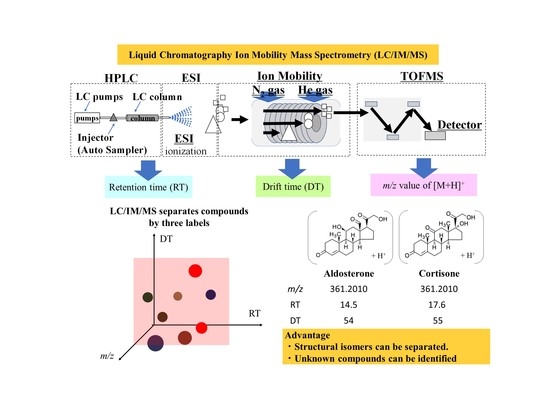
Comprehensive Steroid Assay with Non-Targeted Analysis Using Liquid Chromatography Ion Mobility Mass Spectrometry
Abstract
1. Introduction
2. Results
2.1. LC/IM/MS Measurement of Standard Solution Containing Steroids
2.2. LC/IM/MS Measurement of Human Serum
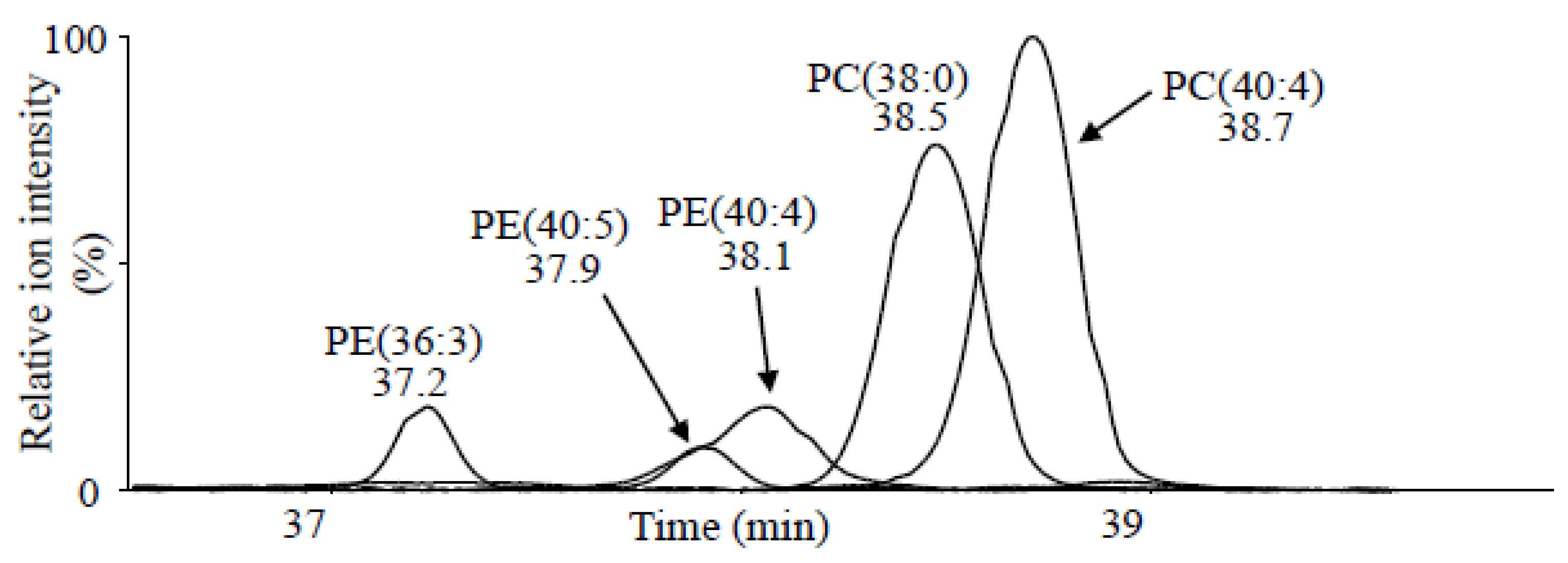
2.3. Non-Targeted Analysis Using LC/IM/MS
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Sample Preparation
4.3. Measurement Apparatus
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Funder, J.W.; Carey, R.M.; Mantero, F.; Murad, M.H.; Reincke, M.; Shibata, H.; Stowasser, M.; Young, W.F., Jr. The management of primary aldosteronism: Case detection, diagnosis, and treatment: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2016, 101, 1889–1916. [Google Scholar] [CrossRef] [PubMed]
- Käyser, S.C.; Dekkers, T.; Groenewoud, H.J.; van der Wilt, G.J.; Carel Bakx, J.; van der Wel, M.C.; Hermus, A.R.; Lenders, J.W.; Deinum, J. Study heterogeneity and estimation of prevalence of primary aldosteronism: A systematic review and meta-regression analysis. J. Clin. Endocrinol. Metab. 2016, 101, 2826–2835. [Google Scholar] [CrossRef] [PubMed]
- Hundemer, G.L.; Curhan, G.C.; Yozamp, N.; Wang, M.; Vaidya, A. Cardiometabolic outcomes and mortality in medically treated primary aldosteronism: A retrospective cohort study. Lancet Diabetes Endocrinol. 2018, 6, 51–59. [Google Scholar] [CrossRef]
- Monticone, S.; D’Ascenzo, F.; Moretti, C.; Williams, T.A.; Veglio, F.; Gaita, F.; Mulatero, P. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: A systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2018, 6, 41–50. [Google Scholar] [CrossRef]
- Choi, M.; Scholl, U.I.; Yue, P.; Björklund, P.; Zhao, B.; Nelson-Williams, C.; Ji, W.; Cho, Y.; Patel, A.; Men, C.J. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science 2011, 331, 768–772. [Google Scholar] [CrossRef]
