The Future of Targeted Treatment of Primary Sjögren’s Syndrome: A Focus on Extra-Glandular Pathology
Abstract
1. Introduction
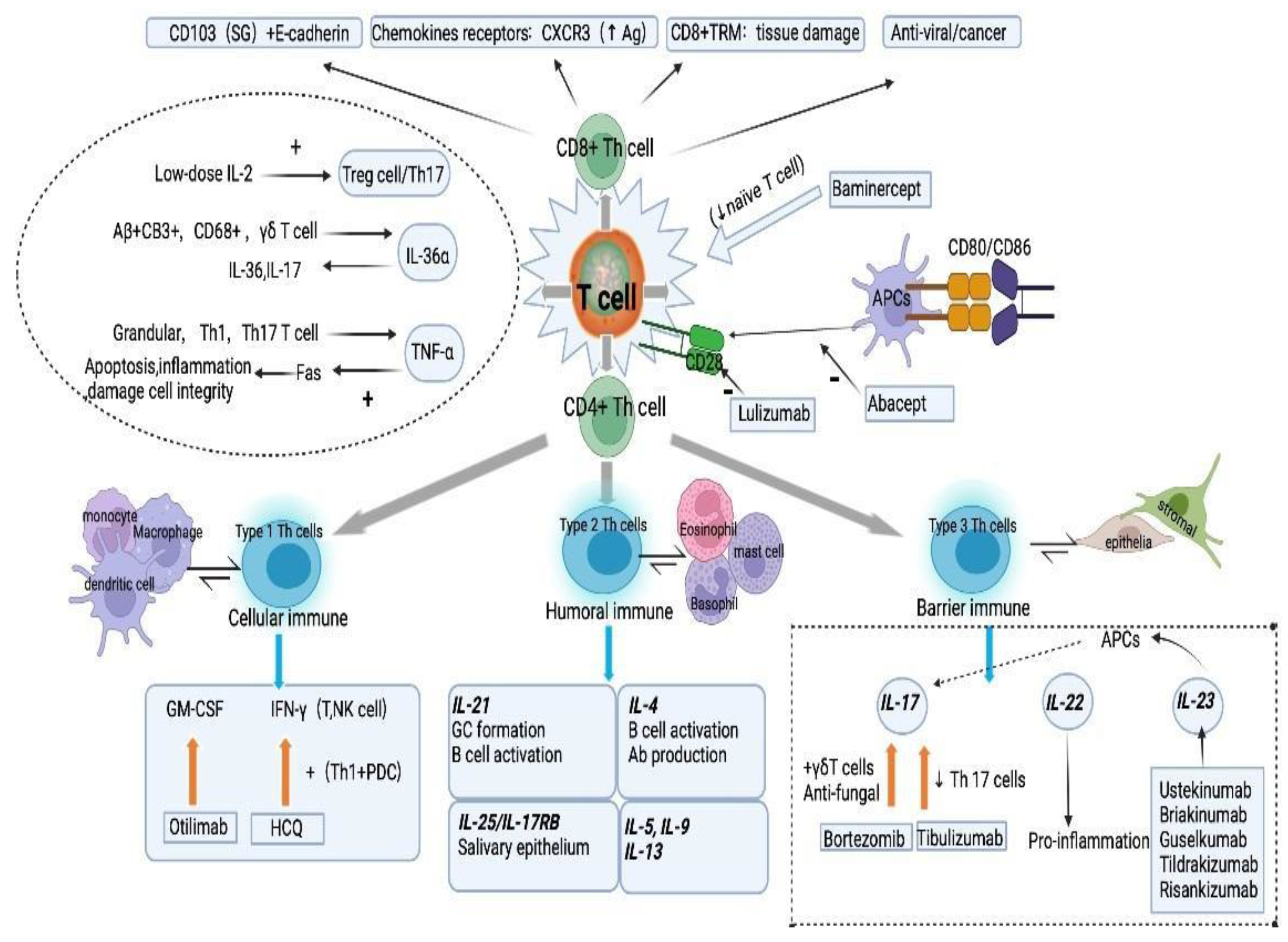
2. Potential T and B Cell Targets for the Development of Treatment Options for pSS
2.1. CD4+ T Helper Cells
2.1.1. Type 1 Th Cells
2.1.2. Type 2 Th Cells
2.1.3. Type 3 Th Cells
2.2. CD8+ Cytotoxic T Cells
2.3. Other Effectors Related with T Cells
2.3.1. IL-1β
2.3.2. TNF-α
2.3.3. IL-36α and γδ T Cells
2.3.4. Treg Cells and IL-2
2.4. B Cells
2.4.1. CD40
2.4.2. CD20
2.4.3. B Cell Activating Factor (BAFF)
2.4.4. IL-6
2.4.5. IL-10
3. Treatment of Exocrine Gland Disease in pSS
3.1. Dry Mouth (Xerostomia)
3.2. Dry Eye
3.3. Vaginal Dryness and Dyspareunia
3.4. Fatigue in pSS
3.4.1. IL-1
3.4.2. IL-36α
3.4.3. Immunoglobulins
3.4.4. Other Mediators
3.4.5. Alternative Medicine
3.4.6. Anti-Inflammatory and Immunosuppressive Treatment for Fatigue
3.5. Depression and Anxiety in pSS
4. Discussion
5. Methods
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Cells | Effector Molecules | References | ||
|---|---|---|---|---|
| T cells | CD4+ T helper cells | Type 1 Th cells | GM-CSF IFN-γ | [10,14,17,18,19] |
| Type 2 Th cells | IL-4 IL-21 IL-25 | [20,21] [9,20,22] [23] | ||
| Type 3 Th cells | IL-17 IL-22 IL-23 | [10,16,25,26,27,28,29] [31,32] [33,34,35] | ||
| CD8+ cytotoxic T cells | CXCR3 CD103 E-cadherin | [36,37,38,39] | ||
| Other effectors related with T cells | αβ+ CB3+, CD68+, γδ T cells | IL-36α | [48,49] | |
| Treg /Th17 balance | IL-2 | [51,52] | ||
| Th1/Th17 balance | TNF-α | [43,44,45,46,47] | ||
| B cells | B effector cells | CD40 | [53,54,55,56,57] | |
| CD20 | [58,59,60,61] | |||
| BAFF | [63,64,65,66] | |||
| IL-6 | [67,68,69] | |||
| Regulatory B cells | IL-10 | [70] | ||
| NCT Number | Drug | Phase | Target or Route | Duration (Years) | N | Primary End Point |
|---|---|---|---|---|---|---|
| NCT04093531 | Ustekinumab | 1 | Th-1/IL12 and Th-17/IL23 | 2 years (15 January 2020–December 2021) | 15 | ESSPRI |
| NCT01601028 | Hydroxychloroquine | 3 | TLR9 and TLR7, IL-6,IL-17,BAFF, etc. | 2 years (July 2011–August 2013) | 39 | Serum cytokine level |
| NCT04546542 | Hydroxychloroquine | - | - | 3 years (1 October 2020–1 December 2023) | 75 | HCQ blood levels |
| NCT02067910 | Abatacept | 3 | - | 5 years (August 2014–August 2019) | 80 | ESSDAI |
| NCT02915159 | Abatacept | 3 | CRP | 2–3 years (6 December 2016–23 July 2019) | 250 | ESSDAI |
| NCT04186871 | Branebrutinib and abatacept | 2 | 3 years (7 January 2020–10 February 2023) | 185 | SLE: mCLASI activity score response; pSS-ESSDAI; RA: ACR50 response | |
| NCT01693393 | Cyclosporin A | 2 | T cell and cytokines | 4 years (March 2010–October 2014) | 30 | Painful and/or swollen joints at end of treatment (EOT) |
| NCT04916756 | Baricitinib | 1,2 | JAK inhibitor, JAK/STAT | 1 year (16 January 2020–25 March 2021) | 11 | ESSDAI |
| NCT04496960 | Tofacitinib | 1b-2a | JAK inhibitor, IL-6 | 3 years (18 May 2021–5 February 2024) | 30 | Safety and tolerability of tofacitinib and ESSDAI |
| NCT02701985 | RO5459072 | 2 | Cathepsin S inhibitor, LIP 10,SSA/SSB, TNF-α,IL-10 | 1 year (5 July 2016–10 July 2017) | 75 | ESSDAI |
| NCT03627065 | Parsaclisib(INCB050465) | 2 | PI3Kδ inhibitor, B cell, BAFF, dsDNA, SSA, SSB, inhibition of AKT phosphorylation | 1 year (28 February 2019–2 January 2020) | 10 | Salivary gland ultrasound (SGUS) score for parotid and submandibular glands |
| NCT02775916 | Leniolisib(CDZ173) | 2 | PI3Kδ inhibitor, B cell, Abs | 1 year (1 June 2016–17 May 2017) | 30 | AEs, death, ESSPRI, ESSDAI |
| NCT00101829 | Rituximab | 1 | B cell, anti-CD20 | 5 years (April 2004–August 2009) | 12 | Grade 3 or higher AEs |
| NCT00363350 | Rituximab | 1,2 | B cell, anti-CD20 | 2 years (August 2006–October 2008) | 30 | Stimulated salivary gland function, histological/molecular parameters |
| NCT00426543 | Rituximab (Mabthera) | 2 | B cell, anti-CD20 | 3–4 years (January 2007–August 2010) | 21 | Disease symptoms (oral/ocular dryness, myoartralgia and fatigue) |
| NCT02631538 | Rituximab and Belimumab | 2 | B cell, anti-CD20 | 3–4 years (17 February 2016–23 June 2020) | 86 | - |
| NCT00740948 | Rituximab | 2,3 | B cell, anti-CD20 | 5 years (March 2008–January 2013) | 122 | 30% improvement between in the values on 2 of the 4 VAS |
| NCT04981145 | Iguratimod (IGU) | 4 | BAFF–BCMA/TACI | 2 years (July 2021–July 2023) | 78 | SSRI-30, ESSPRI, Schirmer test, IgG, C3, C4 |
| NCT04830644 | Iguratimod (IGU) | 2 | BAFF–BCMA/TACI | 1 year (22 March 2021–June 2022) | 144 | ESSDAI, ESSPRI |
| NCT03023592 | Iguratimod (IGU) | 1,2 | BAFF, antibodies | 1 year (February 2017–April 2018) | 30 | ESSDAI, ESSPRI |
| NCT02962895 | Ianalumab (VAY736) | 2 | - | 4 years (27 June 2017–30 September 2021) | 192 | Multi-dimensional disease activity |
| NCT02149420 | Ianalumab (VAY736) | 2 | BAFF-R inhibitor, ADCC | 4 years (23 May 2014–7 February 2018) | 27 | ESSDAI and AEs |
| NCT04078386 | Telitacicept (RC18) | 2 | B cell, BLyS/APRIL pathway, CD11c+age-associated B cells (ABCs)-targeted | 2 years (21 November 2019–23 June 2021) | 42 | ESSDAI |
