcinoma | HSF2 regulates transcription of SERPINA1 and FosB genes | patients | [111] |
| Esophageal Squamous Cell Carcinoma | Expression of HSF2 is down regulated by miR-202 via binding site in the 3′-UTR of HSF2 mRNA | cell line | [124] |
| Prostate Cancer | Homozygous deletion of HSF2 was occurred in 18% of patient samples | patients | [134] |
| Prostatic Adenocarcinoma | mRNA expression of HSF2 in non-invasive cells were higher than invasive cells | cell lines | [134] |
6. Mutation in Human HSF2 Gene Causes Male Infertility
6.1. Discovery of Spermatogenesis Defects in HSF2-Deficient Male Mice
6.2. Discovery of Azoospermic Patients with HSF2 Mutation
7. HSF2 Is an Essential Mediator in Fetal Alcohol Spectrum Disorder (FASD) and a Critical Response Factor to Ethanol-Induced Stress
7.1. Maternal Consumption of Alcohol during Pregnancy Causes Fetal Alcohol Spectrum Disorder (FASD) and Fetal Alcohol Syndrome (FAS)
7.2. HSF2 Is a Master HSF Working during Embryo Growth and Its Deficiency Causes Abnormalities in CNS Development
7.3. HSF2 Is Involved in FASD and Its Deficiency Mitigates the Influence of EtOH Exposure
7.4. HSF2 Is Involved in Synaptic Plasticity Disturbance Caused by EtOH Consumption
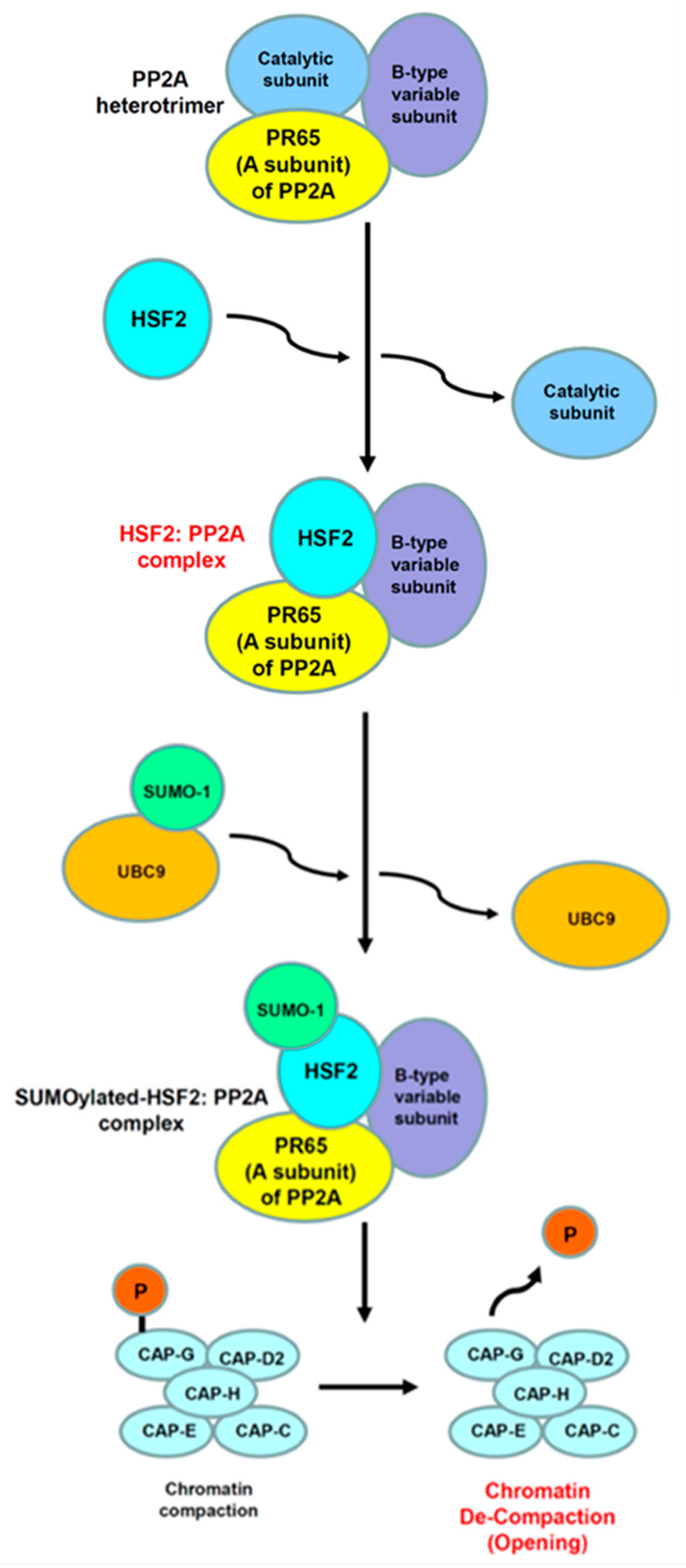
8. Gene Bookmarking Is Indispensable Function of HSF2 in Cell Division, Cell Cycle, and Cell Proliferation
8.1. HSF2 Is the First Transcription Factor Demonstrated Its Gene Bookmarking Function
8.2. HSP70 Gene Bookmarking of HSF2 Is Essential for Cell Survival Immediately after Cell Division
8.3. Now That the Gene Bookmarking Function Is Discovered in Various Transcription Factors
9. Molecular Mechanisms of Transcriptional Activation of HSF2
9.1. HSF2 Interacts with HSF1 and Several HSPs
9.2. HSF2 and HSF1 Form Heterotrimer Complex and Activate Essential Genes in Specific Conditions
9.3. HSF2 Interacts with Set1/MLL Histone H3 Lysine 4 Methyltransferase Complex and Induces Gene Activation
10. Conclusions and Perspectives
10.1. HSF2 Is Involved in Various Important Diseases through Its Functions
10.2. Is Gene Bookmarking the Most Important Function of HSF2?
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saunders, L.R.; Verdin, E. Cell biology. Stress response and aging. Science 2009, 323, 1021–1022. [Google Scholar] [CrossRef]
- Hayashida, N. Set1/MLL complex is indispensable for the transcriptional ability of heat shock transcription factor 2. Biochem. Biophys. Res. Commun. 2015, 467, 805–812. [Google Scholar] [CrossRef]
- Ritossa, F. New puffing pattern induced by temperature shock and DNP in Drosophila. Experientia 1962, 18, 571–573. [Google Scholar] [CrossRef]
- Ritossa, F.M. Behavior of RNA and DNA synthesis at the puff level in salivary gland chromosomes of Drosopila. Exp. Cell Res. 1964, 36, 515–523. [Google Scholar] [CrossRef]
- Ritossa, F. Discovery of the heat shock response. Cell Stress Chaperones 1996, 1, 97–98. [Google Scholar] [CrossRef]
- Tokunaga, Y.; Otsuyama, K.I.; Hayashida, N. Cell Cycle Regulation by Heat Shock Transcription Factors. Cells 2022, 11, 203. [Google Scholar] [CrossRef]
- Tissières, A.; Mitchell, H.K.; Tracy, U.M. Protein synthesis in salivary glands of Drosophila melanogaster: Relation to chromosome puffs. J. Mol. Biol. 1974, 84, 389–398. [Google Scholar] [CrossRef]
- Schedl, P.; Artavanis-Tsakonas, S.; Steward, R.; Gehring, W.J.; Mirault, M.E.; Goldschmidt-Clermont, M.; Moran, L.; Tissières, A. Two hybrid plasmids with D. melanogaster DNA sequences complementary to mRNA coding for the major heat shock protein. Cell 1978, 14, 921–929. [Google Scholar] [CrossRef]
- Wu, C. Two protein-binding sites in chromatin implicated in the activation of heat-shock genes. Nature 1984, 309, 229–234. [Google Scholar] [CrossRef]
- Wu, C. Activating protein factor binds in vitro to upstream control sequences in heat shock gene chromatin. Nature 1984, 311, 81–84. [Google Scholar] [CrossRef]
- Wu, C. An exonuclease protection assay reveals heat-shock element and TATA box DNA-binding proteins in crude nuclear extracts. Nature 1985, 317, 84–87. [Google Scholar] [CrossRef]
- Kingston, R.E.; Schuetz, T.J.; Larin, Z. Heat-inducible human factor that binds to a human hsp70 promoter. Mol. Cell. Biol. 1987, 7, 1530–1534. [Google Scholar]
- Sorger, P.K.; Pelham, H.R. Purification and characterization of a heat-shock element binding protein from yeast. EMBO J. 1987, 6, 3035–3041. [Google Scholar] [CrossRef]
- Sorger, P.K.; Lewis, M.J.; Pelham, H.R. Heat shock factor is regulated differently in yeast and HeLa cells. Nature 1987, 329, 81–84. [Google Scholar] [CrossRef]
- Wiederrecht, G.; Shuey, D.J.; Kibbe, W.A.; Parker, C.S. The Saccharomyces and Drosophila heat shock transcription factors are identical in size and DNA binding properties. Cell 1987, 48, 507–515. [Google Scholar] [CrossRef]
- Rabindran, S.K.; Giorgi, G.; Clos, J.; Wu, C. Molecular cloning and expression of a human heat shock factor, HSF1. Proc. Natl. Acad. Sci. USA 1991, 88, 6906–6910. [Google Scholar] [CrossRef]
- Schuetz, T.J.; Gallo, G.J.; Sheldon, L.; Tempst, P.; Kingston, R.E. Isolation of a cDNA for HSF2: Evidence for two heat shock factor genes in humans. Proc. Natl. Acad. Sci. USA 1991, 88, 6911–6915. [Google Scholar] [CrossRef]
- Sarge, K.D.; Zimarino, V.; Holm, K.; Wu, C.; Morimoto, R.I. Cloning and characterization of two mouse heat shock factors with distinct inducible and constitutive DNA-binding ability. Genes Dev. 1991, 5, 1902–1911. [Google Scholar] [CrossRef]
- Nakai, A.; Tanabe, M.; Kawazoe, Y.; Inazawa, J.; Morimoto, R.I.; Nagata, K. HSF4, a new member of the human heat shock factor family which lacks properties of a transcriptional activator. Mol. Cell. Biol. 1997, 17, 469–481. [Google Scholar] [CrossRef]
- Fujimoto, M.; Hayashida, N.; Katoh, T.; Oshima, K.; Shinkawa, T.; Prakasam, R.; Tan, K.; Inouye, S.; Takii, R.; Nakai, A. A novel mouse HSF3 has the potential to activate nonclassical heat-shock genes during heat shock. Mol. Biol. Cell 2010, 21, 106–116. [Google Scholar] [CrossRef]
- Xiao, X.; Zuo, X.; Davis, A.A.; McMillan, D.R.; Curry, B.B.; Richardson, J.A.; Benjamin, I.J. HSF1 is required for extra-embryonic development, postnatal growth and protection during inflammatory responses in mice. EMBO J. 1999, 18, 5943–5952. [Google Scholar] [CrossRef]
- Christians, E.; Davis, A.A.; Thomas, S.D.; Benjamin, I.J. Maternal effect of Hsf1 on reproductive success. Nature 2000, 407, 693–694. [Google Scholar] [CrossRef]
- Sarge, K.D.; Park-Sarge, O.K.; Kirby, J.D.; Mayo, K.E.; Morimoto, R.I. Expression of heat shock factor 2 in mouse testis: Potential role as a regulator of heat-shock protein gene expression during spermatogenesis. Biol. Reprod. 1994, 50, 1334–1343. [Google Scholar] [CrossRef]
