Mechanisms of Male Reproductive Toxicity of Polybrominated Diphenyl Ethers
Abstract
1. Introduction
2. Results
2.1. Reproductive Health Studies in Humans
2.2. Reproductive Health Outcomes in Animal Studies
2.2.1. Developmental Effects
2.2.2. Adult Effects
2.3. Most Sensitive Outcomes
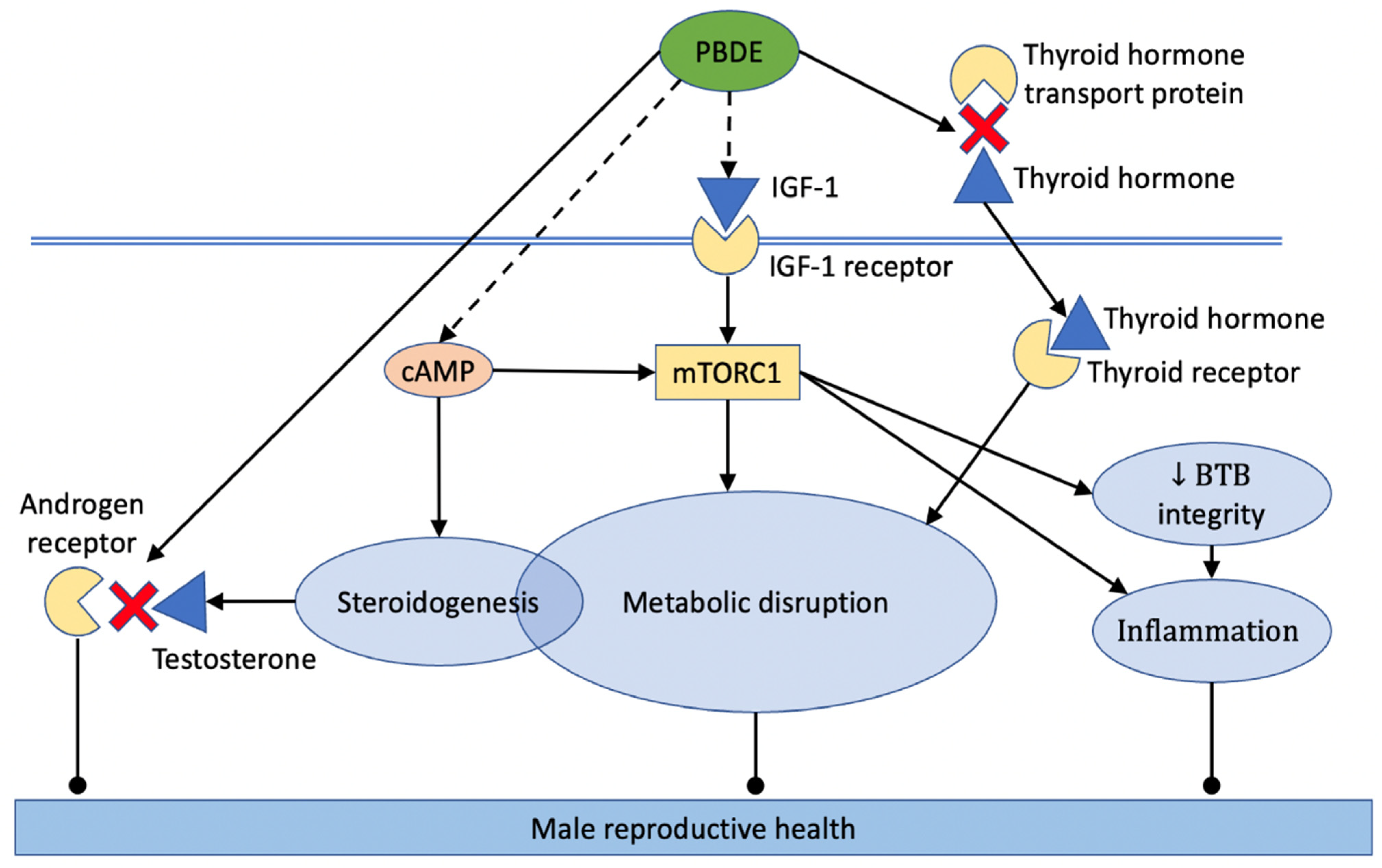
2.4. Mechanisms That Mediate Male Reproductive Toxicity of PBDE
2.4.1. Induction of Oxidative Stress
2.4.2. Metabolic Disruption
2.4.3. Inflammatory Response
2.4.4. Disruption of Blood–Testis Barrier (BTB)
2.4.5. Endocrine Disruption: Testosterone Signaling
2.4.6. Endocrine Disruption: Estrogen Signaling
2.4.7. Endocrine Disruption: Luteinizing Hormone (LH) Signaling
2.4.8. Endocrine Disruption: Follicle-Stimulating Hormone (FSH) Signaling
2.4.9. Endocrine Disruption: Inhibin-B and Sex Hormone-Binding Globulin (SHBG)
2.4.10. Thyroid Hormone Signaling
2.4.11. Insulin-like Growth Factor (IGF) and Mechanistic Target of Rapamycin (mTOR)
2.4.12. Disruption of Steroidogenesis
2.4.13. Mitochondria Disruption and Cell Apoptosis
3. Materials and Methods
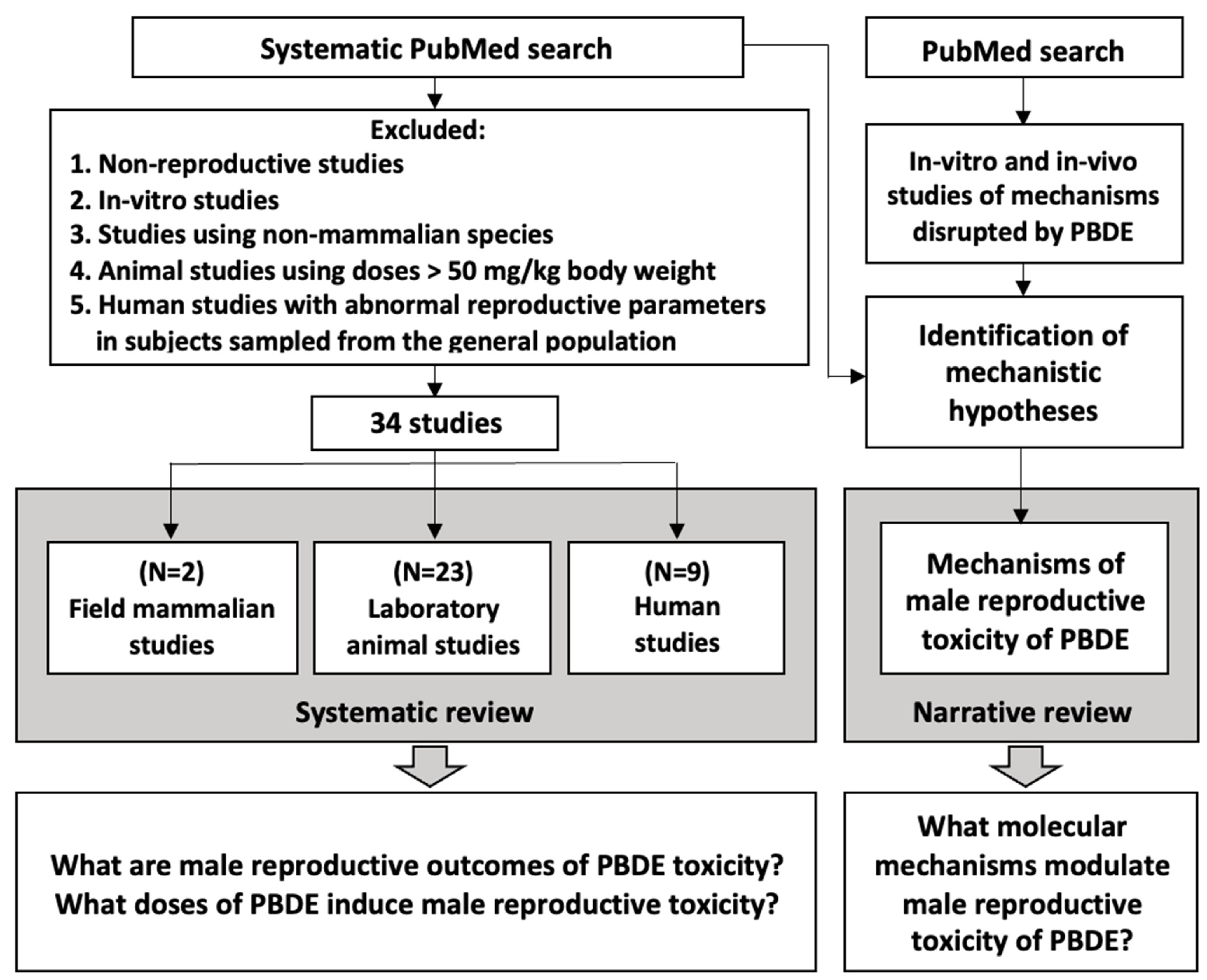
3.1. Identification of Male Reproductive Outcomes Sensitive to PBDE
3.2. Narrative Approach
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Saghir, S.A. Polybrominated Biphenyls (PBBs). In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 978-0-12-801238-3. [Google Scholar]
- Allen, J.G.; Gale, S.; Zoeller, R.T.; Spengler, J.D.; Birnbaum, L.; McNeely, E. PBDE Flame Retardants, Thyroid Disease, and Menopausal Status in U.S. Women. Environ. Health 2016, 15, 60. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Kim, G.B. An Overview of Polybrominated Diphenyl Ethers (PBDEs) in the Marine Environment. Ocean Sci. J. 2015, 50, 119–142. [Google Scholar] [CrossRef]
- Siddiqi, M.A.; Laessig, R.H.; Reed, K.D. Polybrominated Diphenyl Ethers (PBDEs): New Pollutants-Old Diseases. Clin. Med. Res. 2003, 1, 281–290. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, X.; Lin, Z.; Na, G.; Yao, Z. Congener Specific Distributions of Polybrominated Diphenyl Ethers (PBDEs) in Sediment and Mussel (Mytilus Edulis) of the Bo Sea, China. Chemosphere 2009, 74, 896–901. [Google Scholar] [CrossRef] [PubMed]
- Suvorov, A.; Takser, L. Facing the challenge of data transfer from animal models to humans: The case of persistent organohalogens. Environmental Health 2008, 7, 58. [Google Scholar] [CrossRef]
- Portet-Koltalo, F.; Guibert, N.; Morin, C.; de Mengin-Fondragon, F.; Frouard, A. Evaluation of Polybrominated Diphenyl Ether (PBDE) Flame Retardants from Various Materials in Professional Seating Furnishing Wastes from French Flows. Waste Manag. 2021, 131, 108–116. [Google Scholar] [CrossRef]
- Abbasi, G.; Li, L.; Breivik, K. Global Historical Stocks and Emissions of PBDEs. Environ. Sci. Technol. 2019, 53, 6330–6340. [Google Scholar] [CrossRef]
- Sjödin, A.; Mueller, J.F.; Jones, R.; Schütze, A.; Wong, L.-Y.; Caudill, S.P.; Harden, F.A.; Webster, T.F.; Toms, L.-M. Serum Elimination Half-Lives Adjusted for Ongoing Exposure of Tri-to Hexabrominated Diphenyl Ethers: Determined in Persons Moving from North America to Australia. Chemosphere 2020, 248, 125905. [Google Scholar] [CrossRef]
- Ether, R.D. An Alternatives Assessment for the Flame Retardant Decabromodiphenyl Ether (DecaBDE). 2014. Available online: https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/alternatives-assessment-flame-retardant (accessed on 15 November 2021).
