Indirect Immobilised Jagged-1 Enhances Matrisome Proteins Associated with Osteogenic Differentiation of Human Dental Pulp Stem Cells: A Proteomic Study
Abstract
:1. Introduction
2. Results
2.1. Characterisation of hDPSCs
2.2. Jagged-1 Enhanced Osteogenic Differentiation of hDPSCs
2.3. Subcellular Fractionation Showed the Existence of Matrisome Proteins
2.4. Each Cellular Compartment Exhibited a Different Amount of Matrisome Core and Matrisome-Associated Proteins
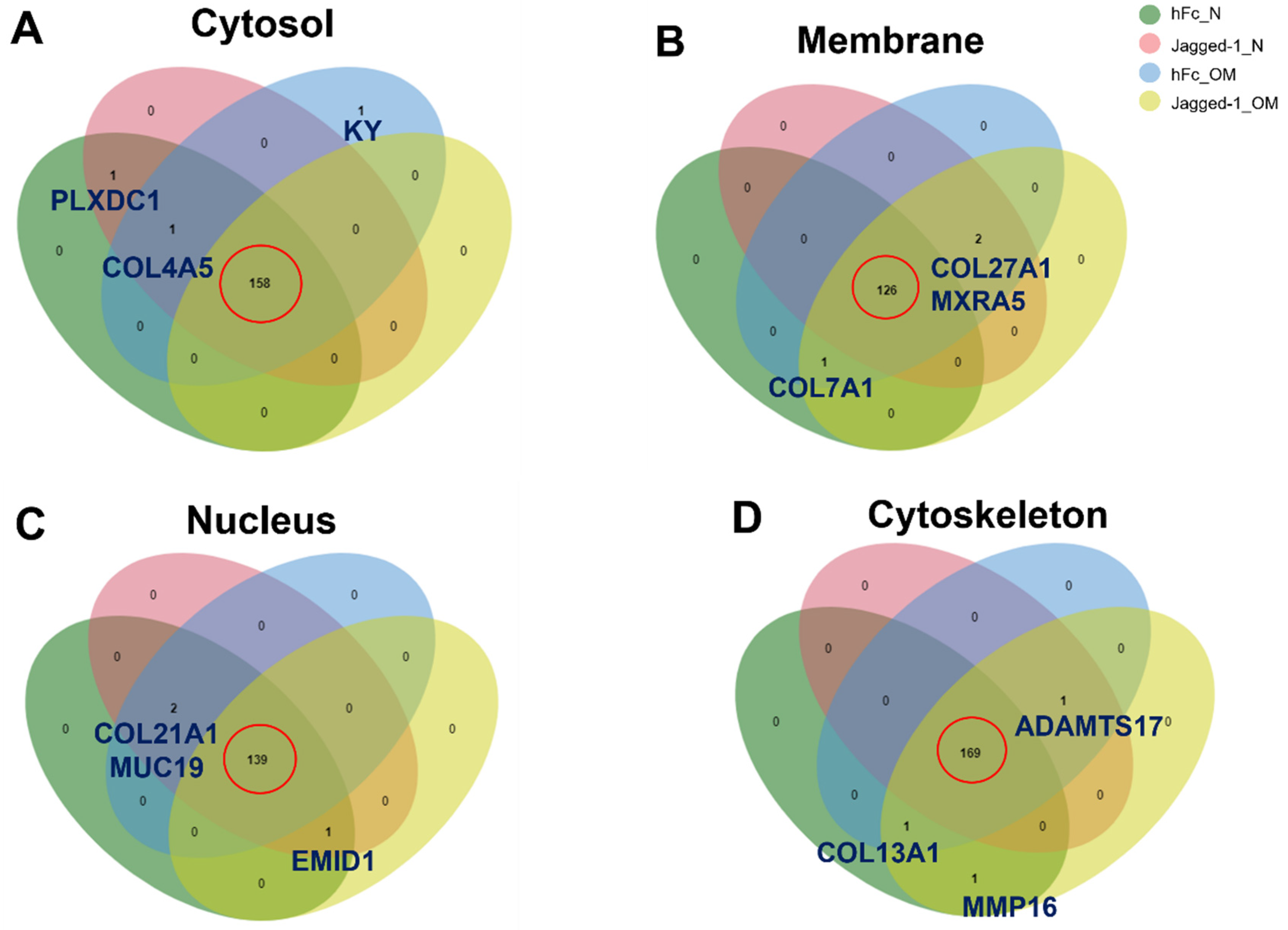
2.5. A Venn Diagram Illustrated the Common and Uncommon Matrisome Proteins from Different Culture Conditions
2.6. Metascape Gene List Analysis Reported the Relationship of Matrisome Proteins with Osteogenesis/Osteogenic Differentiation
2.7. Protein–Protein Interaction Enrichment Analysis of Common Matrisome Proteins Demonstrated Distinctive Interaction among Different Cellular Compartments
2.8. GO from Different Culture Conditions in Each Cellular Component Displayed Several Unique Gene Ontologies
3. Discussion
4. Materials and Methods
4.1. hDPSCs Isolation and Culture
4.2. Jagged-1 Immobilisation
4.3. Cell Preparation
4.4. Mineralisation Assay
4.5. Subcellular Extraction
4.6. Sample Preparation for Shotgun Proteomics
4.7. Sample Preparation
4.8. Liquid Chromatography–Tandem Mass Spectrometry (LC-MS)
4.9. Bioinformatics and Data Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADAMTS17 | A disintegrin and metalloproteinase with thrombospondin motifs 17 |
| ADM | Adipogenic differentiation medium |
| Akt | Protein Kinase B |
| ALP | Alkaline phosphatase |
| AML | Acute myelogenous leukaemia |
| ARS | Alizarin Red S |
| BMPs | Bone morphogenetic proteins |
| BMSCs | Bone marrow stem cells |
| Cbfa1 | Core-binding factor subunit alpha-1 |
| Cluster ID | Cluster identification |
| COL7A1 | Collagen alpha-1 (VII) chain |
| COL13A1 | Collagen alpha-1 (XIII) chain |
| COL21A1 | Collagen alpha-1 (XXI) chain |
| COL27A1 | Collagen alpha-1 (XXVII) chain |
| COL4A5 | Collagen alpha-5 (IV) chain |
| DOP1B | Protein dopey-2 |
| ECM | Extracellular matrix |
| EMID1 | EMI domain-containing protein 1 |
| ERK | Extracellular signal-regulated kinase |
| GO | Gene ontology |
| hDPs | Human dental pulp cells |
| hDPSCs | Human dental pulp stem cells |
| hFc | Human IgG Fc fragment |
| IGF | Insulin-like growth factor |
| IGFBPs | Insulin-like growth factor-binding proteins |
| Jagged-1 | Indirect immobilised Jagged-1 |
| JNK | Jun kinases |
| KY | Kyphoscoliosis peptidase |
| LC-MS | Liquid Chromatography–Tandem Mass Spectrometry |
| MAPK | Mitogen-activated protein kinase |
| MCODE | Molecular Complex Detection |
| MeV | MultiExperiment Viewer |
| MMP16 | Matrix metalloproteinase-16 |
| MSCs | Mesenchymal stem cells |
| mTOR | Mammalian target of rapamycin |
| MUC19 | Mucin-19 |
| MXRA5 | Matrix-remodeling-associated protein 5 |
| NanoLC-MS/MS | Nano-liquid chromatography–tandem mass spectrometry |
| NCAM | Neural cell adhesion molecule |
| NICD | Notch intracellular domain |
| OCN | Osteocalcin |
| OM | Osteogenic differentiation medium |
| OPN | Osteopontin |
