Insights into the 3D In Vitro Permeability and In Vivo Antioxidant Protective Effects of Kiwiberry Leaf Extract: A Step Forward to Human Nutraceutical Use
Abstract
:1. Introduction
2. Results and Discussion
2.1. In Vitro Cell Viability of Kiwiberry Leaf Extract
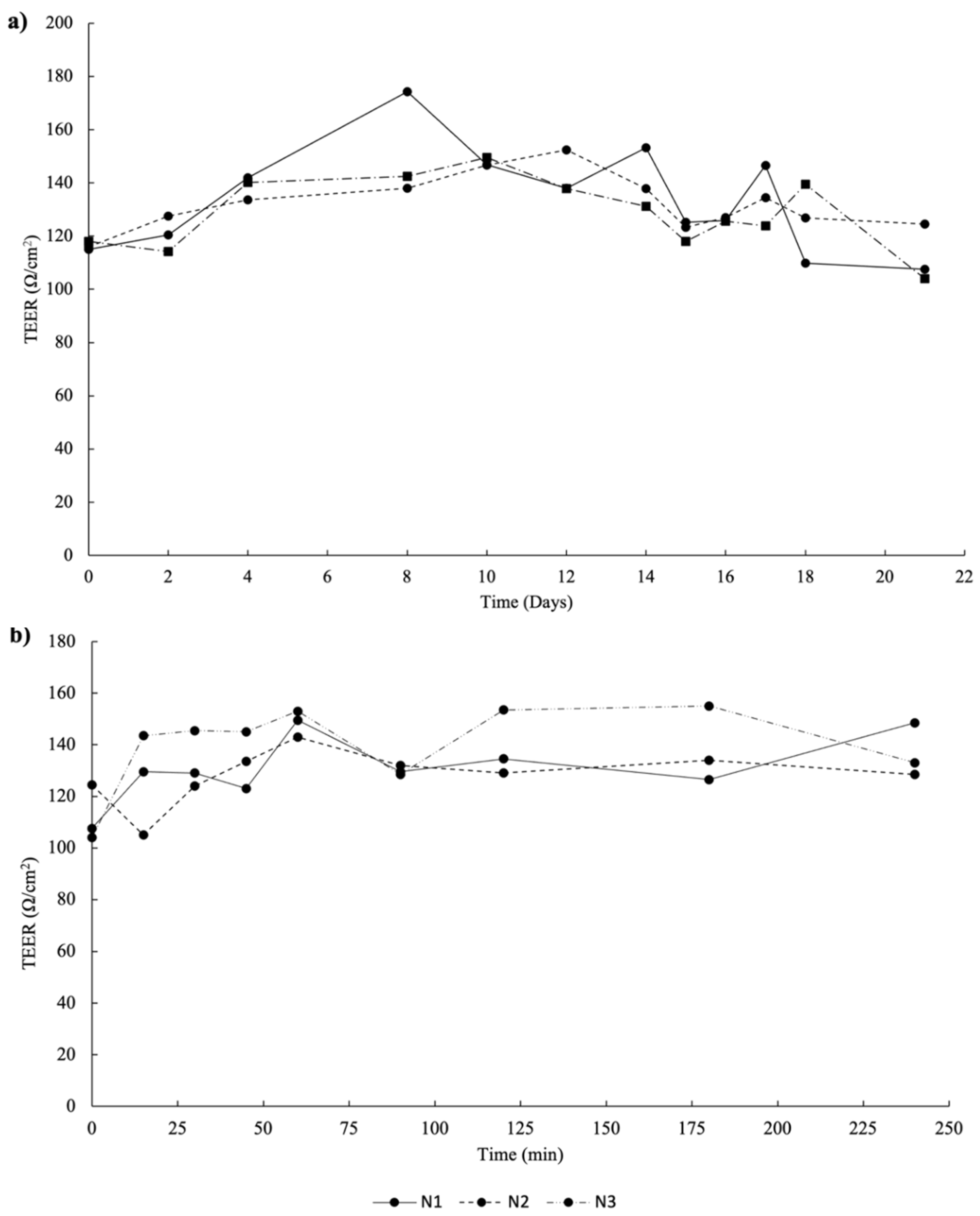
2.2. 3D Intestinal Permeation Assay
2.3. In Vivo Animal Assays
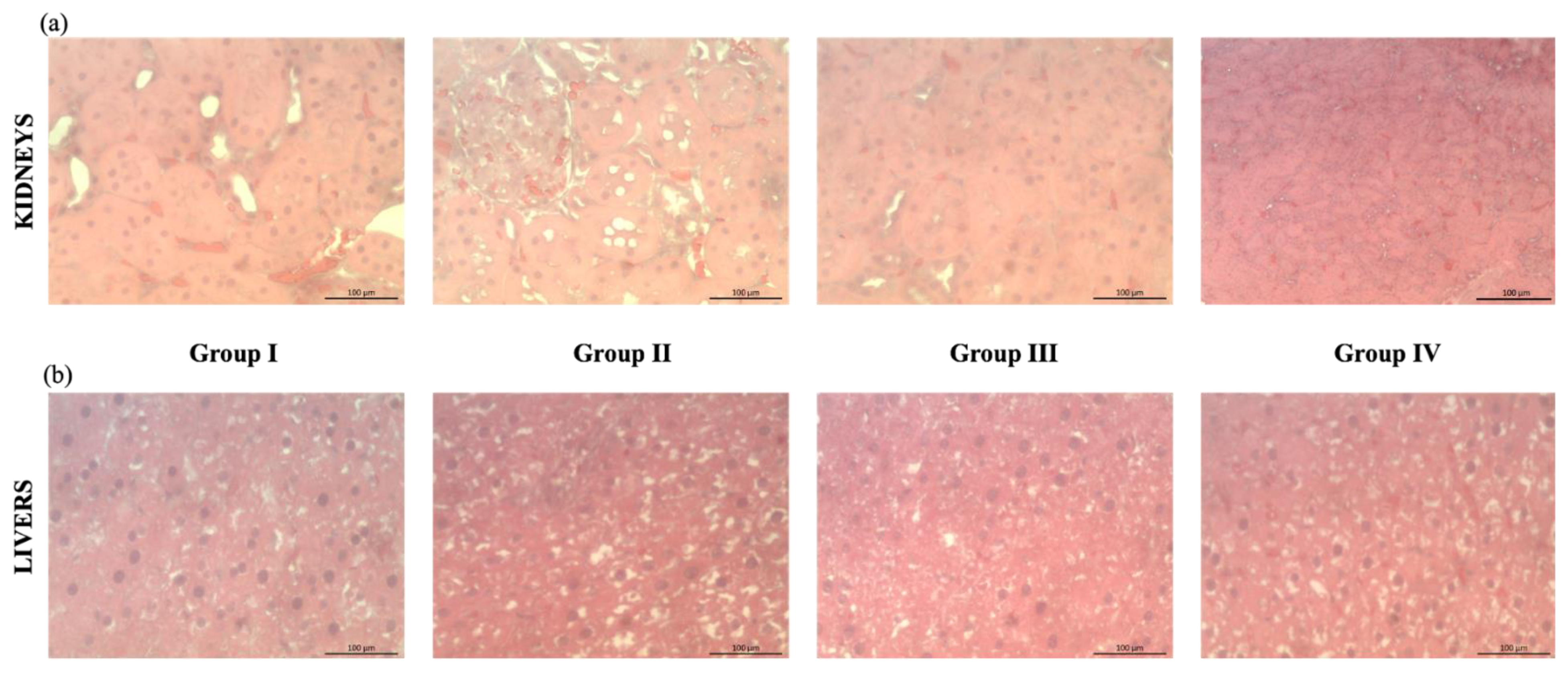
2.3.1. Effects of the Extract on the Morphological Conditions
2.3.2. Effect of the Extract on the 2,2′-Azobis(2-amidinopropane) Dihydrochloride (AAPH)-Induced Hemolysis
2.3.3. Effects of the Extract on Biochemical Parameters
2.3.4. Effects of the Extract on the Antioxidant Parameters
3. Materials and Methods
3.1. Chemicals
3.2. Samples
3.3. Kiwiberry Leaves Ultrasound-Assisted Extraction (UAE)
3.4. In Vitro Cell Assays
3.4.1. Cell Viability Assay
3.4.2. 3D Intestinal Permeability Assay
3.5. LC/DAD-ESI-MS Analysis
- Chlorogenic acid titration curve: y = 198.01x + 20.138 (R2 = 1)
- Rutin titration curve: y = 58.564x + 41.752 (R2 = 0.9998)
- Chlorogenic acid: 353.5 > 191.2
- Rutin: 609.4 > 301.3
- Coumaroyl quinic acid: 337.3 > 191.3
3.6. In Vivo Animal Study and Treatment
- Group I–Normal control group treated with water (4 mL/kg of body weight (bw))
- Group II–Kiwiberry leaf extract prepared in water (50 mg/kg bw)
- Group III–Kiwiberry leaf extract prepared in water (75 mg/kg bw)
- Group IV–Positive control group treated with vitamin C (50 mg/kg bw).
