Advanced Sperm Selection Strategies as a Treatment for Infertile Couples: A Systematic Review
Abstract
:1. Introduction
2. Material and Methods
2.1. Data Sources and Search Strategy
2.2. Study Eligibility
2.3. Study Selection Procedure
2.4. Additional Article Quality Screening
2.5. Data Extraction for Systematic Review and Statistics
2.6. Statistical Analysis
3. Results
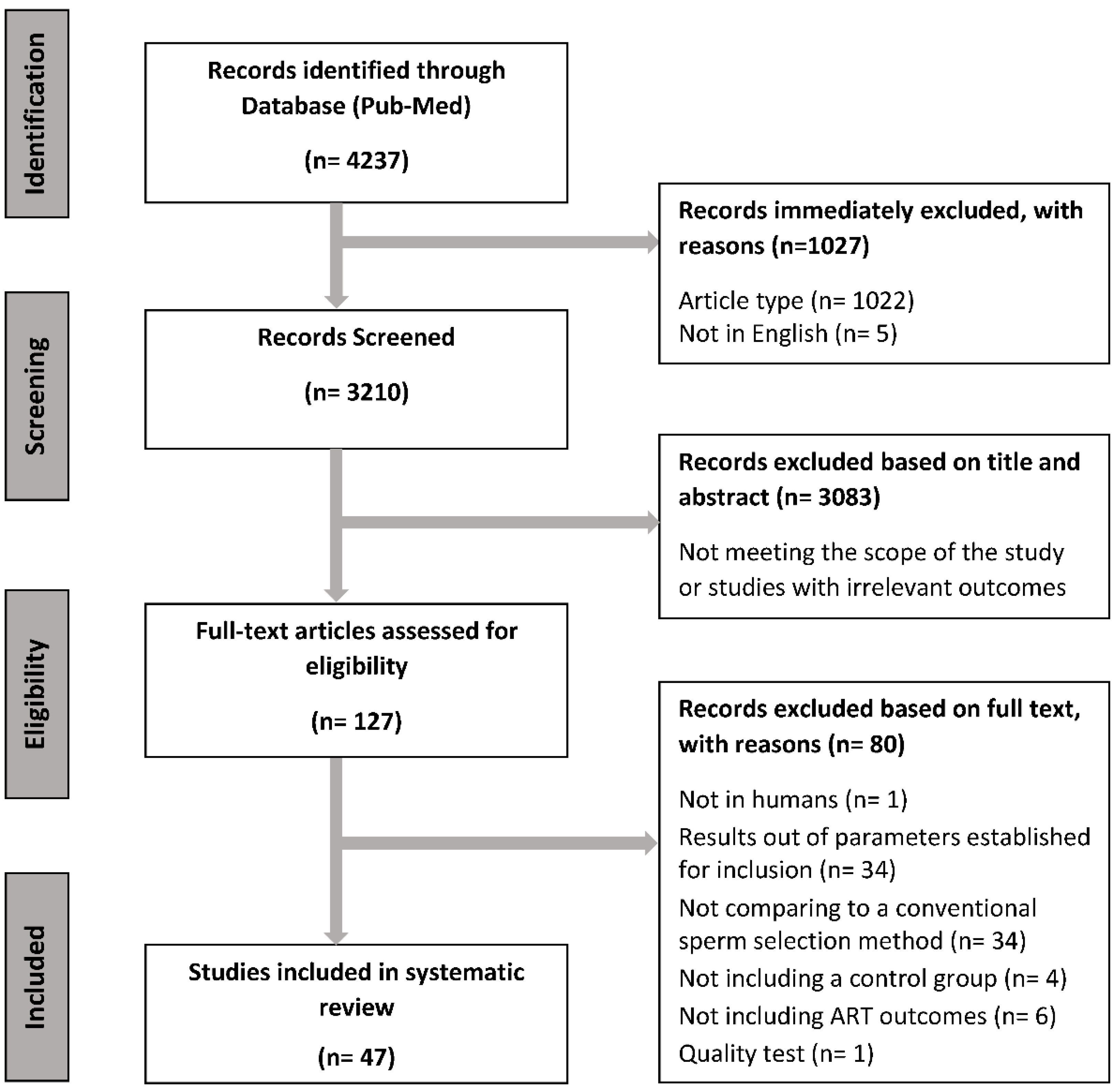
3.1. Identification and Selection of Studies
3.2. Systematic Review: Qualitative Analysis
3.3. Influence of Sperm Selection Methods on Sperm Quality and ART Outcomes
4. Discussion
4.1. Using Advanced Sperm Selection Techniques Improves ART Outcomes
4.2. Advanced Sperm Selection Techniques Increase ART Outcomes through an Improvement of Sperm Quality/Functionality Variables
4.3. Strengths and Limitations
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dyer, S.; Chambers, G.M.; de Mouzon, J.; Nygren, K.G.; Zegers-Hochschild, F.; Mansour, R.; Ishihara, O.; Banker, M.; Adamson, G.D. International Committee for Monitoring Assisted Reproductive Technologies World Report: Assisted Reproductive Technology 2008, 2009 and 2010. Hum. Reprod. 2016, 31, 1588–1609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Datta, J.; Palmer, M.J.; Tanton, C.; Gibson, L.J.; Jones, K.G.; Macdowall, W.; Glasier, A.; Sonnenberg, P.; Field, N.; Mercer, C.H.; et al. Prevalence of Infertility and Help Seeking among 15 000 Women and Men. Hum. Reprod. 2016, 31, 2108–2118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, A.; Mulgund, A.; Hamada, A.; Chyatte, M.R. A Unique View on Male Infertility around the Globe. Reprod. Biol. Endocrinol. 2015, 13, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, N.; Singh, A.K. Trends of Male Factor Infertility, an Important Cause of Infertility: A Review of Literature. J. Hum. Reprod. Sci. 2015, 8, 191–196. [Google Scholar] [CrossRef]
- Wyns, C.; De Geyter, C.; Calhaz-Jorge, C.; Kupka, M.; Motrenko, T.; Smeenk, J.; Bergh, C.; Tandler-Schneider, A.; Rugescu, I.; Goossens, V. ART in Europe, 2018: Results Generated from European Registries by ESHRE. Hum. Reprod. Open 2022, 2022, hoac022. [Google Scholar] [CrossRef]
- Sakkas, D.; Manicardi, G.C.; Tomlinson, M.; Mandrioli, M.; Bizzaro, D.; Bianchi, P.G.; Bianchi, U. The Use of Two Density Gradient Centrifugation Techniques and the Swim-up Method to Separate Spermatozoa with Chromatin and Nuclear DNA Anomalies. Hum. Reprod. 2000, 15, 1112–1116. [Google Scholar] [CrossRef] [Green Version]
- Loutradi, K.E.; Tarlatzis, B.C.; Goulis, D.G.; Zepiridis, L.; Pagou, T.; Chatziioannou, E.; Grimbizis, G.F.; Papadimas, I.; Bontis, I. The Effects of Sperm Quality on Embryo Development after Intracytoplasmic Sperm Injection. J. Assist. Reprod. Genet. 2006, 23, 69–74. [Google Scholar] [CrossRef] [Green Version]
- Ribas-Maynou, J.; Yeste, M.; Becerra-Tomás, N.; Aston, K.K.I.; James, E.E.R.; Salas-Huetos, A. Clinical Implications of Sperm DNA Damage in IVF and ICSI: Updated Systematic Review and Meta-Analysis. Biol. Rev. 2021, 96, 1284–1300. [Google Scholar] [CrossRef]
- Messerlian, C.; Gaskins, A.J. Epidemiologic Approaches for Studying Assisted Reproductive Technologies: Design, Methods, Analysis and Interpretation. Curr. Epidemiol. Rep. 2017, 4, 124–132. [Google Scholar] [CrossRef]
- Cho, C.-L.; Agarwal, A. Role of Sperm DNA Fragmentation in Male Factor Infertility: A Systematic Review. Arab. J. Urol. 2018, 16, 21–34. [Google Scholar] [CrossRef]
- Baldini, D.; Ferri, D.; Baldini, G.M.; Lot, D.; Catino, A.; Vizziello, D.; Vizziello, G. Sperm Selection for ICSI: Do We Have a Winner? Cells 2021, 10, 3566. [Google Scholar] [CrossRef] [PubMed]
- West, R.; Coomarasamy, A.; Frew, L.; Hutton, R.; Kirkman-Brown, J.; Lawlor, M.; Lewis, S.; Partanen, R.; Payne-Dwyer, A.; Román-Montañana, C.; et al. Sperm Selection with Hyaluronic Acid Improved Live Birth Outcomes among Older Couples and Was Connected to Sperm DNA Quality, Potentially Affecting All Treatment Outcomes. Hum. Reprod. 2022, 37, 1106–1125. [Google Scholar] [CrossRef] [PubMed]
- Huszar, G.; Ozkavukcu, S.; Jakab, A.; Celik-Ozenci, C.; Sati, G.L.; Cayli, S. Hyaluronic Acid Binding Ability of Human Sperm Reflects Cellular Maturity and Fertilizing Potential: Selection of Sperm for Intracytoplasmic Sperm Injection. Curr. Opin. Obstet. Gynecol. 2006, 18, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Hasanen, E.; Elqusi, K.; ElTanbouly, S.; Hussin, A.E.G.; AlKhadr, H.; Zaki, H.; Henkel, R.; Agarwal, A. PICSI vs. MACS for Abnormal Sperm DNA Fragmentation ICSI Cases: A Prospective Randomized Trial. J. Assist. Reprod. Genet. 2020, 37, 2605–2613. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- Antinori, M.; Licata, E.; Dani, G.; Cerusico, F.; Versaci, C.; d’Angelo, D.; Antinori, S. Intracytoplasmic Morphologically Selected Sperm Injection: A Prospective Randomized Trial. Reprod. Biomed. Online 2008, 16, 835–841. [Google Scholar] [CrossRef]
- Balaban, B.; Yakin, K.; Alatas, C.; Oktem, O.; Isiklar, A.; Urman, B. Clinical Outcome of Intracytoplasmic Injection of Spermatozoa Morphologically Selected under High Magnification: A Prospective Randomized Study. Reprod. Biomed. Online 2011, 22, 472–476. [Google Scholar] [CrossRef] [Green Version]
- Bartoov, B.; Berkovitz, A.; Eltes, F.; Kogosovsky, A.; Yagoda, A.; Lederman, H.; Artzi, S.; Gross, M.; Barak, Y. Pregnancy Rates Are Higher with Intracytoplasmic Morphologically Selected Sperm Injection than with Conventional Intracytoplasmic Injection. Fertil. Steril. 2003, 80, 1413–1419. [Google Scholar] [CrossRef]
- Berkovitz, A.; Eltes, F.; Ellenbogen, A.; Peer, S.; Feldberg, D.; Bartoov, B. Does the Presence of Nuclear Vacuoles in Human Sperm Selected for ICSI Affect Pregnancy Outcome? Hum. Reprod. 2006, 21, 1787–1790. [Google Scholar] [CrossRef] [Green Version]
- Berkovitz, A.; Eltes, F.; Lederman, H.; Peer, S.; Ellenbogen, A.; Feldberg, B.; Bartoov, B. How to Improve IVF-ICSI Outcome by Sperm Selection. Reprod. Biomed. Online 2006, 12, 634–638. [Google Scholar] [CrossRef]
- Boediono, A.; Handayani, N.; Sari, H.N.; Yusup, N.; Indrasari, W.; Polim, A.A.; Sini, I. Morphokinetics of Embryos after IMSI versus ICSI in Couples with Sub-Optimal Sperm Quality: A Time-Lapse Study. Andrologia 2021, 53, e14002. [Google Scholar] [CrossRef]