| PBS | Phosphate-buffered saline |
| PCR | Polymerase chain reaction |
| PDLSCs | Periodontal ligament stem cells |
| PI3K | Phosphatidylinositol 3-kinase |
| PLXDC1 | Plexin domain-containing protein 1 |
| PPI | Protein–protein interaction |
| Runx2 | Runt-related transcription factor 2 |
| SDS | Sodium Dodecyl Sulfate |
| SMAD | Suppressor of mothers against decapentaplegic |
| TGF-β | Transforming growth factor-beta |
References
- Nuti, N.; Corallo, C.; Chan, B.M.; Ferrari, M.; Gerami-Naini, B. Multipotent Differentiation of Human Dental Pulp Stem Cells: A Literature Review. Stem Cell Rev. Rep. 2016, 12, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Fujimoto, A.; Ito, A.; Yoshimi, R.; Ueda, M. Cluster analysis and gene expression profiles: A cDNA microarray system-based comparison between human dental pulp stem cells (hDPSCs) and human mesenchymal stem cells (hMSCs) for tissue engineering cell therapy. Biomaterials 2006, 27, 3766–3781. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Kawase-Koga, Y.; Hojo, H.; Yano, F.; Sato, M.; Chung, U.-i.; Ohba, S.; Chikazu, D. Bone regeneration by human dental pulp stem cells using a helioxanthin derivative and cell-sheet technology. Stem Cell Res. Ther. 2018, 9, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, L.; Yang, F.; Shen, H.; Hu, X.; Mochizuki, C.; Sato, M.; Wang, S.; Zhang, Y. The effect of composition of calcium phosphate composite scaffolds on the formation of tooth tissue from human dental pulp stem cells. Biomaterials 2011, 32, 7053–7059. [Google Scholar] [CrossRef] [PubMed]
- Bronckaers, A.; Hilkens, P.; Fanton, Y.; Struys, T.; Gervois, P.; Politis, C.; Martens, W.; Lambrichts, I. Angiogenic Properties of Human Dental Pulp Stem Cells. PLoS ONE 2013, 8, e71104. [Google Scholar] [CrossRef] [Green Version]
- Potdar, P.D.; Jethmalani, Y.D. Human dental pulp stem cells: Applications in future regenerative medicine. World J. Stem Cells 2015, 7, 839–851. [Google Scholar] [CrossRef]
- Ohkoshi, S.; Hara, H.; Hirono, H.; Watanabe, K.; Hasegawa, K. Regenerative medicine using dental pulp stem cells for liver diseases. World J. Gastrointest. Pharmacol. Ther. 2017, 8, 1–6. [Google Scholar] [CrossRef]
- Gronthos, S.; Brahim, J.; Li, W.; Fisher, L.W.; Cherman, N.; Boyde, A.; DenBesten, P.; Robey, P.G.; Shi, S. Stem Cell Properties of Human Dental Pulp Stem Cells. J. Dent. Res. 2002, 81, 531–535. [Google Scholar] [CrossRef]
- Luke, A.M.; Patnaik, R.; Kuriadom, S.; Abu-Fanas, S.; Mathew, S.; Shetty, K.P. Human dental pulp stem cells differentiation to neural cells, osteocytes and adipocytes-An in vitro study. Heliyon 2020, 6, e03054. [Google Scholar] [CrossRef] [Green Version]
- Luzuriaga, J.; Polo, Y.; Pastor-Alonso, O.; Pardo-Rodríguez, B.; Larrañaga, A.; Unda, F.; Sarasua, J.-R.; Pineda, J.R.; Ibarretxe, G. Advances and Perspectives in Dental Pulp Stem Cell Based Neuroregeneration Therapies. Int. J. Mol. Sci. 2021, 22, 3546. [Google Scholar] [CrossRef]
