Toll-like Receptor 9 Induced Dendritic Cell Activation Promotes Anti-Myeloperoxidase Autoimmunity and Glomerulonephritis
Abstract
1. Introduction
2. Results
2.1. TLR9 Ligation Enhances MPO Pulsed Dendritic Cell Activation Upregulating Dendritic Cell CD40 and CD80 Expression
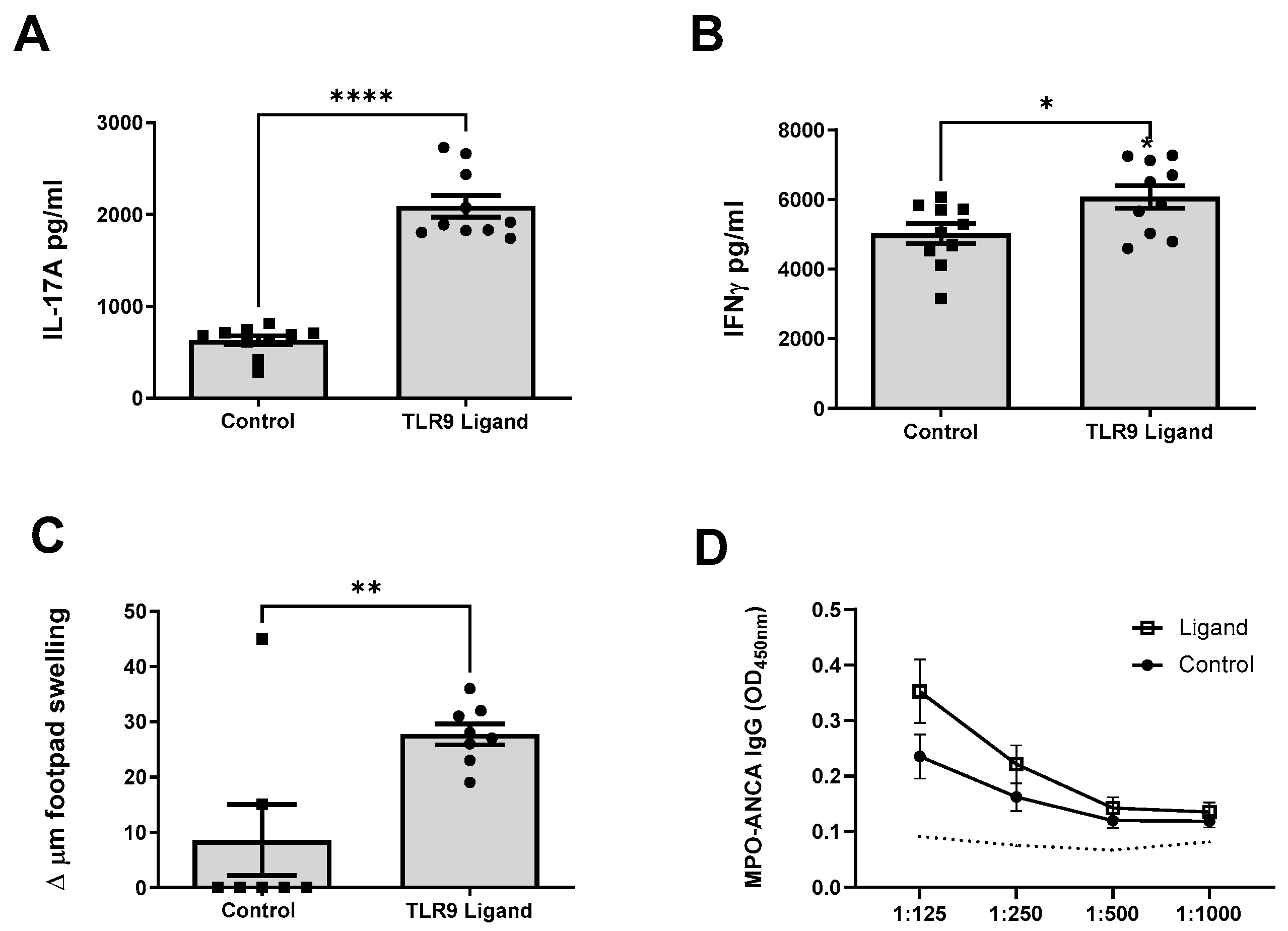
2.2. TLR9 Ligation of MPO Pulsed Dendritic Cells Promotes Systemic Anti-MPO Autoimmunity, T Cell Recall Responses and Subsequent Kidney Injury
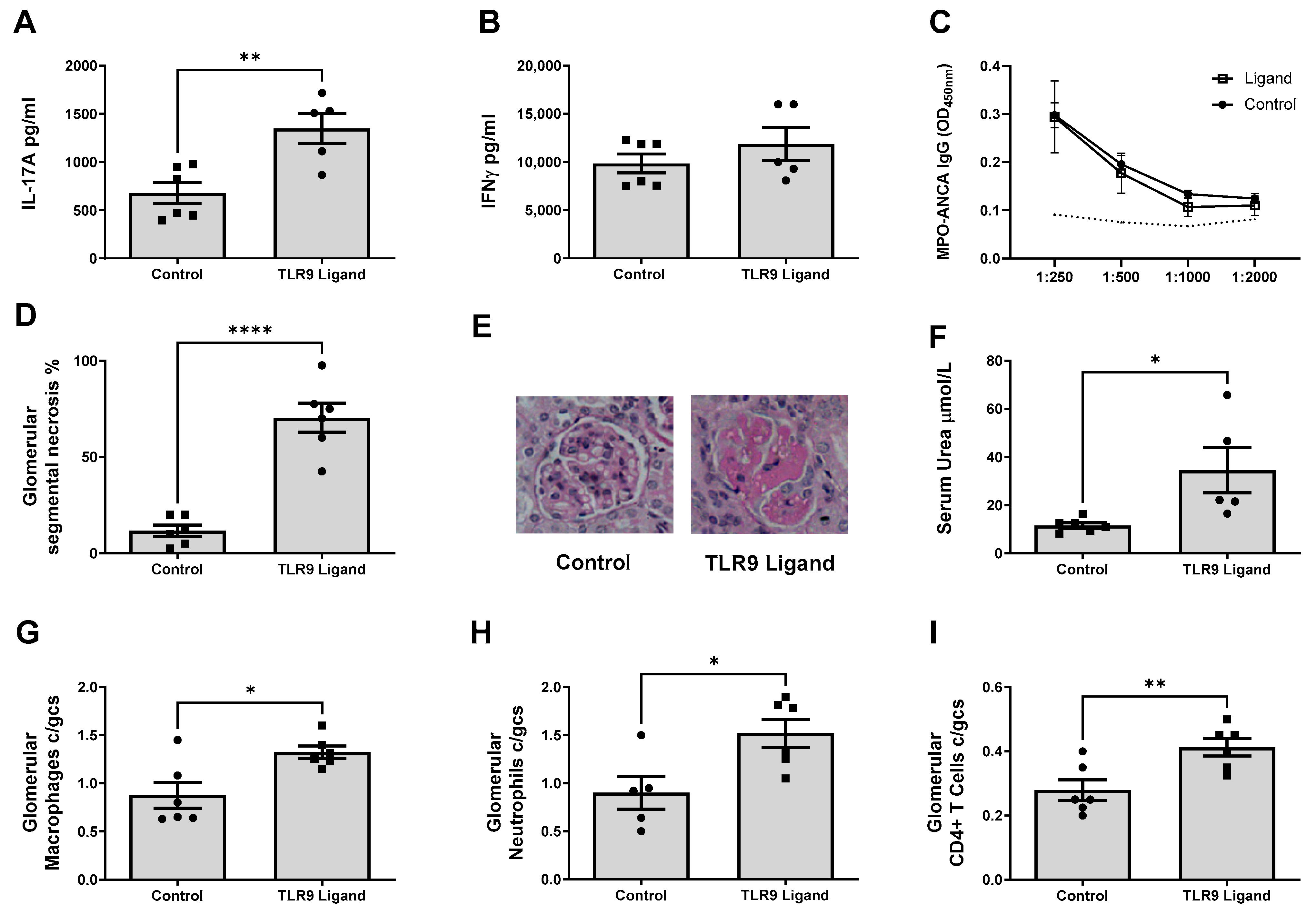
2.3. CD40 Is a Critical Second Signal for the Induction of Nephritogenic Autoimmunity
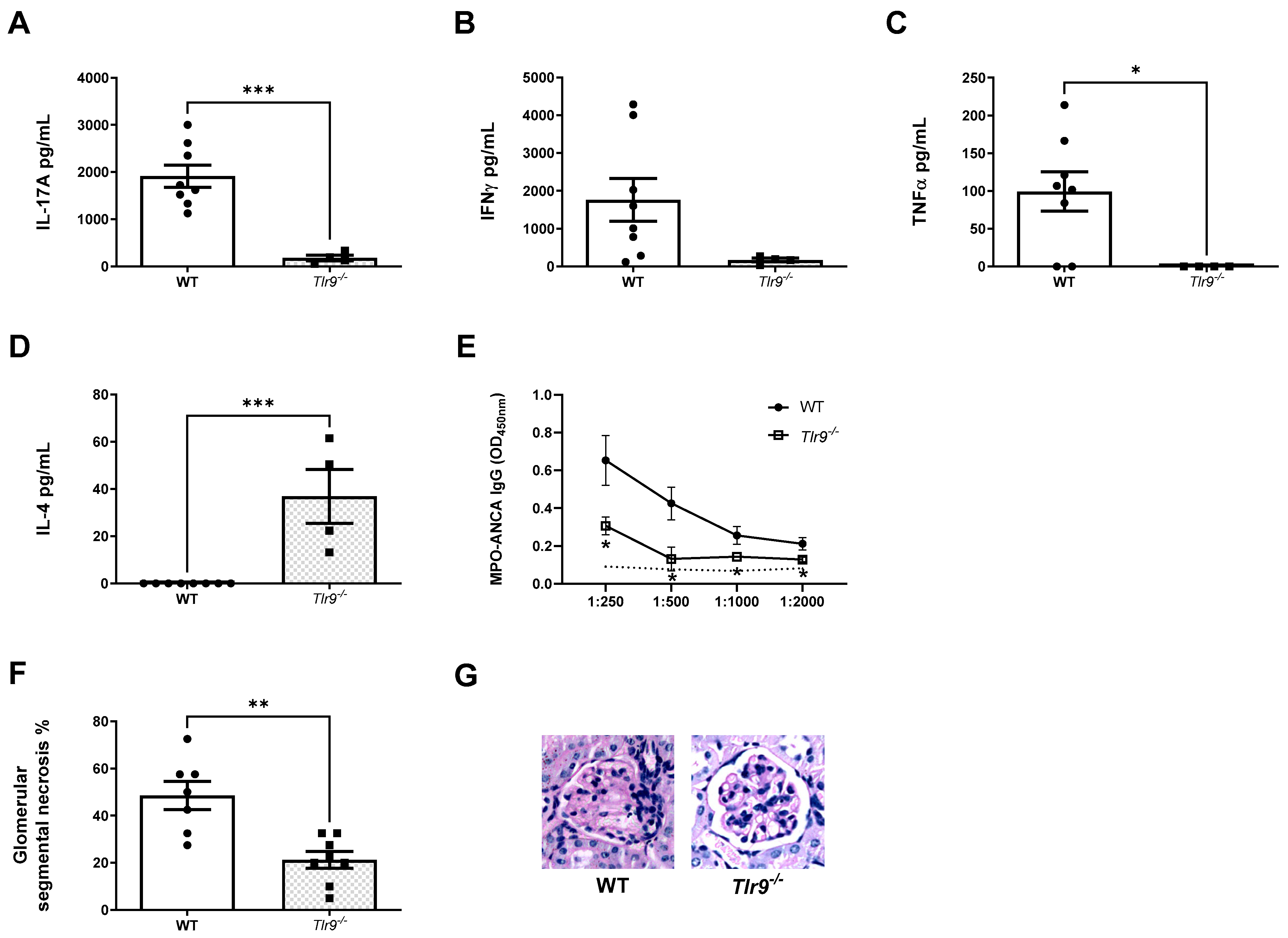
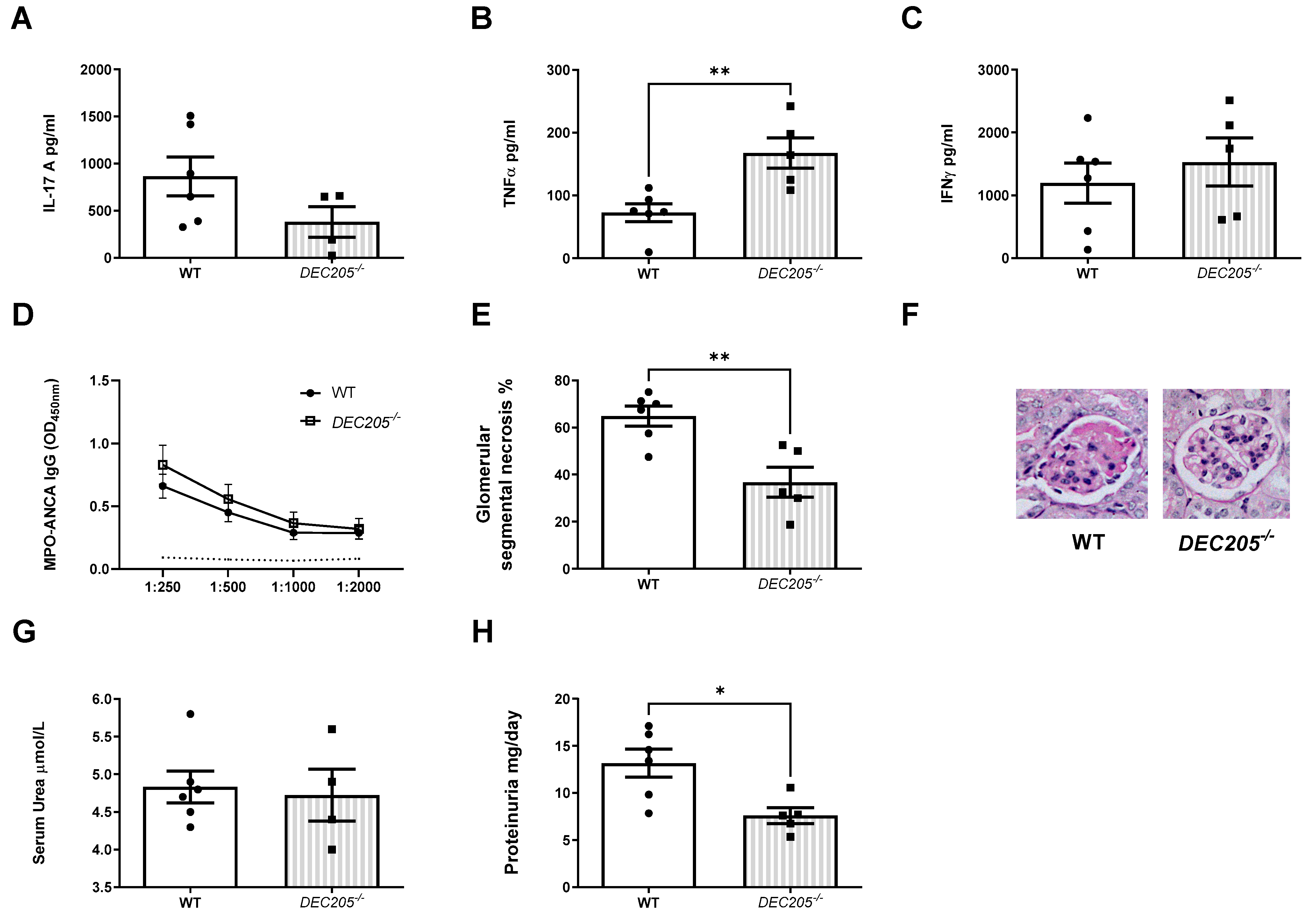
2.4. DEC205, a Cotransporter of the TLR9 Ligand, Facilitates the Development of Th17 Nephritogenic Autoimmunity and Kidney Injury
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Reagents
4.3. Experimental Design
4.4. Fluorescence Activated Cell Sorting (FACS)
4.5. Assessment of Systemic Autoimmune Response to MPO
4.6. Assessment of Functional and Histological Kidney Injury
4.7. Immunohistochemical Analysis of Kidney Leukocyte Accumulation
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walton, E.W. Giant-cell Granuloma of the Respiratory Tract (Wegener’s Granulomatosis). BMJ 1958, 2, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, G.S.; Kerr, G.S.; Leavitt, R.Y.; Hallahan, C.W.; Lebovics, R.S.; Travis, W.D.; Rottem, M.; Fauci, A.S. Wegener Granulomatosis: An Analysis of 158 Patients. Ann. Intern. Med. 1992, 116, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Kitching, A.R.; Anders, H.-J.; Basu, N.; Brouwer, E.; Gordon, J.; Jayne, D.R.; Kullman, J.; Lyons, P.A.; Merkel, P.A.; Savage, C.O.S.; et al. ANCA-associated vasculitis. Nat. Rev. Dis. Prim. 2020, 6, 71. [Google Scholar] [CrossRef]
- Wijngaarden, R.A.D.L.V.; Van Rijn, L.; Hagen, E.C.; Watts, R.A.; Gregorini, G.; Tervaert, J.W.C.; Mahr, A.; Niles, J.L.; De Heer, E.; Bruijn, J.A.; et al. Hypotheses on the Etiology of Antineutrophil Cytoplasmic Autoantibody–Associated Vasculitis: The Cause Is Hidden, but the Result Is Known. Clin. J. Am. Soc. Nephrol. 2007, 3, 237–252. [Google Scholar] [CrossRef] [PubMed]