- Delaroche, L.; Yazbeck, C.; Gout, C.; Kahn, V.; Oger, P.; Rougier, N. Intracytoplasmic Morphologically Selected Sperm Injection (IMSI) after Repeated IVF or ICSI Failures: A Prospective Comparative Study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 167, 76–80. [Google Scholar] [CrossRef]
- Gaspard, O.; Vanderzwalmen, P.; Wirleitner, B.; Ravet, S.; Wenders, F.; Eichel, V.; Mocková, A.; Spitzer, D.; Jouan, C.; Gridelet, V.; et al. Impact of High Magnification Sperm Selection on Neonatal Outcomes: A Retrospective Study. J. Assist. Reprod. Genet. 2018, 35, 1113–1121. [Google Scholar] [CrossRef]
- Gatimel, N.; Parinaud, J.; Leandri, R.D. Intracytoplasmic Morphologically Selected Sperm Injection (IMSI) Does Not Improve Outcome in Patients with Two Successive IVF-ICSI Failures. J. Assist. Reprod. Genet. 2016, 33, 349–355. [Google Scholar] [CrossRef] [Green Version]
- Hazout, A.; Dumont-Hassan, M.; Junca, A.M.; Bacrie, P.C.; Tesarik, J. High-Magnification ICSI Overcomes Paternal Effect Resistant to Conventional ICSI. Reprod. Biomed. Online 2006, 12, 19–25. [Google Scholar] [CrossRef]
- Karabulut, S.; Aksunger, O.; Korkmaz, O.; Eren Gozel, H.; Keskin, I. Intracytoplasmic Morphologically Selected Sperm Injection, but for Whom? Zygote 2019, 27, 299–304. [Google Scholar] [CrossRef]
- Knez, K.; Zorn, B.; Tomazevic, T.; Vrtacnik-Bokal, E.; Virant-Klun, I. The IMSI Procedure Improves Poor Embryo Development in the Same Infertile Couples with Poor Semen Quality: A Comparative Prospective Randomized Study. Reprod. Biol. Endocrinol. 2011, 9, 123. [Google Scholar] [CrossRef] [Green Version]
- Knez, K.; Tomazevic, T.; Zorn, B.; Vrtacnik-Bokal, E.; Virant-Klun, I. Intracytoplasmic Morphologically Selected Sperm Injection Improves Development and Quality of Preimplantation Embryos in Teratozoospermia Patients. Reprod. Biomed. Online 2012, 25, 168–179. [Google Scholar] [CrossRef]
- Leandri, R.D.; Gachet, A.; Pfeffer, J.; Celebi, C.; Rives, N.; Carre-Pigeon, F.; Kulski, O.; Mitchell, V.; Parinaud, J. Is Intracytoplasmic Morphologically Selected Sperm Injection (IMSI) Beneficial in the First ART Cycle? A Multicentric Randomized Controlled Trial. Andrology 2013, 1, 692–697. [Google Scholar] [CrossRef]
- Mangoli, E.; Khalili, M.A.; Talebi, A.R.; Mehdi Kalantar, S.; Montazeri, F.; Agharahimi, A.; Woodward, B.J. Association between Early Embryo Morphokinetics plus Transcript Levels of Sperm Apoptotic Genes and Clinical Outcomes in IMSI and ICSI Cycles of Male Factor Patients. J. Assist. Reprod. Genet. 2020, 37, 2555–2567. [Google Scholar] [CrossRef]
- Mauri, A.L.; Petersen, C.G.; Oliveira, J.B.A.; Massaro, F.C.; Baruffi, R.L.R.; Franco, J.G. Comparison of Day 2 Embryo Quality after Conventional ICSI versus Intracytoplasmic Morphologically Selected Sperm Injection (IMSI) Using Sibling Oocytes. Eur. J. Obstet. Gynecol. Reprod. Biol. 2010, 150, 42–46. [Google Scholar] [CrossRef]
- Oliveira, J.B.A.; Cavagna, M.; Petersen, C.G.; Mauri, A.L.; Massaro, F.C.; Silva, L.F.I.; Baruffi, R.L.R.; Franco, J.G. Pregnancy Outcomes in Women with Repeated Implantation Failures after Intracytoplasmic Morphologically Selected Sperm Injection (IMSI). Reprod. Biol. Endocrinol. 2011, 9, 99. [Google Scholar] [CrossRef] [Green Version]
- Setti, A.S.; Figueira, R.C.S.; Braga, D.P.A.F.; Aoki, T.; Iaconelli, A.J.; Borges, E.J. Intracytoplasmic Morphologically Selected Sperm Injection Is Beneficial in Cases of Advanced Maternal Age: A Prospective Randomized Study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 171, 286–290. [Google Scholar] [CrossRef]
- Setti, A.S.; Braga, D.P.A.F.; Figueira, R.C.S.; Iaconelli, A.; Borges, E. Poor-Responder Patients Do Not Benefit from Intracytoplasmic Morphologically Selected Sperm Injection. J. Assist. Reprod. Genet. 2015, 32, 445–450. [Google Scholar] [CrossRef] [Green Version]
- Shalom-Paz, E.; Anabusi, S.; Michaeli, M.; Karchovsky-Shoshan, E.; Rothfarb, N.; Shavit, T.; Ellenbogen, A. Can Intra Cytoplasmatic Morphologically Selected Sperm Injection (IMSI) Technique Improve Outcome in Patients with Repeated IVF-ICSI Failure? A Comparative Study. Gynecol. Endocrinol. 2015, 31, 247–251. [Google Scholar] [CrossRef]
- Wilding, M.; Coppola, G.; Di Matteo, L.; Palagiano, A.; Fusco, E.; Dale, B. Intracytoplasmic Injection of Morphologically Selected Spermatozoa (IMSI) Improves Outcome after Assisted Reproduction by Deselecting Physiologically Poor Quality Spermatozoa. J. Assist. Reprod. Genet. 2011, 28, 253–262. [Google Scholar] [CrossRef] [Green Version]
- Choe, S.A.; Tae, J.C.; Shin, M.Y.; Kim, H.J.; Kim, C.H.; Lee, J.Y.; Hwang, D.; Kim, K.C.; Suh, C.S.; Jee, B.C. Application of Sperm Selection Using Hyaluronic Acid Binding in Intracytoplasmic Sperm Injection Cycles: A Sibling Oocyte Study. J. Korean Med. Sci. 2012, 27, 1569–1573. [Google Scholar] [CrossRef] [Green Version]
- Erberelli, R.F.; de Salgado, R.M.; Pereira, D.H.M.; Wolff, P. Hyaluronan-Binding System for Sperm Selection Enhances Pregnancy Rates in ICSI Cycles Associated with Male Factor Infertility. J. Bras. Reprod. Assist. 2017, 21, 2–6. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, H.; Kim, T.H.; Jeong, J.; Lee, W.S.; Lyu, S.W. Effect of Sperm Selection Using Hyaluronan on Fertilization and Quality of Cleavage-Stage Embryos in Intracytoplasmic Sperm Injection (ICSI) Cycles of Couples with Severe Teratozoospermia. Gynecol. Endocrinol. 2020, 36, 456–459. [Google Scholar] [CrossRef]
- Liu, Y.; Feenan, K.; Chapple, V.; Roberts, P.; Matson, P. Intracytoplasmic Sperm Injection Using Hyaluronic Acid or Polyvinylpyrrolidone: A Time-Lapse Sibling Oocyte Study. Hum. Fertil. 2019, 22, 39–45. [Google Scholar] [CrossRef]
- Majumdar, G.; Majumdar, A. A Prospective Randomized Study to Evaluate the Effect of Hyaluronic Acid Sperm Selection on the Intracytoplasmic Sperm Injection Outcome of Patients with Unexplained Infertility Having Normal Semen Parameters. J. Assist. Reprod. Genet. 2013, 30, 1471–1475. [Google Scholar] [CrossRef] [Green Version]
- Miller, D.; Pavitt, S.; Sharma, V.; Forbes, G.; Hooper, R.; Bhattacharya, S.; Kirkman-Brown, J.; Coomarasamy, A.; Lewis, S.; Cutting, R.; et al. Physiological, Hyaluronan-Selected Intracytoplasmic Sperm Injection for Infertility Treatment (HABSelect): A Parallel, Two-Group, Randomised Trial. Lancet 2019, 393, 416–422. [Google Scholar] [CrossRef] [Green Version]
