Emerging Role of NLRP3 Inflammasome and Pyroptosis in Liver Transplantation
Abstract
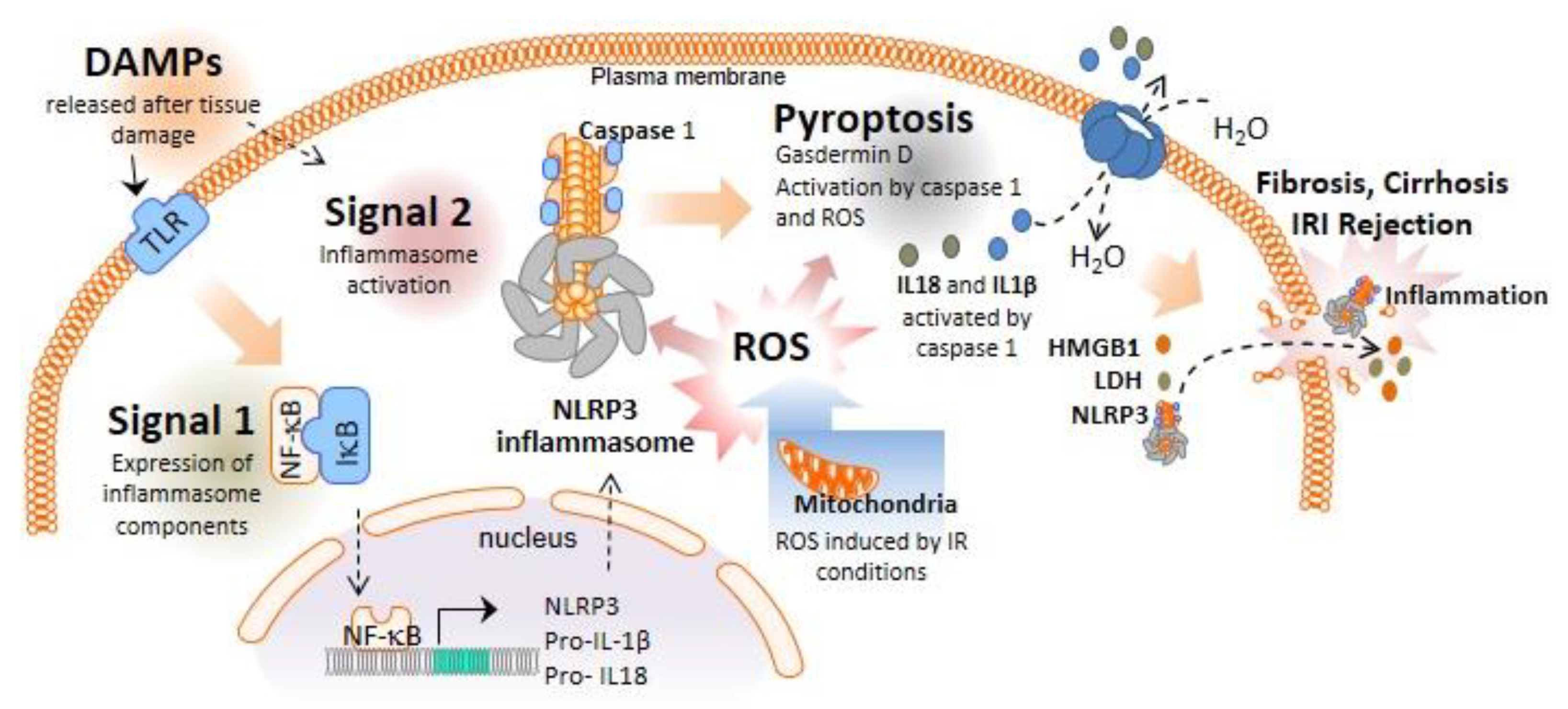
:1. Inflammasomes and Pyroptotic Cell Death
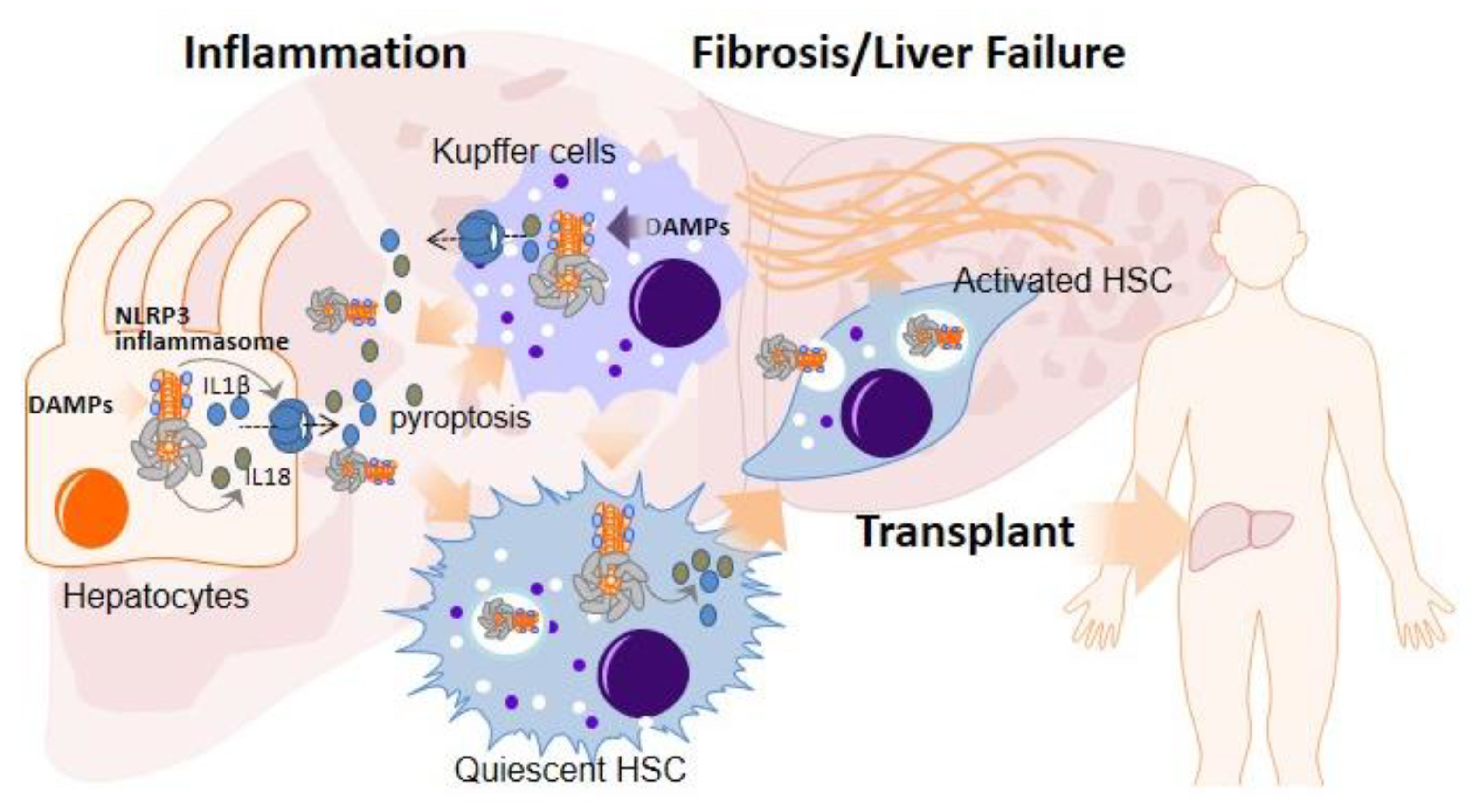
2. Role of the NLRP3 Inflammasome and Pyroptosis in Liver Diseases Necessitating Transplantation
3. Role of NLRP3 Inflammasome and Pyroptosis in Ischemia–Reperfusion Injury
4. NLRP3 Inflammasome Activation in the Early Inflammation Stage after LT: Implications in Acute Rejection
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martinon, F.; Burns, K.; Tschopp, J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell 2002, 10, 417–426. [Google Scholar] [CrossRef]
- Ting, J.P.; Lovering, R.C.; Alnemri, E.S.; Bertin, J.; Boss, J.M.; Davis, B.K.; Flavell, R.A.; Girardin, S.E.; Godzik, A.; Harton, J.A.; et al. The NLR gene family: A standard nomenclature. Immunity 2008, 28, 285–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, A.N.R.; Bittner, Z.A.; Shankar, S.; Liu, X.; Chang, T.H.; Jin, T.; Tapia-Abellan, A. Recent insights into the regulatory networks of NLRP3 inflammasome activation. J. Cell Sci. 2020, 133, jcs248344. [Google Scholar] [CrossRef] [PubMed]
- Broz, P.; Dixit, V.M. Inflammasomes: Mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 2016, 16, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Baroja-Mazo, A.; Martin-Sanchez, F.; Gomez, A.I.; Martinez, C.M.; Amores-Iniesta, J.; Compan, V.; Barbera-Cremades, M.; Yague, J.; Ruiz-Ortiz, E.; Anton, J.; et al. The NLRP3 inflammasome is released as a particulate danger signal that amplifies the inflammatory response. Nat. Immunol. 2014, 15, 738–748. [Google Scholar] [CrossRef]
- Broz, P.; Pelegrin, P.; Shao, F. The gasdermins, a protein family executing cell death and inflammation. Nat. Rev. Immunol. 2020, 20, 143–157. [Google Scholar] [CrossRef]
- Tacke, F. Targeting hepatic macrophages to treat liver diseases. J. Hepatol. 2017, 66, 1300–1312. [Google Scholar] [CrossRef]
- Pelegrin, P.; Alegre, F.; Feldstein, A. Inflammasomes in Liver Fibrosis. Semin. Liver Dis. 2017, 37, 119–127. [Google Scholar]
- Zhu, M.; Barbas, A.S.; Lin, L.; Scheuermann, U.; Bishawi, M.; Brennan, T.V. Mitochondria Released by Apoptotic Cell Death Initiate Innate Immune Responses. Immunohorizons 2018, 2, 384–397. [Google Scholar] [CrossRef] [Green Version]
- Gieling, R.G.; Wallace, K.; Han, Y.P. Interleukin-1 participates in the progression from liver injury to fibrosis. Am. J. physiol. Gastroint. Liver Physiol. 2009, 296, G1324–G1331. [Google Scholar] [CrossRef] [Green Version]
- Gaul, S.; Leszczynska, A.; Alegre, F.; Kaufmann, B.; Johnson, C.D.; Adams, L.A.; Wree, A.; Damm, G.; Seehofer, D.; Calvente, C.J.; et al. Hepatocyte pyroptosis and release of inflammasome particles induce stellate cell activation and liver fibrosis. J. Hepatol. 2021, 74, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Al Mamun, A.; Akter, A.; Hossain, S.; Sarker, T.; Safa, S.A.; Mustafa, Q.G.; Muhammad, S.A.; Munir, F. Role of NLRP3 inflammasome in liver disease. J. Dig. Dis. 2020, 21, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Jiang, M.; Chu, Y.; Wang, W.; Chen, D.; Li, X.; Zhang, Z.; Zhang, D.; Fan, D.; Nie, Y.; et al. Gasdermin D plays a key role as a pyroptosis executor of non-alcoholic steatohepatitis in humans and mice. J. Hepatol. 2018, 68, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, T.; He, K.; Xu, M.; Gong, J.P. Cardiolipin inhibitor ameliorates the non-alcoholic steatohepatitis through suppressing NLRP3 inflammasome activation. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8158–8167. [Google Scholar] [PubMed]
