Effects of Alhagi Honey Polysaccharides as Feed Supplement on Intestine Function and Microbiome, Immune Function, and Growth Performance in Chicken
Abstract
:1. Introduction
2. Results
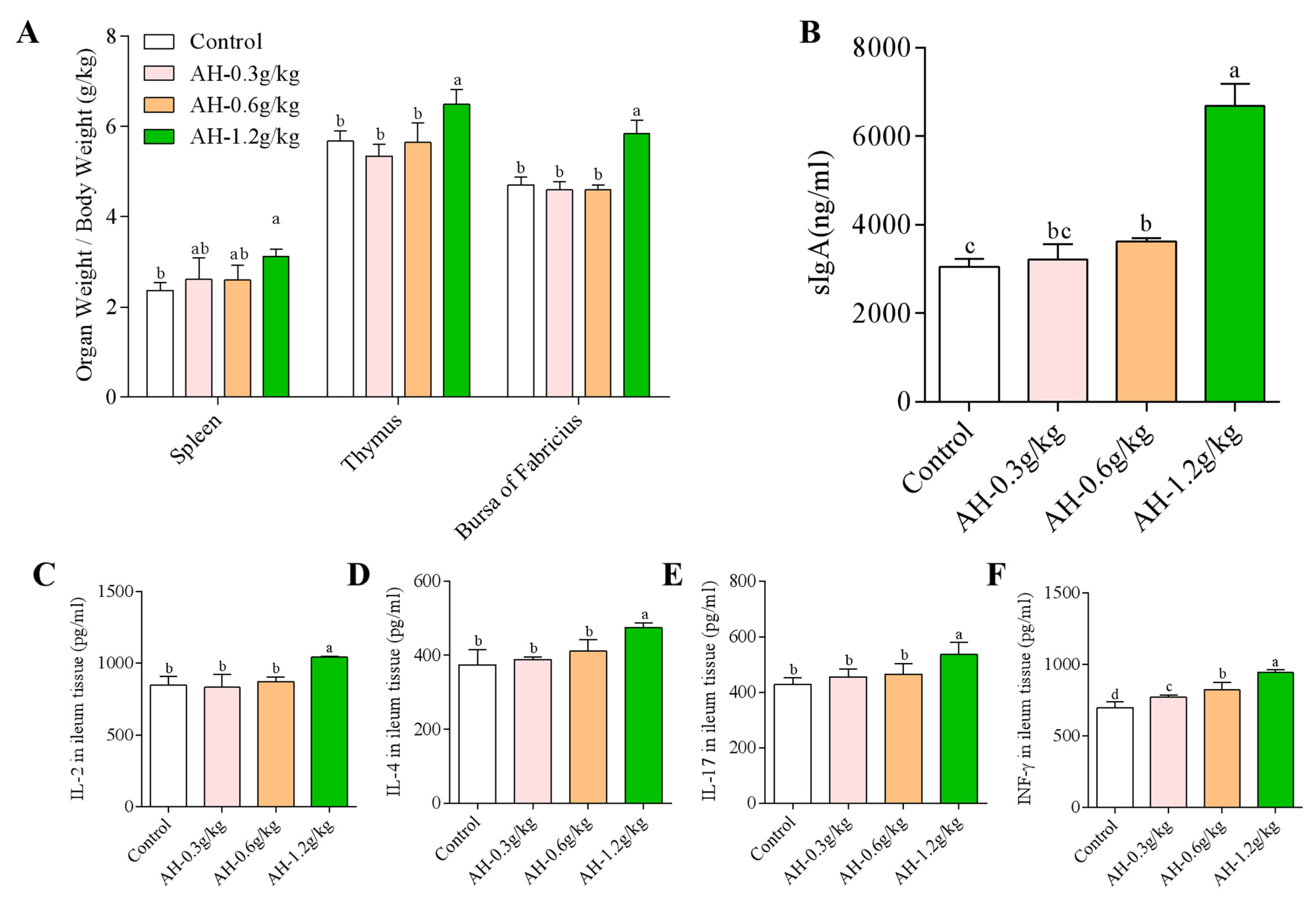
2.1. Influence of Alhagi Honey Polysaccharides on the Growth Performance and the Index of Immune Organs
2.2. Influence of Alhagi Honey Polysaccharides on the Secretion Level of sIgA in Duodenal
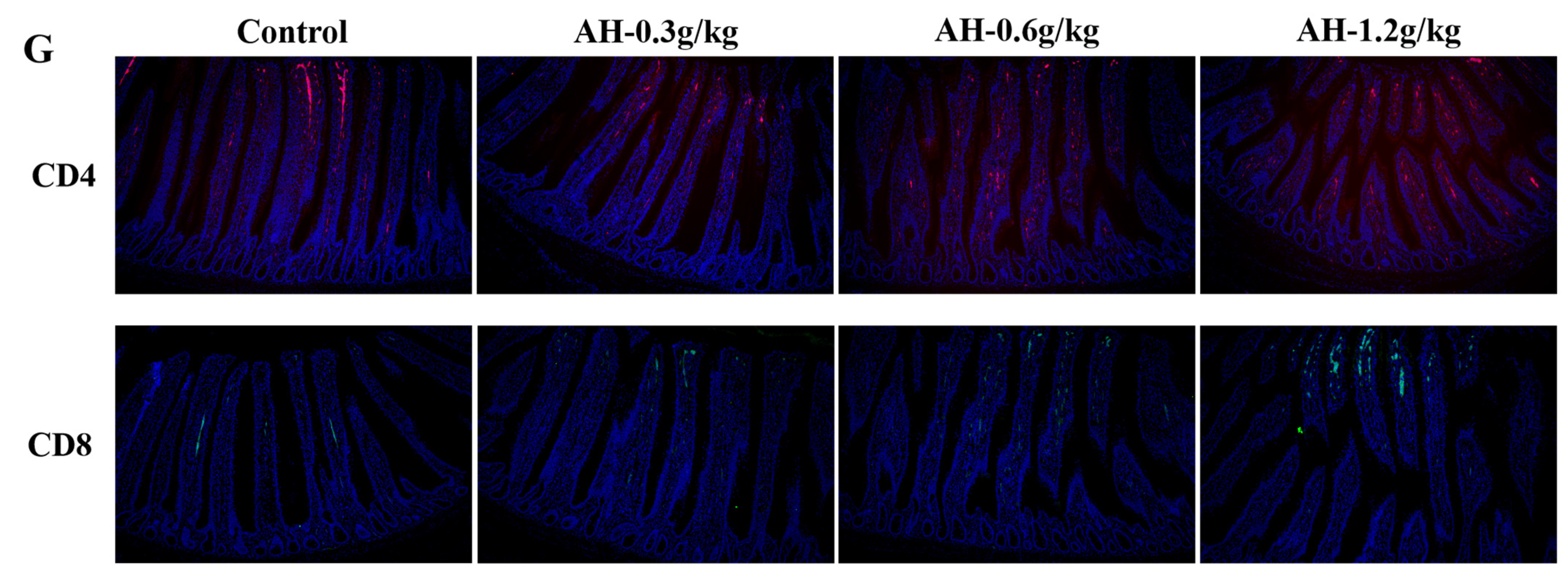
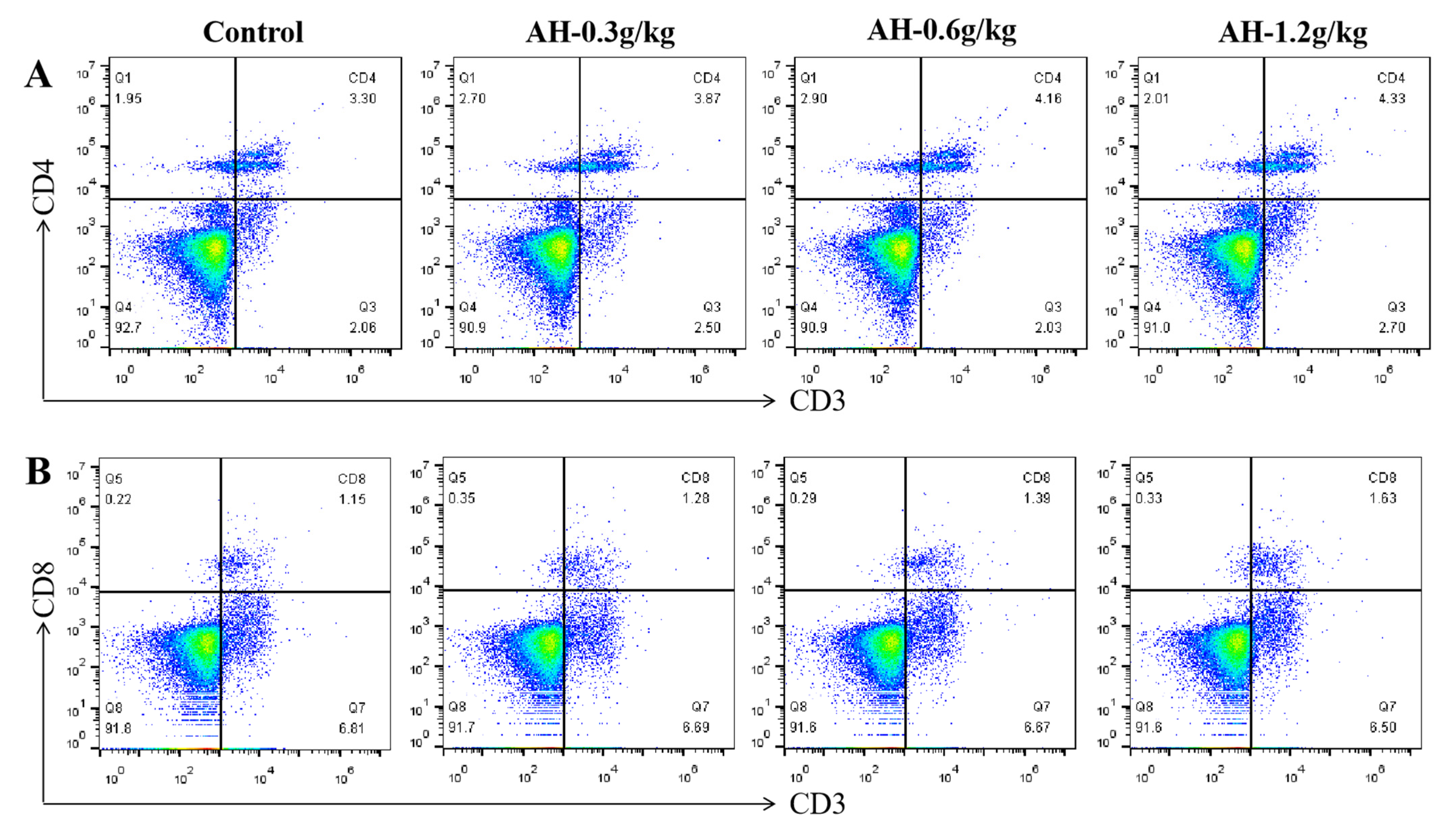
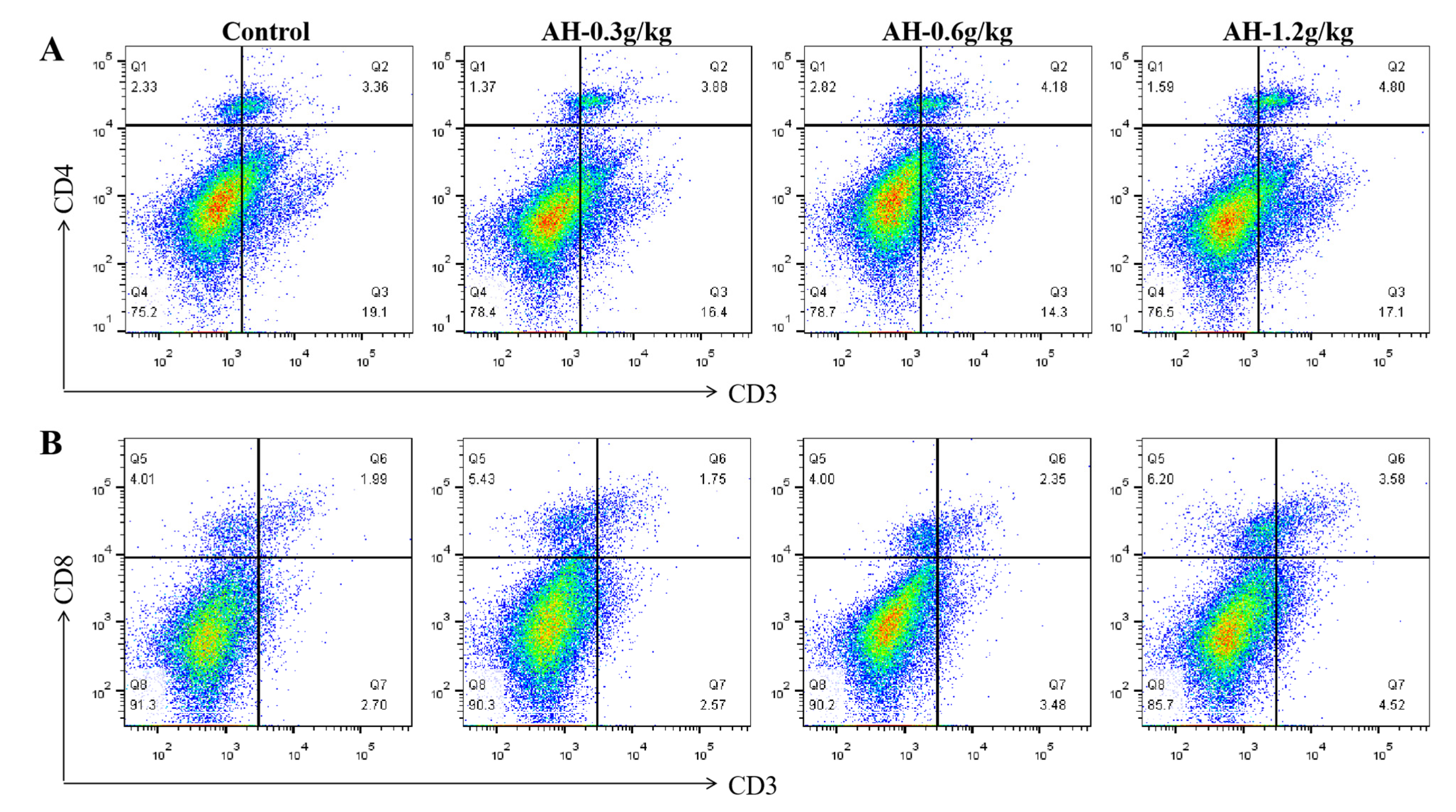
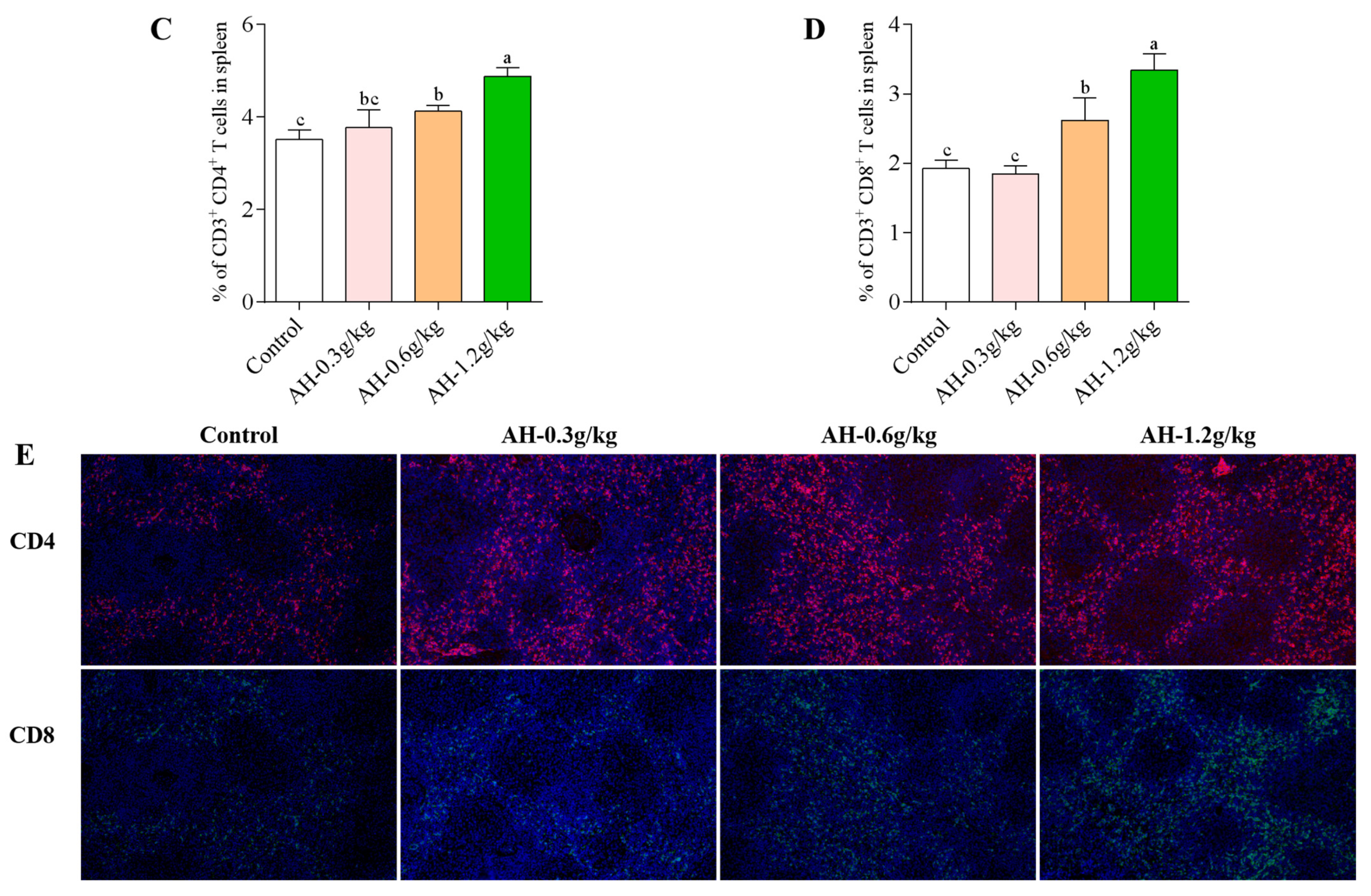
2.3. Influence of Alhagi Honey Polysaccharides on the Secretion Level of Cytokines in Duodenum and the Ratio of CD8+ and CD4+ T Cells in Ileum
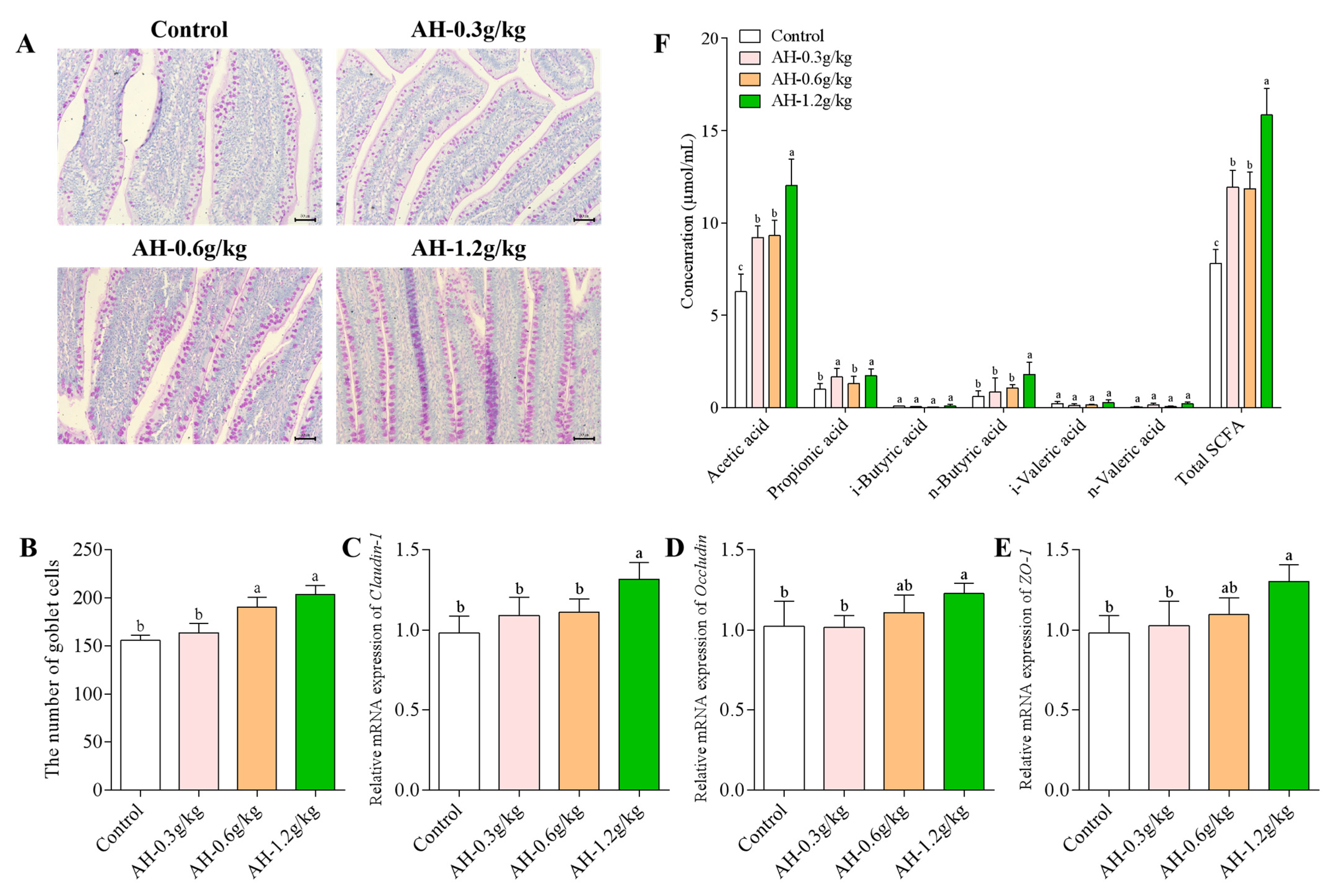
2.4. Influence of Alhagi Honey Polysaccharides on Intestinal Morphology and the Number of IELs
2.5. Influence of Alhagi Honey Polysaccharides on the Number of Goblet Cells, the mRNA Expression of Claudin-1, Occludin, and ZO-1 proteins in Ileum, and the SCFAs Production in Cecum
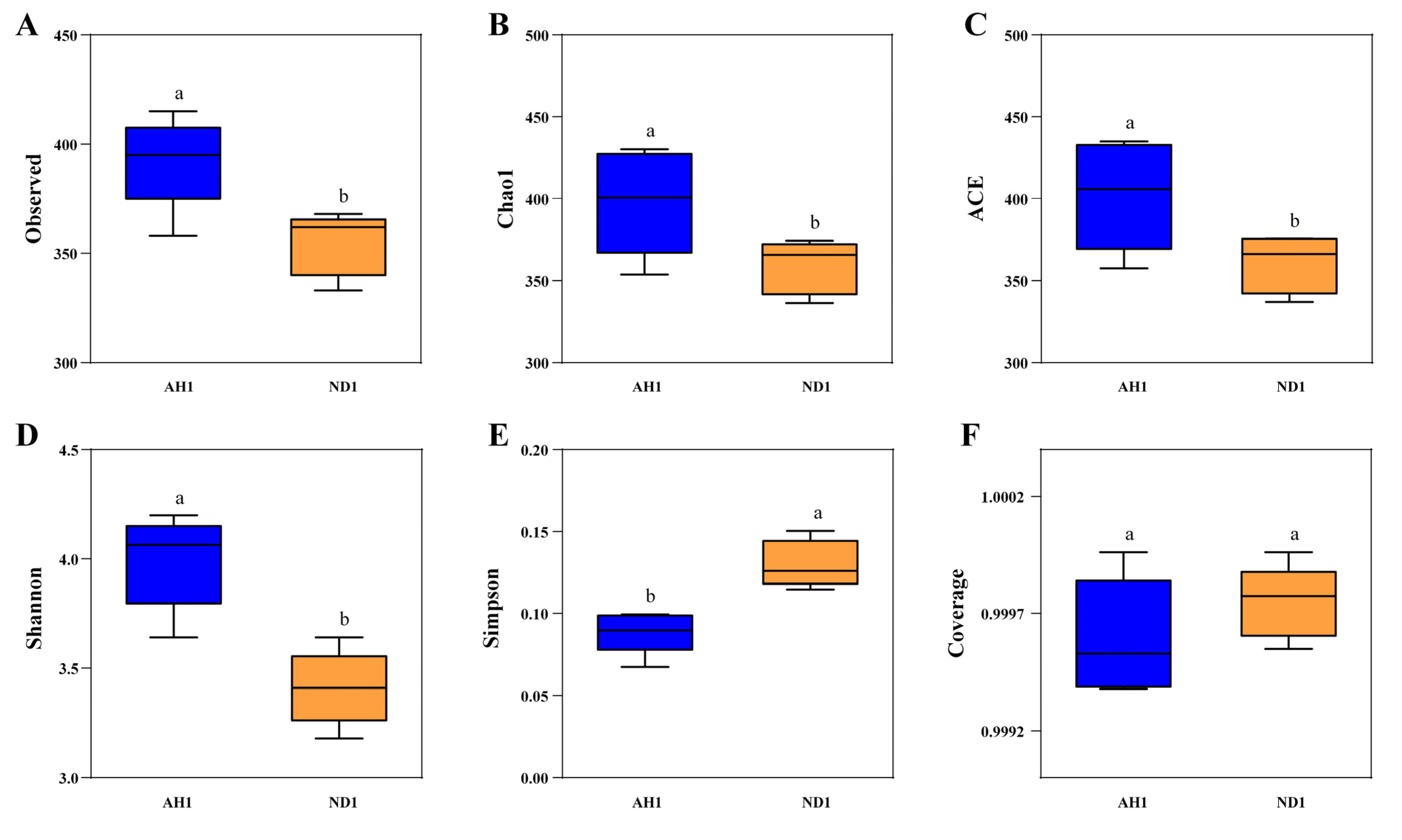
2.6. Influence of Alhagi Honey Polysaccharides on the Gut Microbiome
2.7. Influence of Alhagi Honey Polysaccharides on Intestinal Flora at the Phylum
2.8. Influence of Alhagi Honey Polysaccharides on Activation of CD4+ and CD8+ T Cells in Spleen and Peripheral Blood
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Alhagi Honey Polysaccharides
4.3. Animal Experimental Design
4.4. Measurement of Growth Performance and the Index of Spleen, Thymus, and Bursa of Fabricius
4.5. Determination of sIgA in Duodenal Washing Liquor
4.6. Detection of Cytokines in Duodenum
4.7. CD4, CD8 Immunohistochemistry
4.8. Intestinal Morphology
