Effect of the Production Parameters and In Vitro Digestion on the Content of Polyphenolic Compounds, Phenolic Acids, and Antiradical Properties of Innovative Snacks Enriched with Wild Garlic (Allium ursinum L.) Leaves
Abstract
1. Introduction
2. Results and Discussion
2.1. Influence of A. ursinum Addition and Screw Speed on Polyphenols Content, Free Phenolic Acid Content and Antioxidant Properties of Snacks
2.2. The Digestability of the Snack’s Polyphenols Using Two-Stage In Vitro Human Digestion Model
3. Materials and Methods
3.1. Plant Materials
3.2. Extrusion-Cooking Procedure
3.3. Extraction Procedure
3.4. Hydrolysis of the Samples
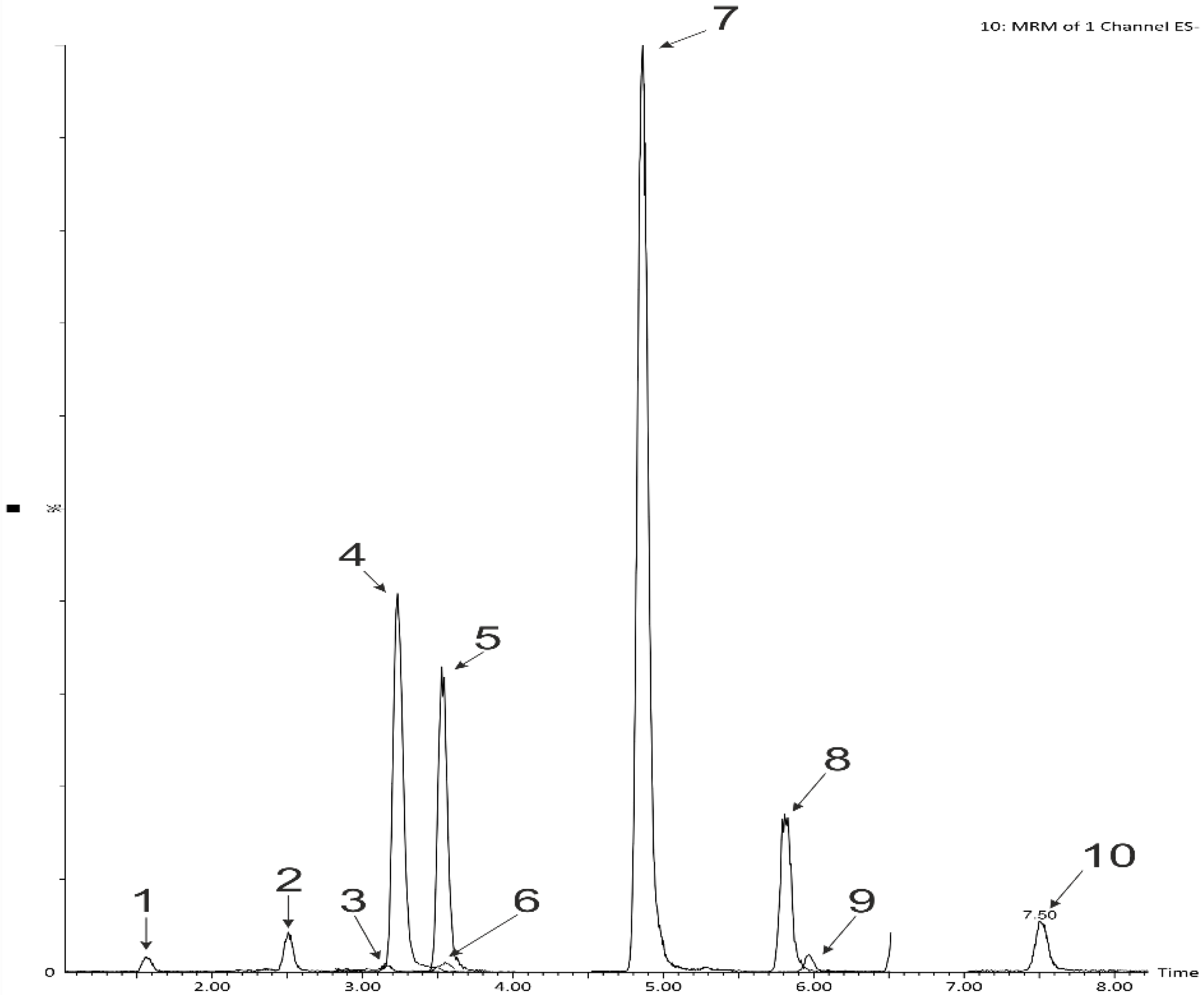
3.5. Determination of Phenolic Acids
3.6. Determination of the Total Content of Polyphenolic Compounds (TPC)
3.7. Ability to Scavenge DPPH
3.8. In Vitro Two-Stages Digestion Model
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A Concise Overview on the Chemistry, Occurrence, and Human Health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.C.F.R.; Barros, L. (Eds.) Functional Food Ingredients from Plants, 1st ed.; Academic Press: Cambridge, MA, USA, 2019; ISBN 978-0-12-816567-6. [Google Scholar]
- Olszowy, M. What Is Responsible for Antioxidant Properties of Polyphenolic Compounds from Plants? Plant Physiol. Biochem. 2019, 144, 135–143. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic Acids: Natural Versatile Molecules with Promising Therapeutic Applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, D.E.; Hurst, R.D. Polyphenolic Phytochemicals—Just Antioxidants or Much More? Cell. Mol. Life Sci. 2007, 64, 2900–2916. [Google Scholar] [CrossRef]
- Brewer, M.S. Natural Antioxidants: Sources, Compounds, Mechanisms of Action, and Potential Applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and Antioxidant Methods: An Updated Overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed]
- Seraglio, S.K.T.; Valese, A.C.; Daguer, H.; Bergamo, G.; Azevedo, M.S.; Nehring, P.; Gonzaga, L.V.; Fett, R.; Costa, A.C.O. Effect of in Vitro Gastrointestinal Digestion on the Bioaccessibility of Phenolic Compounds, Minerals, and Antioxidant Capacity of Mimosa Scabrella Bentham Honeydew Honeys. Food Res. Int. 2017, 99, 670–678. [Google Scholar] [CrossRef]
- Kasprzak, K.; Oniszczuk, T.; Wójtowicz, A.; Waksmundzka-Hajnos, M.; Olech, M.; Nowak, R.; Polak, R.; Oniszczuk, A. Phenolic Acid Content and Antioxidant Properties of Extruded Corn Snacks Enriched with Kale. J. Anal. Methods Chem. 2018, 2018, e7830546. [Google Scholar] [CrossRef]
- Jucá, M.M.; Filho, F.M.S.C.; De Almeida, J.C.; Mesquita, D.D.S.; Barriga, J.R.D.M.; Dias, K.C.F.; Barbosa, T.M.; Vasconcelos, L.C.; Leal, L.K.A.M.; Ribeiro, J.E.; et al. Flavonoids: Biological Activities and Therapeutic Potential. Nat. Prod. Res. 2020, 34, 692–705. [Google Scholar] [CrossRef]
