DPP-IV Inhibitory Peptides GPF, IGL, and GGGW Obtained from Chicken Blood Hydrolysates
Abstract
1. Introduction
2. Results and Discussion
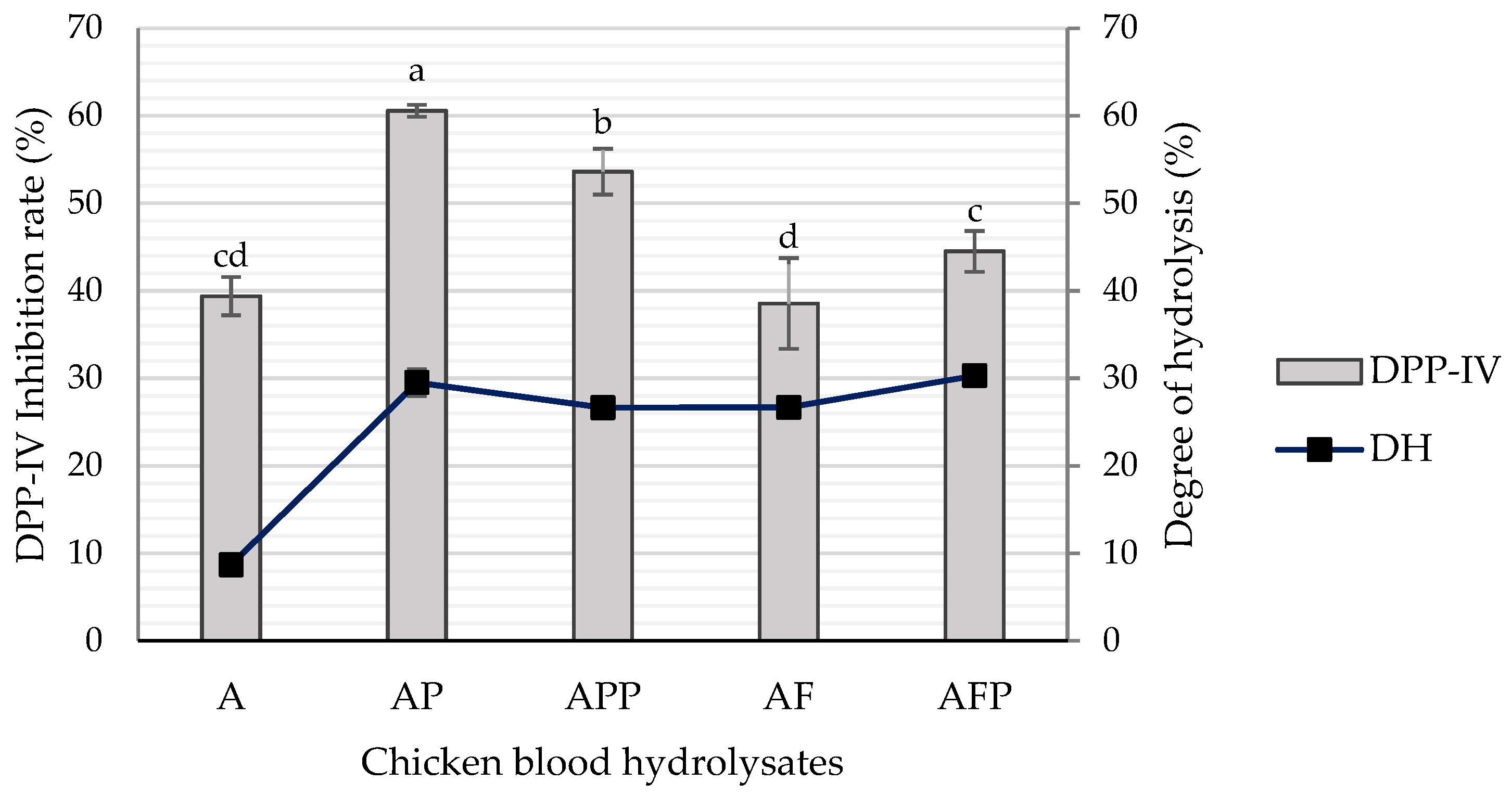
2.1. Degree of Hydrolysis and Inhibitory Activity of DPP-IV
2.2. Determination of Free Amino Acids (FAAs)
2.3. In Silico Analysis of the Identified Peptides
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Enzymatic Hydrolysis
3.3. Degree of Hydrolysis Determination
3.4. DPP-IV Inhibitory Activity
3.5. Peptide Separation by RP-HPLC
3.6. Determination of Free Amino Acids (FAAs)
3.7. Peptide Sequence Identification by LC-MS/MS
3.8. In Silico Analysis of Identified Peptides
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- IDF. International Diabetes Federation. Available online: www.diabetesatlas.org (accessed on 15 July 2022).