- Knorr, J.; Wree, A.; Tacke, F.; Feldstein, A.E. The NLRP3 Inflammasome in Alcoholic and Nonalcoholic Steatohepatitis. Semin. Liver Dis. 2020, 40, 298–306. [Google Scholar] [CrossRef]
- Wang, S.; Qing, D. Progress in inflammasome activation and pyrolysis in alcoholic liver disease. Zhong Nan Da Xue Xue Bao. Yi Xue Ban J. Cent. South University. Med. Sci. 2020, 45, 999–1004. [Google Scholar]
- Zhou, Y.; Wang, S.; Wan, T.; Huang, Y.; Pang, N.; Jiang, X.; Gu, Y.; Zhang, Z.; Luo, J.; Yang, L. Cyanidin-3-O-β-glucoside inactivates NLRP3 inflammasome and alleviates alcoholic steatohepatitis via SirT1/NF-κB signaling pathway. Free Radic. Biol. Med. 2020, 160, 334–341. [Google Scholar] [CrossRef]
- De Miguel, C.; Pelegrin, P.; Baroja-Mazo, A.; Cuevas, S. Emerging Role of the Inflammasome and Pyroptosis in Hypertension. Int. J. Mol. Sci. 2021, 22, 1064. [Google Scholar] [CrossRef]
- Iracheta-Vellve, A.; Petrasek, J.; Satishchandran, A.; Gyongyosi, B.; Saha, B.; Kodys, K.; Fitzgerald, K.A.; Kurt-Jones, E.A.; Szabo, G. Inhibition of sterile danger signals, uric acid and ATP, prevents inflammasome activation and protects from alcoholic steatohepatitis in mice. J. Hepatol. 2015, 63, 1147–1155. [Google Scholar] [CrossRef] [Green Version]
- Cho, I.J.; Oh, D.H.; Yoo, J.; Hwang, Y.C.; Ahn, K.J.; Chung, H.Y.; Jeong, S.W.; Moon, J.Y.; Lee, S.H.; Lim, S.J.; et al. Allopurinol ameliorates high fructose diet induced hepatic steatosis in diabetic rats through modulation of lipid metabolism, inflammation, and ER stress pathway. Sci. Rep. 2021, 11, 9894. [Google Scholar] [CrossRef]
- Ding, X.Q.; Wu, W.Y.; Jiao, R.Q.; Gu, T.T.; Xu, Q.; Pan, Y.; Kong, L.D. Curcumin and allopurinol ameliorate fructose-induced hepatic inflammation in rats via miR-200a-mediated TXNIP/NLRP3 inflammasome inhibition. Pharmacol. Res. 2018, 137, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Baeza-Raja, B.; Goodyear, A.; Liu, X.; Lam, K.; Yamamoto, L.; Li, Y.; Dodson, G.S.; Takeuchi, T.; Kisseleva, T.; Brenner, D.A.; et al. Pharmacological inhibition of P2RX7 ameliorates liver injury by reducing inflammation and fibrosis. PLoS ONE 2020, 15, e0234038. [Google Scholar] [CrossRef] [PubMed]
- Dabbagh, K.; Steven Dodson, G.; Yamamoto, L.; Baeza-Raja, B.; Goodyear, A.W. PS-111—Preclinical and first-in human development of SGM-1019, a first-in-class novel small molecule modulator of inflammasome activity for the treatment of nonalcoholic steatohepatitis (NASH). J. Hepatol. 2018, 68, S60. [Google Scholar] [CrossRef]
- Wei, J.; Shi, M.; Wu, W.Q.; Xu, H.; Wang, T.; Wang, N.; Ma, J.L.; Wang, Y.G. IkappaB kinase-beta inhibitor attenuates hepatic fibrosis in mice. World J. Gastroenterol. 2011, 17, 5203–5213. [Google Scholar] [CrossRef]
- He, X.; Song, Y.; Wang, L.; Xu, J. Protective effect of pyrrolidine dithiocarbamate on isoniazid/rifampicininduced liver injury in rats. Mol. Med. Rep. 2020, 21, 463–469. [Google Scholar]