- Kumari, P.; Kumar, V.; Kumar, R.; Pahuja, S.K. Sorghum Polyphenols: Plant Stress, Human Health Benefits, and Industrial Applications. Planta 2021, 254, 47. [Google Scholar] [CrossRef]
- Kasprzak, K.; Wojtunik-Kulesza, K.; Oniszczuk, T.; Kuboń, M.; Oniszczuk, A. Secondary Metabolites, Dietary Fiber and Conjugated Fatty Acids as Functional Food Ingredients against Overweight and Obesity. Nat. Prod. Commun. 2018, 13, 1934578X1801300836. [Google Scholar] [CrossRef]
- Sobolewska, D.; Podolak, I.; Makowska-Wąs, J. Allium Ursinum: Botanical, Phytochemical and Pharmacological Overview. Phytochem. Rev. 2015, 14, 81–97. [Google Scholar] [CrossRef]
- Rejewski, M. Pochodzenie Łacińskich Nazw Roślin Polskich. In Przewodnik Botaniczny; Książka i Wiedza: Warszawa, Poland, 1996. [Google Scholar]
- Oszmiański, J.; Kolniak-Ostek, J.; Wojdyło, A. Characterization and Content of Flavonol Derivatives of Allium ursinum L. Plant. J. Agric. Food Chem. 2013, 61, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Tomšik, A.; Pavlić, B.; Vladić, J.; Ramić, M.; Brindza, J.; Vidović, S. Optimization of Ultrasound-Assisted Extraction of Bioactive Compounds from Wild Garlic (Allium ursinum L.). Ultrason. Sonochem 2016, 29, 502–511. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.; Oniszczuk, A.; Oniszczuk, T.; Combrzyński, M.; Nowakowska, D.; Matwijczuk, A. Influence of In Vitro Digestion on Composition, Bioaccessibility and Antioxidant Activity of Food Polyphenols—A Non-Systematic Review. Nutrients 2020, 12, 1401. [Google Scholar] [CrossRef] [PubMed]
- Papillo, V.A.; Vitaglione, P.; Graziani, G.; Gokmen, V.; Fogliano, V. Release of Antioxidant Capacity from Five Plant Foods during a Multistep Enzymatic Digestion Protocol. J. Agric. Food Chem. 2014, 62, 4119–4126. [Google Scholar] [CrossRef]
- Olech, M.; Kasprzak, K.; Wójtowicz, A.; Oniszczuk, T.; Nowak, R.; Waksmundzka-Hajnos, M.; Combrzyński, M.; Gancarz, M.; Kowalska, I.; Krajewska, A.; et al. Polyphenol Composition and Antioxidant Potential of Instant Gruels Enriched with Lycium barbarum L. Fruit. Molecules 2020, 25, 4538. [Google Scholar] [CrossRef]
- Oniszczuk, A.; Wójtowicz, A.; Oniszczuk, T.; Olech, M.; Wojtunik-Kulesza, K.; Klimek, M.; Krawczyk, W.; Hajnos, M. Extruded Corn Gruels Containing Linden Flowers: Quantitation of Phenolic Compounds and Selected Quality Characteristics. Open Chem. 2015, 13, 1209–1217. [Google Scholar] [CrossRef]
- Djurdjevic, L.; Dinic, A.; Pavlovic, P.; Mitrovic, M.; Karadzic, B.; Tesevic, V. Allelopathic Potential of Allium ursinum L. Biochem. Syst. Ecol. 2004, 6, 533–544. [Google Scholar] [CrossRef]