- Rallu, M.; Loones, M.; Lallemand, Y.; Morimoto, R.; Morange, M.; Mezger, V. Function and regulation of heat shock factor 2 during mouse embryogenesis. Proc. Natl. Acad. Sci. USA 1997, 94, 2392–2397. [Google Scholar] [CrossRef]
- Brown, I.R.; Rush, S.J. Cellular localization of the heat shock transcription factors HSF1 and HSF2 in the rat brain during postnatal development and following hyperthermia. Brain Res. 1999, 821, 333–340. [Google Scholar] [CrossRef]
- Eriksson, M.; Jokinen, E.; Sistonen, L.; Leppä, S. Heat shock factor 2 is activated during mouse heart development. Int. J. Dev. Biol. 2000, 44, 471–477. [Google Scholar]
- Kallio, M.; Chang, Y.; Manuel, M.; Alastalo, T.P.; Rallu, M.; Gitton, Y.; Pirkkala, L.; Loones, M.T.; Paslaru, L.; Larney, S.; et al. Brain abnormalities, defective meiotic chromosome synapsis and female subfertility in HSF2 null mice. EMBO J. 2002, 21, 2591–2601. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, J.; Moskophidis, D.; Mivechi, N.F. Targeted disruption of the heat shock transcription factor (hsf)-2 gene results in increased embryonic lethality, neuronal defects, and reduced spermatogenesis. Genesis 2003, 36, 48–61. [Google Scholar] [CrossRef]
- Chang, Y.; Ostling, P.; Akerfelt, M.; Trouillet, D.; Rallu, M.; Gitton, Y.; El Fatimy, R.; Fardeau, V.; Le Crom, S.; Morange, M.; et al. Role of heat-shock factor 2 in cerebral cortex formation and as a regulator of p35 expression. Genes Dev. 2006, 20, 836–847. [Google Scholar] [CrossRef]
- Fujimoto, M.; Izu, H.; Seki, K.; Fukuda, K.; Nishida, T.; Yamada, S.; Kato, K.; Yonemura, S.; Inouye, S.; Nakai, A. HSF4 is required for normal cell growth and differentiation during mouse lens development. EMBO J. 2004, 23, 4297–4306. [Google Scholar] [CrossRef]
- Li, Y.; Chia, J.M.; Bartfai, R.; Christoffels, A.; Yue, G.H.; Ding, K.; Ho, M.Y.; Hill, J.A.; Stupka, E.; Orban, L. Comparative analysis of the testis and ovary transcriptomes in zebrafish by combining experimental and computational tools. Comp. Funct. Genom. 2004, 5, 403–418. [Google Scholar] [CrossRef] [PubMed]
- Saju, J.M.; Hossain, M.S.; Liew, W.C.; Pradhan, A.; Thevasagayam, N.M.; Tan, L.S.E.; Anand, A.; Olsson, P.E.; Orbán, L. Heat shock factor 5 is essential for spermatogenesis in zebrafish. Cell Rep. 2018, 25, 3252–3261.e4. [Google Scholar] [CrossRef] [PubMed]
- Hemati, A.; Modarressi, M.H.; Kolivand, S.; Azarnia, M. Heat shock factor 5 is essential for spermatogenesis in mice: Detected by a new monoclonal antibody. Iran. J. Basic Med. Sci. 2020, 23, 293–297. [Google Scholar] [PubMed]
- Brugh, V.M., 3rd; Lipshultz, L.I. Male factor infertility: Evaluation and management. Med. Clin. N. Am. 2004, 88, 367–385. [Google Scholar] [CrossRef]
- Shinka, T.; Sato, Y.; Chen, G.; Naroda, T.; Kinoshita, K.; Unemi, Y.; Tsuji, K.; Toida, K.; Iwamoto, T.; Nakahori, Y. Molecular characterization of heat shock-like factor encoded on the human Y chromosome, and implications for male infertility. Biol. Reprod. 2004, 71, 297–306. [Google Scholar] [CrossRef]
- Tessari, A.; Salata, E.; Ferlin, A.; Bartoloni, L.; Slongo, M.L.; Foresta, C. Characterization of HSFY, a novel AZFb gene on the Y chromosome with a possible role in human spermatogenesis. Mol. Hum. Reprod. 2004, 10, 253–258. [Google Scholar] [CrossRef]
- Stahl, P.J.; Mielnik, A.N.; Barbieri, C.E.; Schlegel, P.N.; Paduch, D.A. Deletion or underexpression of the Y-chromosome genes CDY2 and HSFY is associated with maturation arrest in American men with nonobstructive azoospermia. Asian J. Androl. 2012, 14, 676–682. [Google Scholar] [CrossRef]
- Jurivich, D.A.; Sistonen, L.; Kroes, R.A.; Morimoto, R.I. Effect of sodium salicylate on the human heat shock response. Science 1992, 255, 1243–1245. [Google Scholar] [CrossRef]
- Sistonen, L.; Sarge, K.D.; Phillips, B.; Abravaya, K.; Morimoto, R.I. Activation of heat shock factor 2 during hemin-induced differentiation of human erythroleukemia cells. Mol. Cell. Biol. 1992, 12, 4104–4111. [Google Scholar]
- Rutherford, T.R.; Clegg, J.B.; Weatherall, D.J. K562 human leukaemic cells synthesise embryonic haemoglobin in response to haemin. Nature 1979, 280, 164–165. [Google Scholar] [CrossRef]
- Rutherford, T.R.; Weatherall, D.J. Deficient heme synthesis as the cause of noninducibility of hemoglobin synthesis in a Friend erythroleukemia cell line. Cell 1979, 16, 415–423. [Google Scholar] [CrossRef]
- Rutherford, T.; Clegg, J.B.; Higgs, D.R.; Jones, R.W.; Thompson, J.; Weatherall, D.J. Embryonic erythroid differentiation in the human leukemic cell line K562. Proc. Natl. Acad. Sci. USA 1981, 78, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Stenzel, K.H.; Rubin, A.L.; Novogrodsky, A. Mitogenic and co-mitogenic properties of hemin. J. Immunol. 1981, 127, 2469–2473. [Google Scholar] [PubMed]
- Singh, M.K.; Yu, J. Accumulation of a heat shock-like protein during differentiation of human erythroid cell line K562. Nature 1984, 309, 631–633. [Google Scholar] [CrossRef]
- Mathew, A.; Mathur, S.K.; Morimoto, R.I. Heat shock response and protein degradation: Regulation of HSF2 by the ubiquitin-proteasome pathway. Mol. Cell. Biol. 1998, 18, 5091–5098. [Google Scholar] [CrossRef] [PubMed]
- Mita, S.; Yasuda, H.; Marunouchi, T.; Ishiko, S.; Yamada, M. A temperature-sensitive mutant of cultured mouse cells defective in chromosome condensation. Exp. Cell Res. 1980, 126, 407–416. [Google Scholar] [CrossRef]
- Sudha, T.; Tsuji, H.; Sameshima, M.; Matsuda, Y.; Kaneda, S.; Nagai, Y.; Yamao, F.; Seno, T. Abnormal integrity of the nucleolus associated with cell cycle arrest owing to the temperature-sensitive ubiquitin-activating enzyme E1. Chromosome Res. 1995, 3, 115–123. [Google Scholar] [CrossRef]
- Iwaki, T.; Iwaki, A.; Miyazono, M.; Goldman, J.E. Preferential expression of αB-crystallin in astrocytic elements of neuroectodermal tumors. Cancer 1991, 68, 2230–2240. [Google Scholar] [CrossRef]
- Iwaki, T.; Iwaki, A.; Fukumaki, Y.; Tateishi, J. Alpha B-crystallin in C6 glioma cells supports their survival in elevated extracellular K+: The implication of a protective role of alpha B-crystallin accumulation in reactive glia. Brain Res. 1995, 673, 47–52. [Google Scholar] [CrossRef]
- Hitotsumatsu, T.; Iwaki, T.; Fukui, M.; Tateishi, J. Distinctive immunohistochemical profiles of small heat shock proteins (Heat shock protein 27 and αB-crystallin) in human brain tumors. Cancer 1996, 77, 352–361. [Google Scholar] [CrossRef]
- Sadamitsu, C.; Nagano, T.; Fukumaki, Y.; Iwaki, A. Heat shock factor 2 is involved in the upregulation of alphaB-crystallin by high extracellular potassium. J. Biochem. 2001, 129, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Iwaki, T.; Iwaki, A.; Tateishi, J.; Sakaki, Y.; Goldman, J.E. Alpha B-crystallin and 27-kd heat shock protein are regulated by stress conditions in the central nervous system and accumulate in Rosenthal fibers. Am. J. Pathol. 1993, 143, 487–495. [Google Scholar] [PubMed]