| NCT01160666 | Belimumab | 2 | BAFF, cytokines | 2 years (March 2010–June 2012) | 20 | dryness, fatigue, musculoskeletal pain, systemic activity VAS and biomarkers |
| NCT04186871 | Branebrutinib (BMS-986195) | 2 | BTK inhibitor, BCR and FcR | 3 years (7 January 2020–10 February 2023) | 185 | SLE:mCLASI activity score response; pSS: ESSPRI, ESSDAI, ocular staining, salivary flow and serological marker |
| NCT02705989 | Branebrutinib (BMS-986195) | 1 | BTK inhibitor, BCR and FcR | 1 year (18 August 2016–16 August 2017) | 439 | AEs |
| NCT02843659 | BMS-986142 and BMS-931699 (Lulizumab) | 2 | BTK inhibitor, CD69 | 1 year (18 October 2016–24 July 2017) | 45 | ESSDAI |
| NCT02610543 | Seletalisib | 2 | PI3Kδ inhibitor, | 2 years (October 2015–5 December 2017) | 27 | ESSDAI |
| NCT04572841 | SAR441344 | 2 | T/B cell, anti-CD40L | 2 years (12 November 2020–October 2022) | 88 | ESSDAI |
| NCT02291029 | Iscalimab (CFZ533) | 2 | B cell, anti-CD40/CD154, TDARs (T cell–dependent antibody production) and GCS | 4 years (22 October 2014–29 June 2018) | 69 | ESSDAI |
| NCT02334306 | AMG 557 | 2a | Anti-BAFF and ICOSL | 3 years (8 June 2015–13 August 2018) | 32 | Baseline and day 99 |
| NCT01552681 | Baminercept | 2 | B cell, LTβR-Ig | 3 years (July 2012–June 2015) | 52 | Stimulated whole salivary flow |
| NCT01782235 | Tocilizumab | 2.3 | Anti-IL 6 | 5 years (24 July 2013–16 July 2018) | 110 | ESSDAI |
| NCT04968912 | Nipocalimab (M281) | 2 | Anti-FcRn, Ig | 2 years (21 September 2021–1 December 2023) | 150 | ESSDAI |
| NCT00683345 | Anakinra | 2 | Anti-IL 1 | 2 years (January 2008–April 2010) | 28 | Fatigue severity scale |
References
- Mavragani, C.P. Mechanisms and New Strategies for Primary Sjögren’s Syndrome. Annu. Rev. Med. 2017, 68, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Perera, S.; Ma, L.; Punwaney, R.; Ramachandran, S. Clinical and Cost Burden of Primary Sjögren’s Syndrome: Descriptive Analysis Using a US Administrative Claims Database. J. Health Econ. Outcomes Res. 2017, 5, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Nocturne, G.; Mariette, X. B cells in the pathogenesis of primary Sjögren syndrome. Nat. Rev. Rheumatol. 2018, 14, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Fox, R.I.; Fox, C.M.; Gottenberg, J.E.; Dörner, T. Treatment of Sjögren’s syndrome: Current therapy and future directions. Rheumatology 2021, 60, 2066–2074. [Google Scholar] [CrossRef]
- Oliveira, F.R.; Valim, V.; Pasoto, S.G.; Fernandes, M.L.M.S.; Lopes, M.L.L.; de Magalhães Souza Fialho, S.C.; Pinheiro, A.C.; Dos Santos, L.C.; Appenzeller, S.; Fidelix, T.; et al. 2021 recommendations of the Brazilian Society of Rheumatology for the gynecological and obstetric care of patients with Sjogren’s syndrome. Adv. Rheumatol. 2021, 61, 54. [Google Scholar] [CrossRef] [PubMed]
- van Nimwegen, J.F.; van der Tuuk, K.; Liefers, S.C.; Verstappen, G.M.; Visser, A.; Wijnsma, R.F.; Vissink, A.; Hollema, H.; Mourits, M.J.E.; Bootsma, H.; et al. Vaginal dryness in primary Sjögren’s syndrome: A histopathological case-control study. Rheumatology 2020, 59, 2806–2815. [Google Scholar] [CrossRef] [PubMed]
- Mæland, E.; Miyamoto, S.T.; Hammenfors, D.; Valim, V.; Jonsson, M.V. Understanding Fatigue in Sjögren’s Syndrome: Outcome Measures, Biomarkers and Possible Interventions. Front. Immunol. 2021, 12, 703079. [Google Scholar] [CrossRef]
- Cui, Y.; Xia, L.; Li, L.; Zhao, Q.; Chen, S.; Gu, Z. Anxiety and depression in primary Sjögren’s syndrome: A cross-sectional study. BMC Psychiatry 2018, 18, 131. [Google Scholar] [CrossRef]
- Verstappen, G.M.; Kroese, F.G.M.; Bootsma, H. T cells in primary Sjögren’s syndrome: Targets for early intervention. Rheumatology 2021, 60, 3088–3098. [Google Scholar] [CrossRef]
- Yao, Y.; Ma, J.F.; Chang, C.; Xu, T.; Gao, C.Y.; Gershwin, M.E.; Lian, Z.X. Immunobiology of T Cells in Sjögren’s Syndrome. Clin. Rev. Allergy Immunol. 2021, 60, 111–131. [Google Scholar] [CrossRef]
- Mielle, J.; Tison, A.; Cornec, D.; Le Pottier, L.; Daien, C.; Pers, J.O. B cells in Sjögren’s syndrome: From pathophysiology to therapeutic target. Rheumatology (Oxford, England). Rheumatology 2019, 60, 2545–2560. [Google Scholar] [CrossRef] [PubMed]
- Chatzileontiadou, D.S.M.; Sloane, H.; Nguyen, A.T.; Gras, S.; Grant, E.J. The Many Faces of CD4+ T Cells: Immunological and Structural Characteristics. Int. J. Mol. Sci. 2020, 22, 73. [Google Scholar] [CrossRef] [PubMed]
- Maehara, T.; Moriyama, M.; Hayashida, J.N.; Tanaka, A.; Shinozaki, S.; Kubo, Y.; Matsumura, K.; Nakamura, S. Selective localization of T helper subsets in labial salivary glands from primary Sjögren’s syndrome patients. Clin. Exp. Immunol. 2012, 169, 89–99. [Google Scholar] [CrossRef]
- Tuzlak, S.; Tuzlak, S.; Dejean, A.S.; Iannacone, M.; Quintana, F.J.; Waisman, A.; Ginhoux, F.; Korn, T.; Becher, B. Repositioning T cell polarization from single cytokines to complex help. Nat. Immunol. 2021, 22, 1210–1217. [Google Scholar] [CrossRef] [PubMed]
- Dohlman, T.; Ding, J.; Dana, R.; Chauhan, S.K. T Cell–Derived Granulocyte-Macrophage Colony-Stimulating Factor Contributes to Dry Eye Disease Pathogenesis by Promoting CD11b+ Myeloid Cell Maturation and Migration. Investig. Opthalmol. Vis. Sci. 2017, 58, 1330–1336. [Google Scholar] [CrossRef] [PubMed]