4.9. Determination of the Number of Goblet Cells in Ileum
4.10. Quantitative PCR for Proteins of Claudin-1, Occludin, and ZO-1 in Ileum
4.11. Measurement of SCFAs Concentrations in the Contents of Cecal
4.12. Extraction of Fecal DNA and 16S rDNA Sequencing Analysis
4.13. The Ratio of CD4+ and CD8+ T Cells in Peripheral Blood and Spleen
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sha, Z.; Shang, H.; Miao, Y.; Huang, J.; Niu, X.; Chen, R.; Peng, D.; Wei, K.; Zhu, R. Polysaccharides from Pinus massoniana pollen improve intestinal mucosal immunity in chickens. Poult. Sci. 2021, 100, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Si, C.; Zhao, Z.-T.; Meng, Z.; Yi, H.; Ye, X.-M.; Qi, A.; Ouyang, K.-H.; Wang, W.-J. Effects of polysaccharides from Yingshan Yunwu tea on meat quality, immune status and intestinal microflora in chickens. Int. J. Biol. Macromol. 2020, 155, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Wusiman, A.; Gu, P.; Mao, N.; Xu, S.; Zhu, T.; He, J.; Liu, Z.; Wang, D. Supplementation of Alhagi honey polysaccharides contributes to the improvement of the intestinal immunity regulating the structure of intestinal flora in mice. Food Funct 2021, 12, 9693–9707. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Wu, Y.; Wusiman, A.; Gu, P.; Mao, N.; Xu, S.; Zhu, T.; Feng, Z.; Liu, Z.; Wang, D. Alhagi honey polysaccharides attenuate intestinal injury and immune suppression in cyclophosphamide-induced mice. Food Funct 2021, 12, 6863–6877. [Google Scholar] [CrossRef]
- Guo, S.; Liu, L.; Lei, J.; Qu, X.; He, C.; Tang, S.; Xiao, B.; Li, P.; Gao, Q.; Lan, F.; et al. Modulation of intestinal morphology and microbiota by dietary Macleaya cordata extract supplementation in Xuefeng Black-boned Chicken. Animal 2021, 15, 100399. [Google Scholar] [CrossRef]