- Leonetti, C.; Butt, C.M.; Hoffman, K.; Miranda, M.L.; Stapleton, H.M. Concentrations of Polybrominated Diphenyl Ethers (PBDEs) and 2,4,6-Tribromophenol in Human Placental Tissues. Environ. Int. 2016, 88, 23–29. [Google Scholar] [CrossRef]
- Linares, V.; Bellés, M.; Domingo, J.L. Human Exposure to PBDE and Critical Evaluation of Health Hazards. Arch. Toxicol. 2015, 89, 335–356. [Google Scholar] [CrossRef]
- Byrne, S.C.; Miller, P.; Seguinot-Medina, S.; Waghiyi, V.; Buck, C.L.; von Hippel, F.A.; Carpenter, D.O. Associations between Serum Polybrominated Diphenyl Ethers and Thyroid Hormones in a Cross Sectional Study of a Remote Alaska Native Population. Sci. Rep. 2018, 8, 2198. [Google Scholar] [CrossRef] [PubMed]
- Moreira Bastos, P.; Eriksson, J.; Vidarson, J.; Bergman, A. Oxidative Transformation of Polybrominated Diphenyl Ether Congeners (PBDEs) and of Hydroxylated PBDEs (OH-PBDEs). Environ. Sci. Pollut. Res. Int. 2008, 15, 606–613. [Google Scholar] [CrossRef]
- Lavandier, R.; Quinete, N.; Hauser-Davis, R.A.; Dias, P.S.; Taniguchi, S.; Montone, R.; Moreira, I. Polychlorinated Biphenyls (PCBs) and Polybrominated Diphenyl Ethers (PBDEs) in Three Fish Species from an Estuary in the Southeastern Coast of Brazil. Chemosphere 2013, 90, 2435–2443. [Google Scholar] [CrossRef]
- Kim, J.S.; Klösener, J.; Flor, S.; Peters, T.M.; Ludewig, G.; Thorne, P.S.; Robertson, L.W.; Luthe, G. Toxicity Assessment of Air-Delivered Particle-Bound Polybrominated Diphenyl Ethers. Toxicology 2014, 317, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Trudel, D.; Scheringer, M.; von Goetz, N.; Hungerbühler, K. Total Consumer Exposure to Polybrominated Diphenyl Ethers in North America and Europe. Environ. Sci. Technol. 2011, 45, 2391–2397. [Google Scholar] [CrossRef]
- Geyer, H.J.; Schramm, K.-W.; Darnerud, P.O.; Aune, M.; Feicht, A.; Fried, K.W.; Henkelmann, B.; Lenoir, D.; Schmid, P.; McDonald, T.A. Terminal Elimination Half-Lives of the Brominated Flame Retardants TBBPA, HBCD, and Lower Brominated PBDEs in Humans. Organohalogen Compd. 2004, 66, 6. [Google Scholar]
- Johnson-Restrepo, B.; Kannan, K.; Rapaport, D.P.; Rodan, B.D. Polybrominated Diphenyl Ethers and Polychlorinated Biphenyls in Human Adipose Tissue from New York. Environ. Sci. Technol. 2005, 39, 5177–5182. [Google Scholar] [CrossRef]
- Hurley, S.; Goldberg, D.; Nelson, D.O.; Guo, W.; Wang, Y.; Baek, H.-G.; Park, J.-S.; Petreas, M.; Bernstein, L.; Anton-Culver, H.; et al. Temporal Evaluation of Polybrominated Diphenyl Ether (PBDE) Serum Levels in Middle-Aged and Older California Women, 2011–2015. Environ. Sci. Technol. 2017, 51, 4697–4704. [Google Scholar] [CrossRef]
- Turyk, M.E.; Persky, V.W.; Imm, P.; Knobeloch, L.; Chatterton, R.; Anderson, H.A. Hormone Disruption by PBDEs in Adult Male Sport Fish Consumers. Environ. Health Perspect. 2008, 116, 1635–1641. [Google Scholar] [CrossRef]
- Abdelouahab, N.; Ainmelk, Y.; Takser, L. Polybrominated Diphenyl Ethers and Sperm Quality. Reprod. Toxicol. 2011, 31, 546–550. [Google Scholar] [CrossRef]
- Kodavanti, P.R.S.; Coburn, C.G.; Moser, V.C.; MacPhail, R.C.; Fenton, S.E.; Stoker, T.E.; Rayner, J.L.; Kannan, K.; Birnbaum, L.S. Developmental Exposure to a Commercial PBDE Mixture, DE-71: Neurobehavioral, Hormonal, and Reproductive Effects. Toxicol. Sci. 2010, 116, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhou, X.; Xu, A.; Tian, Y.; Wang, Y.; Zhang, Y.; Gu, Q.; Wang, S.; Wang, Z. Toxicity of Polybrominated Diphenyl Ethers (PBDEs) on Rodent Male Reproductive System: A Systematic Review and Meta-Analysis of Randomized Control Studies. Sci. Total Environ. 2020, 720, 137419. [Google Scholar] [CrossRef] [PubMed]
- Mumford, S.L.; Kim, S.; Chen, Z.; Gore-Langton, R.E.; Boyd Barr, D.; Buck Louis, G.M. Persistent Organic Pollutants and Semen Quality: The LIFE Study. Chemosphere 2015, 135, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Akutsu, K.; Takatori, S.; Nozawa, S.; Yoshiike, M.; Nakazawa, H.; Hayakawa, K.; Makino, T.; Iwamoto, T. Polybrominated Diphenyl Ethers in Human Serum and Sperm Quality. Bull. Environ. Contam. Toxicol. 2008, 80, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Toft, G.; Lenters, V.; Vermeulen, R.; Heederik, D.; Thomsen, C.; Becher, G.; Giwercman, A.; Bizzaro, D.; Manicardi, G.C.; Spanò, M.; et al. Exposure to Polybrominated Diphenyl Ethers and Male Reproductive Function in Greenland, Poland and Ukraine. Reprod. Toxicol. 2014, 43, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Albert, O.; Huang, J.Y.; Aleksa, K.; Hales, B.F.; Goodyer, C.G.; Robaire, B.; Chevrier, J.; Chan, P. Exposure to Polybrominated Diphenyl Ethers and Phthalates in Healthy Men Living in the Greater Montreal Area: A Study of Hormonal Balance and Semen Quality. Environ. Int. 2018, 116, 165–175. [Google Scholar] [CrossRef]
- Goodyer, C.G.; Poon, S.; Aleksa, K.; Hou, L.; Atehortua, V.; Carnevale, A.; Koren, G.; Jednak, R.; Emil, S.; Bagli, D.; et al. A Case-Control Study of Maternal Polybrominated Diphenyl Ether (PBDE) Exposure and Cryptorchidism in Canadian Populations. Environ. Health Perspect. 2017, 125, 057004. [Google Scholar] [CrossRef]
- Yu, Y.-J.; Lin, B.-G.; Liang, W.-B.; Li, L.-Z.; Hong, Y.; Chen, X.-C.; Xu, X.-Y.; Xiang, M.-D.; Huang, S. Associations between PBDEs Exposure from House Dust and Human Semen Quality at an E-Waste Areas in South China-A Pilot Study. Chemosphere 2018, 198, 266–273. [Google Scholar] [CrossRef]
- Koren, G.; Carnevale, A.; Ling, J.; Ozsarfati, J.; Kapur, B.; Bagli, D. Fetal Exposure to Polybrominated Diphenyl Ethers and the Risk of Hypospadias: Focus on the Congeners Involved. J. Pediatr. Urol. 2019, 15, 405.e1–405.e6. [Google Scholar] [CrossRef]
- Poon, S.; Koren, G.; Carnevale, A.; Aleksa, K.; Ling, J.; Ozsarfati, J.; Kapur, B.M.; Bagli, D. Association of In Utero Exposure to Polybrominated Diphenyl Ethers With the Risk of Hypospadias. JAMA Pediatr. 2018, 172, 851–856. [Google Scholar] [CrossRef]
- Carmichael, S.L.; Herring, A.H.; Sjödin, A.; Jones, R.; Needham, L.; Ma, C.; Ding, K.; Shaw, G.M. Hypospadias and Halogenated Organic Pollutant Levels in Maternal Mid-Pregnancy Serum Samples. Chemosphere 2010, 80, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Suvorov, A.; Shershebnev, A.; Wu, H.; Medvedeva, Y.; Sergeyev, O.; Pilsner, J.R. Perinatal Exposure to Low Dose 2,2′,4,4′-Tetrabromodiphenyl Ether (BDE-47) Alters Sperm DNA Methylation in Adult Rats. Reprod. Toxicol. 2018, 75, 136–143. [Google Scholar] [CrossRef]
- Suvorov, A.; Pilsner, J.R.; Naumov, V.; Shtratnikova, V.; Zheludkevich, A.; Gerasimov, E.; Logacheva, M.; Sergeyev, O. Aging Induces Profound Changes in SncRNA in Rat Sperm and These Changes Are Modified by Perinatal Exposure to Environmental Flame Retardant. Int. J. Mol. Sci. 2020, 21, E8252. [Google Scholar] [CrossRef] [PubMed]
- Pilsner, J.R.; Shershebnev, A.; Wu, H.; Marcho, C.; Dribnokhodova, O.; Shtratnikova, V.; Sergeyev, O.; Suvorov, A. Aging-Induced Changes in Sperm DNA Methylation Are Modified by Low Dose of Perinatal Flame Retardants. Epigenomics 2021, 13, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gao, H.; Li, P.; Chen, W.; Tang, S.; Liu, L.; Zhou, G.; Xia, T.; Wang, A.; Zhang, S. Impaired Sperm Quantity and Motility in Adult Rats Following Gestational and Lactational Exposure to Environmentally Relevant Levels of PBDE-47: A Potential Role of Thyroid Hormones Disruption. Environ. Pollut. 2021, 268, 115773. [Google Scholar] [CrossRef]
- Li, Z.; Li, H.; Li, C.; Yan, H.; Ying, Y.; Li, X.; Zhu, Q.; Ge, R.-S.; Wang, Y. Low Dose of Fire Retardant, 2,2′,4,4′-Tetrabromodiphenyl Ether (BDE47), Stimulates the Proliferation and Differentiation of Progenitor Leydig Cells of Male Rats during Prepuberty. Toxicol. Lett. 2021, 342, 6–19. [Google Scholar] [CrossRef]
- Khalil, A.; Parker, M.; Brown, S.E.; Cevik, S.E.; Guo, L.W.; Jensen, J.; Olmsted, A.; Portman, D.; Wu, H.; Suvorov, A. Perinatal Exposure to 2,2′,4′4′ -Tetrabromodiphenyl Ether Induces Testicular Toxicity in Adult Rats. Toxicology 2017, 389, 21–30. [Google Scholar] [CrossRef]
- Stoker, T.E.; Laws, S.C.; Crofton, K.M.; Hedge, J.M.; Ferrell, J.M.; Cooper, R.L. Assessment of DE-71, a Commercial Polybrominated Diphenyl Ether (PBDE) Mixture, in the EDSP Male and Female Pubertal Protocols. Toxicol. Sci. Off. J. Soc. Toxicol. 2004, 78, 144–155. [Google Scholar] [CrossRef]
