Immunogenicity and Immunoprotection of PCV2 Virus-like Particles Incorporating Dominant T and B Cell Antigenic Epitopes Paired with CD154 Molecules in Piglets and Mice
Abstract
:1. Introduction
2. Results
2.1. Screening of Antigenic Epitopes and Construction of Shuttle Plasmids
2.2. Identification of Recombinant Protein Expression in Baculovirus and Optimization of Conditions
2.3. Structural Modeling and Assembly of PCV2 VLP
2.4. Screening of Immune Adjuvants and Immune Response in Mice
2.5. Quality Test of PCV2 Subunit Vaccine
2.5.1. Physicochemical Property Examination
2.5.2. Security Checks
2.6. Immunogenicity and Attack Protection Experiments in Piglets
2.6.1. Immunogenicity of Piglets
2.6.2. Temperature and Clinical Signs
2.6.3. PCV2 Viremia and Viral Load in Lymph Node Tissue
2.6.4. Histopathological Observations on Piglets after Challenge
2.7. Study on the Immunological Activity of Porcine-Derived CD154 Protein
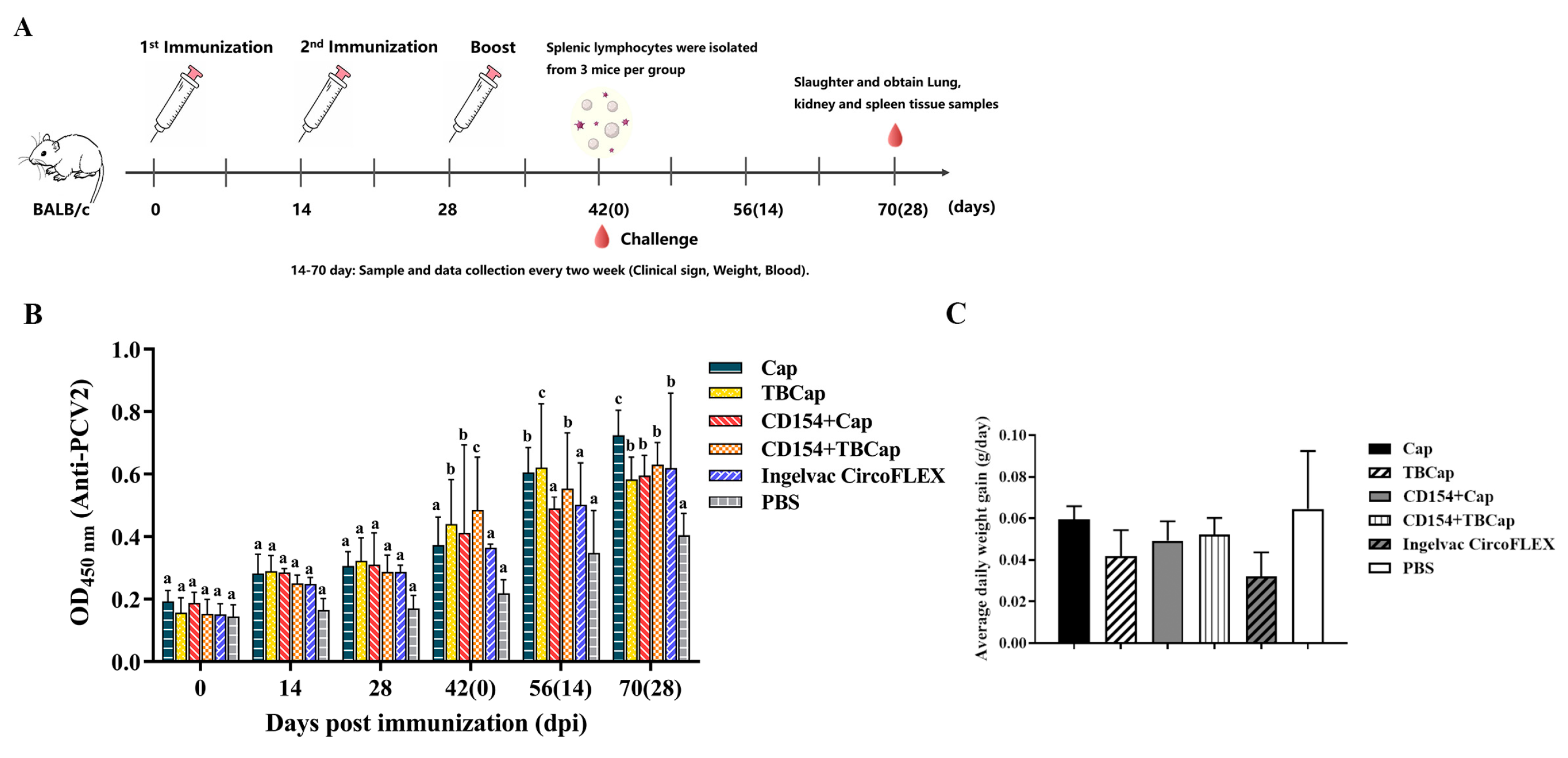
2.7.1. Immunization Strategies and Immunogenicity in Mice
2.7.2. Average Daily Weight Gain
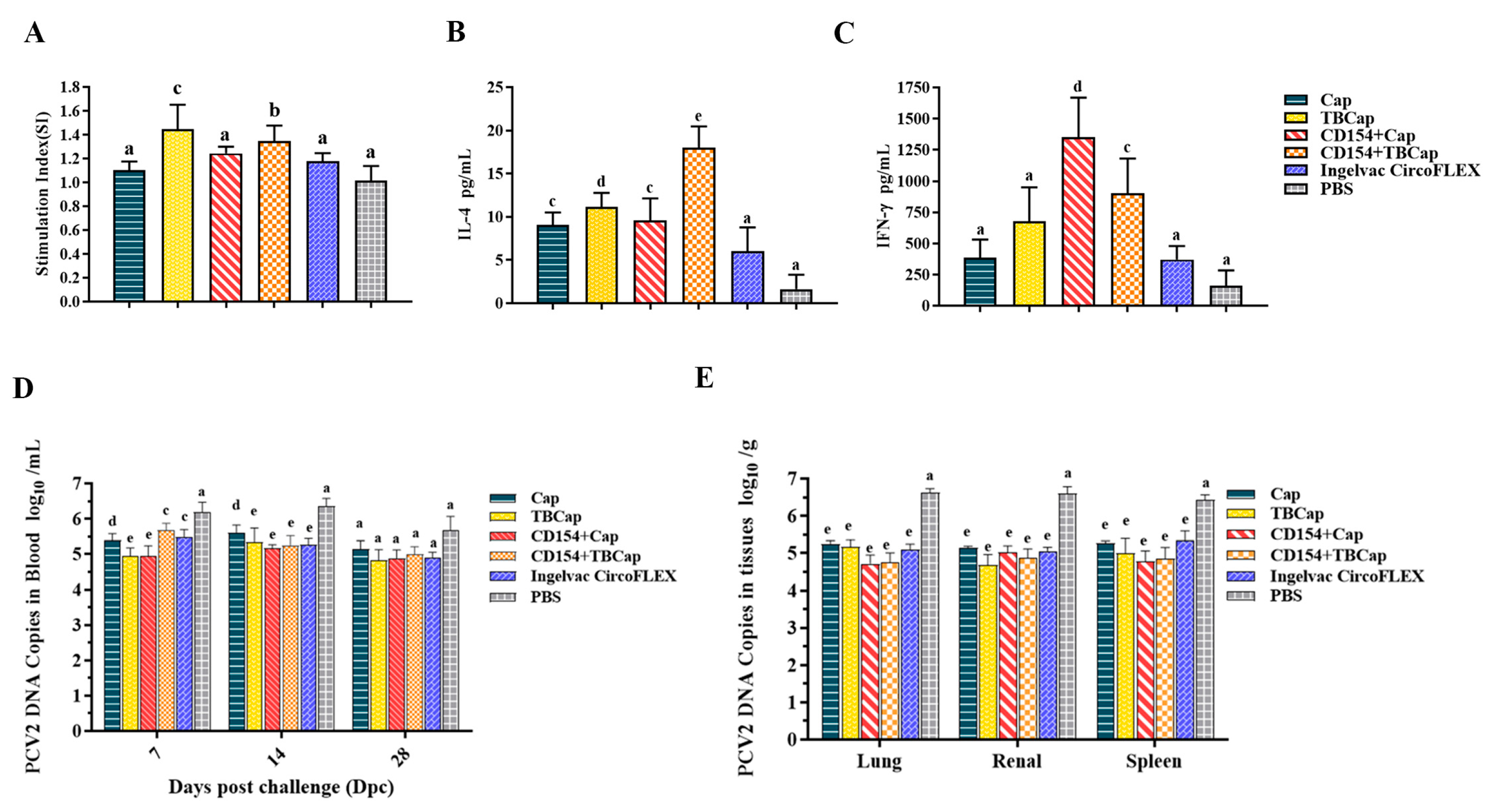
2.7.3. Levels of Lymphocyte Proliferation
2.7.4. The Expression Levels of Cytokines
2.7.5. PCV2 Viremia and Viral Load in Mice after Challenge
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Virus
4.2. Immunofluorescence Assay and Western Blot Analysis
4.3. Assembly and Identification of Virus-like Particles
4.4. Preparation of the Vaccine
4.5. Animal Immunization and Challenge Experiments
4.6. Lymphocyte Proliferation Assay
4.7. Cytokines Release Assay
4.8. Pathological and Histopathology Studies
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tischer, I.; Gelderblom, H.; Vettermann, W.; Koch, M.A. A very small porcine virus with circular single-stranded DNA. Nature 1982, 295, 64–66. [Google Scholar] [CrossRef] [PubMed]
- Fort, M.; Sibila, M.; Nofrarías, M.; Pérez-Martín, E.; Olvera, A.; Mateu, E.; Segalés, J. Porcine circovirus type 2 (PCV2) Cap and Rep proteins are involved in the development of cell-mediated immunity upon PCV2 infection. Veter. Immunol. Immunopathol. 2010, 137, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Du, Q.; Wu, B.; Li, J.; Chang, L.; Zhao, X.; Huang, Y.; Tong, D. Immunogenicity of adenovirus vaccines expressing the PCV2 capsid protein in pigs. Vaccine 2017, 35, 4722–4729. [Google Scholar] [CrossRef] [PubMed]