- Scholl, U.I.; Goh, G.; Stölting, G.; de Oliveira, R.C.; Choi, M.; Overton, J.D.; Fonseca, A.L.; Korah, R.; Starker, L.F.; Kunstman, J.W. Somatic and germline CACNA1D calcium channel mutations in aldosterone-producing adenomas and primary aldosteronism. Nat. Genet. 2013, 45, 1050–1054. [Google Scholar] [CrossRef]
- Azizan, E.A.; Poulsen, H.; Tuluc, P.; Zhou, J.; Clausen, M.V.; Lieb, A.; Maniero, C.; Garg, S.; Bochukova, E.G.; Zhao, W. Somatic mutations in ATP1A1 and CACNA1D underlie a common subtype of adrenal hypertension. Nat. Genet. 2013, 45, 1055–1060. [Google Scholar] [CrossRef]
- Beuschlein, F.; Boulkroun, S.; Osswald, A.; Wieland, T.; Nielsen, H.N.; Lichtenauer, U.D.; Penton, D.; Schack, V.R.; Amar, L.; Fischer, E.; et al. Somatic mutations in ATP1A1 and ATP2B3 lead to aldosterone-producing adenomas and secondary hypertension. Nat. Genet. 2013, 45, 440–444. [Google Scholar] [CrossRef]
- Williams, T.A.; Peitzsch, M.; Dietz, A.S.; Dekkers, T.; Bidlingmaier, M.; Riester, A.; Treitl, M.; Rhayem, Y.; Beuschlein, F.; Lenders, J.W.; et al. Genotype-Specific Steroid Profiles Associated With Aldosterone-Producing Adenomas. Hypertension 2016, 67, 139–145. [Google Scholar] [CrossRef]
- Hattangady, N.G.; Karashima, S.; Yuan, L.; Ponce-Balbuena, D.; Jalife, J.; Gomez-Sanchez, C.E.; Auchus, R.J.; Rainey, W.E.; Else, T. Mutated KCNJ5 activates the acute and chronic regulatory steps in aldosterone production. J. Mol. Endocrinol. 2016, 57, 1–11. [Google Scholar] [CrossRef]
- Karashima, S.; Osaka, I. Rapidity and Precision of Steroid Hormone Measurement. J. Clin. Med. 2022, 11, 956. [Google Scholar] [CrossRef] [PubMed]
