A Case-Control Study on the Changes in High-Sensitivity C-Reactive Protein and Tumor Necrosis Factor-Alpha Levels with Surgical Treatment of OSAS
Abstract
1. Introduction
2. Results
2.1. Characteristics of Groups Based on Age, Gender, BMI, and Laryngological Examination
2.2. Type of Surgery
- 10 modified uvulopalatopharyngoplasty (modified U3P) treatments with tongue base tissue reduction;
- 10 modified U3P procedures with radiofrequency induced thermotherapy of the tongue base;
- 5 expansion sphincter pharyngoplasty (ESP) treatments with tongue base tissue reduction.
2.3. OSAS Severity, ODI, MOS, LOS
2.4. ESS, VAS, SF-36
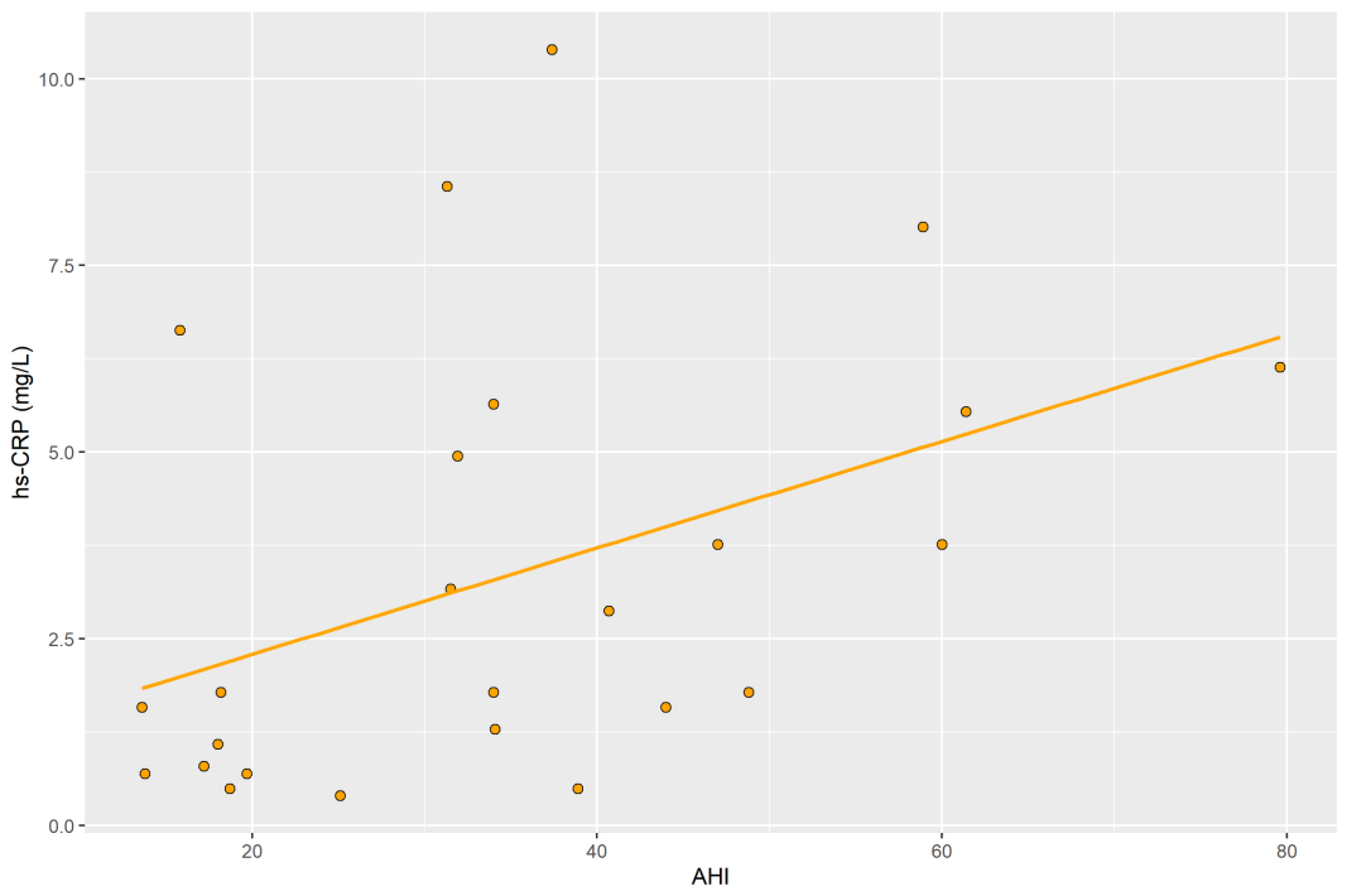
2.5. hs-CRP
2.6. TNF-α
2.7. Generalized Estimating Equations
3. Discussion
4. Materials and Methods
4.1. Study Protocol
- adult patients, between the ages of 18–70 years
- OSAS diagnosed based on polygraphic examination (sleep study type III), following the guidelines of the American Academy of Sleep Medicine, medical history, and physical examination;
- lack of tolerance of PAP therapy
- no surgical treatment for snoring or OSAS minimum 6 months prior to the enrollment;
- written informed consent to participate in the study
4.2. Determination of hs-CRP and TNF-α in Serum
4.3. Surgical Procedures
4.4. Statistical Analysis
5. Conclusions
- OSAS promotes an inflammatory state.
- Surgical treatment reduces inflammation expressed by TNF-α levels in OSAS patients.
6. Study Limitations
- The main limitation of our study was the size of the study group (n = 25 patients). Despite our best efforts, of the 35 patients included, only 25 of them showed up for follow-up. The limited sample was also due to the need to eliminate factors that could have influenced the results (including unregulated diabetes, enteritis, thyroid diseases, and chronic liver diseases). Due to time limitations, resulting from the period of reagents used for the determinations of concentration of particular compounds, it was difficult to obtain a fully homogeneous control group, that met the inclusion, and did not meet the exclusion criteria of the study. Moreover, not all patients included in the study group consented to repeat blood sampling after surgery.