- McInnis, E.A.; Badhwar, A.K.; Muthigi, A.; Lardinois, O.M.; Allred, S.C.; Yang, J.; Free, M.E.; Jennette, J.C.; Preston, G.A.; Falk, R.J.; et al. Dysregulation of Autoantigen Genes in ANCA-Associated Vasculitis Involves Alternative Transcripts and New Protein Synthesis. J. Am. Soc. Nephrol. 2014, 26, 390–399. [Google Scholar] [CrossRef]
- Pendergraft, W.F.; Preston, G.A.; Shah, R.R.; Tropsha, A.; Carter, C.W.; Jennette, J.C.; Falk, R.J. Autoimmunity is triggered by cPR-3(105–201), a protein complementary to human autoantigen proteinase-3. Nat. Med. 2003, 10, 72–79. [Google Scholar] [CrossRef]
- Yang, J.; Bautz, D.J.; Lionaki, S.; Hogan, S.L.; Chin, H.; Tisch, R.M.; Schmitz, J.L.; Pressler, B.; Jennette, J.C.; Falk, R.J.; et al. ANCA patients have T cells responsive to complementary PR-3 antigen. Kidney Int. 2008, 74, 1159–1169. [Google Scholar] [CrossRef][Green Version]
- Bautz, D.J.; Preston, G.A.; Lionaki, S.; Hewins, P.; Wolberg, A.S.; Yang, J.J.; Hogan, S.L.; Chin, H.; Moll, S.; Jennette, J.C.; et al. Antibodies with Dual Reactivity to Plasminogen and Complementary PR3 in PR3-ANCA Vasculitis. J. Am. Soc. Nephrol. 2008, 19, 2421–2429. [Google Scholar] [CrossRef]
- Preston, G.A.; Pendergraft, W.F., 3rd; Falk, R.J. New insights that link microbes with the generation of antineutrophil cytoplasmic autoantibodies: The theory of autoantigen complementarity. Curr. Opin. Nephrol. Hypertens 2005, 14, 217–222. [Google Scholar] [CrossRef]
- Lange, C.; Csernok, E.; Moosig, F.; Holle, J.U. Immune stimulatory effects of neutrophil extracellular traps in granulomatosis with polyangiitis. Clin. Exp. Rheumatol. 2017, 35 (Suppl. 103), 33–39. [Google Scholar]
- Söderberg, D.; Segelmark, M. Neutrophil Extracellular Traps in ANCA-Associated Vasculitis. Front. Immunol. 2016, 7, 256. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, A.; Rousselle, A.; Becker, J.U.; von Mässenhausen, A.; Linkermann, A.; Kettritz, R. Necroptosis controls NET generation and mediates complement activation, endothelial damage, and autoimmune vasculitis. Proc. Natl. Acad. Sci. USA 2017, 114, E9618–E9625. [Google Scholar] [CrossRef]