- Handelsman, D.J.; Wartofsky, L. Requirement for Mass Spectrometry Sex Steroid Assays in the Journal of Clinical Endocrinology and Metabolism. J. Clin. Endocrinol. Metab. 2013, 98, 3971–3973. [Google Scholar] [CrossRef] [PubMed]
- Paglia, G.; Smith, A.J.; Astarita, G. Ion mobility mass spectrometry in the omics era: Challenges and opportunities for metabolomics and lipidomics. Mass Spectrom. Rev. 2022, 41, 722–765. [Google Scholar] [CrossRef]
- Hernandez-Mesa, M.; Bizec, B.L.; Monteau, F.; García-Campañ, A.M.; Dervilly-Pinel, G. Collision Cross Section (CCS) Database: An Additional Measure to Characterize Steroids. Anal. Chem. 2018, 90, 4616–4625. [Google Scholar] [CrossRef] [PubMed]
- Arthur, K.L.; Turner, M.A.; Brailsford, A.D.; Kicman, A.T.; Cowan, D.A.; Reynolds, J.C.; Creaser, C.S. Rapid Analysis of Anabolic Steroid Metabolites in Urine by Combining Field Asymmetric Waveform Ion Mobility Spectrometry with Liquid Chromatography and Mass Spectrometry. Anal. Chem. 2017, 89, 7431–7437. [Google Scholar] [CrossRef]
- Turcu, A.F.; Wannachalee, T.; Tsodikov, A.; Nanba, A.T.; Ren, J.; Shields, J.J.; O’Day, P.J.; Giacherio, D.; Rainey, W.E.; Auchus, R.J. Comprehensive Analysis of Steroid Biomarkers for Guiding Primary Aldosteronism Subtyping. Hypertension 2020, 75, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Eisenhofer, G.; Durán, C.; Cannistraci, C.V.; Peitzsch, M.; Williams, T.A.; Riester, A.; Burrello, J.; Buffolo, F.; Prejbisz, A.; Beuschlein, F.; et al. Use of Steroid Profiling Combined With Machine Learning for Identification and Subtype Classification in Primary Aldosteronism. JAMA Netw. Open 2020, 3, e2016209. [Google Scholar] [CrossRef] [PubMed]
- Verbeck, G.F.; Ruotolo, B.T.; Sawyer, H.A.; Gillig, K.J.; Russell, D.H. A fundamental introduction to ion mobility mass spectrometry applied to the analysis of biomolecules. J. Biomol. Tech. 2002, 2, 56–61. [Google Scholar]