- Long, L.N.; Kang, B.J.; Jiang, Q.; Chen, J.S. Effects of dietary Lycium barbarum polysaccharides on growth performance, digestive enzyme activities, antioxidant status, and immunity of broiler chickens. Poult. Sci. 2020, 99, 744–751. [Google Scholar] [CrossRef]
- Long, L.N.; Zhang, H.H.; Wang, F.; Yin, Y.X.; Yang, L.Y.; Chen, J.S. Research Note: Effects of polysaccharide-enriched Acanthopanax senticosus extract on growth performance, immune function, antioxidation, and ileal microbial populations in broiler chickens. Poult. Sci. 2021, 100, 101028. [Google Scholar] [CrossRef]
- Zhao, R.; Ji, Y.; Chen, X.; Su, A.; Ma, G.; Chen, G.; Hu, Q.; Zhao, L. Effects of a beta-type glycosidic polysaccharide from Flammulina velutipes on anti-inflammation and gut microbiota modulation in colitis mice. Food Funct 2020, 11, 4259–4274. [Google Scholar] [CrossRef]
- Wusiman, A.; Xu, S.; Ni, H.; Gu, P.; Liu, Z.; Zhang, Y.; Qiu, T.; Hu, Y.; Liu, J.; Wu, Y.; et al. Immunomodulatory effects of Alhagi honey polysaccharides encapsulated into PLGA nanoparticles. Carbohydr. Polym. 2019, 211, 217–226. [Google Scholar] [CrossRef]
- Wusiman, A.; He, J.; Zhu, T.; Liu, Z.; Gu, P.; Hu, Y.; Liu, J.; Wang, D. Macrophage immunomodulatory activity of the cationic polymer modified PLGA nanoparticles encapsulating Alhagi honey polysaccharide. Int. J. Biol. Macromol. 2019, 134, 730–739. [Google Scholar] [CrossRef]
- Li, G.; Xiang, Y.; Zhao, J.; Chang, J. Saccharum Alhagi polysaccharide-1 and -2 promote the immunocompetence of RAW264.7 macrophages in vitro. Exp. Ther. Med. 2018, 15, 3556–3562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maimaitimin, K.; Jiang, Z.; Aierken, A.; Shayibuzhati, M.; Zhang, X. Hepatoprotective effect of Alhagi sparsifolia against Alcoholic Liver injury in mice. Braz. J. Pharm. Sci. 2018, 54, 29–37. [Google Scholar] [CrossRef] [Green Version]
