Abstract
Phosphate (P) deficiency severely limits the growth and production of plants. Adventitious root development plays an essential role in responding to low phosphorus stress for apple plants. However, the molecular mechanisms regulating adventitious root growth and development in response to low phosphorus stress have remained elusive. In this study, a mutation (C-T) in the coding region of the apple AUXIN/INDOLE-3-ACETIC ACID 27 (IAA27) gene was identified. MdIAA27T-overexpressing transgenic apple improved the tolerance to phosphorus deficiency, which grew longer and denser adventitious roots and presented higher phosphorous content than the control plants under low phosphorus conditions, while the overexpression of MdIAA27C displayed the opposite trend. Moreover, the heterologous overexpression of MdIAA27 in tobacco yielded the same results, supporting the aforementioned findings. In vitro and in vivo assays showed that MdIAA27 directly interacted with AUXIN RESPONSE FACTOR (ARF8), ARF26 and ARF27, which regulated Small Auxin-Up RNA 76 (MdSAUR76) and lateral organ boundaries domain 16 (MdLBD16) transcription. The mutation in IAA27 resulted in altered interaction modes, which in turn promoted the release of positive ARFs to upregulate SAUR76 and LBD16 expression in low phosphorus conditions. Altogether, our studies provide insights into how the allelic variation of IAA27 affects adventitious root development in response to low phosphorus stress.
1. Introduction
Phosphate (P) is a vital macronutrient that plays an indispensable role in plant growth, development and major metabolic processes. Nevertheless, a large proportion of P interacts with cations (Fe3+, Mg2+, Ca2+ and Al3+), resulting in unavailability for plant uptake. The P content in soil is less than 10 μM [1,2], which can lead to P starvation and limit plant growth and survival. In agricultural production, phosphorus deficiency leads to an imbalance of soil nutrients, which in turn limits the yield potential of multiple crops. Wheat yield is frequently constrained by low phosphorus stress, such as in Australia and other tropical and subtropical regions [3,4,5]. It was also found that phosphorus deficiency could reduce the yield of cotton [6] and soybean crops [7]. Production losses caused by phosphorus are widely reported, especially in some populous countries in the world, such as India, China and the USA [8]. Under phosphorus deficiency conditions, plants have adapted in multiple pathways, such as changing the root growth and architecture [9,10,11], regulating lipid remodeling [12] and enhancing the excretion of organic acids and RNases [13]. A particularly sophisticated strategy is for plants to modify their root structure under phosphorus deficiency. Previous reports had shown that phosphorus stress has a significant influence on modulating the root system architecture (RSA), including the suppression of primary root growth [14], increments in the length and number of lateral roots (LRs) or adventitious roots (ARs) [15], production of a number of cluster roots [16] and enhancement of the density of root hairs [17]. In various plants, roots that derive from non-root tissues, such as stems and leaves, are called adventitious roots. Adventitious roots play a critical role in plant survival under biotic and abiotic stresses [18]. In field conditions, the soil nutrient uptake ability of trees with primary and adventitious roots was higher than that with only initial roots [19]. It has been reported that the initiation and elongation of AR is promoted in many plant species under low phosphorus stress, such as Solanum lycopersicum [20], Populus ussuriensis [21], Oryza sativa [22,23] and Hordeum vulgare [24]. The effect of low phosphorus stress on rice root growth is found to stimulate adventitious root growth, which increases the available root surface area and contributes to nutrient uptake [25]. Most of the previous studies on adventitious root development have centered on herbaceous plants, with little research on woody plants.
Auxin signaling is closely linked with the remodeling of the root architecture under P deficiency. The AUXIN/INDOLE-3-ACETIC ACID (Aux/IAA) proteins, as responsive factors, play vital roles in auxin signaling. Aux/IAA proteins have been revealed to bind with AUXIN RESPONSE FACTOR (ARFs) and suppress the expression of auxin-response genes in the absence of auxin. In contrast, under high auxin levels, Aux/IAA proteins interact with SCFTIR1/AFB, which labels and triggers their ubiquitination and degradation via the 26S proteasome and subsequently releases ARF to activate the expression of auxin downstream responsive genes [26]. Recently, studies on various plants uncovered that the Aux/IAA transcription factors (TFs) are indispensable for the auxin signaling mechanism to regulate root development. For example, in maize, ZmIAA10 played a critical role in the regulation of seminal and lateral root initiation [27]. In Populus, puIAA4, downstream of bZIP53, inhibited adventitious root development [28]. Additionally, Aux/IAA TFs are also involved in biotic and abiotic stress responses. AtIAA5, AtIAA6 and AtIAA19 positively regulated the drought tolerance in Arabidopsis [29]. In rice, overexpression of OsIAA20 positively increased the content of proline to improve drought and salt tolerance [30]. However, few studies have clarified the role of Aux/IAA in regulating adventitious root growth and development in response to P stress.
Apple (Malus domestica), which belongs to the Rosaceae family, occupies an important position in terms of global cultivation area and yield. Previous evidence has demonstrated that transcription factors play a pivotal role in the regulation of P-deficient responses in apple. In a previous study, MdMYB2 positively mediated the phosphate stress response by regulating P starvation-induced (PSI) genes [31]. MdPHR1 improved the phosphorus deficiency of apple by regulating the expression of MdPAP10 [32]. However, the regulatory mechanism of apple Aux/IAA transcription factors in response to phosphorus stress has not been reported. Hence, it is greatly important to explore and reveal the molecular mechanism of the Aux/IAA genes in adventitious root development in apple rootstocks for the breeding of seedlings with more developed roots and increased resistance to phosphorus stress. In our previous bulked segregant sequence (BSA-seq) analysis (NCBI accession number: PRJNA810276, https://www.ncbi.nlm.nih.gov/bioproject/PRJNA810276 accessed on 10 November 2022) of 1002 rootstock progenies crossed from tolerant to phosphorus deficiency apple rootstocks ‘Baleng Crab’ (Malus robusta Rehd.; ‘BC’) and sensitive to phosphorus deficiency apple rootstocks ‘M9’ (Malus pumila Mill.), an auxin-responsive Aux/IAA gene, MdIAA27, was identified.
In this study, MdIAA27 was isolated and investigated in apple ‘Baleng Crab’ (Malus robusta Rehd.; ‘BC’) and ‘M9’ (Malus pumila Mill.) plants. Gene expression profiles demonstrated that MdIAA27 could be induced by low phosphorus treatments. Furthermore, the stable transformation experiment revealed that two linked single-nucleotide polymorphisms (SNPs) in the CDS of MdIAA27 accounted for differences in the length and number of adventitious roots in apple and tobacco, which influenced the phosphorus uptake by roots and thus affected the low phosphorus tolerance. Moreover, IAA27 interacted with ARF8, ARF26 and ARF27, However, under low phosphorus conditions, IAA27 was degraded and thereafter released ARF26 and ARF27, which further upregulated the expression of Small Auxin-Up RNA 76 (SAUR76) and lateral organ boundaries domain 16 (LBD16) to improve the length and number of adventitious roots. In the present study, the allele mutations in IAA27 altered their interactions with ARFs and thereafter influenced adventitious root development in response to low phosphorus stress. This provided basic insights into the molecular mechanism of apple root length and root number responses to low phosphorus stress and offered valuable information for the breeding of phosphorus-tolerant apple rootstocks. Throughout the study, it was found that allele variations significantly altered the function of the IAA27 protein. Previous reports have shown that Aux/IAA was regulated by multiple transcription factors; however, whether allele variation in IAA27 would alter its interaction with other proteins is still unclear. Thus, further investigation remains to verify whether the allele variation in IAA27 affects the binding to other proteins, such as TIR1 and MYB.
2. Results
2.1. An IAA27 Mutant Allele Was Associated with Apple Rootstock Rooting in Low P Conditions
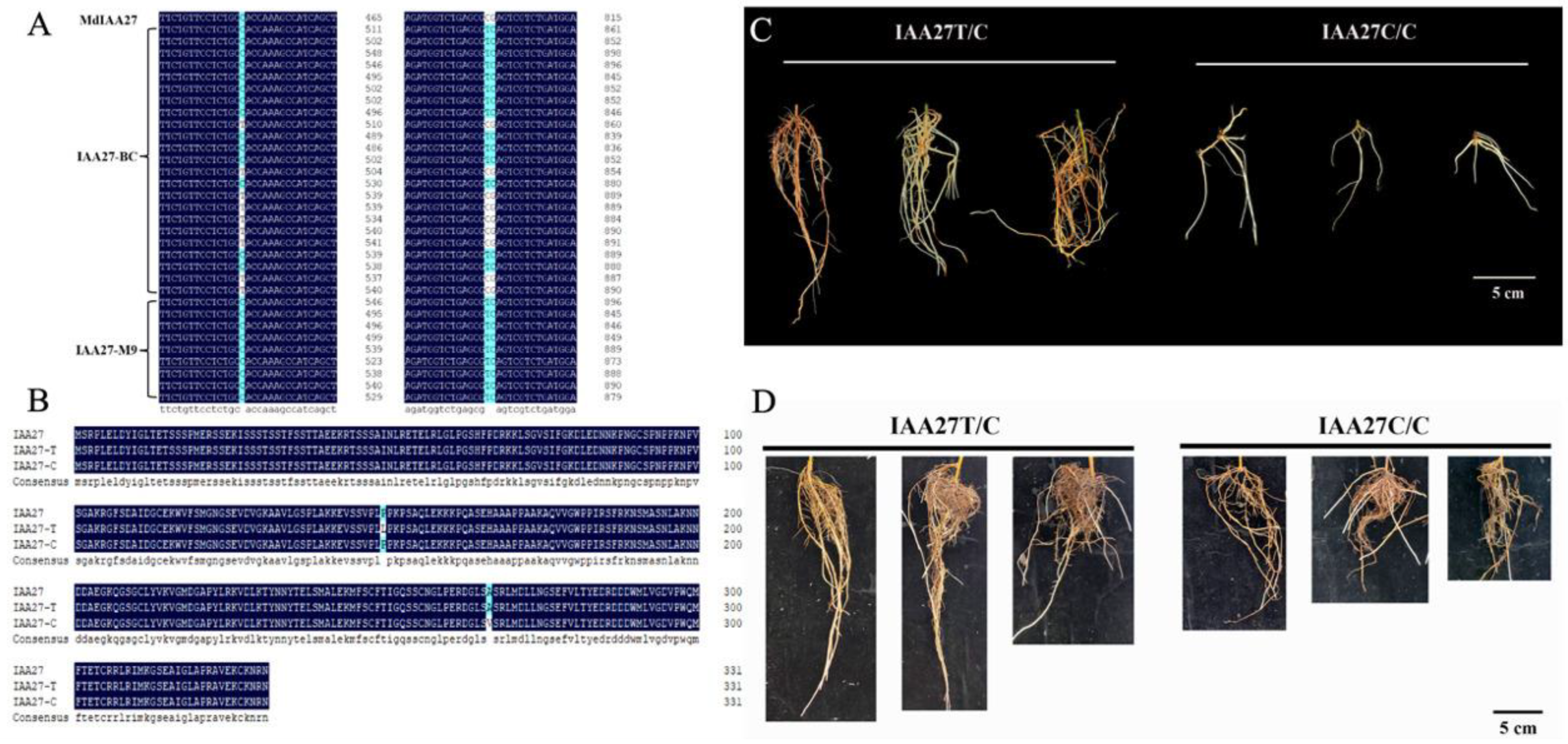
Aux/IAA TF plays vital roles in plant growth and development and is also involved in responses to abiotic stresses. Based on the results of previous BSA analyses, the MdIAA27 (MD17G1189100) gene was potentially related to the regulation of the root length and number in response to phosphorus stress. To investigate the molecular mechanisms of adventitious root development in low phosphorus conditions, the IAA27 gene sequences from the parents ‘BC’ and ‘M9’ were obtained. This revealed a C-T SNP (Figure 1A), resulting in a non-synonymous mutation (Pro to Thr) of the protein sequence (Figure 1B), in a nuclear localization signal (NLS) contributing to degradation [33,34]. Another non-synonymous SNP TC/CG (Figure 1A) caused an amino acid substitution (Val to Ala) (Figure 1B). There was a linkage relationship between the two variants. Adventitious root development in the progenies of ‘BC’ and ‘M9’ displayed a clear pattern: T/C > C/C. We determined that the progenies with longer and denser adventitious roots were homozygous for the T nucleotide (termed the IAA27T/C allele); however, the progenies with less adventitious roots were heterozygous for the IAA27C/C allele (named the IAA27C/C allele) (Figure 1C). Moreover, the longer adventitious roots and higher root number were associated with increased tolerance to low phosphorus stress. After low phosphorus treatment, the adventitious root lengths and root number of IAA27T/C progenies were significantly higher than those of IAA27C/C progenies (Figure 1D).
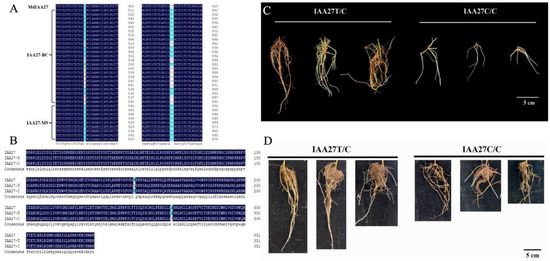
Figure 1.
