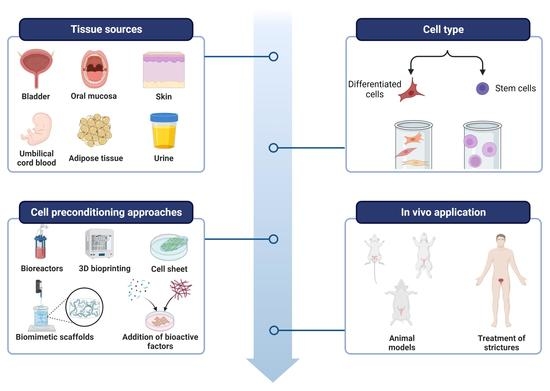
Sources, Selection, and Microenvironmental Preconditioning of Cells for Urethral Tissue Engineering
Abstract
:1. Introduction
2. Overview of the Structure and Function of the Male Urethra
3. Sources of Differentiated Cells
3.1. Urinary Mucosal Keratinocytes
3.2. Oral Mucosal Keratinocytes and Fibroblasts
3.3. Epidermal Keratinocytes
3.4. Mesothelial Cells
3.5. Endothelial Cells
3.6. Smooth Muscle Cells
| Cell Type | Source | Advantages | Limitations | Refs. |
|---|---|---|---|---|
| Mucosal keratinocytes | Urethra | -Accurate urethral phenotype | -Trauma to the urethra -Limited supply and expansion capacity | [21,22] |
| Bladder (biopsy) | -Successful preclinical results -Minimally invasive | -Trauma to the bladder -Invasive procedure | [23,24] | |
| Bladder (washings) | -Ease of harvest -Readily accepted by patients -Successful clinical results | -Limited supply -Requires feeder cells to establish culture -Unavailable in certain patient groups | [25,26,27,28] | |
| Oral mucosa | -Ease of harvest -Phenotype adapted to wet environment -Successful clinical results | -Limited supply -Poor proliferative capacity | [29,30,31,32,33,34] | |
| Fibroblasts | Oral dermis | -Support survival and adhesion of keratinocytes -Produce growth factors | -Limited supply | [35,36,37,38] |
| Skin | -Minimally invasive harvest | -Not extensively characterized | [39] | |
| Epidermal keratinocytes | Foreskin/skin | -Great proliferative capacity -Adapted to wet environment | -Failure to develop transitional epithelium -Biopsy may leave a scar -Unavailable in circumcised patients | [40,41] |
| Mesothelial cells | Omentum | -Successful preclinical results -Phenotypic plasticity | -Limited supply and expansion capacity | [43,44] |
| Endothelial Cells (ECs) | Blood vessels | -Promote angiogenesis | -Phenotypic and physiologic variability -Few studies in relation to urethral repair | [46,47,48,49] |
| Smooth muscle cells (SMCs) | Bladder/ corpus spongiosum | -Improve graft mechanical properties -Promote angiogenesis and epithelial maturation | -Trauma to the bladder/urethra -Invasive procedure | [52,54,55,56] |
4. Sources of Stem Cells
4.1. Embryonic Stem Cells (ESCs)
4.2. Induced Pluripotent Stem Cells (iPSCs)
4.3. Mesenchymal Stem Cells (MSCs)
| Cell Type | Source | Advantages | Limitations | Refs. |
|---|---|---|---|---|
| Embryonic stem cells (ESCs) | Human embryos | -Can be differentiated to any cell type in the urethra -Suitable for in vitro models | -Ethical issues -Malignant potential -Time-consuming differentiation process | [57,58,59,60,61] |
| Induced pluripotent stem cells (iPSCs) | Reprogrammed cells from adult tissues | -Can be differentiated to any cell type in the urethra -Suitable for in vitro models -No ethical issues -Can be used for patient-specific grafts | -Low reprogramming and differentiation efficiency -Malignant potential -Time-consuming differentiation process | [58,64,65] |
| Mesenchymal stem cells (MSCs) | Bone marrow (BMSCs) | -Extensively characterized -Promote neovascularization | -Invasive procedure -Some donor morbidity -Low yield | [67,68] |
| Adipose tissue (ASCs) | -Extensively characterized -Easy to harvest -Highly abundant -Low donor morbidity -Broad paracrine effects | -Inhomogeneous cell population | [74,75,76,77,78,79] | |
| Urine (USCs) | -Non-invasive harvest -Great proliferative capacity | -Compromised viability after long exposure to urine -Not extensively characterized | [86,90,91] | |
| Hair follicles (HFSCs) | -Minimally-invasive harvest -Great proliferative capacity | -Limited supply -Not extensively characterized -More studies are needed in relation to urethral repair | [92,93] | |
| Amniotic fluid (AF-MSCs) | -Great proliferative capacity -Differentiation potential toward urothelial lineage | -Limited supply -Not extensively characterized | [95] | |
| Umbilical cord blood (UCB-MSCs) | -Mostly similar to BMSCs -Easy to harvest -Great proliferative capacity | -More studies are needed in relation to urethral repair | [96] |
5. Cell Preconditioning Approaches
5.1. Biomimetic Microenvironmental Approaches
5.2. Surface Modification and Cell Seeding Technology
5.3. Scaffold-Free Approaches
5.4. Bioprinting
5.5. Bioreactors
5.6. Addition of Bioactive Factors
6. Concluding Remarks and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Santucci, R.A.; Joyce, G.F.; Wise, M. Male urethral stricture disease. J. Urol. 2007, 177, 1667–1674. [Google Scholar] [CrossRef]
- Fenton, A.S.; Morey, A.F.; Aviles, R.; Garcia, C.R. Anterior urethral strictures: Etiology and characteristics. Urology 2005, 65, 1055–1058. [Google Scholar] [CrossRef] [PubMed]