- Davies, O.G.; Cooper, P.R.; Shelton, R.M.; Smith, A.J.; Scheven, B.A. A comparison of the in vitro mineralisation and dentinogenic potential of mesenchymal stem cells derived from adipose tissue, bone marrow and dental pulp. J. Bone Miner. Metab. 2015, 33, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Osathanon, T.; Nowwarote, N.; Pavasant, P. Expression and influence of Notch signaling in oral squamous cell carcinoma. J. Oral Sci. 2016, 58, 283–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phothichailert, S.; Nowwarote, N.; Fournier, B.P.J.; Trachoo, V.; Roytrakul, S.; Namangkalakul, W.; Osathanon, T. Effects of decellularized extracellular matrix derived from Jagged1-treated human dental pulp stem cells on biological responses of stem cells isolated from apical papilla. Front. Cell Dev. Biol. 2022, 10, 948812. [Google Scholar] [CrossRef] [PubMed]
- Pongjantarasatian, S.; Nowwarote, N.; Rotchanakitamnuai, V.; Srirodjanakul, W.; Saehun, R.; Janebodin, K.; Manokawinchoke, J.; Fournier, B.P.J.; Osathanon, T. A gamma-Secretase Inhibitor Attenuates Cell Cycle Progression and Invasion in Human Oral Squamous Cell Carcinoma: An In Vitro Study. Int. J. Mol. Sci. 2022, 23, 8869. [Google Scholar] [CrossRef] [PubMed]
- Zieba, J.T.; Chen, Y.T.; Lee, B.H.; Bae, Y. Notch Signaling in Skeletal Development, Homeostasis and Pathogenesis. Biomolecules 2020, 10, 332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Sato, C.; Cerletti, M.; Wagers, A. Notch signaling in the regulation of stem cell self-renewal and differentiation. Curr. Top. Dev. Biol. 2010, 92, 367–409. [Google Scholar] [CrossRef]
- Ballhause, T.M.; Jiang, S.; Baranowsky, A.; Brandt, S.; Mertens, P.R.; Frosch, K.H.; Yorgan, T.; Keller, J. Relevance of Notch Signaling for Bone Metabolism and Regeneration. Int. J. Mol. Sci. 2021, 22, 1325. [Google Scholar] [CrossRef]
- Campbell, L.J.; Levendusky, J.L.; Steines, S.A.; Hyde, D.R. Retinal regeneration requires dynamic Notch signaling. Neural Regen. Res. 2022, 17, 1199–1209. [Google Scholar] [CrossRef]
- Osathanon, T.; Ritprajak, P.; Nowwarote, N.; Manokawinchoke, J.; Giachelli, C.; Pavasant, P. Surface-bound orientated Jagged-1 enhances osteogenic differentiation of human periodontal ligament-derived mesenchymal stem cells. J. Biomed. Mater. Res. A 2013, 101, 358–367. [Google Scholar] [CrossRef]
- Kopan, R. Notch signaling. Cold Spring Harb. Perspect. Biol. 2012, 4, a011213. [Google Scholar] [CrossRef]
- Sukarawan, W.; Peetiakarawach, K.; Pavasant, P.; Osathanon, T. Effect of Jagged-1 and Dll-1 on osteogenic differentiation by stem cells from human exfoliated deciduous teeth. Arch. Oral Biol. 2016, 65, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Osathanon, T.; Manokawinchoke, J.; Egusa, H.; Pavasant, P. Notch signaling partly regulates the osteogenic differentiation of retinoic acid-treated murine induced pluripotent stem cells. J. Oral Sci. 2017, 59, 405–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manokawinchoke, J.; Nattasit, P.; Thongngam, T.; Pavasant, P.; Tompkins, K.A.; Egusa, H.; Osathanon, T. Indirect immobilized Jagged1 suppresses cell cycle progression and induces odonto/osteogenic differentiation in human dental pulp cells. Sci. Rep. 2017, 7, 10124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kornsuthisopon, C.; Manokawinchoke, J.; Sonpoung, O.; Osathanon, T.; Damrongsri, D. Interleukin 15 participates in Jagged1-induced mineralization in human dental pulp cells. Arch. Oral Biol. 2021, 128, 105163. [Google Scholar] [CrossRef]
