Serum Biomarkers of Renal Fibrosis: A Systematic Review
Abstract
1. Introduction
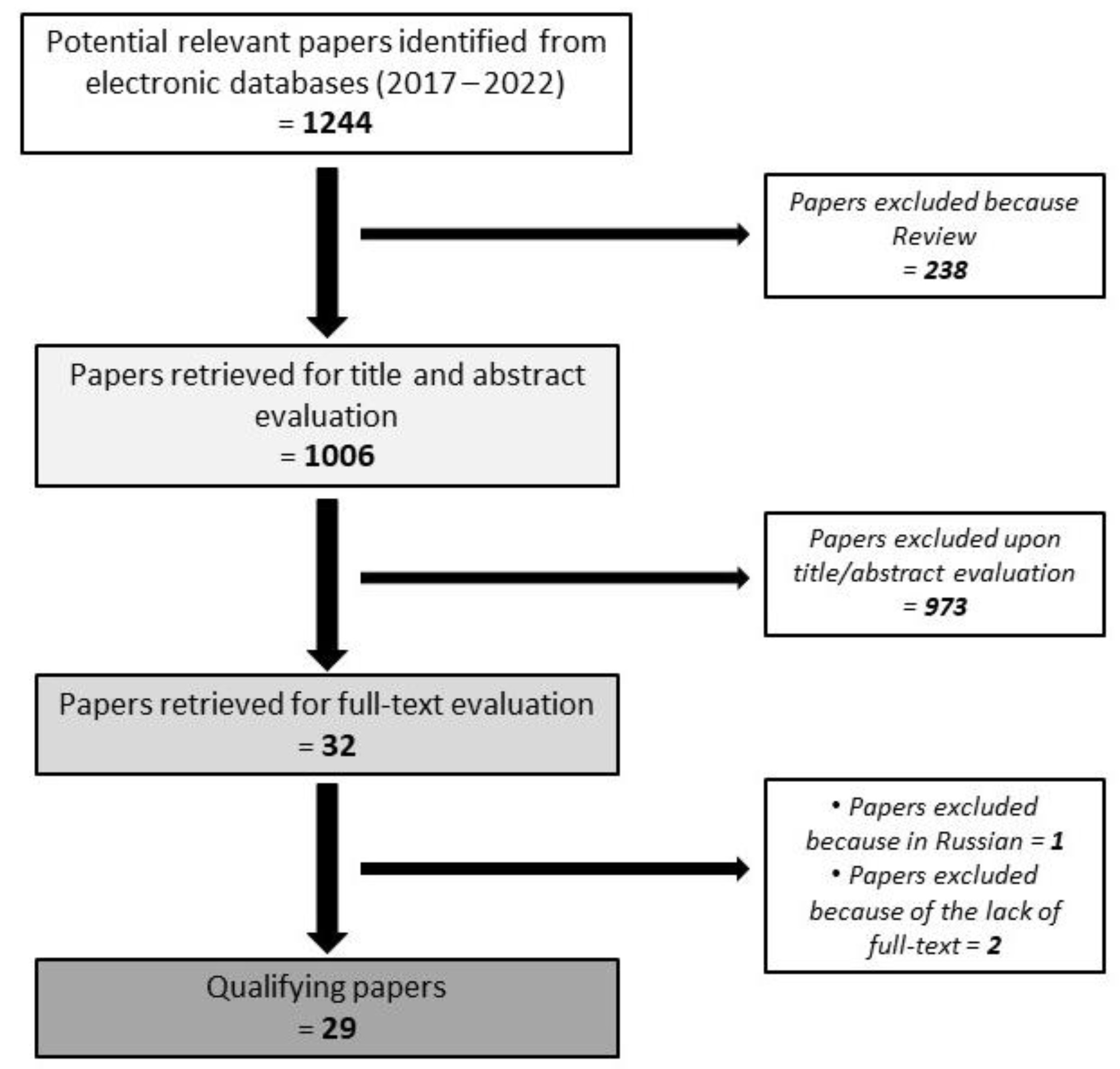
2. Methods
- inclusion of at least 25 patients;
- publication in the last five years;
- presentation of clinical related data (not just in vitro analysis).