- Verstappen, G.M.; Corneth, O.B.J.; Bootsma, H.; Kroese, F.G.M. Th17 cells in primary Sjögren’s syndrome: Pathogenicity and plasticity. J. Autoimmun. 2018, 87, 16–25. [Google Scholar] [CrossRef]
- Crotti, C.; Agape, E.; Becciolini, A.; Biggioggero, M.; Favalli, E.G. Targeting Granulocyte-Monocyte Colony-Stimulating Factor Signaling in Rheumatoid Arthritis: Future Prospects. Drugs 2019, 79, 1741–1755. [Google Scholar] [CrossRef]
- Cha, S.; Brayer, J.; Gao, J.; Brown, V.; Killedar, S.; Yasunari, U.; Peck, A.B. A dual role for interferon-gamma in the pathogenesis of Sjogren’s syndrome-like autoimmune exocrinopathy in the nonobese diabetic mouse. Scand. J. Immunol. 2004, 60, 552–565. [Google Scholar] [CrossRef]
- Ogawa, Y.; Shimizu, E.; Tsubota, K. Interferons and Dry Eye in Sjögren’s Syndrome. Int. J. Mol. Sci. 2018, 19, 3548. [Google Scholar] [CrossRef]
- Bryant, V.L.; Ma, C.S.; Avery, D.T.; Li, Y.; Good, K.L.; Corcoran, L.M.; Malefyt, R.D.W.; Tangye, S.G. Cytokine-Mediated Regulation of Human B Cell Differentiation into Ig-Secreting Cells: Predominant Role of IL-21 Produced by CXCR5+ T Follicular Helper Cells. J. Immunol. 2007, 179, 8180–8190. [Google Scholar] [CrossRef]
- Gao, J.; Killedar, S.; Cornelius, J.G.; Nguyen, C.; Cha, S.; Peck, A.B. Sjögren’s syndrome in the NOD mouse model is an interleukin-4 time-dependent, antibody isotype-specific autoimmune disease. J. Autoimmun. 2006, 26, 90–103. [Google Scholar] [CrossRef]
- Scofield, R.H. IL-21 and Sjögren’s syndrome. Arthritis Res. Ther. 2011, 13, 137. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guggino, G.; Lin, X.; Rizzo, A.; Xiao, F.; Saieva, L.; Raimondo, S.; Di Liberto, D.; Candore, G.; Ruscitti, P.; Cipriani, P.; et al. Interleukin-25 Axis Is Involved in the Pathogenesis of Human Primary and Experimental Murine Sjögren’s Syndrome. Arthritis Rheumatol. 2018, 70, 1265–1275. [Google Scholar] [CrossRef] [PubMed]
- Ciccia, F.; Guggino, G.; Rizzo, A.; Ferrante, A.; Raimondo, S.; Giardina, A.; Dieli, F.; Campisi, G.; Alessandro, R.; Triolo, G. Potential involvement of IL-22 and IL-22-producing cells in the inflamed salivary glands of patients with Sjogren’s syndrome. Ann. Rheum. Dis. 2012, 71, 295–301. [Google Scholar] [CrossRef]
- Katsifis, G.E.; Rekka, S.; Moutsopoulos, N.M.; Pillemer, S.; Wahl, S.M. Systemic and Local Interleukin-17 and Linked Cytokines Associated with Sjögren’s Syndrome Immunopathogenesis. Am. J. Pathol. 2009, 175, 1167–1177. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Rui, K.; Deng, J.; Tian, J.; Wang, X.; Wang, S.; Ko, K.H.; Jiao, Z.; Chan, V.S.; Lau, C.S.; et al. Th17 cells play a critical role in the development of experimental Sjögren’s syndrome. Ann. Rheum. Dis. 2015, 74, 1302–1310. [Google Scholar] [CrossRef]
- Xiao, F.; Lin, X.; Tian, J.; Wang, X.; Chen, Q.; Rui, K.; Ma, J.; Wang, S.; Wang, Q.; Wang, X.; et al. Proteasome inhibition suppresses Th17 cell generation and ameliorates autoimmune development in experimental Sjögren’s syndrome. Cell. Mol. Immunol. 2017, 14, 924–934. [Google Scholar] [CrossRef]
- Fasano, S.; Mauro, D.; Macaluso, F.; Xiao, F.; Zhao, Y.; Lu, L.; Guggino, G.; Ciccia, F. Pathogenesis of primary Sjögren’s syndrome beyond B lymphocytes. Clin. Exp. Rheumatol. 2020, 38 (Suppl. 126), 315–323. [Google Scholar]
- Sparber, F.; LeibundGut-Landmann, S. Interleukin-17 in Antifungal Immunity. Pathogens 2019, 8, 54. [Google Scholar] [CrossRef]
- Dudakov, J.A.; Hanash, A.M.; Brink, M.R.V.D. Interleukin-22: Immunobiology and Pathology. Annu. Rev. Immunol. 2015, 33, 747–785. [Google Scholar] [CrossRef]
- Ciccia, F.; Guggino, G.; Giardina, A.; Ferrante, A.; Carrubbi, F.; Giacomelli, R.; Triolo, G. The role of innate and lymphoid IL-22-producing cells in the immunopathology of primary Sjögren’s syndrome. Expert Rev. Clin. Immunol. 2014, 10, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Barone, F.; Nayar, S.; Campos, J.; Cloake, T.; Withers, D.R.; Toellner, K.-M.; Zhang, Y.; Fouser, L.; Fisher, B.; Bowman, S.; et al. IL-22 regulates lymphoid chemokine production and assembly of tertiary lymphoid organs. Proc. Natl. Acad. Sci. USA 2015, 112, 11024–11029. [Google Scholar] [CrossRef] [PubMed]
- Hasan, Z.; Koizumi, S.-I.; Sasaki, D.; Yamada, H.; Arakaki, N.; Fujihara, Y.; Okitsu, S.; Shirahata, H.; Ishikawa, H. JunB is essential for IL-23-dependent pathogenicity of Th17 cells. Nat. Commun. 2017, 8, 15628. [Google Scholar] [CrossRef] [PubMed]
- Schinocca, C.; Rizzo, C.; Fasano, S.; Grasso, G.; La Barbera, L.; Ciccia, F.; Guggino, G. Role of the IL-23/IL-17 Pathway in Rheumatic Diseases: An Overview. Front. Immunol. 2021, 12, 637829. [Google Scholar] [CrossRef]
- Najm, A.; McInnes, I.B. IL-23 orchestrating immune cell activation in arthritis. Rheumatology 2021, 60, iv4–iv15. [Google Scholar] [CrossRef]
- Caldeira-Dantas, S.; Furmanak, T.; Smith, C.; Quinn, M.; Teos, L.Y.; Ertel, A.; Kurup, D.; Tandon, M.; Alevizos, I.; Snyder, C.M. The Chemokine Receptor CXCR3 Promotes CD8+ T Cell Accumulation in Uninfected Salivary Glands but Is Not Necessary after Murine Cytomegalovirus Infection. J. Immunol. 2017, 200, 1133–1145. [Google Scholar] [CrossRef]
