Solvent-Dependent Fluorescence Properties of CH2-bis(BODIPY)s
Abstract
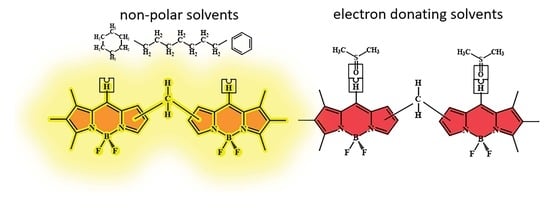
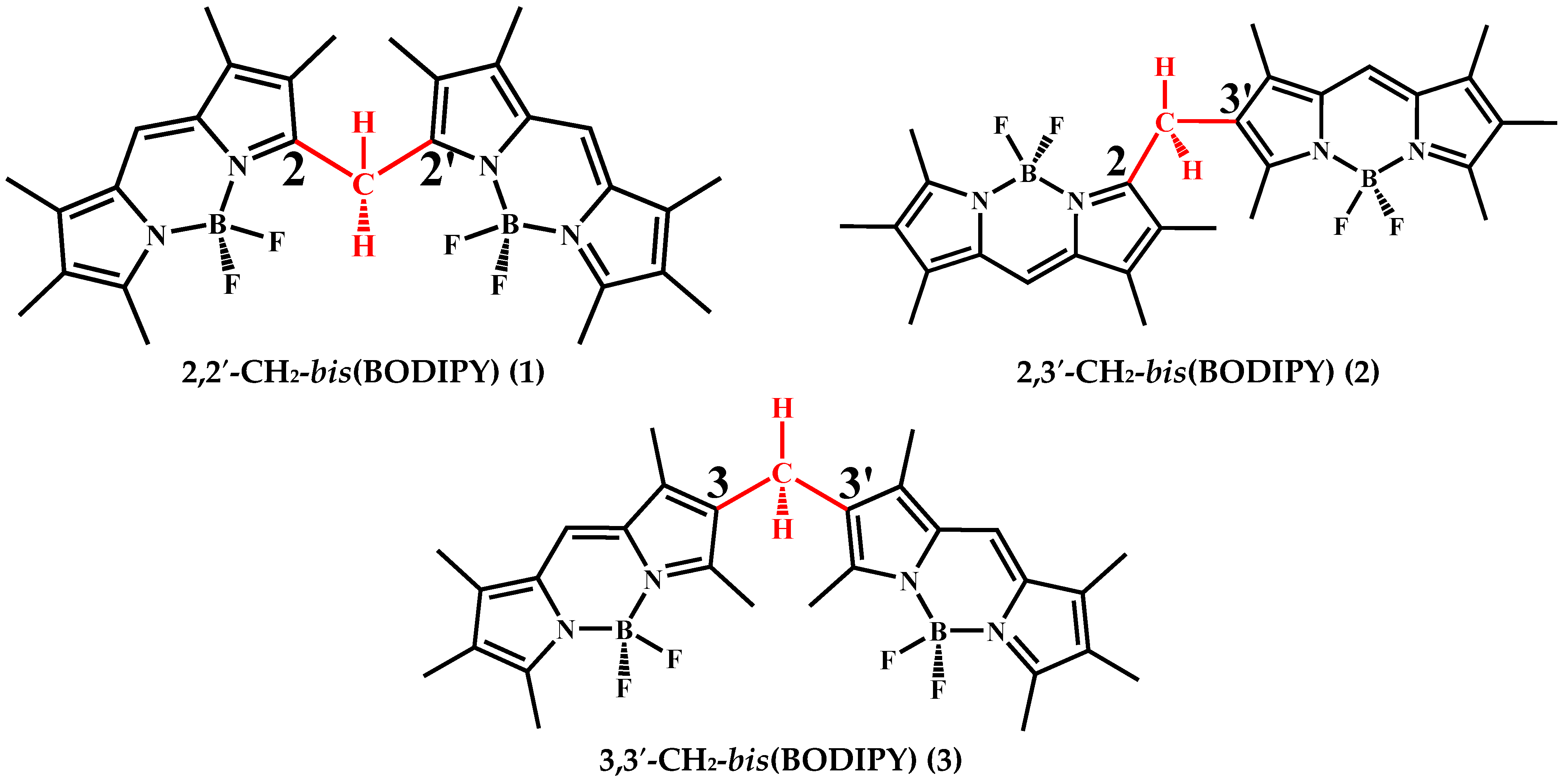
:1. Introduction
2. Results and Discussion
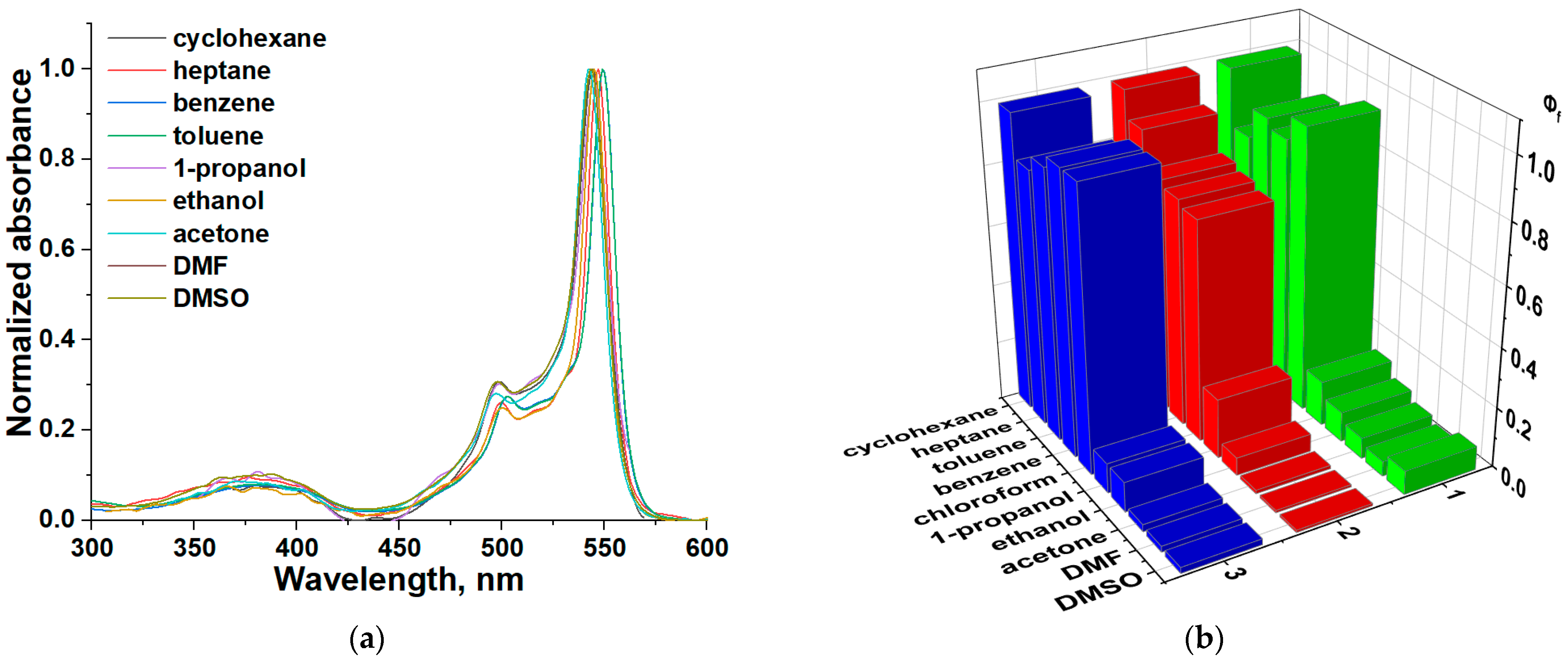
2.1. Effect of the Nature of Polar Electron-Donating Solvents on CH2-bis(BODIPY)s 1–3 Spectral Properties
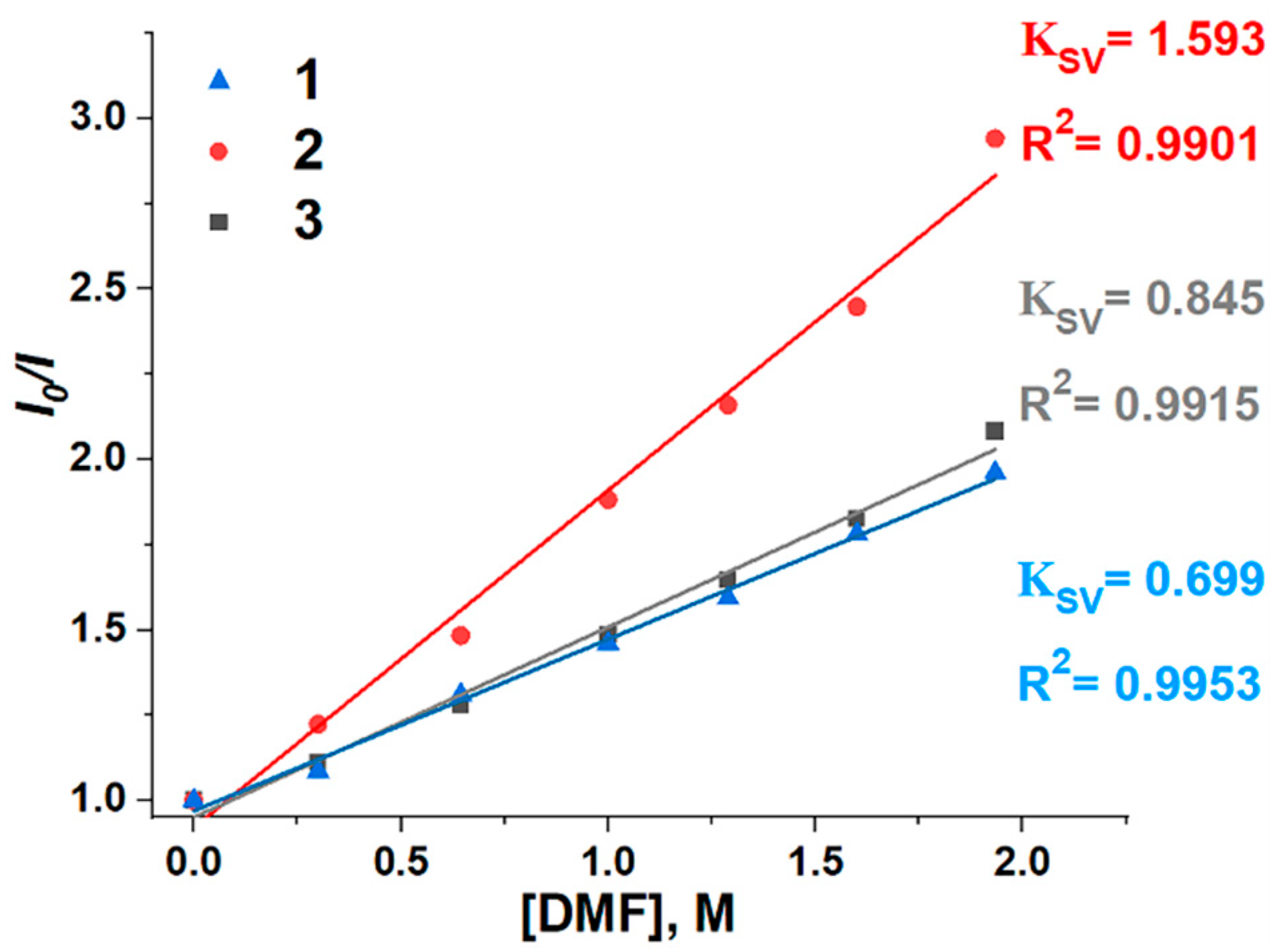
2.2. Investigation of Supramolecular Complexes of 1–3 with Electron-Donating Solvents (Acetone, DMF, and DMSO)
2.3. DFT Calculations
3. Materials and Methods
3.1. Spectroscopic Experiments
3.2. Computational Modeling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chaturvedi, P.; Majumder, S.K.; Krishna, H.; Muttagi, S.; Gupta, P.K. Fluorescence spectroscopy for noninvasive early diagnosis of oral mucosal malignant and potentially malignant lesions. J. Cancer Res. Ther. 2010, 6, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Francisco, A.L.N.; Correr, W.R.; Azevedo, L.H.; Kern, V.G.; Pinto, C.A.L.; Kowalski, L.P.; Kurachi, C. Fluorescence spectroscopy for the detection of potentially malignant disorders and squamous cell carcinoma of the oral cavity. Photodiagnosis Photodyn. Ther. 2014, 11, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Kaniyala