3. Results and Discussion
3.1. MCP-1 (Monocyte Chemoattractant Protein-1) and KIM-1 (Kidney Injury Molecule-1)
3.2. MMP-7 (Matrix Metalloproteinase 7)
3.3. Pro-C3 (Pro-Peptide of Type III Collagen)
3.4. Pro-C6 (Pro-Peptide of Type VI Collagen)
3.5. TNFR-1 (Tumor Necrosis Factor Receptor 1) and TNFR-2 (Tumor Necrosis Factor Receptor 2)
3.6. Serum Biomakers and Their Relationship with Histologic Findings at Kidney Biopsy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jha, V.; Garcia-Garcia, G.; Iseki, K.; Li, Z.; Naicker, S.; Plattner, B.; Saran, R.; Wang, A.Y.-M.; Yang, C.-W. Chronic kidney disease: Global dimension and perspectives. Lancet 2013, 382, 260–272. [Google Scholar] [CrossRef]
- Levey, A.S.; Atkins, R.; Coresh, J.; Cohen, E.P.; Collins, A.J.; Eckardt, K.U.; Nahas, M.E.; Jaber, B.L.; Jadoul, M.; Levin, A.; et al. Chronic kidney disease as a global public health problem: Approaches and initiatives—A position statement from Kidney Disease Improving Global Outcomes. Kidney Int. 2007, 72, 247–259. [Google Scholar] [CrossRef]
- Charles, C.; Ferris, A.H. Chronic Kidney Disease. Prim. Care 2020, 47, 585–595. [Google Scholar] [CrossRef]
- Wang, Y.N.; Ma, S.X.; Chen, Y.Y.; Chen, L.; Liu, B.L.; Liu, Q.Q.; Zhao, Y.Y. Chronic kidney disease: Biomarker diagnosis to therapeutic targets. Clin. Chim. Acta 2019, 499, 54–63. [Google Scholar] [CrossRef]
- Wang, V.; Vilme, H.; Maciejewski, M.L.; Boulware, L.E. The Economic Burden of Chronic Kidney Disease and End-Stage Renal Disease. Semin Nephrol. 2016, 36, 319–330. [Google Scholar] [CrossRef]
- Lv, J.C.; Zhang, L.X. Prevalence and Disease Burden of Chronic Kidney Disease. Adv. Exp. Med. Biol. 2019, 1165, 3–15. [Google Scholar]
- Bikbov, B.; Purcell, C.A.; Levey, A.S.; Smith, M.; Abdoli, A.; Abebe, M.; Adebayo, O.M.; Afarideh, M.; Agarwal, S.K.; Agudelo-Botero, M.; et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef]
- Couser, W.G.; Remuzzi, G.; Mendis, S.; Tonelli, M. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 2011, 80, 1258–1270. [Google Scholar] [CrossRef]
- Liu, M.; Li, X.-C.; Lu, L.; Cao, Y.; Sun, R.-R.; Chen, S.; Zhang, P.-Y. Cardiovascular disease and its relationship with chronic kidney disease. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 2918–2926. [Google Scholar]
- Jankowski, J.; Floege, J.; Fliser, D.; Böhm, M.; Marx, N. Cardiovascular Disease in Chronic Kidney Disease: Pathophysiological Insights and Therapeutic Options. Circulation 2021, 143, 1157–1172. [Google Scholar] [CrossRef]
- Panizo, S.; Martínez-Arias, L.; Alonso-Montes, C.; Cannata, P.; Martín-Carro, B.; Fernández-Martín, J.; Naves-Díaz, M.; Carrillo-López, N.; Cannata-Andía, J. Fibrosis in Chronic Kidney Disease: Pathogenesis and Consequences. Int. J. Mol. Sci. 2021, 22, 408. [Google Scholar] [CrossRef]
- Nogueira, A.; Pires, M.J.; Oliveira, P.A. Pathophysiological Mechanisms of Renal Fibrosis: A Review of Animal Models and Therapeutic Strategies. In Vivo 2017, 31, 1. [Google Scholar] [CrossRef]
- Henderson, N.C.; Rieder, F.; Wynn, T.A. Fibrosis: From mechanisms to medicines. Nature 2020, 587, 555–566. [Google Scholar] [CrossRef]
- Weiskirchen, R.; Weiskirchen, S.; Tacke, F. Organ and tissue fibrosis: Molecular signals, cellular mechanisms and translational implications. Mol. Asp. Med. 2019, 65, 2–15. [Google Scholar] [CrossRef]
- Djudjaj, S.; Boor, P. Cellular and molecular mechanisms of kidney fibrosis. Mol. Asp. Med. 2019, 65, 16–36. [Google Scholar] [CrossRef]
- Gu, Y.Y.; Liu, X.S.; Huang, X.R.; Yu, X.Q.; Lan, H.Y. TGF-β in renal fibrosis: Triumphs and challenges. Future Med. Chem. 2020, 12, 853–866. [Google Scholar] [CrossRef]