- Ellis-Hutchings, R.G.; Cherr, G.N.; Hanna, L.A.; Keen, C.L. Polybrominated Diphenyl Ether (PBDE)-Induced Alterations in Vitamin A and Thyroid Hormone Concentrations in the Rat during Lactation and Early Postnatal Development. Toxicol. Appl. Pharmacol. 2006, 215, 135–145. [Google Scholar] [CrossRef]
- Kuriyama, S.N.; Talsness, C.E.; Grote, K.; Chahoud, I. Developmental Exposure to Low Dose PBDE 99: Effects on Male Fertility and Neurobehavior in Rat Offspring. Environ. Health Perspect. 2005, 113, 149–154. [Google Scholar] [CrossRef]
- Ramhøj, L.; Mandrup, K.; Hass, U.; Svingen, T.; Axelstad, M. Developmental Exposure to the DE-71 Mixture of Polybrominated Diphenyl Ether (PBDE) Flame Retardants Induce a Complex Pattern of Endocrine Disrupting Effects in Rats. PeerJ 2022, 10, e12738. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Tang, X.; Li, D.; Zhao, J.; Zhou, R.; Shu, F.; Jia, W.; Fu, W.; Xia, H.; Liu, G. Prenatal Exposure to Environmentally Relevant Levels of PBDE-99 Leads to Testicular Dysgenesis with Steroidogenesis Disorders. J. Hazard. Mater. 2022, 424, 127547. [Google Scholar] [CrossRef] [PubMed]
- Tseng, L.-H.; Lee, C.-W.; Pan, M.-H.; Tsai, S.-S.; Li, M.-H.; Chen, J.-R.; Lay, J.-J.; Hsu, P.-C. Postnatal Exposure of the Male Mouse to 2,2′,3,3′,4,4′,5,5′,6,6′-Decabrominated Diphenyl Ether: Decreased Epididymal Sperm Functions without Alterations in DNA Content and Histology in Testis. Toxicology 2006, 224, 33–43. [Google Scholar] [CrossRef]
- Miyaso, H.; Nakamura, N.; Matsuno, Y.; Kawashiro, Y.; Komiyama, M.; Mori, C. Postnatal Exposure to Low-Dose Decabromodiphenyl Ether Adversely Affects Mouse Testes by Increasing Thyrosine Phosphorylation Level of Cortactin. J. Toxicol. Sci. 2012, 37, 987–999. [Google Scholar] [CrossRef]
- Miyaso, H.; Nakamura, N.; Naito, M.; Hirai, S.; Matsuno, Y.; Itoh, M.; Mori, C. Early Postnatal Exposure to a Low Dose of Decabromodiphenyl Ether Affects Expression of Androgen and Thyroid Hormone Receptor-Alpha and Its Splicing Variants in Mouse Sertoli Cells. PLoS ONE 2014, 9, e114487. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, X.; Sun, Z.; Dong, H.; Qiu, L.; Gu, J.; Zhou, J.; Wang, X.; Wang, S.-L. Cytochrome P450 3A1 Mediates 2,2′,4,4′-Tetrabromodiphenyl Ether-Induced Reduction of Spermatogenesis in Adult Rats. PLoS ONE 2013, 8, e66301. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yu, Y.; Xu, H.; Wang, C.; Ji, M.; Gu, J.; Yang, L.; Zhu, J.; Dong, H.; Wang, S.-L. High-Fat Diet Aggravates 2,2′,4,4′-Tetrabromodiphenyl Ether-Inhibited Testosterone Production via DAX-1 in Leydig Cells in Rats. Toxicol. Appl. Pharmacol. 2017, 323, 1–8. [Google Scholar] [CrossRef]
- Stoker, T.E.; Cooper, R.L.; Lambright, C.S.; Wilson, V.S.; Furr, J.; Gray, L.E. In Vivo and in Vitro Anti-Androgenic Effects of DE-71, a Commercial Polybrominated Diphenyl Ether (PBDE) Mixture. Toxicol. Appl. Pharmacol. 2005, 207, 78–88. [Google Scholar] [CrossRef]
- Van der Ven, L.T.M.; van de Kuil, T.; Leonards, P.E.G.; Slob, W.; Cantón, R.F.; Germer, S.; Visser, T.J.; Litens, S.; Håkansson, H.; Schrenk, D.; et al. A 28-Day Oral Dose Toxicity Study in Wistar Rats Enhanced to Detect Endocrine Effects of Decabromodiphenyl Ether (DecaBDE). Toxicol. Lett. 2008, 179, 6–14. [Google Scholar] [CrossRef]
- Wei, Z.; Xi, J.; Gao, S.; You, X.; Li, N.; Cao, Y.; Wang, L.; Luan, Y.; Dong, X. Metabolomics Coupled with Pathway Analysis Characterizes Metabolic Changes in Response to BDE-3 Induced Reproductive Toxicity in Mice. Sci. Rep. 2018, 8, 5423. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, J.; Li, L.; Liu, D.; Li, L.; Tang, C.; Li, J. Adverse Effects of 2,2′,4,4′-Tetrabromodiphenyl Ether on Semen Quality and Spermatogenesis in Male Mice. Bull. Environ. Contam. Toxicol. 2013, 90, 51–54. [Google Scholar] [CrossRef]
- Xu, L.; Gao, S.; Zhao, H.; Wang, L.; Cao, Y.; Xi, J.; Zhang, X.; Dong, X.; Luan, Y. Integrated Proteomic and Metabolomic Analysis of the Testes Characterizes BDE-47-Induced Reproductive Toxicity in Mice. Biomolecules 2021, 11, 821. [Google Scholar] [CrossRef]
- Li, X.; Zhu, Y.; Zhang, C.; Liu, J.; Zhou, G.; Jing, L.; Shi, Z.; Sun, Z.; Zhou, X. BDE-209 Induces Male Reproductive Toxicity via Cell Cycle Arrest and Apoptosis Mediated by DNA Damage Response Signaling Pathways. Environ. Pollut. 2019, 255, 113097. [Google Scholar] [CrossRef]
- Li, S.; Che, S.; Chen, S.; Ruan, Z.; Zhang, L. Hesperidin Partly Ameliorates the Decabromodiphenyl Ether-Induced Reproductive Toxicity in Pubertal Mice. Environ. Sci. Pollut. Res. Int. 2022, 1–13. [Google Scholar] [CrossRef]
- Sumner, R.N.; Byers, A.; Zhang, Z.; Agerholm, J.S.; Lindh, L.; England, G.C.W.; Lea, R.G. Environmental Chemicals in Dog Testes Reflect Their Geographical Source and May Be Associated with Altered Pathology. Sci. Rep. 2021, 11, 7361. [Google Scholar] [CrossRef]
- Sonne, C.; Leifsson, P.S.; Dietz, R.; Born, E.W.; Letcher, R.J.; Hyldstrup, L.; Riget, F.F.; Kirkegaard, M.; Muir, D.C.G. Xenoendocrine Pollutants May Reduce Size of Sexual Organs in East Greenland Polar Bears (Ursus Maritimus). Environ. Sci. Technol. 2006, 40, 5668–5674. [Google Scholar] [CrossRef]
- Huwe, J.; Hakk, H.; Lorentzsen, M. Bioavailability and Mass Balance Studies of a Commercial Pentabromodiphenyl Ether Mixture in Male Sprague–Dawley Rats. Chemosphere 2007, 66, 259–266. [Google Scholar] [CrossRef]
- Orn, U.; Klasson-Wehler, E. Metabolism of 2, 2, 4, 4-Tetrabromodiphenyl Ether in Rat and Mouse. Xenobiotica 1998, 28, 199–211. [Google Scholar]
- Sanders, J.M.; Chen, L.-J.; Lebetkin, E.H.; Burka, L.T. Metabolism and Disposition of 2,2′,4,4′- Tetrabromodiphenyl Ether Following Administration of Single or Multiple Doses to Rats and Mice. Xenobiotica 2006, 36, 103–117. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Auten, R.L.; Davis, J.M. Oxygen Toxicity and Reactive Oxygen Species: The Devil Is in the Details. Pediatr. Res. 2009, 66, 121–127. [Google Scholar] [CrossRef]
- Li, R.; Jia, Z.; Trush, M.A. Defining ROS in Biology and Medicine. React. Oxyg. Species Apex NC 2016, 1, 9–21. [Google Scholar] [CrossRef]
- Allamaneni, S.S.R.; Agarwal, A.; Nallella, K.P.; Sharma, R.K.; Thomas, A.J.; Sikka, S.C. Characterization of Oxidative Stress Status by Evaluation of Reactive Oxygen Species Levels in Whole Semen and Isolated Spermatozoa. Fertil. Steril. 2005, 83, 800–803. [Google Scholar] [CrossRef]
- Fingerova, H.; Oborna, I.; Novotny, J.; Svobodova, M.; Brezinova, J.; Radova, L. The Measurement of Reactive Oxygen Species in Human Neat Semen and in Suspended Spermatozoa: A Comparison. Reprod. Biol. Endocrinol. 2009, 7, 118. [Google Scholar] [CrossRef]
- Henkel, R.; Kierspel, E.; Stalf, T.; Mehnert, C.; Menkveld, R.; Tinneberg, H.-R.; Schill, W.-B.; Kruger, T.F. Effect of Reactive Oxygen Species Produced by Spermatozoa and Leukocytes on Sperm Functions in Non-Leukocytospermic Patients. Fertil. Steril. 2005, 83, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Mupfiga, C.; Fisher, D.; Kruger, T.; Henkel, R. The Relationship between Seminal Leukocytes, Oxidative Status in the Ejaculate, and Apoptotic Markers in Human Spermatozoa. Syst. Biol. Reprod. Med. 2013, 59, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Mack, S.R.; Everingham, J.; Zaneveld, L.J. Isolation and Partial Characterization of the Plasma Membrane from Human Spermatozoa. J. Exp. Zool. 1986, 240, 127–136. [Google Scholar] [CrossRef]
- Poulos, A.; White, I.G. The phospholipid composition of human spermatozoa and seminal plasma. Reproduction 1973, 35, 265–272. [Google Scholar] [CrossRef]
- Sanocka, D.; Kurpisz, M. Reactive Oxygen Species and Sperm Cells. Reprod. Biol. Endocrinol. 2004, 2, 12. [Google Scholar] [CrossRef]
- Aitken, R.J.; Clarkson, J.S. Cellular Basis of Defective Sperm Function and Its Association with the Genesis of Reactive Oxygen Species by Human Spermatozoa. J. Reprod. Fertil. 1987, 81, 459–469. [Google Scholar] [CrossRef]
- Alvarez, J.G.; Touchstone, J.C.; Blasco, L.; Storey, B.T. Spontaneous Lipid Peroxidation and Production of Hydrogen Peroxide and Superoxide in Human Spermatozoa. Superoxide Dismutase as Major Enzyme Protectant against Oxygen Toxicity. J. Androl. 1987, 8, 338–348. [Google Scholar] [CrossRef]
- Alvarez, J.G.; Storey, B.T. Differential Incorporation of Fatty Acids into and Peroxidative Loss of Fatty Acids from Phospholipids of Human Spermatozoa. Mol. Reprod. Dev. 1995, 42, 334–346. [Google Scholar] [CrossRef]