- Li, P.-C.; Qiao, X.-W.; Zheng, Q.-S.; Hou, J.-B. Immunogenicity and immunoprotection of porcine circovirus type 2 (PCV2) Cap protein displayed by Lactococcus lactis. Vaccine 2016, 34, 696–702. [Google Scholar] [CrossRef]
- Khayat, R.; Brunn, N.; Speir, J.A.; Hardham, J.M.; Ankenbauer, R.G.; Schneemann, A.; Johnson, J.E. The 2.3-Angstrom Structure of Porcine Circovirus 2. J. Virol. 2011, 85, 7856–7862. [Google Scholar] [CrossRef] [Green Version]
- Mo, X.; Li, X.; Yin, B.; Deng, J.; Tian, K.; Yuan, A. Structural roles of PCV2 capsid protein N-terminus in PCV2 particle assembly and identification of PCV2 type-specific neutralizing epitope. PLoS Pathog. 2019, 15, e1007562. [Google Scholar] [CrossRef] [Green Version]
- Baek, J.-H.; Cha, S.-H.; Cho, S.-H.; Lee, M.-S.; Park, C. Minimal Dosage of Porcine Circovirus Type 2d Based Virus-like Particles to Induce Stable Protective Immunity against Infection. Pathogens 2021, 10, 1644. [Google Scholar] [CrossRef]
- Baha, S.; Zhang, M.; Behloul, N.; Liu, Z.; Wei, W.; Meng, J. Efficient production and characterization of immunogenic HEV-PCV2 chimeric virus-like particles. Veter. Microbiol. 2022, 268, 109410. [Google Scholar] [CrossRef]
- Kang, S.-J.; Bae, S.-M.; Lee, H.-J.; Jeong, Y.-J.; Lee, M.-A.; You, S.-H.; Lee, H.-S.; Hyun, B.-H.; Lee, N.; Cha, S.-H. Porcine Circovirus (PCV) Genotype 2d-Based Virus-like Particles (VLPs) Induced Broad Cross-Neutralizing Antibodies against Diverse Genotypes and Provided Protection in Dual-Challenge Infection of a PCV2d Virus and a Type 1 Porcine Reproductive and Respiratory Syndrome Virus (PRRSV). Pathogens 2021, 10, 1145. [Google Scholar] [CrossRef]
- Wu, P.-C.; Chen, T.-Y.; Chi, J.-N.; Chien, M.-S.; Huang, C. Efficient expression and purification of porcine circovirus type 2 virus-like particles in Escherichia coli. J. Biotechnol. 2016, 220, 78–85. [Google Scholar] [CrossRef]