- Collins, D.C.; Lee, M.L. Developments in Ion Mobility Spectrometry-Mass Spectrometry. Anal. Bioanal. Chem. 2002, 372, 66–73. [Google Scholar] [CrossRef]
- Kanu, A.B.; Dwivedi, P.; Tam, M.; Matz, L.; Hill, H.H.J. Ion Mobility-Mass Spectrometry. J. Mass Spectrom. 2008, 43, 1–22. [Google Scholar] [CrossRef]
- Ahonen, L.; Fasciotti, M.; af Gennäs, G.B.; Kotiaho, T.; Daroda, R.J.; Eberlin, M.; Kostiainen, R. Separation of steroid isomers by ion mobility mass spectrometry. J. Chromatogr. A 2013, 1310, 133–137. [Google Scholar] [CrossRef]
- Fritsche, S.; Steinhart, H. Occurrence of hormonally active compounds in food: A review. Eur. Food Res. Technol. 1999, 209, 153–179. [Google Scholar] [CrossRef]
- Blair, I.A. Analysis of estrogens in serum and plasma from postmenopausal women: Past present, and future. Steroids 2010, 75, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Ruotolo, B.T.; Benesch, J.L.; Sandercock, A.M.; Hyung, S.J.; Robinson, C.V. Ion mobility-mass spectrometry analysis of large protein complexes. Nat. Protoc. 2008, 3, 1139–1152. [Google Scholar] [CrossRef] [PubMed]
- Uetrecht, C.; Rose, R.J.; van Duijn, E.; Lorenzen, K.; Heck, A.J. Ion mobility mass spectrometry of proteins and protein assemblies. Chem. Soc. Rev. 2010, 39, 1633–1655. [Google Scholar] [CrossRef]
- Baker, E.S.; Burnum-Johnson, K.E.; Ibrahim, Y.M.; Orton, D.J.; Monroe, M.E.; Kelly, R.T.; Moore, R.J.; Zhang, X.; Théberge, R.; Costello, C.E.; et al. Enhancing bottom-up and top-down proteomic measurements with ion mobility separations. Proteomics 2015, 15, 2766–2776. [Google Scholar] [CrossRef]
- Zhang, X.; Kew, K.; Reisdorph, R.; Sartain, M.; Powell, R.; Armstrong, M.; Quinn, K.; Cruickshank-Quinn, C.; Walmsley, S.; Bokatzian, S.; et al. Performance of a High-Pressure Liquid Chromatography-Ion Mobility-Mass Spectrometry System for Metabolic Profiling. Anal. Chem. 2017, 89, 6384–6391. [Google Scholar] [CrossRef]