- Mokánszki, A.; Tóthné, E.V.; Bodnár, B.; Tándor, Z.; Molnár, Z.; Jakab, A.; Ujfalusi, A.; Oláh, É. Is Sperm Hyaluronic Acid Binding Ability Predictive for Clinical Success of Intracytoplasmic Sperm Injection: PICSI vs. ICSI? Syst. Biol. Reprod. Med. 2014, 60, 348–354. [Google Scholar] [CrossRef] [Green Version]
- Nasr-Esfahani, M.H.; Razavi, S.; Vahdati, A.A.; Fathi, F.; Tavalaee, M. Evaluation of Sperm Selection Procedure Based on Hyaluronic Acid Binding Ability on ICSI Outcome. J. Assist. Reprod. Genet. 2008, 25, 197–203. [Google Scholar] [CrossRef] [Green Version]
- Parmegiani, L.; Cognigni, G.E.; Bernardi, S.; Troilo, E.; Ciampaglia, W.; Filicori, M. “Physiologic ICSI”: Hyaluronic Acid (HA) Favors Selection of Spermatozoa without DNA Fragmentation and with Normal Nucleus, Resulting in Improvement of Embryo Quality. Fertil. Steril. 2010, 93, 598–604. [Google Scholar] [CrossRef]
- Worrilow, K.C.; Eid, S.; Woodhouse, D.; Perloe, M.; Smith, S.; Witmyer, J.; Ivani, K.; Khoury, C.; Ball, G.D.; Elliot, T.; et al. Use of Hyaluronan in the Selection of Sperm for Intracytoplasmic Sperm Injection (ICSI): Significant Improvement in Clinical Outcomes-Multicenter, Double-Blinded and Randomized Controlled Trial. Hum. Reprod. 2013, 28, 306–314. [Google Scholar] [CrossRef]
- Dirican, E.K.; Özgün, O.D.; Akarsu, S.; Akin, K.O.; Ercan, Ö.; Uǧurlu, M.; Çamsari, Ç.; Kanyilmaz, O.; Kaya, A.; Ünsal, A. Clinical Outcome of Magnetic Activated Cell Sorting of Non-Apoptotic Spermatozoa before Density Gradient Centrifugation for Assisted Reproduction. J. Assist. Reprod. Genet. 2008, 25, 375–381. [Google Scholar] [CrossRef] [Green Version]
- Merino-Ruiz, M.; Morales-Martínez, F.A.; Navar-Vizcarra, E.; Valdés-Martínez, O.H.; Sordia-Hernández, L.H.; Saldívar-Rodríguez, D.; Vidal-Gutiérrez, O. The Elimination of Apoptotic Sperm in IVF Procedures and Its Effect on Pregnancy Rate. J. Bras. Reprod. Assist. 2019, 23, 112–116. [Google Scholar] [CrossRef]
- Romany, L.; Garrido, N.; Motato, Y.; Aparicio, B.; Remohí, J.; Meseguer, M. Removal of Annexin V-Positive Sperm Cells for Intracytoplasmic Sperm Injection in Ovum Donation Cycles Does Not Improve Reproductive Outcome: A Controlled and Randomized Trial in Unselected Males. Fertil. Steril. 2014, 102, 1567–1575.e1. [Google Scholar] [CrossRef]
- Sánchez-Martín, P.; Dorado-Silva, M.; Sánchez-Martín, F.; González Martínez, M.; Johnston, S.D.; Gosálvez, J. Magnetic Cell Sorting of Semen Containing Spermatozoa with High DNA Fragmentation in ICSI Cycles Decreases Miscarriage Rate. Reprod. Biomed. Online 2017, 34, 506–512. [Google Scholar] [CrossRef]
- Sheikhi, A.; Jalali, M.; Gholamian, M.; Jafarzadeh, A.; Jannati, S.; Mousavifar, N. Elimination of Apoptotic Spermatozoa by Magnetic-Activated Cell Sorting Improves the Fertilization Rate of Couples Treated with ICSI Procedure. Andrology 2013, 1, 845–849. [Google Scholar] [CrossRef]
- Stimpfel, M.; Verdenik, I.; Zorn, B.; Virant-Klun, I. Magnetic-Activated Cell Sorting of Non-Apoptotic Spermatozoa Improves the Quality of Embryos According to Female Age: A Prospective Sibling Oocyte Study. J. Assist. Reprod. Genet. 2018, 35, 1665–1674. [Google Scholar] [CrossRef]
- Ziarati, N.; Tavalaee, M.; Bahadorani, M.; Nasr Esfahani, M.H. Clinical Outcomes of Magnetic Activated Sperm Sorting in Infertile Men Candidate for ICSI. Hum. Fertil. 2019, 22, 118–125. [Google Scholar] [CrossRef]
- Ozcan, P.; Takmaz, T.; Yazici, M.G.K.; Alagoz, O.A.; Yesiladali, M.; Sevket, O.; Ficicioglu, C. Does the Use of Microfluidic Sperm Sorting for the Sperm Selection Improve in Vitro Fertilization Success Rates in Male Factor Infertility? J. Obstet. Gynaecol. Res. 2021, 47, 382–388. [Google Scholar] [CrossRef]
- Parrella, A.; Keating, D.; Cheung, S.; Xie, P.; Stewart, J.D.; Rosenwaks, Z.; Palermo, G.D. A Treatment Approach for Couples with Disrupted Sperm DNA Integrity and Recurrent ART Failure. J. Assist. Reprod. Genet. 2019, 36, 2057–2066. [Google Scholar] [CrossRef] [Green Version]
- Yalcinkaya Kalyan, E.; Can Celik, S.; Okan, O.; Akdeniz, G.; Karabulut, S.; Caliskan, E. Does a Microfluidic Chip for Sperm Sorting Have a Positive Add-on Effect on Laboratory and Clinical Outcomes of Intracytoplasmic Sperm Injection Cycles? A Sibling Oocyte Study. Andrologia 2019, 51, e13403. [Google Scholar] [CrossRef]
- Yetkinel, S.; Kilicdag, E.B.; Aytac, P.C.; Haydardedeoglu, B.; Simsek, E.; Cok, T. Effects of the Microfluidic Chip Technique in Sperm Selection for Intracytoplasmic Sperm Injection for Unexplained Infertility: A Prospective, Randomized Controlled Trial. J. Assist. Reprod. Genet. 2019, 36, 403–409. [Google Scholar] [CrossRef]
- Yildiz, K.; Yuksel, S. Use of Microfluidic Sperm Extraction Chips as an Alternative Method in Patients with Recurrent in Vitro Fertilisation Failure. J. Assist. Reprod. Genet. 2019, 36, 1423–1429. [Google Scholar] [CrossRef]
- Duarte, C.; Núñez, V.; Wong, Y.; Vivar, C.; Benites, E.; Rodriguez, U.; Vergara, C.; Ponce, J. Impact of the Z Potential Technique on Reducing the Sperm DNA Fragmentation Index, Fertilization Rate and Embryo Development. J. Bras. Reprod. Assist. 2017, 21, 351–355. [Google Scholar] [CrossRef]
- Kheirollahi-Kouhestani, M.; Razavi, S.; Tavalaee, M.; Deemeh, M.R.; Mardani, M.; Moshtaghian, J.; Nasr-Esfahani, M.H. Selection of Sperm Based on Combined Density Gradient and Zeta Method May Improve ICSI Outcome. Hum. Reprod. 2009, 24, 2409–2416. [Google Scholar] [CrossRef]
- Gianaroli, L.; Magli, M.C.; Collodel, G.; Moretti, E.; Ferraretti, A.P.; Baccetti, B. Sperm Head’s Birefringence: A New Criterion for Sperm Selection. Fertil. Steril. 2008, 90, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Aktan, T.M.; Montag, M.; Duman, S.; Gorkemli, H.; Rink, K.; Yurdakul, T. Use of a Laser to Detect Viable but Immotile Spermatozoa. Andrologia 2004, 36, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, D.M.; Hadyme Miyague, A.; Barbosa, M.A.; Navarro, P.A.; Raine-Fenning, N.; Nastri, C.O.; Martins, W.P. Regular (ICSI) versus Ultra-High Magnification (IMSI) Sperm Selection for Assisted Reproduction. Cochrane Database Syst. Rev. 2020, 2, CD010167. [Google Scholar] [CrossRef] [PubMed]