- Meerts, P.; Misinzo, G.; Lefebvre, D.; Nielsen, J.; Bøtner, A.; Kristensen, C.S.; Nauwynck, H.J. Correlation between the presence of neutralizing antibodies against porcine circovirus 2 (PCV2) and protection against replication of the virus and development of PCV2-associated disease. BMC Veter. Res. 2006, 2, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meerts, P.; Van Gucht, S.; Cox, E.; Vandebosch, A.; Nauwynck, H. Correlation Between Type of Adaptive Immune Response Against Porcine Circovirus Type 2 and Level of Virus Replication. Viral Immunol. 2005, 18, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Sun, Z.; Xia, D.; Wei, Y.; Sun, E.; Liu, C.; Zhu, H.; Bian, H.; Wu, H.; Feng, L.; et al. Neutralization Mechanism of a Monoclonal Antibody Targeting a Porcine Circovirus Type 2 Cap Protein Conformational Epitope. J. Virol. 2020, 94, e01836-19. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Guo, J.; Zhang, G.; Jin, Q.; Liu, Y.; Jia, R.; Wang, A. Fine mapping of linear B cell epitopes on capsid protein of porcine circovirus 3. Appl. Microbiol. Biotechnol. 2020, 104, 6223–6234. [Google Scholar] [CrossRef] [PubMed]
- Koinig, H.C.; Talker, S.C.; Stadler, M.; Ladinig, A.; Graage, R.; Ritzmann, M.; Hennig-Pauka, I.; Gerner, W.; Saalmüller, A. PCV2 vaccination induces IFN-γ/TNF-α co-producing T cells with a potential role in protection. Veter. Res. 2015, 46, 20. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Meng, X.; Wang, S.; Li, Z.; Yang, L.; Tu, L.; Diao, W.; Yu, C.; Yu, Y.; Yan, C.; et al. Virus-like particles of recombinant PCV2b carrying FMDV-VP1 epitopes induce both anti-PCV and anti-FMDV antibody responses. Appl. Microbiol. Biotechnol. 2018, 102, 10541–10550. [Google Scholar] [CrossRef]
- Luo, Q.; Ahmed, W.; Dai, Y.; Mohsin, A.; Hang, H.; Zhuang, Y.; Guo, M. Evaluation of a Virus-like Nanoparticle Porcine Circovirus Type-2 (PCV2) Capsid Protein Fused with the Pig Immunoglobulin Fc Fragment as a Novel Vaccine Candidate against PCV2 in Mice. Vaccines 2021, 9, 1128. [Google Scholar] [CrossRef]