- Jones, R.; Mann, T.; Sherins, R. Peroxidative Breakdown of Phospholipids in Human Spermatozoa, Spermicidal Properties of Fatty Acid Peroxides, and Protective Action of Seminal Plasma. Fertil. Steril. 1979, 31, 531–537. [Google Scholar] [CrossRef]
- Aitken, R.J.; Curry, B.J. Redox Regulation of Human Sperm Function: From the Physiological Control of Sperm Capacitation to the Etiology of Infertility and DNA Damage in the Germ Line. Antioxid. Redox Signal. 2011, 14, 367–381. [Google Scholar] [CrossRef]
- de Lamirande, E.; Gagnon, C. Impact of Reactive Oxygen Species on Spermatozoa: A Balancing Act between Beneficial and Detrimental Effects. Hum. Reprod. 1995, 10 (Suppl. 1), 15–21. [Google Scholar] [CrossRef]
- Plante, M.; de Lamirande, E.; Gagnon, C. Reactive Oxygen Species Released by Activated Neutrophils, but Not by Deficient Spermatozoa, Are Sufficient to Affect Normal Sperm Motility. Fertil. Steril. 1994, 62, 387–393. [Google Scholar] [CrossRef]
- Shi, T.-Y.; Chen, G.; Huang, X.; Yuan, Y.; Wu, X.; Wu, B.; Li, Z.; Shun, F.; Chen, H.; Shi, H. Effects of Reactive Oxygen Species from Activated Leucocytes on Human Sperm Motility, Viability and Morphology. Andrologia 2012, 44, 696–703. [Google Scholar] [CrossRef]
- Morielli, T.; O’Flaherty, C. Oxidative Stress Impairs Function and Increases Redox Protein Modifications in Human Spermatozoa. Reprod. Camb. Engl. 2015, 149, 113–123. [Google Scholar] [CrossRef]
- Ichikawa, T.; Oeda, T.; Ohmori, H.; Schill, W.B. Reactive Oxygen Species Influence the Acrosome Reaction but Not Acrosin Activity in Human Spermatozoa. Int. J. Androl. 1999, 22, 37–42. [Google Scholar] [CrossRef]
- Homa, S.T.; Vessey, W.; Perez-Miranda, A.; Riyait, T.; Agarwal, A. Reactive Oxygen Species (ROS) in Human Semen: Determination of a Reference Range. J. Assist. Reprod. Genet. 2015, 32, 757–764. [Google Scholar] [CrossRef]
- Agarwal, A.; Virk, G.; Ong, C.; du Plessis, S.S. Effect of Oxidative Stress on Male Reproduction. World J. Mens Health 2014, 32, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Asadi, N.; Bahmani, M.; Kheradmand, A.; Rafieian-Kopaei, M. The Impact of Oxidative Stress on Testicular Function and the Role of Antioxidants in Improving It: A Review. J. Clin. Diagn. Res. JCDR 2017, 11, IE01–IE05. [Google Scholar] [CrossRef]
- Bansal, A.K.; Bilaspuri, G.S. Impacts of Oxidative Stress and Antioxidants on Semen Functions. Vet. Med. Int. 2010, 2011, e686137. [Google Scholar] [CrossRef]
- Palani, A.; Alahmar, A. Impact of Oxidative Stress on Semen Parameters in Normozoospermic Infertile Men: A Case–Control Study. Afr. J. Urol. 2020, 26, 50. [Google Scholar] [CrossRef]
- Deavall, D.G.; Martin, E.A.; Horner, J.M.; Roberts, R. Drug-Induced Oxidative Stress and Toxicity. J. Toxicol. 2012, 2012, e645460. [Google Scholar] [CrossRef] [PubMed]
- Lakey, P.S.J.; Berkemeier, T.; Tong, H.; Arangio, A.M.; Lucas, K.; Pöschl, U.; Shiraiwa, M. Chemical Exposure-Response Relationship between Air Pollutants and Reactive Oxygen Species in the Human Respiratory Tract. Sci. Rep. 2016, 6, 32916. [Google Scholar] [CrossRef]
- Manuguerra, S.; Espinosa Ruiz, C.; Santulli, A.; Messina, C.M. Sub-Lethal Doses of Polybrominated Diphenyl Ethers, in Vitro, Promote Oxidative Stress and Modulate Molecular Markers Related to Cell Cycle, Antioxidant Balance and Cellular Energy Management. Int. J. Environ. Res. Public. Health 2019, 16, 588. [Google Scholar] [CrossRef]
- Zhong, Y.F.; Wang, L.L.; Yin, L.L.; An, J.; Hou, M.L.; Zheng, K.W.; Zhang, X.Y.; Wu, M.H.; Yu, Z.Q.; Sheng, G.Y.; et al. Cytotoxic Effects and Oxidative Stress Response of Six PBDE Metabolites on Human L02 Cells. J. Environ. Sci. Health Part A 2011, 46, 1320–1327. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Jing, L.; Li, X.; Zheng, D.; Zhou, G.; Zhang, Y.; Sang, Y.; Shi, Z.; Sun, Z.; Zhou, X. Decabromodiphenyl Ether Disturbs Hepatic Glycolipid Metabolism by Regulating the PI3K/AKT/GLUT4 and MTOR/PPARγ/RXRα Pathway in Mice and L02 Cells. Sci. Total Environ. 2021, 763, 142936. [Google Scholar] [CrossRef]
- Alonso, V.; Linares, V.; Bellés, M.; Albina, M.L.; Pujol, A.; Domingo, J.L.; Sánchez, D.J. Effects of BDE-99 on Hormone Homeostasis and Biochemical Parameters in Adult Male Rats. Food Chem. Toxicol. 2010, 48, 2206–2211. [Google Scholar] [CrossRef]
- Zhai, J.-X.; Wang, X.-H.; Zhang, Z.-X.; Zou, L.-W.; Ding, S.-S. Studying the lipid peroxidation index, morphology and apoptosis in testis of male BALB/c mice exposed to polybrominated diphenyl ether (BDE-209). Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi Zhonghua Laodong Weisheng Zhiyebing Zazhi Chin. J. Ind. Hyg. Occup. Dis. 2011, 29, 294–298. [Google Scholar]
- Tseng, L.-H.; Hsu, P.-C.; Lee, C.-W.; Tsai, S.-S.; Pan, M.-H.; Li, M.-H. Developmental Exposure to Decabrominated Diphenyl Ether (BDE-209): Effects on Sperm Oxidative Stress and Chromatin DNA Damage in Mouse Offspring. Environ. Toxicol. 2013, 28, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.; Singh, S.K. Maternal Exposure to Polybrominated Diphenyl Ether (BDE-209) during Lactation Affects Germ Cell Survival with Altered Testicular Glucose Homeostasis and Oxidative Status through down-Regulation of Cx43 and P27Kip1 in Prepubertal Mice Offspring. Toxicology 2017, 386, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.; Singh, S.K. Decabromodiphenyl Ether (BDE-209) Exposure to Lactating Mice Perturbs Steroidogenesis and Spermatogenesis in Adult Male Offspring. Ecotoxicol. Environ. Saf. 2021, 209, 111783. [Google Scholar] [CrossRef] [PubMed]
- Boutot, M.E.; Whitcomb, B.W.; Abdelouahab, N.; Baccarelli, A.A.; Boivin, A.; Caku, A.; Gillet, V.; Martinez, G.; Pasquier, J.-C.; Zhu, J.; et al. In Utero Exposure to Persistent Organic Pollutants and Childhood Lipid Levels. Metabolites 2021, 11, 657. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.G.; Giordano, G.; Tagliaferri, S.; Caglieri, A.; Mutti, A. Polybrominated Diphenyl Ether (PBDE) Flame Retardants: Environmental Contamination, Human Body Burden and Potential Adverse Health Effects. Acta Bio-Med. Atenei Parm. 2008, 79, 172–183. [Google Scholar]
- Jiang, Y.; Yuan, L.; Lin, Q.; Ma, S.; Yu, Y. Polybrominated Diphenyl Ethers in the Environment and Human External and Internal Exposure in China: A Review. Sci. Total Environ. 2019, 696, 133902. [Google Scholar] [CrossRef]
- Khalil, A.; Parker, M.; Mpanga, R.; Cevik, S.E.; Thorburn, C.; Suvorov, A. Developmental Exposure to 2,2′,4,4′–Tetrabromodiphenyl Ether Induces Long-Lasting Changes in Liver Metabolism in Male Mice. J. Endocr. Soc. 2017, 1, 323. [Google Scholar] [CrossRef]
- Khalil, A.; Cevik, S.E.; Hung, S.; Kolla, S.; Roy, M.A.; Suvorov, A. Developmental Exposure to 2,2′,4,4′-Tetrabromodiphenyl Ether Permanently Alters Blood-Liver Balance of Lipids in Male Mice. Front. Endocrinol. 2018, 9, 548. [Google Scholar] [CrossRef]
- Wang, D.; Yan, J.; Teng, M.; Yan, S.; Zhou, Z.; Zhu, W. In Utero and Lactational Exposure to BDE-47 Promotes Obesity Development in Mouse Offspring Fed a High-Fat Diet: Impaired Lipid Metabolism and Intestinal Dysbiosis. Arch. Toxicol. 2018, 92, 1847–1860. [Google Scholar] [CrossRef]
- Kozlova, E.V.; Chinthirla, B.D.; Pérez, P.A.; DiPatrizio, N.V.; Argueta, D.A.; Phillips, A.L.; Stapleton, H.M.; González, G.M.; Krum, J.M.; Carrillo, V.; et al. Maternal Transfer of Environmentally Relevant Polybrominated Diphenyl Ethers (PBDEs) Produces a Diabetic Phenotype and Disrupts Glucoregulatory Hormones and Hepatic Endocannabinoids in Adult Mouse Female Offspring. Sci. Rep. 2020, 10, 18102. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, A.A.; Carey, G.B. Polybrominated Diphenyl Ethers as Endocrine Disruptors of Adipocyte Metabolism. Obesity 2007, 15, 2942–2950. [Google Scholar] [CrossRef] [PubMed]
- Helaleh, M.; Diboun, I.; Al-Tamimi, N.; Al-Sulaiti, H.; Al-Emadi, M.; Madani, A.; Mazloum, N.A.; Latiff, A.; Elrayess, M.A. Association of Polybrominated Diphenyl Ethers in Two Fat Compartments with Increased Risk of Insulin Resistance in Obese Individuals. Chemosphere 2018, 209, 268–276. [Google Scholar] [CrossRef]