- Ousman, S.S.; Tomooka, B.H.; van Noort, J.M.; Wawrousek, E.F.; O’Connor, K.C.; Hafler, D.A.; Sobel, R.A.; Robinson, W.H.; Steinman, L. Protective and therapeutic role for alphaB-crystallin in autoimmune demyelination. Nature 2007, 448, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, Q.; Tang, M.; Fu, N.; Shao, W.; Zhang, S.; Yin, Y.; Zeng, R.; Wang, X.; Hu, G.; et al. Upregulation of alphaB-crystallin expression in the substantia nigra of patients with Parkinson’s disease. Neurobiol. Aging 2015, 36, 1686–1691. [Google Scholar] [CrossRef]
- Bartelt-Kirbach, B.; Slowik, A.; Beyer, C.; Golenhofen, N. Upregulation and phosphorylation of HspB1/Hsp25 and HspB5/αB-crystallin after transient middle cerebral artery occlusion in rats. Cell Stress Chaperones 2017, 22, 653–663. [Google Scholar] [CrossRef]
- Sistonen, L.; Sarge, K.D.; Morimoto, R.I. Human heat shock factors 1 and 2 are differentially activated and can synergistically induce hsp70 gene transcription. Mol. Cell. Biol. 1994, 14, 2087–2099. [Google Scholar]
- Zhong, Y.H.; Cheng, H.Z.; Peng, H.; Tang, S.C.; Wang, P. Heat shock factor 2 is associated with the occurrence of lung cancer by enhancing the expression of heat shock proteins. Oncol. Lett. 2016, 12, 5106–5112. [Google Scholar] [CrossRef][Green Version]
- Le Masson, F.; Christians, E. HSFs and regulation of Hsp70.1 (Hspa1b) in oocytes and preimplantation embryos: New insights brought by transgenic and knockout mouse models. Cell Stress Chaperones 2011, 16, 275–285. [Google Scholar] [CrossRef]
- Rimoldi, M.; Servadio, A.; Zimarino, V. Analysis of heat shock transcription factor for suppression of polyglutamine toxicity. Brain Res. Bull. 2001, 56, 353–362. [Google Scholar] [CrossRef]
- Fujimoto, M.; Takaki, E.; Hayashi, T.; Kitaura, Y.; Tanaka, Y.; Inouye, S.; Nakai, A. Active HSF1 significantly suppresses polyglutamine aggregate formation in cellular and mouse models. J. Biol. Chem. 2005, 280, 34908–34916. [Google Scholar] [CrossRef]
- Hayashida, N.; Fujimoto, M.; Tan, K.; Prakasam, R.; Shinkawa, T.; Li, L.; Ichikawa, H.; Takii, R.; Nakai, A. Heat shock factor 1 ameliorates proteotoxicity in cooperation with the transcription factor NFAT. EMBO J. 2010, 29, 3459–3469. [Google Scholar] [CrossRef] [PubMed]
- Shinkawa, T.; Tan, K.; Fujimoto, M.; Hayashida, N.; Yamamoto, K.; Takaki, E.; Takii, R.; Prakasam, R.; Inouye, S.; Mezger, V.; et al. Heat shock factor 2 is required for maintaining proteostasis against febrile-range thermal stress and polyglutamine aggregation. Mol. Biol. Cell 2011, 22, 3571–3583. [Google Scholar] [CrossRef] [PubMed]
- Boelens, W.C. Cell biological roles of αB-crystallin. Prog. Biophys. Mol. Biol. 2014, 115, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, J.; Carver, J.A. The multifaceted nature of αB-crystallin. Cell Stress Chaperones 2020, 25, 639–654. [Google Scholar] [CrossRef]
- Balch, W.E.; Morimoto, R.I.; Dillin, A.; Kelly, J.W. Adapting proteostasis for disease intervention. Science 2008, 319, 916–919. [Google Scholar] [CrossRef]
- Joutsen, J.; Da Silva, A.J.; Luoto, J.C.; Budzynski, M.A.; Nylund, A.S.; de Thonel, A.; Concordet, J.P.; Mezger, V.; Sabéran-Djoneidi, D.; Henriksson, E.; et al. Heat Shock Factor 2 Protects against Proteotoxicity by Maintaining Cell-Cell Adhesion. Cell Rep. 2020, 30, 583–597.e6. [Google Scholar] [CrossRef]
- Kanugovi Vijayavittal, A.; Kumar, P.; Sugunan, S.; Joseph, C.; Devaki, B.; Paithankar, K.; Amere Subbarao, S. Heat shock transcription factor HSF2 modulates the autophagy response through the BTG2-SOD2 axis. Biochem. Biophys. Res. Commun. 2022, 600, 44–50. [Google Scholar] [CrossRef]
- Kawalec, P. Indirect costs of inflammatory bowel diseases: Crohn’s disease and ulcerative colitis. A systematic review. Arch. Med. Sci. 2016, 12, 295–302. [Google Scholar] [CrossRef]
- Seyedian, S.S.; Nokhostin, F.; Malamir, M.D. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J. Med. Life 2019, 12, 113–122. [Google Scholar]
- Wang, K.; Zhang, H.; Kugathasan, S.; Annese, V.; Bradfield, J.P.; Russell, R.K.; Sleiman, P.M.; Imielinski, M.; Glessner, J.; Hou, C.; et al. Diverse genome-wide association studies associate the IL12/IL23 pathway with Crohn Disease. Am. J. Hum. Genet. 2009, 84, 399–405. [Google Scholar] [CrossRef]
- Anderson, C.A.; Boucher, G.; Lees, C.W.; Franke, A.; D’Amato, M.; Taylor, K.D.; Lee, J.C.; Goyette, P.; Imielinski, M.; Latiano, A.; et al. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat. Genet. 2011, 43, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Glas, J.; Stallhofer, J.; Ripke, S.; Wetzke, M.; Pfennig, S.; Klein, W.; Epplen, J.T.; Griga, T.; Schiemann, U.; Lacher, M.; et al. Novel genetic risk markers for ulcerative colitis in the IL2/IL21 region are in epistasis with IL23R and suggest a common genetic background for ulcerative colitis and celiac disease. Am. J. Gastroenterol. 2009, 104, 1737–1744. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Niu, J.; Wang, K.; Xiao, Y.; Du, Y.; Zhou, L.; Duan, L.; Li, S.; Yang, G.; Chen, L.; et al. Heat shock factor 2 levels are associated with the severity of ulcerative colitis. PLoS ONE 2014, 9, e88822. [Google Scholar] [CrossRef]
- Pathirana, W.G.W.; Chubb, S.P.; Gillett, M.J.; Vasikaran, S.D. Faecal Calprotectin. Clin. Biochem. Rev. 2018, 39, 77–90. [Google Scholar]
- Wen, Y.; Niu, J.; Zhang, F.; Wu, J.; Li, M.; Sun, Y.; Wang, W.; Xia, S.; Tan, Y.; Wang, K.; et al. Heat shock transcription factor 2 predicts mucosal healing and promotes mucosal repair of ulcerative colitis. Scand. J. Gastroenterol. 2020, 55, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, F.; Li, X.; Luo, J.; Sun, Y.; Wu, J.; Li, M.; Wen, Y.; Liang, H.; Wang, K.; et al. Heat shock transcription factor 2 inhibits intestinal epithelial cell apoptosis through the mitochondrial pathway in ulcerative colitis. Biochem. Biophys. Res. Commun. 2020, 527, 173–179. [Google Scholar] [CrossRef]
- Lang, B.J.; Guerrero-Giménez, M.E.; Prince, T.L.; Ackerman, A.; Bonorino, C.; Calderwood, S.K. Heat Shock Proteins Are Essential Components in Transformation and Tumor Progression: Cancer Cell Intrinsic Pathways and Beyond. Int. J. Mol. Sci. 2019, 20, 4507. [Google Scholar] [CrossRef]
- Prince, T.L.; Lang, B.J.; Guerrero-Gimenez, M.E.; Fernandez-Muñoz, J.M.; Ackerman, A.; Calderwood, S.K. HSF1: Primary Factor in Molecular Chaperone Expression and a Major Contributor to Cancer Morbidity. Cells 2020, 9, 1046. [Google Scholar] [CrossRef]
- Puustinen, M.C.; Sistonen, L. Molecular Mechanisms of Heat Shock Factors in Cancer. Cells 2020, 9, 1202. [Google Scholar] [CrossRef]
- Hoang, A.T.; Huang, J.; Rudra-Ganguly, N.; Zheng, J.; Powell, W.C.; Rabindran, S.K.; Wu, C.; Roy-Burman, P. A novel association between the human heat shock transcription factor 1 (HSF1) and prostate adenocarcinoma. Am. J. Pathol. 2000, 156, 857–864. [Google Scholar] [CrossRef]