- Hampson, L.A.; McAninch, J.W.; Breyer, B.N. Male urethral strictures and their management. Nat. Rev. Urol. 2014, 11, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Wong, S.S.; Aboumarzouk, O.M.; Narahari, R.; O’Riordan, A.; Pickard, R. Simple urethral dilatation, endoscopic urethrotomy, and urethroplasty for urethral stricture disease in adult men. Cochrane Database Syst. Rev. 2012, 12, CD006934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rashidbenam, Z.; Jasman, M.H.; Hafez, P.; Tan, G.H.; Goh, E.H.; Fam, X.I.; Ho, C.C.K.; Zainuddin, Z.M.; Rajan, R.; Nor, F.M.; et al. Overview of urethral reconstruction by tissue engineering: Current strategies, clinical status and future direction. Tissue Eng. Regen. Med. 2019, 16, 365–384. [Google Scholar] [CrossRef] [PubMed]
- Dublin, N.; Stewart, L.H. Oral complications after buccal mucosal graft harvest for urethroplasty. BJU Int. 2004, 94, 867–869. [Google Scholar] [CrossRef]
- Horiguchi, A. Substitution urethroplasty using oral mucosa graft for male anterior urethral stricture disease: Current topics and reviews. Int. J. Urol. 2017, 24, 493–503. [Google Scholar] [CrossRef] [Green Version]
- Chan, Y.Y.; Bury, M.I.; Yura, E.M.; Hofer, M.D.; Cheng, E.Y.; Sharma, A.K. The current state of tissue engineering in the management of hypospadias. Nat. Rev. Urol. 2020, 17, 162–175. [Google Scholar] [CrossRef]
- Xue, J.D.; Gao, J.; Fu, Q.; Feng, C.; Xie, H. Seeding cell approach for tissue-engineered urethral reconstruction in animal study: A systematic review and meta-analysis. Exp. Biol. Med. 2016, 241, 1416–1428. [Google Scholar] [CrossRef] [Green Version]
- Abbas, T.O.; Mahdi, E.; Hasan, A.; AlAnsari, A.; Pennisi, C.P. Current status of tissue engineering in the management of severe hypospadias. Front. Pediatr. 2018, 5, 283. [Google Scholar] [CrossRef]
- De Filippo, R.E.; Yoo, J.J.; Atala, A. Urethral replacement using cell seeded tubularized collagen matrices. J. Urol. 2002, 168, 1789–1793. [Google Scholar] [CrossRef]
- Osman, N.I.; Hillary, C.; Bullock, A.J.; MacNeil, S.; Chapple, C.R. Tissue engineered buccal mucosa for urethroplasty: Progress and future directions. Adv. Drug Deliv. Rev. 2015, 82, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Palminteri, E.; Berdondini, E.; Fusco, F.; De Nunzio, C.; Salonia, A. Long-term results of small intestinal submucosa graft in bulbar urethral reconstruction. Urology 2012, 79, 695–701. [Google Scholar] [CrossRef]
- Versteegden, L.R.; De Jonge, P.K.; IntHout, J.; Van Kuppevelt, T.H.; Oosterwijk, E.; Feitz, W.F.; De Vries, R.B.M.; Daamen, W.F. Tissue engineering of the urethra: A systematic review and meta-analysis of preclinical and clinical studies. Eur. Urol. 2017, 72, 594–606. [Google Scholar] [CrossRef] [PubMed]
- De Kemp, V.; De Graaf, P.; Fledderus, J.O.; Ruud Bosch, J.L.H.; De Kort, L.M. Tissue engineering for human urethral reconstruction: Systematic review of recent literature. PLoS ONE 2015, 10, e0118653. [Google Scholar] [CrossRef]
- Mangera, A.; Chapple, C.R. Tissue engineering in urethral reconstruction—An update. Asian J Androl 2013, 15, 89–92. [Google Scholar] [CrossRef] [Green Version]
- Abbas, T.O.; Yalcin, H.C.; Pennisi, C.P. From acellular matrices to smart polymers: Degradable scaffolds that are transforming the shape of urethral tissue engineering. Int. J. Mol. Sci. 2019, 20, 1763. [Google Scholar] [CrossRef] [Green Version]
- Hickling, D.R.; Sun, T.T.; Wu, X.R. Anatomy and physiology of the urinary tract: Relation to host defense and microbial infection. Microbiol. Spectr. 2017, 3, 1–25. [Google Scholar]
- Wessells, H.; Lue, T.F.; McAninch, J.W. Penile length in the flaccid and erect states: Guidelines for penile augmentation. J. Urol. 1996, 156, 995–997. [Google Scholar] [CrossRef]
- Tiemessen, D.; de Jonge, P.; Daamen, W.; Feitz, W.; Geutjes, P.; Oosterwijk, E. The effect of a cyclic uniaxial strain on urinary bladder cells. World J. Urol. 2017, 35, 1531–1539. [Google Scholar] [CrossRef] [Green Version]
- Romagnoli, G.; De Luca, M.; Faranda, F.; Franzi, A.T.; Cancedda, R. One-step treatment of proximal hypospadias by the autologous graft of cultured urethral epithelium. J. Urol. 1993, 150, 1204–1207. [Google Scholar] [CrossRef]
- Wang, F.; Liu, T.; Yang, L.; Zhang, G.; Liu, H.; Yi, X.; Yang, X.; Lin, T.; Qin, W.; Yuan, J. Urethral reconstruction with tissue-engineered human amniotic scaffold in rabbit urethral injury models. Med. Sci. Monit. 2014, 20, 2430–2438. [Google Scholar]
