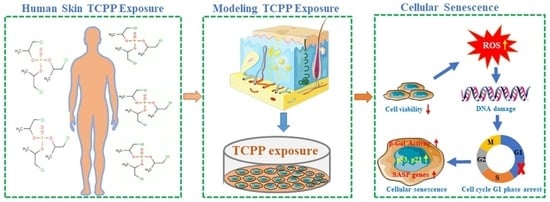
Organophosphorus Flame Retardant TCPP Induces Cellular Senescence in Normal Human Skin Keratinocytes: Implication for Skin Aging
Abstract
:1. Introduction
2. Results and Discussion
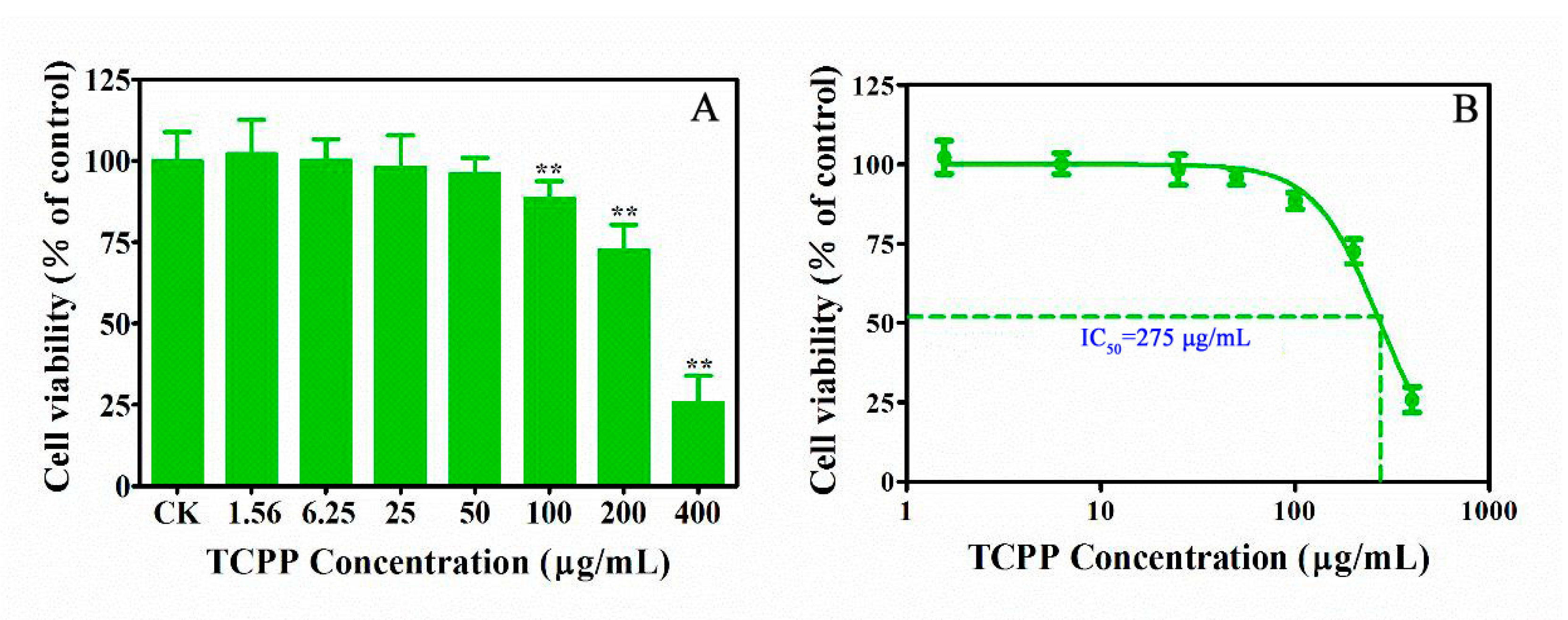
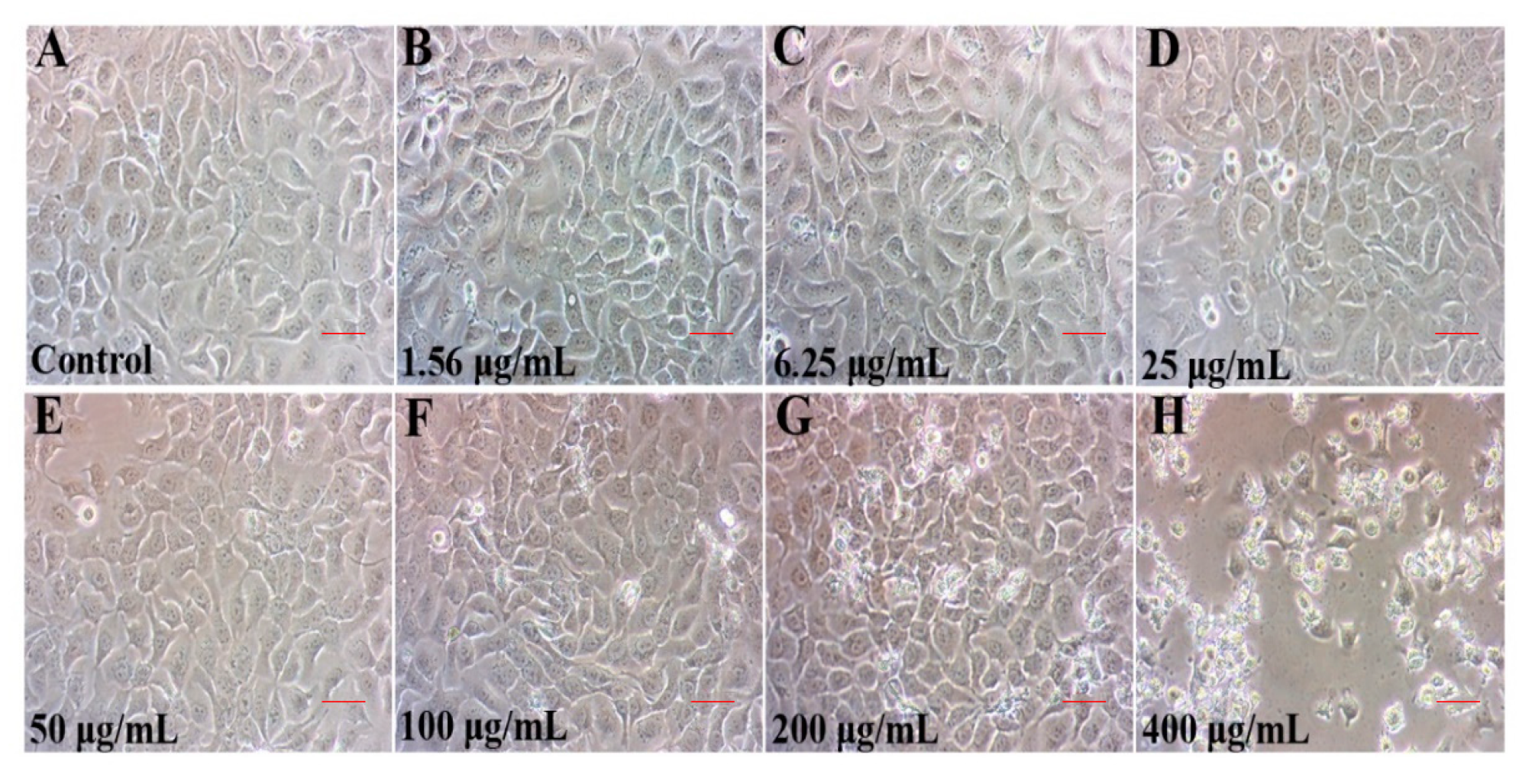
2.1. TCPP Suppressed Cell Viability and Altered Cell Morphology
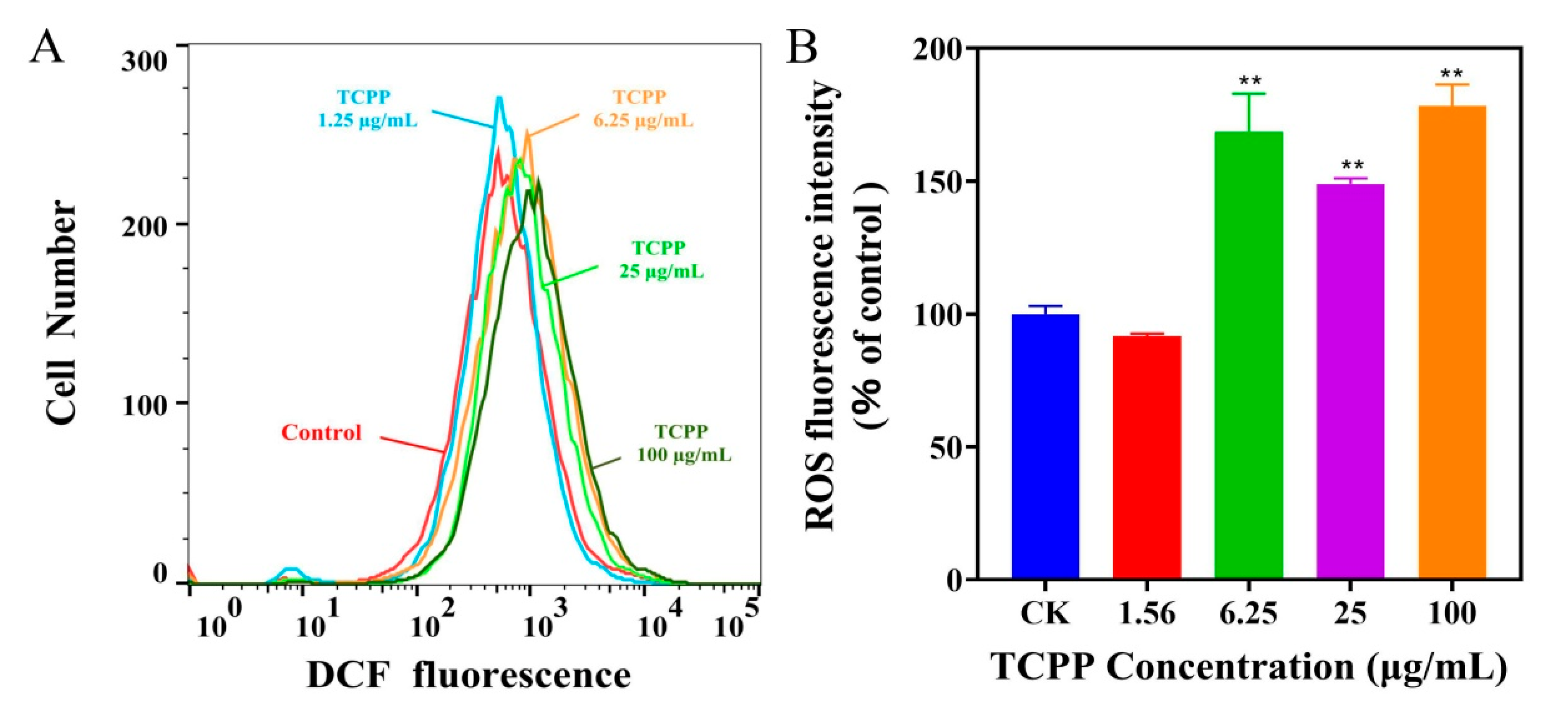
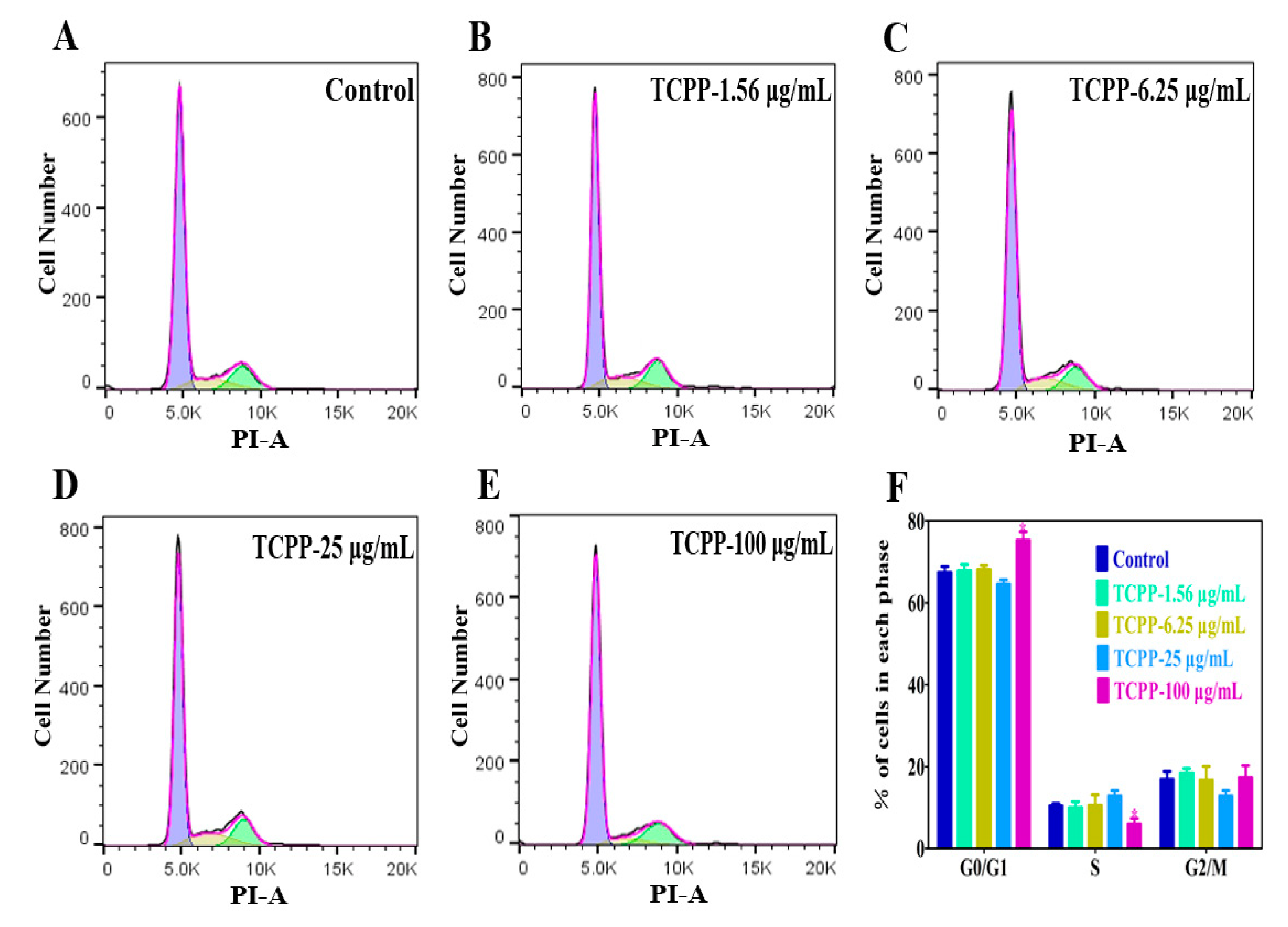
2.2. TCPP Increased Intracellular ROS, Induced DNA Damage and Cell Cycle Arrest in HaCaT Cells
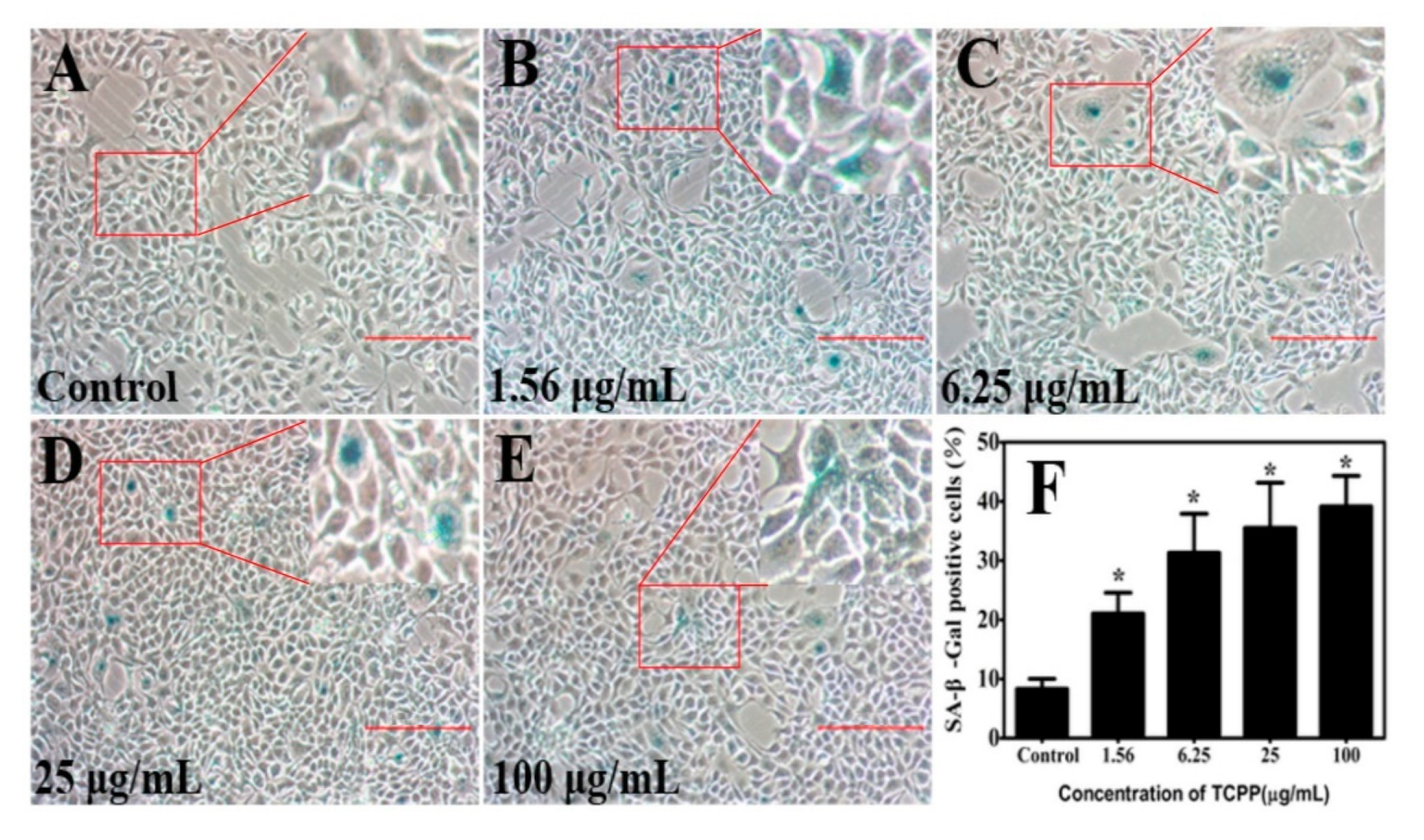
2.3. TCPP Enhanced Cellular SA-β-Gal Activity in HaCaT Cells
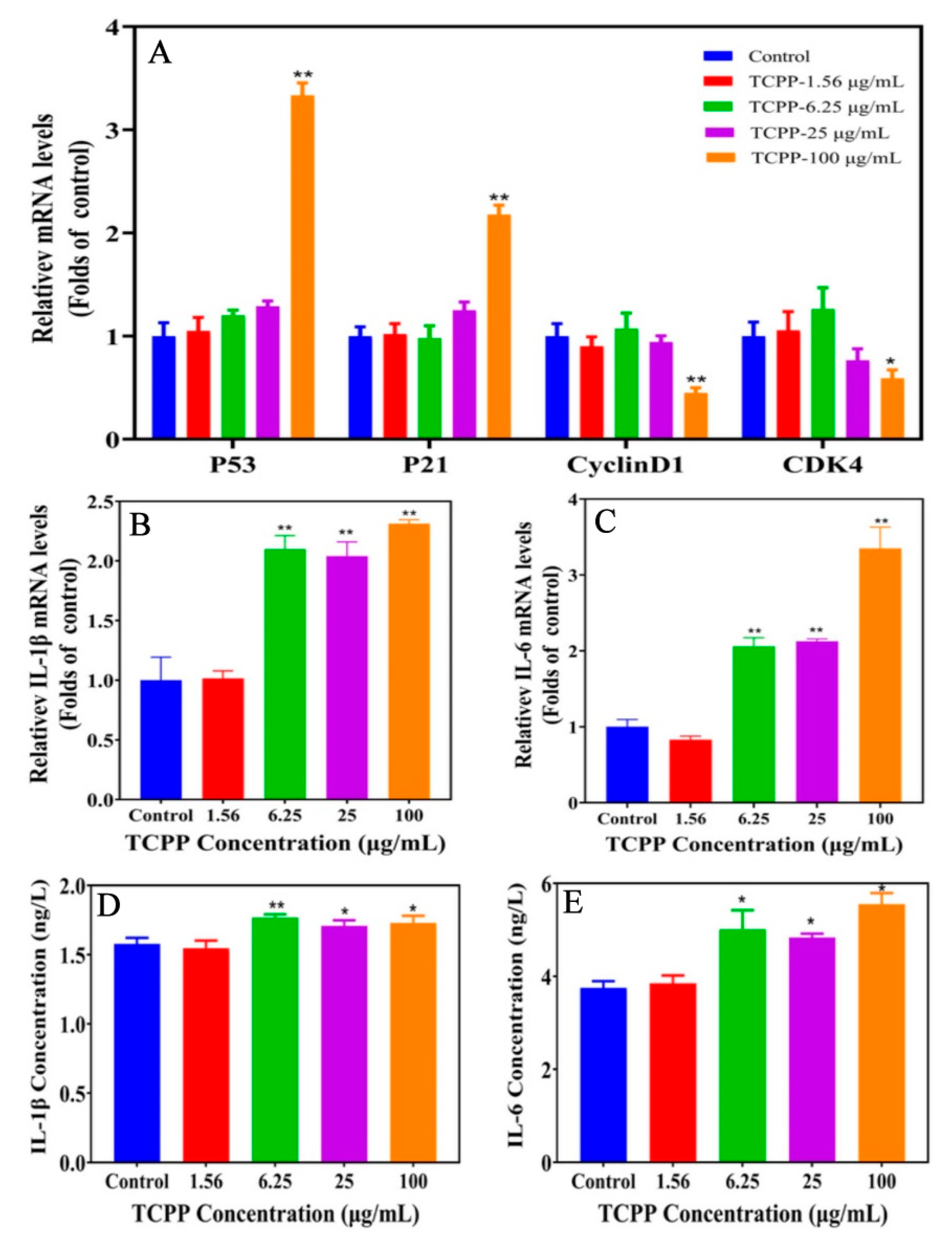
2.4. TCPP Altered Gene Expression of Senescence Markers
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Cell Culture and Treatment
3.3. Cell Viability Analysis
3.4. Measurement of Intracellular Reactive Oxygen Species (ROS)
3.5. Immunofluorescence Staining
3.6. Cell Cycle Analysis
3.7. Senescence β-Galactosidase Staining
3.8. Total RNA Extraction and Quantitative Real-Time PCR (qRT-PCR) Assay
3.9. Enzyme-Linked Immunosorbent Assay (ELISA)
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chokwe, T.B.; Abafe, O.A.; Mbelu, S.P.; Okonkwo, J.O.; Sibali, L.L. A review of sources, fate, levels, toxicity, exposure and transformations of organophosphorus flameretardants and plasticizers in the environment. Emerg. Contam. 2020, 6, 345–366. [Google Scholar] [CrossRef]
- Hoffman, K.; Butt, C.M.; Chen, A.; Limkakeng, A.T.; Stapleton, H.M. High exposure to organophosphate flame retardants in infants: Associations with baby products. Environ. Sci. Technol. 2015, 49, 14554–14559. [Google Scholar] [CrossRef] [PubMed]