- Wu, S. Effect of dietary Astragalus membranaceus polysaccharide on the growth performance and immunity of juvenile broilers. Poult. Sci. 2018, 97, 3489–3493. [Google Scholar] [CrossRef]
- Farahat, M.; Ibrahim, D.; Kishawy, A.T.Y.; Abdallah, H.M.; Hernandez-Santana, A.; Attia, G. Effect of cereal type and plant extract addition on the growth performance, intestinal morphology, caecal microflora, and gut barriers gene expression of broiler chickens. Animal 2021, 15, 100056. [Google Scholar] [CrossRef]
- Xie, S.Z.; Liu, B.; Ye, H.Y.; Li, Q.M.; Pan, L.H.; Zha, X.Q.; Liu, J.; Duan, J.; Luo, J.P. Dendrobium huoshanense polysaccharide regionally regulates intestinal mucosal barrier function and intestinal microbiota in mice. Carbohydr. Polym. 2019, 206, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Shang, Q.H.; Liu, S.J.; He, T.F.; Liu, H.S.; Mahfuz, S.; Ma, X.K.; Piao, X.S. Effects of wheat bran in comparison to antibiotics on growth performance, intestinal immunity, barrier function, and microbial composition in broiler chickens. Poult. Sci. 2020, 99, 4929–4938. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.M.; Miller, M.E.B.; Pence, B.D.; Whitlock, K.; Nehra, V.; Gaskins, H.R.; White, B.A.; Fryer, J.D.; Woods, J.A. Voluntary and forced exercise differentially alters the gut microbiome in C57BL/6J mice. J. Appl. Physiol. 2015, 118, 1059–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ying, M.; Yu, Q.; Zheng, B.; Wang, H.; Wang, J.; Chen, S.; Nie, S.; Xie, M. Cultured Cordyceps sinensis polysaccharides modulate intestinal mucosal immunity and gut microbiota in cyclophosphamide-treated mice. Carbohydr. Polym. 2020, 235, 115957. [Google Scholar] [CrossRef]