- Sartoneva, R.; Haaparanta, A.M.; Lahdes-Vasama, T.; Mannerström, B.; Kellomäki, M.; Salomäki, M.; Sándor, G.; Seppänen, R.; Miettinen, S.; Haimi, S. Characterizing and optimizing poly-L-lactide-co-ε-caprolactone membranes for urothelial tissue engineering. J. R Soc. Interface 2012, 9, 3444–3454. [Google Scholar] [CrossRef] [PubMed]
- Sartoneva, R.; Nordback, P.H.; Haimi, S.; Grijpma, D.W.; Lehto, K.; Rooney, N.; Seppänen, R.; Miettinen, S.; Lahdes-Vasama, T. Comparison of poly (l-lactide-co-ε-caprolactone) and poly (trimethylene carbonate) membranes for urethral regeneration: An in vitro and in vivo study. Tissue Eng. Part A 2018, 24, 117–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fossum, M.; Gustafson, C.J.; Nordenskjld, A.; Kratz, G. Isolation and in vitro cultivation of human urothelial cells from bladder washings of adult patients and children. Scand. J. Plast. Reconstr. Surg. Hand. Surg. 2003, 37, 41–45. [Google Scholar] [CrossRef]
- Fossum, M.; Lundberg, F.; Holmberg, K.; Schoumans, J.; Kratz, G.; Nordenskjöld, A. Long-term culture of human urothelial cells–a qualitative analysis. Cell Tissues Organs. 2005, 181, 11–22. [Google Scholar] [CrossRef]
- Fossum, M.; Svensson, J.; Kratz, G.; Nordenskjöld, A. Autologous in vitro cultured urothelium in hypospadias repair. J. Pediatr. Urol. 2007, 3, 10–18. [Google Scholar] [CrossRef]
- Amesty, M.V.; Chamorro, C.I.; López-Pereira, P.; Martínez-Urrutia, M.J.; Sanz, B.; Rivas, S.; Lobaro, R.; Fossum, M. Creation of Tissue-Engineered Urethras for Large Urethral Defect Repair in a Rabbit Experimental Model. Front. Pediatr. 2021, 9, 691131. [Google Scholar] [CrossRef]
- Zhou, S.; Yang, R.; Zou, Q.; Zhang, K.; Yin, T.; Zhao, W.; Shapter, J.G.; Gao, G.; Fu, Q. Fabrication of tissue-engineered bionic urethra using cell sheet technology and labeling by ultrasmall superparamagnetic iron oxide for full-thickness urethral reconstruction. Theranostics 2017, 7, 2509–2523. [Google Scholar] [CrossRef]
- Huang, J.W.; Lv, X.G.; Li, Z.; Song, L.J.; Feng, C.; Xie, M.K.; Li, C.; Li, H.B.; Wang, J.H.; Zhu, W.D.; et al. Urethral reconstruction with a 3D porous bacterial cellulose scaffold seeded with lingual keratinocytes in a rabbit model. Biomed Mater. 2015, 10, 055005. [Google Scholar] [CrossRef]
- Lazzeri, M.; Barbagli, G.; Fahlenkamp, D.; Romano, G.; Balsmeyer, U.; Knispel, H.; Ram-Liebig, G. MP9-04 preclinical and clinical examination of tissue-engineered graft for urethral reconstruction (mukocell®) with regard to its safety. J. Urol. 2014, 191, e122–e123. [Google Scholar] [CrossRef]
- Barbagli, G.; Ram Liebig, G.; Fahlenkamp, D.; Lazzeri, M. 2 new bulbar urethroplasty using tissue-engineered oral mucosal graft: A preliminary clinical report. J. Urol. 2013, 189, e1. [Google Scholar] [CrossRef]
- Ram-Liebig, G.; Barbagli, G.; Heidenreich, A.; Fahlenkamp, D.; Romano, G.; Rebmann, U.; Standhaft, D.; Van Ahlen, H.; Schakaki, S.; Balsmeyer, U.; et al. Results of use of tissue-engineered autologous oral mucosa graft for urethral reconstruction: A multicenter, prospective, observational trial. EBioMedicine 2017, 23, 185–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbagli, G.; Akbarov, I.; Heidenreich, A.; Zugor, V.; Olianas, R.; Aragona, M.; Romano, G.; Balsmeyer, U.; Fahlenkamp, D.; Lazzeri, M.; et al. Anterior urethroplasty using a new tissue engineered oral mucosa graft: Surgical techniques and outcomes. J. Urol. 2018, 200, 448–456. [Google Scholar] [CrossRef]
- Werner, S.; Krieg, T.; Smola, H. Keratinocyte–fibroblast interactions in wound healing. J. Invest. Derm. 2007, 127, 998–1008. [Google Scholar] [CrossRef] [Green Version]
- Cattan, V.; Bernard, G.; Rousseau, A.; Bouhout, S.; Chabaud, S.; Auger, F.A.; Bolduc, S. Mechanical stimuli-induced urothelial differentiation in a human tissue-engineered tubular genitourinary graft. Eur. Urol. 2011, 60, 1291–1298. [Google Scholar] [CrossRef]
- Xie, M.; Xu, Y.; Song, L.; Wang, J.; Lv, X.; Zhang, Y. Tissue-engineered buccal mucosa using silk fibroin matrices for urethral reconstruction in a canine model. J. Surg. Res. 2014, 188, 1–7. [Google Scholar] [CrossRef]
- Bhargava, S.; Patterson, J.M.; Inman, R.D.; MacNeil, S.; Chapple, C.R. Tissue-engineered buccal mucosa urethroplasty—Clinical outcomes. Eur. Urol. 2008, 53, 1263–1271. [Google Scholar] [CrossRef]
- Osman, N.I.; Patterson, J.M.; MacNeil, S.; Chapple, C.R. Long-term follow-up after tissue-engineered buccal mucosa urethroplasty. Eur. Urol. 2014, 66, 790–791. [Google Scholar] [CrossRef]
- Fu, Q.; Deng, C.L.; Liu, W.; Cao, Y.L. Urethral replacement using epidermal cell-seeded tubular acellular bladder collagen matrix. BJU Int. 2007, 99, 1162–1165. [Google Scholar] [CrossRef]