- Gutnisky, C.; Dalvit, G.C.; Pintos, L.N.; Thompson, J.G.; Beconi, M.T.; Cetica, P.D. Influence of Hyaluronic Acid Synthesis and Cumulus Mucification on Bovine Oocyte in Vitro Maturation, Fertilisation and Embryo Development. Reprod. Fertil. Dev. 2007, 19, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Keefe, D.; Kumar, M.; Kalmbach, K. Oocyte Competency Is the Key to Embryo Potential. Fertil. Steril. 2015, 103, 317–322. [Google Scholar] [CrossRef]
- Chun, Y.P.; Sang, J.U.; Sang, J.S.; Kwang, S.K.; Seung, B.H.; Kil, S.C.; Park, C.; Lee, H.T. Increase of ICSI Efficiency with Hyaluronic Acid Binding Sperm for Low Aneuploidy Frequency in Pig. Theriogenology 2005, 64, 1158–1169. [Google Scholar] [CrossRef]
- Gil, M.; Sar-Shalom, V.; Melendez Sivira, Y.; Carreras, R.; Checa, M.A. Sperm Selection Using Magnetic Activated Cell Sorting (MACS) in Assisted Reproduction: A Systematic Review and Meta-Analysis. J. Assist. Reprod. Genet. 2013, 30, 479–485. [Google Scholar] [CrossRef]
- Nosrati, R.; Graham, P.J.; Zhang, B.; Riordon, J.; Lagunov, A.; Hannam, T.G.; Escobedo, C.; Jarvi, K.; Sinton, D. Microfluidics for Sperm Analysis and Selection. Nat. Rev. Urol. 2017, 14, 707–730. [Google Scholar] [CrossRef]
- Franco, J.G.; Mauri, A.L.; Petersen, C.G.; Massaro, F.C.; Silva, L.F.I.; Felipe, V.; Cavagna, M.; Pontes, A.; Baruffi, R.L.R.; Oliveira, J.B.A.; et al. Large Nuclear Vacuoles Are Indicative of Abnormal Chromatin Packaging in Human Spermatozoa. Int. J. Androl. 2012, 35, 46–51. [Google Scholar] [CrossRef]
- Perdrix, A.; Travers, A.; Chelli, M.H.; Escalier, D.; Do Rego, J.L.; Milazzo, J.P.; Mousset-Simon, N.; Mac, B.; Rives, N. Assessment of Acrosome and Nuclear Abnormalities in Human Spermatozoa with Large Vacuoles. Hum. Reprod. 2011, 26, 47–58. [Google Scholar] [CrossRef]
- Tarozzi, N.; Nadalini, M.; Stronati, A.; Bizzaro, D.; Dal Prato, L.; Coticchio, G.; Borini, A. Anomalies in Sperm Chromatin Packaging: Implications for Assisted Reproduction Techniques. Reprod. Biomed. Online 2009, 18, 486–495. [Google Scholar] [CrossRef]
- Lee, T.H.; Liu, C.H.; Shih, Y.T.; Tsao, H.M.; Huang, C.C.; Chen, H.H.; Lee, M.S. Magnetic-Activated Cell Sorting for Sperm Preparation Reduces Spermatozoa with Apoptotic Markers and Improves the Acrosome Reaction in Couples with Unexplained Infertility. Hum. Reprod. 2010, 25, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Deemeh, M.R.; Mesbah-Namin, S.A.; Movahedin, M. Selecting Motile, Non-Apoptotic and Induced Spermatozoa for Capacitation without Centrifuging by MACS-Up Method. Andrologia 2022, 54, e14405. [Google Scholar] [CrossRef] [PubMed]
- Quinn, M.M.; Jalalian, L.; Ribeiro, S.; Ona, K.; Demirci, U.; Cedars, M.I.; Rosen, M.P. Microfluidic Sorting Selects Sperm for Clinical Use with Reduced DNA Damage Compared to Density Gradient Centrifugation with Swim-up in Split Semen Samples. Hum. Reprod. 2018, 33, 1388–1393. [Google Scholar] [CrossRef] [Green Version]
- Göde, F.; Gürbüz, A.S.; Tamer, B.; Pala, I.; Isik, A.Z. The Effects of Microfluidic Sperm Sorting, Density Gradient and Swim-up Methods on Semen Oxidation Reduction Potential. Urol. J. 2020, 17, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, A.; Tavalaee, M.; Deemeh, M.R.; Azadi, L.; Fazilati, M.; Nasr-Esfahani, M.H. Zeta Potential vs Apoptotic Marker: Which Is More Suitable for ICSI Sperm Selection? J. Assist. Reprod. Genet. 2013, 30, 1181–1186. [Google Scholar] [CrossRef] [Green Version]
- Razavi, S.H.; Nasr-Esfahani, M.H.; Deemeh, M.R.; Shayesteh, M.; Tavalaee, M. Evaluation of Zeta and HA-Binding Methods for Selection of Spermatozoa with Normal Morphology, Protamine Content and DNA Integrity. Andrologia 2010, 42, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.J.A.; Barnhart, K.T.K.; Schlegel, P.P.N. Do Sperm DNA Integrity Tests Predict Pregnancy with in Vitro Fertilization? Fertil. Steril. 2008, 89, 823–831. [Google Scholar] [CrossRef]
- Meseguer, M.; Santiso, R.; Garrido, N.; García-Herrero, S.; Remohí, J.; Fernandez, J.L. Effect of Sperm DNA Fragmentation on Pregnancy Outcome Depends on Oocyte Quality. Fertil. Steril. 2011, 95, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Garrido, N.; Remohi, J.; Martínez-Conejero, J.A.; García-Herrero, S.; Pellicer, A.; Meseguer, M. Contribution of Sperm Molecular Features to Embryo Quality and Assisted Reproduction Success. Reprod. Biomed. Online 2008, 17, 855–865. [Google Scholar] [CrossRef]
- Garrido, N.; Martínez-Conejero, J.A.; Jauregui, J.; Horcajadas, J.A.; Simón, C.; Remohí, J.; Meseguer, M. Microarray Analysis in Sperm from Fertile and Infertile Men without Basic Sperm Analysis Abnormalities Reveals a Significantly Different Transcriptome. Fertil. Steril. 2009, 91, 1307–1310. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Inclusion | Exclusion | Keywords |
|---|---|---|---|
| Population |
|
| Human, Homo sapiens, male, men, man, mammals, infertile, infertility, fertility |
| Intervention |
|
| Sperm selection, sperm quality, sperm function, sperm selection methods, sperm selection techniques,MSOME, motile sperm organelle morphology examination, birefringence, polarized light microscope, Raman microscopy, microfluidics, IMSI, intra-cytoplasmic morphologically selected sperm injection, hyaluronic acid, MACS, magnetic-activated cell sorting, swim up, density gradients, Zeta-potential, electrophoresisAI, artificial insemination, insemination, IVF, in vitro fertilization, ICSI, intracytoplasmic sperm injection, PICSI, physiological intra-cytoplasmic sperm injection. |
| Comparison |
|
| |
| Outcomes |
| Sperm quality, morphology, oxidative, free radicals, ROS, oxidative stress, DNA damage, DNA fragmentation, oxidative damage, motility, viability, embryo, blastocyst, zygote, fertility, pregnancy, implantation, live birth, fertilization | |
| Study design |
|
| Classical Article, Clinical Study, Clinical Trial, Clinical Trial, Phase I, Clinical Trial, Phase II, Clinical Trial, Phase III, Clinical Trial, Phase IV, Research study, Comparative Study, Corrected and Republished Article, English Abstract, Journal Article, Observational Study, English longitudinal study, cross-sectional study, Multicenter Study, Observational Study, Randomized Controlled Trial |
| Reference | Aim | Advanced Sperm Selection Technique | Sample Size | Female/Male Inclusion/Exclusion Factors | Fertility Parameters Assessed | Main Results | Conclusions | Does It Improve Fertility Outcomes? |
|---|---|---|---|---|---|---|---|---|