- Nasri, M. Protein Hydrolysates and Biopeptides: Production, biological activities, and applications in foods and health benefits. A Review. Adv. Food Nutr. Res. 2017, 81, 109–159. [Google Scholar] [CrossRef] [PubMed]
- Wajchenberg, B.L. β-Cell failure in diabetes and preservation by clinical treatment. Endocr. Rev. 2007, 28, 187–218. [Google Scholar] [CrossRef] [PubMed]
- Elam, E.; Feng, J.; Lv, Y.M.; Ni, Z.J.; Sun, P.; Thakur, K.; Zhang, J.G.; Ma, Y.L.; Wei, Z.J. Recent advances on bioactive food derived anti-diabetic hydrolysates and peptides from natural resources. J. Funct. Foods. 2021, 86, 104674. [Google Scholar] [CrossRef]
- Gallego, M.; Aristoy, M.C.; Toldrá, F. Dipeptidyl peptidase IV inhibitory peptides generated in Spanish dry-cured ham. Meat Sci. 2014, 96, 757–761. [Google Scholar] [CrossRef]
- Power, O.; Nongonierma, A.B.; Jakeman, P.; Fitzgerald, R.J. Food protein hydrolysates as a source of dipeptidyl peptidase IV inhibitory peptides for the management of type 2 diabetes. Proc. Nutr. Soc. 2014, 73, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fan, Y.; Xu, F.; Xie, J.; Gao, X.; Li, L.; Tian, Y.; Sheng, J. Characterization of the structure, stability, and activity of hypoglycemic peptides from Moringa oleifera seed protein hydrolysates. Food Funct. 2022, 13, 3481–3494. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, R.; Cheng, C.; Zhang, Y.; Ma, Y.; Lu, W. Identification of two novel dipeptidyl peptidase-IV inhibitory peptides from sheep whey protein and inhibition mechanism revealed by molecular docking. Food Biosci. 2022, 48, 101733. [Google Scholar] [CrossRef]
- Rodriguez, H.; Albericio, F.; Santiago Vispo, N. Iberoamerican network for the development of therapeutic peptides, REDIPEPT. Rev. Bionatura 2020, 5, 1177–1180. [Google Scholar] [CrossRef]
- Toldrá, F.; Gallego, M.; Reig, M.; Aristoy, M.C.; Mora, L. Recent progress in enzymatic release of peptides in foods of animal origin and assessment of bioactivity. J. Agric. Food Chem. 2020, 68, 12842–12855. [Google Scholar] [CrossRef]
- Lacroix, I.M.E.; Li-Chan, E.C.Y. Inhibition of dipeptidyl peptidase (DPP)-IV and α-glucosidase activities by pepsin-treated whey proteins. J. Agric. Food Chem. 2013, 61, 7500–7506. [Google Scholar] [CrossRef]
- Mojica, L.; Gonzalez de Mejia, E.; Granados-Silvestre, M.Á.; Menjivar, M. Evaluation of the hypoglycemic potential of a black bean hydrolyzed protein isolate and its pure peptides using in silico, in vitro and in vivo approaches. J. Funct. Foods 2017, 31, 274–286. [Google Scholar] [CrossRef]
- Vilcacundo, R.; Martínez-Villaluenga, C.; Hernández-Ledesma, B. Release of dipeptidyl peptidase IV, α-amylase and α-glucosidase inhibitory peptides from quinoa (Chenopodium quinoa Willd.) during in vitro simulated gastrointestinal digestion. J. Funct. Foods 2017, 35, 531–539. [Google Scholar] [CrossRef]
- Daliri, E.B.M.; Lee, B.H.; Oh, D.H. Current trends and perspectives of bioactive peptides. Crit. Rev. Food Sci. Nutr. 2017, 58, 2273–2284. [Google Scholar] [CrossRef] [PubMed]
- Wongngam, W.; Mitani, T.; Katayama, S.; Nakamura, S.; Yongsawatdigul, J. Production and characterization of chicken blood hydrolysate with antihypertensive properties. Poult. Sci. 2020, 99, 5163–5174. [Google Scholar] [CrossRef] [PubMed]