- Rogovaya, O.S.; Fayzulin, A.K.; Vasiliev, A.V.; Kononov, A.V.; Terskikh, V.V. Reconstruction of rabbit urethral epithelium with skin keratinocytes. Acta Nat. 2015, 7, 70–77. [Google Scholar] [CrossRef]
- Dauleh, S.; Santeramo, I.; Fielding, C.; Ward, K.; Herrmann, A.; Murray, P.; Wilm, B. Characterisation of cultured mesothelial cells derived from the murine adult omentum. PLoS ONE 2016, 11, e0158997. [Google Scholar] [CrossRef] [PubMed]
- Gu, G.L.; Xia, S.J.; Zhang, J.; Liu, G.H.; Yan, L.; Xu, Z.H.; Zhu, Y.J. Tubularized urethral replacement using tissue-engineered peritoneum-like tissue in a rabbit model. Urol. Int. 2012, 89, 358–364. [Google Scholar] [CrossRef]
- Jiang, S.; Xu, Z.; Zhao, Y.; Yan, L.; Zhou, Z.; Gu, G. Urethral reconstruction using mesothelial cell-seeded autogenous granulation tissue tube: An experimental study in male rabbits. Biomed Res. Int. 2017, 2017, 1850256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krüger-Genge, A.; Blocki, A.; Franke, R.P.; Jung, F. Vascular endothelial cell biology: An update. Int. J. Mol. Sci. 2019, 20, 4411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imbeault, A.; Bernard, G.; Rousseau, A.; Morissette, A.; Chabaud, S.; Bouhout, S.; Bolduc, S. An endothelialized urothelial cell-seeded tubular graft for urethral replacement. Can. Urol. Assoc. J. 2013, 7, E4–E9. [Google Scholar] [CrossRef] [Green Version]
- Heller, M.; Frerick-Ochs, E.V.; Bauer, H.K.; Schiegnitz, E.; Flesch, D.; Brieger, J.; Stein, R.; Al-Nawas, B.; Brochhausen, C.; Thüroff, J.W.; et al. Tissue engineered pre-vascularized buccal mucosa equivalents utilizing a primary triculture of epithelial cells, endothelial cells and fibroblasts. Biomaterials 2016, 77, 207–215. [Google Scholar] [CrossRef]
- Park, H.J.; Yoo, J.J.; Kershen, R.T.; Moreland, R.; Atala, A. Reconstitution of human corporal smooth muscle and endothelial cells in vivo. J. Urol. 1999, 162 Pt 2, 1106–1109. [Google Scholar] [CrossRef]
- Falke, G.; Yoo, J.J.; Kwon, T.G.; Moreland, R.; Atala, A. Formation of corporal tissue architecture in vivo using human cavernosal muscle and endothelial cells seeded on collagen matrices. Tissue Eng. 2003, 9, 871–879. [Google Scholar] [CrossRef]
- Winiarski, B.K.; Acheson, N.; Gutowski, N.J.; McHarg, S.; Whatmore, J.L. An improved and reliable method for isolation of microvascular endothelial cells from human omentum. Microcirculation 2011, 18, 635–645. [Google Scholar] [CrossRef]
- Paschalaki, K.E.; Randi, A.M. Recent advances in endothelial colony forming cells toward their use in clinical translation. Front. Med. 2018, 5, 295. [Google Scholar] [CrossRef] [Green Version]
- Orabi, H.; AbouShwareb, T.; Zhang, Y.; Yoo, J.J.; Atala, A. Cell-seeded tubularized scaffolds for reconstruction of long urethral defects: A preclinical study. Eur. Urol. 2013, 63, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Bouhout, S.; Chabaud, S.; Bolduc, S. Organ-specific matrix self-assembled by mesenchymal cells improves the normal urothelial differentiation in vitro. World J. Urol. 2016, 34, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Xu, Y.M.; Fu, Q.; Zhu, W.D.; Cui, L. Reconstruction of three-dimensional neourethra using lingual keratinocytes and corporal smooth muscle cells seeded acellular corporal spongiosum. Tissue Eng. Part A 2011, 17, 3011–3019. [Google Scholar] [CrossRef] [PubMed]
- Micol, L.A.; Da Silva, L.F.A.; Geutjes, P.J.; Oosterwijk, E.; Hubbell, J.A.; Feitz, W.F.; Frey, P. In-vivo performance of high-density collagen gel tubes for urethral regeneration in a rabbit model. Biomaterials 2012, 33, 7447–7455. [Google Scholar] [CrossRef]
- Raya-Rivera, A.; Esquiliano, D.R.; Yoo, J.J.; Lopez-Bayghen, E.; Soker, S.; Atala, A. Tissue-engineered autologous urethras for patients who need reconstruction: An observational study. Lancet 2011, 377, 1175–1182. [Google Scholar] [CrossRef] [Green Version]
- Oottamasathien, S.; Wang, Y.; Williams, K.; Franco, O.E.; Wills, M.L.; Thomas, J.C.; Saba, K.; Sharif-Afshar, A.R.; Makari, J.H.; Matusik, R.J. Directed differentiation of embryonic stem cells into bladder tissue. Dev. Biol. 2007, 304, 556–566. [Google Scholar] [CrossRef] [Green Version]
- Mauney, J.R.; Ramachandran, A.; Yu, R.N.; Daley, G.Q.; Adam, R.M.; Estrada, C.R. All-trans retinoic acid directs urothelial specification of murine embryonic stem cells via GATA4/6 signaling mechanisms. PLoS ONE 2010, 5, e11513. [Google Scholar] [CrossRef] [Green Version]
