Neural Regulations in Tooth Development and Tooth–Periodontium Complex Homeostasis: A Literature Review
Abstract
:1. Introduction
2. Anatomical and Embryological Basis
3. Tooth Influences Neurophysiology during Development Process
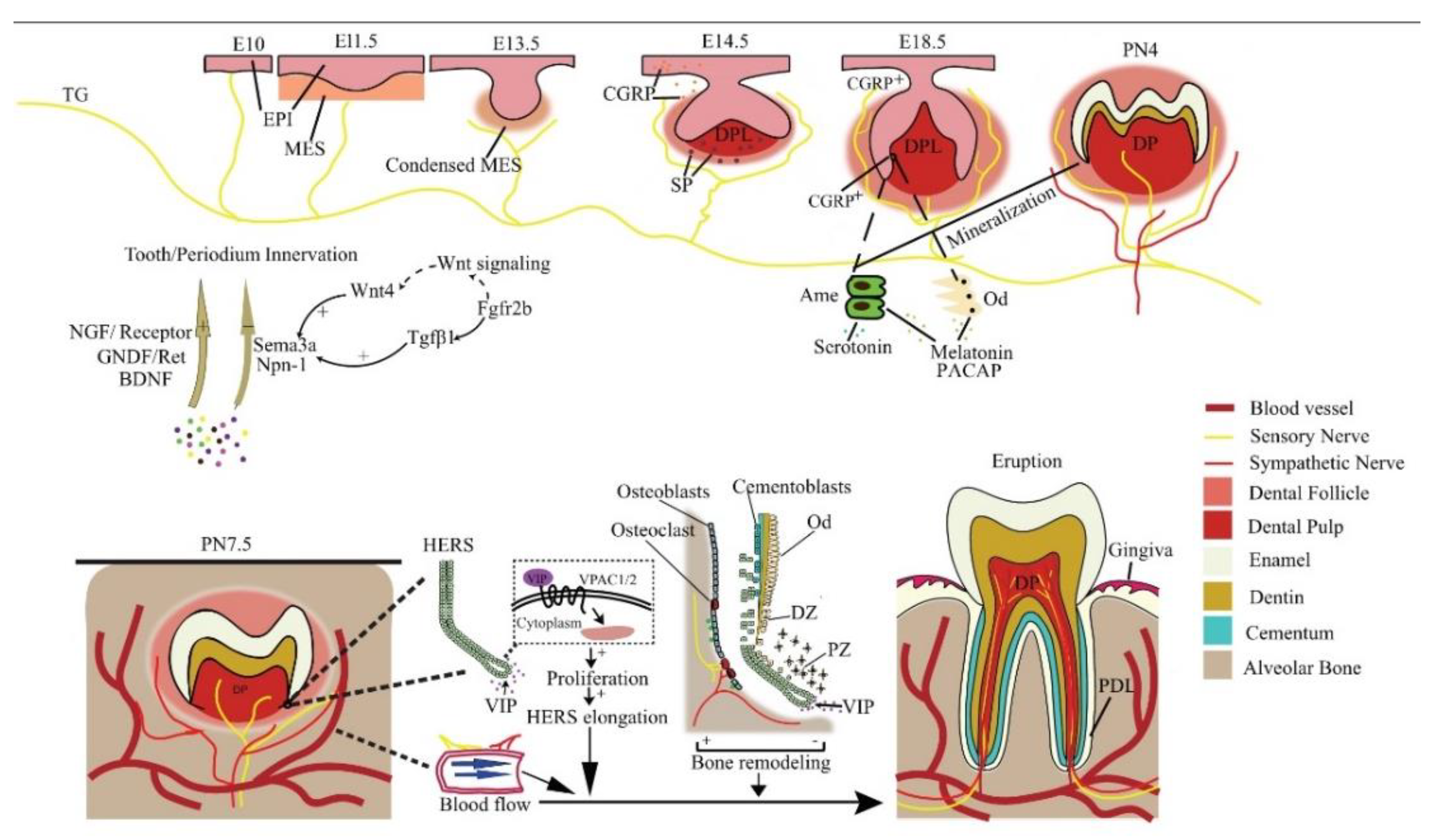
3.1. Tooth Innervation Is Spatiotemporally Regulated
3.2. The Molecular Guidance Cues for Tooth–Periodontium Innervation
3.2.1. Neurotrophins
3.2.2. Semaphorins
3.3. Related Application in Tooth Regeneration
4. The Nervous System Regulates Tooth Development
4.1. Neural Regulations in Tooth Development at Pre-Eruptive Stage
4.2. Neural Regulations in Tooth Eruption
4.3. Related Application in Tooth Regeneration
5. Dental Disease Causes Neurophysiological Changes
5.1. Morphological Changes in Local Dental Nerves
5.2. Molecular Changes in Neural Cells
5.3. Related Application in Tooth Regeneration
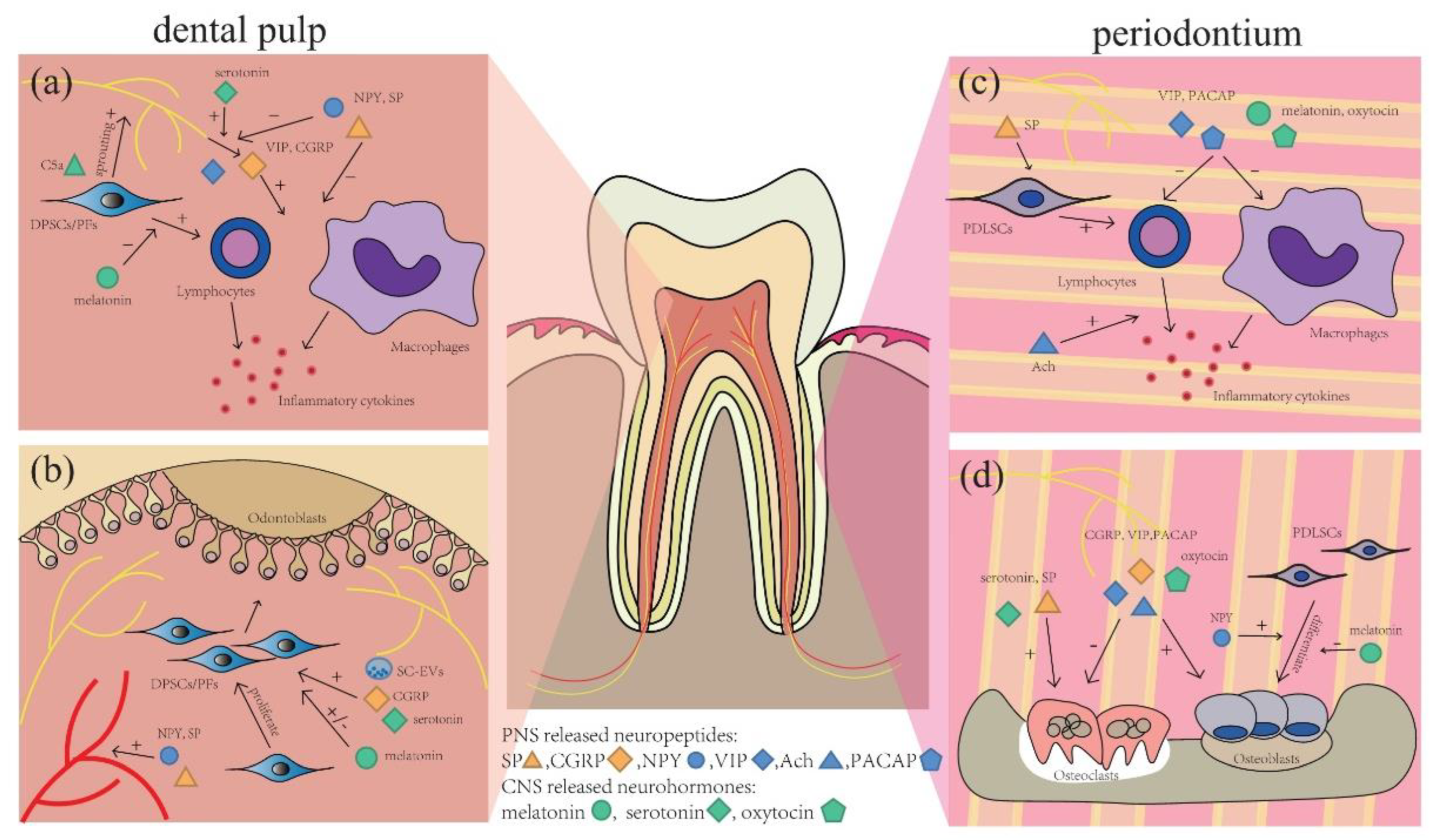
6. The Nervous System Influences the Pathology of Dental Diseases
6.1. SP
6.2. VIP
6.3. ACh and Cholinergic System
6.4. PACAP
6.5. NPY
6.6. CGRP
6.7. CNS-Released Neurohormones
6.8. Related Applications in Tooth Regeneration
7. The Nervous System Regulates Dental Stem Cells
7.1. Neural Regulations and Incisor Stem Cells
7.2. PNS-Derived Neuropeptides on Dental Stem Cells
7.3. The Role of Peripheral Non-Neuron Cells in Dental Stem Cells
7.4. CNS-Released Neurohormones and Stem Cells
7.5. Related Application in Tooth Regeneration
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ruge-Peña, N.O.; Valencia, C.; Cabrera, D.; Aguirre, D.C.; Lopera, F. Moebius syndrome: Craniofacial clinical manifestations and their association with prenatal exposure to misoprostol. Laryngoscope Investig. Otolaryngol. 2020, 5, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-E.; Su, Y.-X.; Zheng, G.-S.; Liang, Y.-J.; Liao, G.-Q. Reinnervated nerves contribute to the secretion function and regeneration of denervated submandibular glands in rabbits. Eur. J. Oral Sci. 2014, 122, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Luukko, K.; Kettunen, P. Coordination of tooth morphogenesis and neuronal development through tissue interactions: Lessons from mouse models. Exp. Cell Res. 2014, 325, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Zhan, C.; Huang, M.; Yang, X.; Hou, J. Dental nerves: A neglected mediator of pulpitis. Int. Endod. J. 2021, 54, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, S.; Kitaura, H.; Kimura, K.; Ishida, M.; Takano-Yamamoto, T. Expression of pituitary adenylate cyclase-activating peptide (PACAP) and PAC1 in the periodontal ligament after tooth luxation. Cell. Mol. Neurobiol. 2013, 33, 885–892. [Google Scholar] [CrossRef]
- Diogenes, A. Trigeminal Sensory Neurons and Pulp Regeneration. J. Endod. 2020, 46, S71–S80. [Google Scholar] [CrossRef]
- Nakao, K.; Morita, R.; Saji, Y.; Ishida, K.; Tomita, Y.; Ogawa, M.; Saitoh, M.; Tomooka, Y.; Tsuji, T. The development of a bioengineered organ germ method. Nat. Methods 2007, 4, 227–230. [Google Scholar] [CrossRef]
- Ikeda, E.; Morita, R.; Nakao, K.; Ishida, K.; Nakamura, T.; Takano-Yamamoto, T.; Ogawa, M.; Mizuno, M.; Kasugai, S.; Tsuji, T. Fully functional bioengineered tooth replacement as an organ replacement therapy. Proc. Natl. Acad. Sci. USA 2009, 106, 13475–13480. [Google Scholar] [CrossRef] [Green Version]
- Duailibi, M.T.; Duailibi, S.E.; Young, C.S.; Bartlett, J.D.; Vacanti, J.P.; Yelick, P.C. Bioengineered teeth from cultured rat tooth bud cells. J. Dent. Res. 2004, 83, 523–528. [Google Scholar] [CrossRef]
- Hung, C.-N.; Mar, K.; Chang, H.-C.; Chiang, Y.-L.; Hu, H.-Y.; Lai, C.-C.; Chu, R.-M.; Ma, C.M. A comparison between adipose tissue and dental pulp as sources of MSCs for tooth regeneration. Biomaterials 2011, 32, 6995–7005. [Google Scholar] [CrossRef]
- Bazina, F.; Brouxhon, S.M.; Graham, U.M.; Kyrkanides, S. Serotonin contributes to the in vitro production of a biomimetic enamel-like material from reprogrammed oral epithelial keratinocytes. Orthod. Craniofac. Res. 2021, 24, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.Q.; Zhang, L.S.; Fei, D.D.; Guo, H.; Wu, M.L.; Liu, J.; He, X.N.; Zhang, Y.J.; Xuan, K.; Li, B. Sensory nerve-deficient microenvironment impairs tooth homeostasis by inducing apoptosis of dental pulp stem cells. Cell Prolif. 2020, 53, e12803. [Google Scholar] [CrossRef] [PubMed]
- Hayano, S.; Fukui, Y.; Kawanabe, N.; Kono, K.; Nakamura, M.; Ishihara, Y.; Kamioka, H. Role of the Inferior Alveolar Nerve in Rodent Lower Incisor Stem Cells. J. Dent. Res. 2018, 97, 954–961. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xia, M.; Chen, T.; Yang, Y.; Fu, G.; Ji, P.; Wu, Q. Inferior alveolar nerve transection disturbs innate immune responses and bone healing after tooth extraction. Ann. N. Y. Acad. Sci. 2019, 1448, 52–64. [Google Scholar] [CrossRef]
- Haug, S.R.; Heyeraas, K.J. Effects of sympathectomy on experimentally induced pulpal inflammation and periapical lesions in rats. Neuroscience 2003, 120, 827–836. [Google Scholar] [CrossRef]
- Kim, Y.; Hamada, N.; Takahashi, Y.; Sasaguri, K.; Tsukinoki, K.; Onozuka, M.; Sato, S. Cervical sympathectomy causes alveolar bone loss in an experimental rat model. J. Periodontal Res. 2009, 44, 695–703. [Google Scholar] [CrossRef]
- Ribeiro, A.B.; Brognara, F.; da Silva, J.F.; Castania, J.A.; Fernandes, P.G.; Tostes, R.C.; Salgado, H.C. Carotid sinus nerve stimulation attenuates alveolar bone loss and inflammation in experimental periodontitis. Sci. Rep. 2020, 10, 19258. [Google Scholar] [CrossRef]
- Kanafi, M.; Majumdar, D.; Bhonde, R.; Gupta, P.; Datta, I. Midbrain cues dictate differentiation of human dental pulp stem cells towards functional dopaminergic neurons. J. Cell Physiol. 2014, 229, 1369–1377. [Google Scholar] [CrossRef]
- Tatarakis, D.; Cang, Z.; Wu, X.; Sharma, P.P.; Karikomi, M.; MacLean, A.L.; Nie, Q.; Schilling, T.F. Single-cell transcriptomic analysis of zebrafish cranial neural crest reveals spatiotemporal regulation of lineage decisions during development. Cell Rep. 2021, 37, 110140. [Google Scholar] [CrossRef]