- Wang, Y.; Theriault, J.R.; He, H.; Gong, J.; Calderwood, S.K. Expression of a dominant negative heat shock factor-1 construct inhibits aneuploidy in prostate carcinoma cells. J. Biol. Chem. 2004, 279, 32651–32659. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Whitesell, L.; Rogers, A.B.; Lindquist, S. Heat shock factor 1 is a powerful multifaceted modifier of carcinogenesis. Cell 2007, 130, 1005–1018. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.P.; Gorzowski, J.J.; Sarge, K.D.; Phillips, B. Characterization of constitutive HSF2 DNA-binding activity in mouse embryonal carcinoma cells. Mol. Cell. Biol. 1994, 14, 5309–5317. [Google Scholar] [PubMed]
- Buc-Caron, M.H.; Condamine, H.; Jacob, F. The presence of F9 antigen on the surface of mouse embryonic cells until day 8 of embryogenesis. J. Embryol. Exp. Morphol. 1978, 47, 149–160. [Google Scholar] [CrossRef]
- Sherman, M.I.; Miller, R.A. F9 embryonal carcinoma cells can differentiate into endoderm-like cells. Dev. Biol. 1978, 63, 27–34. [Google Scholar] [CrossRef]
- Rizzino, A.; Crowley, C. Growth and differentiation of embryonal carcinoma cell line F9 in defined media. Proc. Natl. Acad. Sci. USA 1980, 77, 457–461. [Google Scholar] [CrossRef]
- Hayashida, N.; Inouye, S.; Fujimoto, M.; Tanaka, Y.; Izu, H.; Takaki, E.; Ichikawa, H.; Rho, J.; Nakai, A. A novel HSF1-mediated death pathway that is suppressed by heat shock proteins. EMBO J. 2006, 25, 4773–4783. [Google Scholar] [CrossRef]
- Kavak, E.; Najafov, A.; Ozturk, N.; Seker, T.; Cavusoglu, K.; Aslan, T.; Duru, A.D.; Saygili, T.; Hoxhaj, G.; Hiz, M.C.; et al. Analysis of the Wnt/B-catenin/TCF4 pathway using SAGE, genome-wide microarray and promoter analysis: Identification of BRI3 and HSF2 as novel targets. Cell. Signal. 2010, 22, 1523–1535. [Google Scholar] [CrossRef]
- Satoh, S.; Daigo, Y.; Furukawa, Y.; Kato, T.; Miwa, N.; Nishiwaki, T.; Kawasoe, T.; Ishiguro, H.; Fujita, M.; Tokino, T.; et al. AXIN1 mutations in hepatocellular carcinomas, and growth suppression in cancer cells by virus-mediated transfer of AXIN1. Nat. Genet. 2000, 24, 245–250. [Google Scholar] [CrossRef]
- Yang, L.N.; Ning, Z.Y.; Wang, L.; Yan, X.; Meng, Z.Q. HSF2 regulates aerobic glycolysis by suppression of FBP1 in hepatocellular carcinoma. Am. J. Cancer Res. 2019, 9, 1607–1621. [Google Scholar]
- Grasmann, G.; Smolle, E.; Olschewski, H.; Leithner, K. Gluconeogenesis in cancer cells—Repurposing of a starvation-induced metabolic pathway? Biochim. Biophys. Acta Rev. Cancer 2019, 1872, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, X.; Zhang, J.; Lam, E.K.Y.; Shin, V.Y.; Cheng, A.S.L.; Yu, J.; Chan, F.K.L.; Sung, J.J.Y.; Jin, H.C. Warburg effect revisited: An epigenetic link between glycolysis and gastric carcinogenesis. Oncogene 2010, 29, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhu, S.; Yang, H.; Fan, P.; Meng, Z.; Zhao, J.; Zhang, K.; Jin, X. FBP1 binds to the bromodomain of BRD4 to inhibit pancreatic cancer progression. Am. J. Cancer Res. 2020, 10, 523–535. [Google Scholar] [PubMed]
- Li, G.; He, Y.; Liu, H.; Liu, D.; Chen, L.; Luo, Y.; Chen, L.; Qi, L.; Wang, Y.; Wang, Y.; et al. DNAJC24 is a potential therapeutic target in hepatocellular carcinoma through affecting ammonia metabolism. Cell Death Dis. 2022, 13, 490. [Google Scholar] [CrossRef]
- Heubeck, B.; Wendler, O.; Bumm, K.; Schäfer, R.; Müller-Vogt, U.; Häusler, M.; Meese, E.; Iro, H.; Steinhart, H. Tumor-associated antigenic pattern in squamous cell carcinomas of the head and neck—Analysed by SEREX. Eur. J. Cancer 2013, 49, e1–e7. [Google Scholar] [CrossRef]
- Li, P.; Sheng, C.; Huang, L.; Zhang, H.; Huang, L.; Cheng, Z.; Zhu, Q. MiR-183/-96/-182 cluster is up-regulated in most breast cancers and increases cell proliferation and migration. Breast Cancer Res. 2014, 16, 473. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, Y.; Xiong, X.; Huang, M.; Ying, X.; Wang, M. ALG3 Is Activated by Heat Shock Factor 2 and Promotes Breast Cancer Growth. Med. Sci. Monit. 2018, 24, 3479–3487. [Google Scholar] [CrossRef]
- Verostek, M.F.; Atkinson, P.H.; Trimble, R.B. Structure of Saccharomyces cerevisiae alg3, sec18 mutant oligosaccharides. J. Biol. Chem. 1991, 266, 5547–5551. [Google Scholar] [CrossRef]
- Choi, Y.W.; Bae, S.M.; Kim, Y.W.; Lee, H.N.; Kim, Y.W.; Park, T.C.; Ro, D.Y.; Shin, J.C.; Shin, S.J.; Seo, J.S.; et al. Gene expression profiles in squamous cell cervical carcinoma using array-based comparative genomic hybridization analysis. Int. J. Gynecol. Cancer 2007, 17, 687–696. [Google Scholar] [CrossRef]
- Liu, B.; Ma, X.; Liu, Q.; Xiao, Y.; Pan, S.; Jia, L. Aberrant mannosylation profile and FTX/miR-342/ALG3-axis contribute to development of drug resistance in acute myeloid leukemia. Cell Death Dis. 2018, 9, 688. [Google Scholar] [CrossRef]
- Zhou, H.; Cao, T.; Li, W.P.; Wu, G. Combined expression and prognostic significance of PPFIA1 and ALG3 in head and neck squamous cell carcinoma. Mol. Biol. Rep. 2019, 46, 2693–2701. [Google Scholar] [CrossRef] [PubMed]
- Ke, S.B.; Qiu, H.; Chen, J.M.; Shi, W.; Han, C.; Gong, Y.; Chen, Y.S. ALG3 contributes to the malignancy of non-small cell lung cancer and is negatively regulated by MiR-98-5p. Pathol. Res. Pract. 2020, 216, 152761. [Google Scholar] [CrossRef] [PubMed]
- Shao, P.; Wei, C.; Wang, Y. ALG3 contributes to the malignant properties of OSCC cells by regulating CDK-Cyclin pathway. Oral Dis. 2021, 27, 1426–1434. [Google Scholar] [CrossRef] [PubMed]
- Kaigorodova, E.V.; Bogatyuk, M.V. Heat shock proteins as prognostic markers of cancer. Curr. Cancer Drug Targets 2014, 14, 713–726. [Google Scholar] [CrossRef]
- Saini, J.; Sharma, P.K. Clinical, Prognostic and Therapeutic Significance of Heat Shock Proteins in Cancer. Curr. Drug Targets 2018, 19, 1478–1490. [Google Scholar] [CrossRef]
- Mittal, S.; Rajala, M.S. Heat shock proteins as biomarkers of lung cancer. Cancer Biol. Ther. 2020, 21, 477–485. [Google Scholar] [CrossRef]
- Tomasovic, S.P.; Steck, P.A.; Heitzman, D. Heat-stress proteins and thermal resistance in rat mammary tumor cells. Radiat. Res. 1983, 95, 399–413. [Google Scholar] [CrossRef]
- Delpino, A.; Falcioni, R.; Ferrini, U. Modulation of heat shock protein synthesis in two human melanoma cell lines. Tumori J. 1984, 70, 393–398. [Google Scholar] [CrossRef]
- Wallin, G.; Brönnegård, M.; Grimelius, L.; McGuire, J.; Tørring, O. Expression of the thyroid hormone receptor, the oncogenes c-myc and H-ras, and the 90 kD heat shock protein in normal, hyperplastic, and neoplastic human thyroid tissue. Thyroid 1992, 2, 307–313. [Google Scholar] [CrossRef]
- Têtu, B.; Lacasse, B.; Bouchard, H.L.; Lagacé, R.; Huot, J.; Landry, J. Prognostic influence of HSP-27 expression in malignant fibrous histiocytoma: A clinicopathological and immunohistochemical study. Cancer Res. 1992, 52, 2325–2328. [Google Scholar]