- Davis, D.E., Jr.; Leaptrot, K.L.; Koomen, D.C.; May, J.C.; Cavalcanti, G.A.; Padilha, M.C.; Pereira, H.M.G.; McLean, J.A. Multidimensional Separations of Intact Phase II Steroid Metabolites Utilizing LC-Ion Mobility-HRMS. Anal. Chem. 2021, 93, 10990–10998. [Google Scholar] [CrossRef]
- Velosa, D.C.; Rivera, M.E.; Neal, S.P.; Olsen, S.S.H.; Burkus-Matesevac, A.; Chouinard, C.D. Toward Routine Analysis of Anabolic Androgenic Steroids in Urine Using Ion Mobility-Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2022, 33, 54–61. [Google Scholar] [CrossRef]
- Krone, N.; Hughes, B.A.; Lavery, G.G.; Stewart, P.M.; Arlt, W.; Shackleton, C.H. Gas chromatography/mass spectrometry (GC/MS) remains a pre-eminent discovery tool in clinical steroid investigations even in the era of fast liquid chromatography tandem mass spectrometry (LC/MS/MS). J. Steroid Biochem. Mol. Biol. 2010, 121, 496–504. [Google Scholar] [CrossRef]
- Carvalho, V.M. The coming of age of liquid chromatography coupled to tandem mass spectrometry in the endocrinology laboratory. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012, 883–884, 50–58. [Google Scholar] [CrossRef]
- Rauh, M. Steroid measurement with LC-MS/MS. Application examples in pediatrics. J. Steroid Biochem. Mol. Biol. 2010, 121, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Klåvus, A.; Kokla, M.; Noerman, S.; Koistinen, V.M.; Tuomainen, M.; Zarei, I.; Meuronen, T.; Häkkinen, M.R.; Rummukainen, S.; Farizah Babu, A.; et al. “Notame”: Workflow for Non-Targeted LC-MS Metabolic Profiling. Metabolites 2020, 10, 135. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cruickshank, C.; Armstrong, M.; Mahaffey, S.; Reisdorph, R.; Reisdorph, N. New sample preparation approach for mass spectrometry-based profiling of plasma results in improved coverage of metabolome. J. Chromatogr. A 2013, 1300, 217–226. [Google Scholar] [CrossRef] [PubMed]