- Cockburn, D.W.; Koropatkin, N.M. Polysaccharide Degradation by the Intestinal Microbiota and Its Influence on Human Health and Disease. J. Mol. Biol. 2016, 428, 3230–3252. [Google Scholar] [CrossRef]
- Hooper, L.V. Bacterial contributions to mammalian gut development. Trends. Microbiol. 2004, 12, 129–134. [Google Scholar] [CrossRef]
- Singh, K.M.; Shah, T.; Deshpande, S.; Jakhesara, S.J.; Koringa, P.G.; Rank, D.N.; Joshi, C.G. High through put 16S rRNA gene-based pyrosequencing analysis of the fecal microbiota of high FCR and low FCR broiler growers. Mol. Biol. Rep. 2012, 39, 10595–10602. [Google Scholar] [CrossRef] [PubMed]
- Bi, S.; Zhang, J.; Qu, Y.; Zhou, B.; He, X.; Ni, J. Yeast cell wall product enhanced intestinal IgA response and changed cecum microflora species after oral vaccination in chickens. Poult. Sci. 2020, 99, 6576–6585. [Google Scholar] [CrossRef] [PubMed]
- Cheroutre, H. IELs: Enforcing law and order in the court of the intestinal epithelium. Immunol. Rev. 2005, 206, 114–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Tan, M.T.; Keegan, B.P.; Barry, M.A.; Heffernan, M.J. Time course study of the antigen-specific immune response to a PLGA microparticle vaccine formulation. Biomaterials 2014, 35, 8385–8393. [Google Scholar] [CrossRef]
- Gu, P.; Wusiman, A.; Zhang, Y.; Cai, G.; Xu, S.; Zhu, S.; Liu, Z.; Hu, Y.; Liu, J.; Wang, D. Polyethylenimine-coated PLGA nanoparticles-encapsulated Angelica sinensis polysaccharide as an adjuvant for H9N2 vaccine to improve immune responses in chickens compared to Alum and oil-based adjuvants. Vet. Microbiol. 2020, 251, 108894. [Google Scholar] [CrossRef]
