Microbiota-Derived Extracellular Vesicles Detected in Human Blood from Healthy Donors
Abstract
:1. Introduction
2. Results
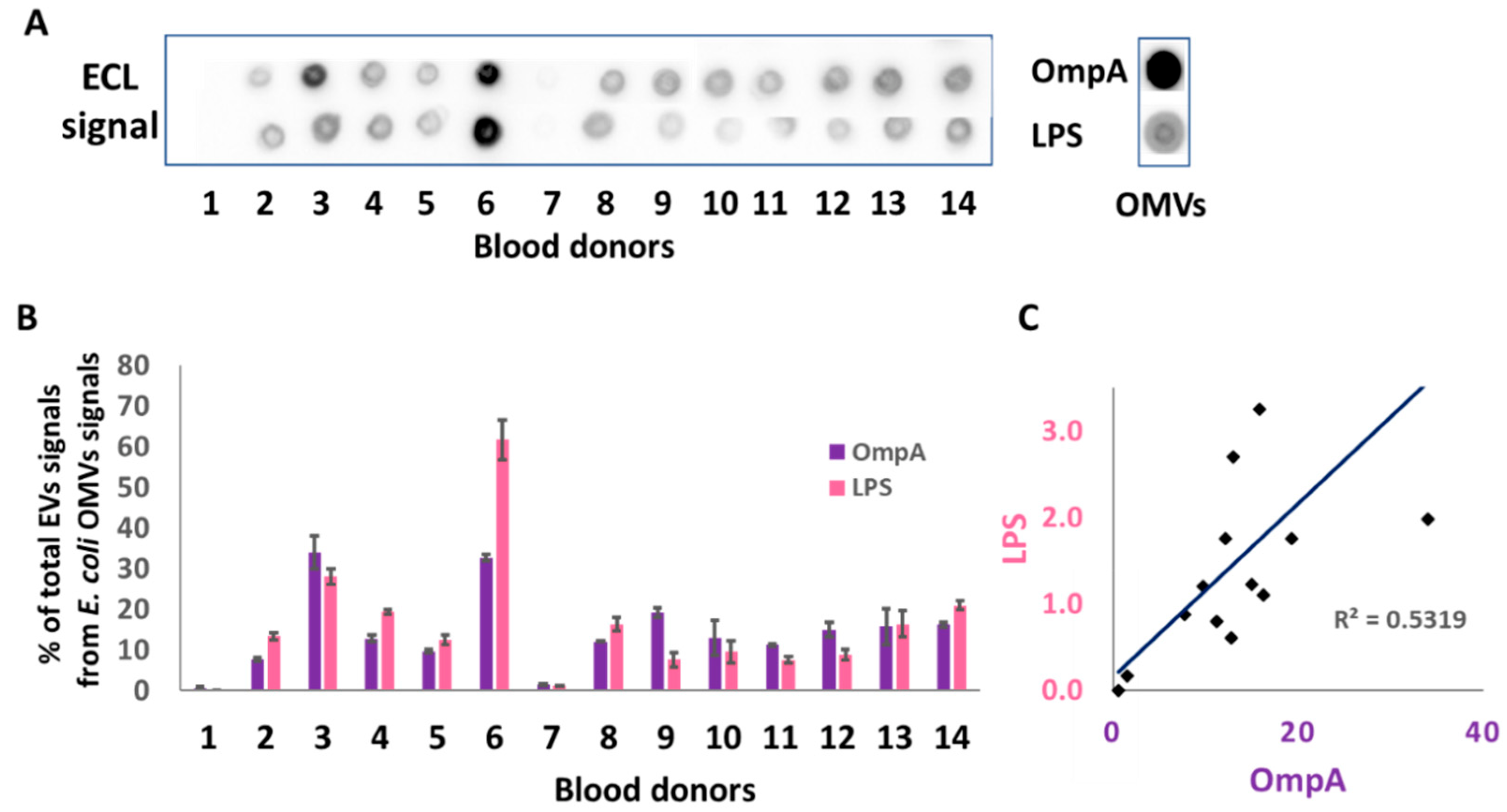
2.1. Ex Vivo Demonstration of the Presence of Enterobacteriaceae OMV Components in Human Red Blood Cell Concentrates
2.2. E. coli Fluorescently Labeled OMVs Are Capable of Fusion with Monocytes from Normal Human Blood
3. Discussion
4. Materials and Methods
4.1. Production of E. coli OMVs
4.2. Preparation of Human Peripheral Blood Mononuclear Cells (PBMCs), Red Blood Cell Concentrates, and Extracellular Vesicles
4.3. Quantification of the Lipids Present in OMVs and EVs by Measurement of the Fluorescence Emitted by Incorporated 1-Anilinonaphthalene-8-Sulfonic Acid (ANS)
4.4. Dot Blots
4.5. Immunoblots
4.6. Fusion Experiments between OMVs and Peripheral Blood Mononuclear Cells
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef] [Green Version]
- Zegarra-Ruiz, D.F.; Kim, D.V.; Norwood, K.; Kim, M.; Wu, W.-J.H.; Saldana-Morales, F.B.; Hill, A.A.; Majumdar, S.; Orozco, S.; Bell, R.; et al. Thymic development of gut-microbiota-specific T cells. Nature 2021, 594, 413–417. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, X.; Wu, Y.; Peng, J.; Wei, H. Effect of the Microbiome on Intestinal Innate Immune Development in Early Life and the Potential Strategy of Early Intervention. Front. Immunol. 2022, 13, 936300. [Google Scholar] [CrossRef]
- Lee, K.-E.; Kim, J.-K.; Han, S.-K.; Lee, D.Y.; Lee, H.-J.; Yim, S.-V.; Kim, D.-H. The extracellular vesicle of gut microbial Paenalcaligenes hominis is a risk factor for vagus nerve-mediated cognitive impairment. Microbiome 2020, 8, 1–18. [Google Scholar] [CrossRef]
- Chronopoulos, A.; Kalluri, R. Emerging role of bacterial extracellular vesicles in cancer. Oncogene 2020, 39, 6951–6960. [Google Scholar] [CrossRef]
- Stentz, R.; Carvalho, A.L.; Jones, E.J.; Carding, S.R. Fantastic voyage: The journey of intestinal microbiota-derived microvesicles through the body. Biochem. Soc. Trans. 2018, 46, 1021–1027. [Google Scholar] [CrossRef] [Green Version]