- Shoeib, T.; Webster, G.M.; Hassan, Y.; Tepe, S.; Yalcin, M.; Turgut, C.; Kurt-Karakuş, P.B.; Jantunen, L. Organophosphate esters in house dust: A comparative study between Canada, Turkey and Egypt. Sci. Total Environ. 2019, 650, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Yang, J.; Bekele, T.G.; Zhao, S.J.; Zhao, H.X.; Li, J.; Wang, M.J.; Zhao, H.D. Organophosphate esters in human serum in Bohai Bay, North China. Environ. Sci. Pollut. Res. Int. 2020, 27, 2721–2729. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.L.; Zhao, X.Z.; Shi, Z.X. Organophosphorus flame retardants in breast milk from Beijing, China: Occurrence, nursing infant’s exposure and risk assessment. Sci. Total Environ. 2021, 771, 145404. [Google Scholar] [CrossRef]
- Zhang, B.; Lu, S.Y.; Huang, M.Z.; Zhou, M.Z.; Zhou, Z.Q.; Zheng, H.C.; Jiang, Y.C.; Bai, X.Y.; Zhang, T. Urinary metabolites of organophosphate flame retardants in 0-5-year-old children: Potential exposure risk for inpatients and home-stay infants. Environ. Pollut. 2018, 243, 318–325. [Google Scholar] [CrossRef]
- Abdallah, M.A.E.; Harrad, S. Dermal uptake of chlorinated organophosphate flame retardants via contact with furniture fabrics; implications for human exposure. Environ. Res. 2022, 209, 112847. [Google Scholar] [CrossRef]
- Abdallah, M.A.E.; Pawar, G.; Harrad, S. Evaluation of in vitro vs. in vivo methods for assessment of dermal absorption of organic flame retardants: A review. Environ. Int. 2015, 74, 13–22. [Google Scholar] [CrossRef]