- Ongono, J.S.; Dow, C.; Gambaretti, J.; Severi, G.; Boutron-Ruault, M.-C.; Bonnet, F.; Fagherazzi, G.; Mancini, F.R. Dietary Exposure to Brominated Flame Retardants and Risk of Type 2 Diabetes in the French E3N Cohort. Environ. Int. 2019, 123, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Eslami, B.; Naddafi, K.; Rastkari, N.; Rashidi, B.H.; Djazayeri, A.; Malekafzali, H. Association between Serum Concentrations of Persistent Organic Pollutants and Gestational Diabetes Mellitus in Primiparous Women. Environ. Res. 2016, 151, 706–712. [Google Scholar] [CrossRef]
- He, Z.; Yin, G.; Li, Q.Q.; Zeng, Q.; Duan, J. Diabetes Mellitus Causes Male Reproductive Dysfunction: A Review of the Evidence and Mechanisms. In Vivo Athens Greece 2021, 35, 2503–2511. [Google Scholar] [CrossRef]
- Kahn, B.E.; Brannigan, R.E. Obesity and Male Infertility. Curr. Opin. Urol. 2017, 27, 441–445. [Google Scholar] [CrossRef]
- Katib, A. Mechanisms Linking Obesity to Male Infertility. Cent. Eur. J. Urol. 2015, 68, 79–85. [Google Scholar] [CrossRef]
- Leisegang, K.; Sengupta, P.; Agarwal, A.; Henkel, R. Obesity and Male Infertility: Mechanisms and Management. Andrologia 2021, 53, e13617. [Google Scholar] [CrossRef]
- Zota, A.R.; Geller, R.J.; Romano, L.E.; Coleman-Phox, K.; Adler, N.E.; Parry, E.; Wang, M.; Park, J.-S.; Elmi, A.F.; Laraia, B.A.; et al. Association between Persistent Endocrine-Disrupting Chemicals (PBDEs, OH-PBDEs, PCBs, and PFASs) and Biomarkers of Inflammation and Cellular Aging during Pregnancy and Postpartum. Environ. Int. 2018, 115, 9–20. [Google Scholar] [CrossRef]
- Arita, Y.; Yeh, C.; Thoma, T.; Getahun, D.; Menon, R.; Peltier, M.R. Effect of Polybrominated Diphenyl Ether Congeners on Placental Cytokine Production. J. Reprod. Immunol. 2018, 125, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Peltier, M.R.; Klimova, N.G.; Arita, Y.; Gurzenda, E.M.; Murthy, A.; Chawala, K.; Lerner, V.; Richardson, J.; Hanna, N. Polybrominated Diphenyl Ethers Enhance the Production of Proinflammatory Cytokines by the Placenta. Placenta 2012, 33, 745–749. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Robinson, J.F.; Kapidzic, M.; Hamilton, E.G.; Chen, H.; Puckett, K.W.; Zhou, Y.; Ona, K.; Parry, E.; Wang, Y.; Park, J.-S.; et al. Genomic Profiling of BDE-47 Effects on Human Placental Cytotrophoblasts. Toxicol. Sci. Off. J. Soc. Toxicol. 2019, 167, 211–226. [Google Scholar] [CrossRef] [PubMed]
- Azenabor, A.; Ekun, A.O.; Akinloye, O. Impact of Inflammation on Male Reproductive Tract. J. Reprod. Infertil. 2015, 16, 123–129. [Google Scholar] [PubMed]
- Dutta, S.; Sengupta, P.; Slama, P.; Roychoudhury, S. Oxidative Stress, Testicular Inflammatory Pathways, and Male Reproduction. Int. J. Mol. Sci. 2021, 22, 10043. [Google Scholar] [CrossRef]
- Leisegang, K.; Henkel, R. The in Vitro Modulation of Steroidogenesis by Inflammatory Cytokines and Insulin in TM3 Leydig Cells. Reprod. Biol. Endocrinol. 2018, 16, 26. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape Provides a Biologist-Oriented Resource for the Analysis of Systems-Level Datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef]
- Belloni, V.; Sorci, G.; Paccagnini, E.; Guerreiro, R.; Bellenger, J.; Faivre, B. Disrupting Immune Regulation Incurs Transient Costs in Male Reproductive Function. PLoS ONE 2014, 9, e84606. [Google Scholar] [CrossRef]
- Suvorov, A.; Takser, L. Delayed Response in the Rat Frontal Lobe Transcriptome to Perinatal Exposure to the Flame Retardant BDE-47. J. Appl. Toxicol. JAT 2011, 31, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Suvorov, A.; Takser, L. Global Gene Expression Analysis in the Livers of Rat Offspring Perinatally Exposed to Low Doses of 2,2′,4,4′-Tetrabromodiphenyl Ether. Environ. Health Perspect. 2010, 118, 97–102. [Google Scholar] [CrossRef]
- Fan, L.; Di Ciano-Oliveira, C.; Weed, S.A.; Craig, A.W.B.; Greer, P.A.; Rotstein, O.D.; Kapus, A. Actin Depolymerization-Induced Tyrosine Phosphorylation of Cortactin: The Role of Fer Kinase. Biochem. J. 2004, 380, 581–591. [Google Scholar] [CrossRef]
- Anahara, R.; Toyama, Y.; Maekawa, M.; Kai, M.; Ishino, F.; Toshimori, K.; Mori, C. Flutamide Depresses Expression of Cortactin in the Ectoplasmic Specialization between the Sertoli Cells and Spermatids in the Mouse Testis. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2006, 44, 1050–1056. [Google Scholar] [CrossRef]
- Anahara, R.; Toyama, Y.; Mori, C. Flutamide Induces Ultrastructural Changes in Spermatids and the Ectoplasmic Specialization between the Sertoli Cell and Spermatids in Mouse Testes. Reprod. Toxicol. 2004, 18, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Berruti, G.; Paiardi, C. The Dynamic of the Apical Ectoplasmic Specialization between Spermatids and Sertoli Cells: The Case of the Small GTPase Rap1. BioMed Res. Int. 2014, 2014, 635979. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Tang, E.I.; Li, N.; Mruk, D.D.; Lee, W.M.; Silvestrini, B.; Cheng, C.Y. Regulation of Blood-Testis Barrier (BTB) Dynamics, Role of Actin-, and Microtubule-Based Cytoskeletons. Methods Mol. Biol. Clifton NJ 2018, 1748, 229–243. [Google Scholar] [CrossRef]
- Vanholder, R.; Ringoir, S. Artificial Organs—An Overview. Int. J. Artif. Organs 1991, 14, 613–618. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Mruk, D.D. The Blood-Testis Barrier and Its Implications for Male Contraception. Pharmacol. Rev. 2012, 64, 16–64. [Google Scholar] [CrossRef]
- Gouesse, R.-J.; Lavoie, M.; Dianati, E.; Wade, M.; Hales, B.; Robaire, B.; Plante, I. Gestational and Lactational Exposure to an Environmentally-Relevant Mixture of Brominated Flame Retardants Down-Regulates Junctional Proteins, Thyroid Hormone Receptor A1 Expression and the Proliferation-Apoptosis Balance in Mammary Glands Post Puberty. Toxicol. Sci. 2019, 171, 13–31. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ao, H.; Chen, L.; Sottas, C.M.; Ge, R.-S.; Zhang, Y. Effect of Brominated Flame Retardant BDE-47 on Androgen Production of Adult Rat Leydig Cells. Toxicol. Lett. 2011, 205, 209–214. [Google Scholar] [CrossRef]
- Eskenazi, B.; Rauch, S.A.; Tenerelli, R.; Huen, K.; Holland, N.T.; Lustig, R.H.; Kogut, K.; Bradman, A.; Sjödin, A.; Harley, K.G. In Utero and Childhood DDT, DDE, PBDE and PCBs Exposure and Sex Hormones in Adolescent Boys: The CHAMACOS Study. Int. J. Hyg. Environ. Health 2017, 220, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.; Chowdhury, J.P.; Singh, S.K. Effect of Polybrominated Diphenyl Ether (BDE-209) on Testicular Steroidogenesis and Spermatogenesis through Altered Thyroid Status in Adult Mice. Gen. Comp. Endocrinol. 2016, 239, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.I.; Stapleton, H.M.; Mukherjee, B.; Hauser, R.; Meeker, J.D. Associations between Brominated Flame Retardants in House Dust and Hormone Levels in Men. Sci. Total Environ. 2013, 445–446, 177–184. [Google Scholar] [CrossRef]
- Hamers, T.; Kamstra, J.H.; Sonneveld, E.; Murk, A.J.; Kester, M.H.A.; Andersson, P.L.; Legler, J.; Brouwer, A. In Vitro Profiling of the Endocrine-Disrupting Potency of Brominated Flame Retardants. Toxicol. Sci. Off. J. Soc. Toxicol. 2006, 92, 157–173. [Google Scholar] [CrossRef] [PubMed]
- Kojima, H.; Takeuchi, S.; Uramaru, N.; Sugihara, K.; Yoshida, T.; Kitamura, S. Nuclear Hormone Receptor Activity of Polybrominated Diphenyl Ethers and Their Hydroxylated and Methoxylated Metabolites in Transactivation Assays Using Chinese Hamster Ovary Cells. Environ. Health Perspect. 2009, 117, 1210–1218. [Google Scholar] [CrossRef]
- Liu, H.; Hu, W.; Sun, H.; Shen, O.; Wang, X.; Lam, M.H.W.; Giesy, J.P.; Zhang, X.; Yu, H. In Vitro Profiling of Endocrine Disrupting Potency of 2,2′,4,4′-Tetrabromodiphenyl Ether (BDE47) and Related Hydroxylated Analogs (HO-PBDEs). Mar. Pollut. Bull. 2011, 63, 287–296. [Google Scholar] [CrossRef]
- Hu, W.; Liu, H.; Sun, H.; Shen, O.; Wang, X.; Lam, M.H.W.; Giesy, J.P.; Zhang, X.; Yu, H. Endocrine Effects of Methoxylated Brominated Diphenyl Ethers in Three in Vitro Models. Mar. Pollut. Bull. 2011, 62, 2356–2361. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, I.A. Endocrine-Disrupting Potential of Polybrominated Diphenyl Ethers (PBDEs) on Androgen Receptor Signaling: A Structural Insight. Struct. Chem. 2021, 32, 887–897. [Google Scholar] [CrossRef]
- Meijer, L.; Martijn, A.; Melessen, J.; Brouwer, A.; Weiss, J.; de Jong, F.H.; Sauer, P.J.J. Influence of Prenatal Organohalogen Levels on Infant Male Sexual Development: Sex Hormone Levels, Testes Volume and Penile Length. Hum. Reprod. Oxf. Engl. 2012, 27, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Meerts, I.A.; Letcher, R.J.; Hoving, S.; Marsh, G.; Bergman, A.; Lemmen, J.G.; van der Burg, B.; Brouwer, A. In Vitro Estrogenicity of Polybrominated Diphenyl Ethers, Hydroxylated PDBEs, and Polybrominated Bisphenol A Compounds. Environ. Health Perspect. 2001, 109, 399–407. [Google Scholar] [CrossRef]