- Millet, A.; Martin, K.R.; Bonnefoy, F.; Saas, P.; Mocek, J.; Alkan, M.; Terrier, B.; Kerstein, A.; Tamassia, N.; Satyanarayanan, S.K.; et al. Proteinase 3 on apoptotic cells disrupts immune silencing in autoimmune vasculitis. J. Clin. Investig. 2015, 125, 4107–4121. [Google Scholar] [CrossRef] [PubMed]
- Abdgawad, M.; Pettersson, A.; Gunnarsson, L.; Bengtsson, A.A.; Geborek, P.; Nilsson, L.; Segelmark, M.; Hellmark, T. Decreased Neutrophil Apoptosis in Quiescent ANCA-Associated Systemic Vasculitis. PLoS ONE 2012, 7, e32439. [Google Scholar] [CrossRef] [PubMed]
- Gabillet, J.; Millet, A.; Pederzoli-Ribeil, M.; Tacnet-Delorme, P.; Guillevin, L.; Mouthon, L.; Frachet, P.; Witko-Sarsat, V. Proteinase 3, the Autoantigen in Granulomatosis with Polyangiitis, Associates with Calreticulin on Apoptotic Neutrophils, Impairs Macrophage Phagocytosis, and Promotes Inflammation. J. Immunol. 2012, 189, 2574–2583. [Google Scholar] [CrossRef]
- Munoz, L.; Lauber, K.; Schiller, M.; Manfredi, A.A.; Herrmann, M. The role of defective clearance of apoptotic cells in systemic autoimmunity. Nat. Rev. Rheumatol. 2010, 6, 280–289. [Google Scholar] [CrossRef]
- Rovere-Querini, P.; Capobianco, A.; Scaffidi, P.; Valentinis, B.; Catalanotti, F.; Giazzon, M.; Dumitriu, I.E.; Müller-Knapp, S.; Iannacone, M.; Traversari, C.; et al. HMGB1 is an endogenous immune adjuvant released by necrotic cells. EMBO Rep. 2004, 5, 825–830. [Google Scholar] [CrossRef]
- Linkermann, A.; Stockwell, B.R.; Krautwald, S.; Anders, H.-J. Regulated cell death and inflammation: An auto-amplification loop causes organ failure. Nat. Rev. Immunol. 2014, 14, 759–767. [Google Scholar] [CrossRef]
- Goodnow, C.C. Multistep Pathogenesis of Autoimmune Disease. Cell 2007, 130, 25–35. [Google Scholar] [CrossRef]
- Stegeman, C.A.; Tervaert, J.W.C.; Sluiter, W.J.; Manson, W.L.; De Jong, P.E.; Kallenberg, C.G.M. Association of Chronic Nasal Carriage of Staphylococcus aureus and Higher Relapse Rates in Wegener Granulomatosis. Ann. Intern. Med. 1994, 120, 12–17. [Google Scholar] [CrossRef]