Identification of IAA27 related to root development in the parents ‘BC’ and ‘M9’ and their progenies. (A) Variation in the IAA27 CDS sequence of the parents ‘BC’ and ‘M9’. (B) Amino acid sequence analysis of IAA27 transcription factors. (C) Phenotypes of adventitious roots from six progenies crossed from ‘BC’ and ‘M9’. The progenies were harvested for adventitious root trait analysis at 30 days after cutting. Scale bars: 5 cm. (D) After low phosphorus treatment for 30 days, adventitious roots of progenies were photographed. Scale bars: 5 cm.
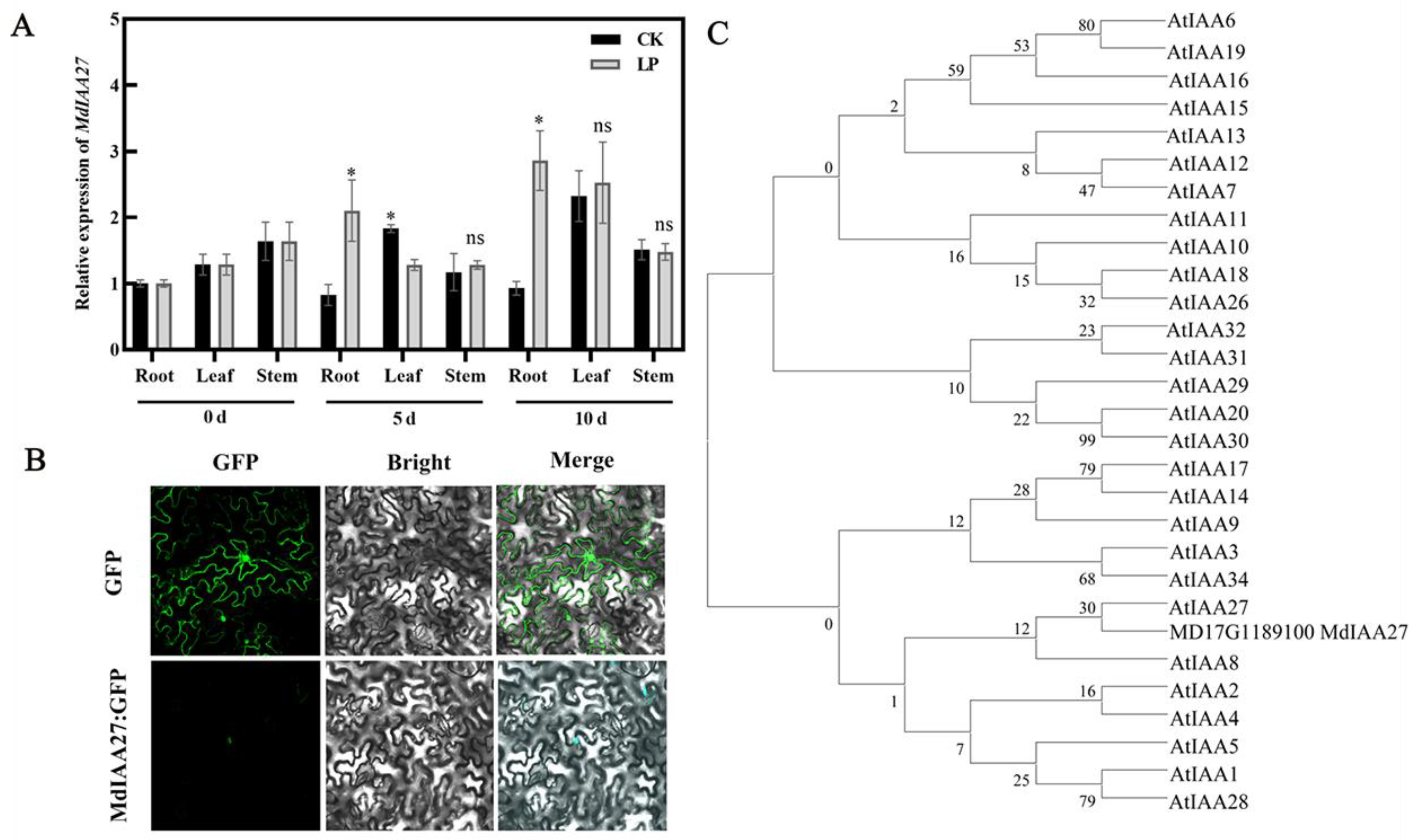
To investigate the expression pattern of MdIAA27, the expression levels of MdIAA27 in various tissues of ‘M9’ under phosphorus deficiency conditions were examined by qRT-PCR. The results showed that MdIAA27 was expressed predominantly in the adventitious roots, and it had a similar expression trend in the leaf and stem, indicating that MdIAA27 is mainly involved in the root response to low phosphorus stress (Figure 2A). The full-length coding sequence lacking the stop codon of MdIAA27 was fused with green fluorescence protein (GFP) and transiently expressed under control of the 35S promoter in tobacco to verify the subcellular location of MdIAA27. The construct was introduced into tobacco leaf cells and the subcellular GFP signal was observed. The result indicated that the MdIAA27-GFP protein was localized in the nucleus (Figure 2B), showing that MdIAA27, similar to other Aux/IAA paralogs, such as SlIAA9 and GmIAA27 [35,36], is localized in the nuclei to affect the auxin response pathways. Phylogenetic analyses of MdIAA27 with the Aux/IAA gene family from A. thaliana were performed. The phylogenetic tree determined that MdIAA27 is closely related to AtIAA27 (Figure 2C).
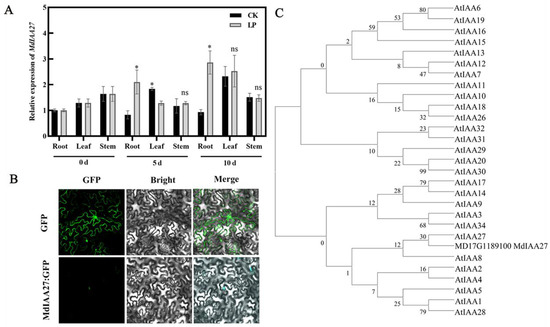
Figure 2.
Expression characteristics of MdIAA27. (A) The qRT-PCR analysis of MdIAA27 expression in root, leaf and stem under low phosphorus conditions. * indicates statistically significant differences at p ≤ 0.05. ns indicates no significant difference between each group. Error bars indicate standard deviation (s.d.) from three biological replicates. (B) Subcellular localization of MdIAA27 in tobacco leaf cells. Scale bars: 10 μm. (C) Phylogenetic analysis of MdIAA27 with Aux/IAA proteins from Arabidopsis.
2.2. Heterologous Expression of MdIAA27 Influenced Tolerance to Phosphate Stress in Transgenic Tobacco
To further verify the function of MdIAA27, it was overexpressed in the tobacco heterologous system. The overexpression lines (MdIAA27T-OE and MdIAA27C-OE) were generated and selected by qRT-PCR and DNA sequence analysis (Supplemental Figure S1A–C). As shown in Figure 3A, all overexpression lines exhibited slightly longer root lengths and denser root numbers than WT under phosphorus-sufficient conditions. However, MdIAA27T-overexpressing lines developed longer and more roots than the WT, but the root development in MdIAA27C-overexpressing lines was suppressed compared with the WT under phosphorus stress treatment conditions (Figure 3A–C). Consistent with the observed root phenotype, P content in the leaves and roots of MdIAA27T transgenic plants was also higher than that of MdIAA27C transgenic lines after low phosphorus treatment (Figure 3D). These results demonstrated that MdIAA27 overexpression in tobacco influenced low phosphorus tolerance.
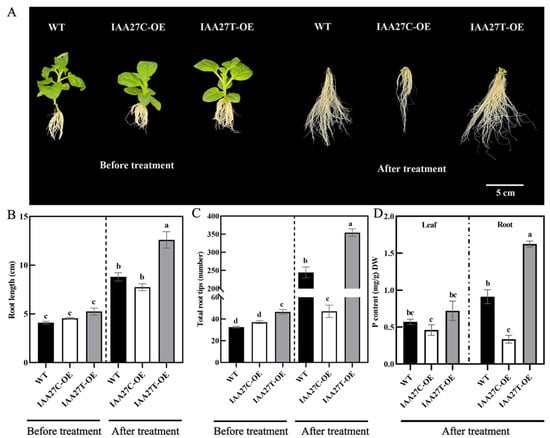
Figure 3.
Ectopic expression of MdIAA27 influenced tolerance to phosphate stress in transgenic tobacco. (A) The phenotypes of MdIAA27 transgenic and WT tobacco in the presence of auxin (0.5 mol/L) for 15 days on MS solid medium. (B,C) Statistical analysis of total root tips and root lengths. (D) Phosphorus content of transgenic and WT lines after treatment with LP (10 μM KH2PO4) for 10 days. Different letters represent significant differences (p < 0.05). Error bars show standard deviation (s.d.) from three biological replicates.
2.3. An IAA27 Mutant Allele Affected Adventitious Root Development and Changed P Deficiency Tolerance in Apple Rootstocks
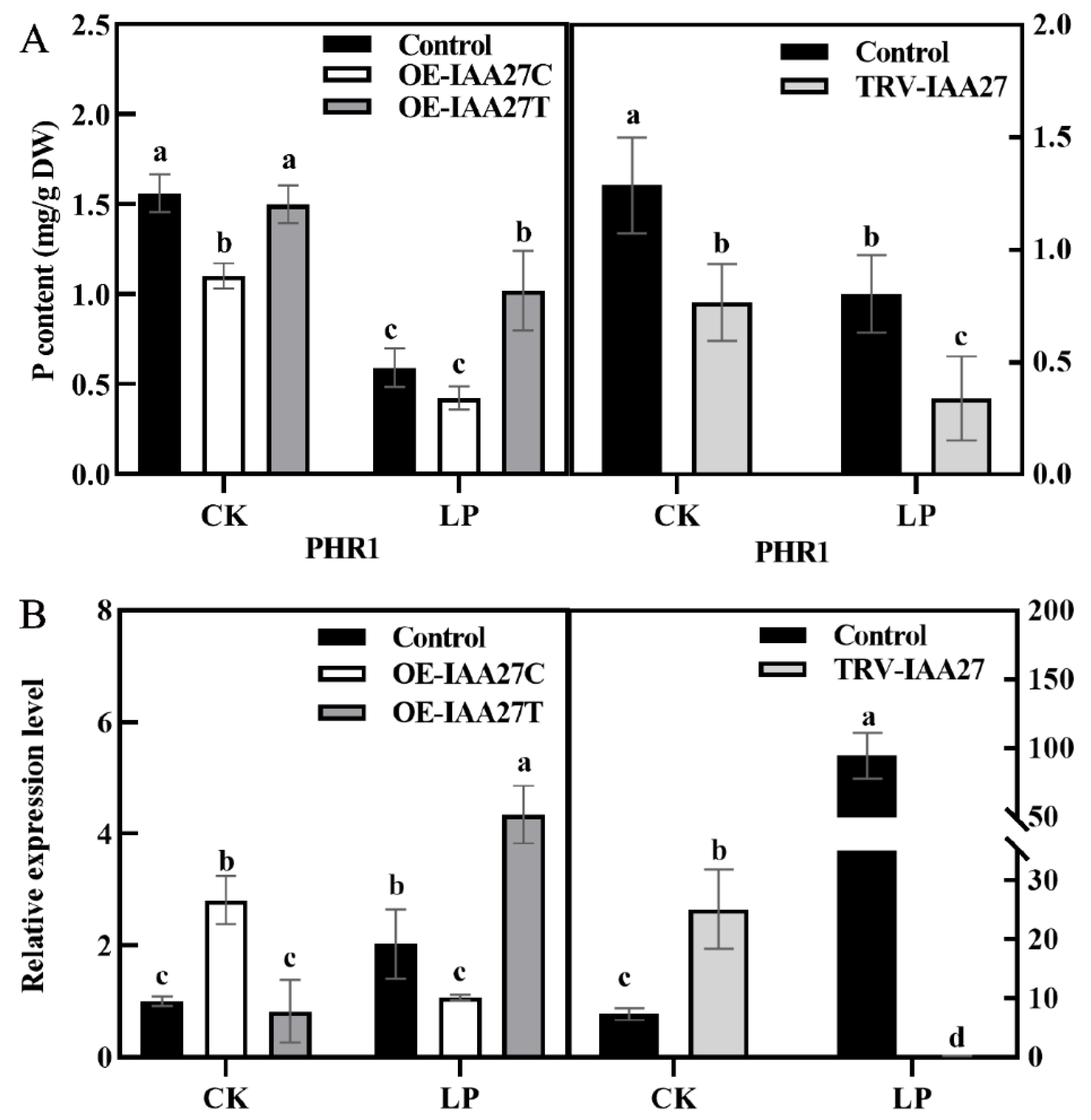
The expression level of MdIAA27 was induced by P deficiency stress, indicating that it may play an important role in low phosphorus conditions. To investigate MdIAA27’s function under phosphorus deficiency, a transient expression system was used to overexpress or silence MdIAA27 in the roots of ‘M9’. The MdIAA27-overexpressed lines (OE-IAA27T, OE-IAA27C) and MdIAA27-silenced lines (TRV-IAA27) were obtained (Supplemental Figure S2). To further clarify the function of MdIAA27 to cope with phosphorus deficiency conditions, the expression of the P uptake gene PHOSPHATE STARVATIO RESPONSE 1 (PHR1) was analyzed in the rootstocks. Five days after phosphorus deficiency treatment, the expression of MdPHR1 was upregulated, and the phosphorus content was increased in the roots of IAA27T-OE lines compared with control lines (Figure 4A,B). Conversely, the expression of MdPHR1 was downregulated, and the phosphorus content of roots was decreased in IAA27C-OE and IAA27-TRV lines in contrast to control lines (Figure 4A,B).