| [16] | To compare IMSI vs. conventional ICSI in patients with severe oligoasthenoteratozoospermia. | IMSI | 446 couples (IMSI: 227; ICSI: 219) | Inclusion:
|
| Patients with severe infertility subjected to IMSI showed significantly higher clinical pregnancy rates than when subjected to conventional ICSI. | IMSI leads to higher pregnancy rates compared to conventional ICSI in patients with severe oligoasthenoteratozoospermia. | Yes |
| [17] | To compare the clinical outcome of IMSI vs. conventional ICSI in unselected infertile couples. | IMSI | 168 cycles (IMSI: 87; ICSI: 81) | NA |
| Although IMSI did not improve overall clinical outcomes, a positive effect in implantation rates, clinical pregnancy, and live births was observed. | IMSI and conventional ICSI procedures provide similar clinical and laboratory results in an unselected infertile population. | No |
| [18] | To evaluate whether IMSI improves ICSI pregnancy rate in infertile couples with repeated failure. | IMSI | 112 couples (IMSI: 62; ICSI: 50) | Inclusion:
|
| Fertilization rate, percentage of high-quality embryos, number of embryos transferred, and pregnancy rate were significantly higher in IMSI than in conventional ICSI. | Fertility outcomes are greater in IMSI than in conventional ICSI, in infertile couples with repeated failure. | Yes |
| [19] | To evaluate whether microinjection of sperm with a normal nuclear shape but large vacuoles affect pregnancy outcome | IMSI | 56 couples (IMSI: 28; ICSI: 28) | Inclusion:
|
| Microinjection of sperm with a normal nuclear shape but large vacuoles led to a significantly lower pregnancy rate per cycle and higher miscarriage rate per pregnancy compared to microinjection of sperm with normal nuclear shape. | Microinjection of sperm with vacuoles reduces pregnancy rates and is associated with early abortion. | No |
| [20] | To assess whether spermatozoa with strictly normal nucleus improves ICSI outcomes. | IMSI | 160 couples (IMSI: 80 couples; ICSI: 80 couples) | NA |
| The percentage of high-quality embryos, implantation, and pregnancy rates were significantly higher and miscarriage rate lower in the IMSI group compared to the ICSI group. | IMSI improves reproductive outcomes. | Yes |
| [21] | To evaluate IMSI vs. conventional ICSI on ART outcomes. | IMSI | 84 couples (IMSI: 51 couples; ICSI: 33 couples) | Exclusion:
|
| Embryonic developmental parameters, clinical pregnancy rate, and the proportion of euploid embryos did not differ between IMSI and conventional ICSI groups. | IMSI does not improve embryo kinetics and quality, or clinical pregnancy rate compared to conventional ICSI. | No |
| [22] | To compare the reproductive outcomes of IMSI vs. IVF and ICSI. | IMSI | 75 couples (Previous IVF failures: 22; Previous ICSI failures: 53) | Inclusion:
|
| Fertilization rates were significantly higher after IMSI than after IVF, but similar to ICSI. The percentage of high-quality embryos, the average number of blastocysts and transferred embryos were significantly higher after IMSI than after conventional IVF or ICSI. | IMSI leads to increase embryo quality and the number of transferred embryos compared to IVF or ICSI. | Yes |
| [23] | To compare IMSI vs. conventional ICSI in terms of neonatal outcomes. | IMSI | 848 couples (IMSI: 275; ICSI: 573) | NA |
| A significantly higher rate of multiple pregnancies was found in the IMSI compared to the ICSI group. A lower, but not statistically significant, proportion of congenital malformations was observed in the IMSI compared to the ICSI group. | IMSI could improve neonatal outcomes. | No |
| [24] | To define the indications for IMSI vs. conventional ICSI in infertile couples with two previous ICSI failures. | IMSI | 216 couples (IMSI: 89; ICSI: 127) | Inclusion:
|
| Fertilization rate and the number of mature oocytes were significantly higher after IMSI than after ICSI. No differences were observed in the other fertility parameters. | In couples with two previous ICSI failures, IMSI does not improve clinical outcomes. | No |
| [25] | To assess the usefulness of IMSI in couples with repeated ICSI failure. | IMSI | 125 couples | Inclusion:
|
| IMSI resulted in significantly higher clinical pregnancy, clinical implantation, delivery, and birth rates compared to the last attempt of conventional ICSI in the same couples. | IMSI improves reproductive outcomes in couples with repeated ICSI failure. | Yes |
| [26] | To analyze the effect of IMSI in infertile couples for selecting patients who may benefit from this procedure. | IMSI | 142 cycles (IMSI: 72; ICSI: 70) | Inclusion:
|
| IMSI resulted in a significant increase in fertilization and high-quality embryo rates compared to ICSI in male factor infertility and in repeated implantation failure patients. No effect, however, was observed in the unselected group of patients. | The application of IMSI is beneficial for a selected group of patients with male factor infertility and repeated implantation failure. | Yes |
| [27] | To compare IMSI in infertile couples with male factor infertility and poor embryo development in their previous ICSI attempts. | IMSI | 57 couples (IMSI: 20; ICSI: 37) | Exclusion:
|
| IMSI was better than ICSI in relation to the number of blastocysts/cycle, number of cycles with all embryos arrested and cycles without embryo transfer. No miscarriages were found in the IMSI group, but two out of three pregnancies in the ICSI group ended in miscarriage. | IMSI improves ART outcomes compared to ICSI, leading to a higher number of transferable embryos in infertile couples with male infertility and poor embryo development. | Yes |
| [28] | To compare clinical outcomes between IMSI vs. ICSI in patients with isolated teratozoospermia. | IMSI | 122 cycles (IMSI: 52; ICSI: 70) | Inclusion:
|
| Compared to ICSI, a significantly lower number of embryos arrested at early developmental stages, and a greater clinical pregnancy rate was observed when IMSI was applied. | IMSI improves ART outcomes in patients with isolated teratozoospermia. | Yes |
| [29] | To determine whether IMSI improves the semen characteristics and reproductive outcomes. | IMSI | 255 couples, (IMSI: 116; ICSI: 139) | Exclusion:
|
| IMSI did not improve clinical outcomes (implantation, clinical pregnancy, and live birth rates) compared to ICSI. | IMSI has no benefit in the first ART attempt. | No |