- Marshak-Rothstein, A. Toll-like receptors in systemic autoimmune disease. Nat. Rev. Immunol. 2006, 6, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Shirali, A.C.; Goldstein, D. Tracking the Toll of Kidney Disease. J. Am. Soc. Nephrol. 2008, 19, 1444–1450. [Google Scholar] [CrossRef] [PubMed]
- Wörnle, M.; Schmid, H.; Banas, B.; Merkle, M.; Henger, A.; Roeder, M.; Blattner, S.; Bock, E.; Kretzler, M.; Gröne, H.-J.; et al. Novel Role of Toll-Like Receptor 3 in Hepatitis C-Associated Glomerulonephritis. Am. J. Pathol. 2006, 168, 370–385. [Google Scholar] [CrossRef]
- Song, J.; Abraham, S.N. TLR-mediated immune responses in the urinary tract. Curr. Opin. Microbiol. 2008, 11, 66–73. [Google Scholar] [CrossRef]
- Cirl, C.; Wieser, A.; Yadav, M.; Duerr, S.; Schubert, S.; Fischer, H.; Stappert, D.; Wantia, N.; Rodriguez, N.; Wagner, H.; et al. Subversion of Toll-like receptor signaling by a unique family of bacterial Toll/interleukin-1 receptor domain–containing proteins. Nat. Med. 2008, 14, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Guay, H.M.; Andreyeva, T.A.; Garcea, R.L.; Welsh, R.M.; Szomolanyi-Tsuda, E. MyD88 Is Required for the Formation of Long-Term Humoral Immunity to Virus Infection. J. Immunol. 2007, 178, 5124–5131. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Carballo, C.; Guerrero-Hue, M.; García-Caballero, C.; Rayego-Mateos, S.; Opazo-Ríos, L.; Morgado-Pascual, J.L.; Herencia-Bellido, C.; Vallejo-Mudarra, M.; Cortegano, I.; Gaspar, M.L.; et al. Toll-Like Receptors in Acute Kidney Injury. Int. J. Mol. Sci. 2021, 22, 816. [Google Scholar] [CrossRef]
- Summers, S.; Hoi, A.; Steinmetz, O.; O’Sullivan, K.; Ooi, J.; Odobasic, D.; Akira, S.; Kitching, A.; Holdsworth, S. TLR9 and TLR4 are required for the development of autoimmunity and lupus nephritis in pristane nephropathy. J. Autoimmun. 2010, 35, 291–298. [Google Scholar] [CrossRef]