- Lu, J.C.; Zhang, Y.P. E2F, HSF2, and miR-26 in thyroid carcinoma: Bioinformatic analysis of RNA-sequencing data. Genet. Mol. Res. 2016, 15, 15017576. [Google Scholar]
- Griffith, O.L.; Melck, A.; Jones, S.J.; Wiseman, S.M. Meta-analysis and meta-review of thyroid cancer gene expression profiling studies identifies important diagnostic biomarkers. J. Clin. Oncol. 2006, 24, 5043–5051. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Hu, L.G. Serpin peptidase inhibitor clade A member 1-overexpression in gastric cancer promotes tumor progression in vitro and is associated with poor prognosis. Oncol. Lett. 2020, 20, 278. [Google Scholar] [CrossRef] [PubMed]
- Rabekova, Z.; Frankova, S.; Jirsa, M.; Neroldova, M.; Lunova, M.; Fabian, O.; Kveton, M.; Varys, D.; Chmelova, K.; Adamkova, V.; et al. Alpha-1 Antitrypsin and Hepatocellular Carcinoma in Liver Cirrhosis: SERPINA1 MZ or MS Genotype Carriage Decreases the Risk. Int. J. Mol. Sci. 2021, 22, 10560. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Lu, Y.T.; Yeh, T.S.; Chan, Y.H.; Dash, S.; Yu, J.S. Identification of Fucosylated SERPINA1 as a Novel Plasma Marker for Pancreatic Cancer Using Lectin Affinity Capture Coupled with iTRAQ-Based Quantitative Glycoproteomics. Int. J. Mol. Sci. 2021, 22, 6079. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Zheng, W.; Chen, C.; Sun, S. Searching for essential genes and drug discovery in breast cancer and periodontitis via text mining and bioinformatics analysis. Anticancer Drugs 2021, 32, 1038–1045. [Google Scholar] [CrossRef]
- Schneider, M.A.; Richtmann, S.; Gründing, A.R.; Wrenger, S.; Welte, T.; Meister, M.; Kriegsmann, M.; Winter, H.; Muley, T.; Janciauskiene, S. Transmembrane serine protease 2 is a prognostic factor for lung adenocarcinoma. Int. J. Oncol. 2022, 60, 39. [Google Scholar] [CrossRef]
- Wang, B.; Chao, S.; Guo, B. Integrated weighted gene co-expression network analysis reveals biomarkers associated with prognosis of high-grade serous ovarian cancer. J. Clin. Lab. Anal. 2022, 36, e24165. [Google Scholar] [CrossRef]
- Zhang, Z.; Cui, F.; Zhou, M.; Wu, S.; Zou, Q.; Gao, B. Single-cell RNA Sequencing Analysis Identifies Key Genes in Brain Metastasis from Lung Adenocarcinoma. Curr. Gene Ther. 2021, 21, 338–348. [Google Scholar] [CrossRef]
- Milde-Langosch, K. The Fos family of transcription factors and their role in tumorigenesis. Eur. J. Cancer 2005, 41, 2449–2461. [Google Scholar] [CrossRef]
- Papoudou-Bai, A.; Hatzimichael, E.; Barbouti, A.; Kanavaros, P. Expression patterns of the activator protein-1 (AP-1) family members in lymphoid neoplasms. Clin. Exp. Med. 2017, 17, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Gazon, H.; Barbeau, B.; Mesnard, J.M.; Peloponese, J.M., Jr. Hijacking of the AP-1 Signaling Pathway during Development of ATL. Front. Microbiol. 2018, 8, 2686. [Google Scholar] [CrossRef] [PubMed]
- Holmes, R.S.; Vaughan, T.L. Epidemiology and pathogenesis of esophageal cancer. Semin. Radiat. Oncol. 2007, 17, 2–9. [Google Scholar] [CrossRef]
- Meng, X.; Chen, X.; Lu, P.; Ma, W.; Yue, D.; Song, L.; Fan, Q. miR-202 Promotes Cell Apoptosis in Esophageal Squamous Cell Carcinoma by Targeting HSF2. Oncol. Res. 2017, 25, 215–223. [Google Scholar] [CrossRef]
- Smyth, E.C.; Lagergren, J.; Fitzgerald, R.C.; Lordick, F.; Shah, M.A.; Lagergren, P.; Cunningham, D. Oesophageal cancer. Nat. Rev. Dis. Prim. 2017, 3, 17048. [Google Scholar] [CrossRef]
- Farazi, T.A.; Hoell, J.I.; Morozov, P.; Tuschl, T. MicroRNAs in human cancer. Adv. Exp. Med. Biol. 2013, 774, 1–20. [Google Scholar] [PubMed]
- Meng, X.R.; Lu, P.; Mei, J.Z.; Liu, G.J.; Fan, Q.X. Expression analysis of miRNA and target mRNAs in esophageal cancer. Braz. J. Med. Biol. Res. 2014, 47, 811–817. [Google Scholar] [CrossRef]
- Farhana, L.; Dawson, M.I.; Fontana, J.A. Down regulation of miR-202 modulates Mxd1 and Sin3A repressor complexes to induce apoptosis of pancreatic cancer cells. Cancer Biol. Ther. 2015, 16, 115–124. [Google Scholar] [CrossRef]
- Jiang, J.; Huang, J.; Wang, X.R.; Quan, Y.H. MicroRNA-202 induces cell cycle arrest and apoptosis in lung cancer cells through targeting cyclin D1. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 2278–2284. [Google Scholar]
- Lin, Z.; Song, D.; Wei, H.; Yang, X.; Liu, T.; Yan, W.; Xiao, J. TGF-β1-induced miR-202 mediates drug resistance by inhibiting apoptosis in human osteosarcoma. J. Cancer Res. Clin. Oncol. 2016, 142, 239–246. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, J.; Yang, G.; Li, H.; Guo, X. miR-202 Inhibits Cell Proliferation, Migration, and Invasion by Targeting Epidermal Growth Factor Receptor in Human Bladder Cancer. Oncol. Res. 2018, 26, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, X.Y.; Hu, P.; Ding, Y.S. lncRNA NORAD Contributes to Colorectal Cancer Progression by Inhibition of miR-202-5p. Oncol. Res. 2018, 26, 1411–1418. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Cai, J.; Xie, W.; Luo, H.; Yang, F. miR-202 suppresses prostate cancer growth and metastasis by targeting PIK3CA. Exp. Ther. Med. 2018, 16, 1499–1504. [Google Scholar] [CrossRef] [PubMed]
- Björk, J.K.; Åkerfelt, M.; Joutsen, J.; Puustinen, M.C.; Cheng, F.; Sistonen, L.; Nees, M. Heat-shock factor 2 is a suppressor of prostate cancer invasion. Oncogene 2016, 35, 1770–1784. [Google Scholar] [CrossRef]
- Taylor, B.S.; Schultz, N.; Hieronymus, H.; Gopalan, A.; Xiao, Y.; Carver, B.S.; Arora, V.K.; Kaushik, P.; Cerami, E.; Reva, B.; et al. Integrative genomic profiling of human prostate cancer. Cancer Cell 2010, 18, 11–22. [Google Scholar] [CrossRef]
- Gleason, D.F.; Mellinger, G.T. Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J. Urol. 1974, 111, 58–64. [Google Scholar] [CrossRef]
- Seo, H.R.; Chung, D.Y.; Lee, Y.J.; Lee, D.H.; Kim, J.I.; Bae, S.; Chung, H.Y.; Lee, S.J.; Jeoung, D.; Lee, Y.S. Heat shock protein 25 or inducible heat shock protein 70 activates heat shock factor 1: Dephosphorylation on serine 307 through inhibition of ERK1/2 phosphorylation. J. Biol. Chem. 2006, 281, 17220–17227. [Google Scholar] [CrossRef]
- Morimoto, R.I. Regulation of the heat shock transcriptional response: Cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 1998, 12, 3788–3796. [Google Scholar] [CrossRef]
- Zou, J.; Guo, Y.; Guettouche, T.; Smith, D.F.; Voellmy, R. Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP90 complex) that forms a stress-sensitive complex with HSF1. Cell 1998, 94, 471–480. [Google Scholar] [CrossRef]
- Smith, R.S.; Takagishi, S.R.; Amici, D.R.; Metz, K.; Gayatri, S.; Alasady, M.J.; Wu, Y.; Brockway, S.; Taiberg, S.L.; Khalatyan, N.; et al. HSF2 cooperates with HSF1 to drive a transcriptional program critical for the malignant state. Sci. Adv. 2022, 8, 6526. [Google Scholar] [CrossRef]