- The control group, who were selected from patients undergoing surgery for otosclerosis, was quite different with respect to age and BMI than the study group, who were selected from those that needed sleep surgery due to the presence of OSAS. Although minimally invasive, the burden due to the tests and samples required for the study enrollment as a control subject limited the potentially eligible population. Due to relative convenience, and to reduce the burden, control subjects were selected among those that needed surgery, but also did not have OSAS. This limited further the ability to find control group candidates with age and BMI similar to the study subjects with older age and BMI who needed sleep surgery associated with such factors. Lower age range and BMI in the control group limited their true role as control when such major variables were not comparable to the study group. Those factors may independently impact the levels of inflammatory markers. In addition, the condition of the control group that qualified the enrollment was otosclerosis disease, which is also considered an inflammatory condition. Even though there are reports on the presence of increased expression of TNF-α in the histological samples of the stapes footplate, we did not find literature indicating elevated levels of these markers in the serum. However, we cannot completely rule out the potential effect of otosclerosis or any other inflammatory conditions in the subjects in the control group on the levels of TNF-α and hs-CRP.
- Based on the literature, the difference in the magnitude of BMI in the study group and the control group may affect the results of the study. Nevertheless, analysis of the blood concentrations of selected compounds in patients before and after surgery indicates a lack of significant change in BMI values in these groups of patients. Thus, we suppose that the influence of the factor related to the change in body weight on the results of our study was small.
- The levels of the inflammatory markers in the blood of patients after surgery may have been influenced by lifestyle, diet, and physical activity in the period between determinations.
- The results of our study were not compared to the results of the study carried out on a group of patients undergoing non-surgical treatment.
- In our experiment we used type III sleep study (polygraphy) in the diagnostic procedure. During polygraphy, sleep structure and arousal identification are not analyzed. However, according to the American Academy of Sleep Medicine, the results of this examination allow qualifying patients for surgery, especially when the study group does not include patients with coexisting diseases [58].