- Beckstead, B.L.; Santosa, D.M.; Giachelli, C.M. Mimicking cell-cell interactions at the biomaterial-cell interface for control of stem cell differentiation. J. Biomed. Mater. Res. A 2006, 79, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Manokawinchoke, J.; Watcharawipas, T.; Ekmetipunth, K.; Jiamjirachart, M.; Osathanon, T. Dorsomorphin attenuates Jagged1-induced mineralization in human dental pulp cells. Int. Endod. J. 2021, 54, 2229–2242. [Google Scholar] [CrossRef] [PubMed]
- Kornsuthisopon, C.; Chansaenroj, A.; Manokawinchoke, J.; Tompkins, K.A.; Pirarat, N.; Osathanon, T. Non-canonical Wnt signaling participates in Jagged1-induced osteo/odontogenic differentiation in human dental pulp stem cells. Sci. Rep. 2022, 12, 7583. [Google Scholar] [CrossRef]
- Manokawinchoke, J.; Sumrejkanchanakij, P.; Subbalekha, K.; Pavasant, P.; Osathanon, T. Jagged1 inhibits osteoprotegerin expression by human periodontal ligament cells. J. Periodontal. Res. 2016, 51, 789–799. [Google Scholar] [CrossRef]
- Karamanos, N.K.; Theocharis, A.D.; Piperigkou, Z.; Manou, D.; Passi, A.; Skandalis, S.S.; Vynios, D.H.; Orian-Rousseau, V.; Ricard-Blum, S.; Schmelzer, C.E.H.; et al. A guide to the composition and functions of the extracellular matrix. FEBS J. 2021, 288, 6850–6912. [Google Scholar] [CrossRef]
- Nowwarote, N.; Petit, S.; Ferre, F.C.; Dingli, F.; Laigle, V.; Loew, D.; Osathanon, T.; Fournier, B.P.J. Extracellular Matrix Derived From Dental Pulp Stem Cells Promotes Mineralization. Front. Bioeng. Biotechnol. 2021, 9, 740712. [Google Scholar] [CrossRef]
- Bardou, P.; Mariette, J.; Escudié, F.; Djemiel, C.; Klopp, C. jvenn: An interactive Venn diagram viewer. BMC Bioinform. 2014, 15, 293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- McGough, I.J.; de Groot, R.E.A.; Jellett, A.P.; Betist, M.C.; Varandas, K.C.; Danson, C.M.; Heesom, K.J.; Korswagen, H.C.; Cullen, P.J. SNX3-retromer requires an evolutionary conserved MON2:DOPEY2:ATP9A complex to mediate Wntless sorting and Wnt secretion. Nat. Commun. 2018, 9, 3737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.H.; Trivedi, V.; Diz-Muñoz, A. Understanding the interplay of membrane trafficking, cell surface mechanics, and stem cell differentiation. Semin. Cell Dev. Biol. 2022. [Google Scholar] [CrossRef]
- Shilo, B.-Z.; Schejter, E.D. Regulation of developmental intercellular signalling by intracellular trafficking. EMBO J. 2011, 30, 3516–3526. [Google Scholar] [CrossRef] [Green Version]
- Kandachar, V.; Roegiers, F. Endocytosis and control of Notch signaling. Curr. Opin. Cell Biol. 2012, 24, 534–540. [Google Scholar] [CrossRef] [Green Version]
- Nair, R.; Santos, L.; Awasthi, S.; von Erlach, T.; Chow, L.W.; Bertazzo, S.; Stevens, M.M. Extracellular Vesicles Derived from Preosteoblasts Influence Embryonic Stem Cell Differentiation. Stem Cells Dev. 2014, 23, 1625–1635. [Google Scholar] [CrossRef]