- Santopolo, S.; Riccio, A.; Rossi, A.; Santoro, M.G. The proteostasis guardian HSF1 directs the transcription of its paralog and interactor HSF2 during proteasome dysfunction. Cell. Mol. Life Sci. 2021, 78, 1113–1129. [Google Scholar] [CrossRef] [PubMed]
- Roos-Mattjus, P.; Sistonen, L. Interplay between mammalian heat shock factors 1 and 2 in physiology and pathology. FEBS J. 2021. Epub ahead of print. [Google Scholar] [CrossRef]
- Prince, T.; Ackerman, A.; Cavanaugh, A.; Schreiter, B.; Juengst, B.; Andolino, C.; Danella, J.; Chernin, M.; Williams, H. Dual targeting of HSP70 does not induce the heat shock response and synergistically reduces cell viability in muscle invasive bladder cancer. Oncotarget 2018, 9, 32702–32717. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Bose, S.; Chakrabarti, S. Identification of Cross-Pathway Connections via Protein-Protein Interactions Linked to Altered States of Metabolic Enzymes in Cervical Cancer. Front. Med. 2021, 8, 736495. [Google Scholar] [CrossRef]
- Cloutier, J.M.; Turner, J.M. Meiotic sex chromosome inactivation. Curr. Biol. 2010, 20, R962–R963. [Google Scholar] [CrossRef] [PubMed]
- Akerfelt, M.; Vihervaara, A.; Laiho, A.; Conter, A.; Christians, E.S.; Sistonen, L.; Henriksson, E. Heat shock transcription factor 1 localizes to sex chromatin during meiotic repression. J. Biol. Chem. 2010, 285, 34469–34476. [Google Scholar] [CrossRef]
- McMillan, D.R.; Christians, E.; Forster, M.; Xiao, X.; Connell, P.; Plumier, J.C.; Zuo, X.; Richardson, J.; Morgan, S.; Benjamin, I.J. Heat shock transcription factor 2 is not essential for embryonic development, fertility, or adult cognitive and psychomotor function in mice. Mol. Cell. Biol. 2002, 22, 8005–8014. [Google Scholar] [CrossRef]
- Morrison, A.J.; Rush, S.J.; Brown, I.R. Heat shock transcription factors and the hsp70 induction response in brain and kidney of the hyperthermic rat during postnatal development. J. Neurochem. 2000, 75, 363–372. [Google Scholar] [CrossRef]
- Schlegel, P.N. Causes of azoospermia and their management. Reprod. Fertil. Dev. 2004, 16, 561–572. [Google Scholar] [CrossRef]
- Lee, J.Y.; Dada, R.; Sabanegh, E.; Carpi, A.; Agarwal, A. Role of Genetics in Azoospermia. Urology 2011, 77, 598–601. [Google Scholar] [CrossRef]
- Kasak, L.; Laan, M. Monogenic Causes of non-Obstructive Azoospermia: Challenges, Established Knowledge, Limitations and Perspectives. Hum. Genet. 2021, 140, 135–154. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, H.; Xi, Q.; Li, L.; Zhu, H.; Hu, X.; Liu, R. Case report: A non-obstructive azoospermia patient with heat shock factor-2 mutation. Medicine 2020, 99, e21107. [Google Scholar] [CrossRef] [PubMed]
- Akerman, J.P.; Hayon, S.; Coward, R.M. Sperm Extraction in Obstructive Azoospermia: What’s Next? Urol. Clin. N. Am. 2020, 47, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Mou, L.; Wang, Y.; Li, H.; Huang, Y.; Jiang, T.; Huang, W.; Li, Z.; Chen, J.; Xie, J.; Liu, Y.; et al. A dominant-negative mutation of HSF2 associated with idiopathic azoospermia. Hum. Genet. 2013, 132, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Kervern, M.; Silvestre de Ferron, B.; Alaux-Cantin, S.; Fedorenko, O.; Antol, J.; Naassila, M.; Pierrefiche, O. Aberrant NMDA-dependent LTD after perinatal ethanol exposure in young adult rat hippocampus. Hippocampus 2015, 25, 912–923. [Google Scholar] [CrossRef]
- Thompson, B.L.; Levitt, P.; Stanwood, G.D. Prenatal exposure to drugs: Effects on brain development and implications for policy and education. Nat. Rev. Neurosci. 2009, 10, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Heller, M.; Burd, L. Review of ethanol dispersion, distribution, and elimination from the fetal compartment. Birth Defects Res. A Clin. Mol. Teratol. 2014, 100, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Hoyme, H.E.; Kalberg, W.O.; Elliott, A.J.; Blankenship, J.; Buckley, D.; Marais, A.S.; Manning, M.A.; Robinson, L.K.; Adam, M.P.; Abdul-Rahman, O.; et al. Updated Clinical Guidelines for Diagnosing Fetal Alcohol Spectrum Disorders. Pediatrics 2016, 138, e20154256. [Google Scholar] [CrossRef]
- Wozniak, J.R.; Riley, E.P.; Charness, M.E. Clinical presentation, diagnosis, and management of fetal alcohol spectrum disorder. Lancet Neurol. 2019, 18, 760–770. [Google Scholar] [CrossRef]
- Gressens, P.; Mesples, B.; Sahir, N.; Marret, S.; Sola, A. Environmental factors and disturbances of brain development. Semin. Neonatol. 2001, 6, 185–194. [Google Scholar] [CrossRef]
- Clarke, M.E.; Gibbard, W.B. Overview of fetal alcohol spectrum disorders for mental health professionals. Can. Child Adolesc. Psychiatr. Rev. 2003, 12, 57–63. [Google Scholar]
- El Fatimy, R.; Miozzo, F.; Le Mouël, A.; Abane, R.; Schwendimann, L.; Sabéran-Djoneidi, D.; de Thonel, A.; Massaoudi, I.; Paslaru, L.; Hashimoto-Torii, K.; et al. Heat shock factor 2 is a stress-responsive mediator of neuronal migration defects in models of fetal alcohol syndrome. EMBO Mol. Med. 2014, 6, 1043–1061. [Google Scholar] [CrossRef] [PubMed]
- Swayze, V.W., 2nd; Johnson, V.P.; Hanson, J.W.; Piven, J.; Sato, Y.; Giedd, J.N.; Mosnik, D.; Andreasen, N.C. Magnetic resonance imaging of brain anomalies in fetal alcohol syndrome. Pediatrics 1997, 99, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Dunty, W.C., Jr.; Chen, S.Y.; Zucker, R.M.; Dehart, D.B.; Sulik, K.K. Selective vulnerability of embryonic cell populations to ethanol-induced apoptosis: Implications for alcohol-related birth defects and neurodevelopmental disorder. Alcohol. Clin. Exp. Res. 2001, 25, 1523–1535. [Google Scholar] [CrossRef] [PubMed]
- Mezger, V.; Renard, J.P.; Christians, E.; Morange, M. Detection of heat shock element-binding activities by gel shift assay during mouse preimplantation development. Dev. Biol. 1994, 165, 627–638. [Google Scholar] [CrossRef]
- Mezger, V.; Rallu, M.; Morimoto, R.I.; Morange, M.; Renard, J.P. Heat shock factor 2-like activity in mouse blastocysts. Dev. Biol. 1994, 166, 819–822. [Google Scholar] [CrossRef]
- Ayala, R.; Shu, T.; Tsai, L.H. Trekking across the brain: The journey of neuronal migration. Cell 2007, 128, 29–43. [Google Scholar] [CrossRef]
- Deuel, T.A.; Liu, J.S.; Corbo, J.C.; Yoo, S.Y.; Rorke-Adams, L.B.; Walsh, C.A. Genetic interactions between doublecortin and doublecortin-like kinase in neuronal migration and axon outgrowth. Neuron 2006, 49, 41–53. [Google Scholar] [CrossRef]
- Friocourt, G.; Liu, J.S.; Antypa, M.; Rakic, S.; Walsh, C.A.; Parnavelas, J.G. Both doublecortin and doublecortin-like kinase play a role in cortical interneuron migration. J. Neurosci. 2007, 27, 3875–3883. [Google Scholar] [CrossRef]
- Boekhoorn, K.; Sarabdjitsingh, A.; Kommerie, H.; de Punder, K.; Schouten, T.; Lucassen, P.J.; Vreugdenhil, E. Doublecortin (DCX) and doublecortin-like (DCL) are differentially expressed in the early but not late stages of murine neocortical development. J. Comp. Neurol. 2008, 507, 1639–1652. [Google Scholar] [CrossRef]