- Kettunen, P.; Løes, S.; Furmanek, T.; Fjeld, K.; Kvinnsland, I.H.; Behar, O.; Yagi, T.; Fujisawa, H.; Vainio, S.; Taniguchi, M.; et al. Coordination of trigeminal axon navigation and patterning with tooth organ formation: Epithelial-mesenchymal interactions, and epithelial Wnt4 and Tgfbeta1 regulate semaphorin 3a expression in the dental mesenchyme. Development 2005, 132, 323–334. [Google Scholar] [CrossRef]
- Moe, K.; Kettunen, P.; Kvinnsland, I.H.; Luukko, K. Development of the pioneer sympathetic innervation into the dental pulp of the mouse mandibular first molar. Arch. Oral Biol. 2008, 53, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Erdélyi, G.; Fried, K.; Hildebrand, C. Nerve growth to tooth buds after homotopic or heterotopic autotransplantation. Brain Res. 1987, 430, 39–47. [Google Scholar] [CrossRef]
- Fried, K.; Erdélyi, G. Inferior alveolar nerve regeneration and incisor pulpal reinnervation following intramandibular neurotomy in the cat. Brain Res. 1982, 244, 259–268. [Google Scholar] [CrossRef]
- Holland, G.R.; Robinson, P.P. Reinnervation of the canine tooth pulp after section of the inferior alveolar nerve in the cat. Brain Res. 1985, 329, 300–303. [Google Scholar] [CrossRef]
- Lillesaar, C.; Fried, K. Neurites from trigeminal ganglion explants grown in vitro are repelled or attracted by tooth-related tissues depending on developmental stage. Neuroscience 2004, 125, 149–161. [Google Scholar] [CrossRef]
- Mahdee, A.; Eastham, J.; Whitworth, J.M.; Gillespie, J.I. Evidence for changing nerve growth factor signalling mechanisms during development, maturation and ageing in the rat molar pulp. Int. Endod. J. 2019, 52, 211–222. [Google Scholar] [CrossRef]
- Qian, X.B.; Naftel, J.P. Effects of neonatal exposure to anti-nerve growth factor on the number and size distribution of trigeminal neurones projecting to the molar dental pulp in rats. Arch. Oral. Biol. 1996, 41, 359–367. [Google Scholar] [CrossRef]
- Mitsiadis, T.A.; Pagella, P. Expression of Nerve Growth Factor (NGF), TrkA, and p75(NTR) in Developing Human Fetal Teeth. Front. Physiol. 2016, 7, 338. [Google Scholar] [CrossRef] [Green Version]
- Matsuo, S.; Ichikawa, H.; Henderson, T.A.; Silos-Santiago, I.; Barbacid, M.; Arends, J.J.; Jacquin, M.F. trkA modulation of developing somatosensory neurons in oro-facial tissues: Tooth pulp fibers are absent in trkA knockout mice. Neuroscience 2001, 105, 747–760. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, Y.; Li, G.; Li, J.; Yang, K.; Liu, C.; Wen, X.; Song, J. The role and potential mechanism of p75NTR in mineralization via in vivo p75NTR knockout mice and in vitro ectomesenchymal stem cells. Cell Prolif. 2020, 53, e12758. [Google Scholar] [CrossRef]
- Zhao, M.; Wen, X.; Li, G.; Ju, Y.; Wang, Y.; Zhou, Z.; Song, J. The spatiotemporal expression and mineralization regulation of p75 neurotrophin receptor in the early tooth development. Cell Prolif. 2019, 52, e12523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, K.; Wang, Y.; Ju, Y.; Li, G.; Liu, C.; Liu, J.; Liu, Q.; Wen, X.; Liu, L.C. p75 neurotrophin receptor regulates differential mineralization of rat ectomesenchymal stem cells. Cell Prolif. 2017, 50, e12290. [Google Scholar] [CrossRef] [PubMed]
- Shan, P.; Wang, X.; Zhang, Y.; Teng, Z.; Zhang, Y.; Jin, Q.; Liu, J.; Ma, J.; Nie, X. P75 neurotrophin receptor positively regulates the odontogenic/osteogenic differentiation of ectomesenchymal stem cells via nuclear factor kappa-B signaling pathway. Bioengineered 2022, 13, 11201–11213. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, C.R.; Shah, A.A.; Suh, E.B.; Pierchala, B.A. Ret Signaling Is Required for Tooth Pulp Innervation during Organogenesis. J. Dent. Res. 2019, 98, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.K.; Jue, S.S.; Dong, X. Projection of non-peptidergic afferents to mouse tooth pulp. J. Dent. Res. 2012, 91, 777–782. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.Y.; Chung, G.; Jo, H.J.; Kim, Y.S.; Bae, Y.C.; Jung, S.J.; Kim, J.S.; Oh, S.B. Characterization of dental nociceptive neurons. J. Dent. Res. 2011, 90, 771–776. [Google Scholar] [CrossRef]
- Vang, H.; Chung, G.; Kim, H.Y.; Park, S.-B.; Jung, S.J.; Kim, J.-S.; Oh, S.B. Neurochemical properties of dental primary afferent neurons. Exp. Neurobiol. 2012, 21, 68–74. [Google Scholar] [CrossRef]
- Won, J.; Vang, H.; Lee, P.R.; Kim, Y.H.; Kim, H.W.; Kang, Y.; Oh, S.B. Piezo2 Expression in Mechanosensitive Dental Primary Afferent Neurons. J. Dent. Res. 2017, 96, 931–937. [Google Scholar] [CrossRef]
- Hoshino, N.; Harada, F.; Alkhamrah, B.A.; Aita, M.; Kawano, Y.; Hanada, K.; Maeda, T. Involvement of brain-derived neurotrophic factor (BDNF) in the development of periodontal Ruffini endings. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2003, 274, 807–816. [Google Scholar] [CrossRef]
- Maruyama, Y.; Harada, F.; Jabbar, S.; Saito, I.; Aita, M.; Kawano, Y.; Suzuki, A.; Nozawa-Inoue, K.; Maeda, T. Neurotrophin-4/5-depletion induces a delay in maturation of the periodontal Ruffini endings in mice. Arch. Histol. Cytol. 2005, 68, 267–288. [Google Scholar] [CrossRef]
- Jongbloets, B.C.; Pasterkamp, R.J. Semaphorin signalling during development. Development 2014, 141, 3292–3297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shrestha, A.; Moe, K.; Luukko, K.; Taniguchi, M.; Kettunen, P. Sema3A chemorepellant regulates the timing and patterning of dental nerves during development of incisor tooth germ. Cell Tissue Res. 2014, 357, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Moe, K.; Sijaona, A.; Shrestha, A.; Kettunen, P.; Taniguchi, M.; Luukko, K. Semaphorin 3A controls timing and patterning of the dental pulp innervation. Differentiation 2012, 84, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Nakashima, T.; Taniguchi, M.; Kodama, T.; Kumanogoh, A.; Takayanagi, H. Osteoprotection by semaphorin 3A. Nature 2012, 485, 69–74. [Google Scholar] [CrossRef]
- Kettunen, P.; Spencer-Dene, B.; Furmanek, T.; Kvinnsland, I.H.; Dickson, C.; Thesleff, I.; Luukko, K. Fgfr2b mediated epithelial-mesenchymal interactions coordinate tooth morphogenesis and dental trigeminal axon patterning. Mech. Dev. 2007, 124, 868–883. [Google Scholar] [CrossRef]