Melanthota, S.; Kistenev, Y.V.; Borisova, E.; Ivanov, D.; Zakharova, O.; Boyko, A.; Vrazhnov, D.; Gopal, D.; Chakrabarti, S.; K, S.P.; et al. Types of spectroscopy and microscopy techniques for cancer diagnosis: A review. Lasers Med. Sci. 2022, 37, 3067–3084. [Google Scholar] [CrossRef]
- Ramanujam, N. Fluorescence spectroscopy of neoplastic and non-neoplastic tissues. Neoplasia 2000, 2, 89–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, Q.-S.; Wang, T.; Zhang, K.-H. Biomedical optical spectroscopy for the early diagnosis of gastrointestinal neoplasms. Tumour Biol. 2017, 39, 1010428317717984. [Google Scholar] [CrossRef] [Green Version]
- Lu, P.; Chung, K.-Y.; Stafford, A.; Kiker, M.; Kafle, K.; Page, Z.A. Boron dipyrromethene (BODIPY) in polymer chemistry. Polym. Chem. 2021, 12, 327–348. [Google Scholar] [CrossRef]
- Lin, W.; Colombani-Garay, D.; Huang, L.; Duan, C.; Han, G. Tailoring nanoparticles based on boron dipyrromethene for cancer imaging and therapy. WIREs Nanomed. Nanobiotechnol. 2020, 12, e1627. [Google Scholar] [CrossRef] [PubMed]
- Kue, C.S.; Ng, S.Y.; Voon, S.H.; Kamkaew, A.; Chung, L.Y.; Kiew, L.V.; Lee, H.B. Recent strategies to improve boron dipyrromethene (BODIPY) for photodynamic cancer therapy: An updated review. Photochem. Photobiol. Sci. 2018, 17, 1691–1708. [Google Scholar] [CrossRef] [PubMed]