- Proudfoot, A.E.; Bonvin, P.; Power, C.A. Targeting chemokines: Pathogens can, why can’t we? Cytokine 2015, 74, 259–267. [Google Scholar] [CrossRef]
- Gao, C.-Y.; Yao, Y.; Li, L.; Yang, S.-H.; Chu, H.; Tsuneyama, K.; Li, X.-M.; Gershwin, M.E.; Lian, Z.-X. Tissue-Resident Memory CD8+ T Cells Acting as Mediators of Salivary Gland Damage in a Murine Model of Sjögren’s Syndrome. Arthritis Rheumatol. 2019, 71, 121–132. [Google Scholar] [CrossRef]
- Thom, J.T.; Weber, T.C.; Walton, S.M.; Torti, N.; Oxenius, A. The Salivary Gland Acts as a Sink for Tissue-Resident Memory CD8(+) T Cells, Facilitating Protection from Local Cytomegalovirus Infection. Cell Rep. 2015, 13, 1125–1136. [Google Scholar] [CrossRef]
- Willeke, P.; Schotte, H.; Schlüter, B.; Erren, M.; Becker, H.; Dyong, A.; Mickholz, E.; Domschke, W.; Gaubitz, M. Interleukin 1beta and tumour necrosis factor alpha secreting cells are increased in the peripheral blood of patients with primary Sjögren’s syndrome. Ann. Rheum. Dis. 2003, 62, 359–362. [Google Scholar] [CrossRef]
- Lopalco, G.; Cantarini, L.; Vitale, A.; Iannone, F.; Anelli, M.G.; Andreozzi, L.; Lapadula, G.; Galeazzi, M.; Rigante, D. Interleukin-1 as a Common Denominator from Autoinflammatory to Autoimmune Disorders: Premises, Perils, and Perspectives. Mediat. Inflamm. 2015, 2015, 194864. [Google Scholar] [CrossRef] [PubMed]
- Azuma, M.; Motegi, K.; Aota, K.; Hayashi, Y.; Sato, M. Role of cytokines in the destruction of acinar structure in Sjögren’s syndrome salivary glands. Lab. Investig. 1997, 77, 269–280. [Google Scholar]
- Matsumura, R.; Umemiya, K.; Goto, T.; Nakazawa, T.; Ochiai, K.; Kagami, M.; Tomioka, H.; Tanabe, E.; Sugiyama, T.; Sueishi, M. Interferon gamma and tumor necrosis factor alpha induce Fas expression and anti-Fas mediated apoptosis in a salivary ductal cell line. Clin. Exp. Rheumatol. 2000, 18, 311–318. [Google Scholar]
- Limaye, A.; Hall, B.; Zhang, L.; Cho, A.; Prochazkova, M.; Zheng, C.; Walker, M.; Adewusi, F.; Burbelo, P.; Sun, Z.; et al. Targeted TNF-α Overexpression Drives Salivary Gland Inflammation. J. Dent. Res. 2019, 98, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Mignogna, M.D.; Fedele, S.; Lo Russo, L.; Lo Muzio, L.; Wolff, A. Sjögren’s syndrome: The diagnostic potential of early oral manifestations preceding hyposalivation/xerostomia. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 2005, 34, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Andréu Sánchez, J.L.; Fernández Castro, M.; Del Campo Fontecha, P.D.; Corominas, H.; Narváez García, F.J.; Gómez de Salazar, J.R.; Rua-Figueroa, Í.; Abad Hernández, M.Á.; Álvarez Rivas, M.N.; Montes, J.D.P.; et al. SER recommendations on the use of biological drugs in primary Sjögren’s syndrome. Reumatol. Clin. 2019, 15, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Sada, P.R.; Isenberg, D.; Ciurtin, C. Biologic treatment in Sjögren’s syndrome. Rheumatology 2015, 54, 219–230. [Google Scholar] [CrossRef]
- Ding, L.; Wang, X.; Hong, X.; Lu, L.; Liu, D. IL-36 cytokines in autoimmunity and inflammatory disease. Oncotarget 2017, 9, 2895–2901. [Google Scholar] [CrossRef]
- Ciccia, F.; Accardo-Palumbo, A.; Alessandro, R.; Alessandri, C.; Priori, R.; Guggino, G.; Raimondo, S.; Carubbi, F.; Valesini, G.; Giacomelli, R.; et al. Interleukin-36α axis is modulated in patients with primary Sjögren’s syndrome. Clin. Exp. Immunol. 2015, 181, 230–238. [Google Scholar] [CrossRef]
- Chinen, T.; Kannan, A.K.; Levine, A.G.; Fan, X.; Klein, U.; Zheng, Y.; Gasteiger, G.; Feng, Y.; Fontenot, J.D.; Rudensky, A.Y. An essential role for the IL-2 receptor in T cell function. Nat. Immunol. 2016, 17, 1322–1333. [Google Scholar] [CrossRef]
- Miao, M.; Hao, Z.; Guo, Y.; Zhang, X.; Zhang, S.; Luo, J.; Zhao, X.; Zhang, C.; Liu, X.; Wu, X.; et al. Short-term and low-dose IL-2 therapy restores the Th17/Treg balance in the peripheral blood of patients with primary Sjögren’s syndrome. Ann. Rheum. Dis. 2018, 77, 1838–1840. [Google Scholar] [CrossRef] [PubMed]
- Graßhoff, H.; Comdühr, S.; Monne, L.R.; Müller, A.; Lamprecht, P.; Riemekasten, G.; Humrich, J.Y. Low-Dose IL-2 Therapy in Autoimmune and Rheumatic Diseases. Front. Immunol. 2021, 12, 648408. [Google Scholar] [CrossRef] [PubMed]
- Elgueta, R.; Benson, M.J.; de Vries, V.C.; Wasiuk, A.; Guo, Y.; Noelle, R.J. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol. Rev. 2009, 229, 152–172. [Google Scholar] [CrossRef] [PubMed]
- Jobling, K.; Ng, W.F. CD40 as a therapeutic target in Sjögren’s syndrome. Expert Rev. Clin. Immunol. 2018, 14, 535–537. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, G.; Bigaud, M.; Pfister, S.; Ceci, M.; McMichael, K.; Afatsawo, C.; Hamburger, M.; Texier, C.; Henry, M.; Cojean, C.; et al. Blockade of CD40–CD154 pathway interactions suppresses ectopic lymphoid structures and inhibits pathology in the NOD/ShiLtJ mouse model of Sjögren’s syndrome. Ann. Rheum. Dis. 2019, 78, 974–978. [Google Scholar] [CrossRef]
- Mahmoud, T.I.; Wang, J.; Karnell, J.L.; Wang, Q.; Wang, S.; Naiman, B.; Gross, P.; Brohawn, P.Z.; Morehouse, C.; Aoyama, J.; et al. Autoimmune manifestations in aged mice arise from early-life immune dysregulation. Sci. Transl. Med. 2016, 8, 361ra137. [Google Scholar] [CrossRef]