- Rommel, A.; Wrolstad, R. Influence of Acid and Base Hydrolysis on the Phenolic Composition of Red Raspberry Juice. J. Agric. Food Chem. 1993, 41, 1237–1241. [Google Scholar] [CrossRef]
- Pop, R.M.; Bocsan, I.C.; Buzoianu, A.D.; Chedea, V.S.; Socaci, S.A.; Pecoraro, M.; Popolo, A. Evaluation of the Antioxidant Activity of Nigella Sativa L. and Allium Ursinum Extracts in a Cellular Model of Doxorubicin-Induced Cardiotoxicity. Molecules 2020, 25, 5259. [Google Scholar] [CrossRef] [PubMed]
- Multari, S.; Marsol-Vall, A.; Keskitalo, M.; Yang, B.; Suomela, J.P. Effects of Different Drying Temperatures on the Content of Phenolic Compounds and Carotenoids in Quinoa Seeds (Chenopodium Quinoa) from Finland. J. Food Compos. Anal. 2018, 72, 75–82. [Google Scholar] [CrossRef]
- Sani, I.M.; Iqbal, S.; Chan, K.W.; Ismail, M. Effect of Acid and Base Catalyzed Hydrolysis on the Yield of Phenolics and Antioxidant Activity of Extracts from Germinated Brown Rice (GBR). Molecules 2012, 17, 7584–7594. [Google Scholar] [CrossRef] [PubMed]
- Patrón-Vázquez, J.; Baas-Dzul, L.; Medina-Torres, N.; Ayora-Talavera, T.; Sánchez-Contreras, Á.; García-Cruz, U.; Pacheco, N. The Effect of Drying Temperature on the Phenolic Content and Functional Behavior of Flours Obtained from Lemon Wastes. Agronomy 2019, 9, 474. [Google Scholar] [CrossRef]
- Korus, J.; Gumul, D.; Czechowska, K. Effect of Extrusion on the Phenolic Composition and Antioxidant Activity of Dry Beans of Phaseolus vulgaris L. Food Technol. Biotechnol. 2007, 45, 277375. [Google Scholar]
- Bernardino-Nicanor, A.; Montañéz-Soto, J.L.; Vivar-Vera, M.A.; Juárez-Goiz, J.M.; Acosta-García, G.; González-Cruz, L. Effect of Drying on the Antioxidant Capacity and Concentration of Phenolic Compounds in Different Parts of the Erythrina Americana Tree. BioResources 2016, 11, 9741–9755. [Google Scholar] [CrossRef]
- Guerrero, B.G.; Montero-Montero, J.C.; Fernández-Quintero, A.; Rivera-Agredo, Y.J.; Ospina-Patiño, B.; Gallego-Castillo, S. Assessing the Effect of Adding Maize and Rice Brans in the Development by Twin-Screw Extrusion of a Ready-to-Eat Cereal Formulated with Flours of Quality Protein Maize and Zinc Biofortified Rice. DYNA 2019, 86, 298–303. [Google Scholar] [CrossRef]
- Schmid, V.; Trabert, A.; Schäfer, J.; Bunzel, M.; Karbstein, H.P.; Emin, M.A. Modification of Apple Pomace by Extrusion Processing: Studies on the Composition, Polymer Structures, and Functional Properties. Foods 2020, 9, 1385. [Google Scholar] [CrossRef]
- Khanal, R.C.; Howard, L.R.; Prior, R.L. Procyanidin Content of Grape Seed and Pomace, and Total Anthocyanin Content of Grape Pomace as Affected by Extrusion Processing. J. Food Sci. 2009, 74, H174–H182. [Google Scholar] [CrossRef]