- Black, L.M.; Lever, J.M.; Agarwal, A. Renal Inflammation and Fibrosis: A Double-edged Sword. J. Histochem. Cytochem. 2019, 67, 663–681. [Google Scholar] [CrossRef]
- Wong, M.G.; Pollock, C.A. Biomarkers in kidney fibrosis: Are they useful? Kidney Int. Suppl. 2014, 4, 79–83. [Google Scholar] [CrossRef]
- Ix, J.H.; Shlipak, M.G. The Promise of Tubule Biomarkers in Kidney Disease: A Review. Am. J. Kidney Dis. 2021, 78, 719–727. [Google Scholar] [CrossRef]
- Bagnasco, S.M.; Rosenberg, A.Z. Biomarkers of Chronic Renal Tubulointerstitial Injury. J. Histochem. Cytochem. 2019, 67, 633–641. [Google Scholar] [CrossRef]
- Restrepo-Escobar, M.; Granda-Carvajal, P.A.; Jaimes, F. Systematic review of the literature on reproducibility of the interpretation of renal biopsy in lupus nephritis. Lupus 2017, 26, 1502–1512. [Google Scholar] [CrossRef]
- Poggio, E.D.; McClelland, R.L.; Blank, K.N.; Hansen, S.; Bansal, S.; Bomback, A.S.; Canetta, P.A.; Khairallah, P.; Kiryluk, K.; Lecker, S.H.; et al. Systematic Review and Meta-Analysis of Native Kidney Biopsy Complications. Clin. J. Am. Soc. Nephrol. 2020, 15, 1595–1602. [Google Scholar] [CrossRef]
- Zhang, J.; Ren, P.; Wang, Y.; Feng, S.; Wang, C.; Shen, X.; Weng, C.; Lang, X.; Chen, Z.; Jiang, H.; et al. Serum Matrix Metalloproteinase-7 Level is Associated with Fibrosis and Renal Survival in Patients with IgA Nephropathy. Kidney Blood Press Res. 2017, 42, 541–552. [Google Scholar] [CrossRef]
- Stribos, E.G.D.; Nielsen, S.H.; Brix, S.; Karsdal, M.A.; Seelen, M.A.; Van Goor, H.; Bakker, S.J.L.; Olinga, P.; Mutsaers, H.A.M.; Genovese, F. Non-invasive quantification of collagen turnover in renal transplant recipients. PLoS ONE 2017, 12, e0175898. [Google Scholar] [CrossRef]
- Akin, D.; Ozmen, S.; Yilmaz, M.E. Hyaluronic Acid as a New Biomarker to Differentiate Acute Kidney Injury from Chronic Kidney Disease. Iran. J. Kidney Dis. 2017, 11, 409–413. [Google Scholar]
- Chen, R.; Wang, L.; Liu, S.; Chen, X.; Hu, Y.; Liu, H.; Zhang, H.; Jiang, Y.; Wang, Q.; Ye, D.; et al. Bcl-3 is a novel biomarker of renal fibrosis in chronic kidney disease. Oncotarget 2017, 8, 97206–97216. [Google Scholar] [CrossRef]
- Cho, N.J.; Han, D.J.; Lee, J.H.; Jang, S.H.; Kang, J.S.; Gil, H.W.; Park, S.; Lee, E.Y. Soluble klotho as a marker of renal fibrosis and podocyte injuries in human kidneys. PLoS ONE 2018, 13, e0194617. [Google Scholar] [CrossRef]
- Luo, J.; Wang, F.; Wan, J.; Ye, Z.; Huang, C.; Cai, Y.; Liu, M.; Wu, B.Q.; Li, L. Serum human epididymis secretory protein 4 as a potential biomarker of renal fibrosis in kidney transplantation recipients. Clin. Chim. Acta 2018, 483, 216–221. [Google Scholar] [CrossRef]
- Holm Nielsen, S.; Guldager Kring Rasmussen, D.; Brix, S.; Fenton, A.; Jesky, M.; Ferro, C.J.; Karsdal, M.; Genovese, F.; Cockwell, P. A novel biomarker of laminin turnover is associated with disease progression and mortality in chronic kidney disease. PLoS ONE 2018, 13, e0204239. [Google Scholar] [CrossRef]
- Yang, X.; Bai, M.; Ning, X.; Ma, F.; Liu, L.; Liu, T.; Liu, M.; Wang, H.; Sun, S. The associations of Bmi-1 with progression of glomerular chronic kidney disease. Clin. Nephrol. 2018, 89, 93–103. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Z.; Heng, Y.; Miao, C. MicroRNA-181 exerts an inhibitory role during renal fibrosis by targeting early growth response factor-1 and attenuating the expression of profibrotic markers. Mol. Med. Rep. 2019, 19, 3305–3313. [Google Scholar] [CrossRef]
- Ren, H.; Shao, Y.; Ma, X.; Yang, M.; Liu, Y.; Wang, Q. Expression levels of serum vasohibin-1 and other biomarkers in type 2 diabetes mellitus patients with different urinary albumin to creatinine ratios. J. Diabetes Complicat. 2019, 33, 477–484. [Google Scholar] [CrossRef]