- Kuchler-Bopp, S.; Bagnard, D.; Van-Der-Heyden, M.; Idoux-Gillet, Y.; Strub, M.; Gegout, H.; Lesot, H.; Benkirane-Jessel, N.; Keller, L. Semaphorin 3A receptor inhibitor as a novel therapeutic to promote innervation of bioengineered teeth. J. Tissue Eng. Regen. Med. 2018, 12, e2151–e2161. [Google Scholar] [CrossRef]
- Mounir, M.M.F.; Rashed, F.M.; Bukhary, S.M. Regeneration of Neural Networks in Immature Teeth with Non-Vital Pulp Following a Novel Regenerative Procedure. Int. J. Stem Cells 2019, 12, 410–418. [Google Scholar] [CrossRef]
- Løes, S.; Kettunen, P.; Kvinnsland, H.; Luukko, K. Mouse rudimentary diastema tooth primordia are devoid of peripheral nerve fibers. Anat. Embryol. 2002, 205, 187–191. [Google Scholar] [CrossRef]
- Lumsden, A.G.; Buchanan, J.A. An experimental study of timing and topography of early tooth development in the mouse embryo with an analysis of the role of innervation. Arch. Oral Biol. 1986, 31, 301–311. [Google Scholar] [CrossRef]
- Stainier, D.Y.; Gilbert, W. Pioneer neurons in the mouse trigeminal sensory system. Proc. Natl. Acad. Sci. USA 1990, 87, 923–927. [Google Scholar] [CrossRef]
- Maeda, Y.; Miwa, Y.; Sato, I. Distribution of the neuropeptide calcitonin gene-related peptide-α of tooth germ during formation of the mouse mandible. Ann. Anat. 2019, 221, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Weil, M.; Itin, A.; Keshet, E. A role for mesenchyme-derived tachykinins in tooth and mammary gland morphogenesis. Development 1995, 121, 2419–2428. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, H.; Sugimoto, T. Pituitary adenylate cyclase-activating polypeptide-immunoreactive nerve fibers in rat and human tooth pulps. Brain Res. 2003, 980, 288–292. [Google Scholar] [CrossRef]
- Sandor, B.; Fintor, K.; Reglodi, D.; Fulop, D.B.; Helyes, Z.; Szanto, I.; Nagy, P.; Hashimoto, H.; Tamas, A. Structural and Morphometric Comparison of Lower Incisors in PACAP-Deficient and Wild-Type Mice. J. Mol. Neurosci. 2016, 59, 300–308. [Google Scholar] [CrossRef] [Green Version]
- Fulop, B.D.; Sandor, B.; Szentleleky, E.; Karanyicz, E.; Reglodi, D.; Gaszner, B.; Zakany, R.; Hashimoto, H.; Juhasz, T.; Tamas, A. Altered Notch Signaling in Developing Molar Teeth of Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP)-Deficient Mice. J. Mol. Neurosci. 2019, 68, 377–388. [Google Scholar] [CrossRef]
- Cai, X.; Gong, P.; Huang, Y.; Lin, Y. Notch signalling pathway in tooth development and adult dental cells. Cell Prolif. 2011, 44, 495–507. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, R.; Tatehara, S.; Kumasaka, S.; Tokuyama, R.; Satomura, K. Effect of melatonin on human dental papilla cells. Int. J. Mol. Sci. 2014, 15, 17304–17317. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.; Chen, H.; Zhang, F.; Yan, T.; Fan, W.; Jiang, L.; He, H.; Huang, F. RORα Regulates Odontoblastic Differentiation and Mediates the Pro-Odontogenic Effect of Melatonin on Dental Papilla Cells. Molecules 2021, 26, 1098. [Google Scholar] [CrossRef]
- Jiang, L.L.; Zhang, F.P.; He, Y.F.; Fan, W.G.; Zheng, M.M.; Kang, J.; Huang, F.; He, H.W. Melatonin regulates mitochondrial function and biogenesis during rat dental papilla cell differentiation. Eur. Rev. Med. Pharm. Sci. 2019, 23, 5967–5979. [Google Scholar] [CrossRef]
- Tao, J.; Zhai, Y.; Park, H.; Han, J.; Dong, J.; Xie, M.; Gu, T.; Lewi, K.; Ji, F.; Jia, W. Circadian Rhythm Regulates Development of Enamel in Mouse Mandibular First Molar. PLoS ONE 2016, 11, e0159946. [Google Scholar] [CrossRef]
- Ren, Q.; Pan, J.; Chen, Y.; Shen, Z.; Yang, Z.; Kwon, K.; Guo, Y.; Wang, Y.; Ji, F. Melatonin-Medicated Neural JNK3 Up-Regulation Promotes Ameloblastic Mineralization. Front. Cell Dev. Biol. 2021, 9, 749642. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Ren, Q.; Yang, Z.; Guo, Y.; Kwon, K.; Shen, C.; Wang, Y.; Ji, F. The effect of melatonin on the mouse ameloblast-lineage cell line ALCs. Sci. Rep. 2022, 12, 8225. [Google Scholar] [CrossRef] [PubMed]
- Moiseiwitsch, J.R.; Lauder, J.M. Stimulation of murine tooth development in organotypic culture by the neurotransmitter serotonin. Arch. Oral Biol. 1996, 41, 161–165. [Google Scholar] [CrossRef]
- Moiseiwitsch, J.R.; Raymond, J.R.; Tamir, H.; Lauder, J.M. Regulation by serotonin of tooth-germ morphogenesis and gene expression in mouse mandibular explant cultures. Arch. Oral Biol. 1998, 43, 789–800. [Google Scholar] [CrossRef]
- Dimitrova-Nakov, S.; Baudry, A.; Harichane, Y.; Collet, C.; Marchadier, A.; Kellermann, O.; Goldberg, M. Deletion of serotonin 2B receptor provokes structural alterations of mouse dental tissues. Calcif. Tissue Int. 2014, 94, 293–300. [Google Scholar] [CrossRef]
- Regueira, L.S.; de Marcelos, P.G.C.L.; Santiago-Jaegger, I.M.; Perez, D.E.d.C.; Evêncio, J.; Baratella-Evêncio, L. Fluoxetine effects on periodontogenesis: Histomorphometrical and immunohistochemical analyses in rats. J. Appl. Oral Sci. 2017, 25, 159–167. [Google Scholar] [CrossRef] [Green Version]
- Kjær, I. Mechanism of human tooth eruption: Review article including a new theory for future studies on the eruption process. Scientifica 2014, 2014, 341905. [Google Scholar] [CrossRef]
- Yang, L.; Kang, M.; He, R.; Meng, B.; Pal, A.; Chen, L.; Jheon, A.H.; Ho, S.P. Microanatomical changes and biomolecular expression at the PDL-entheses during experimental tooth movement. J. Periodontal Res. 2019, 54, 251–258. [Google Scholar] [CrossRef]
- Wan, Q.-Q.; Qin, W.-P.; Ma, Y.-X.; Shen, M.-J.; Li, J.; Zhang, Z.-B.; Chen, J.-H.; Tay, F.R.; Niu, L.-N.; Jiao, K. Crosstalk between Bone and Nerves within Bone. Adv. Sci. 2021, 8, 2003390. [Google Scholar] [CrossRef]
- Cahill, D.R.; Marks, S.C. Tooth eruption: Evidence for the central role of the dental follicle. J. Oral Pathol. 1980, 9, 189–200. [Google Scholar] [CrossRef]