- The polygraph examination also has advantages, which include, among others, that it was conducted in a home setting, which increased the chance of sleep undisturbed by hospital conditions. The report was not automatically generated. In each case, a manual reading of the sleep record by the first and second authors was used to obtain this report.
- Due to a number of factors, although all done within 4 months before the surgery, there was variability in the interval between the sleep study and the surgical treatment, therefore authors cannot rule out a possible change in the severity of the sleep study results during that interval.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AASM | American Academy of Sleep Medicine |
| AHI | apnea/hypopnea index |
| BMI | body mass index |
| C.I. | confidence interval |
| ELISA | enzyme-linked immunosorbent assay |
| ESP | expansion sphincter pharyngoplasty |
| ESS | Epworth Sleep Scale |
| hs-CRP | high-sensitivity C-Reactive Protein |
| IL-6 | interleukin 6 |
| IQR | inter-quartile range |
| LOS | lowest oxygen saturation |
| modified U3P | modified uvulopalatopharyngoplasty |
| MOS | mean oxygen saturation |
| ODI | oxygen saturation index |
| OSAS | obstructive sleep apnea syndrome |
| p | level of statistical significance |
| PAP | positive airway pressure |
| r | correlation coefficient |
| SD | standard deviation |
| SDB | sleep-disordered breathing |
| SF-36 | 36-item Short Form Health Survey |
| TNF-α | tumor necrosis factor α |
| VAS | visual scale of snoring |
| vs. | versus |
References
- Heatley, E.M.; Harris, M.; Battersby, M.; McEvoy, R.D.; Chai-Coetzer, C.L.; Antic, N.A. Obstructive sleep apnoea in adults: A common chronic condition in need of a comprehensive chronic condition management approach. Sleep Med. Rev. 2013, 17, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, N.R.; Peng, Y.-J.; Nanduri, J. Hypoxia-inducible factors and obstructive sleep apnea. J. Clin. Investig. 2020, 130. [Google Scholar] [CrossRef]
- Olszewska, E.; Rogalska, J.; Brzóska, M. The Association of Oxidative Stress in the Uvular Mucosa with Obstructive Sleep Apnea Syndrome: A Clinical Study. J. Clin. Med. 2021, 10, 1132. [Google Scholar] [CrossRef]
- Lavie, L. Oxidative stress in obstructive sleep apnea and intermittent hypoxia—Revisited—The bad ugly and good: Implica-tions to the heart and brain. Sleep Med Rev. 2015, 20, 27–45. [Google Scholar] [CrossRef]
- Navarro, S.L.; Kantor, E.D.; Song, X.; Milne, G.L.; Lampe, J.W.; Kratz, M.; White, E. Factors Associated with Multiple Biomarkers of Systemic Inflammation. Cancer Epidemiol. Biomark. Prev. 2016, 25, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Boudjeltia, K.Z.; van Meerhaeghe, A.; Doumit, S.; Guillaume, M.; Cauchie, P.; Brohée, D.; Vanhaeverbeek, M.; Kerkhofs, M. Sleep Apnoea-Hypopnoea Index Is an Independent Predictor of High-Sensitivity C-Reactive Protein Elevation. Respiration 2006, 73, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Tauman, R.; O’Brien, L.M.; Gozal, D. Hypoxemia and obesity modulate plasma C-reactive protein and interleukin-6 levels in sleep-disordered breathing. Sleep Breath. 2006, 11, 77–84. [Google Scholar] [CrossRef]