- Krutmann, J.; Schikowski, T.; Morita, A.; Berneburg, M. Environmentally-Induced (Extrinsic) Skin Aging: Exposomal Factors and Underlying Mechanisms. J. Investig. Dermatol. 2021, 141, 1096–1103. [Google Scholar] [CrossRef]
- Wang, A.S.; Dreesen, O. Biomarkers of Cellular Senescence and Skin Aging. Front. Genet. 2018, 9, 247. [Google Scholar] [CrossRef] [Green Version]
- Krutmann, J.; Bouloc, A.; Sore, G.; Bernard, B.A.; Passeron, T. The skin aging exposome. J. Dermatol. Sci. 2017, 85, 152–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koohgoli, R.; Hudson, L.; Naidoo, K.; Wilkinson, S.; Chavan, B.; Birch-Machin, M.A. Bad air gets under your skin. Exp. Dermatol. 2018, 26, 384–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, L.; Chen, Y.; Feng, J.; Sun, W.; Li, S.; Ou, M.; Tang, L. Cellular senescence regulated by SWI/SNF complex subunits through p53/p21 and p16/pRB pathway. Int. J. Biochem. Cell Biol. 2017, 90, 29–37. [Google Scholar] [CrossRef]
- Li, H.; Petersen, S.; Garcia Mariscal, A.; Brakebusch, C. Negative Regulation of p53-Induced Senescence by N-WASP Is Crucial for DMBA/TPA-Induced Skin Tumor Formation. Cancer Res. 2019, 79, 2167–2181. [Google Scholar] [CrossRef] [Green Version]
- Behnia, F.; Peltier, M.R.; Saade, G.R.; Menon, R. Environmental Pollutant Polybrominated Diphenyl ether, a flame retardant, induces primary amnion cell senescence. Am. J. Reprod. Immunol. 2015, 74, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.J.; Zhang, Y.J.; Hou, J.; Xu, T.; Yin, W.J.; Xiong, W.; Lu, W.H.; Zheng, H.Y.; Chen, J.; Yuan, J. Tris (2-chloroethyl) phosphate induces senescence-like phenotype of hepatocytes via the p21Waf1/Cip1-Rb pathway in a p53-independent manner. Environ. Toxicol. Pharmacol. 2017, 56, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Salotti, J.; Johnson, P.F. Regulation of senescence and the SASP by the transcription factor C/EBP. Exp. Gerontol. 2019, 128, 110752. [Google Scholar] [CrossRef]