- Xu, J.; Kawashima, N.; Fujiwara, N.; Harada, H.; Ota, M.S.; Suda, H. Promotional effects of vasoactive intestinal peptide on the development of rodent Hertwig’s epithelial root sheath. Congenit. Anom. 2012, 52, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ge, Y.; Chen, G.; Yan, Z.; Yu, M.; Feng, L.; Jiang, Z.; Guo, W.; Tian, W. Hertwig’s epithelial root sheath cells regulate osteogenic differentiation of dental follicle cells through the Wnt pathway. Bone 2014, 63, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Shimada, A.; Komatsu, K.; Chiba, M. Effects of local injections of vasoactive drugs on eruption rate of incisor teeth in anaesthetized rats. Arch. Oral Biol. 2006, 51, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Shimada, A.; Komatsu, K.; Shibata, T.; Chiba, M. Effects of an intravenous infusion of Ringer’s solution on eruption rates of incisor teeth in anesthetized rats. Acta Odontol. Scand. 2006, 64, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Couve, E.; Osorio, R.; Schmachtenberg, O. Reactionary Dentinogenesis and Neuroimmune Response in Dental Caries. J. Dent. Res. 2014, 93, 788–793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chmilewsky, F.; About, I.; Chung, S.H. Pulp Fibroblasts Control Nerve Regeneration through Complement Activation. J. Dent. Res. 2016, 95, 913–922. [Google Scholar] [CrossRef]
- Chmilewsky, F.; Ayaz, W.; Appiah, J.; About, I.; Chung, S.-H. Nerve Growth Factor Secretion From Pulp Fibroblasts is Modulated by Complement C5a Receptor and Implied in Neurite Outgrowth. Sci. Rep. 2016, 6, 31799. [Google Scholar] [CrossRef] [Green Version]
- Irfan, M.; Kim, J.H.; Druzinsky, R.E.; Ravindran, S.; Chung, S. Complement C5aR/LPS-induced BDNF and NGF modulation in human dental pulp stem cells. Sci. Rep. 2022, 12, 2042. [Google Scholar] [CrossRef]
- Byers, M.R. Dynamic plasticity of dental sensory nerve structure and cytochemistry. Arch. Oral Biol. 1994, 39, 13S–21S. [Google Scholar] [CrossRef]
- Zhang, J.; Lian, M.; Cao, P.; Bao, G.; Xu, G.; Sun, Y.; Wang, L.; Chen, J.; Wang, Y.; Feng, G.; et al. Effects of Nerve Growth Factor and Basic Fibroblast Growth Factor Promote Human Dental Pulp Stem Cells to Neural Differentiation. Neurochem. Res. 2017, 42, 1015–1025. [Google Scholar] [CrossRef]
- Suwanchai, A.; Theerapiboon, U.; Chattipakorn, N.; Chattipakorn, S.C. NaV 1.8, but not NaV 1.9, is upregulated in the inflamed dental pulp tissue of human primary teeth. Int. Endod. J. 2012, 45, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, A.; Akhavan, A.; Bouzari, M.; Mousavi, S.B.; Torabinia, N.; Adibi, S. Temporal expression pattern of sodium channel Nav 1.8 messenger RNA in pulpitis. Int. Endod. J. 2011, 44, 499–504. [Google Scholar] [CrossRef]
- Wells, J.E.; Rose, E.T.; Rowland, K.C.; Hatton, J.F. Kv1.4 subunit expression is decreased in neurons of painful human pulp. J. Endod. 2007, 33, 827–829. [Google Scholar] [CrossRef] [PubMed]
- Caterina, M.J.; Julius, D. The vanilloid receptor: A molecular gateway to the pain pathway. Annu. Rev. Neurosci. 2001, 24, 487–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hossain, M.Z.; Bakri, M.M.; Yahya, F.; Ando, H.; Unno, S.; Kitagawa, J. The Role of Transient Receptor Potential (TRP) Channels in the Transduction of Dental Pain. Int. J. Mol. Sci. 2019, 20, 526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.; Ramsey, A.; De Brito-Gariepy, H.; Michot, B.; Podborits, E.; Melnyk, J.; Gibbs, J.L.G. Molecular, cellular and behavioral changes associated with pathological pain signaling occur after dental pulp injury. Mol. Pain 2017, 13, 1744806917715173. [Google Scholar] [CrossRef]
- Akbal Dincer, G.; Erdemir, A.; Kisa, U. Comparison of Neurokinin A, Substance P, Interleukin 8, and Matrix Metalloproteinase-8 Changes in Pulp tissue and Gingival Crevicular Fluid Samples of Healthy and Symptomatic Irreversible Pulpitis Teeth. J. Endod. 2020, 46, 1428–1437. [Google Scholar] [CrossRef]
- Caviedes-Bucheli, J.; Lopez-Moncayo, L.F.; Muñoz-Alvear, H.D.; Gomez-Sosa, J.F.; Diaz-Barrera, L.E.; Curtidor, H.; Munoz, H.R. Expression of substance P, calcitonin gene-related peptide and vascular endothelial growth factor in human dental pulp under different clinical stimuli. BMC Oral Health 2021, 21, 152. [Google Scholar] [CrossRef]
- Burns, L.E.; Ramsey, A.A.; Emrick, J.J.; Janal, M.N.; Gibbs, J.L. Variability in Capsaicin-stimulated Calcitonin Gene-related Peptide Release from Human Dental Pulp. J. Endod. 2016, 42, 542–546. [Google Scholar] [CrossRef] [Green Version]
- Sone, P.P.; Kaneko, T.; Zaw, S.Y.M.; Sueyama, Y.; Gu, B.; Murano, H.; Zaw, Z.C.T.; Okada, Y.; Han, P.; Katsube, K.-I.; et al. Neural Regeneration/Remodeling in Engineered Coronal Pulp Tissue in the Rat Molar. J. Endod. 2020, 46, 943–949. [Google Scholar] [CrossRef]
- Hodo, T.W.; de Aquino, M.T.P.; Shimamoto, A.; Shanker, A. Critical Neurotransmitters in the Neuroimmune Network. Front. Immunol. 2020, 11, 1869. [Google Scholar] [CrossRef] [PubMed]
- Chavarría-Bolaños, D.; Martinez-Zumaran, A.; Lombana, N.; Flores-Reyes, H.; Pozos-Guillen, A. Expression of substance P, calcitonin gene-related peptide, β-endorphin and methionine-enkephalin in human dental pulp tissue after orthodontic intrusion: A pilot study. Angle Orthod. 2014, 84, 521–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Killough, S.A.; Lundy, F.T.; Irwin, C.R. Substance P expression by human dental pulp fibroblasts: A potential role in neurogenic inflammation. J. Endod. 2009, 35, 73–77. [Google Scholar] [CrossRef]
- Park, S.H.; Hsiao, G.Y.W.; Huang, G.T.J. Role of substance P and calcitonin gene-related peptide in the regulation of interleukin-8 and monocyte chemotactic protein-1 expression in human dental pulp. Int. Endod. J. 2004, 37, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Delgado, A.V.; McManus, A.T.; Chambers, J.P. Production of tumor necrosis factor-alpha, interleukin 1-beta, interleukin 2, and interleukin 6 by rat leukocyte subpopulations after exposure to substance P. Neuropeptides 2003, 37, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Mehboob, R.; Hassan, S.; Gilani, S.A.; Hassan, A.; Tanvir, I.; Waseem, H.; Hanif, A. Enhanced Neurokinin-1 Receptor Expression Is Associated with Human Dental Pulp Inflammation and Pain Severity. BioMed Res. Int. 2021, 2021, 5593520. [Google Scholar] [CrossRef]