- Nilsson, J. CRP—Marker or maker of cardiovascular disease? Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1527–1528. [Google Scholar] [CrossRef]
- Bouloukaki, I.; Mermigkis, C.; Kallergis, E.M.; Moniaki, V.; Mauroudi, E.; E Schiza, S. Obstructive sleep apnea syndrome and cardiovascular disease: The influence of C-reactive protein. World J. Exp. Med. 2015, 5, 77–83. [Google Scholar] [CrossRef]
- Cao, Y.; Song, Y.; Ning, P.; Zhang, L.; Wu, S.; Quan, J.; Li, Q. Association between tumor necrosis factor alpha and obstructive sleep apnea in adults: A meta-analysis update. BMC Pulm. Med. 2020, 20, 215. [Google Scholar] [CrossRef]
- Kubota, T.; Li, N.; Guan, Z.; Brown, R.A.; Krueger, J.M. Intrapreoptic microinjection of TNF-α enhances non-REM sleep in rats. Brain Res. 2002, 932, 37–44. [Google Scholar] [CrossRef]
- Rockstrom, M.D.; Chen, L.; Taishi, P.; Nguyen, J.T.; Gibbons, C.M.; Veasey, S.C.; Krueger, J.M. Tumor necrosis factor alpha in sleep regulation. Sleep Med. Rev. 2018, 40, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Vgontzas, A.N.; Papanicolaou, D.A.; Bixler, E.O.; Kales, A.; Tyson, K.; Chrousos, G.P. Elevation of plasma cytokines in disorders of excessive daytime sleepiness: Role of sleep disturbance and obesity. J. Clin. Endocrinol. Metab. 1997, 82, 1313–1316. [Google Scholar] [CrossRef]
- Vgontzas, A.N.; Zoumakis, E.; Lin, H.-M.; Bixler, E.O.; Trakada, G.; Chrousos, G.P. Marked Decrease in Sleepiness in Patients with Sleep Apnea by Etanercept, a Tumor Necrosis Factor-? Antagonist. J. Clin. Endocrinol. Metab. 2004, 89, 4409–4413. [Google Scholar] [CrossRef]
- National Institutes of Health, National Heart, Lung, and Blood Institute. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults–the evidence report. Obes. Res. 1998, 6 (Suppl. S2), 51S–209S. [Google Scholar]
- Friedman, M.; Salapatas, A.M.; Bonzelaar, L.B. Updated Friedman Staging System for Obstructive Sleep Apnea. In Sleep Related Breathing Disorders; Karger Publishers: Basel, Switzerland, 2017; Volume 80, pp. 41–48. [Google Scholar] [CrossRef]
- Nadeem, R.; Molnar, J.; Madbouly, E.M.; Nida, M.; Aggarwal, S.; Sajid, H.; Naseem, J.; Loomba, R. Serum Inflammatory Markers in Obstructive Sleep Apnea: A Meta-Analysis. J. Clin. Sleep Med. 2013, 9, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Rifai, N.; Pfeffer, M.; Sacks, F.; Lepage, S.; Braunwald, E. Elevation of Tumor Necrosis Factor-α and Increased Risk of Recurrent Coronary Events After Myocardial Infarction. Circulation 2000, 101, 2149–2153. [Google Scholar] [CrossRef]
- Patel, N.; Donahue, C.; Shenoy, A.; Patel, A.; El-Sherif, N. Obstructive sleep apnea and arrhythmia: A systemic review. Int. J. Cardiol. 2016, 228, 967–970. [Google Scholar] [CrossRef]
- Ming, H.; Tian, A.; Liu, B.; Hu, Y.; Liu, C.; Chen, R.; Cheng, L. Inflammatory cytokines tumor necrosis factor-α, interleukin-8 and sleep monitoring in patients with obstructive sleep apnea syndrome. Exp. Ther. Med. 2018, 17, 1766–1770. [Google Scholar] [CrossRef]