- Soroka, Y.; Ma’or, Z.; Leshem, Y.; Verochovsky, L.; Neuman, R.; Brégégère, F.M.; Milner, Y. Aged keratinocyte phenotyping: Morphology, biochemical markers and effects of Dead Sea minerals. Exp. Gerontol. 2008, 43, 947–957. [Google Scholar] [CrossRef]
- Ma, J.Y.; Bao, X.C.; Tian, W.; Cui, D.L.; Zhang, M.Y.; Yang, J.; Xiang, P.; Ma, L.Q. Effects of soil-extractable metals Cd and Ni from an e-waste dismantling site on human colonic epithelial cells Caco-2: Mechanisms and implications. Chemosphere 2022, 292, 133361. [Google Scholar] [CrossRef]
- Mokra, K.; Bukowski, K.; Woźniak, K. Effects of tris(1-chloro-2-propyl) phosphate and tris(2-chloroethyl) phosphate on cell viability and morphological changes in peripheral blood mononuclear cells (in vitro study). Hum. Exp. Toxicol. 2018, 37, 1336–1345. [Google Scholar] [CrossRef]
- Neuberg, S.L.; Kenrick, D.T.; Schaller, M. Human threat management systems: Self-protection and disease avoidance. Neurosci. Biobehav. R. 2011, 35, 1042–1051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.S.; Wang, M.; Wang, Y.L.; Guo, Y.T.; Tao, X.X.; Wang, X.C.; Cao, Y.; Tian, S.S.; Li, Q.L. p53-mediated ferroptosis is required for 1-methyl-4-phenylpyridinium-induced senescence of PC12 cells. Toxicol. In Vitro 2021, 73, 105146. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Liu, J.H.; Zhou, G.Q.; Sang, Y.J.; Yue Zhang, Y.; Jing, L.; Shi, Z.X.; Zhou, X.Q.; Sun, Z.W. BDE-209 and DBDPE induce male reproductive toxicity through telomere-related cell senescence and apoptosis in SD rat. Environ. Int. 2021, 146, 106307. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative Stress: Eustress and Distress in Redox Homeostasis. Stress Physiol. Biochem. Pathol. 2019, 3, 153–163. [Google Scholar]
- Saquib, Q.; Siddiqui, M.A.; Ahmad, J.; Ansari, S.M.; Al-Wathnani, H.A.; Rensing, C. 6-OHBDE-47 induces transcriptomic alterations of CYP1A1, XRCC2, HSPA1A, EGR1 genes and trigger apoptosis in HepG2 cells. Toxicology 2018, 400, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.W.; Wang, Y.T.; Fang, C.Y.; Song, E.Q.; Song, Y. Polybrominated diphenyl ether quinone exposure leads to ROS-driven lysosomal damage, mitochondrial dysfunction and NLRP3 inflammasome activation. Environ. Pollut. 2022, 311, 119846. [Google Scholar] [CrossRef]
- Zhao, Z.C.; Shang, D.S.; Qiu, L.P.; Guo, C.; Li, Y.Y.; Liu, H.Q.; Yuan, G.Y.; Tu, Z.G. 4,5-Diphenyl-2-methyl picolinate induces cellular senescence by accumulating DNA damage and activating associated signaling pathways in gastric cancer. Life Sci. 2019, 238, 116973. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.X.; Zhu, Q.S.; Song, E.Q.; Song, Y. Polybrominated diphenyl ethers quinone exhibits neurotoxicity by inducing DNA damage, cell cycle arrest, apoptosis and p53-driven adaptive response in microglia BV2 cells. Toxicology 2021, 457, 152807. [Google Scholar] [CrossRef]
- Oberdoerffer, P.; Miller, K.M. Histone H2A variants: Diversifying chromatin to ensure genome integrity. Semin. Cell Dev. Biol. 2022, in press. [Google Scholar] [CrossRef]