- Clarke, J. CD40 blockade shows promise in pSS trial. Nat. Rev. Rheumatol. 2020, 16, 126. [Google Scholar] [CrossRef]
- Pavlasova, G.; Mraz, M. The regulation and function of CD20: An “enigma” of B-cell biology and targeted therapy. Haematologica 2020, 105, 1494–1506. [Google Scholar] [CrossRef]
- Bowman, S.J.; Everett, C.C.; O’Dwyer, J.L.; Emery, P.; Pitzalis, C.; Ng, W.F.; Pease, C.T.; Price, E.J.; Sutcliffe, N.; Gendi, N.S.T.; et al. Randomized Controlled Trial of Rituximab and Cost-Effectiveness Analysis in Treating Fatigue and Oral Dryness in Primary Sjögren’s Syndrome. Arthritis Rheumatol. 2017, 69, 1440–1450. [Google Scholar] [CrossRef]
- Pinto, A. Management of xerostomia and other complications of Sjögren’s syndrome. Oral Maxillofac. Surg. Clin. North Am. 2014, 26, 63–73. [Google Scholar] [CrossRef]
- Chen, Y.H.; Wang, X.Y.; Jin, X.; Yang, Z.; Xu, J. Rituximab Therapy for Primary Sjögren’s Syndrome. Front. Pharmacol. 2021, 12, 731122. [Google Scholar] [CrossRef] [PubMed]
- Thompson, N.; Isenberg, D.A.; Jury, E.C.; Ciurtin, C. Exploring BAFF: Its expression, receptors and contribution to the immunopathogenesis of Sjögren’s syndrome. Rheumatology 2016, 55, 1548–1555. [Google Scholar] [CrossRef] [PubMed]
- 63. Mariette, X.; Seror, R.; Quartuccio, L.; Baron, G.; Salvin, S.; Fabris, M.; Desmoulins, F.; Nocturne, G.; Ravaud, P.; De Vita, S. Efficacy and safety of belimumab in primary Sjögren’s syndrome: Results of the BELISS open-label phase II study. Ann. Rheum. Dis. 2015, 74, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Benschop, R.J.; Chow, C.-K.; Tian, Y.; Nelson, J.; Barmettler, B.; Atwell, S.; Clawson, D.; Chai, Q.; Jones, B.; Fitchett, J.; et al. Development of tibulizumab, a tetravalent bispecific antibody targeting BAFF and IL-17A for the treatment of autoimmune disease. mAbs 2019, 11, 1175–1190. [Google Scholar] [CrossRef] [PubMed]
- Varin, M.-M.; Le Pottier, L.; Youinou, P.; Saulep, D.; Mackay, F.; Pers, J.-O. B-cell tolerance breakdown in Sjögren’s Syndrome: Focus on BAFF. Autoimmun. Rev. 2010, 9, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Gandolfo, S.; De Vita, S. Double anti-B cell and anti-BAFF targeting for the treatment of primary Sjögren’s syndrome. Clin. Exp. Rheumatol. 2019, 37 (Suppl. 118), 199–208. [Google Scholar]
- Villar-Fincheira, P.; Sanhueza-Olivares, F.; Norambuena-Soto, I.; Cancino-Arenas, N.; Hernandez-Vargas, F.; Troncoso, R.; Gabrielli, L.; Chiong, M. Role of Interleukin-6 in Vascular Health and Disease. Front. Mol. Biosci. 2021, 8, 641734. [Google Scholar] [CrossRef]
- Barr, T.A.; Shen, P.; Brown, S.; Lampropoulou, V.; Roch, T.; Lawrie, S.; Fan, B.; O’Connor, R.A.; Anderton, S.M.; Bar-Or, A.; et al. B cell depletion therapy ameliorates autoimmune disease through ablation of IL-6–producing B cells. J. Exp. Med. 2012, 209, 1001–1010. [Google Scholar] [CrossRef]
- Felten, R.; Devauchelle-Pensec, V.; Seror, R.; Duffau, P.; Saadoun, D.; Hachulla, E.; Yves, H.P.; Salliot, C.; Perdriger, A.; Morel, J.; et al. Interleukin 6 receptor inhibition in primary Sjögren syndrome: A multicentre double-blind randomised placebo-controlled trial. Ann. Rheum. Dis. 2020, 80, 329–338. [Google Scholar] [CrossRef]
- Lin, X.; Wang, X.; Xiao, F.; Ma, K.; Liu, L.; Wang, X.; Xu, D.; Wang, F.; Shi, X.; Liu, D.; et al. IL-10-producing regulatory B cells restrain the T follicular helper cell response in primary Sjögren’s syndrome. Cell. Mol. Immunol. 2019, 16, 921–931. [Google Scholar] [CrossRef]
- Assy, Z.; Bikker, F.J.; Picauly, O.; Brand, H.S. The association between oral dryness and use of dry-mouth interventions in Sjögren’s syndrome patients. Clin. Oral Investig. 2021, 26, 1465–1475. [Google Scholar] [CrossRef] [PubMed]
- Mariette, X. [Treatment of oral dryness in Sjögren’s syndrome]. La Rev. Med. Interne 2004, 25, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kim, M.; Kho, H. Oral health-related quality of life and associated factors in patients with xerostomia. Int. J. Dent. Hyg. 2021, 19, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Garlapati, K.; Kammari, A.; Badam, R.K.; Surekha, B.E.; Boringi, M.; Soni, P. Meta-analysis on pharmacological therapies in the management of xerostomia in patients with Sjogren’s syndrome. Immunopharmacol. Immunotoxicol. 2019, 41, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Ship, J.A.; Fox, P.C.; Michalek, J.E.; Cummins, M.J.; Richards, A.B. Treatment of primary Sjögren’s syndrome with low-dose natural human interferon-alpha administered by the oral mucosal route: A phase II clinical trial. IFN Protocol Study Group. J. Interferon Cytokine Res. Off. J. Int. Soc. Interferon Cytokine Res. 1999, 19, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Cummins, M.J.; Papas, A.; Kammer, G.M.; Fox, P.C. Treatment of primary Sjögren’s syndrome with low-dose human interferon alfa administered by the oromucosal route: Combined phase III results. Arthritis Rheum. 2003, 49, 585–593. [Google Scholar] [CrossRef]
- Skopouli, F.N.; Jagiello, P.; Tsifetaki, N.; Moutsopoulos, H.M. Methotrexate in primary Sjögren’s syndrome. Clin. Exp. Rheumatol. 1996, 14, 555–558. [Google Scholar]