- Osborn, S.L.; Thangappan, R.; Luria, A.; Lee, J.H.; Nolta, J.; Kurzrock, E.A. Induction of human embryonic and induced pluripotent stem cells into urothelium. Stem. Cells Transl. Med. 2014, 3, 610–619. [Google Scholar] [CrossRef]
- Wang, Z.; Wen, Y.; Li, Y.H.; Wei, Y.; Green, M.; Wani, P.; Zhang, P.Z.; Pera, R.R.; Chen, B. Smooth muscle precursor cells derived from human pluripotent stem cells for treatment of stress urinary incontinence. Stem. Cells Dev. 2016, 25, 453–461. [Google Scholar] [CrossRef] [Green Version]
- Wilson, H.K.; Canfield, S.G.; Shusta, E.V.; Palecek, S.P. Concise review: Tissue-specific microvascular endothelial cells derived from human pluripotent stem cells. Stem. Cells 2014, 32, 3037–3045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuchs, E.; Segre, J.A. Stem cells: A new lease on life. Cell 2000, 100, 143–155. [Google Scholar] [CrossRef]
- Singh, V.K.; Kalsan, M.; Kumar, N.; Saini, A.; Chandra, R. Induced pluripotent stem cells: Applications in regenerative medicine, disease modeling, and drug discovery. Front. Cell Dev. Biol. 2015, 3. [Google Scholar] [CrossRef] [Green Version]
- Kang, M.; Kim, H.H.; Han, Y.M. Generation of bladder urothelium from human pluripotent stem cells under chemically defined serum-and feeder-free system. Int. J. Mol. Sci. 2014, 15, 7139–7157. [Google Scholar] [CrossRef] [Green Version]
- Moad, M.; Pal, D.; Hepburn, A.C.; Williamson, S.C.; Wilson, L.; Lako, M.; Armstrong, L.; Hayward, S.W.; Franco, O.E.; Heer, R.; et al. A novel model of urinary tract differentiation, tissue regeneration, and disease: Reprogramming human prostate and bladder cells into induced pluripotent stem cells. Eur. Urol. 2013, 64, 753–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yee, J. Turning somatic cells into pluripotent stem cells. Nat. Educ. 2010, 3, 25. [Google Scholar]
- Tian, H.; Bharadwaj, S.; Liu, Y.; Ma, P.X.; Atala, A.; Zhang, Y. Differentiation of human bone marrow mesenchymal stem cells into bladder cells: Potential for urological tissue engineering. Tissue Eng. Part A 2010, 16, 1769–1779. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.S.; Bury, M.I.; Fuller, N.J.; Sturm, R.M.; Ahmad, N.; Sharma, A.K. Bone marrow stem/progenitor cells attenuate the inflammatory milieu following substitution urethroplasty. Sci. Rep. 2016, 6, 35638. [Google Scholar] [CrossRef] [Green Version]
- Mastrolia, I.; Foppiani, E.M.; Murgia, A.; Candini, O.; Samarelli, A.V.; Grisendi, G.; Veronesi, E.; Horwitz, E.M.; Dominici, M. Challenges in clinical development of mesenchymal stromal/stem cells: Concise review. Stem. Cell Transl. Med. 2019, 8, 1135–1148. [Google Scholar] [CrossRef] [Green Version]
- Fraser, J.K.; Wulur, I.; Alfonso, Z.; Hedrick, M.H. Fat tissue: An underappreciated source of stem cells for biotechnology. Trends. Biotechnol. 2006, 24, 150–154. [Google Scholar] [CrossRef]
- Katz, A.J.; Llull, R.; Hedrick, M.H.; Futrell, J.W. Emerging approaches to the tissue engineering of fat. Clin. Plast. Surg. 1999, 26, 587–603. [Google Scholar] [CrossRef]
- Gimble, J.M.; Katz, A.J.; Bunnell, B.A. Adipose-derived stem cells for regenerative medicine. Circ. Res. 2007, 100, 1249–1260. [Google Scholar] [CrossRef]
- Wankhade, U.D.; Shen, M.; Kolhe, R.; Fulzele, S. Advances in adipose-derived stem cells isolation, characterization, and application in regenerative tissue engineering. Stem. Cell Int. 2016, 2016, 3206807. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, M.X.; Zhou, Z.; Zhang, K.; Zhou, J.; Zhao, Y.; Wang, Z.; Lu, M.J. The differentiation of human adipose-derived stem cells towards a urothelium-like phenotype in vitro and the dynamic temporal changes of related cytokines by both paracrine and autocrine signal regulation. PLoS ONE 2014, 9, e95583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Huang, J.; Lin, T.; Zhang, C.; Yin, X. Cell-to-cell contact induces human adipose tissue-derived stromal cells to differentiate into urothelium-like cells in vitro. Biochem. Biophys. Res. Commun. 2009, 390, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Peng, Y.; Zhou, Z.; Zhou, J.; Wang, Z.; Lu, M. Differentiation of human adipose-derived stem cells co-cultured with urothelium cell line toward a urothelium-like phenotype in a nude murine model. Urology 2013, 81, e15–e22. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xu, Y.; Fu, Q.; Li, C. Effects of multiple agents on epithelial differentiation of rabbit adipose-derived stem cells in 3D culture. Tissue Eng. Part A 2012, 18, 1760–1770. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.J.; Li, M.Y.; Huang, W.T.; Lu, M.H.; Hu, C.; Li, K.; Qiu, J.Q.; Gao, X. Repair of urethral defects with polylactid acid fibrous membrane seeded with adipose-derived stem cells in a rabbit model. Connect Tissue Res. 2015, 56, 434–439. [Google Scholar] [CrossRef]