- Bruck, R.; Schey, R.; Aeed, H.; Hochman, A.; Genina, O.; Pines, M. A protective effect of pyrrolidine dithiocarbamate in a rat model of liver cirrhosis. Liver Int. 2004, 24, 169–176. [Google Scholar] [CrossRef]
- Qu, J.; Yuan, Z.; Wang, G.; Wang, X.; Li, K. The selective NLRP3 inflammasome inhibitor MCC950 alleviates cholestatic liver injury and fibrosis in mice. Int. Immunopharmacol. 2019, 70, 147–155. [Google Scholar] [CrossRef]
- Mridha, A.R.; Wree, A.; Robertson, A.A.B.; Yeh, M.M.; Johnson, C.D.; Van Rooyen, D.M.; Haczeyni, F.; Teoh, N.C.; Savard, C.; Ioannou, G.N.; et al. NLRP3 inflammasome blockade reduces liver inflammation and fibrosis in experimental NASH in mice. J. Hepatol. 2017, 66, 1037–1046. [Google Scholar] [CrossRef]
- Yu, Y.; Cheng, Y.; Pan, Q.; Zhang, Y.J.; Jia, D.G.; Liu, Y.F. Effect of the Selective NLRP3 Inflammasome Inhibitor mcc950 on Transplantation Outcome in a Pig Liver Transplantation Model with Organs From Donors After Circulatory Death Preserved by Hypothermic Machine Perfusion. Transplantation 2019, 103, 353–362. [Google Scholar] [CrossRef]
- Zhang, W.J.; Fang, Z.M.; Liu, W.Q. NLRP3 inflammasome activation from Kupffer cells is involved in liver fibrosis of Schistosoma japonicum-infected mice via NF-kappaB. Parasit. Vectors 2019, 12, 29. [Google Scholar] [CrossRef] [Green Version]
- Petrasek, J.; Bala, S.; Csak, T.; Lippai, D.; Kodys, K.; Menashy, V.; Barrieau, M.; Min, S.Y.; Kurt-Jones, E.A.; Szabo, G. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J. Clin. Investig. 2012, 122, 3476–3489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massaro, M.G.; Pompili, M.; Sicignano, L.L.; Pizzolante, F.; Verrecchia, E.; Vecchio, F.M.; Rigante, D.; Manna, R. Improvement of Liver Involvement in Familial Mediterranean Fever After the Introduction of Canakinumab: A Case Report. Mediterr. J. Hematol. Infect. Dis. 2020, 12, e2020059. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.J.; Liu, H.; Wang, Z.H.; Zhu, Y.X.; Su, L.Y.; Zhang, M.X.; Xu, K.; Chen, J.Z. Inflammasome activation involved in early inflammation reaction after liver transplantation. Immunol. Lett. 2017, 190, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Sangineto, M.; Grabherr, F.; Adolph, T.E.; Grander, C.; Reider, S.; Jaschke, N.; Mayr, L.; Schwarzler, J.; Dallio, M.; Moschen, A.R.; et al. Dimethyl fumarate ameliorates hepatic inflammation in alcohol related liver disease. Liver Int. 2020, 40, 1610–1619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Zhao, S.; Fu, Y.; Yan, L.; Feng, Y.; Chen, Y.; Wu, Y.; Deng, Y.; Zhang, G.; Chen, Z.; et al. Computational repositioning of dimethyl fumarate for treating alcoholic liver disease. Cell Death Dis. 2020, 11, 641. [Google Scholar] [CrossRef]
- Xu, W.F.; Zhang, Q.; Ding, C.J.; Sun, H.Y.; Che, Y.; Huang, H.; Wang, Y.; Wu, J.W.; Hao, H.P.; Cao, L.J. Gasdermin E-derived caspase-3 inhibitors effectively protect mice from acute hepatic failure. Acta. Pharmacol. Sin. 2021, 42, 68–76. [Google Scholar] [CrossRef]
- Zhao, J.; He, B.; Zhang, S.; Huang, W.; Li, X. Ginsenoside Rg1 alleviates acute liver injury through the induction of autophagy and suppressing NF-kappaB/NLRP3 inflammasome signaling pathway. Int. J. Med. Sci. 2021, 18, 1382–1389. [Google Scholar] [CrossRef]
- Wang, Q.; Ou, Y.; Hu, G.; Wen, C.; Yue, S.; Chen, C.; Xu, L.; Xie, J.; Dai, H.; Xiao, H.; et al. Naringenin attenuates non-alcoholic fatty liver disease by down-regulating the NLRP3/NF-kappaB pathway in mice. Br. J. Pharmacol. 2020, 177, 1806–1821. [Google Scholar] [CrossRef]