- Loudet, A.; Burgess, K. BODIPY dyes and their derivatives: Syntheses and spectroscopic properties. Chem. Rev. 2007, 107, 4891–4932. [Google Scholar] [CrossRef]
- Requena, S.; Ponomarchuk, O.; Castillo, M.; Rebik, J.; Brochiero, E.; Borejdo, J.; Gryczynski, I.; Dzyuba, S.V.; Gryczynski, Z.; Grygorczyk, R.; et al. Imaging viscosity of intragranular mucin matrix in cystic fibrosis cells. Sci. Rep. 2017, 7, 16761. [Google Scholar] [CrossRef]
- Raut, S.L.; Kimball, J.D.; Fudala, R.; Bora, I.; Chib, R.; Jaafari, H.; Castillo, M.K.; Smith, N.W.; Gryczynski, I.; Dzyuba, S.V.; et al. A triazine-based BODIPY trimer as a molecular viscometer. Phys. Chem. Chem. Phys. 2016, 18, 4535–4540. [Google Scholar] [CrossRef] [Green Version]
- Kimball, J.D.; Raut, S.; Jameson, L.P.; Smith, N.W.; Gryczynski, Z.; Dzyuba, S.V. BODIPY-BODIPY dyad: Assessing the potential as a viscometer for molecular and ionic liquids. RSC Adv. 2015, 5, 19508–19511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bumagina, N.A.; Antina, E.V.; Ksenofontov, A.A.; Antina, L.A.; Kalyagin, A.A.; Berezin, M.B. Basic structural modifications for improving the practical properties of BODIPY. Coord. Chem. Rev. 2022, 469, 214684. [Google Scholar] [CrossRef]