- Ramírez-Anaya, J.D.P.; Samaniego-Sánchez, C.; Castañeda-Saucedo, M.C.; Villalón-Mir, M.; de la Serrana, H.L.-G. Phenols and the Antioxidant Capacity of Mediterranean Vegetables Prepared with Extra Virgin Olive Oil Using Different Domestic Cooking Techniques. Food Chem. 2015, 188, 430–438. [Google Scholar] [CrossRef]
- Alonso, R.; Grant, G.; Dewey, P.; Marzo, F. Nutritional Assessment In Vitro and In Vivo of Raw and Extruded Peas (Pisum sativum L.). J. Agric. Food Chem. 2000, 48, 2286–2290. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, Y.; Toledo, R. Antioxidant Activity of Water-Soluble Maillard Reaction Products. Food Chem. 2005, 93, 273–278. [Google Scholar] [CrossRef]
- Brennan, C.; Brennan, M.; Derbyshire, E.; Tiwari, B.K. Effects of Extrusion on the Polyphenols, Vitamins and Antioxidant Activity of Foods. Trends Food Sci. Technol. 2011, 22, 570–575. [Google Scholar] [CrossRef]
- Singh, S.; Gamlath, S.; Wakeling, L. Nutritional Aspects of Food Extrusion: A Review. Int. J. Food Sci. 2007, 42, 916–929. [Google Scholar] [CrossRef]
- Doniec, J.; Florkiewicz, A.; Dziadek, K.; Filipiak-Florkiewicz, A. Hydrothermal Treatment Effect on Antioxidant Activity and Polyphenols Concentration and Profile of Brussels Sprouts (Brassica Oleracea Var. Gemmifera) in an In Vitro Simulated Gastrointestinal Digestion Model. Antioxidants 2022, 11, 446. [Google Scholar] [CrossRef]
- Schulze, K. Imaging and Modelling of Digestion in the Stomach and the Duodenum. Neurogastroenterol. Motil. 2006, 18, 172–183. [Google Scholar] [CrossRef]
- Clarysse, S.; Tack, J.; Lammert, F.; Duchateau, G.; Reppas, C.; Augustijns, P. Postprandial Evolution in Composition and Characteristics of Human Duodenal Fluids in Different Nutritional States. J. Pharm. Sci. 2009, 98, 1177–1192. [Google Scholar] [CrossRef]
- Dupont, D.; Alric, M.; Blanquet-Diot, S.; Bornhorst, G.; Cueva, C.; Deglaire, A.; Denis, S.; Ferrua, M.; Havenaar, R.; Lelieveld, J.; et al. Can Dynamic In Vitro Digestion Systems Mimic the Physiological Reality? Crit. Rev. Food Sci. Nutr. 2019, 59, 1546–1562. [Google Scholar] [CrossRef]
- Mateo Anson, N.; van den Berg, R.; Havenaar, R.; Bast, A.; Haenen, G.R.M.M. Bioavailability of Ferulic Acid Is Determined by Its Bioaccessibility. J. Cereal Sci. 2009, 49, 296–300. [Google Scholar] [CrossRef]
- Siracusa, L.; Kulisic-Bilusic, T.; Politeo, O.; Krause, I.; Dejanovic, B.; Ruberto, G. Phenolic Composition and Antioxidant Activity of Aqueous Infusions from Capparis spinosa L. and Crithmum maritimum L. before and after Submission to a Two-Step In Vitro Digestion Model. J. Agric. Food Chem. 2011, 59, 12453–12459. [Google Scholar] [CrossRef]