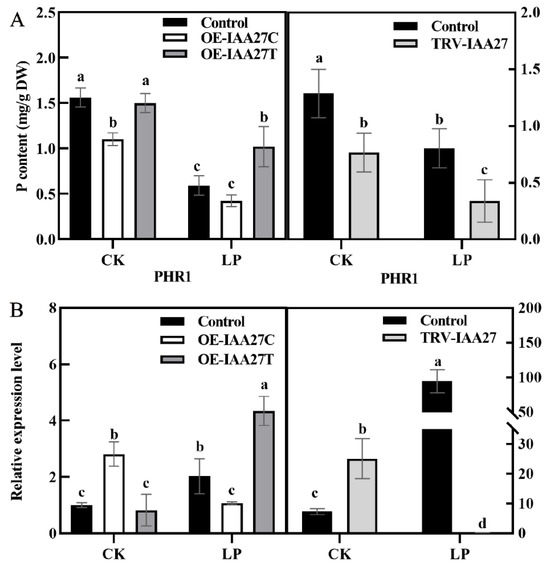
Figure 4.
IAA27 influenced adventitious root development and P uptake. (A) The P content of roots in transgenic and control apples after low phosphorus treatment. (B) PHR1 expression was analyzed by qRT-PCR in MdIAA27-overexpressed and -silenced ‘M9’ plants. Error bars indicate standard deviations (s.d.) from three biological replicates. Different letters represent significant differences (p < 0.05).
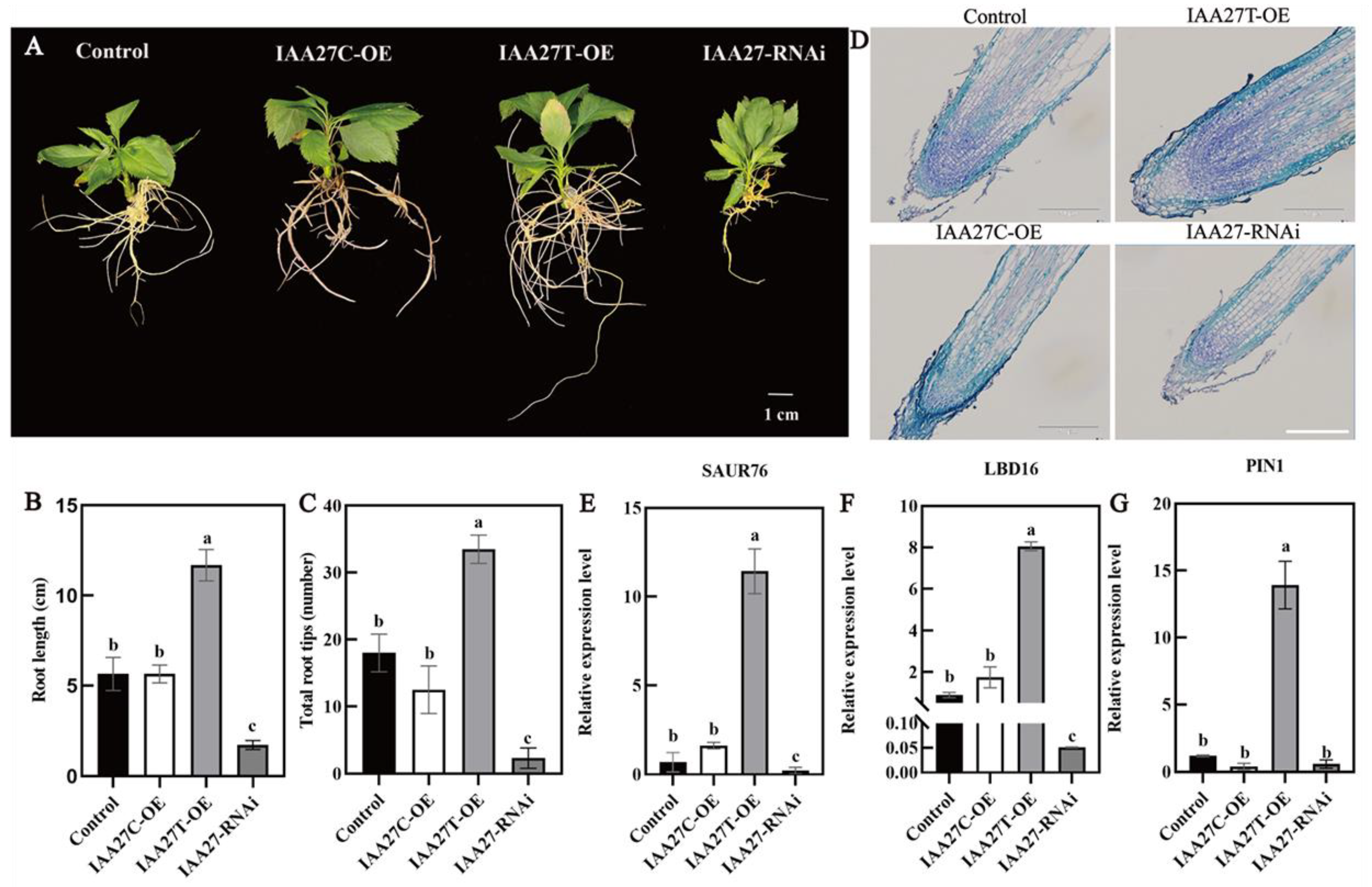
To further determine the role of MdIAA27 in the phosphorus uptake of adventitious roots, IAA27T-OE, IAA27C-OE, and IAA27-RNAi were constructed and stably transformed into ‘Gala3’ (‘GL3’) apple plants, resulting in transgenic plants with significant increases or decreases in IAA27 transcription (Supplemental Figure S3A–C). The empty vector was transformed as the control. Obviously, compared with the control, the numbers and lengths of the ARs were significantly higher when IAA27T was overexpressed, while no obvious change in root development in plants with the overexpression of IAA27C was observed. Following this, the MdIAA27-silenced plants exhibited a significant suppression of root development compared with the control plants (Figure 5A–C). To clarify whether the different root phenotypes of transgenic plants are due to changes in root cell division, adventitious root longitudinal sections for control and transgenic plants were obtained. The results showed that the meristematic activity of OE-MdIAA27T in the root tip was clearly higher than that in the control, while the meristematic activity of the other transgenic lines was significantly lower than that of the control (Figure 5D).
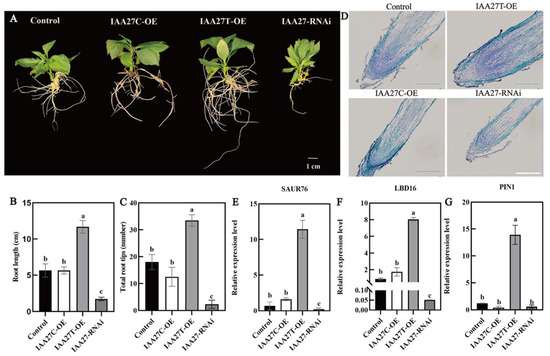
Figure 5.
Overexpression and silenced lines of IAA27 in ‘GL3’. (A–C) The adventitious root phenotypes of transgenic plants and control plants in the presence of auxin (0.5 mol/L) for 50 days on 1/2 MS solid medium; the experiment was repeated three times and the phenotype was consistent. (D) MdIAA27 could influence the meristematic activity of root tips. Scale bars: 10 μm. (E–G) The gene expression of transgenic plants. Error bars indicate standard deviations (s.d.) from three biological replicates. Different letters represent significant differences (p < 0.05).
Auxin-related genes such as SAUR76, LBD16, and PIN-FORMED 1 (PIN1) play a significant role in modulating AR and LR formation. To verify whether MdIAA27 regulates the expression levels of these genes, the transcript levels of MdSAUR76, MdLBD16, and MdPIN1 were measured in MdIAA27 transgenic apple lines and control plants. As shown in Figure 5E, MdSAUR76, whose homologs show a positive influence on meristematic activity in Arabidopsis, had higher expression in overexpressed MdIAA27T lines and had no influence on IAA27C lines compared to control plants. Furthermore, the expression levels of MdLBD16 and MdPIN1 were upregulated in MdIAA27T-OE lines but had no significant change in MdIAA27C-OE lines compared with controls (Figure 5F,G). These results showed that IAA27T positively regulated the expression of MdSAUR76, MdLBD16, and MdPIN1. However, the above auxin-related genes showed the opposite expression trend in IAA27-RNAi lines. Taken together, these results indicated that MdIAA27T activated the transcription of several auxin-related genes and positively modulated root development in apple.
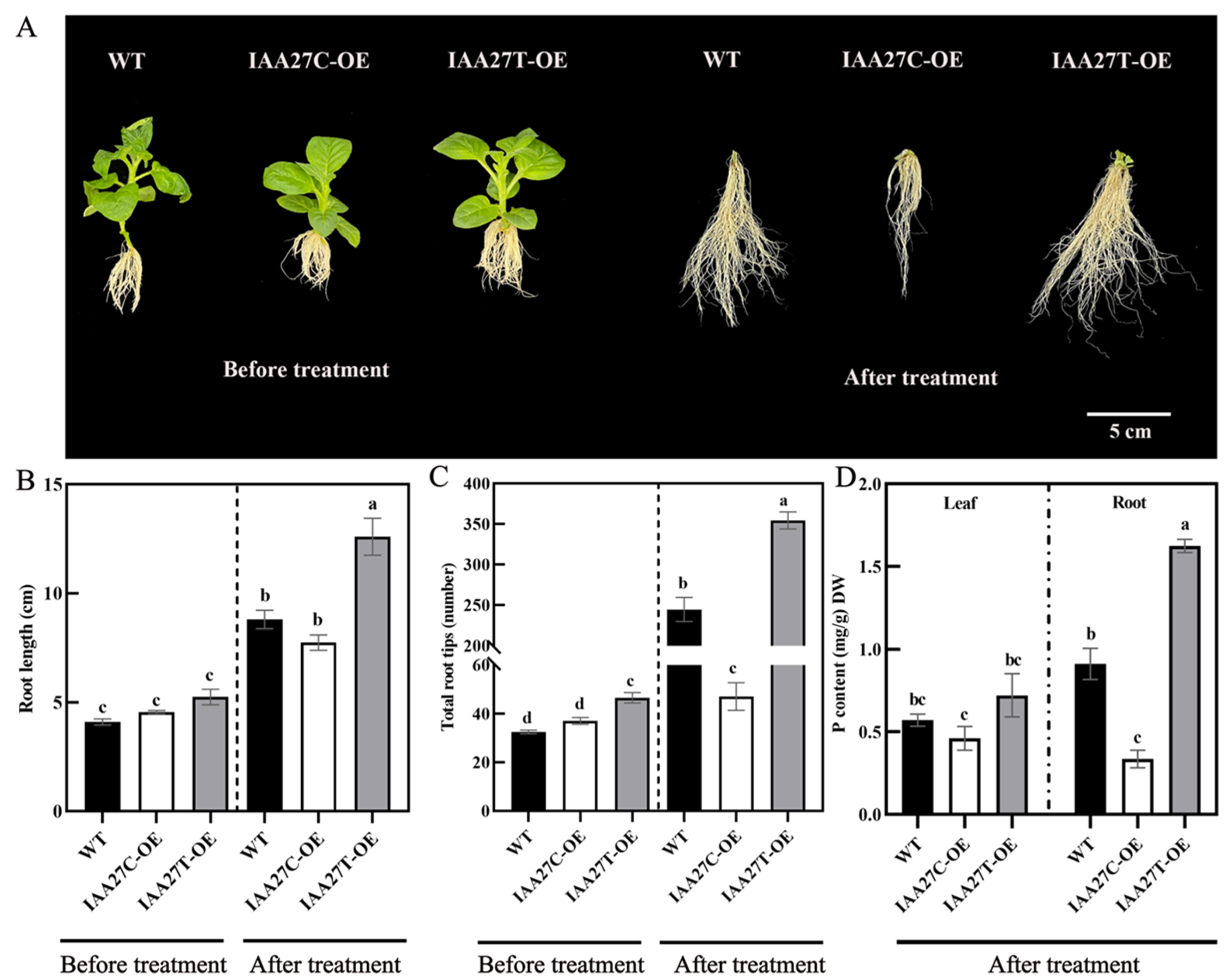
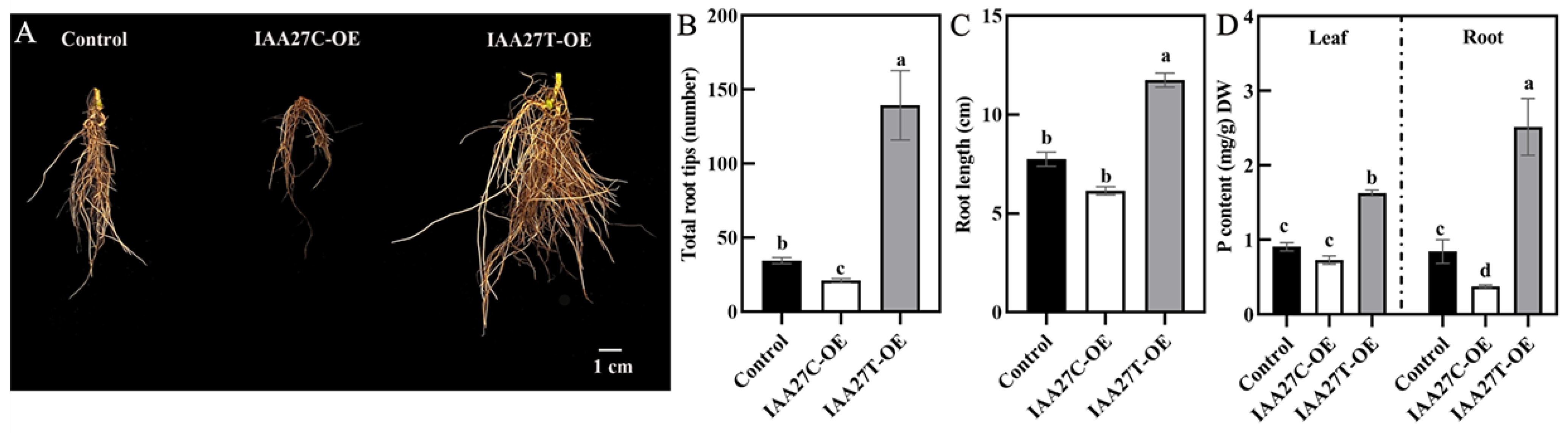
These overexpression lines were further treated with low phosphate. After 10 days of growth in a solution containing 10 µM P, compared with the control plants, the MdIAA27T-OE transgenic apple plants revealed stronger adventitious root development, but the MdIAA27C-OE apples revealed attenuated growth in terms of adventitious root development (Figure 6A–C). Moreover, the P content both in the root and leaf tissue of MdIAA27T-OE plants was obviously the highest, but the lowest P content was observed in the whole plants of MdIAA27C-OE lines (Figure 6D). These results indicated that MdIAA27 is a key regulator involved in apple for responding to P starvation.