3.6.1. Collection of Biological Samples
3.6.2. Histopathological Studies
3.6.3. Determination of In Vivo Antioxidant Parameters
3.6.4. Inhibition of Erythrocyte Hemolysis in Rat Blood
3.6.5. Biochemical Analysis
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silva, A.M.; Costa, P.C.; Delerue-Matos, C.; Latocha, P.; Rodrigues, F. Extraordinary composition of Actinidia arguta by-products as skin ingredients: A new challenge for cosmetic and medical skincare industries. Trends Food Sci. Technol. 2021, 116, 842–853. [Google Scholar] [CrossRef]
- Pinto, D.; Delerue-Matos, C.; Rodrigues, F. Bioactivity, phytochemical profile and pro-healthy properties of Actinidia arguta: A review. Food Res. Int. 2020, 136, 109449. [Google Scholar] [CrossRef] [PubMed]
- Ravipati, A.S.; Zhang, L.; Koyyalamudi, S.R.; Jeong, S.C.; Reddy, N.; Bartlett, J.; Smith, P.T.; Shanmugam, K.; Münch, G.; Wu, M.J. Antioxidant and anti-inflammatory activities of selected Chinese medicinal plants and their relation with antioxidant content. BMC Complement. Altern. Med. 2012, 12, 173. [Google Scholar] [CrossRef] [Green Version]
- Latocha, P. The nutritional and health benefits of kiwiberry (Actinidia arguta)—A review. Plant Foods Hum. Nutr. 2017, 72, 325–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wojdyło, A.; Nowicka, P.; Oszmiański, J.; Golis, T. Phytochemical compounds and biological effects of Actinidia fruits. J. Funct. Foods 2017, 30, 194–202. [Google Scholar] [CrossRef]
- Latocha, P.; Krupa, T.; Wołosiak, R.; Worobiej, E.; Wilczak, J. Antioxidant activity and chemical difference in fruit of different Actinidia sp. Int. J. Food Sci. Nutr. 2010, 61, 381–394. [Google Scholar] [CrossRef]
- Silva, A.M.; Luís, A.S.; Moreira, M.M.; Ferraz, R.; Brezo-Borjan, T.; Švarc-Gajić, J.; Costa, P.C.; Delerue-Matos, C.; Rodrigues, F. Influence of temperature on the subcritical water extraction of Actinidia arguta leaves: A screening of pro-healthy compounds. Sustain. Chem. Pharm. 2022, 25, 100593. [Google Scholar] [CrossRef]
- Almeida, D.; Pinto, D.; Santos, J.; Vinha, A.F.; Palmeira, J.; Ferreira, H.N.; Rodrigues, F.; Oliveira, M.B.P.P. Hardy kiwifruit leaves (Actinidia arguta): An extraordinary source of value-added compounds for food industry. Food Chem. 2018, 259, 113–121. [Google Scholar] [CrossRef]
- Cyboran, S.; Oszmiański, J.; Kleszczyńska, H. Modification of the properties of biological membrane and its protection against oxidation by Actinidia arguta leaf extract. Chem. Biol. Interact. 2014, 222, 50–59. [Google Scholar] [CrossRef]