- Özkan, G.; Güzel, S.; Atar, R.V.; Fidan, Ç.; Kara, S.P.; Ulusoy, Ş. Elevated serum levels of procollagen C-proteinase enhancer-1 in patients with chronic kidney disease is associated with a declining glomerular filtration rate. Nephrology 2019, 24, 938–942. [Google Scholar] [CrossRef]
- Basturk, T.; Ojalvo, D.; Mazi, E.E.; Hasbal, N.B.; Ozagari, A.A.; Ahbap, E.; Sakaci, T.; Koc, Y.; Sevinc, M.; Unsal, A. Pentraxin-2 is Associated with Renal Fibrosis in Patients Undergoing Renal Biopsy. Clinics 2020, 75, 1–5. [Google Scholar] [CrossRef]
- Bieniaś, B.; Sikora, P. Selected Metal Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinases as Potential Biomarkers for Tubulointerstitial Fibrosis in Children with Unilateral Hydronephrosis. Dis. Markers 2020, 2020, 9520309. [Google Scholar] [CrossRef]
- Ihara, K.; Skupien, J.; Kobayashi, H.; Md Dom, Z.I.; Wilson, J.M.; O’neil, K.; Badger, H.S.; Bowsman, L.M.; Satake, E.; Breyer, M.D.; et al. Profibrotic Circulating Proteins and Risk of Early Progressive Renal Decline in Patients With Type 2 Diabetes with and Without Albuminuria. Diabetes Care 2020, 43, 2760–2767. [Google Scholar] [CrossRef]
- Zhang, X.-Q.; Li, X.; Zhou, W.-Q.; Liu, X.; Huang, J.-L.; Zhang, Y.-Y.; Lindholm, B.; Yu, C. Serum Lysyl Oxidase Is a Potential Diagnostic Biomarker for Kidney Fibrosis. Am. J. Nephrol. 2020, 51, 907–918. [Google Scholar] [CrossRef]
- Musiał, K.; Zwolińska, D. Monocyte chemoattractant protein-1, macrophage colony stimulating factor, survivin, and tissue inhibitor of matrix metalloproteinases-2 in analysis of damage and repair related to pediatric chronic kidney injury. Adv. Clin. Exp. Med. 2020, 29, 1083–1090. [Google Scholar] [CrossRef]
- Schrauben, S.J.; Shou, H.; Zhang, X.; Anderson, A.H.; Bonventre, J.V.; Chen, J.; Coca, S.; Furth, S.L.; Greenberg, J.H.; Gutierrez, O.M.; et al. Association of Multiple Plasma Biomarker Concentrations with Progression of Prevalent Diabetic Kidney Disease: Findings from the Chronic Renal Insufficiency Cohort (CRIC) Study. J. Am. Soc. Nephrol. 2021, 32, 115–126. [Google Scholar] [CrossRef]
- Genovese, F.; Rasmussen, D.G.K.; Karsdal, M.A.; Jesky, M.; Fenton, A.; Cockwell, P. Imbalanced turnover of collagen type III is associated with disease progression and mortality in high-risk chronic kidney disease patients. Clin. Kidney J. 2020, 14, 593–601. [Google Scholar] [CrossRef]
- Xie, C.; Yi-Ying, Y.; Miao, C. Correlation of serum uromodulin levels with renal fibrosis and renal function progression in patients with CKD. Pak. J. Pharm. Sci. 2021, 34, 2417–2422. [Google Scholar]
- Schmidt, I.M.; Colona, M.R.; Kestenbaum, B.R.; Alexopoulos, L.G.; Palsson, R.; Srivastava, A.; Liu, J.; Stillman, I.E.; Rennke, H.G.; Vaidya, V.S.; et al. Cadherin-11, Sparc-related modular calcium binding protein-2, and Pigment epithelium-derived factor are promising non-invasive biomarkers of kidney fibrosis. Kidney Int. 2021, 100, 672–683. [Google Scholar] [CrossRef]
- Sun, D.; Xie, N.; Wang, X.; Wu, W.; Li, X.; Chen, X.; Qian, G.; Li, C.; Zhang, H.; Jiang, Y.; et al. Serum RelB is correlated with renal fibrosis and predicts chronic kidney disease progression. Clin. Transl. Med. 2021, 11, e362. [Google Scholar] [CrossRef]
- Sparding, N.; Genovese, F.; Rasmussen, D.G.K.; Karsdal, M.A.; Neprasova, M.; Maixnerova, D.; Satrapova, V.; Frausova, D.; Hornum, M.; Bartonova, L.; et al. Endotrophin, a collagen type VI-derived matrikine, reflects the degree of renal fibrosis in patients with IgA nephropathy and in patients with ANCA-associated vasculitis. Nephrol. Dial. Transplant. 2022, 37, 1099–1108. [Google Scholar] [CrossRef]