- Andersen, H.; Mantegazza, R.; Wang, J.J.; O’Brien, F.; Patra, K.; Howard, J.F. Eculizumab improves fatigue in refractory generalized myasthenia gravis. Qual. Life Res. Int. J. Qual. Life Asp. Treat. Care Rehabil. 2019, 28, 2247–2254. [Google Scholar] [CrossRef]
- Vivino, F.B.; Bunya, V.Y.; Massaro-Giordano, G.; Johr, C.R.; Giattino, S.L.; Schorpion, A.; Shafer, B.; Peck, A.; Sivils, K.; Rasmussen, A.; et al. Sjogren’s syndrome: An update on disease pathogenesis, clinical manifestations and treatment. Clin. Immunol. 2019, 203, 81–121. [Google Scholar] [CrossRef]
- Thulasi, P.; Djalilian, A.R. Update in Current Diagnostics and Therapeutics of Dry Eye Disease. Ophthalmology 2017, 124, S27–S33. [Google Scholar] [CrossRef]
- Haskett, S.; Ding, J.; Zhang, W.; Thai, A.; Cullen, P.; Xu, S.; Petersen, B.; Kuznetsov, G.; Jandreski, L.; Hamann, S.; et al. Identification of Novel CD4+ T Cell Subsets in the Target Tissue of Sjögren’s Syndrome and Their Differential Regulation by the Lymphotoxin/LIGHT Signaling Axis. J. Immunol. 2016, 197, 3806–3819. [Google Scholar] [CrossRef] [PubMed]
- St Clair, E.W.; Baer, A.N.; Wei, C.; Noaiseh, G.; Parke, A.; Coca, A.; Utset, T.O.; Genovese, M.C.; Wallace, D.J.; McNamara, J.; et al. Clinical Efficacy and Safety of Baminercept, a Lymphotoxin β Receptor Fusion Protein, in Primary Sjögren’s Syndrome: Results From a Phase II Randomized, Double-Blind, Placebo-Controlled Trial. Arthritis Rheumatol. 2018, 70, 1470–1480. [Google Scholar] [CrossRef] [PubMed]
- Yoon, C.H.; Lee, H.J.; Lee, E.Y.; Lee, E.B.; Lee, W.-W.; Kim, M.K.; Wee, W.R. Effect of Hydroxychloroquine Treatment on Dry Eyes in Subjects with Primary Sjögren’s Syndrome: A Double-Blind Randomized Control Study. J. Korean Med. Sci. 2016, 31, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-Q.; Zhang, L.-W.; Wei, P.; Hua, H. Is hydroxychloroquine effective in treating primary Sjogren’s syndrome: A systematic review and meta-analysis. BMC Musculoskelet. Disord. 2017, 18, 186. [Google Scholar] [CrossRef]
- Hargreaves, P.; Daoudlarian, D.; Theron, M.; Kolb, F.A.; Young, M.M.; Reis, B.; Tiaden, A.; Bannert, B.; Kyburz, D.; Manigold, T. Differential effects of specific cathepsin S inhibition in biocompartments from patients with primary Sjögren syndrome. Arthritis Res. Ther. 2019, 21, 175. [Google Scholar] [CrossRef]
- Shahin, A.A.; El-Agha, S.; El-Azkalany, G.S. The effect of leflunomide on the eye dryness in secondary Sjögren’s syndrome associated with rheumatoid arthritis and in rheumatoid arthritis patients. Clin. Rheumatol. 2014, 33, 925–930. [Google Scholar] [CrossRef]
- Usuba, F.S.; De Medeiros-Ribeiro, A.C.; Novaes, P.; Aikawa, N.E.; Bonfiglioli, K.; Santo, R.M.; Bonfa, E.; Alves, M.R. Dry eye in rheumatoid arthritis patients under TNF-inhibitors: Conjunctival goblet cell as an early ocular biomarker. Sci. Rep. 2020, 10, 14054. [Google Scholar] [CrossRef]
- Wan, K.H.; Chen, L.J.; Young, A.L. Efficacy and Safety of Topical 0.05% Cyclosporine Eye Drops in the Treatment of Dry Eye Syndrome: A Systematic Review and Meta-analysis. Ocul. Surf. 2015, 13, 213–225. [Google Scholar] [CrossRef]
- Ng, W.-F.; Bowman, S.J. Primary Sjogren’s syndrome: Too dry and too tired. Rheumatology 2010, 49, 844–853. [Google Scholar] [CrossRef]
- Strömbeck, B.; Ekdahl, C.; Manthorpe, R.; Jacobsson, L.T. Physical capacity in women with primary Sjögren’s syndrome: A controlled study. Arthritis Rheum. 2003, 49, 681–688. [Google Scholar] [CrossRef]
- Dinarello, C.A. The IL-1 family of cytokines and receptors in rheumatic diseases. Nat. Rev. Rheumatol. 2019, 15, 612–632. [Google Scholar] [CrossRef] [PubMed]
- Migliorini, P.; Italiani, P.; Pratesi, F.; Puxeddu, I.; Boraschi, D. The IL-1 family cytokines and receptors in autoimmune diseases. Autoimmun. Rev. 2020, 19, 102617. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R. Cytokine-induced sickness behavior: Where do we stand? Brain Behav. Immun. 2001, 15, 7–24. [Google Scholar] [CrossRef] [PubMed]
- Harboe, E.; Tjensvoll, A.B.; Vefring, H.K.; Gøransson, L.G.; Kvaløy, J.T.; Omdal, R. Fatigue in primary Sjögren’s syndrome—A link to sickness behaviour in animals? Brain Behav. Immun. 2009, 23, 1104–1108. [Google Scholar] [CrossRef]
- Bårdsen, K.; Brede, C.; Kvivik, I.; Kvaløy, J.T.; Jonsdottir, K.; Tjensvoll, A.B.; Ruoff, P.; Omdal, R. Interleukin-1-related activity and hypocretin-1 in cerebrospinal fluid contribute to fatigue in primary Sjögren’s syndrome. J. Neuroinflammation 2019, 16, 102. [Google Scholar] [CrossRef]
- Schlesinger, N.; De Meulemeester, M.; Pikhlak, A.; Yücel, A.E.; Richard, D.; Murphy, V.; Arulmani, U.; Sallstig, P.; So, A. Canakinumab relieves symptoms of acute flares and improves health-related quality of life in patients with difficult-to-treat Gouty Arthritis by suppressing inflammation: Results of a randomized, dose-ranging study. Arthritis Res. Ther. 2011, 13, R53. [Google Scholar] [CrossRef]
- Bodewes, I.L.A.; van der Spek, P.J.; Leon, L.G.; Wijkhuijs, A.J.M.; van Helden-Meeuwsen, C.G.; Tas, L.; Schreurs, M.W.J.; van Daele, P.L.A.; Katsikis, P.D.; Versnel, M.A. Fatigue in Sjögren’s Syndrome: A Search for Biomarkers and Treatment Targets. Front. Immunol. 2019, 10, 312. [Google Scholar] [CrossRef]