- Silva, A.M.; Pinto, D.; Fernandes, I.; Freitas, V.d.; Cádiz-Gurrea, M.d.l.L.; Costa, P.; Delerue-Matos, C.; Rodrigues, F. An Insight into Kiwiberry Leaf Valorization: Phenolic Composition, Bioactivity and Health Benefits. Molecules 2021, 26, 2314. [Google Scholar] [CrossRef]
- Marangi, F.; Pinto, D.; de Francisco, L.; Alves, R.C.; Puga, H.; Sut, S.; Dall’Acqua, S.; Rodrigues, F.; Oliveira, M.B.P.P. Hardy kiwi leaves extracted by multi-frequency multimode modulated technology: A sustainable and promising by-product for industry. Food Res. Int. 2018, 112, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.M.; Pinto, D.; Fernandes, I.; Gonçalves Albuquerque, T.; Costa, H.S.; Freitas, V.; Rodrigues, F.; Oliveira, M.B.P.P. Infusions and decoctions of dehydrated fruits of Actinidia arguta and Actinidia deliciosa: Bioactivity, radical scavenging activity and effects on cells viability. Food Chem. 2019, 289, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Wang, Z.; Feng, X.; Irfan, M.; Liu, C. Identification of bioactive compounds and antioxidant activity in leaves and fruits of Actinidia arguta accessions from northeastern China. Preprints 2020, 2020120453. [Google Scholar] [CrossRef]
- Yoo, S.K.; Kang, J.Y.; Lee, U.; Park, S.K.; Kim, J.M.; Han, H.J.; Kim, D.O.; Heo, H.J. Improving effect of Actinidia arguta leaf on hyperglycemia-induced cognitive dysfunction. J. Funct. Foods 2021, 76, 104315. [Google Scholar] [CrossRef]
- Martins, N.; Barros, L.; Ferreira, I.C. In vivo antioxidant activity of phenolic compounds: Facts and gaps. Trends Food Sci. Technol. 2016, 48, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Palafox-Carlos, H.; Ayala-Zavala, J.F.; González-Aguilar, G.A. The role of dietary fiber in the bioaccessibility and bioavailability of fruit and vegetable antioxidants. J. Food Sci. 2011, 76, R6–R15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haminiuk, C.W.; Maciel, G.M.; Plata-Oviedo, M.S.; Peralta, R.M. Phenolic compounds in fruits–an overview. Int. J. Food Sci. Technol. 2012, 47, 2023–2044. [Google Scholar] [CrossRef]
- Silva, A.M.; Pinto, D.; Moreira, M.M.; Costa, P.C.; Delerue-Matos, C.; Rodrigues, F. Valorization of Kiwiberry Leaves Recovered by Ultrasound-Assisted Extraction for Skin Application: A Response Surface Methodology Approach. Antioxidants 2022, 11, 763. [Google Scholar] [CrossRef]
- Sarmento, B.; Andrade, F.; Silva, S.B.d.; Rodrigues, F.; das Neves, J.; Ferreira, D. Cell-based in vitro models for predicting drug permeability. Expert Opin. Drug Metab. Toxicol. 2012, 8, 607–621. [Google Scholar] [CrossRef]