- Kitamura, S.; Shinohara, S.; Iwase, E.; Sugihara, K.; Uramaru, N.; Shigematsu, H.; Fujimoto, N.; Ohta, S. Affinity for Thyroid Hormone and Estrogen Receptors of Hydroxylated Polybrominated Diphenyl Ethers. J. Health Sci. 2008, 54, 607–614. [Google Scholar] [CrossRef]
- Mercado-Feliciano, M.; Bigsby, R.M. Hydroxylated Metabolites of the Polybrominated Diphenyl Ether Mixture DE-71 Are Weak Estrogen Receptor-Alpha Ligands. Environ. Health Perspect. 2008, 116, 1315–1321. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gao, Y.; Guo, L.-H.; Jiang, G. Structure-Dependent Activities of Hydroxylated Polybrominated Diphenyl Ethers on Human Estrogen Receptor. Toxicology 2013, 309, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Hamers, T.; Kamstra, J.H.; Sonneveld, E.; Murk, A.J.; Visser, T.J.; Van Velzen, M.J.M.; Brouwer, A.; Bergman, A. Biotransformation of Brominated Flame Retardants into Potentially Endocrine-Disrupting Metabolites, with Special Attention to 2,2′,4,4′-Tetrabromodiphenyl Ether (BDE-47). Mol. Nutr. Food Res. 2008, 52, 284–298. [Google Scholar] [CrossRef] [PubMed]
- Shanle, E.K.; Xu, W. Endocrine Disrupting Chemicals Targeting Estrogen Receptor Signaling: Identification and Mechanisms of Action. Chem. Res. Toxicol. 2011, 24, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.-Y.; Ren, X.-M.; Yang, Y.; Wan, B.; Guo, L.-H.; Chen, D.; Fan, Y. Hydroxylated Polybrominated Biphenyl Ethers Exert Estrogenic Effects via Non-Genomic G Protein–Coupled Estrogen Receptor Mediated Pathways. Environ. Health Perspect. 2018, 126, 057005. [Google Scholar] [CrossRef]
- Kester, M.H.A.; Bulduk, S.; van Toor, H.; Tibboel, D.; Meinl, W.; Glatt, H.; Falany, C.N.; Coughtrie, M.W.H.; Schuur, A.G.; Brouwer, A.; et al. Potent Inhibition of Estrogen Sulfotransferase by Hydroxylated Metabolites of Polyhalogenated Aromatic Hydrocarbons Reveals Alternative Mechanism for Estrogenic Activity of Endocrine Disrupters. J. Clin. Endocrinol. Metab. 2002, 87, 1142–1150. [Google Scholar] [CrossRef]
- Main, K.M.; Kiviranta, H.; Virtanen, H.E.; Sundqvist, E.; Tuomisto, J.T.; Tuomisto, J.; Vartiainen, T.; Skakkebaek, N.E.; Toppari, J. Flame Retardants in Placenta and Breast Milk and Cryptorchidism in Newborn Boys. Environ. Health Perspect. 2007, 115, 1519–1526. [Google Scholar] [CrossRef]
- Meeker, J.D.; Johnson, P.I.; Camann, D.; Hauser, R. Polybrominated Diphenyl Ether (PBDE) Concentrations in House Dust Are Related to Hormone Levels in Men. Sci. Total Environ. 2009, 407, 3425–3429. [Google Scholar] [CrossRef]
- Makey, C.M.; McClean, M.D.; Braverman, L.E.; Pearce, E.N.; Sjödin, A.; Weinberg, J.; Webster, T.F. Polybrominated Diphenyl Ether Exposure and Reproductive Hormones in North American Men. Reprod. Toxicol. 2016, 62, 46–52. [Google Scholar] [CrossRef]
- Gravel, S.; Lavoué, J.; Bakhiyi, B.; Lavoie, J.; Roberge, B.; Patry, L.; Bouchard, M.F.; Verner, M.-A.; Zayed, J.; Labrèche, F. Multi-Exposures to Suspected Endocrine Disruptors in Electronic Waste Recycling Workers: Associations with Thyroid and Reproductive Hormones. Int. J. Hyg. Environ. Health 2020, 225, 113445. [Google Scholar] [CrossRef]
- Yu, Y.; Lin, B.; Chen, X.; Qiao, J.; Li, L.; Liang, Y.; Zhang, G.; Jia, Y.; Zhou, X.; Chen, C.; et al. Polybrominated Diphenyl Ethers in Human Serum, Semen and Indoor Dust: Effects on Hormones Balance and Semen Quality. Sci. Total Environ. 2019, 671, 1017–1025. [Google Scholar] [CrossRef]
- Guo, L.-C.; Pan, S.; Yu, S.; Liu, T.; Xiao, J.; Zhu, B.; Qu, Y.; Huang, W.; Li, M.; Li, X.; et al. Human Sex Hormone Disrupting Effects of New Flame Retardants and Their Interactions with Polychlorinated Biphenyls, Polybrominated Diphenyl Ethers, a Case Study in South China. Environ. Sci. Technol. 2018, 52, 13935–13941. [Google Scholar] [CrossRef] [PubMed]
- Krassas, G.E.; Poppe, K.; Glinoer, D. Thyroid Function and Human Reproductive Health. Endocr. Rev. 2010, 31, 702–755. [Google Scholar] [CrossRef] [PubMed]
- La Vignera, S.; Vita, R.; Condorelli, R.A.; Mongioì, L.M.; Presti, S.; Benvenga, S.; Calogero, A.E. Impact of Thyroid Disease on Testicular Function. Endocrine 2017, 58, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Ghiasi, H.; Kaiwar, R.; Nesburn, A.B.; Slanina, S.; Wechsler, S.L. Baculovirus-Expressed Glycoprotein E (GE) of Herpes Simplex Virus Type-1 (HSV-1) Protects Mice against Lethal Intraperitoneal and Lethal Ocular HSV-1 Challenge. Virology 1992, 188, 469–476. [Google Scholar] [CrossRef]
- Laslett, A.L.; Li, L.H.; Jester, W.F.; Orth, J.M. Thyroid Hormone Down-Regulates Neural Cell Adhesion Molecule Expression and Affects Attachment of Gonocytes in Sertoli Cell-Gonocyte Cocultures. Endocrinology 2000, 141, 1633–1641. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mendeluk, G.R.; Rosales, M. Thyroxin Is Useful to Improve Sperm Motility. Int. J. Fertil. Steril. 2016, 10, 208–214. [Google Scholar] [CrossRef]
- Suvorov, A.; Girard, S.; Lachapelle, S.; Abdelouahab, N.; Sebire, G.; Takser, L. Perinatal Exposure to Low-Dose BDE-47, an Emergent Environmental Contaminant, Causes Hyperactivity in Rat Offspring. Neonatology 2009, 95, 203–209. [Google Scholar] [CrossRef]
- Abdelouahab, N.; Suvorov, A.; Pasquier, J.-C.; Langlois, M.-F.; Praud, J.-P.; Takser, L. Thyroid Disruption by Low-Dose BDE-47 in Prenatally Exposed Lambs. Neonatology 2009, 96, 120–124. [Google Scholar] [CrossRef]
- Yang, J.; Ma, Y.; Zhang, X.; Liao, X.; Yang, Y.; Sweetman, A.; Li, H. The Potential Association of Polybrominated Diphenyl Ether Concentrations in Serum to Thyroid Function in Patients with Abnormal Thyroids: A Pilot Study. Ann. Palliat. Med. 2021, 10, 9192–9205. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, H.; Li, J.; Shan, Z.; Teng, W.; Teng, X. The Correlation between Polybrominated Diphenyl Ethers (PBDEs) and Thyroid Hormones in the General Population: A Meta-Analysis. PLoS ONE 2015, 10, e0126989. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Lin, Y.; Guo, L.-H.; Zhang, A.-Q.; Wei, Y.; Yang, Y. Structure-Based Investigation on the Binding Interaction of Hydroxylated Polybrominated Diphenyl Ethers with Thyroxine Transport Proteins. Toxicology 2010, 277, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.M.; Guo, L.-H. Assessment of the Binding of Hydroxylated Polybrominated Diphenyl Ethers to Thyroid Hormone Transport Proteins Using a Site-Specific Fluorescence Probe. Environ. Sci. Technol. 2012, 46, 4633–4640. [Google Scholar] [CrossRef]
- Krainick, J.U.; Thoden, U. Methods of pain modulation by electrical stimulation (author’s transl). Langenbecks Arch. Chir. 1976, 342, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Marchesini, G.R.; Meimaridou, A.; Haasnoot, W.; Meulenberg, E.; Albertus, F.; Mizuguchi, M.; Takeuchi, M.; Irth, H.; Murk, A.J. Biosensor Discovery of Thyroxine Transport Disrupting Chemicals. Toxicol. Appl. Pharmacol. 2008, 232, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Bansal, R.; Tighe, D.; Danai, A.; Rawn, D.F.K.; Gaertner, D.W.; Arnold, D.L.; Gilbert, M.E.; Zoeller, R.T. Polybrominated Diphenyl Ether (DE-71) Interferes with Thyroid Hormone Action Independent of Effects on Circulating Levels of Thyroid Hormone in Male Rats. Endocrinology 2014, 155, 4104–4112. [Google Scholar] [CrossRef] [PubMed]
- Lema, S.C.; Dickey, J.T.; Schultz, I.R.; Swanson, P. Dietary Exposure to 2,2′,4,4′-Tetrabromodiphenyl Ether (PBDE-47) Alters Thyroid Status and Thyroid Hormone-Regulated Gene Transcription in the Pituitary and Brain. Environ. Health Perspect. 2008, 116, 1694–1699. [Google Scholar] [CrossRef]
- Roberts, S.C.; Bianco, A.C.; Stapleton, H.M. Disruption of Type 2 Iodothyronine Deiodinase Activity in Cultured Human Glial Cells by Polybrominated Diphenyl Ethers. Chem. Res. Toxicol. 2015, 28, 1265–1274. [Google Scholar] [CrossRef]
- Hull, K.L.; Harvey, S. Growth Hormone and Reproduction: A Review of Endocrine and Autocrine/Paracrine Interactions. Int. J. Endocrinol. 2014, 2014, 234014. [Google Scholar] [CrossRef]