- Vivino, F.B.; Carsons, S.E.; Foulks, G.; Daniels, T.E.; Parke, A.; Brennan, M.T.; Forstot, S.L.; Scofield, R.H.; Hammitt, K.M. New Treatment Guidelines for Sjögren’s Disease. Rheum. Dis. Clin. N. Am. 2016, 42, 531–551. [Google Scholar] [CrossRef]
- Davies, K.; Registry, U.P.S.S.; Mirza, K.; Tarn, J.; Howard-Tripp, N.; Bowman, S.J.; Lendrem, D.; Ng, W.-F. Fatigue in primary Sjögren’s syndrome (pSS) is associated with lower levels of proinflammatory cytokines: A validation study. Rheumatol. Int. 2019, 39, 1867–1873. [Google Scholar] [CrossRef]
- Howard Tripp, N.; Mirza, K.; Tarn, J.; Howard-Tripp, N.; Bowman, S.J.; Lendrem, D. Fatigue in primary Sjögren’s syndrome is associated with lower levels of proinflammatory cytokines. RMD Open 2016, 2, e000282. [Google Scholar] [CrossRef]
- Liu, X.; Li, X.; Li, X.; Li, Z.; Zhao, D.; Liu, S.; Zhang, M.; Zhang, F.; Zhu, P.; Chen, J.; et al. The efficacy and safety of total glucosides of peony in the treatment of primary Sjögren’s syndrome: A multi-center, randomized, double-blinded, placebo-controlled clinical trial. Clin. Rheumatol. 2019, 38, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Wu, N.; Li, T.; Lu, W.; Yu, G. Total glucosides of peony ameliorates Sjögren’s syndrome by affecting Th1/Th2 cytokine balance. Exp. Ther. Med. 2016, 11, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Zhang, H.; Pang, R.; Chen, J.; Liu, Z.; Zhou, X. Acupuncture for Primary Sjögren Syndrome (pSS) on symptomatic improvements: Study protocol for a randomized controlled trial. BMC Complement. Altern. Med. 2017, 17, 61. [Google Scholar] [CrossRef]
- Fox, R.I.; Dixon, R.; Guarrasi, V.; Krubel, S. Treatment of primary Sjögren’s syndrome with hydroxychloroquine: A retrospective, open-label study. Lupus 1996, 5 (Suppl. 1), S31–S36. [Google Scholar] [CrossRef] [PubMed]
- Gottenberg, J.-E.; Ravaud, P.; Puéchal, X.; Le Guern, V.; Sibilia, J.; Goeb, V.; Larroche, C.; Dubost, J.J.; Rist, S.; Saraux, A.; et al. Effects of hydroxychloroquine on symptomatic improvement in primary Sjögren syndrome: The JOQUER randomized clinical trial. JAMA 2014, 312, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Carsons, S.E.; Vivino, F.B.; Parke, A.; Carteron, N.; Sankar, V.; Brasington, R.; Brennan, M.T.; Ehlers, W.; Fox, R.; Scofield, H.; et al. Treatment Guidelines for Rheumatologic Manifestations of Sjögren’s Syndrome: Use of Biologic Agents, Management of Fatigue, and Inflammatory Musculoskeletal Pain. Arthritis Care Res. 2017, 69, 517–527. [Google Scholar] [CrossRef]
- Dass, S.; Bowman, S.J.; Vital, E.M.; Ikeda, K.; Pease, C.T.; Hamburger, J.; Richards, A.; Rauz, S.; Emery, P. Reduction of fatigue in Sjögren syndrome with rituximab: Results of a randomised, double-blind, placebo-controlled pilot study. Ann. Rheum. Dis. 2008, 67, 1541–1544. [Google Scholar] [CrossRef]
- Devauchelle-Pensec, V.; Mariette, X.; Jousse-Joulin, S.; Berthelot, J.-M.; Perdriger, A.; Puéchal, X.; Le Guern, V.; Sibilia, J.; Gottenberg, J.-E.; Chiche, L.; et al. Treatment of primary Sjögren syndrome with rituximab: A randomized trial. Ann. Intern. Med. 2014, 160, 233–242. [Google Scholar] [CrossRef]
- Steinfeld, S.D.; Demols, P.; Salmon, I.; Kiss, R.; Appelboom, T. Infliximab in patients with primary Sjögren’s syndrome: A pilot study. Arthritis Rheum. 2001, 44, 2371–2375. [Google Scholar] [CrossRef]
- Scallon, B.; Cai, A.; Solowski, N.; Rosenberg, A.; Song, X.-Y.; Shealy, D.; Wagner, C. Binding and Functional Comparisons of Two Types of Tumor Necrosis Factor Antagonists. J. Pharmacol. Exp. Ther. 2002, 301, 418–426. [Google Scholar] [CrossRef]
- Norheim, K.B.; Harboe, E.; Gøransson, L.G.; Omdal, R. Interleukin-1 inhibition and fatigue in primary Sjögren’s syndrome—A double blind, randomised clinical trial. PLoS ONE 2012, 7, e30123. [Google Scholar] [CrossRef] [PubMed]
- Meiners, P.M.; Vissink, A.; Kroese, F.G.M.; Spijkervet, F.K.L.; Smitt-Kamminga, N.S.; Abdulahad, W.H.; Bulthuis-Kuiper, J.; Brouwer, E.; Arends, S.; Bootsma, H. Abatacept treatment reduces disease activity in early primary Sjögren’s syndrome (open-label proof of concept ASAP study). Ann. Rheum. Dis. 2014, 73, 1393–1396. [Google Scholar] [CrossRef] [PubMed]
- Steinfeld, S.D.; Tant, L.; Burmester, G.R.; Teoh, N.K.W.; Wegener, W.A.; Goldenberg, D.M.; Pradier, O. Epratuzumab (humanised anti-CD22 antibody) in primary Sjögren’s syndrome: An open-label phase I/II study. Arthritis Res. Ther. 2006, 8, R129. [Google Scholar] [CrossRef] [PubMed]
- Hartkamp, A.; Geenen, R.; Godaert, G.L.R.; Bootsma, H.; Kruize, A.A.; Bijlsma, J.W.J.; Derksen, R.H.W.M. Effect of dehydroepiandrosterone administration on fatigue, well-being, and functioning in women with primary Sjögren syndrome: A randomised controlled trial. Ann. Rheum. Dis. 2008, 67, 91–97. [Google Scholar] [CrossRef]
- Virkki, L.M.; Porola, P.; Forsblad-d’Elia, H.; Valtysdottir, S.; Solovieva, S.A.; Konttinen, Y.T. Dehydroepiandrosterone (DHEA) substitution treatment for severe fatigue in DHEA-deficient patients with primary Sjögren’s syndrome. Arthritis Care Res. 2010, 62, 118–124. [Google Scholar] [CrossRef]