- Ito, T.; Naini, B.V.; Markovic, D.; Aziz, A.; Younan, S.; Lu, M.; Hirao, H.; Kadono, K.; Kojima, H.; DiNorcia, J., 3rd; et al. Ischemia-reperfusion injury and its relationship with early allograft dysfunction in liver transplant patients. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2021, 21, 614–625. [Google Scholar] [CrossRef]
- Jimenez-Castro, M.B.; Cornide-Petronio, M.E.; Gracia-Sancho, J.; Peralta, C. Inflammasome-Mediated Inflammation in Liver Ischemia-Reperfusion Injury. Cells 2019, 8, 1131. [Google Scholar] [CrossRef] [Green Version]
- Pu, J.L.; Huang, Z.T.; Luo, Y.H.; Mou, T.; Li, T.T.; Li, Z.T.; Wei, X.F.; Wu, Z.J. Fisetin mitigates hepatic ischemia-reperfusion injury by regulating GSK3β/AMPK/NLRP3 inflammasome pathway. Hepatobiliary Pancreat. Dis. Int. 2021, 20, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Luo, J.; Xiong, Y.; Liu, Z.; Ye, Q. 25-Hydroxycholesterol mitigates hepatic ischemia reperfusion injury via mediating mitophagy. Int. Immunopharmacol. 2021, 96, 107643. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, H.; Chen, X.; Han, S.; Zhu, Y.; Wang, H.; Cheng, F.; Pu, L. Inhibiting ATP6V0D2 Aggravates Liver Ischemia-Reperfusion Injury by Promoting NLRP3 Activation via Impairing Autophagic Flux Independent of Notch1/Hes1. J. Immunol. Res. 2021, 2021, 6670495. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Han, S.; Chen, X.; Li, X.; Xia, N.; Pu, L. Eva1a inhibits NLRP3 activation to reduce liver ischemia-reperfusion injury via inducing autophagy in kupffer cells. Mol. Immunol. 2021, 132, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Xue, R.; Qiu, J.; Wei, S.; Liu, M.; Wang, Q.; Wang, P.; Sha, B.; Wang, H.; Shi, Y.; Zhou, J.; et al. Lycopene alleviates hepatic ischemia reperfusion injury via the Nrf2/HO-1 pathway mediated NLRP3 inflammasome inhibition in Kupffer cells. Ann. Transl. Med. 2021, 9, 631. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhao, F.; Cao, Y.; Zhang, J.; Shi, P.; Sun, X.; Zhang, F.; Tong, L. DHA attenuates hepatic ischemia reperfusion injury by inhibiting pyroptosis and activating PI3K/Akt pathway. Eur. J. Pharmacol. 2018, 835, 1–10. [Google Scholar] [CrossRef]
- Luo, Y.; Huang, Z.; Mou, T.; Pu, J.; Li, T.; Li, Z.; Yang, H.; Yan, P.; Wu, Z.; Wu, Q. SET8 mitigates hepatic ischemia/reperfusion injury in mice by suppressing MARK4/NLRP3 inflammasome pathway. Life Sci. 2021, 273, 119286. [Google Scholar] [CrossRef]
- Fagenson, A.M.; Xu, K.; Saaoud, F.; Nanayakkara, G.; Jhala, N.C.; Liu, L.; Drummer, C.; Sun, Y.; Lau, K.N.; Di Carlo, A.; et al. Liver Ischemia Reperfusion Injury, Enhanced by Trained Immunity, Is Attenuated in Caspase 1/Caspase 11 Double Gene Knockout Mice. Pathogens 2020, 9, 879. [Google Scholar] [CrossRef]
- Oguz, A.; Boyuk, A.; Ekinci, A.; Alabalik, U.; Turkoglu, A.; Tuncer, M.C.; Ekingen, A.; Deveci, E.; Gulturk, B.; Aday, U. Investigation of antioxidant effects of rosmarinic acid on liver, lung and kidney in rats: A biochemical and histopathological study. Folia Morphol. 2020, 79, 288–295. [Google Scholar] [CrossRef] [Green Version]
- Saidi, R.F.; Hejazii Kenari, S.K. Challenges of organ shortage for transplantation: Solutions and opportunities. Int. J. Organ Transplant. Med. 2014, 5, 87–96. [Google Scholar]