- Karabucak, B.; Walsch, H.; Jou, Y.-T.; Simchon, S.; Kim, S. The role of endothelial nitric oxide in the Substance P induced vasodilation in bovine dental pulp. J. Endod. 2005, 31, 733–736. [Google Scholar] [CrossRef] [PubMed]
- Caviedes-Bucheli, J.; Muñoz, H.R.; Azuero-Holguín, M.M.; Ulate, E. Neuropeptides in dental pulp: The silent protagonists. J. Endod. 2008, 34, 773–788. [Google Scholar] [CrossRef]
- Lundy, F.T.; Mullally, B.H.; Burden, D.J.; Lamey, P.J.; Shaw, C.; Linden, G.J. Changes in substance P and neurokinin A in gingival crevicular fluid in response to periodontal treatment. J. Clin. Periodontol. 2000, 27, 526–530. [Google Scholar] [CrossRef]
- Yan, K.; Lin, Q.; Tang, K.; Liu, S.; Du, Y.; Yu, X.; Li, S. Substance P participates in periodontitis by upregulating HIF-1α and RANKL/OPG ratio. BMC Oral Health 2020, 20, 27. [Google Scholar] [CrossRef]
- El Karim, I.A.; Lamey, P.J.; Ardill, J.; Linden, G.J.; Lundy, F.T. Vasoactive intestinal polypeptide (VIP) and VPAC1 receptor in adult human dental pulp in relation to caries. Arch. Oral Biol. 2006, 51, 849–855. [Google Scholar] [CrossRef] [PubMed]
- de Campos Soriani Azevedo, M.; Garlet, T.P.; Francisconi, C.F.; Colavite, P.M.; Tabanez, A.P.; Melchiades, J.L.; Favaro Trombone, A.P.; Sfeir, C.; Little, S.; Silva, R.M.; et al. Vasoactive Intestinal Peptide Immunoregulatory Role at the Periapex: Associative and Mechanistic Evidences from Human and Experimental Periapical Lesions. J. Endod. 2019, 45, 1228–1236. [Google Scholar] [CrossRef] [PubMed]
- Foster, N.; Cheetham, J.; Taylor, J.J.; Preshaw, P.M. VIP Inhibits Porphyromonas gingivalis LPS-induced immune responses in human monocytes. J. Dent. Res. 2005, 84, 999–1004. [Google Scholar] [CrossRef] [PubMed]
- Foster, N.; Lea, S.R.; Preshaw, P.M.; Taylor, J.J. Pivotal advance: Vasoactive intestinal peptide inhibits up-regulation of human monocyte TLR2 and TLR4 by LPS and differentiation of monocytes to macrophages. J. Leukoc. Biol. 2007, 81, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Gürkan, A.; Emingil, G.; Nizam, N.; Doğanavşargil, B.; Sezak, M.; Kütükçüler, N.; Atilla, G. Therapeutic efficacy of vasoactive intestinal peptide in escherichia coli lipopolysaccharide-induced experimental periodontitis in rats. J. Periodontol. 2009, 80, 1655–1664. [Google Scholar] [CrossRef]
- Macpherson, A.; Zoheir, N.; Awang, R.A.; Culshaw, S.; Ramage, G.; Lappin, D.F.; Nile, C.J. The alpha 7 nicotinic receptor agonist PHA-543613 hydrochloride inhibits Porphyromonas gingivalis-induced expression of interleukin-8 by oral keratinocytes. Inflamm. Res. 2014, 63, 557–568. [Google Scholar] [CrossRef] [Green Version]
- Zoheir, N.; Lappin, D.F.; Nile, C.J. Acetylcholine and the alpha 7 nicotinic receptor: A potential therapeutic target for the treatment of periodontal disease? Inflamm. Res. 2012, 61, 915–926. [Google Scholar] [CrossRef] [Green Version]
- Apatzidou, D.A.; Iskas, A.; Konstantinidis, A.; Alghamdi, A.M.; Tumelty, M.; Lappin, D.F.; Nile, C.J. Clinical associations between acetylcholine levels and cholinesterase activity in saliva and gingival crevicular fluid and periodontal diseases. J. Clin. Periodontol. 2018, 45, 1173–1183. [Google Scholar] [CrossRef]
- Kashiwagi, Y.; Yanagita, M.; Kojima, Y.; Shimabukuro, Y.; Murakami, S. Nicotine up-regulates IL-8 expression in human gingival epithelial cells following stimulation with IL-1β or P. gingivalis lipopolysaccharide via nicotinic acetylcholine receptor signalling. Arch. Oral Biol. 2012, 57, 483–490. [Google Scholar] [CrossRef]
- Du, Y.; Yang, K.; Zhou, Z.; Wu, L.; Wang, L.; Chen, Y.; Ge, X.; Wang, X. Nicotine regulates autophagy of human periodontal ligament cells through α7 nAchR that promotes secretion of inflammatory factors IL-1β and IL-8. BMC Oral Health 2021, 21, 560. [Google Scholar] [CrossRef]
- Toth, D.; Szabo, E.; Tamas, A.; Juhasz, T.; Horvath, G.; Fabian, E.; Opper, B.; Szabo, D.; Maugeri, G.; D’Amico, A.G.; et al. Protective Effects of PACAP in Peripheral Organs. Front. Endocrinol. 2020, 11, 377. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, M.d.C.S.; Fonseca, A.C.; Colavite, P.M.; Melchiades, J.L.; Tabanez, A.P.; Codo, A.C.; de Medeiros, A.I.; Trombone, A.P.F.; Garlet, G.P. Macrophage Polarization and Alveolar Bone Healing Outcome: Despite a Significant M2 Polarizing Effect, VIP and PACAP Treatments Present a Minor Impact in Alveolar Bone Healing in Homeostatic Conditions. Front. Immunol. 2021, 12, 782566. [Google Scholar] [CrossRef] [PubMed]
- Shende, P.; Desai, D. Physiological and Therapeutic Roles of Neuropeptide Y on Biological Functions. Adv. Exp. Med. Biol. 2020, 1237, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Rethnam, S.; Raju, B.; Fristad, I.; Berggreen, E.; Heyeraas, K.J. Differential expression of neuropeptide Y Y1 receptors during pulpal inflammation. Int. Endod. J. 2010, 43, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Killough, S.A.; Lundy, F.T.; Irwin, C.R. Dental pulp fibroblasts express neuropeptide Y Y1 receptor but not neuropeptide Y. Int. Endod. J. 2010, 43, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, J.L.; Hargreaves, K.M. Neuropeptide Y Y1 receptor effects on pulpal nociceptors. J. Dent. Res. 2008, 87, 948–952. [Google Scholar] [CrossRef] [Green Version]
- Winning, L.; El Karim, I.A.; Linden, G.J.; Irwin, C.R.; Killough, S.A.; Lundy, F.T. Differential regulation of NPY and SP receptor expression in STRO-1+ve PDLSCs by inflammatory cytokines. J. Periodontal Res. 2022, 57, 186–194. [Google Scholar] [CrossRef]
- Divaris, K.; Monda, K.L.; North, K.E.; Olshan, A.F.; Reynolds, L.M.; Hsueh, W.-C.; Lange, E.M.; Moss, K.; Barros, S.P.; Weyant, R.J.; et al. Exploring the genetic basis of chronic periodontitis: A genome-wide association study. Hum. Mol. Genet. 2013, 22, 2312–2324. [Google Scholar] [CrossRef]
- Freitag-Wolf, S.; Dommisch, H.; Graetz, C.; Jockel-Schneider, Y.; Harks, I.; Staufenbiel, I.; Meyle, J.; Eickholz, P.; Noack, B.; Bruckmann, C.; et al. Genome-wide exploration identifies sex-specific genetic effects of alleles upstream NPY to increase the risk of severe periodontitis in men. J. Clin. Periodontol. 2014, 41, 1115–1121. [Google Scholar] [CrossRef]