- Koizumi, H.; Fujioka, H.; Togashi, K.; Thompson, J.; Yates, J.R., 3rd; Gleeson, J.G.; Emoto, K. DCLK1 phosphorylates the microtubule-associated protein MAP7D1 to promote axon elongation in cortical neurons. Dev. Neurobiol. 2017, 77, 493–510. [Google Scholar] [CrossRef]
- Holmberg, C.I.; Tran, S.E.; Eriksson, J.E.; Sistonen, L. Multisite phosphorylation provides sophisticated regulation of transcription factors. Trends Biochem. Sci. 2002, 27, 619–627. [Google Scholar] [CrossRef]
- Dayalan Naidu, S.; Dinkova-Kostova, A.T. Regulation of the mammalian heat shock factor 1. FEBS J. 2017, 284, 1606–1627. [Google Scholar] [CrossRef] [PubMed]
- Howland, J.G.; Wang, Y.T. Synaptic plasticity in learning and memory: Stress effects in the hippocampus. Prog. Brain Res. 2008, 169, 145–158. [Google Scholar] [PubMed]
- MacDonald, J.F.; Jackson, M.F.; Beazely, M.A. G protein-coupled receptors control NMDARs and metaplasticity in the hippocampus. Biochim. Biophys. Acta 2007, 1768, 941–951. [Google Scholar] [CrossRef]
- Jahr, C.E.; Lester, R.A. Synaptic excitation mediated by glutamate-gated ion channels. Curr. Opin. Neurobiol. 1992, 2, 270–274. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, Y.; Cheng, X.; Chen, X.; Xie, Y.; Zhang, L.; Wang, L.; Hu, J.; Gao, Z. The differences between GluN2A and GluN2B signaling in the brain. J. Neurosci. Res. 2018, 96, 1430–1443. [Google Scholar] [CrossRef] [PubMed]
- Herring, B.E.; Nicoll, R.A. Long-Term Potentiation: From CaMKII to AMPA Receptor Trafficking. Annu. Rev. Physiol. 2016, 78, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Diering, G.H.; Huganir, R.L. The AMPA Receptor Code of Synaptic Plasticity. Neuron 2018, 100, 314–329. [Google Scholar] [CrossRef]
- Drissi, I.; Deschamps, C.; Fouquet, G.; Alary, R.; Peineau, S.; Gosset, P.; Sueur, H.; Marcq, I.; Debuysscher, V.; Naassila, M.; et al. Memory and plasticity impairment after binge drinking in adolescent rat hippocampus: GluN2A/GluN2B NMDA receptor subunits imbalance through HDAC2. Addict. Biol. 2020, 25, e12760. [Google Scholar] [CrossRef]
- Drissi, I.; Deschamps, C.; Alary, R.; Robert, A.; Dubreuil, V.; Le Mouël, A.; Mohammed, M.; Sabéran-Djoneidi, D.; Mezger, V.; Naassila, M.; et al. Role of heat shock transcription factor 2 in the NMDA-dependent neuroplasticity induced by chronic ethanol intake in mouse hippocampus. Addict. Biol. 2021, 26, e12939. [Google Scholar] [CrossRef]
- Xing, H.; Wilkerson, D.C.; Mayhew, C.N.; Lubert, E.J.; Skaggs, H.S.; Goodson, M.L.; Hong, Y.; Park-Sarge, O.K.; Sarge, K.D. Mechanism of hsp70i gene bookmarking. Science 2005, 307, 421–423. [Google Scholar] [CrossRef]
- Lodhi, N.; Ji, Y.; Tulin, A. Mitotic bookmarking: Maintaining post-mitotic reprogramming of transcription reactivation. Curr. Mol. Biol. Rep. 2016, 2, 10–16. [Google Scholar] [CrossRef] [PubMed]
- John, S.; Workman, J.L. Bookmarking genes for activation in condensed mitotic chromosomes. Bioessays 1998, 20, 275–279. [Google Scholar] [CrossRef]
- Follmer, N.E.; Francis, N.J. Speed reading for genes: Bookmarks set the pace. Dev. Cell 2011, 21, 807–808. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kadauke, S.; Udugama, M.I.; Pawlicki, J.M.; Achtman, J.C.; Jain, D.P.; Cheng, Y.; Hardison, R.C.; Blobel, G.A. Tissue-specific mitotic bookmarking by hematopoietic transcription factor GATA1. Cell 2012, 150, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Kadauke, S.; Blobel, G.A. Mitotic bookmarking by transcription factors. Epigenet. Chromatin 2013, 6, 6. [Google Scholar] [CrossRef]
- Caravaca, J.M.; Donahue, G.; Becker, J.S.; He, X.; Vinson, C.; Zaret, K.S. Bookmarking by specific and nonspecific binding of FoxA1 pioneer factor to mitotic chromosomes. Genes Dev. 2013, 27, 251–260. [Google Scholar] [CrossRef]
- Michelotti, E.F.; Sanford, S.; Levens, D. Marking of active genes on mitotic chromosomes. Nature 1997, 388, 895–899. [Google Scholar] [CrossRef]
- Weintraub, H. Assembly of an active chromatin structure during replication. Nucleic Acids Res. 1979, 7, 781–792. [Google Scholar] [CrossRef]
- Struhl, G. A gene product required for correct initiation of segmental determination in Drosophila. Nature 1981, 293, 36–41. [Google Scholar] [CrossRef]
- Groudine, M.; Weintraub, H. Propagation of globin DNAase I-hypersensitive sites in absence of factors required for induction: A possible mechanism for determination. Cell 1982, 30, 131–139. [Google Scholar] [CrossRef]
- Morano, K.A.; Thiele, D.J. Heat shock factor function and regulation in response to cellular stress, growth, and differentiation signals. Gene Expr. 1999, 7, 271–282. [Google Scholar] [PubMed]
- Jolly, C.; Morimoto, R.I. Role of the heat shock response and molecular chaperones in oncogenesis and cell death. J. Natl. Cancer Inst. 2000, 92, 1564–1572. [Google Scholar] [CrossRef] [PubMed]
- Pirkkala, L.; Nykänen, P.; Sistonen, L. Roles of the heat shock transcription factors in regulation of the heat shock response and beyond. FASEB J. 2001, 15, 1118–1131. [Google Scholar] [CrossRef]
- Hong, Y.; Sarge, K.D. Regulation of protein phosphatase 2A activity by heat shock transcription factor 2. J. Biol. Chem. 1999, 274, 12967–12970. [Google Scholar] [CrossRef]
- Hong, Y.; Lubert, E.J.; Rodgers, D.W.; Sarge, K.D. Molecular basis of competition between HSF2 and catalytic subunit for binding to the PR65/A subunit of PP2A. Biochem. Biophys. Res. Commun. 2000, 272, 84–89. [Google Scholar] [CrossRef]
- Lubert, E.J.; Hong, Y.; Sarge, K.D. Interaction between protein phosphatase 5 and the A subunit of protein phosphatase 2A: Evidence for a heterotrimeric form of protein phosphatase 5. J. Biol. Chem. 2001, 276, 38582–38587. [Google Scholar] [CrossRef]
- Goodson, M.L.; Hong, Y.; Rogers, R.; Matunis, M.J.; Park-Sarge, O.K.; Sarge, K.D. Sumo-1 modification regulates the DNA binding activity of heat shock transcription factor 2, a promyelocytic leukemia nuclear body associated transcription factor. J. Biol. Chem. 2001, 276, 18513–18518. [Google Scholar] [CrossRef]
- Smith, B.J.; Yaffe, M.P. A mutation in the yeast heat-shock factor gene causes temperature-sensitive defects in both mitochondrial protein import and the cell cycle. Mol. Cell. Biol. 1991, 11, 2647–2655. [Google Scholar]
- Jedlicka, P.; Mortin, M.A.; Wu, C. Multiple functions of Drosophila heat shock transcription factor in vivo. EMBO J. 1997, 16, 2452–2462. [Google Scholar] [CrossRef]
- Sents, W.; Ivanova, E.; Lambrecht, C.; Haesen, D.; Janssens, V. The biogenesis of active protein phosphatase 2A holoenzymes: A tightly regulated process creating phosphatase specificity. FEBS J. 2013, 280, 644–661. [Google Scholar] [CrossRef]