- Tenuta, M.; Carlomagno, F.; Cangiano, B.; Kanakis, G.; Pozza, C.; Sbardella, E.; Isidori, A.M.; Krausz, C.; Gianfrilli, D. Somatotropic-Testicular Axis: A Crosstalk between GH/IGF-I and Gonadal Hormones during Development, Transition, and Adult Age. Andrology 2021, 9, 168–184. [Google Scholar] [CrossRef]
- Lee, H.S.; Park, Y.-S.; Lee, J.S.; Seo, J.T. Serum and Seminal Plasma Insulin-like Growth Factor-1 in Male Infertility. Clin. Exp. Reprod. Med. 2016, 43, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Suvorov, A.; Battista, M.-C.; Takser, L. Perinatal Exposure to Low-Dose 2,2′,4,4′-Tetrabromodiphenyl Ether Affects Growth in Rat Offspring: What Is the Role of IGF-1? Toxicology 2009, 260, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yekeen, T.A.; Xiao, Q.; Wang, Y.; Lu, F.; Huo, X. Placental IGF-1 and IGFBP-3 Expression Correlate with Umbilical Cord Blood PAH and PBDE Levels from Prenatal Exposure to Electronic Waste. Environ. Pollut. 2013, 182, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Shy, C.-G.; Huang, H.-L.; Chao, H.-R.; Chang-Chien, G.-P. Cord Blood Levels of Thyroid Hormones and IGF-1 Weakly Correlate with Breast Milk Levels of PBDEs in Taiwan. Int. J. Hyg. Environ. Health 2012, 215, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Dibble, C.C.; Manning, B.D. Signal Integration by MTORC1 Coordinates Nutrient Input with Biosynthetic Output. Nat. Cell Biol. 2013, 15, 555–564. [Google Scholar] [CrossRef]
- Zoncu, R.; Efeyan, A.; Sabatini, D.M. MTOR: From Growth Signal Integration to Cancer, Diabetes and Ageing. Nat. Rev. Mol. Cell Biol. 2011, 12, 21–35. [Google Scholar] [CrossRef]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-Based Map of the Human Proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- Boobes, Y.; Bernieh, B.; Saadi, H.; Raafat Al Hakim, M.; Abouchacra, S. Gonadal Dysfunction and Infertility in Kidney Transplant Patients Receiving Sirolimus. Int. Urol. Nephrol. 2010, 42, 493–498. [Google Scholar] [CrossRef]
- Deutsch, M.A.; Kaczmarek, I.; Huber, S.; Schmauss, D.; Beiras-Fernandez, A.; Schmoeckel, M.; Ochsenkuehn, R.; Meiser, B.; Mueller-Hoecker, J.; Reichart, B.; et al. Sirolimus-Associated Infertility: Case Report and Literature Review of Possible Mechanisms. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2007, 7, 2414–2421. [Google Scholar] [CrossRef]
- Huyghe, E.; Matsuda, T.; Thonneau, P. Increasing Incidence of Testicular Cancer Worldwide: A Review. J. Urol. 2003, 170, 5–11. [Google Scholar] [CrossRef]
- Johnson, E.M.; Anderson, J.K.; Jacobs, C.; Suh, G.; Humar, A.; Suhr, B.D.; Kerr, S.R.; Matas, A.J. Long-Term Follow-up of Living Kidney Donors: Quality of Life after Donation. Transplantation 1999, 67, 717–721. [Google Scholar] [CrossRef]
- Li, N.; Cheng, C.Y. Mammalian Target of Rapamycin Complex (MTOR) Pathway Modulates Blood-Testis Barrier (BTB) Function through F-Actin Organization and Gap Junction. Histol. Histopathol. 2016, 31, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Mok, K.W.; Mruk, D.D.; Cheng, C.Y. Regulation of Blood-Testis Barrier (BTB) Dynamics during Spermatogenesis via the “Yin” and “Yang” Effects of Mammalian Target of Rapamycin Complex 1 (MTORC1) and MTORC2. Int. Rev. Cell Mol. Biol. 2013, 301, 291–358. [Google Scholar] [CrossRef] [PubMed]
- Mok, K.-W.; Mruk, D.D.; Silvestrini, B.; Cheng, C.Y. RpS6 Regulates Blood-Testis Barrier Dynamics by Affecting F-Actin Organization and Protein Recruitment. Endocrinology 2012, 153, 5036–5048. [Google Scholar] [CrossRef] [PubMed]
- Mok, K.-W.; Mruk, D.D.; Lee, W.M.; Cheng, C.Y. Rictor/MTORC2 Regulates Blood-Testis Barrier Dynamics via Its Effects on Gap Junction Communications and Actin Filament Network. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2013, 27, 1137–1152. [Google Scholar] [CrossRef] [PubMed]
- Karandrea, S.; Yin, H.; Liang, X.; Heart, E.A. BDE-47 and BDE-85 Stimulate Insulin Secretion in INS-1 832/13 Pancreatic β-Cells through the Thyroid Receptor and Akt. Environ. Toxicol. Pharmacol. 2017, 56, 29–34. [Google Scholar] [CrossRef]
- Zhang, A.; Li, C.Y.; Kelly, E.J.; Sheppard, L.; Cui, J.Y. Transcriptomic Profiling of PBDE-Exposed HepaRG Cells Unveils Critical LncRNA- PCG Pairs Involved in Intermediary Metabolism. PLoS ONE 2020, 15, e0224644. [Google Scholar] [CrossRef]
- Conn, C.S.; Qian, S.-B. MTOR Signaling in Protein Homeostasis: Less Is More? Cell Cycle 2011, 10, 1940–1947. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. Regulation of MTORC1 and Its Impact on Gene Expression at a Glance. J. Cell Sci. 2013, 126, 1713–1719. [Google Scholar] [CrossRef]
- Miller, W.L.; Auchus, R.J. The Molecular Biology, Biochemistry, and Physiology of Human Steroidogenesis and Its Disorders. Endocr. Rev. 2011, 32, 81–151. [Google Scholar] [CrossRef]
- Whirledge, S.; Cidlowski, J.A. Chapter 5—Steroid Hormone Action. In Yen and Jaffe’s Reproductive Endocrinology, 8th ed.; Strauss, J.F., Barbieri, R.L., Eds.; Elsevier: Philadelphia, PA, USA, 2019; pp. 115–131.e4. ISBN 978-0-323-47912-7. [Google Scholar]
- Bremer, A.A.; Miller, W.L. Chapter 13—Regulation of Steroidogenesis. In Cellular Endocrinology in Health and Disease; Ulloa-Aguirre, A., Conn, P.M., Eds.; Academic Press: Boston, MA, USA, 2014; pp. 207–227. ISBN 978-0-12-408134-5. [Google Scholar]
- Kumar, V.; Chakraborty, A.; Kural, M.R.; Roy, P. Alteration of Testicular Steroidogenesis and Histopathology of Reproductive System in Male Rats Treated with Triclosan. Reprod. Toxicol. 2009, 27, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Rone, M.B.; Fan, J.; Papadopoulos, V. Cholesterol Transport in Steroid Biosynthesis: Role of Protein-Protein Interactions and Implications in Disease States. Biochim. Biophys. Acta 2009, 1791, 646–658. [Google Scholar] [CrossRef]
- Song, R.; He, Y.; Murphy, M.B.; Yeung, L.W.Y.; Yu, R.M.K.; Lam, M.H.W.; Lam, P.K.S.; Hecker, M.; Giesy, J.P.; Wu, R.S.S.; et al. Effects of Fifteen PBDE Metabolites, DE71, DE79 and TBBPA on Steroidogenesis in the H295R Cell Line. Chemosphere 2008, 71, 1888–1894. [Google Scholar] [CrossRef]
- He, Y.; Murphy, M.B.; Yu, R.M.K.; Lam, M.H.W.; Hecker, M.; Giesy, J.P.; Wu, R.S.S.; Lam, P.K.S. Effects of 20 PBDE Metabolites on Steroidogenesis in the H295R Cell Line. Toxicol. Lett. 2008, 176, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.-L.; Hsia, S.-M.; Mao, I.-F.; Chen, M.-L.; Wang, S.-W.; Wang, P.S. Effects of Polybrominated Diphenyl Ethers on Steroidogenesis in Rat Leydig Cells. Hum. Reprod. 2011, 26, 2209–2217. [Google Scholar] [CrossRef] [PubMed]
- Evaul, K.; Hammes, S.R. Cross-Talk between G Protein-Coupled and Epidermal Growth Factor Receptors Regulates Gonadotropin-Mediated Steroidogenesis in Leydig Cells. J. Biol. Chem. 2008, 283, 27525–27533. [Google Scholar] [CrossRef]
- Kim, H.W.; Ha, S.H.; Lee, M.N.; Huston, E.; Kim, D.-H.; Jang, S.K.; Suh, P.-G.; Houslay, M.D.; Ryu, S.H. Cyclic AMP Controls MTOR through Regulation of the Dynamic Interaction between Rheb and Phosphodiesterase 4D. Mol. Cell. Biol. 2010, 30, 5406–5420. [Google Scholar] [CrossRef]
- Liu, D.; Bordicchia, M.; Zhang, C.; Fang, H.; Wei, W.; Li, J.-L.; Guilherme, A.; Guntur, K.; Czech, M.P.; Collins, S. Activation of MTORC1 Is Essential for β-Adrenergic Stimulation of Adipose Browning. J. Clin. Investig. 2016, 126, 1704–1716. [Google Scholar] [CrossRef]
- Kabakci, R.; Yigit, A.A. Effects of Bisphenol A, Diethylhexyl Phthalate and Pentabrominated Diphenyl Ether 99 on Steroid Synthesis in Cultured Bovine Luteal Cells. Reprod. Domest. Anim. 2020, 55, 683–690. [Google Scholar] [CrossRef]
- Vertika, S.; Singh, K.K.; Rajender, S. Mitochondria, Spermatogenesis, and Male Infertility—An Update. Mitochondrion 2020, 54, 26–40. [Google Scholar] [CrossRef]
- Midzak, A.S.; Chen, H.; Aon, M.A.; Papadopoulos, V.; Zirkin, B.R. ATP Synthesis, Mitochondrial Function, and Steroid Biosynthesis in Rodent Primary and Tumor Leydig Cells. Biol. Reprod. 2011, 84, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, S.K.; Agrawal, A.K.; Hakim, B.A.; Vishwakarma, A.L.; Narender, T.; Sachan, R.; Sachdev, M. Mitochondrial Membrane Potential (MMP) Regulates Sperm Motility. In Vitro Cell. Dev. Biol. Anim. 2016, 52, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.L. Steroid Hormone Synthesis in Mitochondria. Mol. Cell. Endocrinol. 2013, 379, 62–73. [Google Scholar] [CrossRef]
- Antico Arciuch, V.G.; Elguero, M.E.; Poderoso, J.J.; Carreras, M.C. Mitochondrial Regulation of Cell Cycle and Proliferation. Antioxid. Redox Signal. 2012, 16, 1150–1180. [Google Scholar] [CrossRef] [PubMed]