- Cirelli, T.; Nepomuceno, R.; Orrico, S.R.P.; Rossa, C.; Cirelli, J.A.; North, K.E.; Graff, M.; Barros, S.P.; Scarel-Caminaga, R.M. Validation in a Brazilian population of gene markers of periodontitis previously investigated by GWAS and bioinformatic studies. J. Periodontol. 2021, 92, 689–703. [Google Scholar] [CrossRef]
- Caviedes-Bucheli, J.; Arenas, N.; Guiza, O.; Moncada, N.A.; Moreno, G.C.; Diaz, E.; Munoz, H.R. Calcitonin gene-related peptide receptor expression in healthy and inflamed human pulp tissue. Int. Endod. J. 2005, 38, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Caviedes-Bucheli, J.; Moreno, G.C.; López, M.P.; Bermeo-Noguera, A.M.; Pacheco-Rodríguez, G.; Cuellar, A.; Muñoz, H.R. Calcitonin gene-related peptide receptor expression in alternatively activated monocytes/macrophages during irreversible pulpitis. J. Endod. 2008, 34, 945–949. [Google Scholar] [CrossRef] [PubMed]
- Kaewpitak, A.; Bauer, C.S.; Seward, E.P.; Boissonade, F.M.; Douglas, C.W.I. Porphyromonas gingivalis lipopolysaccharide rapidly activates trigeminal sensory neurons and may contribute to pulpal pain. Int. Endod. J. 2020, 53, 846–858. [Google Scholar] [CrossRef]
- Toth, C.C.; Willis, D.; Twiss, J.L.; Walsh, S.; Martinez, J.A.; Liu, W.-Q.; Midha, R.; Zochodne, D.W. Locally synthesized calcitonin gene-related Peptide has a critical role in peripheral nerve regeneration. J. Neuropathol. Exp. Neurol. 2009, 68, 326–337. [Google Scholar] [CrossRef] [Green Version]
- Moore, E.R.; Michot, B.; Erdogan, O.; Ba, A.; Gibbs, J.L.; Yang, Y. CGRP and Shh Mediate the Dental Pulp Cell Response to Neuron Stimulation. J. Dent. Res. 2022, 101, 1119–1126. [Google Scholar] [CrossRef]
- Austah, O.N.; Ruparel, N.B.; Henry, M.A.; Fajardo, R.J.; Schmitz, J.E.; Diogenes, A. Capsaicin-sensitive Innervation Modulates the Development of Apical Periodontitis. J. Endod. 2016, 42, 1496–1502. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, H.; Zhang, G.; He, Y.; Zhang, P.; Sun, Z.; Gao, Y.; Tan, Y. Calcitonin gene-related peptide reduces Porphyromonas gingivalis LPS-induced TNF-α release and apoptosis in osteoblasts. Mol. Med. Rep. 2018, 17, 3246–3254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, N.; Matsuda, Y.; Sato, K.; de Jong, P.R.; Bertin, S.; Tabeta, K.; Yamazaki, K. Neuronal TRPV1 activation regulates alveolar bone resorption by suppressing osteoclastogenesis via CGRP. Sci. Rep. 2016, 6, 29294. [Google Scholar] [CrossRef]
- Lundy, F.T.; Shaw, C.; McKinnell, J.; Lamey, P.J.; Linden, G.J. Calcitonin gene-related peptide in gingival crevicular fluid in periodontal health and disease. J. Clin. Periodontol. 1999, 26, 212–216. [Google Scholar] [CrossRef]
- Pang, P.; Shimo, T.; Takada, H.; Matsumoto, K.; Yoshioka, N.; Ibaragi, S.; Sasaki, A. Expression pattern of sonic hedgehog signaling and calcitonin gene-related peptide in the socket healing process after tooth extraction. Biochem. Biophys. Res. Commun. 2015, 467, 21–26. [Google Scholar] [CrossRef]
- Kara, A.; Akman, S.; Ozkanlar, S.; Tozoglu, U.; Kalkan, Y.; Canakci, C.F.; Tozoglu, S. Immune modulatory and antioxidant effects of melatonin in experimental periodontitis in rats. Free Radic. Biol. Med. 2013, 55, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Liu, Q.; Zhang, H.; Fan, W.; Li, J.; Kang, J.; He, H.; Huang, F. Melatonin enhances hydrogen peroxide-induced apoptosis in human dental pulp cells. J. Dent. Sci. 2019, 14, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Fan, W.; He, Y.; Zhang, F.; Guan, X.; Deng, Q.; Lu, X.; He, H.; Huang, F. Effects of melatonin on the proliferation and differentiation of human dental pulp cells. Arch. Oral Biol. 2017, 83, 33–39. [Google Scholar] [CrossRef]
- Kantrong, N.; Jit-Armart, P.; Arayatrakoollikit, U. Melatonin antagonizes lipopolysaccharide-induced pulpal fibroblast responses. BMC Oral Health 2020, 20, 91. [Google Scholar] [CrossRef] [PubMed]
- Loyd, D.R.; Sun, X.X.; Locke, E.E.; Salas, M.M.; Hargreaves, K.M. Sex differences in serotonin enhancement of capsaicin-evoked calcitonin gene-related peptide release from human dental pulp. Pain 2012, 153, 2061–2067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, S.; Hickman, T.M.; Lopez-Ramirez, A.; McDonald, H.; Lockhart, L.M.; Darwish, O.; Averitt, D.L. Estrogen modulation of the pronociceptive effects of serotonin on female rat trigeminal sensory neurons is timing dependent and dosage dependent and requires estrogen receptor alpha. Pain 2022, 163, e899–e916. [Google Scholar] [CrossRef]
- Hakam, A.E.; Duarte, P.M.; Mbadu, M.P.; Aukhil, I.; da Silva, H.D.P.; Chang, J. Association of different antidepressant classes with clinical attachment level and alveolar bone loss in patients with periodontitis: A retrospective study. J. Periodontal Res. 2022, 57, 75–84. [Google Scholar] [CrossRef]
- Colli, V.C.; Okamoto, R.; Spritzer, P.M.; Dornelles, R.C.M. Oxytocin promotes bone formation during the alveolar healing process in old acyclic female rats. Arch. Oral Biol. 2012, 57, 1290–1297. [Google Scholar] [CrossRef] [Green Version]
- Paksoy, T.; Ustaoğlu, G.; Şehirli, A.; Ünsal, R.B.K.; Sayıner, S.; Orhan, K.; Aycı, N.B.; Çetinel, Ş.; Aksoy, U. Evaluation of the oxytocin effect in a rat model with experimental periodontitis. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2022, 395, 1599–1608. [Google Scholar] [CrossRef]
- Zheng, H.; Lim, J.Y.; Kim, Y.; Jung, S.T.; Hwang, S.W. The role of oxytocin, vasopressin, and their receptors at nociceptors in peripheral pain modulation. Front. Neuroendocr. 2021, 63, 100942. [Google Scholar] [CrossRef]
- Kline, L.W.; Yu, D.C. Effects of calcitonin, calcitonin gene-related peptide, human recombinant bone morphogenetic protein-2, and parathyroid hormone-related protein on endodontically treated ferret canines. J. Endod. 2009, 35, 866–869. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Xiang, L.; Yao, Y.; Yuan, Q.; Li, L.; Gong, P. CGRP-alpha application: A potential treatment to improve osseoperception of endosseous dental implants. Med. Hypotheses 2013, 81, 297–299. [Google Scholar] [CrossRef] [PubMed]