- Fortini, M.E.; Bilder, D. Endocytic regulation of Notch signaling. Curr. Opin. Genet. Dev. 2009, 19, 323–328. [Google Scholar] [CrossRef] [Green Version]
- Naba, A.; Clauser, K.R.; Ding, H.; Whittaker, C.A.; Carr, S.A.; Hynes, R.O. The extracellular matrix: Tools and insights for the “omics” era. Matrix Biol. 2016, 49, 10–24. [Google Scholar] [CrossRef]
- Krasny, L.; Huang, P.H. Advances in the proteomic profiling of the matrisome and adhesome. Expert Rev. Proteom. 2021, 18, 781–794. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Krishnan, M.; Ganganahalli, G.; Saraswathy, S.; Johnson, R.; Iyer, S.R. Microarray illustrates enhanced mechanistic action towards osteogenesis for magnesium aluminate spinel ceramic-based polyphasic composite scaffold with mesenchymal stem cells and bone morphogenetic protein 2. J. Biomed. Mater. Res. B Appl. Biomater. 2022. [Google Scholar] [CrossRef] [PubMed]
- Satoh, C.; Kondoh, T.; Shimizu, H.; Kinoshita, A.; Mishima, H.; Nishimura, G.; Miyazaki, M.; Okano, K.; Kumai, Y.; Yoshiura, K.-i. Brothers with novel compound heterozygous mutations in COL27A1 causing dental and genital abnormalities. Eur. J. Med. Genet. 2021, 64, 104125. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.G.; Volkmuth, W. Cell adhesion and matrix remodeling genes identified by co-expression analysis. Gene Funct. Dis. 2002, 3, 109–112. [Google Scholar] [CrossRef]
- Peffers, M.J.; Collins, J.; Loughlin, J.; Proctor, C.; Clegg, P.D. A proteomic analysis of chondrogenic, osteogenic and tenogenic constructs from ageing mesenchymal stem cells. Stem Cell Res. Ther. 2016, 7, 133. [Google Scholar] [CrossRef] [Green Version]
- Poveda, J.; Sanz, A.B.; Fernandez-Fernandez, B.; Carrasco, S.; Ruiz-Ortega, M.; Cannata-Ortiz, P.; Ortiz, A.; Sanchez-Niño, M.D. MXRA5 is a TGF-β1-regulated human protein with anti-inflammatory and anti-fibrotic properties. J. Cell. Mol. Med. 2017, 21, 154–164. [Google Scholar] [CrossRef]
- Wu, M.; Chen, G.; Li, Y.-P. TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016, 4, 16009. [Google Scholar] [CrossRef] [Green Version]
- Mazziotta, C.; Lanzillotti, C.; Iaquinta, M.R.; Taraballi, F.; Torreggiani, E.; Rotondo, J.C.; Otòn-Gonzalez, L.; Mazzoni, E.; Frontini, F.; Bononi, I.; et al. MicroRNAs Modulate Signaling Pathways in Osteogenic Differentiation of Mesenchymal Stem Cells. Int. J. Mol. Sci. 2021, 22, 2362. [Google Scholar] [CrossRef]
- Ducy, P. CBFA1: A molecular switch in osteoblast biology. Dev. Dyn. 2000, 219, 461–471. [Google Scholar] [CrossRef]
- Komori, T.; Yagi, H.; Nomura, S.; Yamaguchi, A.; Sasaki, K.; Deguchi, K.; Shimizu, Y.; Bronson, R.T.; Gao, Y.H.; Inada, M.; et al. Targeted Disruption of Cbfa1 Results in a Complete Lack of Bone Formation owing to Maturational Arrest of Osteoblasts. Cell 1997, 89, 755–764. [Google Scholar] [CrossRef]