- Nagakubo, T.; Nomura, N.; Toyofuku, M. Cracking Open Bacterial Membrane Vesicles. Front. Microbiol. 2019, 10, 3026. [Google Scholar] [CrossRef] [Green Version]
- Schwechheimer, C.; Kuehn, M.J. Outer-membrane vesicles from Gram-negative bacteria: Biogenesis and functions. Nat. Rev. Genet. 2015, 13, 605–619. [Google Scholar] [CrossRef] [Green Version]
- Ellis, T.N.; Leiman, S.A.; Kuehn, M.J. Naturally Produced Outer Membrane Vesicles from Pseudomonas aeruginosa Elicit a Potent Innate Immune Response via Combined Sensing of Both Lipopolysaccharide and Protein Components. Infect. Immun. 2010, 78, 3822–3831. [Google Scholar] [CrossRef] [Green Version]
- Badi, S.A.; Moshiri, A.; Fateh, A.; Jamnani, F.R.; Sarshar, M.; Vaziri, F.; Siadat, S.D. Microbiota-Derived Extracellular Vesicles as New Systemic Regulators. Front. Microbiol. 2017, 8, 1610. [Google Scholar] [CrossRef]
- Tulkens, J.; Vergauwen, G.; Van Deun, J.; Geeurickx, E.; Dhondt, B.; Lippens, L.; De Scheerder, M.-A.; Miinalainen, I.; Rappu, P.; De Geest, B.G.; et al. Increased levels of systemic LPS-positive bacterial extracellular vesicles in patients with intestinal barrier dysfunction. Gut 2020, 69, 191–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tulkens, J.; De Wever, O.; Hendrix, A. Analyzing bacterial extracellular vesicles in human body fluids by orthogonal biophysical separation and biochemical characterization. Nat. Protoc. 2020, 15, 40–67. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, C.-S.; Badia, J.; Bosch, M.; Giménez, R.; Baldomà, L. Outer Membrane Vesicles and Soluble Factors Released by Probiotic Escherichia coli Nissle 1917 and Commensal ECOR63 Enhance Barrier Function by Regulating Expression of Tight Junction Proteins in Intestinal Epithelial Cells. Front. Microbiol. 2016, 7, 1981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, E.J.; Booth, C.; Fonseca, S.; Parker, A.; Cross, K.; Miquel-Clopés, A.; Hautefort, I.; Mayer, U.; Wileman, T.; Stentz, R.; et al. The Uptake, Trafficking, and Biodistribution of Bacteroides thetaiotaomicron Generated Outer Membrane Vesicles. Front. Microbiol. 2020, 11, 57. [Google Scholar] [CrossRef] [Green Version]