- Pereira, C.; Costa, J.; Sarmento, B.; Araújo, F. Cell-based in vitro models for intestinal permeability studies. In Concepts and Models for Drug Permeability Studies; Sarmento, B., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 57–81. [Google Scholar]
- Antunes, F.; Andrade, F.; Araújo, F.; Ferreira, D.; Sarmento, B. Establishment of a triple co-culture in vitro cell models to study intestinal absorption of peptide drugs. Eur. J. Pharm. Biopharm. 2013, 83, 427–435. [Google Scholar] [CrossRef]
- Sun, H.; Chow, E.C.; Liu, S.; Du, Y.; Pang, K.S. The Caco-2 cell monolayer: Usefulness and limitations. Expert Opin. Drug Metab. Toxicol. 2008, 4, 395–411. [Google Scholar] [CrossRef] [PubMed]
- Barros, H.D.d.F.Q.; Junior, M.R.M. Phenolic compound bioavailability using in vitro and in vivo models. In Bioactive Compounds; Campos, M.R.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 3, pp. 113–126. [Google Scholar]
- Lozoya-Agullo, I.; Araújo, F.; González-Álvarez, I.; Merino-Sanjuán, M.; González-Álvarez, M.; Bermejo, M.; Sarmento, B. Usefulness of Caco-2/HT29-MTX and Caco-2/HT29-MTX/Raji B coculture models to predict intestinal and colonic permeability compared to Caco-2 monoculture. Mol. Pharm. 2017, 14, 1264–1270. [Google Scholar] [CrossRef] [PubMed]
- Ravi, G.; Charyulu, R.N.; Dubey, A.; Prabhu, P.; Hebbar, S.; Mathias, A.C. Nano-lipid complex of rutin: Development, characterisation and in vivo investigation of hepatoprotective, antioxidant activity and bioavailability study in rats. AAPS PharmSciTech 2018, 19, 3631–3649. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Xiang, R.; Fu, C.; Qu, Z.; Liu, C. The Regulatory effect of chlorogenic acid on gut-brain function and its mechanism: A systematic review. Biomed. Pharmacother. 2022, 149, 112831. [Google Scholar] [CrossRef]
- Mortelé, O.; Jörissen, J.; Spacova, I.; Lebeer, S.; van Nuijs, A.L.; Hermans, N. Demonstrating the involvement of an active efflux mechanism in the intestinal absorption of chlorogenic acid and quinic acid using a caco-2 bidirectional permeability assay. Food Funct. 2021, 12, 417–425. [Google Scholar] [CrossRef]
- Araújo, F.; Sarmento, B. Towards the characterization of an in vitro triple co-culture intestine cell model for permeability studies. Int. J. Pharm. 2013, 458, 128–134. [Google Scholar] [CrossRef]