- Oliveros Anerillas, L.; Kingham, P.J.; Lammi, M.J.; Wiberg, M.; Kelk, P. Three-Dimensional Osteogenic Differentiation of Bone Marrow Mesenchymal Stem Cells Promotes Matrix Metallopeptidase 13 (MMP13) Expression in Type I Collagen Hydrogels. Int. J. Mol. Sci. 2021, 22, 13594. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.P.H.; Xu, J.; Xue, M.; Jackson, C. Matrix metalloproteinases in bone development and pathology: Current knowledge and potential clinical utility. Met. Med. 2016, 3, 93–102. [Google Scholar] [CrossRef] [Green Version]
- Papadogiannis, F.; Batsali, A.; Klontzas, M.E.; Karabela, M.; Georgopoulou, A.; Mantalaris, A.; Zafeiropoulos, N.E.; Chatzinikolaidou, M.; Pontikoglou, C. Osteogenic differentiation of bone marrow mesenchymal stem cells on chitosan/gelatin scaffolds: Gene expression profile and mechanical analysis. Biomed. Mater. 2020, 15, 064101. [Google Scholar] [CrossRef] [PubMed]
- Youssef, A.; Aboalola, D.; Han, V.K.M. The Roles of Insulin-Like Growth Factors in Mesenchymal Stem Cell Niche. Stem Cells Int. 2017, 2017, 9453108. [Google Scholar] [CrossRef] [Green Version]
- Rosen, C.J. Insulin-like growth factor I and bone mineral density: Experience from animal models and human observational studies. Best Pract. Res. Clin. Endocrinol. Metab. 2004, 18, 423–435. [Google Scholar] [CrossRef]
- Piecewicz, S.M.; Pandey, A.; Roy, B.; Xiang, S.H.; Zetter, B.R.; Sengupta, S. Insulin-like growth factors promote vasculogenesis in embryonic stem cells. PLoS ONE 2012, 7, e32191. [Google Scholar] [CrossRef] [Green Version]
- Lv, T.; Wu, Y.; Mu, C.; Liu, G.; Yan, M.; Xu, X.; Wu, H.; Du, J.; Yu, J.; Mu, J. Insulin-like growth factor 1 promotes the proliferation and committed differentiation of human dental pulp stem cells through MAPK pathways. Arch. Oral Biol. 2016, 72, 116–123. [Google Scholar] [CrossRef]
- Wang, S.; Mu, J.; Fan, Z.; Yu, Y.; Yan, M.; Lei, G.; Tang, C.; Wang, Z.; Zheng, Y.; Yu, J.; et al. Insulin-like growth factor 1 can promote the osteogenic differentiation and osteogenesis of stem cells from apical papilla. Stem Cell Res. 2012, 8, 346–356. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Mu, J.; Fan, Z.; Lei, G.; Yan, M.; Wang, S.; Tang, C.; Wang, Z.; Yu, J.; Zhang, G. Insulin-like growth factor 1 enhances the proliferation and osteogenic differentiation of human periodontal ligament stem cells via ERK and JNK MAPK pathways. Histochem. Cell Biol. 2012, 137, 513–525. [Google Scholar] [CrossRef]
- Xian, L.; Wu, X.; Pang, L.; Lou, M.; Rosen, C.J.; Qiu, T.; Crane, J.; Frassica, F.; Zhang, L.; Rodriguez, J.P.; et al. Matrix IGF-1 maintains bone mass by activation of mTOR in mesenchymal stem cells. Nat. Med. 2012, 18, 1095–1101. [Google Scholar] [CrossRef]
- Feng, X.; Huang, D.; Lu, X.; Feng, G.; Xing, J.; Lu, J.; Xu, K.; Xia, W.; Meng, Y.; Tao, T.; et al. Insulin-like growth factor 1 can promote proliferation and osteogenic differentiation of human dental pulp stem cells via mTOR pathway. Dev. Growth Differ. 2014, 56, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Jiang, W.; Huang, J.; He, B.-C.; Zuo, G.-W.; Zhang, W.; Luo, Q.; Shi, Q.; Zhang, B.