- Jack, G.S.; Zhang, R.; Lee, M.; Xu, Y.; Wu, B.M.; Rodríguez, L.V. Urinary bladder smooth muscle engineered from adipose stem cells and a three dimensional synthetic composite. Biomaterials 2009, 30, 3259–3270. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Jack, G.S.; Rao, N.; Zuk, P.; Ignarro, L.J.; Wu, B.; Rodríguez, L.V. Nuclear fusion-independent smooth muscle differentiation of human adipose-derived stem cells induced by a smooth muscle environment. Stem. Cell 2012, 30, 481–490. [Google Scholar] [CrossRef]
- Fu, Q.; Deng, C.L.; Zhao, R.Y.; Wang, Y.; Cao, Y. The effect of mechanical extension stimulation combined with epithelial cell sorting on outcomes of implanted tissue-engineered muscular urethras. Biomaterials 2014, 35, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Riis, S.; Hansen, A.C.; Johansen, L.; Lund, K.; Pedersen, C.; Pitsa, A.; Hyldig, K.; Zachar, V.; Fink, T.; Pennisi, C.P. Fabrication and characterization of extracellular matrix scaffolds obtained from adipose-derived stem cells. Methods 2020, 171, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Fortier, G.M.; Gauvin, R.; Proulx, M.; Vallée, M.; Fradette, J. Dynamic culture induces a cell type-dependent response impacting on the thickness of engineered connective tissues. J. Tissue Eng. Regen. Med. 2013, 7, 292–301. [Google Scholar] [CrossRef]
- Zhang, D.; Wei, G.; Li, P.; Zhou, X.; Zhang, Y. Urine-derived stem cells: A novel and versatile progenitor source for cell-based therapy and regenerative medicine. Genes Dis. 2014, 1, 8–17. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.Z.; He, T.; Cui, J.; Jiang, Y.L.; Zeng, J.F.; Zhang, W.Q.; Xie, H.Q. Urine-Derived Stem Cells for Regenerative Medicine: Basic Biology, Applications, and Challenges. Tissue Eng. Part B Rev. 2022, 28, 978–994. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, S.; Liu, G.; Shi, Y.; Wu, R.; Yang, B.; He, T.; Fan, Y.; Lu, X.; Zhou, X.; Zhang, Y.; et al. Multipotential differentiation of human urine-derived stem cells: Potential for therapeutic applications in urology. Stem. Cell 2013, 31, 1840–1856. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, G.; Wu, R.; Mack, D.L.; Sun, X.S.; Maxwell, J.; Guan, X.; Atala, A.; Zhang, Y. Differentiation Capacity of Human Urine-Derived Stem Cells to Retain Telomerase Activity. Front. Cell Dev. Biol. 2022, 10, 890574. [Google Scholar] [CrossRef]
- Lang, R.; Liu, G.; Shi, Y.; Bharadwaj, S.; Leng, X.; Zhou, X.; Liu, H.; Atala, A.; Zhang, Y. Self-renewal and differentiation capacity of urine-derived stem cells after urine preservation for 24 hours. PLoS ONE 2013, 8, e53980. [Google Scholar] [CrossRef] [Green Version]
- Abbas, T.O.; Ali, T.A.; Uddin, S. Urine as a main effector in urological tissue engineering—A double-edged sword. Cells 2020, 9, 538. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Wu, R.; Yang, B.; Deng, C.; Lu, X.; Walker, S.J.; Ma, P.X.; Mou, S.; Atala, A.; Zhang, Y. Human urine-derived stem cell differentiation to endothelial cells with barrier function and nitric oxide production. Stem. Cells Transl. Med. 2018, 7, 686–698. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Liu, Y.; Bharadwaj, S.; Atala, A.; Zhang, Y. Human urine-derived stem cells seeded in a modified 3D porous small intestinal submucosa scaffold for urethral tissue engineering. Biomaterials 2011, 32, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Drewa, T.; Joachimiak, R.; Bajek, A.; Gagat, M.; Grzanka, A.; Bodnar, M.; Marszalek, A.; Dębski, R.; Chłosta, P. Hair follicle stem cells can be driven into a urothelial-like phenotype: An experimental study. Int. J. Urol. 2013, 20, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.B.; Liu, C.X.; Xie, Q.; Zhu, B.L. Rabbit hair follicle stem cells and urethral mucosa stem cells used as seed cells for urethra tissue engineering: A comparison study. Acad. J. Second Mil. Med 2013, 33, 388–392. [Google Scholar] [CrossRef]
- Richardson, G.D.; Arnott, E.C.; Whitehouse, C.J.; Lawrence, C.M.; Reynolds, A.J.; Hole, N.; Jahoda, C.A. Plasticity of rodent and human hair follicle dermal cells: Implications for cell therapy and tissue engineering. J. Investig. Derm. Symp. Proc. 2005, 10, 180–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, H.H.; Kang, J.J.; Kang, H.G.; Chung, S.S. Urothelial differentiation of human amniotic fluid stem cells by urothelium specific conditioned medium. Cell Biol. Int. 2014, 38, 531–537. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Cheng, Z.; Liu, G.; Zhao, X.; Zhong, L.; Zhu, Y.; Zhu, J. Urothelial differentiation of human umbilical cord-derived mesenchymal stromal cells in vitro. Anal. Cell Pathol. 2013, 36, 63–69. [Google Scholar] [CrossRef]