- Ciftci, T.U.; Kokturk, O.; Bukan, N.; Bilgihan, A. The relationship between serum cytokine levels with obesity and obstructive sleep apnea syndrome. Cytokine 2004, 28, 87–91. [Google Scholar] [CrossRef]
- De la Peña Bravo, M.; Serpero, L.D.; Barceló, A.; Barbé, F.; Agustí, A.; Gozal, D. Inflammatory proteins in patients with obstructive sleep apnea with and without daytime sleepiness. Sleep Breath. 2007, 11, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Sahlman, J.; Miettinen, K.; Peuhkurinen, K.; Seppä, J.; Peltonen, M.; Herder, C.; Punnonen, K.; Vanninen, E.; Gylling, H.; Partinen, M.; et al. The activation of the inflammatory cytokines in overweight patients with mild obstructive sleep apnoea: Sleep apnea and inflammation. J. Sleep Res. 2010, 19, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Dorkova, Z.; Petrasova, D.; Molcanyiova, A.; Popovnakova, M.; Tkacova, R. Effects of Continuous Positive Airway Pressure on Cardiovascular Risk Profile in Patients With Severe Obstructive Sleep Apnea and Metabolic Syndrome. Chest 2008, 134, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yu, W.; Gao, M.; Zhang, F.; Gu, C.; Yu, Y.; Wei, Y. Impact of obstructive sleep apnea syndrome on endothelial function, arterial stiffening, and serum inflammatory markers: An updated meta-analysis and metaregression of 18 studies. J. Am. Heart Assoc. 2015, 4, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Fornadi, K.; Lindner, A.; Czira, M.E.; Szentkiralyi, A.; Lazar, A.; Zoller, R.; Turanyi, C.Z.; Veber, O.; Novak, M.; Mucsi, I.; et al. Lack of association between objectively assessed sleep disorders and inflammatory markers among kidney transplant recipients. Int. Urol. Nephrol. 2011, 44, 607–617. [Google Scholar] [CrossRef]
- Entzian, P.; Linnemann, K.; Schlaak, M.; Zabel, P. Obstructive sleep apnea syndrome and circadian rhythms of hormones and cytokines. Am. J. Respir. Crit. Care Med. 1996, 153, 1080–1086. [Google Scholar] [CrossRef]
- Kataoka, T.; Enomoto, F.; Kim, R.; Yokoi, H.; Fujimori, M.; Sakai, Y.; Ando, I.; Ichikawa, G.I.; Ikeda, K. The effect of surgical treatment of obstructive sleep apnea syndrome on the plasma TNF-α levels. Tohoku J. Exp. Med. 2004, 204, 267–272. [Google Scholar] [CrossRef]
- Li, Y.; Chongsuvivatwong, V.; Geater, A.; Liu, A. Exhaled breath condensate cytokine level as a diagnostic tool for obstructive sleep apnea syndrome. Sleep Med. 2009, 10, 95–103. [Google Scholar] [CrossRef]
- Zumbach, M.S.; Boehme, J.; Wahl, P.; Stremmel, W.; Ziegler, R.; Nawroth, P.P. Tumor necrosis factor increases serum leptin levels in humans. J. Clin. Endocrinol. Metab. 2015, 82, 4080–4082. [Google Scholar] [CrossRef]
- Blake, G.J.; Ridker, P.M. Inflammatory bio-markers and cardiovascular risk prediction. J. Intern. Med. 2002, 252, 283–294. [Google Scholar] [CrossRef]