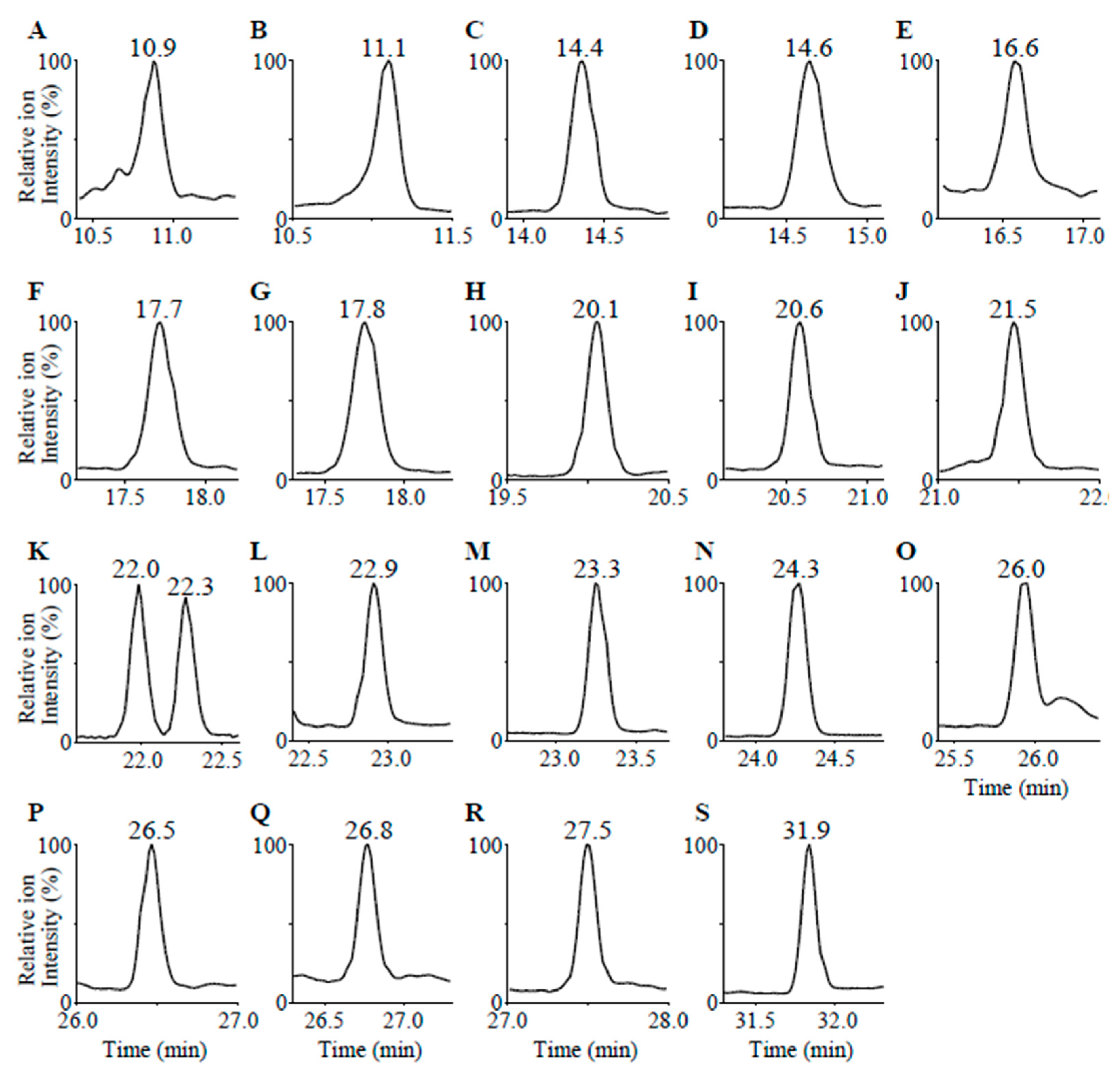

| Compounds | Calculated Mass [M + H]+ | Measured Mass [M + H]+ | RT (min) | DT (bins) | |
|---|---|---|---|---|---|
| a | 18-oxoF | 377.196 | 377.197 | 10.9 | 56 |
| b | 18-OHF | 379.212 | 379.213 | 11.1 | 57 |
| c | 18-OHB | 363.217 | 363.217 | 14.4 | 55 |
| d | Aldosterone | 361.201 | 361.200 | 14.6 | 54 |
| e | 19-OHA4 | 303.195 | 303.196 | 16.6 | 44 |
| f | Cortisone | 361.201 | 361.203 | 17.7 | 55 |
| g | Cortisol | 363.217 | 363.218 | 17.8 | 56 |
| h | 11-KT | 303.195 | 303.196 | 20.1 | 44 |
| i | 18-OH-DOC | 347.222 | 347.222 | 20.6 | 54 |
| j | Adrenosterone | 301.180 | 301.183 | 21.5 | 43 |
| k | 21-Deoxycortisol | 347.222 | 347.223 | 22.0 | 52 |
| l | Corticosterone | 347.222 | 347.223 | 22.3 | 54 |
| m | 11-Deoxycortisol | 347.222 | 347.225 | 22.9 | 55 |
| n | 16α-OHP | 331.227 | 331.228 | 23.3 | 52 |
| o | 11β-OHP | 331.227 | 331.227 | 24.3 | 50 |
| p | Testosterone | 289.216 | 289.217 | 26.0 | 43 |
| q | DOC | 331.227 | 331.229 | 26.5 | 53 |
| r | Androstenedione | 287.201 | 287.202 | 26.8 | 43 |
| s | 17α-OHP | 331.227 | 331.229 | 27.5 | 51 |
| t | Progesterone | 315.232 | 315.233 | 31.8 | 49 |
| Compounds | IS | ISconc. (ng/dL) | LOD (ng/dL) | LOQ (ng/dL) | RSD of LOQ (%) | Recovery (%) | Matrix Effect | |
|---|---|---|---|---|---|---|---|---|
| a | 18-oxoF | 18-OHF-d4 | 300 | 2.0 | 3.0 | 4.7 | 92 | 1.04 |
| b | 18-OHF | 18-OHF-d4 | 300 | 0.5 | 1.0 | 7.5 | 95 | 1.18 |
| c | 18-OHB | Aldosterone-d7 | 400 | 1.0 | 1.0 | 9.2 | 115 | 1.01 |
| d | Aldosterone | Aldosterone-d7 | 400 | 1.0 | 1.0 | 12.5 | 100 | 0.99 |