- Yuan, H.; Zhuang, Y.; Xiong, J.; Zhi, W.; Liu, L.; Wei, Q.; Han, P. Human umbilical mesenchymal stem cells-seeded bladder acellular matrix grafts for reconstruction of bladder defects in a canine model. PLoS ONE 2013, 8, e80959. [Google Scholar] [CrossRef]
- Hare, J.M.; DiFede, D.L.; Rieger, A.C.; Florea, V.; Landin, A.M.; El-Khorazaty, J.; Khan, A.; Mushtaq, M.; Lowery, M.H.; Byrnes, J.J.; et al. Randomized comparison of allogeneic versus autologous mesenchymal stem cells for nonischemic dilated cardiomyopathy: POSEIDON-DCM trial. J. Am. Coll. Cardiol. 2017, 69, 526–537. [Google Scholar] [CrossRef]
- Shah, K.; Shah, N.; Ghassemi, F.; Ly, C.; George, T.; Lutz, C.; Sumer, H. Alloreactivity of Allogeneic Mesenchymal Stem/Stromal Cells and Other Cellular Therapies: A Concise Review. Stem. Cell Int. 2022, 2022, 9589600. [Google Scholar] [CrossRef]
- Horiguchi, A.; Ojima, K.; Shinchi, M.; Mayumi, Y.; Kushibiki, T.; Katoh, S.; Takeda, M.; Iwasaki, M.; Yoshioka, H.; Abraham, S.J.; et al. In Vitro Culture Expansion and Characterization of Buccal Mucosal Epithelial Cells for Tissue Engineering Applications in Urethral Stricture After Transportation Using a Thermoreversible Gelation Polymer. Biopreserv. Biobank 2022, 20, 97–103. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Zamani, M.; Shakhssalim, N.; Ramakrishna, S.; Naji, M. Electrospinning: Application and prospects for urologic tissue engineering. Front Bioeng. Biotechnol. 2020, 8, 579925. [Google Scholar] [CrossRef]
- Xie, M.; Song, L.; Wang, J.; Fan, S.; Zhang, Y.; Xu, Y. Evaluation of stretched electrospun silk fibroin matrices seeded with urothelial cells for urethra reconstruction. J. Surg. Res. 2013, 184, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Simsek, A.; Bullock, A.J.; Roman, S.; Chapple, C.R.; Macneil, S. Developing improved tissue-engineered buccal mucosa grafts for urethral reconstruction. Can. Urol. Assoc. J. 2018, 12, E234–E242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sievert, K.D.; Daum, L.; Maurer, S.; Toomey, P.; Vaegler, M.; Aufderklamm, S.; Amend, B. Urethroplasty performed with an autologous urothelium-vegetated collagen fleece to treat urethral stricture in the minipig model. World J. Urol. 2020, 38, 2123–2131. [Google Scholar] [CrossRef] [PubMed]
- Uchida, N.; Sivaraman, S.; Amoroso, N.J.; Wagner, W.R.; Nishiguchi, A.; Matsusaki, M.; Akashi, M.; Nagatomi, J. Nanometer-sized extracellular matrix coating on polymer-based scaffold for tissue engineering applications. J. Biomed Mater. Res. A 2016, 104, 94–103. [Google Scholar] [CrossRef]
- Gnecchi, M.; Danieli, P.; Malpasso, G.; Ciuffreda, M.C. Paracrine mechanisms of mesenchymal stem cells in tissue repair. Methods Mol. Biol. 2016, 1416, 123–146. [Google Scholar]
- Lv, X.G.; Feng, C.; Fu, Q.; Xie, H.; Wang, Y.; Huang, J.W.; Xie, M.K.; Atala, A.; Xu, Y.M.; Zhao, W.X. Comparative study of different seeding methods based on a multilayer SIS scaffold: Which is the optimal procedure for urethral tissue engineering? J. Biomed Mater. Res. B Appl. Biomater. 2016, 104, 1098–1108. [Google Scholar] [CrossRef]
- Melke, J.; Zhao, F.; Ito, K.; Hofmann, S. Orbital seeding of mesenchymal stromal cells increases osteogenic differentiation and bone-like tissue formation. J. Orthop. Res. 2020, 38, 1228–1237. [Google Scholar] [CrossRef]
- Akiyama, Y.; Kikuchi, A.; Yamato, M.; Okano, T. Ultrathin poly (N-isopropylacrylamide) grafted layer on polystyrene surfaces for cell adhesion/detachment control. Langmuir 2004, 20, 5506–5511. [Google Scholar] [CrossRef]
- Masuda, S.; Shimizu, T. Three-dimensional cardiac tissue fabrication based on cell sheet technology. Adv. Drug Deliv. Rev. 2016, 96, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Lal, H.; Patralekh, M.K. 3D printing and its applications in orthopaedic trauma: A technological marvel. J. Clin. Orthop. Trauma 2018, 9, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Zhao, W.; Zhu, J.M.; Albanna, M.Z.; Yoo, J.J.; Atala, A. Complex heterogeneous tissue constructs containing multiple cell types prepared by inkjet printing technology. Biomaterials 2013, 34, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Fu, Q.; Yoo, J.; Chen, X.; Chandra, P.; Mo, X.; Song, L.; Atala, A.; Zhao, W. 3D bioprinting of urethra with PCL/PLCL blend and dual autologous cells in fibrin hydrogel: An in vitro evaluation of biomimetic mechanical property and cell growth environment. Acta Biomater. 2017, 50, 154–164. [Google Scholar] [CrossRef]