- Banchereau, J.; Bazan, F.; Blanchard, D.; Brière, F.; Galizzi, J.; Van Kooten, C.; Liu, Y.J.; Rousset, F.; Saeland, S. The CD40 Antigen and its Ligand. Annu. Rev. Immunol. 1994, 12, 881–926. [Google Scholar] [CrossRef]
- Noelle, R.J.; Roy, M.; Shepherd, D.M.; Stamenkovic, I.; A Ledbetter, J.; Aruffo, A. A 39-kDa protein on activated helper T cells binds CD40 and transduces the signal for cognate activation of B cells. Proc. Natl. Acad. Sci. USA 1992, 89, 6550–6554. [Google Scholar] [CrossRef] [Green Version]
- Pamukcu, B.; Lip, G.Y.H.; Snezhitskiy, V.; Shantsila, E. The CD40-CD40L system in cardiovascular disease. Ann. Med. 2011, 43, 331–340. [Google Scholar] [CrossRef]
- Cao, J.; Wang, X.; Du, Y.; Li, Y.; Wang, X.; Jiang, P. CD40 ligand expressed in adenovirus can improve the immunogenicity of the GP3 and GP5 of porcine reproductive and respiratory syndrome virus in swine. Vaccine 2010, 28, 7514–7522. [Google Scholar] [CrossRef] [PubMed]
- Gares, S.L.; Fischer, K.P.; Congly, S.E.; Lacoste, S.; Addison, W.R.; Tyrrell, D.L.; Gutfreund, K.S. Immunotargeting with CD154 (CD40 Ligand) Enhances DNA Vaccine Responses in Ducks. Clin. Vaccine Immunol. 2006, 13, 958–965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wienhold, D.; Armengol, E.; Marquardt, A.; Marquardt, C.; Voigt, H.; Büttner, M.; Saalmüller, A.; Pfaff, E. Immunomodulatory effect of plasmids co-expressing cytokines in classical swine fever virus subunit gp55/E2-DNA vaccination. Veter. Res. 2005, 36, 571–587. [Google Scholar] [CrossRef] [Green Version]
- Manoj, S.; Griebel, P.J.; Babiuk, L.A.; Hurk, S.V.D.L.-V.D. Modulation of immune responses to bovine herpesvirus-1 in cattle by immunization with a DNA vaccine encoding glycoprotein D as a fusion protein with bovine CD154. Immunology 2004, 112, 328–338. [Google Scholar] [CrossRef]
- Harcourt, J.L.; Brown, M.P.; Anderson, L.J.; A Tripp, R. CD40 ligand (CD154) improves the durability of respiratory syncytial virus DNA vaccination in BALB/c mice. Vaccine 2003, 21, 2964–2979. [Google Scholar] [CrossRef]
- Wu, Y. Construction, Prokaryotic Expression and Immunogenicity Studies of PCV2 Dominant T and B Cell Epitopes in Tandem with Truncated Cap Genes. Master’s Thesis, South China Agricultural University, Guangzhou, China, 2016; p. 61. [Google Scholar]