- Toldrá, F.; Reig, M.; Mora, L. Management of meat by- and co-products for an improved meat processing sustainability. Meat Sci. 2021, 181, 108608. [Google Scholar] [CrossRef] [PubMed]
- Nongonierma, A.B.; FitzGerald, R.J. Dipeptidyl peptidase IV inhibitory properties of a whey protein hydrolysate: Influence of fractionation, stability to simulated gastrointestinal digestion and food–drug interaction. Int. Dairy J. 2013, 32, 33–39. [Google Scholar] [CrossRef]
- Clemente, A. Enzymatic protein hydrolysates in human nutrition. Trends Food Sci. Technol. 2000, 11, 254–262. [Google Scholar] [CrossRef]
- Nchienzia, H.A.; Morawicki, R.O.; Gadang, V.P. Enzymatic hydrolysis of poultry meal with endo- and exopeptidases. Poult. Sci. 2010, 89, 2273–2280. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; Dellafiora, L.; Paolella, S.; Galaverna, G.; Cozzini, P.; FitzGerald, R.J. In silico approaches applied to the study of peptide analogs of Ile-Pro-Ile in relation to their dipeptidyl peptidase IV inhibitory properties. Front. Endocrinol. 2018, 9, 329. [Google Scholar] [CrossRef]
- Agyei, D. Bioactive proteins and peptides from soybeans. Recent Pat. Food Nutr. Agric. 2015, 7, 100–107. [Google Scholar] [CrossRef]
- Lammi, C.; Aiello, G.; Boschin, G.; Arnoldi, A. Multifunctional peptides for the prevention of cardiovascular disease: A new concept in the area of bioactive food-derived peptides. J. Funct. Foods 2019, 55, 135–145. [Google Scholar] [CrossRef]
- Li-Chan, E.C.Y.; Hunag, S.L.; Jao, C.L.; Ho, K.P.; Hsu, K.C. Peptides derived from Atlantic salmon skin gelatin as dipeptidyl-peptidase IV Inhibitors. J. Agric. Food Chem. 2012, 60, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Mojica, L.; de Mejía, E.G. Optimization of enzymatic production of anti-diabetic peptides from black bean (Phaseolus vulgaris L.) proteins, their characterization and biological potential. Food Funct. 2016, 7, 713–727. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Yao, Y.; Xu, X.; Wang, M.; Pan, M.; Ji, S.; Wu, J.; Jiang, D.; Ju, X.; Wang, L. Identification and quantification of DPP-IV-inhibitory peptides from hydrolyzed-rapeseed-protein-derived napin with analysis of the interactions between key residues and protein domains. J. Agric. Food Chem. 2019, 67, 3679–3690. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.W.; Lee, G.H.; Kim, J.Y.; Kim, C.Y.; Choo, Y.M.; Cho, W.; Han, E.H.; Hwang, Y.P.; Kim, Y.A.; Jeong, H.G. Effect of porcine whole blood protein hydrolysate on slow-twitch muscle fiber expression and mitochondrial biogenesis via the AMPK/SIRT1 pathway. Int. J. Mol. Sci. 2022, 23, 1229. [Google Scholar] [CrossRef]
- Young Park, J.; Kim, M.-Y.; Jeong, Y.-J. Changes in physicochemical characteristics of porcine blood under various conditions of enzyme hydrolysis. Korean J. Food Preserv. 2016, 23, 413–421. [Google Scholar] [CrossRef]
- Sadri, H.; Larki, N.N.; Kolahian, S. Hypoglycemic and hypolipidemic effects of leucine, zinc, and chromium, alone and in combination, in rats with type 2 diabetes. Biol. Trace Elem. Res. 2017, 180, 246–254. [Google Scholar] [CrossRef]