-Q.; Wagner, E.R.; et al. Insulin-like growth factor 2 (IGF-2) potentiates BMP-9-induced osteogenic differentiation and bone formation. J. Bone Mineral. Res. 2010, 25, 2447–2459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansen, J.H.W.; van der Jagt, O.P.; Punt, B.J.; Verhaar, J.A.N.; van Leeuwen, J.P.T.M.; Weinans, H.; Jahr, H. Stimulation of osteogenic differentiation in human osteoprogenitor cells by pulsed electromagnetic fields: An in vitro study. BMC Musculoskelet. Disord. 2010, 11, 188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Tang, Y.; Zhang, X.; Chu, Z.; Liu, Y.; Tang, C. MMP-1 promotes osteogenic differentiation of human bone marrow mesenchymal stem cells via the JNK and ERK pathway. Int. J. Biochem. Cell Biol. 2020, 129, 105880. [Google Scholar] [CrossRef]
- Wu, M.; Cronin, K.; Crane, J.S. Biochemistry, Collagen Synthesis. In StatPearls; StatPearls Publishing Copyright © 2022; StatPearls Publishing LLC.: Tampa, FL, USA, 2022. [Google Scholar]
- Gajko-Galicka, A. Mutations in type I collagen genes resulting in osteogenesis imperfecta in humans. Acta Biochim. Pol. 2002, 49, 433–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kihara, T.; Hirose, M.; Oshima, A.; Ohgushi, H. Exogenous type I collagen facilitates osteogenic differentiation and acts as a substrate for mineralization of rat marrow mesenchymal stem cells in vitro. Biochem. Biophys. Res. Commun. 2006, 341, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Baxter, R.C. IGF binding proteins in cancer: Mechanistic and clinical insights. Nat. Rev. Cancer 2014, 14, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Clemmons, D.R. Role of IGF Binding Proteins in Regulating Metabolism. Trends Endocrinol. Metab. 2016, 27, 375–391. [Google Scholar] [CrossRef]
- Sitar, T.; Popowicz, G.M.; Siwanowicz, I.; Huber, R.; Holak, T.A. Structural basis for the inhibition of insulin-like growth factors by insulin-like growth factor-binding proteins. Proc. Natl. Acad. Sci. USA 2006, 103, 13028–13033. [Google Scholar] [CrossRef] [Green Version]
- Kuznetsov, S.A.; Riminucci, M.; Ziran, N.; Tsutsui, T.W.; Corsi, A.; Calvi, L.; Kronenberg, H.M.; Schipani, E.; Robey, P.G.; Bianco, P. The interplay of osteogenesis and hematopoiesis: Expression of a constitutively active PTH/PTHrP receptor in osteogenic cells perturbs the establishment of hematopoiesis in bone and of skeletal stem cells in the bone marrow. J. Cell Biol. 2004, 167, 1113–1122. [Google Scholar] [CrossRef]
- Le, P.M.; Andreeff, M.; Battula, V.L. Osteogenic niche in the regulation of normal hematopoiesis and leukemogenesis. Haematologica 2018, 103, 1945–1955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Stacy, T.; Binder, M.; Marin-Padilla, M.; Sharpe, A.H.; Speck, N.A. Disruption of the Cbfa2 gene causes necrosis and hemorrhaging in the central nervous system and blocks definitive hematopoiesis. Proc. Natl. Acad. Sci. USA 1996, 93, 3444–3449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manokawinchoke, J.; Pavasant, P.; Sawangmake, C.; Limjeerajarus, N.; Limjeerajarus, C.N.; Egusa, H.; Osathanon, T. Intermittent compressive force promotes osteogenic differentiation in human periodontal ligament cells by regulating the transforming growth factor-beta pathway. Cell Death Dis. 2019, 10, 761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowwarote, N.; Sukarawan, W.; Pavasant, P.; Foster, B.L.; Osathanon, T. Basic