- Potter, K.F.; Cocchiola, B.; Quader, M.A. Donation after circulatory death: Opportunities on the horizon. Curr. Opin. Anaesthesiol. 2021, 34, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Croome, K.P.; Taner, C.B. The Changing Landscapes in DCD Liver Transplantation. Curr. Transplant. Rep. 2020, 7, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Quader, M.; Mezzaroma, E.; Kenning, K.; Toldo, S. Targeting the NLRP3 inflammasome to reduce warm ischemic injury in donation after circulatory death heart. Clin. Transplant. 2020, 34, e14044. [Google Scholar] [CrossRef] [PubMed]
- Florim, G.M.S.; Caldas, H.C.; Goncalves, N.N.; Bueno, G.; Baptista, M.; Fernandes-Charpiot, I.M.M.; Abbud-Filho, M. Activation of HMGB1-TLR4 Pathway and Inflammasome Contribute to Enhanced Inflammatory Response in Extended Criteria and Kidneys with KDPI ≥ 85. Transplantation 2020, 104, 724–730. [Google Scholar] [CrossRef]
- Hessheimer, A.J.; Coll, E.; Torres, F.; Ruiz, P.; Gastaca, M.; Rivas, J.I.; Gomez, M.; Sanchez, B.; Santoyo, J.; Ramirez, P.; et al. Normothermic regional perfusion vs. super-rapid recovery in controlled donation after circulatory death liver transplantation. J. Hepatol. 2019, 70, 658–665. [Google Scholar] [CrossRef]
- Sutherland, A.I.; Oniscu, G.C. Challenges and advances in optimizing liver allografts from donation after circulatory death donors. J. Nat. Sci. Biol. Med. 2016, 7, 10–15. [Google Scholar]
- Watson, C.J.E.; Hunt, F.; Messer, S.; Currie, I.; Large, S.; Sutherland, A.; Crick, K.; Wigmore, S.J.; Fear, C.; Cornateanu, S.; et al. In situ normothermic perfusion of livers in controlled circulatory death donation may prevent ischemic cholangiopathy and improve graft survival. Am. J. Transplant. 2019, 19, 1745–1758. [Google Scholar] [CrossRef] [Green Version]
- Saidi, R.F.; Kenari, S.K. Liver ischemia/reperfusion injury: An overview. J. Investig. Surg. 2014, 27, 366–379. [Google Scholar] [CrossRef]
- Aufhauser, D.D., Jr.; Foley, D.P. Beyond Ice and the Cooler: Machine Perfusion Strategies in Liver Transplantation. Clin. Liver Dis. 2021, 25, 179–194. [Google Scholar] [CrossRef]
- van Beekum, C.J.; Vilz, T.O.; Glowka, T.R.; von Websky, M.W.; Kalff, J.C.; Manekeller, S. Normothermic Machine Perfusion (NMP) of the Liver—Current Status and Future Perspectives. Ann. Transplant. 2021, 26, e931664. [Google Scholar] [CrossRef]
- Schlegel, A.; Muller, X.; Dutkowski, P. Machine perfusion strategies in liver transplantation. Hepatobiliary Surg. Nutr. 2019, 8, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Sadowsky, D.; Zamora, R.; Barclay, D.; Yin, J.; Fontes, P.; Vodovotz, Y. Machine Perfusion of Porcine Livers with Oxygen-Carrying Solution Results in Reprogramming of Dynamic Inflammation Networks. Front Pharmacol. 2016, 7, 413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, W.; Ye, S.; Zeng, C.; Xue, S.; Hu, X.; Zhang, X.; Gao, S.; Xiong, Y.; He, X.; Vivalda, S.; et al. Hypothermic oxygenated perfusion (HOPE) attenuates ischemia/reperfusion injury in the liver through inhibition of the TXNIP/NLRP3 inflammasome pathway in a rat model of donation after cardiac death. FASEB J. 2018, 32, 6212–6227. [Google Scholar] [CrossRef] [Green Version]