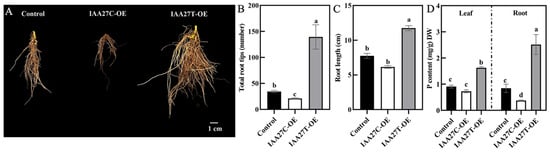
Figure 6.
Effect of MdIAA27 on phosphorus tolerance in transgenic apple plants. (A) The phenotypes and P content of 75-day-old transgenic and control apple plants treated with low phosphorus (LP, 10 μM KH2PO4) for 10 days. Transgenic plants were grown for 14 days with P and then transferred to a hydroponic solution lacking P for 10 days. (B,C) Statistical analysis of total root tips and root lengths. (D) Phosphorus content of transgenic and control lines after treatment with LP (10 μM KH2PO4) for 10 days. Error bars indicate standard deviations (s.d.) from three biological replicates. Different letters represent significant differences (p < 0.05).
2.4. IAA27 Acts as a Regulator of Root Development by Interacting with ARF
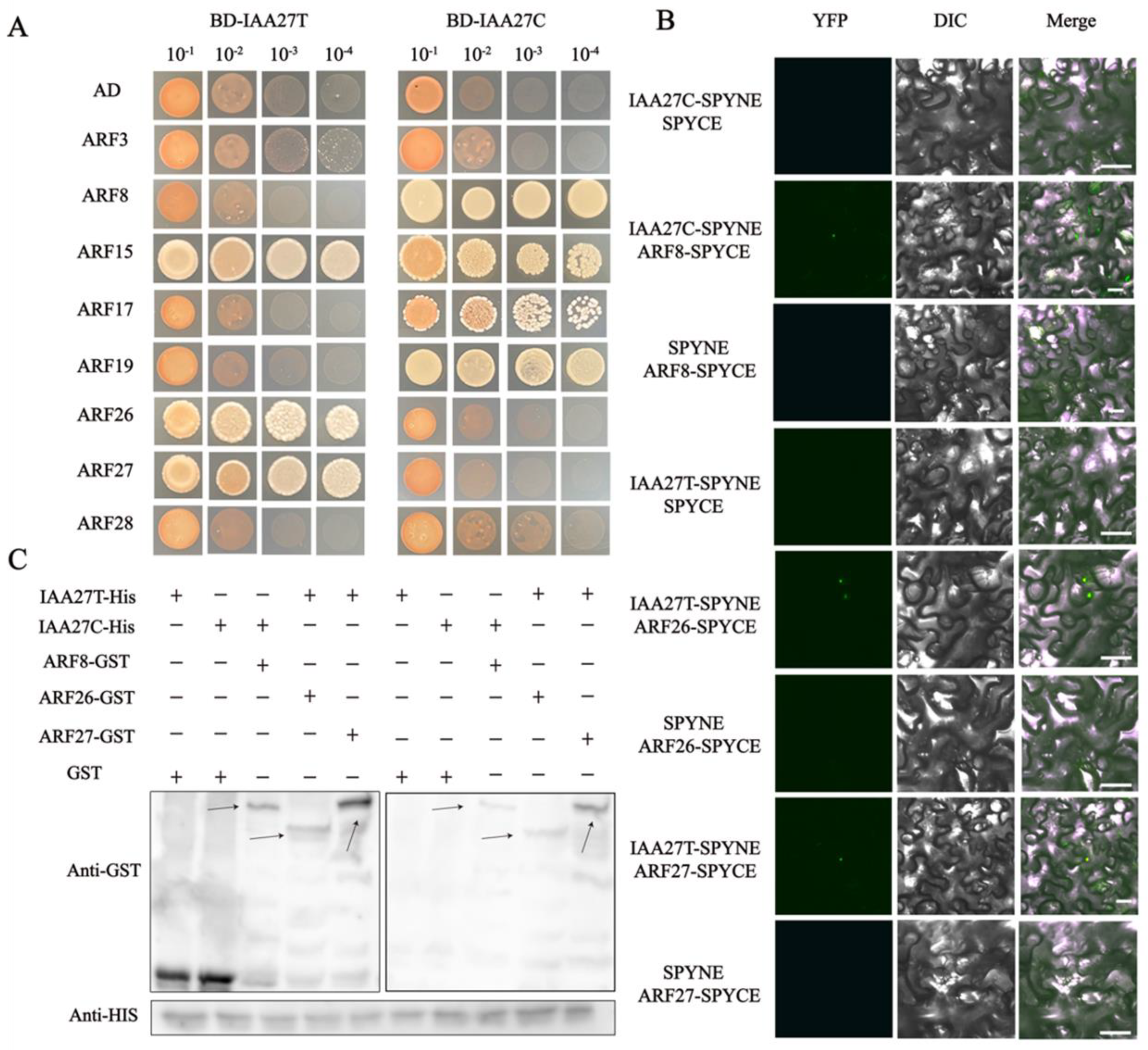
In various plants, Aux/IAA proteins, as transcriptional regulators, mediate auxin signaling through protein–protein interactions with ARF members. The auxin response factors ARF3, ARF8, ARF15, ARF17, ARF19, ARF26, ARF27, and ARF28 were identified as potential MdIAA27-interacting proteins (Supplemental Table S2). Subsequently, Y2H assays were conducted to further validate the interactions between IAA27 and ARFs. The full length of MdIAA27s and MdARFs was inserted into the pGBKT7 (pGBD-MdIAA27T and pGBD-MdIAA27C) and pGADT7 (pGAD-MdARFs) vectors, respectively (Figure 7A,B). The results showed that MdIAA27-T interacted with MdARF15, MdARF26, and MdARF27, while MdIAA27-C interacted with MdARF8, MdARF15, MdARF17, and MdARF19.
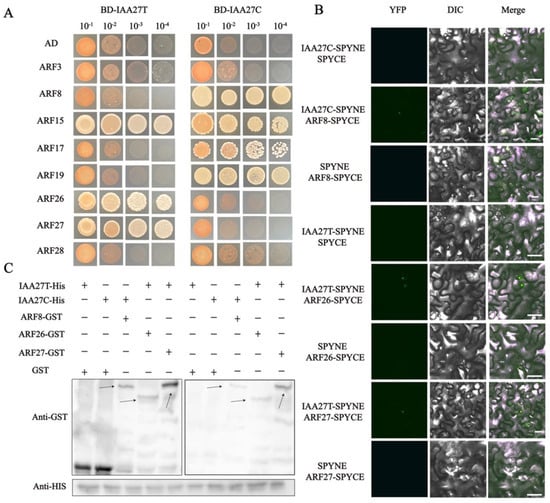
Figure 7.
MdIAA27 interacts with MdARFs, which was identified by Y2H, BiFC, and pull−down assays. (A) Interaction between MdIAA27 and MdARFs in a Y2H assay. The ability of yeast cells to grow on synthetic medium lacking tryptophan, leucine, histidine, and adenine was scored as positive interaction. (B) Interaction between MdIAA27 and MdARFs as determined using a BiFC assay. MdIAA27−SPYNE and MdARFs−SPYCE were performed for the interaction assay. MdIAA27−SPYNE + SPYCE and SPYNE + MdARFs−SPYCE were used as negative controls. MdIAA27−SPYNE + MdARFs−SPYCE was used as a positive control. Scale bar = 10 μm. (C) Pull−down assay analysis of the MdIAA27−MdARFs interaction. The recombinant MdARF−GST and GST proteins were incubated with MdIAA27−HIS protein. The immunoblotting result was tested using anti−HIS and anti−GST antibodies. The plus and minus signs indicate the presence or absence of that protein, respectively. Black arrows indicate the positions of proteins. These assays were repeated three times with identical results.
To further verify the interactions between MdIAA27 and MdARF TFs, a pull-down assay was conducted in vitro (Figure 7C). The MdARFs-GST fusion protein and GST protein were separately incubated with the MdIAA27-HIS protein, and the MdIAA27-HIS protein was pulled down by MdARFs-GST, but not by the GST protein alone. A BiFC assay was carried out in Nicotiana benthamiana leaf cells to further investigate the MdIAA27-MdARFs interactions. The YFP signal was observed only when pSPYNE-MdIAA27 was co-expressed with pSPYCE-MdARF in the same cells, but was not detected in the other vector combination (Figure 7B). Collectively, these results showed that MdIAA27T physically interacted with MdARF26 and MdARF27, while MdIAA27C physically interacted with MdARF8.