- Baskaran, R.; Velmurugan, B.K. Protein phosphatase 2A as therapeutic targets in various disease models. Life Sci. 2018, 210, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Onn, I.; Aono, N.; Hirano, M.; Hirano, T. Reconstitution and subunit geometry of human condensin complexes. EMBO J. 2007, 26, 1024–1034. [Google Scholar] [CrossRef] [PubMed]
- Hara, K.; Kinoshita, K.; Migita, T.; Murakami, K.; Shimizu, K.; Takeuchi, K.; Hirano, T.; Hashimoto, H. Structural basis of HEAT-kleisin interactions in the human condensin I subcomplex. EMBO Rep. 2019, 20, e47183. [Google Scholar] [CrossRef]
- Kimura, K.; Hirano, M.; Kobayashi, R.; Hirano, T. Phosphorylation and activation of 13S condensin by Cdc2 in vitro. Science 1998, 282, 487–490. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, I.; Molliex, A.; Navarro, P. Mitotic memories of gene activity. Curr. Opin. Cell Biol. 2021, 69, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Tachmatzidi, E.C.; Galanopoulou, O.; Talianidis, I. Transcription Control of Liver Development. Cells 2021, 10, 2026. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Vanderford, N.L.; Sarge, K.D. The TBP-PP2A mitotic complex bookmarks genes by preventing condensin action. Nat. Cell Biol. 2008, 10, 1318–1323. [Google Scholar] [CrossRef]
- Murphy, L.A.; Wilkerson, D.C.; Hong, Y.; Sarge, K.D. PRC1 associates with the hsp70i promoter and interacts with HSF2 during mitosis. Exp. Cell Res. 2008, 314, 2224–2230. [Google Scholar] [CrossRef][Green Version]
- Karagianni, P.; Moulos, P.; Schmidt, D.; Odom, D.T.; Talianidis, I. Bookmarking by Non-pioneer Transcription Factors during Liver Development Establishes Competence for Future Gene Activation. Cell Rep. 2020, 30, 1319–1328.e6. [Google Scholar] [CrossRef]
- Inukai, S.; Kock, K.H.; Bulyk, M.L. Transcription factor-DNA binding: Beyond binding site motifs. Curr. Opin. Genet. Dev. 2017, 43, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Nunes, S.L.; Calderwood, S.K. Heat shock factor-1 and the heat shock cognate 70 protein associate in high molecular weight complexes in the cytoplasm of NIH-3T3 cells. Biochem. Biophys. Res. Commun. 1995, 213, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, S.; Ali, A.; Ovsenek, N. Multiple components of the HSP90 chaperone complex function in regulation of heat shock factor 1 in vivo. Mol. Cell. Biol. 1999, 19, 8033–8041. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Feldman, R.A.; Radhakrishnan, V.M.; Carey, S.; Martinez, J.D. Hsf1 is required for the nuclear translocation of p53 tumor suppressor. Neoplasia 2008, 10, 1138–1145. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Westerheide, S.D.; Anckar, J.; Stevens, S.M., Jr.; Sistonen, L.; Morimoto, R.I. Stress-inducible regulation of heat shock factor 1 by the deacetylase SIRT1. Science 2009, 323, 1063–1066. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, M.; Takaki, E.; Takii, R.; Tan, K.; Prakasam, R.; Hayashida, N.; Iemura, S.; Natsume, T.; Nakai, A. RPA assists HSF1 access to nucleosomal DNA by recruiting histone chaperone FACT. Mol. Cell 2012, 48, 182–194. [Google Scholar] [CrossRef]
- Tan, K.; Fujimoto, M.; Takii, R.; Takaki, E.; Hayashida, N.; Nakai, A. Mitochondrial SSBP1 protects cells from proteotoxic stresses by potentiating stress-induced HSF1 transcriptional activity. Nat. Commun. 2015, 6, 6580. [Google Scholar] [CrossRef]
- Takii, R.; Fujimoto, M.; Matsumoto, M.; Srivastava, P.; Katiyar, A.; Nakayama, K.I.; Nakai, A. The pericentromeric protein shugoshin 2 cooperates with HSF1 in heat shock response and RNA Pol II recruitment. EMBO J. 2019, 38, e102566. [Google Scholar] [CrossRef]
- Yoshima, T.; Yura, T.; Yanagi, H. The trimerization domain of human heat shock factor 2 is able to interact with nucleoporin p62. Biochem. Biophys. Res. Commun. 1997, 240, 228–233. [Google Scholar] [CrossRef]
- Smith, L.M.; Bhattacharya, D.; Williams, D.J.; Dixon, I.; Powell, N.R.; Erkina, T.Y.; Erkine, A.M. High-throughput screening system for inhibitors of human Heat Shock Factor 2. Cell Stress Chaperones 2015, 20, 833–841. [Google Scholar] [CrossRef]
- Ostling, P.; Björk, J.K.; Roos-Mattjus, P.; Mezger, V.; Sistonen, L. Heat shock factor 2 (HSF2) contributes to inducible expression of hsp genes through interplay with HSF1. J. Biol. Chem. 2007, 282, 7077–7086. [Google Scholar] [CrossRef] [PubMed]
- Loison, F.; Debure, L.; Nizard, P.; le Goff, P.; Michel, D.; Le Dréan, Y. Up-regulation of the clusterin gene after proteotoxic stress: Implication of HSF1-HSF2 heterocomplexes. Biochem. J. 2006, 395, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Sandqvist, A.; Björk, J.K.; Akerfelt, M.; Chitikova, Z.; Grichine, A.; Vourc’h, C.; Jolly, C.; Salminen, T.A.; Nymalm, Y.; Sistonen, L. Heterotrimerization of heat-shock factors 1 and 2 provides a transcriptional switch in response to distinct stimuli. Mol. Biol. Cell 2009, 20, 1340–1347. [Google Scholar] [CrossRef] [PubMed]
- Schapira, M.; Tyers, M.; Torrent, M.; Arrowsmith, C.H. WD40 repeat domain proteins: A novel target class? Nat. Rev. Drug Discov. 2017, 16, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Chen, G.; Jin, J.; Zhang, B.; Wang, Y.; Cai, Z.; Ye, F. The Development of Inhibitors Targeting the Mixed Lineage Leukemia 1 (MLL1)-WD Repeat Domain 5 Protein (WDR5) Protein- Protein Interaction. Curr. Med. Chem. 2020, 27, 5530–5542. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tokunaga, Y.; Otsuyama, K.-I.; Kakuta, S.; Hayashida, N. Heat Shock Transcription Factor 2 Is Significantly Involved in Neurodegenerative Diseases, Inflammatory Bowel Disease, Cancer, Male Infertility, and Fetal Alcohol Spectrum Disorder: The Novel Mechanisms of Several Severe Diseases. Int. J. Mol. Sci. 2022, 23, 13763. https://doi.org/10.3390/ijms232213763
Tokunaga Y, Otsuyama K-I, Kakuta S, Hayashida N. Heat Shock Transcription Factor 2 Is Significantly Involved in Neurodegenerative Diseases, Inflammatory Bowel Disease, Cancer, Male Infertility, and Fetal Alcohol Spectrum Disorder: The Novel Mechanisms of Several Severe Diseases. International Journal of Molecular Sciences. 2022; 23(22):13763. https://doi.org/10.3390/ijms232213763
Chicago/Turabian StyleTokunaga, Yasuko, Ken-Ichiro Otsuyama, Shigeru Kakuta, and Naoki Hayashida. 2022. "Heat Shock Transcription Factor 2 Is Significantly Involved in Neurodegenerative Diseases, Inflammatory Bowel Disease, Cancer, Male Infertility, and Fetal Alcohol Spectrum Disorder: The Novel Mechanisms of Several Severe Diseases" International Journal of Molecular Sciences 23, no. 22: 13763. https://doi.org/10.3390/ijms232213763
APA StyleTokunaga, Y., Otsuyama, K.-I., Kakuta, S., & Hayashida, N. (2022). Heat Shock Transcription Factor 2 Is Significantly Involved in Neurodegenerative Diseases, Inflammatory Bowel Disease, Cancer, Male Infertility, and Fetal Alcohol Spectrum Disorder: The Novel Mechanisms of Several Severe Diseases. International Journal of Molecular Sciences, 23(22), 13763. https://doi.org/10.3390/ijms232213763