- Baeza, G.; Sarriá, B.; Bravo, L.; Mateos, R. Polyphenol Content, in Vitro Bioaccessibility and Antioxidant Capacity of Widely Consumed Beverages. J. Sci. Food Agric. 2018, 98, 1397–1406. [Google Scholar] [CrossRef]
- Dacrema, M.; Sommella, E.; Santarcangelo, C.; Bruno, B.; Marano, M.G.; Insolia, V.; Saviano, A.; Campiglia, P.; Stornaiuolo, M.; Daglia, M. Metabolic Profiling, In Vitro Bioaccessibility and in Vivo Bioavailability of a Commercial Bioactive Epilobium angustifolium L. Extract. Biomed Pharm. 2020, 131, 110670. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Capanoglu, E.; Bilen, F.D.; Gonzales, G.B.; Grootaert, C.; Van de Wiele, T.; Van Camp, J. Bioaccessibility of Polyphenols from Plant-Processing Byproducts of Black Carrot (Daucus carota L.). J. Agric. Food Chem. 2016, 64, 2450–2458. [Google Scholar] [CrossRef]
- Majdoub, Y.O.E.; Ginestra, G.; Mandalari, G.; Dugo, P.; Mondello, L.; Cacciola, F. The Digestibility of Hibiscus sabdariffa L. Polyphenols Using an In Vitro Human Digestion Model and Evaluation of Their Antimicrobial Activity. Nutrients 2021, 13, 2360. [Google Scholar] [CrossRef]
- Gayoso, L.; Claerbout, A.-S.; Calvo, M.I.; Cavero, R.Y.; Astiasarán, I.; Ansorena, D. Bioaccessibility of rutin, caffeic acid and rosmarinic acid: Influence of the in vitro gastrointestinal digestion models. J. Funct. Foods 2016. [Google Scholar] [CrossRef]
- Tagliazucchi, D.; Verzelloni, E.; Bertolini, D.; Conte, A. In Vitro Bio-Accessibility and Antioxidant Activity of Grape Polyphenols. Food Chem. 2010, 120, 599–606. [Google Scholar] [CrossRef]
- Cristea, E.; Sturza, R.; Jauregi, P.; Niculaua, M.; Ghendov-Moșanu, A.; Patras, A. Influence of PH and Ionic Strength on the Color Parameters and Antioxidant Properties of an Ethanolic Red Grape Marc Extract. J. Food Biochem. 2019, 43, e12788. [Google Scholar] [CrossRef] [PubMed]
- Materska, M.; Olszówka, K.; Chilczuk, B.; Stochmal, A.; Pecio, Ł.; Pacholczyk-Sienicka, B.; Piacente, S.; Pizza, C.; Masullo, M. Polyphenolic Profiles in Lettuce (Lactuca sativa L.) after CaCl2 Treatment and Cold Storage. Eur. Food Res. Technol. 2019, 245, 733–744. [Google Scholar] [CrossRef]
- Altunkaya, A.; Gökmen, V.; Skibsted, L.H. PH Dependent Antioxidant Activity of Lettuce (L. Sativa) and Synergism with Added Phenolic Antioxidants. Food Chem. 2016, 190, 25–32. [Google Scholar] [CrossRef]
- Sun, H.-N.; Mu, T.-H.; Xi, L.-S. Effect of PH, Heat, and Light Treatments on the Antioxidant Activity of Sweet Potato Leaf Polyphenols. Int. J. Food Prop. 2017, 20, 318–332. [Google Scholar] [CrossRef]
- Kong, F.; Singh, R.P. A Human Gastric Simulator (HGS) to Study Food Digestion in Human Stomach. J. Food Sci. 2010, 75, E627–E635. [Google Scholar] [CrossRef] [PubMed]