- Korn, T.; Bettelli, E.; Oukka, M.; Kuchroo, V.K. IL-17 and Th17 Cells. Annu. Rev. Immunol. 2009, 27, 485–517. [Google Scholar] [CrossRef]
- Bai, Y.; Huang, F.; Zhang, R.; Dong, L.; Jia, X.; Liu, L.; Yi, Y.; Zhang, M. Longan pulp polysaccharides relieve intestinal injury in vivo and in vitro by promoting tight junction expression. Carbohydr. Polym. 2020, 229, 115475. [Google Scholar] [CrossRef]
- Lievin-Le Moal, V.; Servin, A.L. The front line of enteric host defense against unwelcome intrusion of harmful microorganisms: Mucins, antimicrobial peptides, and microbiota. Clin. Microbiol. Rev. 2006, 19, 315–337. [Google Scholar] [CrossRef] [Green Version]
- Qasem, M.A.A.; Dkhil, M.A.; Al-Shaebi, E.M.; Murshed, M.; Mares, M.; Al-Quraishy, S. Rumex nervosus leaf extracts enhance the regulation of goblet cells and the inflammatory response during infection of chickens with Eimeria tenella. J. King Saud Univ.-Sci. 2020, 32, 1818–1823. [Google Scholar] [CrossRef]
- Furuse, M.; Hata, M.; Furuse, K.; Yoshida, Y.; Haratake, A.; Sugitani, Y.; Noda, T.; Kubo, A.; Tsukita, S. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: A lesson from claudin-1-deficient mice. J. Cell. Biol. 2002, 156, 1099–1111. [Google Scholar] [CrossRef]
- Ma, T.Y.; Iwamoto, G.K.; Hoa, N.T.; Akotia, V.; Pedram, A.; Boivin, M.A.; Said, H.M. TNF-α-induced increase in intestinal epithelial tight junction permeability requires NF-κB activation. Am. J. Physiol.-Gastrointest. Liver Physiol. 2004, 286, G367–G376. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Liao, J.; Yu, W.; Pei, R.; Qiao, N.; Han, Q.; Hu, L.; Li, Y.; Guo, J.; Pan, J.; et al. Copper induces oxidative stress with triggered NF-κB pathway leading to inflammatory responses in immune organs of chicken. Ecotoxicol. Environ. Saf. 2020, 200, 110715. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T.R.; Sad, S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol. Today 1996, 17, 138–146. [Google Scholar] [CrossRef]
- Liu, Y.; Jiao, F.; Qiu, Y.; Li, W.; Lao, F.; Zhou, G.; Sun, B.; Xing, G.; Dong, J.; Zhao, Y.; et al. The effect of Gd@C82(OH)22 nanoparticles on the release of Th1/Th2 cytokines and induction of TNF-α mediated cellular immunity. Biomaterials 2009, 30, 3934–3945. [Google Scholar] [CrossRef]
- Madkour, M.; Aboelenin, M.M.; Aboelazab, O.; Elolimy, A.A.; El-Azeem, N.A.; El-Kholy, M.S.; Alagawany, M.; Shourrap, M. Hepatic expression responses of DNA methyltransferases, heat shock proteins, antioxidant enzymes, and NADPH 4 to early life thermal conditioning in broiler chickens. Ital. J. Anim. Sci. 2021, 20, 433–446. [Google Scholar] [CrossRef]
- Madkour, M.; Aboelenin, M.M.; Shakweer, W.M.E.; Alfarraj, S.; Alharbi, S.A.; Abdel-Fattah, S.A.; Alagawany, M. Early life thermal stress modulates hepatic expression of thermotolerance related genes and physiological responses in two rabbit breeds. Ital. J. Anim. Sci. 2021, 20, 736–748. [Google Scholar] [CrossRef]



| Items | Control | AH-0.3 g/kg | AH-0.6 g/kg | AH-1.2 g/kg | SEM | p-Value |
|---|---|---|---|---|---|---|
| D1 to D7 after first AH-treatment | ||||||
| ADG, g/d | 8.87 | 8.59 | 9.51 | 9.76 | 0.4 | 0.871 |
| ADFI, g/d | 21.31 | 21.23 | 22.28 | 22.28 | 0.17 | 0.083 |
| F:G ratio | 2.42 | 2.49 | 2.45 | 2.43 | 0.1 | 0.995 |
| D8 to D14 after first AH-treatment | ||||||
| ADG, g/d | 12.01 b | 12.87 ab | 13.65 ab | 14.5 a | 0.33 | 0.024 |