- Skoog, T.; Dichtl, W.; Boquist, S.; Skoglund-Andersson, C.; Karpe, F.; Tang, R.; Bond, M.; De Faire, U.; Nilsson, J.; Eriksson, P.; et al. Plasma tumour necrosis factor-α and early carotid atherosclerosis in healthy middle-aged men. Eur. Heart J. 2002, 23, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Paynter, N.P.; Rifai, N.; Gaziano, J.M.; Cook, N.R. C-reactive protein and parental history improve global cardiovas-cular risk prediction: The Reynolds risk score for men. Circulation 2008, 118, 2243–2251. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.; Nolan, G.M.; Hannigan, E.; Cunningham, S.; Taylor, C.; McNicholas, W.T. Cardiovascular risk markers in obstructive sleep apnoea syndrome and correlation with obesity. Thorax 2007, 62, 509–514. [Google Scholar] [CrossRef]
- Reid, M.B.; Lännergren, J.; Westerblad, H. Respiratory and limb muscle weakness induced by tumor necrosis factor-α: Involvement of muscle myofilaments. Am. J. Respir Crit. Care Med. 2002, 166, 479–484. [Google Scholar] [CrossRef]
- Li, X.; Moody, M.R.; Engel, D.; Walker, S.; Clubb, F.J., Jr.; Sivasubramanian, N.; Mann, D.L.; Reid, M.B. Cardiac-Specific Overexpression of Tumor Necrosis Factor-α Causes Oxidative Stress and Contractile Dysfunction in Mouse Diaphragm. Circulation 2000, 102, 1690–1696. [Google Scholar] [CrossRef]
- Sharma, S.K.; Mishra, H.K.; Sharma, H.; Goel, A.; Sreenivas, V.; Gulati, V.; Tahir, M. Obesity, and not obstructive sleep apnea, is re-sponsible for increased serum hs-CRP levels in patients with sleep-disordered breathing in Delhi. Sleep Med. 2008, 9, 149–156. [Google Scholar] [CrossRef]
- Chung, S.; Yoon, I.-Y.; Shin, Y.-K.; Lee, C.H.; Kim, J.-W.; Lee, T.; Choi, D.-J.; Ahn, H.J. Endothelial Dysfunction and C-Reactive Protein in Relation with the Severity of Obstructive Sleep Apnea Syndrome. Sleep 2007, 30, 997–1001. [Google Scholar] [CrossRef][Green Version]
- Firat Guven, S.; Turkkani, M.H.; Ciftci, B.; Ulukavak Ciftci, T.; Erdogan, Y. The relationship between high-sensitivity C-reactive protein levels and the severity of obstructive sleep apnea. Sleep Breath. 2012, 16, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Van der Touw, T.; Andronicos, N.M.; Smart, N. Is C-reactive protein elevated in obstructive sleep apnea? A systematic review and meta-analysis. Biomarkers 2019, 24, 429–435. [Google Scholar] [CrossRef]
- Kang, K.; Yeh, T.; Hsu, Y.; Ko, J.; Lee, C.; Lin, M.; Hsu, W. Effect of Sleep Surgery on C-Reactive Protein Levels in Adults With Obstructive Sleep Apnea: A Meta-Analysis. Laryngoscope 2020, 131, 1180–1187. [Google Scholar] [CrossRef]
- Shamsuzzaman, A.S.; Winnicki, M.; Lanfranchi, P.; Wolk, R.; Kara, T.; Accurso, V.; Somers, V.K. Elevated C-Reactive Protein in Patients With Obstructive Sleep Apnea. Circulation 2002, 105, 2462–2464. [Google Scholar] [CrossRef] [PubMed]
- Testelmans, D.; Tamisier, R.; Barone-Rochette, G.; Baguet, J.-P.; Roux-Lombard, P.; Pépin, J.-L.; Lévy, P. Profile of circulating cytokines: Impact of OSA, obesity and acute cardiovascular events. Cytokine 2013, 62, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Pasceri, V.; Willerson, J.T.; Yeh, E.T.H. Direct Proinflammatory Effect of C-Reactive Protein on Human Endothelial Cells. Circulation 2000, 102, 2165–2168. [Google Scholar] [CrossRef]