- An, J.; Kim, S.H.; Hwang, D.; Lee, K.E.; Kim, M.J.; Yang, E.G.; Kim, S.Y.; Chung, H.S. Caspase-4 disaggregates lipopolysaccharide micelles via LPS-CARD interaction. Sci. Rep. 2019, 9, 826. [Google Scholar] [CrossRef] [Green Version]
- Palva, A.M. ompA gene in the detection of Escherichia coli and other Enterobacteriaceae by nucleic acid sandwich hybridization. J. Clin. Microbiol. 1983, 18, 92–100. [Google Scholar] [CrossRef] [Green Version]
- Girard, P.; Ponsard, B.; Charles, J.; Chaperot, L.; Aspord, C. Potent Bidirectional Cross-Talk Between Plasmacytoid Dendritic Cells and γδT Cells Through BTN3A, Type I/II IFNs and Immune Checkpoints. Front. Immunol. 2020, 11, 861. [Google Scholar] [CrossRef]
- Park, J.-Y.; Choi, J.; Lee, Y.; Lee, J.-E.; Lee, E.-H.; Kwon, H.-J.; Yang, J.; Jeong, B.-R.; Kim, Y.-K.; Han, P.-L. Metagenome Analysis of Bodily Microbiota in a Mouse Model of Alzheimer Disease Using Bacteria-derived Membrane Vesicles in Blood. Exp. Neurobiol. 2017, 26, 369–379. [Google Scholar] [CrossRef]
- Païssé, S.; Valle, C.; Servant, F.; Courtney, M.; Burcelin, R.; Amar, J.; Lelouvier, B. Comprehensive description of blood microbiome from healthy donors assessed by 16S targeted metagenomic sequencing. Transfusion 2016, 56, 1138–1147. [Google Scholar] [CrossRef]
- Lee, Y.; Park, J.-Y.; Lee, E.-H.; Yang, J.; Jeong, B.-R.; Kim, Y.-K.; Seoh, J.-Y.; Lee, S.; Han, P.-L.; Kim, E.-J. Rapid Assessment of Microbiota Changes in Individuals with Autism Spectrum Disorder Using Bacteria-derived Membrane Vesicles in Urine. Exp. Neurobiol. 2017, 26, 307–317. [Google Scholar] [CrossRef]
- Jang, S.C.; Kim, S.R.; Yoon, Y.J.; Park, K.-S.; Kim, J.H.; Lee, J.; Kim, O.Y.; Choi, E.-J.; Kim, D.-K.; Choi, D.-S.; et al. In vivo Kinetic Biodistribution of Nano-Sized Outer Membrane Vesicles Derived from Bacteria. Small 2014, 11, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Thevaranjan, N.; Puchta, A.; Schulz, C.; Naidoo, A.; Szamosi, J.; Verschoor, C.P.; Loukov, D.; Schenck, L.P.; Jury, J.; Foley, K.P.; et al. Age-Associated