2.5. SAUR76 and LBD16 Act Downstream of ARF TFs to Regulate AR Development
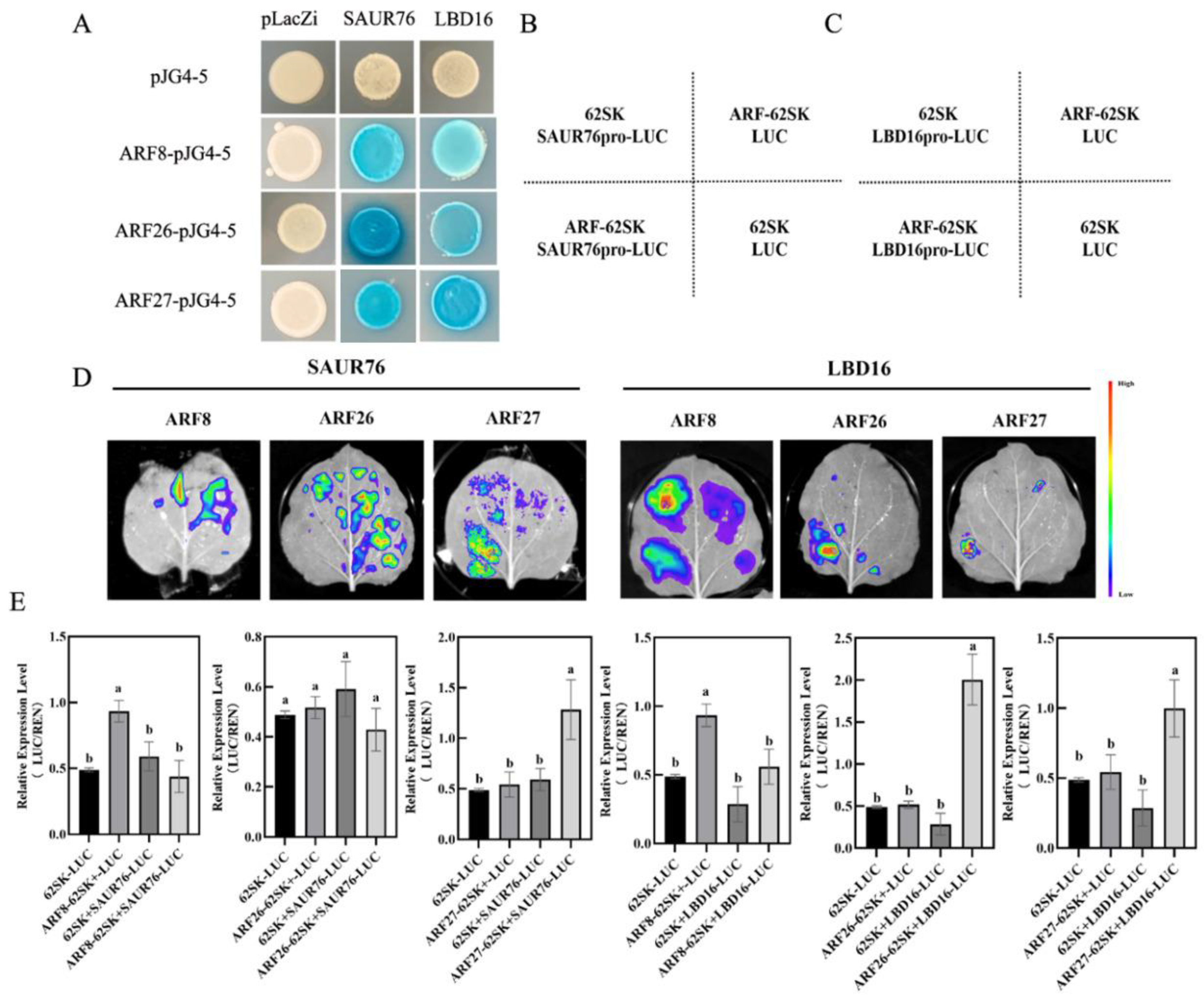
To investigate the molecular mechanism by which IAA27 influences the expression of LBD16 and SAUR76 genes, the 2000-bp upstream region in the promoter before the start translation codon ATG of MdSAUR76 and MdLBD16 was sequenced. There are three MdARFs-binding sites located at the MdSAUR76 and MdLBD16 promoters. Subsequently, yeast one-hybrid (Y1H) assays were conducted to confirm combinations between promoters of MdSAUR76 and MdLBD16 and all ARF genes in vitro. MdARFs bound to the MdSAUR76 and MdLBD16 promoter (Figure 8A).
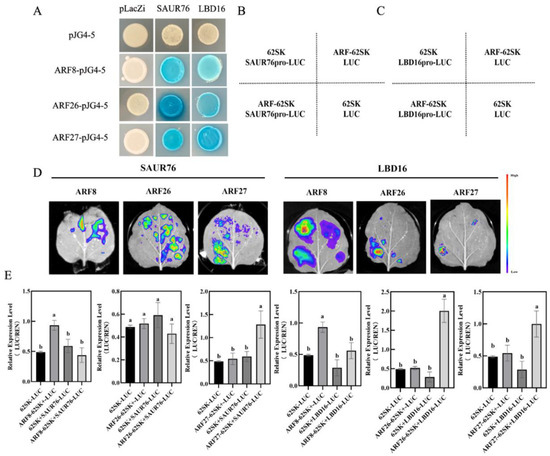
Figure 8.
MdARFs directly bind to the promoter of MdSAUR76 and MdLBD16. (A) Results of the Y1H, showing MdARF8, MdARF26, and MdARF27 binding to the MdSAUR76 and MdLBD16 promoter. (B–E) Effect of MdARFs on the regulation of the MdSAUR76 and MdLBD16 promoter in tobacco leaves and LUC/REN ratio analysis. Red color represents a stronger signal, and violet color represents a weaker signal. Error bars indicate standard deviations (s.d.) from three biological replicates. These assays were repeated three times with the same results. Different letters represent significant differences (p < 0.05).
Moreover, LUC/REN activity was also assessed using the MdARF8, ARF26, and ARF27 cDNAs, which were cloned into the pGreenII62-SK vector to construct effector plasmids, separately. The 1984-bp MdLBD16 and1968-bp MdSAUR76 promoter were cloned into the pGreenII0800-LUC vector to generate the reporter construct. The effector and reporter constructs were introduced into tobacco leaf cells and transformation with the empty reporter plasmid was used as a control. In the LUC assay, MdARF27 significantly activated MdSAUR76 and MdLBD16 expression. MdARF26 clearly activated MdLBD16 expression, but it did not show a significant change in MdSAUR76 expression. By contrast, MdARF8 suppressed MdSAUR76 and MdLBD16 expression (Figure 8B–E). These results showed that ARF27 may play a more positive role in adventitious root development compared with other ARF members.
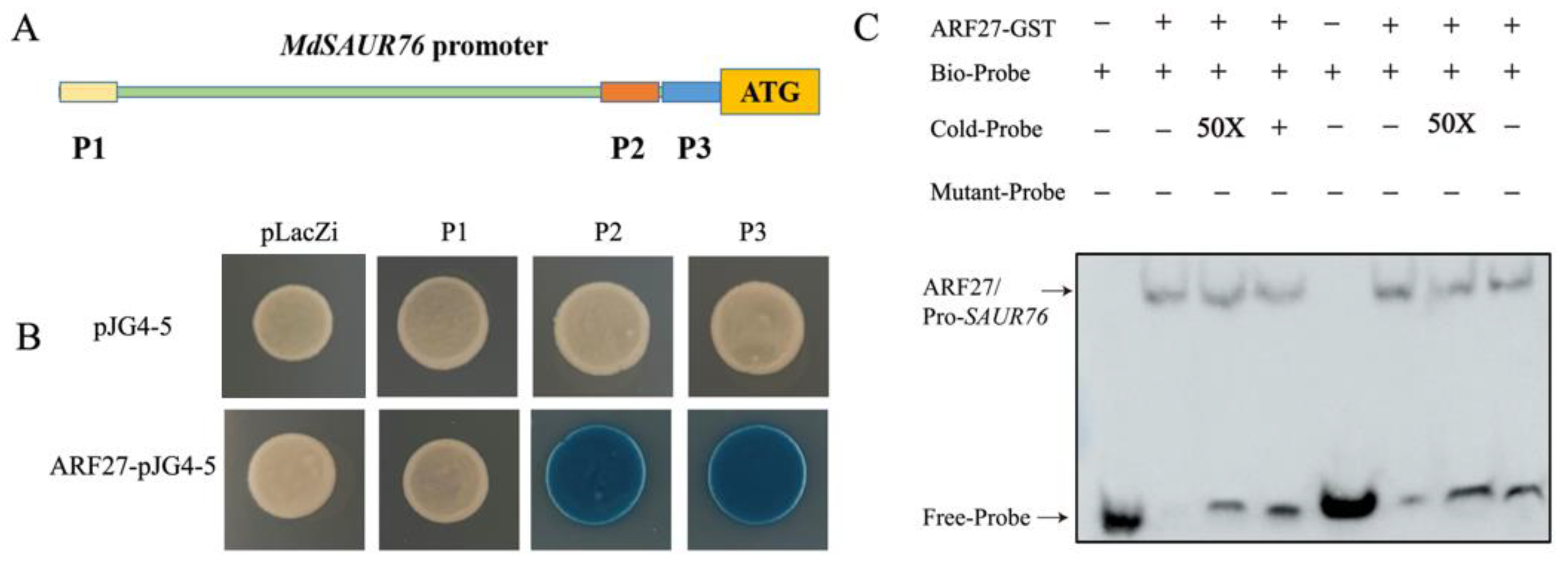
To further investigate the mechanism of ARF27 and SAUR76 regulating the root development in low phosphorus conditions, the promoter fragment was further truncated into three shorter sequences with ARF binding sites, named P1, P2, and P3, respectively (Figure 9A). Y1H assays indicated that these combinations were also observed with both fragments P2 and P3 (Figure 9B). In addition, the electrophoretic mobility shift assay (EMSA) affirmed that ARF27-GST bound to the P2 and P3 segment in the promoter of MdSAUR76 (Figure 9C).
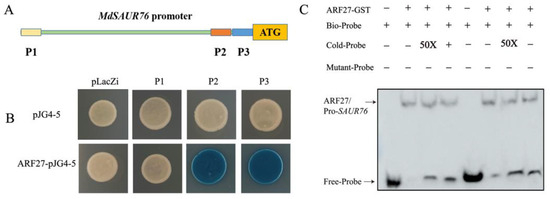
Figure 9.
Interaction of MdIAA27 with the promoter of MdSAUR76. (A) Schematic diagram of the MdSAUR76 promoter region; (B) Y1H confirmation of the binding of MdSAUR76 promoter P2 and P3 fragments by MdARF27; (C) EMSA assay showing that MdARF27 could directly bind to the promoters of MdSAUR76 (P2 and P3). The plus and minus signs indicate the presence or absence of that protein, respectively.
3. Discussion
3.1. MdIAA27 Responds to Phosphorus Deficiency Stress
Numerous strategies to cope with nutrient stress have been suggested, among which transcription factors involved in responsive mechanisms have great potential. The Aux/IAA family is normally involved in responding with biotic and abiotic stress [37]. In previous studies, MdIAA24 was reported to enhance cadmium tolerance by reducing Cd accumulation [38]; MdIAA9 was observed to confer osmotic tolerance [39]; OsIAA6 positively regulated drought resistance [40]. However, reports on the function of Aux/IAA TFs in alleviating phosphorus stress in apple are still rare. Therefore, based on the BSA results of a previous study, we identified and cloned a candidate gene, MdIAA27, from ‘BC’ and ‘M9’ and further investigated the molecular basis underlying the regulation of phosphorus deficiency tolerance in apple. Aux/IAA protein domains are highly conserved and are normally localized in the nucleus, such as SlIAA9 and GmIAA27 [35,36]. The subcellular location indicated that MdIAA27 was located in the nucleus (Figure 2B). Hence, MdIAA27 might act as a transcriptional regulator to affect the expression of downstream genes in the nucleus. The expression pattern of MdIAA27 exposed to phosphorus deficiency showed that MdIAA27 was significantly induced by 10 µM KH2PO4 and markedly upregulated by KH2PO4 treatment for 5 days. In different tissues, the expression patterns of MdIAA27 were differentially induced. In adventitious roots, the expression of MdIAA27 was induced the most (Figure 2A). Taken together, these results suggested that MdIAA27, a transcription factor similar to other Aux/IAA members, was involved in apple’s response to phosphorus stress. The P content determination of MdIAA27 transgenic apple plants after low phosphorus treatment also supported this conclusion (Figure 4A,B). The stable overexpression of MdIAA27T in apple and tobacco showed more tolerance to low phosphorus, as determined by the identification of longer and denser roots, higher P content levels, and better growth outcomes (Figure 3 and Figure 6). These results further demonstrated that MdIAA27 is highly associated with resistance to phosphorus stress in apple.
3.2. MdIAA27 Regulates the Number and Length of Adventitious Roots to Enhance Phosphorus Uptake
Several studies have shown that Aux/IAA TFs are also involved in root growth and development. For instance, SlIAA15-suppressed plants exhibit increased lateral root formation [41]. Recently, gain-of-function mutants of the OsIAA13 gene in rice also displayed defects in the growth of lateral roots, which are closely associated with the transcriptional regulation of the set of genes participating in lateral root initiation [42]. However, few studies have revealed the functions of Aux/IAA in woody plants, especially in apple. In this study, the phenotypes of progenies showed that the adventitious root lengths and number of the MdIAA27T genotype were greatly higher than those of the MdIAA27C genotype after low phosphorus treatment (Figure 1D). Overexpressing MdIAA27C resulted in fewer ARs than the control lines (Figure 5A), which was similar to the overexpression of AtIAA8 [43]. Interestingly, in our study, the number of adventitious roots and root length were greatly higher in IAA27T-OE lines compared with the control plants (Figure 5A). A similar phenotype was observed in OsIAA4 transgenic lines [44]. The phenotype of RNAi-IAA27 lines exhibited significant defects in adventitious root length and number compared to control plants (Figure 5A). These results indicated that MdIAA27 is a critical regulator of adventitious root development. Adventitious roots commonly increase the absorption area of roots and enhance their abilities to assimilate nutrients and support plants [45]. Previous studies have shown that adventitious roots promote phosphorus absorption under phosphorus deficiency conditions. In rice, overexpression of OsMYB2 stimulated adventitious root growth and, subsequently, phosphorus absorption in shoot and roots [22]. In our study, it was also observed that MdIAA27T overexpression lines increased the phosphorus content after low phosphorus treatment (Figure 3D and Figure 6D). The higher phosphorus content of IAA27T-OE plants is due to the enlarged growth of adventitious roots by MdIAA27T, which in turn causes an increased surface area, leading to improved phosphorus uptake in apple.