- Ravikanth, M.; Vellanki, L.; Sharma, R. Functionalized boron-dipyrromethenes and their applications. Rep. Org. Chem. 2016, 6, 1. [Google Scholar] [CrossRef] [Green Version]
- Xiao, H.; Li, P.; Tang, B. Recent progresses in fluorescent probes for detection of polarity. Coord. Chem. Rev. 2021, 427, 213582. [Google Scholar] [CrossRef]
- Saremi, B.; Bandi, V.; Kazemi, S.; Hong, Y.; D’Souza, F.; Yuan, B. Exploring NIR Aza-BODIPY-Based Polarity Sensitive Probes with ON-and-OFF Fluorescence Switching in Pluronic Nanoparticles. Polymers 2020, 12, 540. [Google Scholar] [CrossRef] [Green Version]
- Squeo, B.M.; Pasini, M. BODIPY platform: A tunable tool for green to NIR OLEDs. Supramol. Chem. 2020, 32, 56–70. [Google Scholar] [CrossRef]
- Ivaniuk, K.; Pidluzhna, A.; Stakhira, P.; Baryshnikov, G.V.; Kovtun, Y.P.; Hotra, Z.; Minaev, B.F.; Ågren, H. BODIPY-core 1,7-diphenyl-substituted derivatives for photovoltaics and OLED applications. Dye. Pigment. 2020, 175, 108123. [Google Scholar] [CrossRef]
- Bi, J.; Ji, X.; Guo, M.; Guo, H.; Yang, F. A fluorescent sensor for thymine based on bis-BODIPY containing butanediamido bridges. New J. Chem. 2019, 43, 5890–5896. [Google Scholar] [CrossRef]