| ADFI, g/d | 26.41 | 25.26 | 26.11 | 25.72 | 0.17 | 0.082 |
| F:G ratio | 2.2 a | 1.97 ab | 1.95 ab | 1.78 b | 0.05 | 0.011 |
| Ingredients (%) | Chemical Composition Calculated (%) | ||
|---|---|---|---|
| Corn | 54.69 | Metabolizable energy (MJ/kg) | 2.89 |
| Soybean meal | 32.79 | Crude protein | 22.47 |
| Fish meal | 3.09 | Calcium | 1.05 |
| Soybean oil | 2.36 | Available phosphorus | 0.55 |
| Limestone | 1.03 | Lysine | 1.23 |
| Bicalcium phosphate | 1.89 | Methionine | 0.54 |
| Salt | 0.35 | Methionine + cystine | 0.85 |
| DL-Methionine | 1.75 | ||
| L-Lysine | 0.84 | ||
| Vitamin-mineral premix a | 1.2 | ||
| Total | 100 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, G.; Mao, N.; Gu, P.; Zhu, T.; He, J.; Peng, S.; Yang, Y.; Liu, Z.; Hu, Y.; Wang, D. Effects of Alhagi Honey Polysaccharides as Feed Supplement on Intestine Function and Microbiome, Immune Function, and Growth Performance in Chicken. Int. J. Mol. Sci. 2022, 23, 14332. https://doi.org/10.3390/ijms232214332
Cai G, Mao N, Gu P, Zhu T, He J, Peng S, Yang Y, Liu Z, Hu Y, Wang D. Effects of Alhagi Honey Polysaccharides as Feed Supplement on Intestine Function and Microbiome, Immune Function, and Growth Performance in Chicken. International Journal of Molecular Sciences. 2022; 23(22):14332. https://doi.org/10.3390/ijms232214332
Chicago/Turabian StyleCai, Gaofeng, Ningning Mao, Pengfei Gu, Tianyu Zhu, Jin He, Song Peng, Yang Yang, Zhenguang Liu, Yuanliang Hu, and Deyun Wang. 2022. "Effects of Alhagi Honey Polysaccharides as Feed Supplement on Intestine Function and Microbiome, Immune Function, and Growth Performance in Chicken" International Journal of Molecular Sciences 23, no. 22: 14332. https://doi.org/10.3390/ijms232214332