Microbial Dysbiosis Promotes Intestinal Permeability, Systemic Inflammation, and Macrophage Dysfunction. Cell Host Microbe 2017, 21, 455–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Avila-Calderón, E.D.; Ruiz-Palma, M.D.S.; Aguilera-Arreola, M.G.; Velázquez-Guadarrama, N.; Ruiz, E.A.; Gomez-Lunar, Z.; Witonsky, S.; Contreras-Rodríguez, A. Outer Membrane Vesicles of Gram-Negative Bacteria: An Outlook on Biogenesis. Front. Microbiol. 2021, 12, 557902. [Google Scholar] [CrossRef]
- Bonnington, K.E.; Kuehn, M.J. Protein Selection and Export via Outer Membrane Vesicles. Biochim. Et Biophys. Acta 2014, 1843, 1612–1619. [Google Scholar] [CrossRef] [Green Version]
- Mancini, F.; Rossi, O.; Necchi, F.; Micoli, F. OMV Vaccines and the Role of TLR Agonists in Immune Response. Int. J. Mol. Sci. 2020, 21, 4416. [Google Scholar] [CrossRef]
- Gay, L.; Mezouar, S.; Cano, C.; Frohna, P.; Madakamutil, L.; Mège, J.-L.; Olive, D. Role of Vγ9vδ2 T lymphocytes in infectious diseases. Front. Immunol. 2022, 13, 928441. [Google Scholar] [CrossRef]
- Santos, N.C.; Silva, A.C.; Castanho, M.A.R.B.; Martins-Silva, J.; Saldanha, C. Evaluation of Lipopolysaccharide Aggregation by Light Scattering Spectroscopy. ChemBioChem 2003, 4, 96–100. [Google Scholar] [CrossRef]
- Aurell, C.A.; Wistrom, A.O. Critical Aggregation Concentrations of Gram-Negative Bacterial Lipopolysaccharides (LPS). Biochem. Biophys. Res. Commun. 1998, 253, 119–123. [Google Scholar] [CrossRef]
- Laulagnier, K.; Javalet, C.; Hemming, F.J.; Chivet, M.; Lachenal, G.; Blot, B.; Chatellard, C.; Sadoul, R. Amyloid precursor protein products concentrate in a subset of exosomes specifically endocytosed by neurons. Cell. Mol. Life Sci. 2018, 75, 757–773. [Google Scholar] [CrossRef]
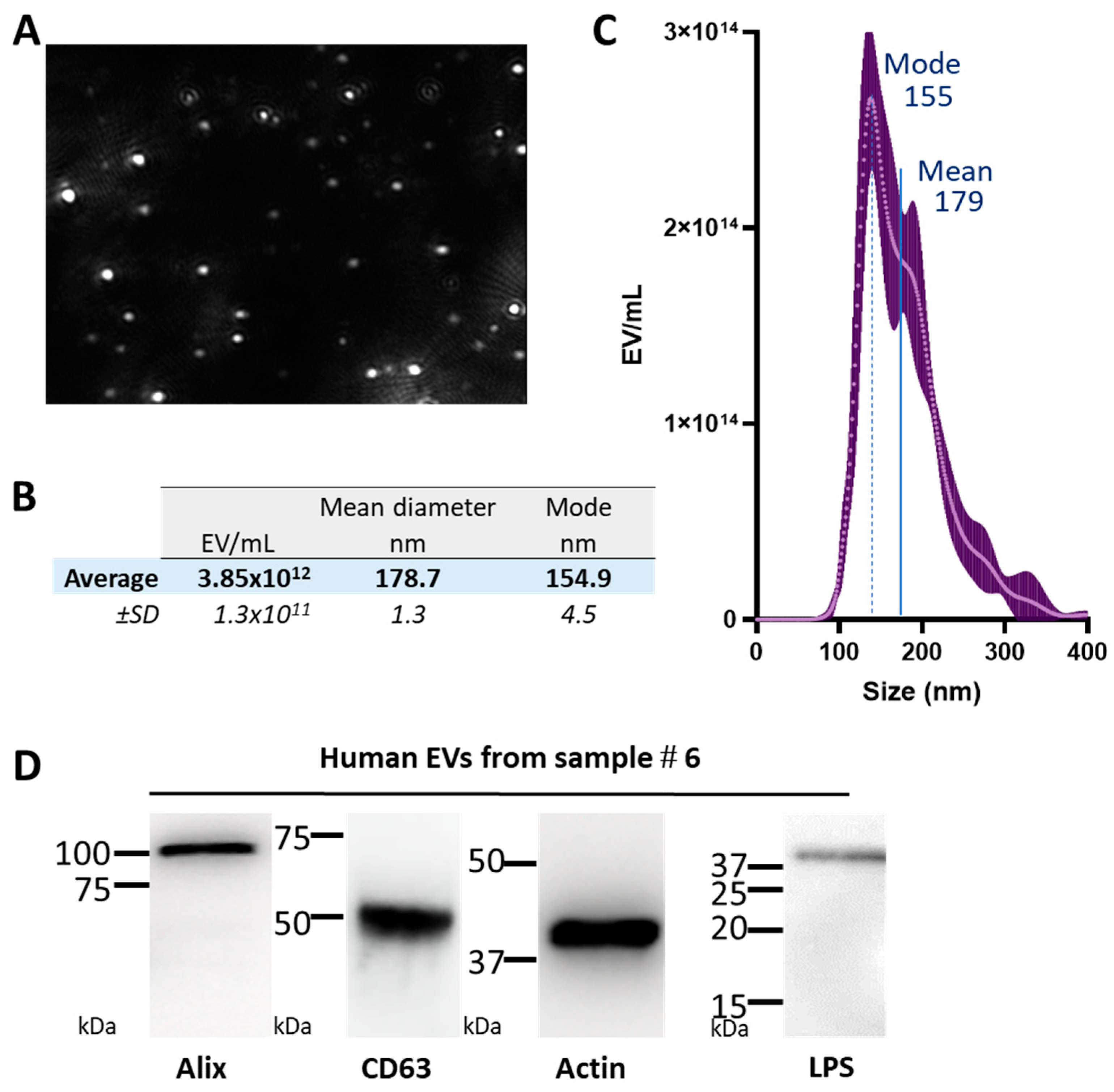

| Blood Donor EV | Concentration (EV/mL) | Mean Size (nm) | ±SD | Mode (nm) | Storage Time (days) | Blood Group | Gender | Age |
|---|---|---|---|---|---|---|---|---|
| #1 | 1.09 × 1010 | 126.4 | 12.9 | 87.4 | 42 | AB+ | M | 48 |
| #2 | 3.90 × 1011 | 183.3 | 0.6 | 183.5 | 9 | A+ | F | 27 |