- Turinetto, V.; Giachino, C. Multiple facets of histone variant H2AX: A DNA double-strand-break marker with several biological functions. Nucleic Acids Res. 2015, 43, 2489–2498. [Google Scholar] [CrossRef] [Green Version]
- Rahmanian, N.; Shokrzadeh, M.; Eskandani, M. Recent advances in γH2AX biomarker-based genotoxicity assays: A marker of DNA damage and repair. DNA Repair 2021, 108, 103243. [Google Scholar] [CrossRef]
- Yang, G.T.; Zhou, X.; Wang, J.; Zhang, W.J.; Zheng, H.Y.; Lu, W.H.; Yuan, J. MEHP-induced oxidative DNA damage and apoptosis in HepG2 cells correlates with p53-mediated mitochondria-dependent signaling pathway. Food Chem. Toxicol. 2012, 50, 2424–2431. [Google Scholar] [CrossRef]
- Calcinotto, A.; Kohli, J.; Zagato, E.; Pellegrini, L.; Demaria, M.; Alimonti, A. Cellular Senescence: Aging, Cancer, and Injury. Physiol. Rev. 2019, 99, 1047–1078. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.L.; Bi, J.; Zhang, Z.N.; Li, M.Y.; Qin, Y.S.; Xiang, P.; Ma, L.Q. Organophosphorus flame retardant TDCPP-induced cytotoxicity and associated mechanisms in normal human skin keratinocytes. Sci. Total Environ. 2020, 726, 138526. [Google Scholar] [CrossRef]
- Zheng, K.W.; Zhong, Y.F.; Yu, Z.Q.; Shang, Y.; An, J. Triphenyl phosphate (TPP) and tris (2-chloroisopropyl) phosphate (TCPP) induced apoptosis and cell cycle arrest in HepG2 cells. SDRP J. Earth Sci. Environ. Stud. 2018, 4, 490–501. [Google Scholar]
- Roger, L.; Tomas, F.; Gire, V. Mechanisms and Regulation of Cellular Senescence. Int. J. Mol. Sci. 2021, 22, 13173. [Google Scholar] [CrossRef] [PubMed]