- Tait, S.W.G.; Green, D.R. Mitochondria and Cell Signalling. J. Cell Sci. 2012, 125, 807–815. [Google Scholar] [CrossRef]
- Mathur, P.P.; D’Cruz, S.C. The Effect of Environmental Contaminants on Testicular Function. Asian J. Androl. 2011, 13, 585–591. [Google Scholar] [CrossRef]
- Huang, S.; Wang, J.; Cui, Y. 2,2′,4,4′-Tetrabromodiphenyl Ether Injures Cell Viability and Mitochondrial Function of Mouse Spermatocytes by Decreasing Mitochondrial Proteins Atp5b and Uqcrc1. Environ. Toxicol. Pharmacol. 2016, 46, 301–310. [Google Scholar] [CrossRef]
- Dong, L.; Li, P.; Yang, K.; Liu, L.; Gao, H.; Zhou, G.; Zhao, Q.; Xia, T.; Wang, A.; Zhang, S. Promotion of Mitochondrial Fusion Protects against Developmental PBDE-47 Neurotoxicity by Restoring Mitochondrial Homeostasis and Suppressing Excessive Apoptosis. Theranostics 2020, 10, 1245–1261. [Google Scholar] [CrossRef]
- Souza, A.O.; Tasso, M.J.; Oliveira, A.M.C.; Pereira, L.C.; Duarte, F.V.; Oliveira, D.P.; Palmeira, C.M.; Dorta, D.J. Evaluation of Polybrominated Diphenyl Ether Toxicity on HepG2 Cells—Hexabrominated Congener (BDE-154) Is Less Toxic than Tetrabrominated Congener (BDE-47). Basic Clin. Pharmacol. Toxicol. 2016, 119, 485–497. [Google Scholar] [CrossRef]
- Suvorov, A.; Naumov, V.; Shtratnikova, V.; Logacheva, M.; Shershebnev, A.; Wu, H.; Gerasimov, E.; Zheludkevich, A.; Pilsner, J.R.; Sergeyev, O. Rat Liver Epigenome Programing by Perinatal Exposure to 2,2′,4′4′-Tetrabromodiphenyl Ether. Epigenomics 2020, 12, 235–249. [Google Scholar] [CrossRef]
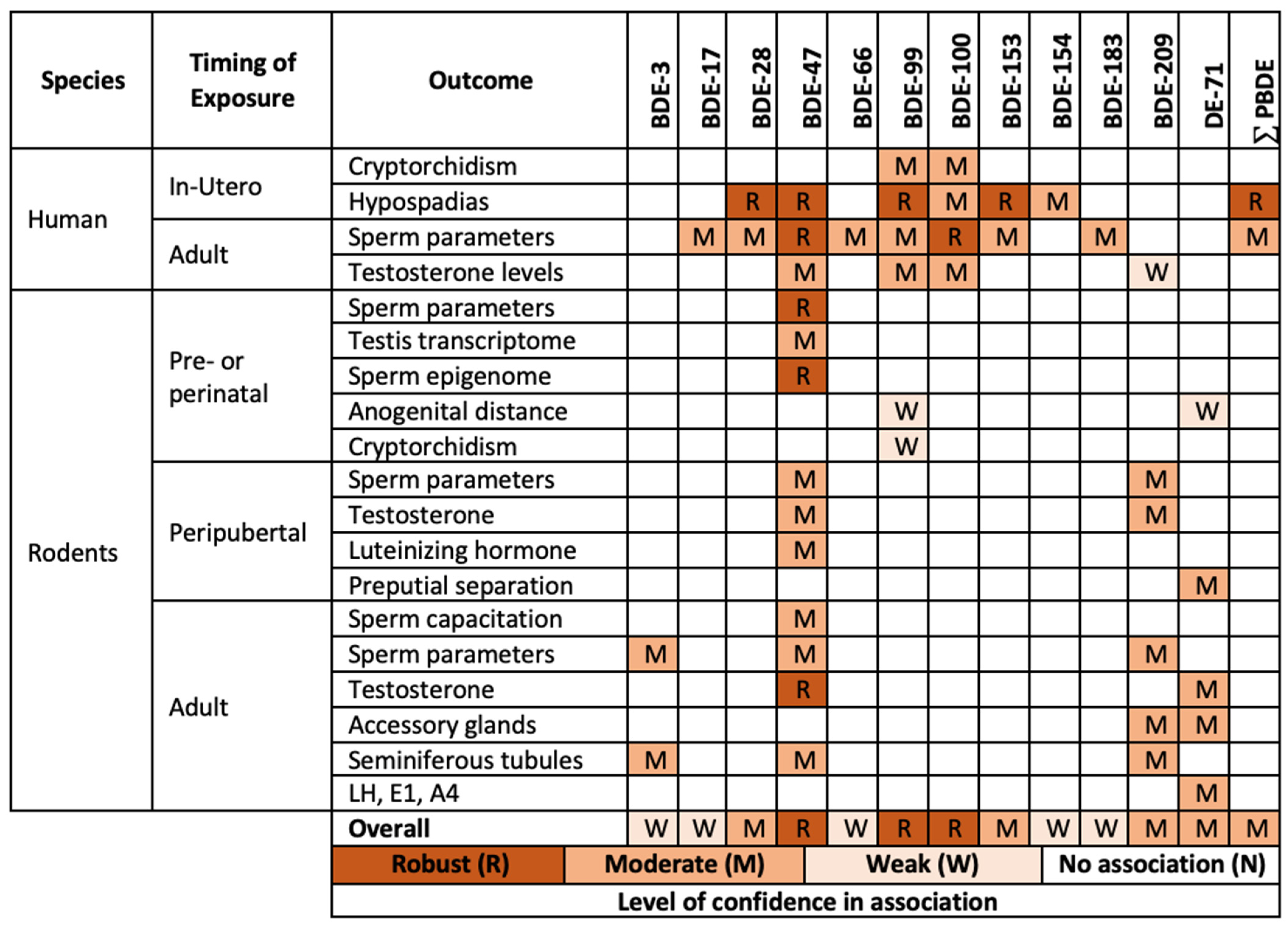
| PBDE Levels | Sample Size | Design and Timing of Exposure | Affected Outcomes | Timing of Outcomes | Study Population | References | ||
|---|---|---|---|---|---|---|---|---|
| Congeners | Concentration | Media | ||||||
| 10 BDEs, including BDE-17, 28, 153 | Individual: range of medians (ng/g): 0.00–0.12 | Serum | 468 men (LIFE study) | Cross-sectional part of prospective study, mean age 31.8 years, 2005–2009 | Negative association with sperm motility and morphology | Mean age 31.8 years | Texas and Michigan states | [25] |
| 29 BDEs, including BDE-47, 99, 100, 153 | Individual: BDE-47 median (ng/g lipid): 0.72 | Serum | 10 men | Cross-sectional, age 18–22 years, 2003 | Inverse correlation with sperm concentration and testis size | Age 18–22 years | Kawasaki, Japan | [26] |
| 7 BDEs, including BDE-47, 153 | Individual: medians (ng/g lipid): BDE-47-Ukraine-0.2, Poland-0.6, Greenland-2.0; BDE-153-Ukraine-0.3, Poland-0.5, Greenland-2.7 | Serum | 299 men | Cross-sectional, IQR age: Ukraine 20.7–38.2; Poland 25.3–36.9; Greenland 21.2–43.6 years 2002–2004 | No effect on sperm quality and serum reproductive hormones | IQR age: Ukraine 20.7–38.2; Poland 25.3–36.9; Greenland 21.2–43.6 years | Ukraine (Kharkiv), Poland (Warsaw), and Greenland | [27] |
| BDE-47, 99, 100 | Total: median (ng/g lipid): 29.1 Individual BDE-47: median: 9.4 | Hair | 153 men | Cross-sectional, age 18–41 years, 2009–2012 | Negative association with sperm motility | Age 18–41 years | Montreal, Canada | [28] |
| BDE-28, 47, 99, 100, 153, 154, 183, 209 | Total: median (ng/g): 53.0. Individual: medians: BDE-99-7.9; BDE-100-7.4; BDE-154-4.0 | Hair | 137 mothers/boys vs. 158 controls | Case-control, age 18–48 years, 2011–2014 | Higher risk of cryptorchidism | Antenatal, age 3–12 months | Montreal, Canada | [29] |
| BDE-28, 47, 153 | Individual: range of medians (pg/g ww): 3.6–6.1 | Semen | 32 men | Cross-sectional, age 20–50 years, 2015–2016 | Negative correlation with sperm concentration and count | Age 20–50 years | Qingyuan, China | [30] |
| BDE-28, 47, 99, 153, 154 | Individual: range of 5 medians (ng/g): 4–10.8 | Hair | 152 mothers/boys vs. 64 controls | Case-control, mothers’ IQR age 29–36 years, 2011–2014 | Higher level in cases of hypospadias | Antenatal, IQR age 5–12 months | Toronto, Canada | [31] |
| BDE-28, 47, 99, 100, 153, 154, 183, 209 | Total: median (ng/g): 51.4 | Hair | 89 mothers/boys vs. 54 controls | Case-control, mothers’ IQR age 29–36 years, 2011–2013 | Higher risk of hypospadias | Antenatal, IQR age 5–12 months | Toronto, Canada | [32] |
| BDE-28, 47, 99, 100, 153 | Individual: range of 5 medians (ng/g lipid): 1.0–19.1 | Serum | 20 mothers/boys vs. 28 controls | Nested case-control, maternal mid-pregnancy, 2003 | No effect on hypospadias | Antenatal | Southern California | [33] |
| PBDE | Lowest Toxic Dose, mg/kg Body Weight | Species | Route of Exposure | Duration of Exposure | Outcome Altered at Lowest Dose | Outcome Assessed | References |
|---|---|---|---|---|---|---|---|
| Developmental Studies | |||||||
| BDE-47 | 0.2 | Rat | Pipette feeding | GD8–PND21 | DNA methylation of sperm | PND65 and PND120 | [34] |
| BDE-47 | 0.2 | Rat | Pipette feeding | GD8–PND21 | Sperm small noncoding RNA | PND65 and PND120 | [35] |
| BDE-47 | 0.2 | Rat | Pipette feeding | GD8–PND21 | DNA methylation of sperm | PND65 and PND120 | [36] |
| BDE-47 | 0.1 | Rat | Oral gavage | 10 days before mating–PND21 | Testis weight | PND88 | [37] |
| BDE-47 | 0.4 | Rat | Oral gavage | PND21–35 | Leydig cell number, serum LH and testosterone levels | PND35 | [38] |
| BDE-47 | 0.2 | Rat | Pipette feeding | GD8–PND21 | Sperm parameters, testes weight, daily sperm production, and testis transcriptome. | PND120 | [39] |
| DE-71 | 30 | Rat | Oral gavage | PND23–53 | Preputial separation | PND53 | [40] |
| DE-71 | 18 | Rat | Oral gavage | GD6–PND18 | Testis weight | PND31 | [41] |
| BDE-99 | 0.06 | Rat | Oral gavage | GD6 | Daily sperm production, sperm and spermatid counts | PND140 | [42] |
| DE-71 | 40 | Rat | Oral gavage | GD7–PND16 | Anogenital distance | PND1 | [43] |
| BDE-99 | 0.2 | Mouse | Oral gavage | GD1–GD21 | Anogenital distance, testosterone levels, testes weight, Leydig cell number, gene and protein expression | PND35 | [44] |
| BDE-209 | 10 | Mouse | Oral gavage | PND21–70 | No significant change at the dose of exposure | PND71 | [45] |
| BDE-209 | 0.025 | Mouse | Sub-cutaneous injection | PND1–5 | Testis weight, sperm count, elongated spermatids | PND84 | [46] |
| BDE-209 | 0.025 | Mouse | Sub-cutaneous injection | PND1–5 | Serum testosterone | PND84 | [47] |
| Adult studies | |||||||
| BDE-47 | 0.03 | Rat | Oral gavage | 48 days | Multinucleated giant cells in testis, serum testosterone levels | 24 h after exposure | [48] |
| BDE-47 | 0.03 | Rat | Oral gavage | 84 days of exposure | Testosterone levels, organization of the seminiferous epithelium | After 84 days of exposure | [49] |
| DE-71 | 3 | Rat | Oral gavage | PND90–93 | Serum LH, estrone, androstenedione, and testosterone levels | PND93 | [50] |
| BDE-209 | 0.2 | Rat | Oral gavage | PND77–105 | Seminal vesicle/coagulation of gland weight | PND105 | [51] |
| BDE-3 | 1.5 | Mouse | Oral gavage | PND105–147 | Sperm count | PND148 | [52] |
| BDE-47 | 0.0015 | Mouse | Oral gavage | PND56–86 | Sperm capacitation and sperm motility | PND86 | [53] |
| BDE-47 | 10 | Mouse | Oral gavage | PND56–92 | Sperm levels in the epididymal lumen | PND93 | [54] |
| BDE-209 | 7.5 | Mouse | Oral gavage | PND42–70 | Sperm number, germinal epithelium | PND70 | [55] |
| BDE-209 | 20 | Mouse | Oral gavage | PND28–140 | Testicular expression of genes and proteins | PND140 | [56] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arowolo, O.; Pilsner, J.R.; Sergeyev, O.; Suvorov, A. Mechanisms of Male Reproductive Toxicity of Polybrominated Diphenyl Ethers. Int. J. Mol. Sci. 2022, 23, 14229. https://doi.org/10.3390/ijms232214229
Arowolo O, Pilsner JR, Sergeyev O, Suvorov A. Mechanisms of Male Reproductive Toxicity of Polybrominated Diphenyl Ethers. International Journal of Molecular Sciences. 2022; 23(22):14229. https://doi.org/10.3390/ijms232214229
Chicago/Turabian StyleArowolo, Olatunbosun, J. Richard Pilsner, Oleg Sergeyev, and Alexander Suvorov. 2022. "Mechanisms of Male Reproductive Toxicity of Polybrominated Diphenyl Ethers" International Journal of Molecular Sciences 23, no. 22: 14229. https://doi.org/10.3390/ijms232214229
APA StyleArowolo, O., Pilsner, J. R., Sergeyev, O., & Suvorov, A. (2022). Mechanisms of Male Reproductive Toxicity of Polybrominated Diphenyl Ethers. International Journal of Molecular Sciences, 23(22), 14229. https://doi.org/10.3390/ijms232214229