- Scheuermann, U.; Zhu, M.; Song, M.; Yerxa, J.; Gao, Q.; Davis, R.P.; Zhang, M.; Parker, W.; Hartwig, M.G.; Kwun, J.; et al. Damage-Associated Molecular Patterns Induce Inflammatory Injury During Machine Preservation of the Liver: Potential Targets to Enhance a Promising Technology. Liver Transpl. 2019, 25, 610–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jassem, W.; Xystrakis, E.; Ghnewa, Y.G.; Yuksel, M.; Pop, O.; Martinez-Llordella, M.; Jabri, Y.; Huang, X.; Lozano, J.J.; Quaglia, A.; et al. Normothermic Machine Perfusion (NMP) Inhibits Proinflammatory Responses in the Liver and Promotes Regeneration. Hepatology 2019, 70, 682–695. [Google Scholar] [CrossRef] [PubMed]
- Ronca, V.; Wootton, G.; Milani, C.; Cain, O. The Immunological Basis of Liver Allograft Rejection. Front Immunol. 2020, 11, 2155. [Google Scholar] [CrossRef] [PubMed]
- Wanderer, A.A. Rationale and timeliness for IL-1beta-targeted therapy to reduce allogeneic organ injury at procurement and to diminish risk of rejection after transplantation. Clin. Transplant. 2010, 24, 307–311. [Google Scholar] [CrossRef]
- Pareja, E.; Cortes, M.; Hervas, D.; Mir, J.; Valdivieso, A.; Castell, J.V.; Lahoz, A. A score model for the continuous grading of early allograft dysfunction severity. Liver Transpl. 2015, 21, 38–46. [Google Scholar] [CrossRef]
- Amores-Iniesta, J.; Barbera-Cremades, M.; Martinez, C.M.; Pons, J.A.; Revilla-Nuin, B.; Martinez-Alarcon, L.; Di Virgilio, F.; Parrilla, P.; Baroja-Mazo, A.; Pelegrin, P. Extracellular ATP Activates the NLRP3 Inflammasome and Is an Early Danger Signal of Skin Allograft Rejection. Cell Rep. 2017, 21, 3414–3426. [Google Scholar] [CrossRef] [Green Version]
- Yoshino, O.; Wong, B.K.L.; Cox, D.R.A.; Lee, E.; Hepworth, G.; Christophi, C.; Jones, R.; Dobrovic, A.; Muralidharan, V.; Perini, M.V. Elevated levels of circulating mitochondrial DNA predict early allograft dysfunction in patients following liver transplantation. J. Gastroenterol. Hepatol. 2021, 36, 3500–3507. [Google Scholar] [CrossRef]
- Lee, D.D.; Croome, K.P.; Shalev, J.A.; Musto, K.R.; Sharma, M.; Keaveny, A.P.; Taner, C.B. Early allograft dysfunction after liver transplantation: An intermediate outcome measure for targeted improvements. Ann. Hepatol. 2016, 15, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Costa, T.J.; Potje, S.R.; Fraga-Silva, T.F.C.; da Silva-Neto, J.A.; Barros, P.R.; Rodrigues, D.; Machado, M.R.; Martins, R.B.; Santos-Eichler, R.A.; Benatti, M.N.; et al. Mitochondrial DNA and TLR9 activation contribute to SARS-CoV-2-induced endothelial cell damage. Vasc. Pharmacol. 2022, 142, 106946. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Dai, Z.; Li, Y.; Zhu, H.; Zhao, L. TLR9 regulates NLRP3 inflammasome activation via the NF-kB signaling pathway in diabetic nephropathy. Diabetol. Metab. Syndr. 2022, 14, 26. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Garcia, J.J.; Martinez-Banaclocha, H.; Angosto-Bazarra, D.; de Torre-Minguela, C.; Baroja-Mazo, A.; Alarcon-Vila, C.; Martinez-Alarcon, L.; Amores-Iniesta, J.; Martin-Sanchez, F.; Ercole, G.A.; et al. P2X7 receptor induces mitochondrial failure in monocytes and compromises NLRP3 inflammasome activation during sepsis. Nat. Commun. 2019, 10, 2711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tupik, J.D.; Markov Madanick, J.W.; Ivester, H.M.; Allen, I.C. Detecting DNA: An Overview of DNA Recognition by Inflammasomes and Protection against Bacterial Respiratory Infections. Cells 2022, 11, 1681. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, H.; Zhang, Q.; Yao, X.; Ni, W.; Zhou, K. Emerging role of STING signalling in CNS injury: Inflammation, autophagy, necroptosis, ferroptosis and pyroptosis. J. Neuroinflamm. 2022, 19, 242. [Google Scholar] [CrossRef]