- Kokturk, O.; Ciftci, T.U.; Mollarecep, E.; Ciftci, B. Elevated C-Reactive Protein Levels and Increased Cardiovascular Risk in Patients With Obstructive Sleep Apnea Syndrome. Int. Heart J. 2005, 46, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Lui, M.M.S.; Lam, J.C.M.; Mak, H.K.F.; Xu, A.; Ooi, C.; Lam, D.C.L.; Mak, J.C.W.; Khong, P.L.; Ip, M.S.M. C-reactive protein is associated with Obstructive sleep apnea independent of visceral obesity. Chest 2009, 135, 950–956. [Google Scholar] [CrossRef]
- Akashiba, T.; Akahoshi, T.; Kawahara, S.; Majima, T.; Horie, T. Effects of Long-term Nasal Continuous Positive Airway Pressure on C-reactive Protein in Patients with Obstructive Sleep Apnea Syndrome. Intern. Med. 2005, 44, 899–900. [Google Scholar] [CrossRef] [PubMed]
- Imani, M.M.; Sadeghi, M.; Khazaie, H.; Emami, M.; Bahmani, D.S.; Brand, S. Evaluation of Serum and Plasma Interleukin-6 Levels in Obstructive Sleep Apnea Syndrome: A Meta-Analysis and Meta-Regression. Front. Immunol. 2020, 11, 1343. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.-A.; Chen, N.-H.; Huang, C.-G.; Lin, S.-W.; Fang, T.; Li, H.-Y. Patients with severe obstructive sleep apnea syndrome and elevated high-sensitivity C-reactive protein need priority treatment. Otolaryngol. Neck Surg. 2010, 143, 72–77. [Google Scholar] [CrossRef]
- Lee, L.A.; Huang, C.G.; Chen, N.H.; Wang, C.L.; Fang, T.J.; Li, H.Y. Severity of Obstruc-tive Sleep Apnea Syndrome and High-Sensitivity C-Reactive Protein Reduced After Relocation Pharyngoplasty. Otolaryngol. Head Neck Surg. 2011, 144, 632–638. [Google Scholar] [CrossRef]
- Garcia, V.P.; Rocha, H.N.M.; Sales, A.R.K.; Rocha, N.G.; da Nóbrega, A.C.L. Diferenças na proteína c reativa ultrassensível associado ao gênero em indivíduos com fatores de risco da síndrome metabólica. Arq. Bras. Cardiol. 2016, 106, 182–187. [Google Scholar]
- Jung, J.H.; Park, J.W.; Kim, D.H.; Kim, S.T. The Effects of Obstructive Sleep Apnea on Risk factors for Cardiovascular diseases. Ear Nose Throat J. 2019, 100, 477S–482S. [Google Scholar] [CrossRef] [PubMed]
- Guilleminault, C.; Kirisoglu, C.; Ohayon, M.M. C-Reactive Protein and Sleep-Disordered Breathing. Sleep 2004, 27, 1507–1517. [Google Scholar] [CrossRef] [PubMed]
- Bouloukaki, I.; Mermigkis, C.; Tzanakis, N.; Kallergis, E.; Moniaki, V.; Mauroudi, E.; Schiza, S.E. Evaluation of Inflammatory Markers in a Large Sample of Obstructive Sleep Apnea Patients without Comorbidities. Mediat. Inflamm. 2017, 2017, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Minoguchi, K.; Yokoe, T.; Tazaki, T.; Minoguchi, H.; Tanaka, A.; Oda, N.; Okada, S.; Ohta, S.; Naito, H.; Adachi, M. Increased Carotid Intima-Media Thickness and Serum Inflammatory Markers in Obstructive Sleep Apnea. Am. J. Respir. Crit. Care Med. 2005, 172, 625–630. [Google Scholar] [CrossRef]
- Greenberg, H.; Ye, X.; Wilson, D.; Htoo, A.K.; Hendersen, T.; Liu, S.F. Chronic intermittent hypoxia activates nuclear factor-κB in cardiovascular tissues in vivo. Biochem. Biophys. Res. Commun. 2006, 343, 591–596. [Google Scholar] [CrossRef]
- Kheirandish-Gozal, L.; Gozal, D. Obstructive Sleep Apnea and Inflammation: Proof of Concept Based on Two Illustrative Cytokines. Int. J. Mol. Sci. 2019, 20, 459. [Google Scholar] [CrossRef]