- Zhu, Q.Y.; Schramm, D.D.; Gross, H.B.; Holt, R.R.; Kim, S.H.; Yamaguchi, T.; Kwik-Uribe, C.L.; Keen, C.L. Influence of cocoa flavanols and procyanidins on free radical-induced human erythrocyte hemolysis. Clin. Dev. Immunol. 2005, 12, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Su, X.-Y.; Wang, Z.-Y.; Liu, J.-R. In vitro and in vivo antioxidant activity of Pinus koraiensis seed extract containing phenolic compounds. Food Chem. 2009, 117, 681–686. [Google Scholar] [CrossRef]
- Barreira, J.C.M.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P.; Pereira, J.A. Antioxidant activities of the extracts from chestnut flower, leaf, skins and fruit. Food Chem. 2008, 107, 1106–1113. [Google Scholar] [CrossRef]
- Kumar, R.; Akhtar, F.; Rizvi, S.I. Hesperidin attenuates altered redox homeostasis in an experimental hyperlipidaemic model of rat. Clin. Exp. Pharmacol. Physiol. 2020, 47, 571–582. [Google Scholar] [CrossRef]
- Madureira, A.R.; Nunes, S.; Campos, D.A.; Fernandes, J.C.; Marques, C.; Zuzarte, M.; Gullon, B.; Rodriguez-Alcala, L.M.; Calhau, C.; Sarmento, B. Safety profile of solid lipid nanoparticles loaded with rosmarinic acid for oral use: In vitro and animal approaches. Int. J. Nanomed. 2016, 11, 3621. [Google Scholar]
- Varghese, S.; Kannappan, P.; Kanakasabapathi, D.; Madathil, S.; Perumalsamy, M. Antidiabetic and antilipidemic effect of Clerodendrum paniculatum flower ethanolic extract. An in vivo investigation in Albino Wistar rats. Biocatal. Agric. Biotechnol. 2021, 36, 102095. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Bhattacharya, A.; Kumar, A.; Ghosal, S. Antioxidant activity ofBacopa monniera in rat frontal cortex, striatum and hippocampus. Phytother. Res. 2000, 14, 174–179. [Google Scholar] [CrossRef]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Góth, L.; Rass, P.; Páy, A. Catalase enzyme mutations and their association with diseases. Mol. Diagn. Ther. 2004, 8, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Drotar, A.; Phelps, P.; Fall, R. Evidence for glutathione peroxidase activities in cultured plant cells. Plant Sci. 1985, 42, 35–40. [Google Scholar] [CrossRef]
- Lee, B.; Jung, J.-H.; Kim, H.-S. Assessment of red onion on antioxidant activity in rat. Food Chem. Toxicol. 2012, 50, 3912–3919. [Google Scholar] [CrossRef]
- González, F.; García-Martínez, E.; del Mar Camacho, M.; Martínez-Navarrete, N.; Sarmento, B.; Fernandes, I.; Freitas, V.; Rodrigues, F.; Oliveira, B. Insights into the development of grapefruit nutraceutical powder by spray drying: Physical characterization, chemical composition and 3D intestinal permeability. J. Sci. Food Agric. 2019, 99, 4686–4694. [Google Scholar] [CrossRef]