Under phosphorus deficiency, root length significantly increased for better P uptake. The activity of meristematic cells in the root meristem influences root elongation [46]. In previous research, overexpression of AtIAA1 in Arabidopsis exhibited a significantly suppressed cell length and number [47]. In rice, the mutant of GLU3 was observed, which had a defect in root cell division to inhibit root development [48]. In our study, differences in root meristematic activity were also observed in these transgenic apple plants. AtSAUR76 was found to positively control root development by influencing meristematic activity and cell elongation [49]. As shown in Figure 5A–E, the long roots of overexpression MdIAA27T lines were due to the increased expression of MdSAUR76 regulated by MdIAA27T, which caused improved meristematic activity, leading to a longer root phenotype. Similarly, the short roots of IAA27-RNAi plants might be due to the suppression of MdSAUR76 expression, which in turn caused the arrest of cell division, resulting in more a severe root development defect phenotype. In addition, the overexpression of MdIAA27T and MdIAA27C in tobacco plants significantly influenced their root length (Figure 3A,B). Collectively, this observation proved that the root length was increased after promoting the root meristematic activity of apple, leading to the improvement in phosphorus uptake in roots.
Previous studies have indicated that auxin-inducible genes PIN1 and LBD16 are required for root development in plants [50,51]. In apple, overexpression of MdPIN1 led to the accumulation of auxin and increased the adventitious root development [52]. Overexpression of AtASL18/LBD16 induced root formation in Arabidopsis [53]. In this study, silenced apple plants led to lower PIN1 and LBD16 expression, while the expression of PIN1 and LBD16 was significantly higher when IAA27T was overexpressed. These results clarified that MdIAA27 directly or indirectly regulated some auxin-related genes, i.e., PIN1 and LBD16, to affect adventitious roots development.
3.3. Low Phosphorus Regulates Adventitious Root Development through the Aux/IAA–ARF–SAUR76/LBD16 Signaling Pathway in Apple
During plant growth, auxin regulates root development through the Aux/IAA–ARF pathway. In Arabidopsis, AtIAA19 together with ARF7 regulated lateral root formation [54]. The ARFs act as regulators of root formation. The AtARF17-overexpressing plants produced fewer adventitious roots, suggesting that ARF17 plays a negative role in the development of adventitious roots [55]. On the contrary, ARF6 and ARF8 act as positive regulators of adventitious root formation [56]. Consistent with previous studies, Y2H, BiFC, and pull-down experiments were performed to reveal that MdIAA27T could interact with the positive regulators, including MdARF26 and MdARF27, while MdIAA27C could interact with the negative regulators MdARF17 and MdARF19 and a positive regulator, MdARF8 (Figure 7). These results indicated that allele mutants (C to T) can result in differences in binding to ARF and, consequently, differences in auxin response genes’ expression levels. In addition, these findings indicate that the roles of the IAA27-ARF TF modules in adventitious root growth are similar to those of other known Aux/IAA–ARF modules.
In plants, ARF TFs directly activate SAUR and LBD genes, regulating lateral and adventitious root development. The AtSAUR15 acts downstream of ARF TFs to promote LR and AR formation in Arabidopsis [57] and the AtLBD16 and AtLBD18 as target genes of ARF7 and ARF19 to control AR formation [58]. In our study, the expression of MdSAUR76 and MdLBD16 in MdIAA27 transgenic plants was significantly upregulated with the overexpression of MdIAA27T, suggesting that MdSAUR76 and MdLBD16, as downstream genes of MdIAA27, regulate AR development in response to phosphorus stress. Y1H, dual-luciferase expression, and EMSA assays were conducted to determine the specific binding site of MdARF TFs to the MdSAUR76 and MdLBD16 promoter. The results fully verified that MdARF26 and 27 activated the expression of MdSAUR76 and MdLBD16, and MdARF8 weakly repressed the expression of MdSAUR76 and MdLBD16 (Figure 8 and Figure 9). In addition, it was observed that phosphate starvation response gene MdPHR1 showed significantly enhanced expression levels in MdIAA27T-OE lines but decreased expression in the MdIAA27C-OE and MdIAA27-TRV lines after LP treatment. These results indicated that the allele mutant of MdIAA27 directly or indirectly influences the expression of PHR1, which potentially further affects the absorption of phosphorus, but the specific mechanism remains to be explored.
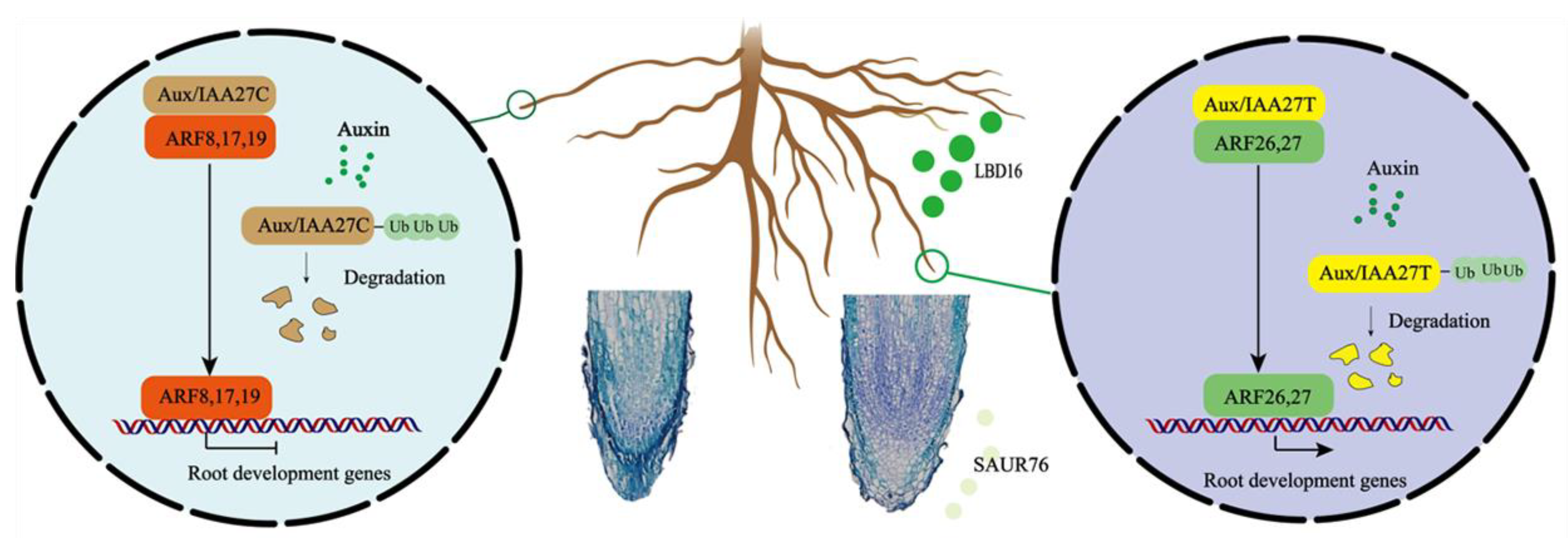
Our results together show a model for MdIAA27 to regulate the development of adventitious roots and increase tolerance to low phosphorus stress in apple rootstocks (Figure 10). The mutation (C to T) in the CDS of IAA27 results in a changed interaction from negative regulators (ARF8, ARF17, and ARF19) to positive regulators (ARF26, ARF27). When low phosphorus stress occurs, it leads to the degradation of IAA27 and release of the ARFs. The degradation of IAA27T can release the positive ARFs, which further upregulates the expression of SAUR76 and LBD16 to improve the length and number of AR, while the degradation of IAA27C releases the negative ARFs to suppress the expression SAUR76 and LBD16 to inhibit the length and number of AR. Therefore, our studies provide new insights into the molecular crosstalk between adventitious root development and P starvation in apple plants.
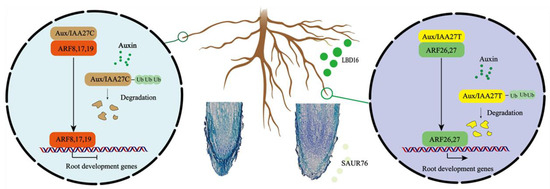
Figure 10.
A working model of MdIAA27-regulated adventitious root growth and development in response to phosphorus stress. Yellow domains denote the IAA27-T mutant proteins and brown domains denote the IAA27-C proteins. Green domains denote the positive ARF proteins and red domains denote the negative ARF proteins. Arrows indicate positive effects, whereas lines ending with a short bar indicate suppressive interactions.
4. Materials and Methods
4.1. Plant Growth and Treatments
Propagation of ‘BC’ × ‘M9’ F1 hybrids was performed by shoot cutting. After rooting, well-growing and healthy progenies were transferred into 1/2 strength Hoagland solution with 500 µM KH2PO4 for 14 days and then recorded by photographing.
To determine the root phenotype under low phosphorus conditions, extreme progenies were cultured in 8 L 1/2 strength Hoagland solution with 10 µM KH2PO4 (LP) for 30 days. To determine the expression levels of IAA27 genes, ‘M9’ was cultured in 8 L 1/2 strength Hoagland solution with 500 µM KH2PO4 (CK) and with 10 µM KH2PO4 (LP), respectively. Roots, stems, and leaves were collected every five days and immediately placed in liquid nitrogen, and then stored at −80 °C.
‘GL3’ (Malus domestica) and tobacco (N. benthamiana) used for the transformation experiment were cultured on Murashige and Skoog solid medium containing 0.2 mg/L indole-3-butyric acid (IBA) and 0.6 mg/L 6-benzyl amino purine (6-BA) with a pH of 5.8–6.0 under a 16 h:8 h (light:dark) photoperiod at 22 °C. Tobacco seedlings were used for subcellular localization, BiFC, and dual-luciferase assays and these plants were grown at 22 °C under long-day conditions (16 h:8 h, light:dark). In order to evaluate the phenotype traits of roots and quantify P content, all transgenic plantlets were treated with 1/2 strength Hoagland solution, and then roots and leaves were harvested at 10 days after treatments.
4.2. Vector Construction and Genetic Transformation
The intact MdIAA27 CDS was inserted into the PRI101 vector containing the 35S promoter to generate the MdIAA27-OE vector. For RNAi constructs, a 400-bp fragment was also inserted into the PRI101-RNAi vector.
Apple hairy root transformation: MdIAA27T-OE, MdIAA27C-OE, MdIAA27-RNAi vectors and empty vectors were transferred into Agrobacterium rhizogenes strain K599 and introduced into ‘GL3’ stems [59,60,61]. The A. rhizogenes positive colonies were transferred to LB liquid medium containing 50 mg/L rifampicin and kanamycin by shaking at 28 °C for approximately 10 h. The bacterial liquid was centrifuged for 8 min at 7000× g and resuspended with MS buffer (4.43 g/L MS, pH 5.6, 30 g/L sucrose, and 100 μM acetosyringone) to a final OD600 of 0.6–0.8.
Tobacco genetic transformation: MdIAA27T-OE and MdIAA27C-OE construct plasmids were transferred into A. tumefaciens strain GV3101 and introduced into tobacco by the Agrobacterium-mediated leaf disk method [62,63]. Transgenic lines were then selected on kanamycin (50 mg/L).
The transgenic apple and tobacco were analyzed by PCR to confirm their identity. Primers are listed in Supplementary Table S1.
4.3. Subcellular Localization
The coding sequence of MdIAA27 was amplified and inserted into the PRI101-GFP expression vector. Vectors were transformed into tobacco leaf cells through PRI101-GFP as a control. Two days after infestation, GFP fluorescence was observed using confocal laser scanning microscopy, FV3000 (Olympus, Tokyo, Japan). Primers are listed in Supplementary Table S1.
4.4. Agrobacterium-Mediated Transient Transformation of Apple Plants and Treatments
For the overexpression of IAA27, the coding sequences of IAA27C and IAA27T were amplified by PCR using the ‘BC’ and ‘M9’ cDNAs as a template, and the fragment was inserted into the PRI101 vector. To silence IAA27 expression using VIGS [64,65], a 400-bp CDS of IAA27 was inserted into the TRV2 vector to construct the pTRV-IAA27 plasmid. The recombinant vectors were introduced into A. tumefaciens strain GV3101, and the resulting positive A. tumefaciens lines containing the plasmids were introduced into ‘M9’ seedlings using vacuum infiltration. The ‘M9’ seedlings were infected under a vacuum (over 0.08 MPa); five seedlings per gene were used for each treatment. The vacuum was released slowly and thereafter plants were washed in deionized water to remove excess bacterium solution and kept in water for 3 days at 22 °C, which was followed by growing in a 1/2 strength Hoagland solution. After 5 days, the fresh roots were used for RNA isolation and qRT-PCR analysis. Primers are listed in Supplementary Table S1.
4.5. Pull-Down Assays
The MdIAA27T and MdIAA27C CDSs were inserted into the pGEX4T-1 vector; MdARF8, ARF26 and ARF27 cDNAs were inserted into pET32a. The MdIAA27-pGEX4T-1, empty pGEX4T-1 vectors, MdARF8-pET32a, MdARF26-pET32a, and MdARF27-pET32a were respectively transformed into BL21 (Weidi Biotechnology Co, Ltd., Shanghai, China). Transformed cells were treated with 0.5 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) for 14 h at 16 °C. Pull-down assays were performed as described in [66]. Finally, the proteins were detected by a Western blot assay with anti-GST and anti-His antibodies (CoWin Biosciences, Beijing, China), as previously reported [65,66]. Primers are listed in Supplementary Table S1.