- Rakibuzzaman, A.; Kolyvushko, O.; Singh, G.; Nara, P.; Piñeyro, P.; Leclerc, E.; Pillatzki, A.; Ramamoorthy, S. Targeted Alteration of Antibody-Based Immunodominance Enhances the Heterosubtypic Immunity of an Experimental PCV2 Vaccine. Vaccines 2020, 8, 506. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, S.; Zou, Y.; Yu, W.; Jiang, Y.; Zhan, Y.; Wang, N.; Dong, Y.; Yang, Y. Structure-Based Design of Porcine Circovirus Type 2 Chimeric VLPs (cVLPs) Displays Foreign Peptides on the Capsid Surface. Front. Cell. Infect. Microbiol. 2018, 8, 232. [Google Scholar] [CrossRef] [Green Version]
- Nie, K.; Wu, Y.; Yi, L.; Wu, Z.; Chen, J.; Zhao, M. Prokaryotic expression and immunogenicity study of porcine circovirus type 2 Cap protein with recombinant dominant T and B cell epitopes. Chin. J. Prev. Vet. Med. 2019, 41, 945–950. [Google Scholar]
- A Luckow, V.; Lee, S.C.; Barry, G.F.; O Olins, P. Efficient generation of infectious recombinant baculoviruses by site-specific transposon-mediated insertion of foreign genes into a baculovirus genome propagated in Escherichia coli. J. Virol. 1993, 67, 4566–4579. [Google Scholar] [CrossRef] [Green Version]
- Manoj, S.; Griebel, P.J.; Babiuk, L.A.; Hurk, S.V.D.L.-V.D. Targeting with Bovine CD154 Enhances Humoral Immune Responses Induced by a DNA Vaccine in Sheep. J. Immunol. 2003, 170, 989–996. [Google Scholar] [CrossRef] [Green Version]
- Bereznaya, N.M.; Chekhun, V.F. Expression of CD40 and CD40L on tumor cells: The role of their interaction and new approach to immunotherapy. Exp. Oncol. 2007, 29, 2–12. [Google Scholar] [PubMed]
- Wang, Y. Immunopotentiation of PCV2-Cap Protein by Cytokines and Identification of Immunogenicity of Cap and FMDV-Vp1 Co-Expression Products. MA Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2013. [Google Scholar]
- Yoshida, T.; Yoshida, R.; Ma, B.Y.; Mikolajczak, S.; Kelvin, D.J.; Ochi, A. A novel mitogen fusion protein against CD40+ cells with potent vaccine adjuvant properties. Vaccine 2010, 28, 3688–3695. [Google Scholar] [CrossRef] [PubMed]