- Silva, A.M.; Garcia, J.; Dall’Acqua, S.; Costa, P.; Delerue-Matos, C.; Rodrigues, F. Eco-friendly insights on kiwiberry leaves valorization through in-vitro and in-vivo studies. Ind. Crops Prod. 2022, 184, 115090. [Google Scholar] [CrossRef]
- Sunil, C.; Ignacimuthu, S. In vitro and in vivo antioxidant activity of Symplocos cochinchinensis S. Moore leaves containing phenolic compounds. Food Chem. Toxicol. 2011, 49, 1604–1609. [Google Scholar] [CrossRef]
- Li, Y.; Zhuang, Y.; Tian, W.; Sun, L. In vivo acute and subacute toxicities of phenolic extract from rambutan (Nephelium lappaceum) peels by oral administration. Food Chem. 2020, 320, 126618. [Google Scholar] [CrossRef]
- Barros, L.; Baptista, P.; Ferreira, I.C.F.R. Effect of Lactarius piperatus fruiting body maturity stage on antioxidant activity measured by several biochemical assays. Food Chem. Toxicol. 2007, 45, 1731–1737. [Google Scholar] [CrossRef]

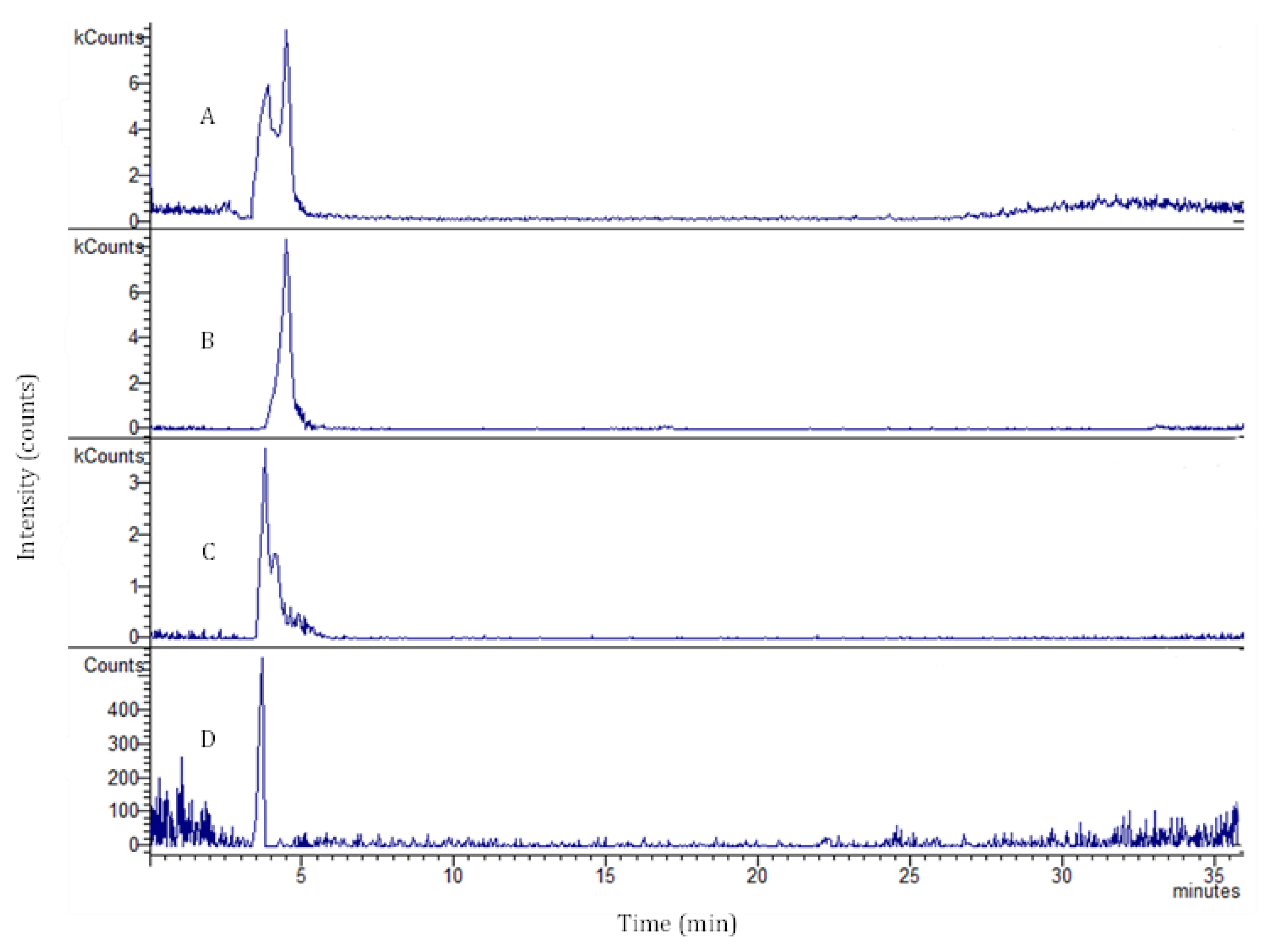
| Time (min) | Permeability (%) | ||
|---|---|---|---|
| Coumaroyl Quinic Acid m/z = 337 | Chlorogenic Acid m/z = 353 | Rutin m/z = 609 | |
| 15 | 1.04 ± 0.05 | <LOD | 6.67 ± 0.33 |
| 30 | 18.81 ± 0.94 | 1.79 ± 0.09 | 10.89 ± 0.54 |
| 45 | 7.99 ± 0.39 | <LOD | 17.01 ± 0.85 |
| 60 | 13.50 ± 0.68 | <LOD | 14.61 ± 0.73 |
| 90 | 19.74 ± 0.99 | <LOD | 14.56 ± 0.73 |
| 120 | 14.60 ± 0.73 | <LOD | 18.50 ± 0.93 |
| 180 | 24.32 ± 1.22 | <LOD | 18.30 ± 0.92 |
| 240 | 34.23 ± 1.71 | 2.49 ± 0.13 | 25.40 ± 1.27 |
| Biochemical Parameters | Group I | Group II | Group III | Group IV |
|---|---|---|---|---|
| TC (mg/dL) | 82.01 ± 22.00 | 88.40 ± 20.40 | 80.66 ± 12.94 | 79.81 ± 15.11 |
| HDL-C (mg/dL) | 198.64 ± 46.02 | 191.97 ± 47.31 | 134.24 ± 24.83 | 155.09 ± 37.71 |
| LDL-C (mg/dL) | 111.37 ± 32.27 | 114.22 ± 30.93 | 131.22 ± 32.17 | 76.24 ± 16.52 |
| TG (mg/dL) | 304.41 ± 80.08 a | 70.28 ± 10.87 b | 86.82 ± 27.59 b | 229.76 ± 33.54 a |
| Antioxidant Parameters | Group I | Group II | Group III | Group IV |
|---|---|---|---|---|
| SOD | ||||
| Kidneys (units/g protein) | 111.01 ± 7.91 c | 168.80 ± 23.77 a,b | 183.36 ± 37.91 a | 123.13 ± 19.27 b,c |
| Liver (units/g protein) | 82.89 ± 11.36 b | 155.42 ± 21.61 a | 175.26 ± 13.99 a | 83.71 ± 6.06 b |
| Serum (units/mL protein) | 148.03 ± 22.92 a | 98.26 ± 14.49 b | 81.57 ± 9.63 b | 79.20 ± 5.76 b |
| CAT | ||||
| Kidneys (nmol/min/g protein) | 344,131 ± 740 b | 3,435,762 ± 636,996 a | 3,638,866 ± 685,222 a | 427,496 ± 78,015 b |