| e | 19-OHA4 | Cortisone-d7 | 50 | 3.0 | 3.0 | 17.8 | 95 | 1.03 |
| f | Cortisone | Cortisone-d7 | 50 | 1.0 | 1.0 | 8.2 | 105 | 0.99 |
| g | Cortisol | Cortisol-d4 | 1000 | 0.5 | 1.0 | 17.4 | 86 | 1.16 |
| h | 11-KT | 11-KT-d3 | 300 | 0.5 | 1.0 | 13.6 | 94 | 0.84 |
| i | 18-OH-DOC | 11-KT-d3 | 300 | 1.0 | 1.0 | 12.6 | 102 | 1.12 |
| j | Adrenosterone | 21-Deoxycortisol-d8 | 300 | 0.5 | 1.0 | 12.8 | 94 | 0.77 |
| k | 21-Deoxycortisol | 21-Deoxycortisol-d8 | 300 | 0.5 | 1.0 | 6.5 | 95 | 0.86 |
| l | Corticosterone | Corticosterone-d8 | 100 | 0.5 | 1.0 | 4.8 | 95 | 1.04 |
| m | 11-Deoxycortisol | 11-Deoxycortisol-d5 | 40 | 1.0 | 1.0 | 8.3 | 98 | 0.89 |
| n | 16α-OHP | 11-Deoxycortisol-d5 | 40 | 0.5 | 1.0 | 9.1 | 98 | 0.83 |
| o | 11β-OHP | Testosterone-d5 | 50 | 0.5 | 1.0 | 8.1 | 87 | 0.68 |
| p | Testosterone | Testosterone-d5 | 50 | 1.0 | 1.0 | 19.6 | 107 | 1.13 |
| q | DOC | DOC-d8 | 25 | 0.5 | 1.0 | 14.6 | 103 | 0.65 |
| R | Androstenedione | Androstenedione-d3 | 30 | 0.5 | 1.0 | 18.9 | 74 | 0.62 |
| S | 17α-OHP | 17α-OHP-d8 | 25 | 0.5 | 1.0 | 18.0 | 116 | 0.67 |
| T | Progesterone | Progesterone-d9 | 30 | 0.5 | 1.0 | 5.0 | 77 | 0.63 |
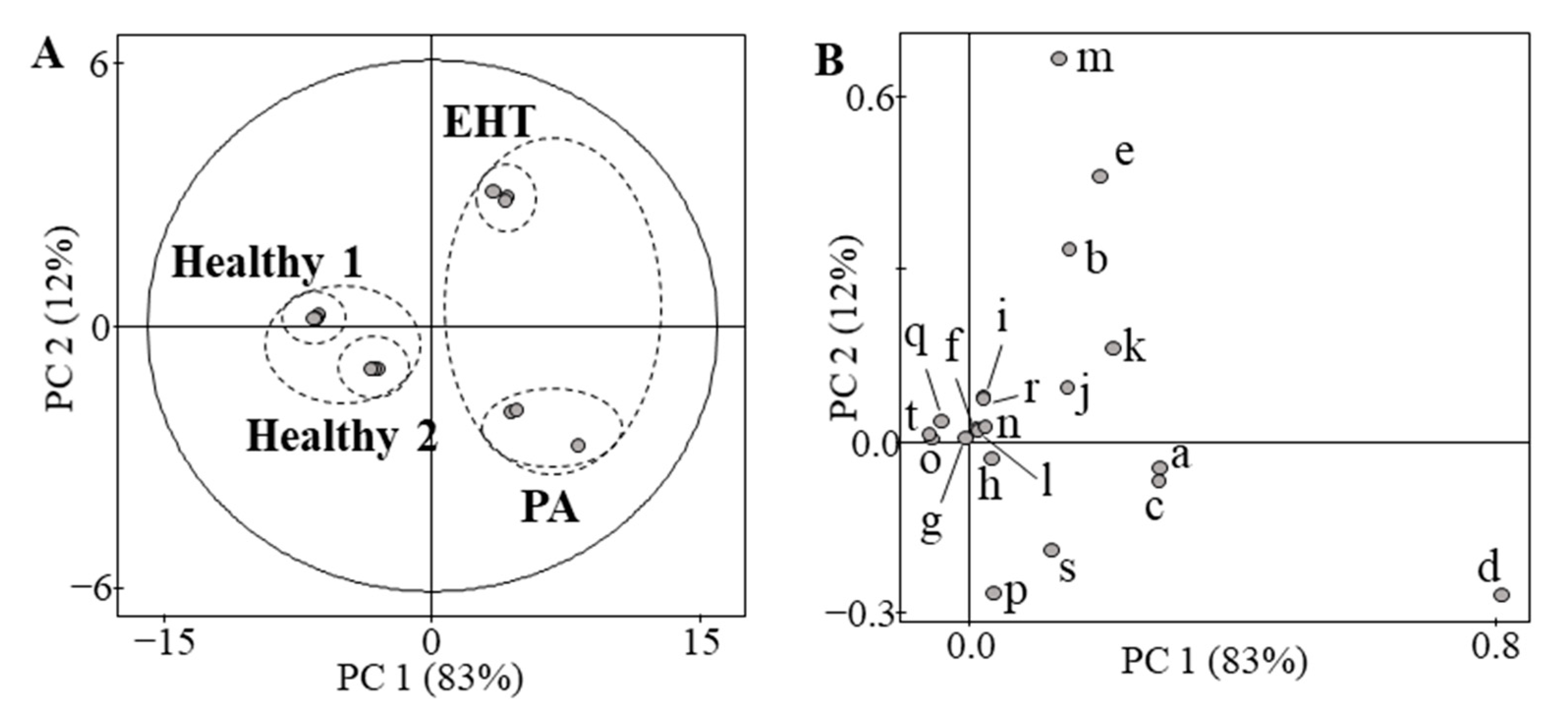
| Compounds | Healthy 1 (ng/dL) | Healthy 2 (ng/dL) | EHT (ng/dL) | PA (ng/dL) | |
|---|---|---|---|---|---|
| a | 18-oxoF | 3.3 | 4.6 | 11 | 12 |
| b | 18-OHF | 35 | 116 | 178 | 98 |
| c | 18-OHB | 11 | 45 | 36 | 41 |
| d | Aldosterone | 1.9 | 8.1 | 12 | 17 |
| e | 19-OHA4 | 5.6 | 7.1 | 30 | 14 |
| f | Cortisone | 1580 | 6030 | 4780 | 3550 |
| g | Cortisol | 9130 | 24,600 | 25,800 | 16,100 |
| h | 11-KT | 17 | 24 | 20 | 19 |
| i | 18-OH-DOC | 11 | 16 | 12 | 8.6 |
| j | Adrenosterone | 16 | 14 | 48 | 33 |
| k | 21-Deoxycortisol | 1.4 | <1 | 3.6 | 3.6 |
| l | Corticosterone | 140 | 480 | 450 | 260 |
| m | 11-Deoxycortisol | 31 | 37 | 134 | 27 |