| #3 | 5.93 × 1011 | 192.3 | 1.1 | 198.9 | 12 | O− | F | 39 |
| #4 | 4.25 × 1011 | 180.7 | 0.1 | 168.2 | 15 | O− | M | 63 |
| #5 | 2.06 × 1011 | 180.4 | 1.5 | 157.9 | 15 | O+ | F | 33 |
| #6 | 3.85 × 1012 | 178.7 | 1.3 | 154.9 | 38 | O+ | M | 62 |
| #7 | 1.05 × 1015 | 183.6 | 1.3 | 174.1 | 21 | A+ | M | 35 |
| #8 | 9.91 × 1011 | 167.7 | 1.3 | 154.3 | 31 | O+ | M | 63 |
| #9 | 9.75 × 1010 | 171.8 | 2.5 | 161.0 | 10 | A+ | M | 51 |
| #10 | 4.27 × 1011 | 157.4 | 0.8 | 140.2 | 20 | AB+ | F | 36 |
| #11 | 7.26 × 1011 | 181.4 | 1.4 | 186.1 | 27 | A− | M | 55 |
| #12 | 4.99 × 1011 | 166.3 | 0.4 | 141.8 | 30 | O+ | M | 35 |
| #13 | 3.94 × 1011 | 161.8 | 1.0 | 144.0 | 31 | O+ | M | 55 |
| #14 | 6.03 × 1011 | 161.7 | 0.9 | 133.4 | 36 | O+ | F | 30 |
| Mean | 7.56 × 1013 | 171.0 | 156.1 | 24.1 | 45 | |||
| SD | 2.80 × 1014 | 16.4 | 27.5 | 11.0 | 13 | |||
| Min. | 1.09 × 1010 | 126.4 | 87.4 | 9.0 | 27 | |||
| Max. | 1.05 × 1015 | 192.3 | 198.9 | 42.0 | 63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schaack, B.; Hindré, T.; Quansah, N.; Hannani, D.; Mercier, C.; Laurin, D. Microbiota-Derived Extracellular Vesicles Detected in Human Blood from Healthy Donors. Int. J. Mol. Sci. 2022, 23, 13787. https://doi.org/10.3390/ijms232213787
Schaack B, Hindré T, Quansah N, Hannani D, Mercier C, Laurin D. Microbiota-Derived Extracellular Vesicles Detected in Human Blood from Healthy Donors. International Journal of Molecular Sciences. 2022; 23(22):13787. https://doi.org/10.3390/ijms232213787
Chicago/Turabian StyleSchaack, Béatrice, Thomas Hindré, Nyamekye Quansah, Dalil Hannani, Corinne Mercier, and David Laurin. 2022. "Microbiota-Derived Extracellular Vesicles Detected in Human Blood from Healthy Donors" International Journal of Molecular Sciences 23, no. 22: 13787. https://doi.org/10.3390/ijms232213787
APA StyleSchaack, B., Hindré, T., Quansah, N., Hannani, D., Mercier, C., & Laurin, D. (2022). Microbiota-Derived Extracellular Vesicles Detected in Human Blood from Healthy Donors. International Journal of Molecular Sciences, 23(22), 13787. https://doi.org/10.3390/ijms232213787