- Elgueta, R.; Benson, M.J.; De Vries, V.C.; Wasiuk, A.; Guo, Y.; Noelle, R.J. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol. Rev. 2009, 229, 152–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breitfeld, D.; Ohl, L.; Kremmer, E.; Ellwart, J.; Sallusto, F.; Lipp, M.; Förster, R. Follicular B Helper T Cells Express Cxc Chemokine Receptor 5, Localize to B Cell Follicles, and Support Immunoglobulin Production. J. Exp. Med. 2000, 192, 1545–1552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.; Cao, J.; Zhou, N.; Jin, Y.; Wu, J. Identification and functional analysis of the novel ORF4 protein encoded by porcine circovirus type 2. J. Virol. 2013, 87, 1420–1429. [Google Scholar] [CrossRef]






| Group | No. | Viraemia | Day with Fever (≥40 ℃) | Clinical Symptoms | |
|---|---|---|---|---|---|
| 14 DPC | 28 DPC | ||||
| Cap | 766 | − | − | + | − |
| 741 | − | − | − | + | |
| 798 | − | − | − | − | |
| 799 | − | − | − | − | |
| 791 | − | − | − | − | |
| Total | 0/5 | 0/5 | 1/5 | 1/5 | |
| TBCap | 780 | − | − | + | − |
| 223 | − | − | − | − | |
| 759 | − | − | − | − | |
| 768 | − | − | − | − | |
| 206 | − | − | − | − | |
| Total | 0/5 | 0/5 | 1/5 | 0/5 | |
| PBS | 790 | + | − | − | − |
| 756 | + | − | − | − | |
| 773 | + | + | − | ++ | |
| 746 | + | + | − | +++ | |
| 334 | + | + | − | ++ | |
| Total | 5/5 | 3/5 | 0/5 | 3/5 | |
| Group | Formulation | Adjuvant | Immunization Time Points (DAI) | Vaccine Dose |
|---|---|---|---|---|
| 1, PBS | PBS | - | 0.14 | 200 μL |
| 2, Cap 201VG | Cap | ISA 201VG | 0.14 | 200 μL |
| 3, Cap Gel 01 | Cap | MontanideTM GEL 01 | 0.14 | 200 μL |
| 4, TBCap 201VG | TBCap | ISA 201VG | 0.14 | 200 μL |
| 5, TBCap Gel 01 | TBCap | MontanideTM GEL 01 | 0.14 | 200 μL |
| 6, Ingelvac Circo FLEX | Ingelvac Circo FLEX | - | 0.14 | 200 μL |
| Group | Formulation | Adjuvant | Vaccine Dose | Challenge Dose (TCID50) |
|---|---|---|---|---|
| 1, Cap | Cap | MontanideTM GEL 01 | 1 mL | 3 mL, 1 × 105.5 |
| 2, TBCap | TBCap | MontanideTM GEL 01 | 1 mL | 3 mL, 1 × 105.5 |
| 3, PBS | PBS | - | 1 mL | 3 mL, 1 × 105.5 |