- Czaban, J.; Sułek, A.; Pecio, Ł.; Zuchowski, J.; Podolska, G. Effect of Genotype and Crop Management Systems on Phenolic Acid Content in Winter Wheat Grain. J. Food Agric. Environ. 2013, 11, 1201–1206. [Google Scholar]
- Burda, S.; Oleszek, W. Antioxidant and Antiradical Activities of Flavonoids. J. Agric. Food Chem. 2001, 49, 2774–2779. [Google Scholar] [CrossRef] [PubMed]


| Wild Garlic Addition | Polyphenols Content, 80 rpm | Polyphenols Content, 120 rpm |
|---|---|---|
| 0% | 0.288 ± 0.008 | 0.381 ± 0.002 |
| 1% | 0.302 ± 0.007 | 0.400 ± 0.003 |
| 2% | 0.388 ± 0.002 | 0.403 ± 0.022 |
| 3% | 0.440 ± 0.011 | 0.445 ± 0.016 |
| 4% | 0.603 ± 0.009 | 0.610 ± 0.018 |
| Content of Phenolic Acid (µg/g d.w.) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Screw Speed | Garlic Addition | Protokatechuic | 4-OH-Benzoic | Vanilic | Caffeic | Syringic | Coumaric | Ferulic | Sinapic | Salicylic | Sum |
| 80 rpm | 0% | 0.406 ± 0.01 | 4.012 ± 0.20 | 0.121 ± 0.00 | 30.358 ± 1.72 | 8.817 ± 0.21 | 64.058 ± 2.36 | 665.751 ± 3.22 | 63.950 ± 1.15 | 0.657 ± 0.02 | 838.130 ± 8.89 |
| 1% | 0.574 ± 0.02 | 3.915 ± 0.16 | 0.873 ± 0.01 | 49.192 ± 2.04 | 11.088 ± 0.54 | 165.700 ± 5.24 | 1159.250 ± 12.06 | 82.192 ± 0.97 | 0.877 ± 0.03 | 1473.677 ± 21.07 | |
| 2% | 0.509 ± 0.02 | 4.961 ± 0.18 | 5.083 ± 0.05 | 63.217 ± 0.39 | 11.191 ± 0.27 | 184.117 ± 2.35 | 1216.350 ± 1.28 | 93.850 ± 0.89 | 0.909 ± 0.03 | 1580.187 ± 5.46 | |
| 3% | 0.624 ± 0.03 | 5.026 ± 0.20 | 21.868 ± 0.19 | 66.137 ± 1.49 | 11.253 ± 0.09 | 196.575 ± 3.28 | 1260.917 ± 11.98 | 93.700 ± 2.08 | 1.065 ± 0.01 | 1657.165 ± 19.35 | |
| 4% | 0.442 ± 0.02 | 5.124 ± 0.01 | 52,142 ± 2.04 | 77.700 ± 0.92 | 12.065 ± 0.02 | 212.340 ± 0.34 | 1322.666 ± 7.64 | 105.342 ± 2.97 | 1.155 ± 0.02 | 1788.975 ± 13.98 | |
| 120 rpm | 0% | 0.443 ± 0.00 | 4.928 ± 0.19 | 0.123 ± 0.01 | 32.083 ± 1.13 | 9.521 ± 0.18 | 66,875 ± 1.14 | 516.583 ± 7.09 | 62.108 ± 2.59 | 0.635 ± 0.01 | 693.299 ± 12.34 |
| 1% | 0.666 ± 0.01 | 4.955 ± 0.11 | 2.609 ± 0.02 | 58.508 ± 2.32 | 11.098 ± 0.31 | 173.200 ± 2.54 | 1161.750 ± 1.59 | 81.883 ± 1.38 | 0.779 ± 0.00 | 1495.448 ± 8.28 | |
| 2% | 0.656 ± 0.01 | 5.050 ± 0.09 | 10.038 ± 0.31 | 66.479 ± 1.22 | 11.266 ± 0.16 | 187.716 ± 1.18 | 1225.083 ± 12.58 | 91.633 ± 2.03 | 0.904 ± 0.00 | 1598.825 ± 17.58 | |
| 3% | 0.714 ± 0.00 | 5.035 ± 0.21 | 25.296 ± 0.29 | 67.554 ± 0.38 | 10.694 ± 0.08 | 200.208 ± 5.19 | 1265.333 ± 7.22 | 89.525 ± 0.50 | 1.053 ± 0.02 | 1665.415 ± 13.89 | |
| 4% | 0.751 ± 0.00 | 5.365 ± 0.12 | 96.375 ± 1.15 | 78.383 ± 3.09 | 11.413 ± 0.02 | 216.650 ± 1.94 | 1323.583 ± 0.93 | 102.700 ± 4.02 | 1.130 ± 0.03 | 1836.350 ± 11.71 | |
| Radical Scavenging towards DPPH (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time (min) | Wild Garlic Addition (%), 80 rpm | Wild Garlic Addition (%), 120 rpm | ||||||||