4.6. Bimolecular Fluorescence Complementation (BiFC) Assay
Full-length MdIAA27T and MdIAA27C CDSs were cloned into the 35S::pSPYNE vector (MdIAA27T-SPYNE, MdIAA27C-SPYNE) and MdARF8, MdARF26, and MdARF27 were cloned into the 35S::pSPYCE vector (MdARF8-SPYCE, MdARF26-SPYCE, and MdARF27-SPYCE). These confirmed constructed vectors were used for transformation into GV3101 Agrobacterium separately. Various combinations of these vectors were co-expressed in tobacco leaves. The tobacco leaves were observed under a FV3000 confocal microscope (Olympus, Tokyo, Japan) to examine the fluorescence signal. Primers are listed in Supplementary Table S1.
4.7. Y1H and Y2H Assays
The MdARF8, MdARF26, and MdARF27 CDSs were inserted into the pJG4-5 vector (Clontech, USA), and the MdSAUR76 promoter (full-length, −2001 to −33 bp; P1 fragment, −2001 to −1850; P2 fragment, −467 to −341; P3 fragment, −341 to −258) and MdLBD16 promoter were cloned into the pLacZi vector. The constructed fusion and empty vectors were introduced into yeast strain EGY48 (Weidi Biotechnology 86 Co, Ltd., Shanghai, China). The Y1H assay was conducted as previously described [67]. The yeast cells were grown on SD/-Leu/-Trp-deficient medium at 28 °C for 3 days.
For the Y2H assay, MdIAA27T and MdIAA27C CDSs were cloned into pGBKT7 and MdARFs CDSs were cloned into pGADT7 vectors. All constructs and empty vectors were introduced into the yeast strain Y2H (Weidi Biotechnology 86 Co, Ltd., China), as described in the Yeast Protocols Handbook (Clontech, Shanghai, China). Yeast cells were cultured on a minimal Leu and Trp medium for 3 days at 28 °C and then plated onto the SD (-Leu, -Trp, -His, and -ade) medium to analyze possible interactions. Primers are listed in Supplementary Table S1.
4.8. Dual-Luciferase Reporter Assay
For the LUC/REN activity assay, MdARF8, MdARF26, and MdARF27 CDSs were cloned into the pGreenII62-SK vector to construct the effector; MdSAUR76 and MdLBD16 promoters were cloned into the pGreenII0800-LUC vector to construct the reporter plasmid. The effector and reporter constructs were introduced into Agrobacterium tumefaciens strain GV3101 (pSoup). After positive colonies were isolated, the bacterial liquid mixture of effector and reporter (9:1) was introduced into leaf cells by agroinfiltration. After 3 days, the LUC imaging was performed using a plant imaging system, LB985 NightSHADE (Berthold Technologies, Wildbad, Germany). The LUC/REN activities were detected and measured using the Dual-Luciferase® Reporter Assay System (Solarbio, Beijing, China) on a Glomax 20/20 Luminometer (Promega, Madison, WI, USA). Primers are listed in Supplementary Table S1.
4.9. EMSA
The MdARF27 CDS was amplified and inserted into the pGEX4T-1 vector. The resulting ARF27-GST fusion plasmids were transformed into the BL21 (DE3) strain of Escherichia coli. The expression of the ARF27-GST fusion protein was induced with 0.5 mM IPTG at 16 °C for 14 h. The recombinant protein ARF27-GST was purified using a GST-Tagged Protein Purification Kit (Soluble Protein) (CWBIO, Beijing, China). The SAUR76 promoter fragments containing two ARF binding sites were synthesized as the Cold Probe and Bio-Probe. EMSA was performed using a Chemiluminescent EMSA kit (Beyotime, Shanghai, China). Primers are listed in Supplementary Table S1.
4.10. Determination of Phosphorus Content
Leaves and roots were harvested on the 10th day after low phosphorus treatment. The leaves and roots were dried for 3 days in an oven at 65 °C until a constant weight was reached, and the dry weight was recorded. Dried plants were pulverized in an electric grinder and then digested with HNO3 in a microwave oven (Mars, CEM, Matthews, NC, USA). The phosphorus content was measured via inductively coupled plasma optical emission spectrometry (ICP-OES, Optima 5300DV, PerkinElmer, Waltham, MA, USA).
4.11. Accession Numbers
The accession numbers for genes investigated in this study are as follows: IAA27 (MD17G1189100), ARF3 (MD16G1239300), ARF8 (MD17G1131500), ARF15 (MD03G1119500), ARF17 (MD15G1359400), ARF19 (MD00G1103900), ARF26 (MD04G1096900), ARF27 (MD15G1221400), LBD16 (MD05G1200900), SAUR76 (MD07G1220700).
5. Conclusions
Overexpression of MdIAA27 affected the root morphologies by increasing the length and number of adventitious roots in response to low phosphorus stress. The allele variation of MdIAA27 directly interacted with ARF8, ARF26, and ARF27, which affected the expression of the downstream MdSAUR76 and MdLBD16 genes involved in adventitious root growth and development. The mechanisms behind the allele variation of IAA27 involved in the apple plants’ root development in response to phosphorus stress were indicated and provide a basis for further investigation relating to root development regulated by the auxin response process under phosphorus stress.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ijms232214029/s1.
Author Contributions
C.Q. and Z.H. conceived the project and designed the experiments. S.Z. conducted the experiments and wrote the manuscript. S.Z. and X.Z. performed data analysis. X.X. participated in revising the manuscript grammatically. S.Z. participated in the apple plant transgenic experiments. All authors have read and agreed to the published version of the manuscript.
Funding
The work was supported by funding from the National Key Research and Development Program of Ministery of Science and Technology of People’s Republic of China (Grant No. 2019YFD1000103), the National Natural Science Foundation of China (Grant No. 31801810), the earmarked fund for China Agriculture Research System (CARS-27), the Construction of Beijing Science and Technology Innovation and Service Capacity in Top Subjects (CEFF-PXM2019_014207_000032), and the 2115 Talent Development Program of China Agricultural University.
Institutional Review Board Statement
These studies did not involve humans or animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no known competing financial interest or personal relationships that could have appeared to influence the work reported in this paper.
References
- Raghothama, K.G.; Karthikeyan, A.S. Phosphate Acquisition. Plant Soil 2005, 274, 37–49. [Google Scholar] [CrossRef]
- Chen, Y.F.; Li, L.Q.; Xu, Q.; Kong, Y.H.; Wang, H.; Wu, W.H. The WRKY6 transcription factor modulates PHOSPHATE1 expression in response to low Pi stress in Arabidopsis. Plant Cell 2009, 21, 3554–3566. [Google Scholar] [CrossRef] [PubMed]
- Calderon-Vazquez, C.; Ibarra-Laclette, E.; Caballero-Perez, J.; Herrera-Estrella, L. Transcript profiling of Zea mays roots reveals gene responses to phosphate deficiency at the plant- and species-specific levels. J. Exp. Bot. 2008, 59, 2479–2497. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Yuan, M.; He, Y.; Li, Y.; Zhang, L. Physiological and transcriptome analysis of He-Ne laser pretreated wheat seedlings in response to drought stress. Sci. Rep. 2017, 7, 6108. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.H.; Derrick, J.W.; Dann, P.R. Grain mineral concentrations and yield of wheat grown under organic and conventional management. J. Sci. Food Agric. 2004, 84, 207–216. [Google Scholar] [CrossRef]
- Vieira, J.L.V.; Nardi, K.T.; Silva, G.R.A.; Moreira, L.A.; Zavaschi, E.; Moura, T.A.; Otto, R. Nutrient Uptake by High-Yielding Cotton Crop in Brazil. Rev. Bras. Ciência Solo 2018, 42. [Google Scholar] [CrossRef]
- Singh, S.K.; Reddy, V.R.; Fleisher, D.H.; Timlin, D.J. Growth, nutrient dynamics, and efficiency responses to carbon dioxide and phosphorus nutrition in soybean. J. Plant Interact. 2014, 9, 838–849. [Google Scholar] [CrossRef]
- Haslam, R.; Darch, T.; Blackwell, M. Phosphorus use efficiency and fertilizers: Future opportunities for improvements. Front. Agric. Sci. Eng. 2019, 6, 332–340. [Google Scholar] [CrossRef]
- Peret, B.; Clement, M.; Nussaume, L.; Desnos, T. Root developmental adaptation to phosphate starvation: Better safe than sorry. Trends Plant Sci. 2011, 16, 442–450. [Google Scholar] [CrossRef]
- López-Bucio, J.; Cruz-Ramírez, A.; Herrera-Estrella, L. The role of nutrient availability in regulating root architecture. Curr. Opin. Plant Biol. 2003, 6, 280–287. [Google Scholar] [CrossRef]
- Osmont, K.S.; Sibout, R.; Hardtke, C.S. Hidden branches: Developments in root system architecture. Annu. Rev. Plant. Biol. 2007, 58, 93–113. [Google Scholar] [CrossRef] [PubMed]
- Pant, B.D.; Burgos, A.; Pant, P.; Cuadros-Inostroza, A.; Willmitzer, L.; Scheible, W.R. The transcription factor PHR1 regulates lipid remodeling and triacylglycerol accumulation in Arabidopsis thaliana during phosphorus starvation. J. Exp. Bot. 2015, 66, 1907–1918. [Google Scholar] [CrossRef] [PubMed]
- Plaxton, W.C.; Tran, H.T. Metabolic adaptations of phosphate-starved plants. Plant Physiol. 2011, 156, 1006–1015. [Google Scholar] [CrossRef] [PubMed]
- Giehl, R.F.; Gruber, B.D.; von Wiren, N. It’s time to make changes: Modulation of root system architecture by nutrient signals. J. Exp. Bot. 2014, 65, 769–778. [Google Scholar] [CrossRef]
- Rogers, E.D.; Benfey, P.N. Regulation of plant root system architecture: Implications for crop advancement. Curr. Opin. Biotechnol. 2015, 32, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Funayama-Noguchi, S.; Noguchi, K.; Terashima, I. Comparison of the response to phosphorus deficiency in two lupin species, Lupinus albus and L. angustifolius, with contrasting root morphology. Plant Cell Environ. 2015, 38, 399–410. [Google Scholar] [CrossRef]
- Haling, R.E.; Brown, L.K.; Bengough, A.G.; Young, I.M.; Hallett, P.D.; White, P.J.; George, T.S. Root hairs improve root penetration, root-soil contact, and phosphorus acquisition in soils of different strength. J. Exp. Bot. 2013, 64, 3711–3721. [Google Scholar] [CrossRef]
- Li, S.; Xue, L.; Xu, S.; Feng, H.; An, L. Hydrogen peroxide acts as a signal molecule in the adventitious root formation of mung bean seedlings. Environ. Exp. Bot. 2009, 65, 63–71. [Google Scholar] [CrossRef]
- Pernot, C.; Thiffault, N.; DesRochers, A. Root system origin and structure influence planting shock of black spruce seedlings in boreal microsites. For. Ecol. Manag. 2019, 433, 594–605. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lynch, J.P.; Brown, K.M. Ethylene insensitivity impedes a subset of responses to phosphorus deficiency in tomato and petunia. Plant Cell Environ. 2008, 31, 1744–1755. [Google Scholar] [CrossRef]
- Wang, H.; Pak, S.; Yang, J.; Wu, Y.; Li, W.; Feng, H.; Yang, J.; Wei, H.; Li, C. Two high hierarchical regulators, PuMYB40 and PuWRKY75, control the low phosphorus driven adventitious root formation in Populus ussuriensis. Plant Biotechnol. J. 2022, 20, 1561–1577. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Wang, Y.; Yang, A.; Zhang, W.H. OsMYB2P-1, an R2R3 MYB transcription factor, is involved in the regulation of phosphate-starvation responses and root architecture in rice. Plant. Physiol. 2012, 159, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Negi, M.; Sanagala, R.; Rai, V.; Jain, A. Deciphering Phosphate Deficiency-Mediated Temporal Effects on Different Root Traits in Rice Grown in a Modified Hydroponic System. Front. Plant Sci. 2016, 7, 550. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Chen, G.; Zhang, X.; Li, T.; Yu, H.; Liu, C. Quantitative trait locus analysis of adventitious and lateral root morphology of barley grown at low and high P. Funct. Plant Biol. 2018, 45, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xia, M.; Wu, P. Effect of phosphorus deficiency stress on rice lateral root growth and nutrient absorption. Zhiwu Xuebao (Acta Bot. Sin.) 2001, 43, 1154–1160. [Google Scholar]