| 4, Negative group | - | - | - | - |
| Group | Formulation | Adjuvant | Vaccine Dose | Challenge Dose (TCID50) |
|---|---|---|---|---|
| 1, Cap | Cap | MontanideTM GEL 01 | 200 μL | 350 μL, 1 × 105.5 |
| 2, TBCap | TBCap | MontanideTM GEL 01 | 200 μL | 350 μL, 1 × 105.5 |
| 3, CD154+Cap | CD154, Cap | MontanideTM GEL 01 | 200 μL | 350 μL, 1 × 105.5 |
| 4, CD154+TBCap | CD154, TBCap | MontanideTM GEL 01 | 200 μL | 350 μL, 1 × 105.5 |
| 5, Ingelvac Circo FLEX | Ingelvac Circo FLEX | - | 200 μL | 350 μL, 1 × 105.5 |
| 6, PBS | PBS | - | 200 μL | 350 μL, 1 × 105.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, K.; Hu, W.; Zhou, B.; Li, B.; Li, X.; Yan, Q.; Chen, W.; Li, Y.; Ding, H.; Zhao, M.; et al. Immunogenicity and Immunoprotection of PCV2 Virus-like Particles Incorporating Dominant T and B Cell Antigenic Epitopes Paired with CD154 Molecules in Piglets and Mice. Int. J. Mol. Sci. 2022, 23, 14126. https://doi.org/10.3390/ijms232214126
Wu K, Hu W, Zhou B, Li B, Li X, Yan Q, Chen W, Li Y, Ding H, Zhao M, et al. Immunogenicity and Immunoprotection of PCV2 Virus-like Particles Incorporating Dominant T and B Cell Antigenic Epitopes Paired with CD154 Molecules in Piglets and Mice. International Journal of Molecular Sciences. 2022; 23(22):14126. https://doi.org/10.3390/ijms232214126
Chicago/Turabian StyleWu, Keke, Wenshuo Hu, Bolun Zhou, Bingke Li, Xiaowen Li, Quanhui Yan, Wenxian Chen, Yuwan Li, Hongxing Ding, Mingqiu Zhao, and et al. 2022. "Immunogenicity and Immunoprotection of PCV2 Virus-like Particles Incorporating Dominant T and B Cell Antigenic Epitopes Paired with CD154 Molecules in Piglets and Mice" International Journal of Molecular Sciences 23, no. 22: 14126. https://doi.org/10.3390/ijms232214126
APA StyleWu, K., Hu, W., Zhou, B., Li, B., Li, X., Yan, Q., Chen, W., Li, Y., Ding, H., Zhao, M., Fan, S., Yi, L., & Chen, J. (2022). Immunogenicity and Immunoprotection of PCV2 Virus-like Particles Incorporating Dominant T and B Cell Antigenic Epitopes Paired with CD154 Molecules in Piglets and Mice. International Journal of Molecular Sciences, 23(22), 14126. https://doi.org/10.3390/ijms232214126