- Gutiérrez, O.M.; Shlipak, M.G.; Katz, R.; Waikar, S.S.; Greenberg, J.H.; Schrauben, S.J.; Coca, S.; Parikh, C.R.; Vasan, R.S.; Feldman, H.I.; et al. Associations of Plasma Biomarkers of Inflammation, Fibrosis, and Kidney Tubular Injury With Progression of Diabetic Kidney Disease: A Cohort Study. Am. J. Kidney Dis. 2022, 79, 849–857.e1. [Google Scholar] [CrossRef]
- Liu, H.; Sridhar, V.S.; Lovblom, L.E.; Lytvyn, Y.; Burger, D.; Burns, K.; Brinc, D.; Lawler, P.R.; Cherney, D.Z. Markers of Kidney Injury, Inflammation, and Fibrosis Associated With Ertugliflozin in Patients With CKD and Diabetes. Kidney Int Rep. 2021, 6, 2095–2104. [Google Scholar] [CrossRef]
- Genovese, F.; Akhgar, A.; Lim, S.S.; Farris, A.B.; Battle, M.; Cobb, J.; Sinibaldi, D.; Karsdal, M.; White, W.I. Collagen Type III and VI Remodeling Biomarkers Are Associated with Kidney Fibrosis in Lupus Nephritis. Kidney360 2021, 2, 1473–1481. [Google Scholar] [CrossRef]
- Naicker, S.; Dix-Peek, T.; Klar, R.M.; Kalunga, G.; Mosiane, P.; Dickens, C.; Duarte, R. Profiling Biomarkers in HIV Glomerular Disease—Potential for the Non-Invasive Diagnosis of HIVAN? Int. J. Nephrol. Renovasc. Dis. 2021, 14, 427–440. [Google Scholar] [CrossRef]
- Enoksen, I.T.; Svistounov, D.; Norvik, J.V.; Stefansson, V.T.; Solbu, M.D.; Eriksen, B.O.; Melsom, T. Serum Matrix Metalloproteinase 7 and accelerated GFR decline in a general non-diabetic population. Nephrol. Dial. Transplant. 2021, 37, 1657–1667. [Google Scholar] [CrossRef]
- Chan, J.; Svensson, M.; Tannæs, T.M.; Waldum-Grevbo, B.; Jenssen, T.; Eide, I.A. Associations of Serum Uromodulin and Urinary Epidermal Growth Factor with Measured Glomerular Filtration Rate and Interstitial Fibrosis in Kidney Transplantation. Am. J. Nephrol. 2022, 53, 108–117. [Google Scholar] [CrossRef]
- Sciascia, S.; Barinotti, A.; Radin, M.; Cecchi, I.; Menegatti, E.; Terzolo, E.; Rossi, D.; Baldovino, S.; Fenoglio, R.; Roccatello, D. Dickkopf Homolog 3 (DKK3) as a Prognostic Marker in Lupus Nephritis: A Prospective Monocentric Experience. J. Clin. Med. 2022, 11, 2977. [Google Scholar] [CrossRef]
- Singh, S.; Anshita, D.; Ravichandiran, V. MCP-1: Function, regulation, and involvement in disease. Int. Immunopharmacol. 2021, 101 Pt B, 107598. [Google Scholar] [CrossRef]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1 (MCP-1): An overview. J Interferon Cytokine Res. 2009, 29, 313–325. [Google Scholar] [CrossRef]
- Karmakova, T.A.; Sergeeva, N.S.; Kanukoev, K.Y.; Alekseev, B.Y.; Kaprin, A.D. Kidney Injury Molecule 1 (KIM-1): A Multifunctional Glycoprotein and Biological Marker (Review). Sovrem Tekhnologii V Meditsine 2021, 13, 64–80. [Google Scholar] [CrossRef]
- Han, W.K.; Bailly, V.; Abichandani, R.; Thadhani, R.; Bonventre, J.V. Kidney Injury Molecule-1 (KIM-1): A novel biomarker for human renal proximal tubule injury. Kidney Int. 2002, 62, 237–244. [Google Scholar] [CrossRef]
- Siddiqui, K.; Joy, S.S.; Al-Rubeaan, K. Association of urinary monocyte chemoattractant protein-1 (MCP-1) and kidney injury molecule-1 (KIM-1) with risk factors of diabetic kidney disease in type 2 diabetes patients. Int. Urol. Nephrol. 2019, 51, 1379–1386. [Google Scholar] [CrossRef]
- Hu, Q.; Lan, J.; Liang, W.; Chen, Y.; Chen, B.; Liu, Z.; Xiong, Y.; Zhong, Z.; Wang, Y.; Ye, Q. MMP7 damages the integrity of the renal tubule epithelium by activating MMP2/9 during ischemia–reperfusion injury. J. Mol. Histol. 2020, 51, 685–700. [Google Scholar] [CrossRef]
- Stene, C.; Polistena, A.; Gaber, A.; Nodin, B.; Ottochian, B.; Adawi, D.; Avenia, N.; Jirström, K.; Johnson, L.B. MMP7 Modulation by Short- and Long-term Radiotherapy in Patients with Rectal Cancer. In Vivo 2018, 32, 133–138. [Google Scholar]