- Summers, S.A.; Steinmetz, O.M.; Gan, P.-Y.; Ooi, J.D.; Odobasic, D.; Kitching, A.R.; Holdsworth, S.R. Toll-like receptor 2 induces Th17 myeloperoxidase autoimmunity while Toll-like receptor 9 drives Th1 autoimmunity in murine vasculitis. Arthritis Rheum. 2010, 63, 1124–1135. [Google Scholar] [CrossRef]
- Fu, Y.; Xie, C.; Chen, J.; Zhu, J.; Zhou, H.; Thomas, J.; Zhou, X.J.; Mohan, C. Innate Stimuli Accentuate End-Organ Damage by Nephrotoxic Antibodies via Fc Receptor and TLR Stimulation and IL-1/TNF-α Production. J. Immunol. 2005, 176, 632–639. [Google Scholar] [CrossRef]
- Anders, H.-J.; Banas, B.; Linde, Y.; Weller, L.; Cohen, C.D.; Kretzler, M.; Martin, S.; Vielhauer, V.; Schlöndorff, D.; Gröne, H.-J. Bacterial CpG-DNA Aggravates Immune Complex Glomerulonephritis: Role of TLR9-Mediated Expression of Chemokines and Chemokine Receptors. J. Am. Soc. Nephrol. 2003, 14, 317–326. [Google Scholar] [CrossRef]
- Hurtado, P.R.; Jeffs, L.; Nitschke, J.; Patel, M.; Sarvestani, G.; Cassidy, J.; Hissaria, P.; Gillis, D.; Peh, C.A. CpG oligodeoxynucleotide stimulates production of anti-neutrophil cytoplasmic antibodies in ANCA associated vasculitis. BMC Immunol. 2008, 9, 34. [Google Scholar] [CrossRef]
- Tadema, H.; Abdulahad, W.H.; Lepse, N.; Stegeman, C.A.; Kallenberg, C.G.M.; Heeringa, P. Bacterial DNA motifs trigger ANCA production in ANCA-associated vasculitis in remission. Rheumatology 2010, 50, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Holle, J.U.; Windmöller, M.; Lange, C.; Gross, W.L.; Herlyn, K.; Csernok, E. Toll-like receptor TLR2 and TLR9 ligation triggers neutrophil activation in granulomatosis with polyangiitis. Rheumatology 2013, 52, 1183–1189. [Google Scholar] [CrossRef]
- Lahoud, M.H.; Ahmet, F.; Zhang, J.-G.; Meuter, S.; Policheni, A.N.; Kitsoulis, S.; Lee, C.-N.; O’Keeffe, M.; Sullivan, L.C.; Brooks, A.G.; et al. DEC-205 is a cell surface receptor for CpG oligonucleotides. Proc. Natl. Acad. Sci. USA 2012, 109, 16270–16275. [Google Scholar] [CrossRef] [PubMed]