| 0 | 1 | 2 | 3 | 4 | 0 | 1 | 2 | 3 | 4 | |
| 0 | 19.21 ± 0.19 | 22.58 ± 0.15 | 24.69 ± 0.12 | 27.92 ± 0.46 | 28.95 ± 0.96 | 24.11 ± 0.44 | 25.39 ± 0.59 | 27.31 ± 0.71 | 28.92 ± 0.96 | 30.48 ± 0.71 |
| 5 | 60.75 ± 0.43 | 67.92 ± 1.72 | 69.95 ± 0.23 | 74.69 ± 1.29 | 77.63 ± 0.79 | 62.28 ± 2.72 | 69.15 ± 0.81 | 72.92 ± 0.84 | 75.97 ± 1.59 | 79.18 ± 0.16 |
| 10 | 62.32 ± 0.01 | 69.65 ± 0.98 | 76.58 ± 1.14 | 78.19 ± 0.21 | 83.16 ± 0.81 | 65.77 ± 1.34 | 71.53 ± 0.02 | 77.95 ± 0.93 | 80.18 ± 2.21 | 87.68 ± 0.93 |
| 15 | 62.32 ± 0.00 | 69.65 ± 0.81 | 76.50 ± 0.56 | 78.19 ± 0.93 | 83.16 ± 1.73 | 65.77 ± 0.20 | 71.53 ± 1.13 | 77.95 ± 1.07 | 80.18 ± 0.93 | 87.68 ± 0.13 |
| Total Polyphenols | Free Phenolic Acid | DPPH Radical Scavenging Activity | |
|---|---|---|---|
| 80 rpm | |||
| Wild garlic content | 0.952 | 0.891 | 0.979 |
| Total polyphenols | 0.749 | 0.903 | |
| Free phenolic acids | 0.945 | ||
| 120 rpm | |||
| Wild garlic content | 0.849 | 0.873 | 0.991 |
| Total polyphenols | 0.623 | 0.859 | |
| Free phenolic acids | 0.887 | ||
| Wild Garlic Addition | Polyphenols Content | ||
|---|---|---|---|
| Before Digestion | Gastric Digestion | Duodendal Digestion | |
| 0% | 0.221 ± 0.005 | 0.124 ± 0.002 | 0.042 ± 0.002 |
| 2% | 0.307 ± 0.003 | 0.213 ± 0.0227 | 0.110 ± 0.0227 |
| 4% | 0.542 ± 0.016 | 0.390 ± 0.0184 | 0.189 ± 0.0184 |
| Content of Phenolic Acid (µg/g d.w.) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Conditions | Garlic Addition | Protokatechuic | 4-OH-benzoic | Vanillic | Caffeic | Syringic | Coumaric | Ferulic | Sinapic | Salicylic | Sum |
| Before digestion | 0% | 0.131 ± 0.003 | 0.235 ± 0.001 | 0.456 ± 0.002 | 1.374 ± 0.022 | 0.196 ± 0.000 | 4.374 ± 0.051 | 2.381 ± 0.003 | 0.324 ± 0.006 | 0.082 ± 0.000 | 9.553 ± 0.088 |
| 2% | 0.170 ± 0.001 | 0.272 ± 0.002 | 0.588 ± 0.008 | 1.387 ± 0.017 | 0.271 ± 0.004 | 6.121 ± 0.009 | 3.697 ± 0.071 | 0.362 ± 0.001 | 0.156 ± 0.004 | 13.024 ± 0.117 | |
| 4% | 0.192 ± 0.002 | 0.296 ± 0.000 | 0.599 ± 0.010 | 1.808 ± 0.041 | 0.336 ± 0.003 | 7.878 ± 0.085 | 5.128 ± 0.011 | 0.556 ± 0.003 | 0.193 ± 0.002 | 16.986 ± 0.002 | |
| After digestion | 0% I | - | - | - | 0.079 ± 0.000 | - | 0.428 ± 0.001 | 0.236 ± 0.000 | - | - | 0.743 ± 0.157 |
| 0% II | - | - | - | - | - | 0.218 ± 0.000 | - | - | - | 0.218 ± 0.000 | |
| 2% I | - | - | - | 0.132 ± 0.001 | - | 1.356 ± 0.023 | 0.397 ± 0.003 | - | - | 1.885 ± 0.027 | |
| 2% II | - | - | - | - | - | 0.477 ± 0.001 | - | - | - | 0.477 ± 0.001 | |
| 4% I | - | - | - | 0.144 ± 0.001 | - | 1.893 ± 0.003 | 0.502 ± 0.007 | - | - | 2.539 ± 0.011 | |
| 4% II | - | - | - | - | - | 0.754 ± 0.008 | - | - | - | 0.754 ± 0.008 | |