| n | 16α-OHP | 26 | 16 | 20 | 22 |
| o | 11β-OHP | 6.7 | <1 | <1 | <1 |
| p | Testosterone | 532 | 1130 | 378 | 1240 |
| q | DOC | 8.6 | 4 | 3.4 | 1.8 |
| r | Androstenedione | 73 | 39 | 72 | 50 |
| s | 17α-OHP | 143 | 106 | 115 | 176 |
| t | Progesterone | 119 | 4.1 | 2.5 | 2.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamakawa, M.; Karashima, S.; Takata, R.; Haba, T.; Kuroiwa, K.; Touyama, H.; Hashimoto, A.; Konishi, S.; Aono, D.; Kometani, M.; et al. Comprehensive Steroid Assay with Non-Targeted Analysis Using Liquid Chromatography Ion Mobility Mass Spectrometry. Int. J. Mol. Sci. 2022, 23, 13858. https://doi.org/10.3390/ijms232213858
Yamakawa M, Karashima S, Takata R, Haba T, Kuroiwa K, Touyama H, Hashimoto A, Konishi S, Aono D, Kometani M, et al. Comprehensive Steroid Assay with Non-Targeted Analysis Using Liquid Chromatography Ion Mobility Mass Spectrometry. International Journal of Molecular Sciences. 2022; 23(22):13858. https://doi.org/10.3390/ijms232213858
Chicago/Turabian StyleYamakawa, Mai, Shigehiro Karashima, Riko Takata, Taichi Haba, Keigo Kuroiwa, Hideaki Touyama, Atsushi Hashimoto, Seigo Konishi, Daisuke Aono, Mitsuhiro Kometani, and et al. 2022. "Comprehensive Steroid Assay with Non-Targeted Analysis Using Liquid Chromatography Ion Mobility Mass Spectrometry" International Journal of Molecular Sciences 23, no. 22: 13858. https://doi.org/10.3390/ijms232213858
APA StyleYamakawa, M., Karashima, S., Takata, R., Haba, T., Kuroiwa, K., Touyama, H., Hashimoto, A., Konishi, S., Aono, D., Kometani, M., Nambo, H., Yoneda, T., & Osaka, I. (2022). Comprehensive Steroid Assay with Non-Targeted Analysis Using Liquid Chromatography Ion Mobility Mass Spectrometry. International Journal of Molecular Sciences, 23(22), 13858. https://doi.org/10.3390/ijms232213858