- Zhou, D.; Tian, Y.; Sun, L.; Zhou, L.; Xiao, L.; Tan, R.J.; Tian, J.; Fu, H.; Hou, F.F.; Liu, Y. Matrix Metalloproteinase-7 Is a Urinary Biomarker and Pathogenic Mediator of Kidney Fibrosis. J. Am. Soc. Nephrol. 2017, 28, 598–611. [Google Scholar] [CrossRef]
- Ke, B.; Fan, C.; Yang, L.; Fang, X. Matrix Metalloproteinases-7 and Kidney Fibrosis. Front. Physiol. 2017, 8, 21. [Google Scholar] [CrossRef]
- Nielsen, M.J.; Nedergaard, A.F.; Sun, S.; Veidal, S.S.; Larsen, L.; Zheng, Q.; Suetta, C.; Henriksen, K.; Christiansen, C.; Karsdal, M.A.; et al. The neo-epitope specific PRO-C3 ELISA measures true formation of type III collagen associated with liver and muscle parameters. Am. J. Transl. Res. 2013, 5, 303–315. [Google Scholar]
- Cescon, M.; Gattazzo, F.; Chen, P.; Bonaldo, P. Collagen VI at a glance. J. Cell Sci. 2015, 128, 3525–3531. [Google Scholar] [CrossRef]
- Fenton, A.; Jesky, M.D.; Ferro, C.J.; Sørensen, J.; Karsdal, M.A.; Cockwell, P.; Genovese, F. Serum endotrophin, a type VI collagen cleavage product, is associated with increased mortality in chronic kidney disease. PLoS ONE 2017, 12, e0175200. [Google Scholar] [CrossRef]
- Al-Lamki, R.S.; Mayadas, T.N. TNF receptors: Signaling pathways and contribution to renal dysfunction. Kidney Int. 2015, 87, 281–296. [Google Scholar] [CrossRef]
- Meldrum, K.K.; Misseri, R.; Metcalfe, P.; Dinarello, C.A.; Hile, K.L.; Meldrum, D.R. TNF-alpha neutralization ameliorates obstruction-induced renal fibrosis and dysfunction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R1456–R1464. [Google Scholar] [CrossRef]
- Taguchi, S.; Azushima, K.; Yamaji, T.; Urate, S.; Suzuki, T.; Abe, E.; Tanaka, S.; Tsukamoto, S.; Kamimura, D.; Kinguchi, S.; et al. Effects of tumor necrosis factor-α inhibition on kidney fibrosis and inflammation in a mouse model of aristolochic acid nephropathy. Sci. Rep. 2021, 11, 23587. [Google Scholar] [CrossRef]
- Gupta, J.; Mitra, N.; Kanetsky, P.A.; Devaney, J.; Wing, M.R.; Reilly, M.; Shah, V.O.; Balakrishnan, V.S.; Guzman, N.J.; Girndt, M.; et al. Association between albuminuria, kidney function, and inflammatory biomarker profile in CKD in CRIC. Clin. J. Am. Soc. Nephrol. 2012, 7, 1938–1946. [Google Scholar] [CrossRef]
- A Working Group of the International IgA Nephropathy Network and the Renal Pathology Society; Cattran, D.C.; Coppo, R.; Cook, H.T.; Feehally, J.; Roberts, I.S.; Troyanov, S.; Alpers, C.E.; Amore, A.; Barratt, J.; et al. The Oxford classification of IgA nephropathy: Rationale, clinicopathological correlations, and classification. Kidney Int. 2009, 76, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Loupy, A.; Mengel, M.; Haas, M. Thirty years of the International Banff Classification for Allograft Pathology: The past, present, and future of kidney transplant diagnostics. Kidney Int. 2022, 101, 678–691. [Google Scholar] [CrossRef] [PubMed]
| Ref | Authors | Year | Design | N Patients | Patients Population | Controls | Tested Biomarkers | Biomarkers Statistically Associated with Fibrosis |
|---|---|---|---|---|---|---|---|---|
| [23] | Zhang et al. | 2017 | R | 244 | IgA nephropathy | 40 HC | MMP-7 | MMP-7 |
| [24] | Stribos et al. | 2017 | R | 78 | Renal transplant recipients | NA | C3M, Pro-C3, C4M, C5M, Pro-C6, C6M | C3M, Pro-C6 |
| [25] | Akin et al. | 2017 | PR | 81 | AKI (44), CKD (37) | NA | HA | HA |
| [26] | Chen et al. | 2017 | R | 31 | CKD | 25 HC | Bcl-3 | Bcl-3 |
| [27] | Cho et al. | 2018 | R | 67 | IgA nephropathy (26), FSGS (12), MCD (7), MN (3), TBMD (3), MPGN (2), post-infectious glomerulonephritis (1), LN (1), GN (1), ATN (1), amyloidosis (1), non-specific findings (9) | NA | Klotho | Klotho |