| Liver (nmol/min/g protein) | 4,843,676 ± 986,453 b,c | 7,840,180 ± 1,733,773 a | 7,526,357 ± 1,638,318 a,b | 3,762,450 ± 434,242 c |
| Serum (nmol/min/mL protein) | 73,018 ± 7656 a | 13,809 ± 3531 c | 17,369 ± 1763 b,c | 34,911 ± 7975 b |
| GSH-Px | ||||
| Kidneys (units/g protein) | 142.53 ± 16.20 b | 205.35 ± 46.08 a | 158.12 ± 15.58 a,b | 136.66 ± 19.62 b |
| Liver (units/g protein) | 55.21 ± 6.20 c | 133.60 ± 23.03 a | 94.36 ± 12.18 b | 71.34 ± 10.94 b,c |
| Serum (units/mL protein) | 36.66 ± 7.70 b | 64.57 ± 15.80 a | 26.84 ± 4.65 b | 20.16 ± 5.29 b |
| MDA | ||||
| Kidneys (nmol/g protein) | 232,452 ± 29,458 a | 67,598 ± 15,061 b | 54,566 ± 9736 b | 196,748 ± 31,125 a |
| Liver (nmol/g protein) | 177,919 ± 3415 a | 54,994 ± 5742 c | 44,968 ± 2907 c | 145,827 ± 10,063 b |
| Serum (nmol/mL protein) | 1457.93 ± 270.27 a | 195.44 ± 41.21 c | 493.13 ± 3.75 b,c | 746.79 ± 123.55 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, A.M.; Almeida, A.; Dall’Acqua, S.; Loschi, F.; Sarmento, B.; Costa, P.C.; Delerue-Matos, C.; Rodrigues, F. Insights into the 3D In Vitro Permeability and In Vivo Antioxidant Protective Effects of Kiwiberry Leaf Extract: A Step Forward to Human Nutraceutical Use. Int. J. Mol. Sci. 2022, 23, 14130. https://doi.org/10.3390/ijms232214130
Silva AM, Almeida A, Dall’Acqua S, Loschi F, Sarmento B, Costa PC, Delerue-Matos C, Rodrigues F. Insights into the 3D In Vitro Permeability and In Vivo Antioxidant Protective Effects of Kiwiberry Leaf Extract: A Step Forward to Human Nutraceutical Use. International Journal of Molecular Sciences. 2022; 23(22):14130. https://doi.org/10.3390/ijms232214130
Chicago/Turabian StyleSilva, Ana Margarida, Andreia Almeida, Stefano Dall’Acqua, Francesca Loschi, Bruno Sarmento, Paulo C. Costa, Cristina Delerue-Matos, and Francisca Rodrigues. 2022. "Insights into the 3D In Vitro Permeability and In Vivo Antioxidant Protective Effects of Kiwiberry Leaf Extract: A Step Forward to Human Nutraceutical Use" International Journal of Molecular Sciences 23, no. 22: 14130. https://doi.org/10.3390/ijms232214130
APA StyleSilva, A. M., Almeida, A., Dall’Acqua, S., Loschi, F., Sarmento, B., Costa, P. C., Delerue-Matos, C., & Rodrigues, F. (2022). Insights into the 3D In Vitro Permeability and In Vivo Antioxidant Protective Effects of Kiwiberry Leaf Extract: A Step Forward to Human Nutraceutical Use. International Journal of Molecular Sciences, 23(22), 14130. https://doi.org/10.3390/ijms232214130