- Mohamad Kamal, N.S.; Safuan, S.; Shamsuddin, S.; Foroozandeh, P. Aging of the cells: Insight into cellular senescence and detection Methods. Eur. J. Cell Biol. 2020, 99, 151108. [Google Scholar] [CrossRef] [PubMed]
- Debacq-Chainiaux, F.; Erusalimsky, J.D.; Campisi, J.; Toussaint, O. Protocols to detect senescence-associated beta-galactosidase (SA-beta-gal) activity, a biomarker of senescent cells in culture and in vivo. Nat. Protoc. 2009, 4, 1798–1806. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front. Cell Dev. Biol. 2021, 9, 645593. [Google Scholar] [CrossRef]
- Chen, Q.Z.; Sun, X.L.; Luo, X.L.; Wang, J.; Hu, J.B.; Feng, Y.D. PIK3R3 inhibits cell senescence through p53/p21 signaling. Cell Death Dis. 2020, 11, 798. [Google Scholar] [CrossRef]
- Chen, C.C.; Chen, J.; Wang, W.L.; Xie, L.; Shao, C.Q.; Zhang, Y.X. Inhibition of the P53/P21 Pathway Attenuates the Effects of Senescent Nucleus Pulposus Cell-Derived Exosomes on the Senescence of Nucleus Pulposus Cells. Orthop Surg. 2021, 13, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.B.; Schumacher, B. p53 in the DNA-Damage-Repair Process. Cold Spring Harb. Perspect. Med. 2016, 6, a026070. [Google Scholar] [CrossRef] [PubMed]
- He, Q.Q.; Au, B.; Kulkarni, M.; Shen, Y.; Lim, K.J.; Maimaiti, J.; Wong, C.K.; Luijten, M.N.H.; Chong, H.C.; Lim, E.H.; et al. Chromosomal instability-induced senescence potentiates cell non-autonomous tumourigenic effects. Oncogenesis 2018, 7, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bitar, S.A.; Gali-Muhtasib, H. The Role of the Cyclin Dependent Kinase Inhibitor p21cip1/waf1 in Targeting Cancer: Molecular Mechanisms and Novel Therapeutics. Cancers 2019, 11, 1475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birch, J.; Gil, J. Senescence and the SASP: Many therapeutic avenues. Genes Dev. 2020, 34, 1565–1576. [Google Scholar] [CrossRef]