fibroblast growth factor regulates phosphate/pyrophosphate regulatory genes in stem cells isolated from human exfoliated deciduous teeth. Stem Cell Res. Ther. 2018, 9, 345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Incharoen, T.; Roytrakul, S.; Likittrakulwong, W. Dietary Germinated Paddy Rice and Stocking Density Affect Egg Performance, Serum Biochemical Properties, and Proteomic and Transcriptomic Response of Laying Hens Exposed to Chronic Heat Stress. Proteomes 2021, 9, 48. [Google Scholar] [CrossRef]
- Johansson, C.; Samskog, J.; Sundström, L.; Wadensten, H.; Björkesten, L.; Flensburg, J. Differential expression analysis of Escherichia coli proteins using a novel software for relative quantitation of LC-MS/MS data. Proteomics 2006, 6, 4475–4485. [Google Scholar] [CrossRef]
- Thorsell, A.; Portelius, E.; Blennow, K.; Westman-Brinkmalm, A. Evaluation of sample fractionation using micro-scale liquid-phase isoelectric focusing on mass spectrometric identification and quantitation of proteins in a SILAC experiment. Rapid Commun. Mass Spectrom. 2007, 21, 771–778. [Google Scholar] [CrossRef]
- Howe, E.A.; Sinha, R.; Schlauch, D.; Quackenbush, J. RNA-Seq analysis in MeV. Bioinformatics 2011, 27, 3209–3210. [Google Scholar] [CrossRef]
















Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chansaenroj, A.; Kornsuthisopon, C.; Roytrakul, S.; Phothichailert, S.; Rochanavibhata, S.; Fournier, B.P.J.; Srithanyarat, S.S.; Nowwarote, N.; Osathanon, T. Indirect Immobilised Jagged-1 Enhances Matrisome Proteins Associated with Osteogenic Differentiation of Human Dental Pulp Stem Cells: A Proteomic Study. Int. J. Mol. Sci. 2022, 23, 13897. https://doi.org/10.3390/ijms232213897
Chansaenroj A, Kornsuthisopon C, Roytrakul S, Phothichailert S, Rochanavibhata S, Fournier BPJ, Srithanyarat SS, Nowwarote N, Osathanon T. Indirect Immobilised Jagged-1 Enhances Matrisome Proteins Associated with Osteogenic Differentiation of Human Dental Pulp Stem Cells: A Proteomic Study. International Journal of Molecular Sciences. 2022; 23(22):13897. https://doi.org/10.3390/ijms232213897
Chicago/Turabian StyleChansaenroj, Ajjima, Chatvadee Kornsuthisopon, Sittiruk Roytrakul, Suphalak Phothichailert, Sunisa Rochanavibhata, Benjamin P. J. Fournier, Supreda Suphanantachat Srithanyarat, Nunthawan Nowwarote, and Thanaphum Osathanon. 2022. "Indirect Immobilised Jagged-1 Enhances Matrisome Proteins Associated with Osteogenic Differentiation of Human Dental Pulp Stem Cells: A Proteomic Study" International Journal of Molecular Sciences 23, no. 22: 13897. https://doi.org/10.3390/ijms232213897
APA StyleChansaenroj, A., Kornsuthisopon, C., Roytrakul, S., Phothichailert, S., Rochanavibhata, S., Fournier, B. P. J., Srithanyarat, S. S., Nowwarote, N., & Osathanon, T. (2022). Indirect Immobilised Jagged-1 Enhances Matrisome Proteins Associated with Osteogenic Differentiation of Human Dental Pulp Stem Cells: A Proteomic Study. International Journal of Molecular Sciences, 23(22), 13897. https://doi.org/10.3390/ijms232213897