- Nielsen, S.D.; Beverly, R.L.; Qu, Y.; Dallas, D.C. Milk bioactive peptide database: A comprehensive database of milk protein-derived bioactive peptides and novel visualization. Food Chem. 2017, 232, 673–682. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; Fitzgerald, R.J. Susceptibility of milk protein-derived peptides to dipeptidyl peptidase IV (DPP-IV) hydrolysis. Food Chem. 2014, 145, 845–852. [Google Scholar] [CrossRef]
- Boots, J.-W.P. US8273710B2—Protein Hydrolysate Enriched in Peptides Inhibiting DPP-IV and Their Use. Europen Patent EP1831361B1, 2006. [Google Scholar]
- Church, F.C.; Swaisgood, H.E.; Porter, D.H.; Catignani, G.L. Spectrophotometric assay using o-phthaldialdehyde for determination of proteolysis in milk and isolated milk proteins. J. Dairy Sci. 1983, 66, 1219–1227. [Google Scholar] [CrossRef]
- Aristoy, M.C.; Toldrá, F. Deproteinization techniques for HPLC amino acid analysis in fresh pork muscle and dry-cured ham. J. Agric. Food Chem. 1991, 39, 1792–1795. [Google Scholar] [CrossRef]
- Flores, M.; Aristoy, M.C.; Spanier, A.M.; Toldrá, F. Non-volatile components effects on quality of “Serrano” dry-cured ham as related to processing time. J. Food Sci. 1997, 62, 1235–1239. [Google Scholar] [CrossRef]
- Mooney, C.; Haslam, N.J.; Pollastri, G.; Shields, D.C. Towards the improved discovery and design of functional peptides: Common features of diverse classes permit generalized prediction of bioactivity. PLoS ONE 2012, 7, e45012. [Google Scholar] [CrossRef] [PubMed]
- Minkiewicz, P.; Iwaniak, A.; Darewicz, M. BIOPEP-UWM Database of bioactive peptides: Current opportunities. Int. J. Mol. Sci. 2019, 20, 5978. [Google Scholar] [CrossRef]
- Dimitrov, I.; Bangov, I.; Flower, D.R.; Doytchinova, I. AllerTOP v.2—A server for in silico prediction of allergens. J. Mol. Model. 2014, 20, 2278. [Google Scholar] [CrossRef]
- Gupta, S.; Kapoor, P.; Chaudhary, K.; Gautam, A.; Kumar, R.; Raghava, G.P.S. In silico approach for predicting toxicity of peptides and proteins. PLoS ONE 2013, 8, e73957. [Google Scholar] [CrossRef]





| Sample | Peptide Ranker 1 | Peptide Sequence | MW (g/mol) | Enzyme Action 2 | Active Fragments | Bioactivity | |
|---|---|---|---|---|---|---|---|
| AP | 0.971435 | GNGGGWGNSGGGGGGGGGGNGDGGGGDGCGNSGGCGGGGG | 2926.42 | GN–GGGW–GN–SGGGGGGGGGGN–GDGGGGDGCGN–SGGCGGGGG | - | - | |
| AP | 0.892122 | NCFAAGF | 728.90 | N–CF–AAGF | CF | ACE inhibitor | |
| AP | 0.864842 | PTWALLGCVLLLPSLR | 1752.44 | PTW–AL–L–GCVL–L–L–PSL–R | AL | Dipeptidyl peptidase IV inhibitor | |
| AP | 0.861194 | FIGLFIGISKFMAT | 1545.13 | F–IGL–IGISK–F–M- AT | AT | Dipeptidyl peptidase IV inhibitor | |
| AP | 0.833521 | LGPFSFSFGPAT | 1227.54 | L–GPF–SF–SF–GPAT | SF | ACE inhibitor, dipeptidyl peptidase IV inhibitor and renin inhibitor | |
| AP | 0.715609 | PVGPMGPLGPA | 992.36 | PVGPM–GPL–GPA | GPL–GPA | ACE inhibitor and dipeptidyl peptidase IV inhibitor | |
| AP | 0.704105 | GPAGDAGAEGKPGIPG | 1350.68 | GPAGDAGAEGK–PGIPG | - | - | |
| AP | 0.690027 | AWIRYSKVKFVSFNF | 1892.68 | AW–IR–Y–SK–VK–F–VSF–N–F | AW | ACE inhibitor, antioxidative, dipeptidyl peptidase IV inhibitor | |