| Gene | Forward Primer (5′–3′) | Reserve Primer (5′–3′) |
|---|---|---|
| P53 | CAGCACATGACGGAGGTTGT | TCATCCAAATACTCCACACGC |
| P21 | TGTCCGTCAGAACCCATGC | AAAGTCGAAGTTCCATCGCTC |
| Cyclin D1 | AGCTGTGCATCTACACCGAC | GAAATCGTGCGGGGTCATTG |
| CDK4 | AGATGGCACTTACACCCGTG | ACATGTCCACAGGTGTTGCA |
| IL-1β | ACAGATGAAGTGCTCCTTCCA | GTCGGAGATTCGTAGCTGGAT |
| IL-6 | CAATCTGGATTCAATGAGGAGAC | CTCTGGCTTGTTCCTCACTACTC |
| β-Actin | GTACCACTGGCATCGTGATGGACT | CCGCTCATTGCCAATGGTGAT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.-X.; Cui, D.-L.; Yang, D.-L.; Li, J.-Y.; Yang, Z.-Y.; Su, J.-Z.; Ren, C.-X.; Niu, Y.-Y.; Xiang, P. Organophosphorus Flame Retardant TCPP Induces Cellular Senescence in Normal Human Skin Keratinocytes: Implication for Skin Aging. Int. J. Mol. Sci. 2022, 23, 14306. https://doi.org/10.3390/ijms232214306
Liu J-X, Cui D-L, Yang D-L, Li J-Y, Yang Z-Y, Su J-Z, Ren C-X, Niu Y-Y, Xiang P. Organophosphorus Flame Retardant TCPP Induces Cellular Senescence in Normal Human Skin Keratinocytes: Implication for Skin Aging. International Journal of Molecular Sciences. 2022; 23(22):14306. https://doi.org/10.3390/ijms232214306
Chicago/Turabian StyleLiu, Jian-Xiang, Dao-Lei Cui, Dan-Lei Yang, Jing-Ya Li, Zi-Yue Yang, Jin-Zhou Su, Cai-Xia Ren, You-Ya Niu, and Ping Xiang. 2022. "Organophosphorus Flame Retardant TCPP Induces Cellular Senescence in Normal Human Skin Keratinocytes: Implication for Skin Aging" International Journal of Molecular Sciences 23, no. 22: 14306. https://doi.org/10.3390/ijms232214306