- Tinto, M.; Sartori, M.; Pizzi, I.; Verga, A.; Longoni, S. Melatonin as host modulating agent supporting nonsurgical periodontal therapy in patients affected by untreated severe periodontitis: A preliminary randomized, triple-blind, placebo-controlled study. J. Periodontal Res. 2020, 55, 61–67. [Google Scholar] [CrossRef]
- Abdelrasoul, M.; El-Fattah, A.A.; Kotry, G.; Ramadan, O.; Essawy, M.; Kamaldin, J.; Kandil, S. Regeneration of critical-sized grade II furcation using a novel injectable melatonin-loaded scaffold. Oral Dis. 2022. [CrossRef] [PubMed]
- Yu, Y.; Li, X.; Li, J.; Li, D.; Wang, Q.; Teng, W. Dopamine-assisted co-deposition of hydroxyapatite-functionalised nanoparticles of polydopamine on implant surfaces to promote osteogenesis in environments with high ROS levels. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 131, 112473. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, X.; He, Y.; Zhao, X.; Lin, J.; Feng, Y.; Chen, J.; Luo, F.; Li, Z.; Li, J.; et al. A bioinspired Janus polyurethane membrane for potential periodontal tissue regeneration. J. Mater. Chem. B 2022, 10, 2602–2616. [Google Scholar] [CrossRef]
- Juriga, D.; Kalman, E.E.; Toth, K.; Barczikai, D.; Szöllősi, D.; Földes, A.; Varga, G.; Zrinyi, M.; Jedlovszky-Hajdu, A.; Nagy, K.S. Analysis of Three-Dimensional Cell Migration in Dopamine-Modified Poly(aspartic acid)-Based Hydrogels. Gels 2022, 8, 65. [Google Scholar] [CrossRef]
- Fioretti, F.; Mendoza-Palomares, C.; Helms, M.; Al Alam, D.; Richert, L.; Arntz, Y.; Rinckenbach, S.; Garnier, F.; Haïkel, Y.; Gangloff, S.C.; et al. Nanostructured assemblies for dental application. ACS Nano 2010, 4, 3277–3287. [Google Scholar] [CrossRef]
- Zhao, H.; Feng, J.; Seidel, K.; Shi, S.; Klein, O.; Sharpe, P.; Chai, Y. Secretion of shh by a neurovascular bundle niche supports mesenchymal stem cell homeostasis in the adult mouse incisor. Cell Stem Cell 2014, 14, 160–173. [Google Scholar] [CrossRef] [Green Version]
- Kaukua, N.; Shahidi, M.K.; Konstantinidou, C.; Dyachuk, V.; Kaucka, M.; Furlan, A.; An, Z.; Wang, L.; Hultman, I.; Ahrlund-Richter, L.; et al. Glial origin of mesenchymal stem cells in a tooth model system. Nature 2014, 513, 551–554. [Google Scholar] [CrossRef]
- Li, Z.; Liang, Y.; Pan, K.; Li, H.; Yu, M.; Guo, W.; Chen, G.; Tian, W. Schwann cells secrete extracellular vesicles to promote and maintain the proliferation and multipotency of hDPCs. Cell Prolif. 2017, 50, e12353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Lyu, Y.; Yang, Y.; Zhang, S.; Chen, G.; Pan, J.; Tian, W. Schwann cell-derived EVs facilitate dental pulp regeneration through endogenous stem cell recruitment via SDF-1/CXCR4 axis. Acta Biomater. 2022, 140, 610–624. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.-H.; Ho, K.-N.; Lee, Y.-C.; Chou, M.-J.; Lew, W.-Z.; Huang, H.-M.; Lai, P.-C.; Feng, S.-W. Melatonin enhances osteogenic differentiation of dental pulp mesenchymal stem cells by regulating MAPK pathways and promotes the efficiency of bone regeneration in calvarial bone defects. Stem Cell Res. 2022, 13, 73. [Google Scholar] [CrossRef] [PubMed]
- García-Bernal, D.; López-García, S.; Sanz, J.L.; Guerrero-Gironés, J.; García-Navarro, E.M.; Moraleda, J.M.; Forner, L.; Rodríguez-Lozano, F.J. Melatonin Treatment Alters Biological and Immunomodulatory Properties of Human Dental Pulp Mesenchymal Stem Cells via Augmented Transforming Growth Factor Beta Secretion. J. Endod. 2021, 47, 424–435. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Deng, Q.; Fan, W.; Zeng, Q.; He, H.; Huang, F. Melatonin-induced suppression of DNA methylation promotes odontogenic differentiation in human dental pulp cells. Bioengineered 2020, 11, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Baysal, E.; Zırh, E.B.; Buber, E.; Jakobsen, T.K.; Zeybek, N.D. The effect of melatonin on Hippo signaling pathway in dental pulp stem cells. Neurochem. Int. 2021, 148, 105079. [Google Scholar] [CrossRef]
- Hosokawa, Y.; Hosokawa, I.; Ozaki, K.; Nakae, H.; Matsuo, T. Increase of CCL20 expression by human gingival fibroblasts upon stimulation with cytokines and bacterial endotoxin. Clin. Exp. Immunol. 2005, 142, 285–291. [Google Scholar] [CrossRef]
- Kook, Y.-A.; Lee, S.-K.; Son, D.-H.; Kim, Y.; Kang, K.-H.; Cho, J.-H.; Kim, S.-C.; Kim, Y.-S.; Lee, H.-J.; Lee, S.-K.; et al. Effects of substance P on osteoblastic differentiation and heme oxygenase-1 in human periodontal ligament cells. Cell Biol. Int. 2009, 33, 424–428. [Google Scholar] [CrossRef]
- Itoyama, T.; Yoshida, S.; Tomokiyo, A.; Hasegawa, D.; Hamano, S.; Sugii, H.; Ono, T.; Fujino, S.; Maeda, H. Possible function of GDNF and Schwann cells in wound healing of periodontal tissue. J. Periodontal Res. 2020, 55, 830–839. [Google Scholar] [CrossRef]
- Tan, Y.-Z.; Xu, X.-Y.; Dai, J.-M.; Yin, Y.; He, X.-T.; Zhang, Y.-L.; Zhu, T.-X.; An, Y.; Tian, B.-M.; Chen, F.-M. Melatonin induces the rejuvenation of long-term ex vivo expanded periodontal ligament stem cells by modulating the autophagic process. Stem Cell Res. 2021, 12, 254. [Google Scholar] [CrossRef]
- Zheng, M.; Zhang, F.; Fan, W.; Jiang, L.; Li, J.; Xie, S.; Huang, F.; He, H. Suppression of osteogenic differentiation and mitochondrial function change in human periodontal ligament stem cells by melatonin at physiological levels. PeerJ 2020, 8, e8663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Le, N.H.; Kang, D. Melatonin alleviates oxidative stress-inhibited osteogenesis of human bone marrow-derived mesenchymal stem cells through AMPK activation. Int. J. Med. Sci. 2018, 15, 1083–1091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, J.; Mantesso, A.; De Bari, C.; Nishiyama, A.; Sharpe, P.T. Dual origin of mesenchymal stem cells contributing to organ growth and repair. Proc. Natl. Acad. Sci. USA 2011, 108, 6503–6508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seidel, K.; Ahn, C.P.; Lyons, D.; Nee, A.; Ting, K.; Brownell, I.; Cao, T.; Carano, R.A.D.; Curran, T.; Schober, M.; et al. Hedgehog signaling regulates the generation of ameloblast progenitors in the continuously growing mouse incisor. Development 2010, 137, 3753–3761. [Google Scholar] [CrossRef] [Green Version]