| IR | ACE inhibitor, antioxidative, renin inhibitor, CaMPDE inhibitor and dipeptidyl peptidase IV inhibitor | ||||||
| SK | Dipeptidyl peptidase IV inhibitor | ||||||
| VK | ACE inhibitor, dipeptidyl peptidase IV inhibitor | ||||||
| AP | 0.671755 | GAGLLLLEALEKGYWV | 1732.31 | GAGL–L–L–L–EAL–EK–GY–W–V | EK–GY | ACE inhibitor, dipeptidyl peptidase IV inhibitor | |
| AP | 0.670104 | GEKGPLGPNGPVGV | 1277.66 | GEK–GPL–GPN–GPVGV | GPL | ACE inhibitor | |
| AP | 0.63634 | SPSKRFRGWRARTERT | 1991.45 | SPSK–R–F–R–GW–R–AR–TER–T | GW | ACE inhibitor, dipeptidyl peptidase IV inhibitor | |
| AR | ACE inhibitor | ||||||
| AP | 0.607254 | RDGPQGPLGPAG | 1121.39 | R–DGPQGPL–GPAG | GPAG | Dipeptidyl peptidase IV inhibitor | |
| AP | 0.571682 | GPQGKVGPTGAPG | 1122.44 | GPQGK–VGPTGAP | - | - | |
| AP | 0.560064 | GPAGAPGFPGAPGSKGEAGPTGARG | 2121.65 | GPAGAPGF–PGAPGSK–GEAGPTGAR -G | - | - | |
| AP | 0.551038 | RAAELRPLR | 1081.40 | R–AAEL–R–PL–R | PL | ACE inhibitor and dipeptidyl peptidase IV inhibitor | |
| AP | 0.518296 | PMADSGCLTEGEMGLIFVN | 1984.57 | PM–ADSGCL–TEGEM–GL–IF–VN | PM | Dipeptidyl peptidase IV inhibitor | |
| GL | ACE inhibitor and dipeptidyl peptidase IV inhibitor | ||||||
| IF | ACE inhibitor | ||||||
| VN | Dipeptidyl peptidase IV inhibitor | ||||||
| AP | 0.50915 | FSFLPQPPQEKAHDGGRYY | 2237.73 | F–SF–L–PQPPQEK–AH–DGGR–Y–Y | SF | ACE inhibitor, dipeptidyl peptidase IV inhibitor and renin inhibitor | |
| AH | ACE inhibitor, antioxidative and dipeptidyl peptidase IV inhibitor | ||||||
| 18 | APP | 0.584356 | KCYTPVCLK | 1054.45 | K–CY–TPVCL–K | - | - |
| Characteristics | GPF | GGGW | IGL | AW | GW |
|---|---|---|---|---|---|
| MW (g/mol) | 319.39 | 375.44 | 301.43 | 275.32 | 261.30 |
| Charge | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Isoelectric point (pI) | 5.88 | 5.88 | 5.88 | 5.88 | 5.88 |
| Steric hindrance | 0.58 | 0.64 | 0.64 | 0.51 | 0.59 |
| Sidebulk | 0.58 | 0.64 | 0.64 | 0.51 | 0.59 |
| Hydrophobicity | 0.23 | 0.21 | 0.47 | 0.31 | 0.27 |
| Hydrophilicity | −0.83 | −0.85 | −1.20 | −1.95 | −1.70 |
| Amphipathicity | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| IC50 (mM) | 0.94 | 2.73 | 2.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrera-Alvarado, G.; Toldrá, F.; Mora, L. DPP-IV Inhibitory Peptides GPF, IGL, and GGGW Obtained from Chicken Blood Hydrolysates. Int. J. Mol. Sci. 2022, 23, 14140. https://doi.org/10.3390/ijms232214140
Carrera-Alvarado G, Toldrá F, Mora L. DPP-IV Inhibitory Peptides GPF, IGL, and GGGW Obtained from Chicken Blood Hydrolysates. International Journal of Molecular Sciences. 2022; 23(22):14140. https://doi.org/10.3390/ijms232214140
Chicago/Turabian StyleCarrera-Alvarado, Gisela, Fidel Toldrá, and Leticia Mora. 2022. "DPP-IV Inhibitory Peptides GPF, IGL, and GGGW Obtained from Chicken Blood Hydrolysates" International Journal of Molecular Sciences 23, no. 22: 14140. https://doi.org/10.3390/ijms232214140
APA StyleCarrera-Alvarado, G., Toldrá, F., & Mora, L. (2022). DPP-IV Inhibitory Peptides GPF, IGL, and GGGW Obtained from Chicken Blood Hydrolysates. International Journal of Molecular Sciences, 23(22), 14140. https://doi.org/10.3390/ijms232214140