| Radical Scavenging (%) before Digestion | Radical Scavenging (%) after Gastric Digestion | Radical Scavenging (%) after Duodendal Digestion | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Time | Wild Garlic Addition (%) | ||||||||
| 0 | 2 | 4 | 0 | 2 | 4 | 0 | 2 | 4 | |
| 0 | 15.41 ± 0.05 | 21.08 ± 0.54 | 25.91 ±1.06 | 11.21 ± 0.08 | 21.08 ± 0.54 | 28.10 ±0.67 | 10.32 ± 0.34 | 20.01 ± 1.15 | 24.91 ±0.39 |
| 5 | 58.73 ± 0.83 | 62.22 ± 0.49 | 75.16 ± 1.78 | 24.07 ± 0.34 | 32.76 ± 0.33 | 36.55 ± 1.17 | 18.07 ± 0.94 | 29.17 ± 0.13 | 31.79 ± 0.45 |
| 10 | 59.15 ± 0.24 | 73.56 ± 2.09 | 82.16 ± 0.78 | 38.23 ± 0.86 | 51.56 ± 0.76 | 59.98 ± 0.98 | 29.23 ± 0.89 | 38.56 ± 1.26 | 46.98 ± 0.57 |
| 15 | 59.15 ± 0.00 | 73.56 ± 0.08 | 82.16 ± 2.73 | 38.23 ± 0.12 | 51.56 ± 2.18 | 59.98 ± 1.11 | 29.23 ± 1.07 | 38.56 ± 1.19 | 46.02 ± 2.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasprzak-Drozd, K.; Oniszczuk, T.; Kowalska, I.; Mołdoch, J.; Combrzyński, M.; Gancarz, M.; Dobrzański, B., Jr.; Kondracka, A.; Oniszczuk, A. Effect of the Production Parameters and In Vitro Digestion on the Content of Polyphenolic Compounds, Phenolic Acids, and Antiradical Properties of Innovative Snacks Enriched with Wild Garlic (Allium ursinum L.) Leaves. Int. J. Mol. Sci. 2022, 23, 14458. https://doi.org/10.3390/ijms232214458
Kasprzak-Drozd K, Oniszczuk T, Kowalska I, Mołdoch J, Combrzyński M, Gancarz M, Dobrzański B Jr., Kondracka A, Oniszczuk A. Effect of the Production Parameters and In Vitro Digestion on the Content of Polyphenolic Compounds, Phenolic Acids, and Antiradical Properties of Innovative Snacks Enriched with Wild Garlic (Allium ursinum L.) Leaves. International Journal of Molecular Sciences. 2022; 23(22):14458. https://doi.org/10.3390/ijms232214458
Chicago/Turabian StyleKasprzak-Drozd, Kamila, Tomasz Oniszczuk, Iwona Kowalska, Jarosław Mołdoch, Maciej Combrzyński, Marek Gancarz, Bohdan Dobrzański, Jr., Adrianna Kondracka, and Anna Oniszczuk. 2022. "Effect of the Production Parameters and In Vitro Digestion on the Content of Polyphenolic Compounds, Phenolic Acids, and Antiradical Properties of Innovative Snacks Enriched with Wild Garlic (Allium ursinum L.) Leaves" International Journal of Molecular Sciences 23, no. 22: 14458. https://doi.org/10.3390/ijms232214458
APA StyleKasprzak-Drozd, K., Oniszczuk, T., Kowalska, I., Mołdoch, J., Combrzyński, M., Gancarz, M., Dobrzański, B., Jr., Kondracka, A., & Oniszczuk, A. (2022). Effect of the Production Parameters and In Vitro Digestion on the Content of Polyphenolic Compounds, Phenolic Acids, and Antiradical Properties of Innovative Snacks Enriched with Wild Garlic (Allium ursinum L.) Leaves. International Journal of Molecular Sciences, 23(22), 14458. https://doi.org/10.3390/ijms232214458