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K. Clinical practice guideline OSA American Academy. J. Clin. Sleep Med. 2017, 13, 479–504. [Google Scholar] [CrossRef]
| Sleep Parameters | Before Surgery | After Surgery | Control |
|---|---|---|---|
| Mean ± SD [Median (IQR)] | Mean ± SD [Median (IQR)] | Mean ± SD [Median (IQR)] | |
| Number of Subjects (n) | 25 | 25 | 18 |
| AHI | 34.9 ± 17.3 [34 (18.5–45.5)] | 18.7 ± 15.3 [13.3 (7.5–27.3)] | 1.5 ± 0.8 [1.4 (1.0–2.1)] |
| ODI | 37.2 ± 18.2 [35 (22.3–47.6)] | 22.6 ± 17.6 [18.1 (9.8–29.7)] | 1.9 ± 0.8 [1.9 (1.2–2.5)] |
| MOS (%) | 91.3 ± 2.9 [91.6 (91.0–92.8)] | 92.6 ± 2.2 [92.9(91.7–93.7)] | 95.6 ± 1.0 [95.9 (95.0–96.1)] |
| LOS (%) | 74.2 ± 11.8 [75.0 (68.5–82.0)] | 80.1 ± 8.3 [82.0 (75.5–86.0)] | 88.4 ± −5.4 [89.5 (87.0–92.3)] |
| Inflammatory Markers | Before Surgery | After Surgery | Control |
|---|---|---|---|
| Mean ± SD [Median (IQR)] | Mean ± SD [Median (IQR)] | Mean ± SD [Median (IQR)] | |
| Number of Subjects (n) | 25 | 25 | 18 |
| hs-CRP (mg/L) | 3.356 ± 2.879 [1.782 (0.941–5.594)] | 3.334 ± 3.160 [1.980 (0.990–5.445)] | 1.775 ± 2.299 [0.891 (0.767–1.436)] |
| TNF-α (pg/mL) | 8.378 ± 3.267 [7.999 (6.137–9.216)] | 6.635 ± 1.390 [6.614 (5.534–7.460)] | 6.119 ± 1.779 [6.000 (5.026–6.823)] |
| Dependent Variable | Independent Variable | B (95% C.I.) | p |
|---|---|---|---|
| hs-CRP (mg/L) | After vs. before surgery | 0.008 (−0.899–−0.916) | 0.985 |
| Patients vs. controls | 2.347 (−1.288–−5.981) | 0.206 | |
| BMI | 0.306 (−0.090–−0.703) | 0.130 | |
| Age | 0.137 (−0.051–−0.324) | 0.153 | |
| TNF-α (pg/mL) | After vs. before surgery | −1.175 (−2.344–−0.005) | 0.049 * |
| Patients vs. controls | −1.081 (−3.352–−1.190) | 0.351 | |
| BMI | 0.143 (0.007–−0.279) | 0.039 * | |
| Age | 0.006 (−0.063–−0.074) | 0.868 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olszewska, E.; Pietrewicz, T.M.; Świderska, M.; Jamiołkowski, J.; Chabowski, A. A Case-Control Study on the Changes in High-Sensitivity C-Reactive Protein and Tumor Necrosis Factor-Alpha Levels with Surgical Treatment of OSAS. Int. J. Mol. Sci. 2022, 23, 14116. https://doi.org/10.3390/ijms232214116
Olszewska E, Pietrewicz TM, Świderska M, Jamiołkowski J, Chabowski A. A Case-Control Study on the Changes in High-Sensitivity C-Reactive Protein and Tumor Necrosis Factor-Alpha Levels with Surgical Treatment of OSAS. International Journal of Molecular Sciences. 2022; 23(22):14116. https://doi.org/10.3390/ijms232214116
Chicago/Turabian StyleOlszewska, Ewa, Tymoteusz Marek Pietrewicz, Magdalena Świderska, Jacek Jamiołkowski, and Adrian Chabowski. 2022. "A Case-Control Study on the Changes in High-Sensitivity C-Reactive Protein and Tumor Necrosis Factor-Alpha Levels with Surgical Treatment of OSAS" International Journal of Molecular Sciences 23, no. 22: 14116. https://doi.org/10.3390/ijms232214116
APA StyleOlszewska, E., Pietrewicz, T. M., Świderska, M., Jamiołkowski, J., & Chabowski, A. (2022). A Case-Control Study on the Changes in High-Sensitivity C-Reactive Protein and Tumor Necrosis Factor-Alpha Levels with Surgical Treatment of OSAS. International Journal of Molecular Sciences, 23(22), 14116. https://doi.org/10.3390/ijms232214116