| [30] | To compare DNA fragmentation, apoptosis and transcript levels in spermatozoa selected using IMSI, compared to conventional ICSI. In addition, embryo kinetics with time-lapse imaging and clinical outcomes were assessed and compared. | IMSI | 80 couples (IMSI: 40; ICSI: 40) | Inclusion:
|
| Fertilization and embryo development were found to be improved after IMSI, in comparison to ICSI. The rates for implantation, biochemical and clinical pregnancy, and live birth were higher in IMSI group; however, only implantation rates showed statistically significant results.Cleavage abnormalities (fragmentation, multinucleation, uneven blastomere, and reverse cleavage) were less frequent in embryos derived from IMSI. Indeed, embryos derived from ICSI cleaved quickly and exhibited a greater number of abnormalities compared to IMSI. | IMSI improves sperm quality in terms of DNA damage and apoptosis. Additionally, IMSI leads to improved clinical outcomes and embryo kinetics in infertile patients. | Yes |
| [31] | To evaluate whether IMSI may influence embryo quality at day 2 compared to conventional ICSI. | IMSI | 331 couples (IMSI: 159; ICSI: 172) | NA |
| No differences in terms of fertilization rate, early embryo cleavage rate, cleavage rate, and day 2 embryo quality were observed between ICSI vs. IMSI. | ICSI and IMSI show similar performance in terms of embryo quality on day 2. | No |
| [32] | To compare the reproductive outcomes of ICSI vs. IMSI in couples with implantation failure. | IMSI | 200 couples (IMSI: 100; ICSI: 100) | Inclusion:
|
| No statistically significant differences were observed between the two groups in all parameters assessed. However, miscarriage, ongoing pregnancy, and live birth rates showed better results (but not statistically significant) in the IMSI group. | IMSI does not improve clinical outcome in couples with implantation failure. | No |
| [33] | To examine whether IMSI improves ART outcomes in cases of advanced maternal age. | IMSI | 66 cycles (IMSI: 33; ICSI: 33) | Inclusion:
|
| Blastocyst formation rate, number of embryos transferred, implantation, and clinical pregnancy rates after IMSI were significantly higher than after ICSI. | IMSI in couples with advanced maternal age increases clinical pregnancy rates. | Yes |
| [34] | To analyze whether IMSI affects ART outcomes in couples with poor ovarian response. | IMSI | 414 cycles (Normal responders, 324 cycles: 164 ICSI and 160 IMSI. Poor responders, 90 cycles: 43 ICSI and 47 IMSI) | Inclusion:
|
| Normal responder group: no differences in terms of cycle outcomes were observed between ICSI- and IMSI-treated couples.Poor responder group: fertilization rate, proportion of cycles with embryo transfer, and number of embryos transferred were significantly lower in IMSI than in ICSI. | IMSI does not improve ART outcomes in couples with poor response to controlled ovarian stimulation. | No |
| [35] | To assess the potential beneficial effect of IMSI in couples with at least three repeated ICSI failure cycles. | IMSI | 207 cycles (IMSI: 53; ICSI:154) | Inclusion:
|
| Rates of implantation rate, clinical pregnancy and delivery were significantly higher in the IMSI than in the IVF–ICSI group. The proportion of miscarriages after IMSI was lower than after IVF of ICSI. | IMSI improves pregnancy outcomes in couples with more than three IVF–ICSI failures. | Yes |
| [36] | To assess whether MSOME improves ART outcomes compared to traditional ICSI. | IMSI | 250 couples (IMSI: 125; ICSI: 125) | Inclusion:
|
| Pregnancy and implantation rates were higher when IMSI was applied compared to ICSI. However, no difference in the proportion of pregnancies that led to a live birth was observed when IMSI and ICSI were compared. | MSOME ameliorates outcomes. | Yes |
| [37] | To investigate whether sperm selection by hyaluronic acid binding (PICSI) could improve ART outcomes in ICSI cycles. | PICSI | 18 couples with 219 oocytes (HA-bound: 107 oocytes; Control: 112 oocytes) | Inclusion:
|
| After IMSI, fertilization and cleavage rates on day 2 in oocytes injected with hyaluronic acid-bound sperm were lower but not significantly different from after conventional ICSI. Blastocyst formation rate and the number of embryos transferred were similar between the two groups. | Sperm selection by hyaluronic acid binding does not improve ART outcomes after ICSI. | No |
| [38] | To compare fertility outcomes in conventional ICSI vs. sperm selection procedure based on hyaluronic acid binding ability in couples with male factor infertility. | PICSI | 56 cycles (PICSI: 19; ICSI: 37) | Inclusion:
|
| Biochemical and clinical pregnancy rates were significantly higher in the PICSI group compared to the ICSI group. No differences in the abortion rate were found between groups. | PICSI increases pregnancy rates in couples with male factor infertility. | Yes |
| [39] | To evaluate the effect of sperm selection procedure based on hyaluronic acid binding ability on ART outcomes in couples with severe teratozoospermia. | PICSI | 152 couples (PICSI: 77; ICSI: 75) | Inclusion:
|
| Fertilization rate per retrieved oocyte, fertilization rate per inseminated oocyte, and the rate of high-quality embryos were significantly higher in the PICSI than in the ICSI group. | PICSI improves fertilization and embryo quality in couples with severe teratozoospermia. | Yes |
| [40] | To analyze ART outcomes comparing the PVP-ICSI and hyaluronic acid-ICSI (PICSI) sperm selection methods. | PICSI | 21 couples with 206 oocytes (PICSI: 103; ICSI: 103) | Exclusion:
|
| A higher incidence of abnormal fertilization rate was observed in the PVP-ICSI group compared to the PICSI group. The proportion of high-quality embryos was similar in both groups. | PICSI improves fertilization rates. | Yes |
| [41] | To examine whether sperm selection by hyaluronic acid binding helps improve ICSI outcomes. | PICSI | 156 couples (PICSI: 78; ICSI: 78) | Exclusion:
|
| No differences in fertilization rate, number of high-quality embryos and clinical pregnancy rates were observed between ICSI and PICSI groups. | PICSI does not improve ART outcomes. | No |