| [28] | Luo et al. | 2018 | R | 103 | Renal transplant recipients | 127 HC | HE4 | HE4 |
| [29] | Nielsen et al. | 2018 | PR | 492 | CKD | NA | LAMC1 | LAMC1 |
| [30] | Yiang et al. | 2018 | post hoc | 230 | CKD | 67 HC | Bmi-1 | Bmi-1 |
| [31] | Zhang et al. | 2019 | R | 58 | Biopsy-proven renal fibrosis | 10 HC | miR-181 | miR-181 |
| [32] | Ren et al. | 2019 | R | 697 | DN | 150 HC | VASH-1, SIRT1, HIF1α, VEGF, CRP, TNF-α, TGF-β1 | VASH-1 |
| [33] | Ozkan et al. | 2019 | R | 131 | CKD | 34 HC | PCPE-1 | PCPE-1 |
| [34] | Basturk et al. | 2020 | R | 45 | CKD | 16 HC | PTX-2 | Pentraxin-2 (PTX-2) |
| [35] | Bieniaś et al. | 2020 | R | 45 | Unilateral hydronephrosis (children) | 21 HC | MMP-1, MMP-2, MMP-9, TIMP-1 and TIMP-2 | MMP-1, MMP-2, MMP-9, TIMP-1 and TIMP-2 |
| [36] | Ihara et al. | 2020 | PR | 1181 | Type II diabetes | NA | WFDC2, MMP-7 | WFDC2 and MMP-7 |
| [37] | Zhang et al. | 2020 | R | 202 | IgA nephropathy (43), MN (42), DN (28), hypertensive nephrosclerosis (21), MCD (16), ANCA-associated nephritis (12), minor histopathology abnormality (11), LN (8), FSGS (5), renal amyloidosis (5), cast nephropathy (5), ORG (2), TMA (2), ATN (1), uric acid nephropathy (1) | 30 HC | LOX | LOX |
| [38] | Musiał et al. | 2020 | R | 70 | Children with CKD: obstructive uropathy (23), hypo-/dysplastic kidneys (15), reflux nephropathy (14), PKD (4), other genetic disorders (5), AKI (4), and unknown factors (5) | 12 children with monosymptomatic nocturnal enuresis and normal kidney function | MCP-1, MCSF, TIMP-2, BIRC5 | MCP-1, MCSF, TIMP-2, BIRC5 |
| [39] | Schrauben et al. | 2020 | PR | 894 | DN | NA | KIM-1, TNFR-1, TNFR-2, MCP-1, suPAR, YKL-40 | KIM-1, TNFR-1, TNFR-2, MCP-1, suPAR, YKL-40 |
| [40] | Genovese et al. | 2020 | PR | 500 | CKD | NA | Pro-C3, C3M | Pro-C3, C3M |
| [41] | Jie et al. | 2021 | R | 168 | CKD | NA | UMOD | UMOD |
| [42] | Schmidt et al. | 2021 | R | 973 | CKD | snRNA-seq dataset derived from 3 healthy kidneys | CDH11, SMOC2, PEDF, MGP, TSP-2 | CDH11, SMOC2, and PEDF |
| [43] | Sun et al. | 2021 | R | 47 | CKD | 60 HC | RelB, HE4 | RelB, HE4 |
| [44] | Sparding et al. | 2021 | R | 96 | IgA nephropathy (49), ANCA-associated vasculitis (47) | 85 IgAN (validation cohort), 10 HC | ETP (Pro-C6) | ETP (Pro-C6) |
| [45] | Gutiérrez et al. | 2021 | PR | 594 | Type II diabetes | NA | TNFR1, TNFR2, suPAR, MCP-1, YKL-40, KIM-1 | TNFR1, TNFR2, YKL-40 |
| [46] | Liu et al. | 2021 | post hoc | 231 | Type II diabetes and stage 3 CKD | NA | PDGF-AA, PDGF-BB, MCD, FGF2, GMCSF, INFα2, MCP-3, IL-12p70, sCD40L, IL-2, IL-6, IL-8, MIP-1α, NGAL, cystatin C | |
| [47] | Genovese et al. | 2021 | R | 40 | LN | SLE without LN (20), HC (20), biopsy-proven histologic kidney inflammation/damage without SLE (10) | Pro-C3, Pro-C6 | Pro-C6 |
| [48] | Naicker et al. | 2021 | R | 25 | HIV-positive CKD | 25 HIV-positive without CKD, 24 HC | NGAL, cystatin C, TGF-β1, TGF-β2, TGF-β3, BMP-7 | NGAL, cystatin C, TGF-β1, TGF-β2, TGF-β3, BMP-7 |
| [49] | Enoksen et al. | 2021 | PR | 1302 | NA, general population | NA | MMP-2, MMP-7, TIMP1 | MMP-7 |
| [50] | Chan et al. | 2022 | R | 132 | Renal transplant recipients | NA | UMOD | UMOD |
| [51] | Sciascia et al. | 2022 | PR | 132 | 75 SLE, 57 SLE with LN | 50 HC | DKK-3 | DKK-3 |
| Diagnosis | Patients Number (%) |
|---|---|
| Diabetic nephropathy | 3625 (40.7) |
| CKD without specifying the underlying cause | 2675 (30.1) |
| IgA nephropathy | 362 (4.1) |
| Renal transplant recipients | 313 (3.5) |
| Lupus nephritis | 106 (1.2) |
| Systemic lupus erythematosus | 75 (0.8) |
| Generically reported as biopsy-proven renal fibrosis | 58 (0.6) |
| Acute kidney injury | 50 (0.5) |
| ANCA-associated vasculitis | 47 (0.5) |