- Binder, M.; Chmielarz, P.; McKinnon, P.J.; Biggs, L.C.; Thesleff, I.; Balic, A. Functionally Distinctive Ptch Receptors Establish Multimodal Hedgehog Signaling in the Tooth Epithelial Stem Cell Niche. Stem Cells 2019, 37, 1238–1248. [Google Scholar] [CrossRef]
- Ladizesky, M.G.; Lama, M.A.; Cutrera, R.A.; Boggio, V.; Giglio, M.J.; Cardinali, D.P. Effect of unilateral superior cervical ganglionectomy on mandibular incisor eruption rate in rats. Auton. Neurosci. 2001, 93, 65–70. [Google Scholar] [CrossRef]
- Michot, B.; Casey, S.M.; Gibbs, J.L. Effects of Calcitonin Gene-related Peptide on Dental Pulp Stem Cell Viability, Proliferation, and Differentiation. J. Endod. 2020, 46, 950–956. [Google Scholar] [CrossRef]
- Michot, B.; Casey, S.M.; Gibbs, J.L. Effects of CGRP-Primed Dental Pulp Stem Cells on Trigeminal Sensory Neurons. J. Dent. Res. 2021, 100, 1273–1280. [Google Scholar] [CrossRef]
- Mao, Q.; Nguyen, P.D.; Shanti, R.M.; Shi, S.; Shakoori, P.; Zhang, Q.; Le, A.D. Gingiva-Derived Mesenchymal Stem Cell-Extracellular Vesicles Activate Schwann Cell Repair Phenotype and Promote Nerve Regeneration. Tissue Eng. Part A 2019, 25, 887–900. [Google Scholar] [CrossRef] [Green Version]
- Baudry, A.; Alleaume-Butaux, A.; Dimitrova-Nakov, S.; Goldberg, M.; Schneider, B.; Launay, J.-M.; Kellermann, O. Essential Roles of Dopamine and Serotonin in Tooth Repair: Functional Interplay Between Odontogenic Stem Cells and Platelets. Stem Cells 2015, 33, 2586–2595. [Google Scholar] [CrossRef]
- Tumedei, M.; Mancinelli, R.; Di Filippo, E.S.; Marrone, M.; Iezzi, G.; Piattelli, A.; Fulle, S. Osteogenic Potential of Human Dental Pulp Stem Cells Co-Cultured with Equine Bone Substitute Combined with Melatonin. Int. J. Periodontics Restor. Dent. 2022, 42, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Pan, F.; Prpic, V.; Wise, G.E. Differentiation of stem cells in the dental follicle. J. Dent. Res. 2008, 87, 767–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonoyama, W.; Liu, Y.; Fang, D.; Yamaza, T.; Seo, B.-M.; Zhang, C.; Liu, H.; Gronthos, S.; Wang, C.-Y.; Wang, S.; et al. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS ONE 2006, 1, e79. [Google Scholar] [CrossRef] [Green Version]
- Yokoi, T.; Saito, M.; Kiyono, T.; Iseki, S.; Kosaka, K.; Nishida, E.; Tsubakimoto, T.; Harada, H.; Eto, K.; Noguchi, T.; et al. Establishment of immortalized dental follicle cells for generating periodontal ligament in vivo. Cell Tissue Res. 2007, 327, 301–311. [Google Scholar] [CrossRef] [PubMed]
| Nerve Fibers | Regional Distribution | Function |
|---|---|---|
| sensory | Raschkow plexus Throughout the pulp In the dentinal tubule | Highly specialized nociceptors |
| sympathetic | SCG→TG→sensory fibers→pulp SCG→inferior/superior alveolar artery→pulp Around pulp arterioles Raschkow plexus | Regulate pulp blood flow |
| Nerve Fibers | Regional Distribution | Function |
|---|---|---|
| sensory | Throughout the PDL As bundles around blood vessels near the alveolar bone As free endings near the cementum | Nociceptors and mechanoreceptors Regulate blood flow |
| autonomic | Few | Regulate blood flow |
| Stem cells | Neural Regulations | Biological Effects | Molecular Mechanism | Ref. |
|---|---|---|---|---|
| MSCs (Incisor) | IAN secretion | Promoting MSC proliferation | Wnt signaling | [149] |
| Sustaining Gli1 expression and odontogenic commitment of MSCs | Shh signaling | [149] | ||
| SCs/SCPs | Giving rise to MSCs that generate odontoblasts and pulp cells | SCs and SCPs differentiation | [150] | |
| ESCs (Incisor) | IAN | Regulating ESC niches and differentiation | Mesenchymal–epithelial interaction | [13] |
| DPSCs | Shh & CGRP | Shh promotes odontoblastic differentiation and CGRP promotes proliferation of DPSCs | CGRP/Shh signaling | [125] |
| SC-EVs | Promoting DPSC multipotency | Oct4/Sox2/Nanog | [151] | |
| Promoting DPSC proliferation | TGF-β/Smad signaling TGF-β/MAPK signaling | [151] | ||
| Promoting DPSC migration and osteogenic differentiation | SDF-1/CXCR4 | [152] | ||
| Melatonin (intermediate concentration) | Promoting DPSC proliferation and osteogenic differentiation | COX-2/NF-κB signaling p38/ERK MAPK signaling | [153] | |
| Promoting DPSC migration and proliferation | - | [154] | ||
| Increasing TGF-b production by DPSCs to suppress T-cell proliferation upon stimuli | - | [154] | ||
| Promoting DPSC osteogenic differentiation | Suppressing DNA methylation | [155] | ||
| Melatonin (low concentration) | Inhibiting DPSC proliferation and inducing DPSC neuronal differentiation | Hippo pathway | [156] | |
| PDLSCs | SP | PDLSCs produce CCL20/MIP-3α to recruit T cells | p38 and ERK/MAPK signaling | [157] |
| SP | Promoting PDLSC osteogenic differentiation | SP/HO-1/Nrf-2 | [158] | |
| NPY | Moderate osteogenic effects | - | [117] | |
| SCs | Promoting osteogenic differentiation of PDLSCs | ERK1/2 signaling | [159] | |
| Melatonin (low concentration) | Rejuvenating long-term ex vivo expanded PDLSCs by restoring their autophagy | PI3K/AKT/mTOR signaling | [160] | |
| Suppressing PDLSC osteogenic differentiation | Increasing mitochondrial fission | [161] | ||
| BMSCs | Melatonin (intermediate concentration) | Promoting BMSCs osteogenic differentiation | AMPK/FOXO3a/RUNX2 signaling | [162] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, Y.; Liang, Y.; Yang, F.; Ma, Y. Neural Regulations in Tooth Development and Tooth–Periodontium Complex Homeostasis: A Literature Review. Int. J. Mol. Sci. 2022, 23, 14150. https://doi.org/10.3390/ijms232214150
Duan Y, Liang Y, Yang F, Ma Y. Neural Regulations in Tooth Development and Tooth–Periodontium Complex Homeostasis: A Literature Review. International Journal of Molecular Sciences. 2022; 23(22):14150. https://doi.org/10.3390/ijms232214150
Chicago/Turabian StyleDuan, Yihong, Yongfeng Liang, Fangyi Yang, and Yuanyuan Ma. 2022. "Neural Regulations in Tooth Development and Tooth–Periodontium Complex Homeostasis: A Literature Review" International Journal of Molecular Sciences 23, no. 22: 14150. https://doi.org/10.3390/ijms232214150