- Milic, V.; Grujic, M.; Barisic, J.; Marinkovic-Eric, J.; Duisin, D.; Cirkovic, A.; Damjanov, N. Personality, depression and anxiety in primary Sjogren’s syndrome—Association with sociodemographic factors and comorbidity. PLoS ONE 2019, 14, e0210466. [Google Scholar] [CrossRef]
- Liu, Z.; Dong, Z.; Liang, X.; Liu, J.; Xuan, L.; Wang, J.; Zhang, G.; Hao, W. Health-related quality of life and psychological status of women with primary Sjögren’s syndrome: A cross-sectional study of 304 Chinese patients. Medicine 2017, 96, e9208. [Google Scholar] [CrossRef]
- Hsieh, M.-C.; Hsu, C.-W.; Lu, M.-C.; Koo, M. Increased risks of psychiatric disorders in patients with primary Sjögren’s syndrome-a secondary cohort analysis of nationwide, population-based health claim data. Clin. Rheumatol. 2019, 38, 3195–3203. [Google Scholar] [CrossRef]
- Milin, M.; Cornec, D.; Chastaing, M.; Griner, V.; Berrouiguet, S.; Nowak, E.; Marhadour, T.; Saraux, A.; Devauchelle-Pensec, V. Sicca symptoms are associated with similar fatigue, anxiety, depression, and quality-of-life impairments in patients with and without primary Sjögren’s syndrome. Jt. Bone Spine 2016, 83, 681–685. [Google Scholar] [CrossRef]
- Peng, G.-J.; Tian, J.-S.; Gao, X.-X.; Zhou, Y.-Z.; Qin, X.-M. Research on the Pathological Mechanism and Drug Treatment Mechanism of Depression. Curr. Neuropharmacol. 2015, 13, 514–523. [Google Scholar] [CrossRef]
- Karaiskos, D.; Mavragani, C.P.; Sinno, M.H.; Déchelotte, P.; Zintzaras, E.; Skopouli, F.N.; Fetissov, S.O.; Moutsopoulos, H.M. Psychopathological and personality features in primary Sjogren’s syndrome—Associations with autoantibodies to neuropeptides. Rheumatology 2010, 49, 1762–1769. [Google Scholar] [CrossRef] [PubMed]
- Hammett, E.K.; Fernandez-Carbonell, C.; Crayne, C.; Boneparth, A.; Cron, R.Q.; Radhakrishna, S.M. Adolescent Sjogren’s syndrome presenting as psychosis: A case series. Pediatr. Rheumatol. 2020, 18, 15. [Google Scholar] [CrossRef]
- Crupi, R.; Cambiaghi, M.; Spatz, L.; Hen, R.; Thorn, M.; Friedman, E.; Vita, G.; Battaglia, F. Reduced Adult Neurogenesis and Altered Emotional Behaviors in Autoimmune-Prone B-Cell Activating Factor Transgenic Mice. Biol. Psychiatry 2010, 67, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Crupi, R.; Cambiaghi, M.; Deckelbaum, R.; Hansen, I.; Mindes, J.; Spina, E.; Battaglia, F. n−3 fatty acids prevent impairment of neurogenesis and synaptic plasticity in B-cell activating factor (BAFF) transgenic mice. Prev. Med. 2012, 54, S103–S108. [Google Scholar] [CrossRef] [PubMed]
- Murrough, J.W.; Yaqubi, S.; Sayed, S.; Charney, D.S. Emerging drugs for the treatment of anxiety. Expert Opin. Emerg. Drugs 2015, 20, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.-L.; Li, T.-Y. Focus on Effects of Chinese Medicine on Improving Anxiety-Depression and Quality of Life of Patients with Primary Sjögren’s Syndrome. Chin. J. Integr. Med. 2020, 26, 486–489. [Google Scholar] [CrossRef]
- Miyamoto, S.T.; Valim, V.; Carletti, L.; Ng, W.F.; Perez, A.J.; Lendrem, D.W.; Trennel, M.; Giovelli, R.A.; Dias, L.H.; Serrano, É.V.; et al. Supervised walking improves cardiorespiratory fitness, exercise tolerance, and fatigue in women with primary Sjögren’s syndrome: A randomized-controlled trial. Rheumatol. Int. 2019, 39, 227–238. [Google Scholar] [CrossRef]
- Quock, R.L. Xerostomia: Current streams of investigation. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 122, 53–60. [Google Scholar] [CrossRef]
- Baum, B.J.; Alevizos, I.; Zheng, C.; Cotrim, A.P.; Liu, S.; McCullagh, L.; Goldsmith, C.M.; Burbelo, P.D.; Citrin, D.E.; Mitchell, J.B.; et al. Early responses to adenoviral-mediated transfer of the aquaporin-1 cDNA for radiation-induced salivary hypofunction. Proc. Natl. Acad. Sci. USA 2012, 109, 19403–19407. [Google Scholar] [CrossRef]
- Petitdemange, A.; Blaess, J.; Sibilia, J.; Felten, R.; Arnaud, L. Shared development of targeted therapies among autoimmune and inflammatory diseases: A systematic repurposing analysis. Ther. Adv. Musculoskelet. Dis. 2020, 12, 1759720X20969261. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, W.; Zhou, X.; Yu, S.; Liu, R.; Quek, C.W.N.; Yu, H.; Tay, R.Y.K.; Lin, X.; Feng, Y. The Future of Targeted Treatment of Primary Sjögren’s Syndrome: A Focus on Extra-Glandular Pathology. Int. J. Mol. Sci. 2022, 23, 14135. https://doi.org/10.3390/ijms232214135
Zeng W, Zhou X, Yu S, Liu R, Quek CWN, Yu H, Tay RYK, Lin X, Feng Y. The Future of Targeted Treatment of Primary Sjögren’s Syndrome: A Focus on Extra-Glandular Pathology. International Journal of Molecular Sciences. 2022; 23(22):14135. https://doi.org/10.3390/ijms232214135
Chicago/Turabian StyleZeng, Weizhen, Xinyao Zhou, Sulan Yu, Ruihua Liu, Chrystie Wan Ning Quek, Haozhe Yu, Ryan Yong Kiat Tay, Xiang Lin, and Yun Feng. 2022. "The Future of Targeted Treatment of Primary Sjögren’s Syndrome: A Focus on Extra-Glandular Pathology" International Journal of Molecular Sciences 23, no. 22: 14135. https://doi.org/10.3390/ijms232214135
APA StyleZeng, W., Zhou, X., Yu, S., Liu, R., Quek, C. W. N., Yu, H., Tay, R. Y. K., Lin, X., & Feng, Y. (2022). The Future of Targeted Treatment of Primary Sjögren’s Syndrome: A Focus on Extra-Glandular Pathology. International Journal of Molecular Sciences, 23(22), 14135. https://doi.org/10.3390/ijms232214135