| [42] | To investigate the efficiency of sperm selection based on hyaluronic acid binding ability vs. standard ICSI in terms of live birth rate. | PICSI | 2766 couples (PICSI: 1386; ICSI: 1380) | Inclusion:
|
| Miscarriage rates were significantly lower in the PICSI than in the ICSI group. Clinical pregnancy or preterm birth were different between the two groups. | PICSI does not improve clinical pregnancy rates but reduces miscarriage. | No |
| [43] | To examine the effect of sperm selection procedure based on hyaluronic acid binding ability on ART outcomes compared to conventional ICSI. | PICSI | 250 couples (PICSI: 110; ICSI: 140) | Inclusion:
|
| Fertilization, implantation, clinical pregnancy, and live birth rates were significantly higher and pregnancy loss significantly lower in PICSI compared to conventional ICSI. | Sperm selection based on hyaluronic acid binding is useful to improve clinical pregnancy rates. | Yes |
| [44] | To assess whether sperm selection based on hyaluronic acid binding ability affects ICSI outcomes | PICSI | 50 couples (PICSI: 25; ICSI: 25) | Exclusion:
|
| Fertilization rate was significantly higher in the hyaluronic acid group compared to conventional ICSI. No differences, nevertheless, were found in the other fertility parameters assessed. | Sperm selection based on the ability to bind hyaluronic acid improves ICSI outcomes, in terms of fertilization rate. | Yes |
| [45] | To test whether the sperm selection procedure based on hyaluronic acid binding ability improves ART outcomes. | PICSI | 379 couples (PICSI: 293; ICSI: 86) | Inclusion:
|
| High-quality embryo and implantation rates were significantly higher in PICSI than in the control group. A trend towards a better pregnancy rate per transfer was also found in the PICSI compared to the control group. | Sperm selection based on the ability to bind hyaluronic acid is beneficial in ICSI treatments. | Yes |
| [46] | To evaluate whether the sperm selection procedure based on hyaluronic acid binding ability affects ICSI outcomes. | PICSI | 680 couples (PICSI: 269; ICSI: 411) | Exclusion:
|
| Sperm selection procedure based on the ability to bind hyaluronic acid led to a significantly higher implantation rate and lower pregnancy loss compared to the control group. | Sperm selection through hyaluronic acid binding is beneficial for patients subjected to ICSI. | Yes |
| [47] | To evaluate clinical and embryo outcomes after sperm selection with MACS. | MACS | 196 couples (MACS: 122; Control: 74) | Inclusion:
|
| Cleavage and pregnancy rates were significantly higher in MACS group than in the control. Implantation rates did not increase after sperm selection by MACS. | MACS improves pregnancy rates in ICSI treatments. | Yes |
| [48] | To analyze the effectiveness of MACS in the removal of apoptotic sperm, in a population of patients subject to IVF/ICSI. | MACS | 92 couples (MACS: 46; Control: 46) | NA |
| No differences in ART outcomes were found between MACS and the control. In spite of this, when couples were split into two groups based on the involvement of own or donated oocytes, MACS led to higher fertilization rates in the own oocyte group and greater clinical pregnancy in the donor oocyte group. | MACS improves fertilization and clinical pregnancy rates, in the case of donated oocytes. | Yes |
| [49] | To determine the impact of MACS on live-birth delivery rates after ICSI in couples with oocyte donation. | MACS | 263 couples (MACS: 138; Control: 125) | Inclusion:
|
| Similar results were obtained for all fertility parameters assessed in the two groups. | Sperm selection by MACS technology does not improve the reproductive outcome of ICSI in couples undergoing oocyte donation. | No |
| [50] | To determine whether MACS increases live birth rate in couples presenting a high level of sperm DNA fragmentation. | MACS | 305 couples (MACS: 87; Control: 218) | Inclusion:
|
| No differences in live birth rates were observed between MACS and the control. There was no evidence of miscarriage in the MACS group, whereas there were 10 miscarriages in the control. | Density gradient centrifugation followed by MACS in combination with ICSI has the potential to reduce the incidence of miscarriage in ICSI-derived pregnancies. | Yes |
| [51] | To evaluate whether the elimination of apoptotic sperm through MACS improves ICSI outcomes. | MACS | 74 couples (MACS: 37; Control: 37) | Inclusion:
|
| Fertilization and blastocyst rates were increased in MACS compared to the control. No differences were observed in pregnancy rates. | MACS increases fertilization and blastocyst rates. | Yes |
| [52] | To evaluate the beneficial effect of MACS on ICSI in patients with teratozoospermia. | MACS | 26 couples (Half of the mature oocytes were fertilized with conventional ICSI, and the second half after MACS) | Inclusion:
|
| A significantly higher percentage of high-quality blastocysts was found in MACS compared to the control in women older than 30 years. | Sperm selection of non-apoptotic spermatozoa by MACS may be a useful method in couples with male infertility due to teratozoospermia and when the female is older than 30 years. | Yes |
| [53] | To assess the efficiency of sperm selection by MACS in a prospective randomized trial. | MACS | 62 couples (MACS: 29; Control: 33) | Exclusion:
|
| High quality embryos, implantation, and pregnancy rates were higher in MACS than in the control. No differences were found for fertilization rates. | Sperm selection by MACS can improve clinical ICSI outcomes. | Yes |
| [54] | To evaluate whether a microfluidic device improves embryo and clinical outcomes in ICSI cycles. | Microfluidic sperm sorting | 181 couples (Microfluidics: 91; Control: 90) | Inclusion:
|
| No significant differences in clinical pregnancy and ongoing pregnancy rates were reported between groups. | Microfluidic device does not enhance ART outcomes. | No |
| [55] | To evaluate whether a microfluidic sperm sorting device is useful to select sperm with high chromatin fragmentation. | Microfluidic sperm sorting | 15 couples (Microfluidics: 4; Control: 11) | NA |
| A higher clinical pregnancy rate was observed when the microfluidic device was used, leading patients with repeated ART failure and compromised sperm DNA integrity to achieve pregnancy. | Microfluidic device improves pregnancy rates and is particularly useful in patients with repeated ART failure and disrupted sperm DNA integrity. | Yes |