- Gaidt, M.M.; Ebert, T.S.; Chauhan, D.; Ramshorn, K.; Pinci, F.; Zuber, S.; O’Duill, F.; Schmid-Burgk, J.L.; Hoss, F.; Buhmann, R.; et al. The DNA Inflammasome in Human Myeloid Cells Is Initiated by a STING-Cell Death Program Upstream of NLRP3. Cell 2017, 171, 1110–1124. [Google Scholar] [CrossRef]
- Coll, R.C. Role reversal: Adaptive immunity instructs inflammasome activation for anti-viral defence. EMBO J. 2019, 38, e103533. [Google Scholar] [CrossRef]
- Seoane, P.I.; Lee, B.; Hoyle, C.; Yu, S.; Lopez-Castejon, G.; Lowe, M.; Brough, D. The NLRP3-inflammasome as a sensor of organelle dysfunction. J. Cell Biol. 2020, 219, e202006194. [Google Scholar] [CrossRef]
- Wang, Q.; Bu, Q.; Liu, M.; Zhang, R.; Gu, J.; Li, L.; Zhou, J.; Liang, Y.; Su, W.; Liu, Z.; et al. XBP1-mediated activation of the STING signalling pathway in macrophages contributes to liver fibrosis progression. JHEP Rep. Innov. Hepatol. 2022, 4, 100555. [Google Scholar] [CrossRef]
- Land, W.G. Emerging role of innate immunity in organ transplantation part II: Potential of damage-associated molecular patterns to generate immunostimulatory dendritic cells. Transplant. Rev. 2012, 26, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Pollara, J.; Edwards, R.W.; Lin, L.; Bendersky, V.A.; Brennan, T.V. Circulating mitochondria in deceased organ donors are associated with immune activation and early allograft dysfunction. JCI Insight 2018, 3, e121622. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Xu, H.; Bishawi, M.; Feng, F.; Samy, K.; Truskey, G.; Barbas, A.S.; Kirk, A.D.; Brennan, T.V. Circulating mitochondria in organ donors promote allograft rejection. Am. J. Transplant. 2019, 19, 1917–1929. [Google Scholar] [CrossRef] [PubMed]
- Moeller-Gorman, R. Pharma Looks to Inflammasome Inhibitors as All-Around Therapies. In The Scientist; Bio Business: Belfast, Ireland, 2021. [Google Scholar]
- Dengu, F.; Abbas, S.H.; Ebeling, G.; Nasralla, D. Normothermic Machine Perfusion (NMP) of the Liver as a Platform for Therapeutic Interventions during Ex-Vivo Liver Preservation: A Review. J. Clin. Med. 2020, 9, 1046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Buchwald, J.E.; Martins, P.N. Review of Current Machine Perfusion Therapeutics for Organ Preservation. Transplantation 2020, 104, 1792–1803. [Google Scholar] [CrossRef]
- Bruggenwirth, I.M.A.; Martins, P.N. RNA interference therapeutics in organ transplantation: The dawn of a new era. Am. J. Transplant. 2020, 20, 931–941. [Google Scholar] [CrossRef]
- Thijssen, M.F.; Bruggenwirth, I.M.A.; Gillooly, A.; Khvorova, A.; Kowalik, T.F.; Martins, P.N. Gene Silencing With siRNA (RNA Interference): A New Therapeutic Option During Ex Vivo Machine Liver Perfusion Preservation. Liver Transpl. 2019, 25, 140–151. [Google Scholar] [CrossRef] [Green Version]
- Nickkholgh, A.; Nikdad, M.; Shafie, S.; Abbasi Dezfouli, S.; Mehrabi, A.; Eason, J.D.; Mas, V.R.; Maluf, D.G. Ex Situ Liver Machine Perfusion as an Emerging Graft Protective Strategy in Clinical Liver Transplantation: The Dawn of a New Era. Transplantation 2019, 103, 2003–2011. [Google Scholar] [CrossRef]