- Mockaitis, K.; Estelle, M. Auxin receptors and plant development: A new signaling paradigm. Annu. Rev. Cell Dev. Biol. 2008, 24, 55–80. [Google Scholar] [CrossRef]
- Zhang, Y.; von Behrens, I.; Zimmermann, R.; Ludwig, Y.; Hey, S.; Hochholdinger, F. LATERAL ROOT PRIMORDIA 1 of maize acts as a transcriptional activator in auxin signalling downstream of the Aux/IAA gene rootless with undetectable meristem. J. Exp. Bot. 2015, 66, 3855–3863. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, X.; Cao, P.; Xiao, Z.; Zhan, C.; Liu, M.; Nvsvrot, T.; Wang, N. The bZIP53-IAA4 module inhibits adventitious root development in Populus. J. Exp. Bot. 2020, 71, 3485–3498. [Google Scholar] [CrossRef]
- Salehin, M.; Li, B.; Tang, M.; Katz, E.; Song, L.; Ecker, J.R.; Kliebenstein, D.J.; Estelle, M. Auxin-sensitive Aux/IAA proteins mediate drought tolerance in Arabidopsis by regulating glucosinolate levels. Nat. Commun. 2019, 10, 4021. [Google Scholar] [CrossRef]
- Zhang, A.; Yang, X.; Lu, J.; Song, F.; Sun, J.; Wang, C.; Lian, J.; Zhao, L.; Zhao, B. OsIAA20, an Aux/IAA protein, mediates abiotic stress tolerance in rice through an ABA pathway. Plant. Sci. 2021, 308, 110903. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Ren, Y.R.; Zheng, P.F.; Qu, F.J.; Song, L.Q.; You, C.X.; Wang, X.F.; Hao, Y.J. Functional identification of apple MdMYB2 gene in phosphate-starvation response. J. Plant. Physiol. 2020, 244, 153089. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; An, J.-P.; You, C.-X.; Wang, X.-F.; Hao, Y.-J. Overexpression of MdPHR1 Enhanced Tolerance to Phosphorus Deficiency by Increasing MdPAP10 Transcription in Apple (Malus × Domestica). J. Plant Growth Regul. 2020, 40, 1753–1763. [Google Scholar] [CrossRef]
- Colón-Carmona, A.; Chen, D.L.; Yeh, K.-C.; Abel, S. Aux/IAA proteins are phosphorylated by phytochrome in vitro. Plant Physiol. 2000, 124, 1728–1738. [Google Scholar] [CrossRef] [PubMed]
- Worley, C.K.; Zenser, N.; Ramos, J.; Rouse, D.; Leyser, O.; Theologis, A.; Callis, J. Degradation of Aux/IAA proteins is essential for normal auxin signalling. Plant J. 2000, 21, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jones, B.; Li, Z.; Frasse, P.; Delalande, C.; Regad, F.; Chaabouni, S.; Latche, A.; Pech, J.C.; Bouzayen, M. The tomato Aux/IAA transcription factor IAA9 is involved in fruit development and leaf morphogenesis. Plant Cell 2005, 17, 2676–2692. [Google Scholar] [CrossRef]
- Su, B.; Wu, H.; Guo, Y.; Gao, H.; Wei, Z.; Zhao, Y.; Qiu, L. GmIAA27 Encodes an AUX/IAA Protein Involved in Dwarfing and Multi-Branching in Soybean. Int. J. Mol. Sci. 2022, 23, 8643. [Google Scholar] [CrossRef]
- Shani, E.; Salehin, M.; Zhang, Y.; Sanchez, S.E.; Doherty, C.; Wang, R.; Mangado, C.C.; Song, L.; Tal, I.; Pisanty, O.; et al. Plant Stress Tolerance Requires Auxin-Sensitive Aux/IAA Transcriptional Repressors. Curr. Biol. 2017, 27, 437–444. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, D.; Niu, D.; Deng, J.; Ma, F.; Liu, C. Overexpression of auxin response gene MdIAA24 enhanced cadmium tolerance in apple (Malus domestica). Ecotoxicol. Environ. Saf. 2021, 225, 112734. [Google Scholar] [CrossRef]
- Huang, D.; Wang, Q.; Duan, D.; Dong, Q.; Zhao, S.; Zhang, M.; Jing, G.; Liu, C.; van Nocker, S.; Ma, F.; et al. Overexpression of MdIAA9 confers high tolerance to osmotic stress in transgenic tobacco. PeerJ 2019, 7, e7935. [Google Scholar] [CrossRef]
- Jung, H.; Lee, D.K.; Choi, Y.D.; Kim, J.K. OsIAA6, a member of the rice Aux/IAA gene family, is involved in drought tolerance and tiller outgrowth. Plant Sci. 2015, 236, 304–312. [Google Scholar] [CrossRef]
- Deng, W.; Yang, Y.; Ren, Z.; Audran-Delalande, C.; Mila, I.; Wang, X.; Song, H.; Hu, Y.; Bouzayen, M.; Li, Z. The tomato SlIAA15 is involved in trichome formation and axillary shoot development. New Phytol. 2012, 194, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Kitomi, Y.; Inahashi, H.; Takehisa, H.; Sato, Y.; Inukai, Y. OsIAA13-mediated auxin signaling is involved in lateral root initiation in rice. Plant Sci. 2012, 190, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Arase, F.; Nishitani, H.; Egusa, M.; Nishimoto, N.; Sakurai, S.; Sakamoto, N.; Kaminaka, H. IAA8 involved in lateral root formation interacts with the TIR1 auxin receptor and ARF transcription factors in Arabidopsis. PLoS ONE 2012, 7, e43414. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Xu, Z.F. Ectopic overexpression of an AUXIN/INDOLE-3-ACETIC ACID (Aux/IAA) gene OsIAA4 in rice induces morphological changes and reduces responsiveness to Auxin. Int. J. Mol. Sci. 2013, 14, 13645–13656. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Bian, B.; Zhang, M.; Wang, C.; Li, C.; Liao, W. The role and proteomic analysis of ethylene in hydrogen gas-induced adventitious rooting development in cucumber (Cucumis sativus L.) explants. PeerJ 2020, 8, e8896. [Google Scholar] [CrossRef]
- Blakeslee, J.J.; Peer, W.A.; Murphy, A.S. Auxin transport. Curr. Opin. Plant Biol. 2005, 8, 494–500. [Google Scholar] [CrossRef]
- Ku, S.J.; Park, J.Y.; Ha, S.B.; Kim, J. Overexpression of IAA1 with domain II mutation impairs cell elongation and cell division in inflorescences and leaves of Arabidopsis. J. Plant Physiol. 2009, 166, 548–553. [Google Scholar] [CrossRef]
- Zhang, J.W.; Xu, L.; Wu, Y.R.; Chen, X.A.; Liu, Y.; Zhu, S.H.; Ding, W.N.; Wu, P.; Yi, K.K. OsGLU3, a putative membrane-bound endo-1,4-beta-glucanase, is required for root cell elongation and division in rice (Oryza sativa L.). Mol. Plant 2012, 5, 176–186. [Google Scholar] [CrossRef]
- Ren, H.; Gray, W.M. SAUR Proteins as Effectors of Hormonal and Environmental Signals in Plant Growth. Mol. Plant 2015, 8, 1153–1164. [Google Scholar] [CrossRef]
- Xu, M.; Zhu, L.; Shou, H.; Wu, P. A PIN1 family gene, OsPIN1, involved in auxin-dependent adventitious root emergence and tillering in rice. Plant Cell Physiol. 2005, 46, 1674–1681. [Google Scholar] [CrossRef]
- Liu, W.; Yu, J.; Ge, Y.; Qin, P.; Xu, L. Pivotal role of LBD16 in root and root-like organ initiation. Cell Mol. Life Sci. 2018, 75, 3329–3338. [Google Scholar] [CrossRef]
- Gan, Z.; Wang, Y.; Wu, T.; Xu, X.; Zhang, X.; Han, Z. MdPIN1b encodes a putative auxin efflux carrier and has different expression patterns in BC and M9 apple rootstocks. Plant Mol. Biol. 2018, 96, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.J.; Wang, J.; Lin, S.; Tian, Z.; Zhou, K.; Luan, H.Y.; Lyu, C.; Zhang, X.Z.; Xu, R.G. A genome-wide analysis of the ASYMMETRIC LEAVES2/LATERAL ORGAN BOUNDARIES (AS2/LOB) gene family in barley (Hordeum vulgare L.). J. Zhejiang Univ. Sci. B 2016, 17, 763–774. [Google Scholar] [CrossRef]
- Tatematsu, K.; Kumagai, S.; Muto, H.; Sato, A.; Watahiki, M.K.; Harper, R.M.; Liscum, E.; Yamamoto, K.T. MASSUGU2 encodes Aux/IAA19, an auxin-regulated protein that functions together with the transcriptional activator NPH4/ARF7 to regulate differential growth responses of hypocotyl and formation of lateral roots in Arabidopsis thaliana. Plant Cell 2004, 16, 379–393. [Google Scholar] [CrossRef]
- Sorin, C.; Bussell, J.D.; Camus, I.; Ljung, K.; Kowalczyk, M.; Geiss, G.; McKhann, H.; Garcion, C.; Vaucheret, H.; Sandberg, G.; et al. Auxin and light control of adventitious rooting in Arabidopsis require ARGONAUTE. Plant Cell 2005, 17, 1343–1359. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, L.; Bussell, J.D.; Pacurar, D.I.; Schwambach, J.; Pacurar, M.; Bellini, C. Phenotypic plasticity of adventitious rooting in Arabidopsis is controlled by complex regulation of AUXIN RESPONSE FACTOR transcripts and microRNA abundance. Plant Cell 2009, 21, 3119–3132. [Google Scholar] [CrossRef]
- Yin, H.; Li, M.; Lv, M.; Hepworth, S.R.; Li, D.; Ma, C.; Li, J.; Wang, S.M. SAUR15 Promotes Lateral and Adventitious Root Development via Activating H(+)-ATPases and Auxin Biosynthesis. Plant Physiol. 2020, 184, 837–851. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Cho, C.; Pandey, S.K.; Park, Y.; Kim, M.J.; Kim, J. LBD16 and LBD18 acting downstream of ARF7 and ARF19 are involved in adventitious root formation in Arabidopsis. BMC Plant Biol. 2019, 19, 46. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.J.; Zhang, C.L.; Zhang, R.F.; Wang, G.L.; Li, Y.Y.; Hao, Y.J. The SUMO E3 Ligase MdSIZ1 Targets MdbHLH104 to Regulate Plasma Membrane H(+)-ATPase Activity and Iron Homeostasis. Plant Physiol. 2019, 179, 88–106. [Google Scholar] [CrossRef]
- Zhang, C.L.; Wang, G.L.; Zhang, Y.L.; Hu, X.; Zhou, L.J.; You, C.X.; Li, Y.Y.; Hao, Y.J. Apple SUMO E3 ligase MdSIZ1 facilitates SUMOylation of MdARF8 to regulate lateral root formation. New. Phytol. 2021, 229, 2206–2222. [Google Scholar] [CrossRef]
- Liu, L.; Yu, Z.; Xu, Y.; Guo, C.; Zhang, L.; Wu, C.; Yang, G.; Huang, J.; Yan, K.; Shu, H.; et al. Function identification of MdTIR1 in apple root growth benefited from the predicted MdPPI network. J. Integr. Plant Biol. 2020, 63, 723–739. [Google Scholar] [CrossRef]
- Horsch, R.; Fry, J.; Hoffmann, N.; Wallroth, M.; Eichholtz, D.; Rogers, S.; Fraley, R. A simple and general method for transferring genes into plants. Science 1985, 227, 1229–1231. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, X.; Hu, X.; Wu, T.; Wang, Y.; Xu, X.; Zhang, X.; Han, Z. High miR156 Expression Is Required for Auxin-Induced Adventitious Root Formation via MxSPL26 Independent of PINs and ARFs in Malus xiaojinensis. Front. Plant Sci. 2017, 8, 1059. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wu, T.; Li, Q.; Zhang, X.; Xu, X.; Li, T.; Han, Z.; Wang, Y. An ethylene response factor (MxERF4) functions as a repressor of Fe acquisition in Malus xiaojinensis. Sci. Rep. 2018, 8, 1068. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liu, W.; Feng, Y.; Li, D.; Li, K.; Sun, Q.; Zhai, L.; Wu, T.; Zhang, X.; Xu, X.; et al. Ethylene Response Factors MbERF4 and MbERF72 Suppress Iron Uptake in Woody Apple Plants by Modulating Rhizosphere pH. Plant Cell Physiol. 2020, 61, 699–711. [Google Scholar] [CrossRef]
- Hu, Y.; Han, Z.; Wang, T.; Li, H.; Li, Q.; Wang, S.; Tian, J.; Wang, Y.; Zhang, X.; Xu, X.; et al. Ethylene response factor MdERF4 and histone deacetylase MdHDA19 suppress apple fruit ripening through histone deacetylation of ripening-related genes. Plant Physiol. 2022, 188, 2166–2181. [Google Scholar] [CrossRef]
- Li, X.; Shen, F.; Xu, X.; Zheng, Q.; Wang, Y.; Wu, T.; Li, W.; Qiu, C.; Xu, X.; Han, Z.; et al. An HD-ZIP transcription factor, MxHB13, integrates auxin-regulated and juvenility-determined control of adventitious rooting in Malus xiaojinensis. Plant J. 2021, 107, 1663–1680. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).