- Ruth, A.-J.; Kitching, A.R.; Kwan, R.Y.; Odobasic, D.; Ooi, J.D.; Timoshanko, J.R.; Hickey, M.J.; Holdsworth, S.R. Anti-Neutrophil Cytoplasmic Antibodies and Effector CD4+ Cells Play Nonredundant Roles in Anti-Myeloperoxidase Crescentic Glomerulonephritis. J. Am. Soc. Nephrol. 2006, 17, 1940–1949. [Google Scholar] [CrossRef] [PubMed]
- Lúdvíksson, B.R.; Sneller, M.C.; Chua, K.S.; Talar-Williams, C.; Langford, C.A.; Ehrhardt, R.O.; Fauci, A.S.; Strober, W. Active Wegener’s granulomatosis is associated with HLA-DR+ CD4+ T cells exhibiting an unbalanced Th1-type T cell cytokine pattern: Reversal with IL-10. J. Immunol. 1998, 160, 3602–3609. [Google Scholar] [CrossRef]
- Holdsworth, S.R.; Kitching, A.R.; Tipping, P.G. Th1 and Th2T helper cell subsets affect patterns of injury and outcomes in glomerulonephritis. Kidney Int. 1999, 55, 1198–1216. [Google Scholar] [CrossRef]
- Nogueira, E.; Hamour, S.; Sawant, D.; Henderson, S.; Mansfield, N.; Chavele, K.-M.; Pusey, C.D.; Salama, A.D. Serum IL-17 and IL-23 levels and autoantigen-specific Th17 cells are elevated in patients with ANCA-associated vasculitis. Nephrol. Dial. Transplant. 2010, 25, 2209–2217. [Google Scholar] [CrossRef] [PubMed]
- Abdulahad, W.H.; Stegeman, C.A.; Limburg, P.C.; Kallenberg, C.G.M. Skewed distribution of Th17 lymphocytes in patients with Wegener’s granulomatosis in remission. Arthritis Rheum. 2008, 58, 2196–2205. [Google Scholar] [CrossRef]
- Pinching, A.J.; Rees, A.J.; Pussell, B.A.; Lockwood, C.M.; Mitchison, R.S.; Peters, D.K. Relapses in Wegener’s granulomatosis: The role of infection. BMJ 1980, 281, 836–838. [Google Scholar] [CrossRef] [PubMed]
- Capizzi, S.A.; Specks, U. Does infection play a role in the pathogenesis of pulmonary vasculitis? Semin. Respir. Infect. 2003, 18, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Arimura, Y.; Minoshima, S.; Kamiya, Y.; Tanaka, U.; Nakabayashi, K.; Kitamoto, K.; Nagasawa, T.; Sasaki, T.; Suzuki, K. Serum myeloperoxidase and serum cytokines in anti-myeloperoxidase antibody-associated glomerulonephritis. Clin. Nephrol. 1993, 40, 256–264. [Google Scholar] [PubMed]