| Unilateral hydronephrosis | 45 children (0.5) |
| Membranous nephropathy | 45 (0.5) |
| HIV-positive CKD | 25 (0.3) |
| Minimal change disease | 23 (0.25) |
| Obstructive uropathy | 23 (0.25) |
| Hypertensive nephrosclerosis | 21 (0.2) |
| Focal segmental glomerulosclerosis | 17 (0.2) |
| Hypo/dysplastic kidney | 15 (0.2) |
| Reflux nephropathy | 14 (0.15) |
| ANCA-associated nephritis | 12 (0.1) |
| Minor histopathologic abnormality | 11 (0.1) |
| Renal amyloidosis | 6 (0.05) |
| Cast nephropathy | 5 (0.05) |
| Polycystic kidney disease | 4 (0.05) |
| Thin basement membrane disease | 3 (0.03) |
| Thrombotic microangiopathy | 2 (0.02) |
| Membranous proliferative glomerulonephritis | 2 (0.02) |
| Post-infectious glomerulonephritis | 1 (0.01) |
| Crescentic glomerulonephritis | 1 (0.01) |
| Uric acid nephropathy | 1 (0.01) |
| General population (prospectively followed) | 1302 (14.6) |
| Ref | Author | Year | N Patients | Biomarkers Tested | Biomarkers Statistically Associated with Fibrosis | Fibrosis Grade Assessment |
|---|---|---|---|---|---|---|
| [23] | Zhang et al. | 2017 | 244 | MMP-7 | MMP-7 | MEST-C (Oxford classification) [68] |
| [24] | Stribos et al. | 2017 | 78 | C3M, Pro-C3, C4M, C5M, Pro-C6, C6M | C3M, Pro-C6 | Not specified |
| [27] | Cho et al. | 2020 | 67 | Klotho | Klotho |
|
| [28] | Luo et al. | 2018 | 103 | HE4 | HE4 | Banff classification [69] |
| [31] | Zhang et al. | 2019 | 58 | miR-181 | miR-181 | Not specified |
| [34] | Basturk et al. | 2020 | 45 | PTX-2 | PTX-2 | Not specified |
| [37] | Zhang et al. | 2020 | 202 | LOX | LOX | Not specified |
| [42] | Schmidt et al. | 2021 | 973 | CDH11, SMOC2, PEDF, MGP, TSP2 | CDH11, SMOC2, PEDF | IFTA was graded as involvement of <10%, 11–25%, 26–50%, or >50% of total cortical volume. |
| [43] | Sun et al. | 2021 | 47 | RelB, HE4 | RelB, HE4 | Not specified |
| [44] | Sparding et al. | 2021 | 96 | ETP | ETP | MEST-C (Oxford classification) [68], Banff classification [69] |
| [47] | Genovese et al. | 2021 | 40 | Pro-C3, Pro-C6 | Pro-C6 |
|
| [48] | Naicker et al. | 2021 | 25 | NGAL, cystatin C, TGF-β1, TGF-β2, TGF-β3, BMP-7 | NGAL, cystatin C, TGF-β1, TGF-β2, TGF-β3, BMP-7 | Not specified |
| [50] | Chan et al. | 2022 | 132 | Uromodulin | Uromodulin | Areas with fibrosis were determined at 5% level for each visual field and 1% level for averaged values. There was a high degree of concordance in IF% scores between the investigators, with intra- and inter-observer variability <5% in all but three cases. |
| [51] | Sciascia et al. | 2022 | 132 | DKK-3 | DKK-3 | ISN/RPS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barinotti, A.; Radin, M.; Cecchi, I.; Foddai, S.G.; Rubini, E.; Roccatello, D.; Sciascia, S. Serum Biomarkers of Renal Fibrosis: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 14139. https://doi.org/10.3390/ijms232214139
Barinotti A, Radin M, Cecchi I, Foddai SG, Rubini E, Roccatello D, Sciascia S. Serum Biomarkers of Renal Fibrosis: A Systematic Review. International Journal of Molecular Sciences. 2022; 23(22):14139. https://doi.org/10.3390/ijms232214139
Chicago/Turabian StyleBarinotti, Alice, Massimo Radin, Irene Cecchi, Silvia Grazietta Foddai, Elena Rubini, Dario Roccatello, and Savino Sciascia. 2022. "Serum Biomarkers of Renal Fibrosis: A Systematic Review" International Journal of Molecular Sciences 23, no. 22: 14139. https://doi.org/10.3390/ijms232214139
APA StyleBarinotti, A., Radin, M., Cecchi, I., Foddai, S. G., Rubini, E., Roccatello, D., & Sciascia, S. (2022). Serum Biomarkers of Renal Fibrosis: A Systematic Review. International Journal of Molecular Sciences, 23(22), 14139. https://doi.org/10.3390/ijms232214139