- Pi, Q.; Maharjan, S.; Yan, X.; Liu, X.; Singh, B.; van Genderen, A.M.; Robledo-padilla, F.; Parra-Saldivar, R.; Hu, N.; Zhang, Y.S.; et al. Digitally tunable microfluidic bioprinting of multilayered cannular tissues. Adv. Mater. 2018, 30, e1706913. [Google Scholar] [CrossRef]
- Davis, N.F.; Mooney, R.; Piterina, A.V.; Callanan, A.; McGuire, B.B.; Flood, H.D.; McGloughlin, T.M. Construction and evaluation of urinary bladder bioreactor for urologic tissue-engineering purposes. Urology 2011, 78, 954–960. [Google Scholar] [CrossRef]
- Bakhshandeh, B.; Ranjbar, N.; Abbasi, A.; Amiri, E.; Abedi, A.; Mehrabi, M.R.; Dehghani, Z.; Pennisi, C.P. Recent progress in the manipulation of biochemical and biophysical cues for engineering functional tissues. Bioeng. Transl. Med. 2022, e10383. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, Q.; Zhao, R.Y.; Deng, C.L. Muscular tubes of urethra engineered from adipose-derived stem cells and polyglycolic acid mesh in a bioreactor. Biotechnol. Lett. 2014, 36, 1909–1916. [Google Scholar] [CrossRef]
- Yang, P.J.; Pham, J.; Choo, J.; Hu, D.L. Duration of urination does not change with body size. Proc. Natl. Acad. Sci. USA 2014, 111, 11932–11937. [Google Scholar] [CrossRef] [Green Version]
- Versteegden, L.R.; Van Kampen, K.A.; Janke, H.P.; Tiemessen, D.M.; Hoogenkamp, H.R.; Hafmans, T.G.; Roozen, E.A.; Lomme, R.M.; Van Goor, H.; Daamen, W.F.; et al. Tubular collagen scaffolds with radial elasticity for hollow organ regeneration. Acta Biomater. 2017, 52, 1–8. [Google Scholar] [CrossRef]
- Guan, Y.; Ou, L.; Hu, G.; Wang, H.; Xu, Y.; Chen, J.; Zhang, J.; Yu, Y.; Kong, D. Tissue engineering of urethra using human vascular endothelial growth factor gene-modified bladder urothelial cells. Artif. Organs 2008, 32, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.J.; Pandolfi, L.; Wang, X.; Minardi, S.; Lupo, C.; Evangelopoulos, M.; Hendrickson, T.; Shi, A.; Storci, G.; Taraballi, F.; et al. Electrospun patch functionalized with nanoparticles allows for spatiotemporal release of VEGF and PDGF-BB promoting in vivo neovascularization. ACS Appl. Mater. Interfaces 2018, 10, 44344–44353. [Google Scholar] [CrossRef] [PubMed]
- Loai, Y.; Yeger, H.; Coz, C.; Antoon, R.; Islam, S.S.; Moore, K.; Farhat, W.A. Bladder tissue engineering: Tissue regeneration and neovascularization of HA-VEGF-incorporated bladder acellular constructs in mouse and porcine animal models. J. Biomed Mater. Res. A 2010, 94, 1205–1215. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Tang, H.; Wu, J.; Hou, X.; Chen, B.; Chen, W.; Zhao, Y.; Shi, C.; Zhou, F.; Yu, W.; et al. Urethral tissue regeneration using collagen scaffold modified with collagen binding VEGF in a beagle model. Biomaterials 2015, 69, 45–55. [Google Scholar] [CrossRef]
- Cheng, J.H.; She, H.; Han, Y.P.; Wang, J.; Xiong, S.; Asahina, K.; Tsukamoto, H. Wnt antagonism inhibits hepatic stellate cell activation and liver fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G39–G49. [Google Scholar] [CrossRef] [PubMed]
- Conidi, A.; Van den Berghe, V.; Huylebroeck, D. Aptamers and Their Potential to Selectively Target Aspects of EGF, Wnt/β-Catenin and TGFβ–Smad Family Signaling. Int. J. Mol. Sci. 2013, 14, 6690–6719. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Guo, X.; Zhao, W.; Niu, G.; Mo, X.; Fu, Q. Application of Wnt pathway inhibitor delivering scaffold for inhibiting fibrosis in urethra strictures: In vitro and in vivo study. Int. J. Mol. Sci. 2015, 16, 27659–27676. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Xu, Y.M.; Liu, Z.S.; Li, H.B. Urethral reconstruction with tissue engineering and RNA interference techniques in rabbits. Urology 2013, 81, 1075–1080. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xuan, Z.; Zachar, V.; Pennisi, C.P. Sources, Selection, and Microenvironmental Preconditioning of Cells for Urethral Tissue Engineering. Int. J. Mol. Sci. 2022, 23, 14074. https://doi.org/10.3390/ijms232214074
Xuan Z, Zachar V, Pennisi CP. Sources, Selection, and Microenvironmental Preconditioning of Cells for Urethral Tissue Engineering. International Journal of Molecular Sciences. 2022; 23(22):14074. https://doi.org/10.3390/ijms232214074
Chicago/Turabian StyleXuan, Zongzhe, Vladimir Zachar, and Cristian Pablo Pennisi. 2022. "Sources, Selection, and Microenvironmental Preconditioning of Cells for Urethral Tissue Engineering" International Journal of Molecular Sciences 23, no. 22: 14074. https://doi.org/10.3390/ijms232214074