- Stegeman, C.A.; Tervaert, J.W.C.; de Jong, P.E.; Kallenberg, C.G. Trimethoprim–Sulfamethoxazole (Co-Trimoxazole) for the Prevention of Relapses of Wegener’s Granulomatosis. Dutch Co-Trimoxazole Wegener Study Group. N. Eng. J. Med. 1996, 335, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Prinz, M.; Garbe, F.; Schmidt, H.; Mildner, A.; Gutcher, I.; Wolter, K.; Piesche, M.; Schroers, R.; Weiss, E.; Kirschning, C.J.; et al. Innate immunity mediated by TLR9 modulates pathogenicity in an animal model of multiple sclerosis. J. Clin. Investig. 2006, 116, 456–464. [Google Scholar] [CrossRef]
- Husmann, C.A.; Holle, J.U.; Moosig, F.; Mueller, S.; Wilde, B.; Tervaert, J.W.C.; Harper, L.; Assmann, G.; Gross, W.L.; Epplen, J.T.; et al. Genetics of toll like receptor 9 in ANCA associated vasculitides. Ann. Rheum. Dis. 2013, 73, 890–896. [Google Scholar] [CrossRef]
- Grewal, I.S.; Flavell, R.A. CD40 and CD154 in cell-mediated immunity. Annu. Rev. Immunol. 1998, 16, 111–135. [Google Scholar] [CrossRef]
- Peters, A.L.; Stunz, L.L.; Bishop, G.A. CD40 and autoimmunity: The dark side of a great activator. Semin. Immunol. 2009, 21, 293–300. [Google Scholar] [CrossRef]
- Tomasson, G.; Lavalley, M.; Tanriverdi, K.; Finkielman, J.D.; Davis, J.C.; Hoffman, G.S.; McCUNE, W.J.; Clair, E.W.S.; Specks, U.; Spiera, R.; et al. Relationship Between Markers of Platelet Activation and Inflammation with Disease Activity in Wegener’s Granulomatosis. J. Rheumatol. 2011, 38, 1048–1054. [Google Scholar] [CrossRef]
- Gan, P.-Y.; Chan, A.; Ooi, J.D.; Dick, J.; Nagai, K.; O’Sullivan, K.M.; Oudin, V.; Shim, R.; Kitching, A.R.; Holdsworth, S.R. Biologicals targeting T helper cell subset differentiating cytokines are effective in the treatment of murine anti-myeloperoxidase glomerulonephritis. Kidney Int. 2019, 96, 1121–1133. [Google Scholar] [CrossRef]
- Sulczewski, F.B.; Martino, L.A.; Almeida, B.D.S.; Zaneti, A.B.; Ferreira, N.S.; Amorim, K.N.D.S.; Yamamoto, M.M.; Apostolico, J.D.S.; Rosa, D.S.; Boscardin, S.B. Conventional type 1 dendritic cells induce T H 1, T H 1-like follicular helper T cells and regulatory T cells after antigen boost via DEC205 receptor. Eur. J. Immunol. 2020, 50, 1895–1911. [Google Scholar] [CrossRef] [PubMed]