| [56] | To investigate the putative beneficial effect of microfluidic sperm sorting device on clinical outcomes. | Microfluidic sperm sorting | 81 couples (Half of the embryos were produced after microfluidics and the other half served as a control) | Inclusion:
|
| No differences in terms of clinical pregnancy, live birth, and miscarriage rates were found between groups. | Neither laboratory results nor clinical outcomes are improved by sperm selection through microfluidics. | No |
| [57] | To analyze the effect of microfluidics sperm selection on the results of ICSI cycles in patients with unexplained infertility. | Microfluidic sperm sorting | 122 couples (Microfluidics: 61; Control: 61) | Inclusion:
|
| The number of high-quality embryos was significantly greater in the microfluidics than in the control group. No differences between groups were found in the other fertility parameters. | Sperm selection through microfluidics prior to IVF does not alter fertilization, clinical pregnancy, or live birth rates in couples with unexplained infertility. | No |
| [58] | To compare the effect of conventional sperm selection method vs. microfluidics selection on ART outcomes. | Microfluidic sperm sorting | 428 couples (Microfluidics: 116; Control: 312) | NA |
| In recurrent ART failure patients, fertilization rate was higher in the microfluidic group compared to the control. No differences in pregnancy rates were observed. | Microfluidics can improve fertilization rates in patients with repeated ART failure. | Yes |
| [59] | To evaluate whether Zeta-potential can be used to select sperm with intact DNA in non-normospermic patients, and to assess the impact of this selection on fertility parameters. | Zeta-potential | 54 couples who used oocyte donors | Inclusion:
|
| No differences in embryo development parameters were found when sperm were selected by zeta-potential. | Zeta-potential reduces DNA fragmentation but does not improve laboratory outcomes. | No |
| [60] | To evaluate the efficacy of Zeta-potential to recover sperm with intact chromatin, and to assess whether this procedure improves ICSI outcomes. | Zeta-potential | 30 couples (Half of the oocytes fertilized with sperm selected by zeta-potential and the second half served as a control) | Exclusion:
|
| Fertilization rates were significantly higher when sperm were selected by Zeta-potential. Pregnancy and implantation rates in the Zeta-potential group did not differ from the control. | Selection of sperm through Zeta-potential may lead to higher fertilization rates but does not improve pregnancy or implantation rates. | Yes |
| [61] | To evaluate polarization microscopy as a method for sperm selection before ICSI. | Birefringence | 231 couples (Birefringence method + ICSI: 112; Conventional ICSI: 119) | Exclusion:
|
| The proportion of high-quality embryos on day 3 and their ability to implant and progress beyond 16 weeks of gestation were higher when sperm were selected by birefringence. | Not only is birefringence a diagnostic tool, but it is also an accurate and novel method for sperm selection that improves ART outcomes. | Yes |
| [62] | To evaluate the effectiveness of laser to detect those viable sperm among immotile sperm for their use in ICSI cycles. | Laser beam | 77 couples (Laser method: 45; Control: 32) | Inclusion:
|
| Fertilization and cleavage rates were significantly higher when sperm were selected by laser, compared to conventional sperm selection. | Application of a single laser shot for sperm selection improves fertilization and cleavage rates. | Yes |
| Reference | Advanced Sperm Selection Technique | Primary Sperm Quality/Functionality Parameter Assessed | Main Results | Does It Improve Fertility Outcomes? |
|---|---|---|---|---|
| [29] | IMSI |
| No differences for DNA fragmentation index, chromatin condensation and sperm morphology were found. | No |
| [30] | IMSI |
| A lower percentage of DNA fragmentation and transcript levels of apoptotic genes was observed in MSOME-selected spermatozoa. | Yes |
| [36] | IMSI |
| Incidence of DNA fragmentation was lower in sperm selected through IMSI. | Yes |
| [47] | MACS |
| The percentage of sperm with normal morphology increased after selection by MACS. | Yes |
| [48] | MACS |
| Sperm motility, viability and morphology were better after selection through MACS | Yes |
| [49] | MACS |
| Sperm selection by MACS did not alter the proportions of motile sperm. | No |
| [44] | PICSI |
| A significant negative correlation between sperm bound to hyaluronic acid and DNA fragmentation, chromatin condensation, and sperm morphology was observed. | Yes |
| [60] | Zeta-potential |
| The percentage of DNA-damaged sperm and chromatin condensation were significantly reduced when Zeta-potential was applied for sperm selection. | Yes |
| [55] | Microfluidic sperm sorting |
| Sperm selected through a microfluidic device exhibited better motility and less DNA damage. | Yes |
| [58] | Microfluidic sperm sorting |
| Microfluidics sperm sorting led to an increase in the percentage of sperm with low DNA fragmentation. | Yes |
| [62] | Laser beam |
| The percentage of sperm classified as viable by the HOS test was comparable to the percentage of sperm that exhibited a movement upon laser incidence. | Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribas-Maynou, J.; Barranco, I.; Sorolla-Segura, M.; Llavanera, M.; Delgado-Bermúdez, A.; Yeste, M. Advanced Sperm Selection Strategies as a Treatment for Infertile Couples: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 13859. https://doi.org/10.3390/ijms232213859
Ribas-Maynou J, Barranco I, Sorolla-Segura M, Llavanera M, Delgado-Bermúdez A, Yeste M. Advanced Sperm Selection Strategies as a Treatment for Infertile Couples: A Systematic Review. International Journal of Molecular Sciences. 2022; 23(22):13859. https://doi.org/10.3390/ijms232213859
Chicago/Turabian StyleRibas-Maynou, Jordi, Isabel Barranco, Maria Sorolla-Segura, Marc Llavanera, Ariadna Delgado-Bermúdez, and Marc Yeste. 2022. "Advanced Sperm Selection Strategies as a Treatment for Infertile Couples: A Systematic Review" International Journal of Molecular Sciences 23, no. 22: 13859. https://doi.org/10.3390/ijms232213859