- Jochmans, I.; Hessheimer, A.J.; Neyrinck, A.P.; Paredes, D.; Bellini, M.I.; Dark, J.H.; Kimenai, H.; Pengel, L.H.M.; Watson, C.J.E. Consensus statement on normothermic regional perfusion in donation after circulatory death: Report from the European Society for Organ Transplantation’s Transplant Learning Journey. Transpl. Int. 2021, 34, 2019–2030. [Google Scholar] [CrossRef]
- Hessheimer, A.J.; de la Rosa, G.; Gastaca, M.; Ruiz, P.; Otero, A.; Gomez, M.; Alconchel, F.; Ramirez, P.; Bosca, A.; Lopez-Andujar, R.; et al. Abdominal normothermic regional perfusion in controlled donation after circulatory determination of death liver transplantation: Outcomes and risk factors for graft loss. Am. J. Transplant. 2021, 22, 1169–1181. [Google Scholar] [CrossRef]

| Drug | Target | Action | Liver Toxicity | Clinical Trial | References |
|---|---|---|---|---|---|
| Allopurinol | DAMPs inhibitor | Alcoholic hepatitis. NASH | Minor. Rare acute liver injury | [19,20,21] | |
| Probenecid | DAMPs inhibitor | Alcoholic hepatitis | Minor | [19] | |
| SGM-1019 | P2X7 Antagonist | Liver fibrosis; NASH | n.d. | NCT03676231 | [22,23] |
| IMD-0354 | NF-κB inhibitor | Liver fibrosis | n.d. | [24] | |
| Pyrrolidine dithiocarbamate | NF-κB inhibitor | Liver injury; liver cirrhosis | n.d. | [25,26] | |
| MCC950 | NLRP3 inhibitor | NASH; liver fibrosis; cholestatic liver injury; rejection | n.d. | [27,28,29,30] | |
| Anakinra | IL-1 receptor antagonist | Alcoholic hepatitis | Rare acute liver injury | NCT04072822 NCT01809132 | [31] |
| Canakinumab | IL-1β blocking antibody | Liver fibrosis | Rare acute liver injury | NCT03775109 | [32] |
| Ac-YVAD-CMK | Caspase-1 inhibitor | Rejection | n.d. | [33] | |
| Dimethyl fumarate | Gasdermin inhibitor | ALD | No | [34,35] | |
| Ac-DMPD/DMLD-CMK | Gasdermin inhibitor | Acute liver injury | n.d. | [36] | |
| Ginsenoside-Rg1 | Antioxidant | Acute liver injury | No | [37] | |
| Naringenin | Antioxidant | NAFLD | n.d. | [38] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucas-Ruiz, F.; Peñín-Franch, A.; Pons, J.A.; Ramírez, P.; Pelegrín, P.; Cuevas, S.; Baroja-Mazo, A. Emerging Role of NLRP3 Inflammasome and Pyroptosis in Liver Transplantation. Int. J. Mol. Sci. 2022, 23, 14396. https://doi.org/10.3390/ijms232214396
Lucas-Ruiz F, Peñín-Franch A, Pons JA, Ramírez P, Pelegrín P, Cuevas S, Baroja-Mazo A. Emerging Role of NLRP3 Inflammasome and Pyroptosis in Liver Transplantation. International Journal of Molecular Sciences. 2022; 23(22):14396. https://doi.org/10.3390/ijms232214396
Chicago/Turabian StyleLucas-Ruiz, Fernando, Alejandro Peñín-Franch, José Antonio Pons, Pablo Ramírez, Pablo Pelegrín, Santiago Cuevas, and Alberto Baroja-Mazo. 2022. "Emerging Role of NLRP3 Inflammasome and Pyroptosis in Liver Transplantation" International Journal of Molecular Sciences 23, no. 22: 14396. https://doi.org/10.3390/ijms232214396
APA StyleLucas-Ruiz, F., Peñín-Franch, A., Pons, J. A., Ramírez, P., Pelegrín, P., Cuevas, S., & Baroja-Mazo, A. (2022). Emerging Role of NLRP3 Inflammasome and Pyroptosis in Liver Transplantation. International Journal of Molecular Sciences, 23(22), 14396. https://doi.org/10.3390/ijms232214396