- Apostolopoulos, J.; Ooi, J.D.; Odobasic, D.; Holdsworth, S.R.; Kitching, A.R. The isolation and purification of biologically active recombinant and native autoantigens for the study of autoimmune disease. J. Immunol. Methods 2006, 308, 167–178. [Google Scholar] [CrossRef]
- Kitching, A.R.; Holdsworth, S.R.; Tipping, P.G. IFN-gamma mediates crescent formation and cell-mediated immune injury in murine glomerulonephritis. J. Am. Soc. Nephrol. 1999, 10, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Lutz, M.B.; Kukutsch, N.; Ogilvie, A.L.; Rößner, S.; Koch, F.; Romani, N.; Schuler, G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods 1999, 223, 77–92. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ford, S.L.; O’Sullivan, K.M.; Kitching, A.R.; Holdsworth, S.R.; Gan, P.Y.; Summers, S.A. Toll-like Receptor 9 Induced Dendritic Cell Activation Promotes Anti-Myeloperoxidase Autoimmunity and Glomerulonephritis. Int. J. Mol. Sci. 2023, 24, 1339. https://doi.org/10.3390/ijms24021339
Ford SL, O’Sullivan KM, Kitching AR, Holdsworth SR, Gan PY, Summers SA. Toll-like Receptor 9 Induced Dendritic Cell Activation Promotes Anti-Myeloperoxidase Autoimmunity and Glomerulonephritis. International Journal of Molecular Sciences. 2023; 24(2):1339. https://doi.org/10.3390/ijms24021339
Chicago/Turabian StyleFord, Sharon L., Kim M. O’Sullivan, A. Richard Kitching, Stephen R. Holdsworth, Poh Yi Gan, and Shaun A. Summers. 2023. "Toll-like Receptor 9 Induced Dendritic Cell Activation Promotes Anti-Myeloperoxidase Autoimmunity and Glomerulonephritis" International Journal of Molecular Sciences 24, no. 2: 1339. https://doi.org/10.3390/ijms24021339
APA StyleFord, S. L., O’Sullivan, K. M., Kitching, A. R., Holdsworth, S. R., Gan, P. Y., & Summers, S. A. (2023). Toll-like Receptor 9 Induced Dendritic Cell Activation Promotes Anti-Myeloperoxidase Autoimmunity and Glomerulonephritis. International Journal of Molecular Sciences, 24(2), 1339. https://doi.org/10.3390/ijms24021339