- Boens, N.; Leen, V.; Dehaen, W. Fluorescent indicators based on BODIPY. Chem. Soc. Rev. 2012, 41, 1130–1172. [Google Scholar] [CrossRef]
- Antina, L.A.; Ksenofontov, A.A.; Kalyagin, A.A.; Antina, E.V.; Berezin, M.B.; Khodov, I.A. Luminescent properties of new 2,2-, 2,3- and 3,3-CH2-bis(BODIPY)s dyes: Structural and solvation effects. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 218, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Ksenofontov, A.A.; Guseva, G.B.; Antina, E.V.; V’yugin, A.I. Molecular structure of bis(dipyrrolylmethanates) of d-metals according to the quantum chemical calculations by the PM6 method. J. Struct. Chem. 2014, 55, 418–423. [Google Scholar] [CrossRef]
- Ksenofontov, A.A.; Antina, E.V.; Guseva, G.B.; Berezin, M.B.; Antina, L.A.; Vyugin, A.I. Prospects of applications of fluorescent sensors based on zinc(II) and boron(III) bis(dipyrromethenate)s. J. Mol. Liq. 2019, 274, 681–689. [Google Scholar] [CrossRef]
- Ksenofontov, A.A.; Guseva, G.B.; Antina, E.V.; Nuraneeva, E.N. «On-off» fluorescent sensors for aromatic analytes based on zinc(II) bis(dipyrromethenate)s. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 173, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, J.; Cordes, B.; Wicht, R.; Wolfram, B.; Bröring, M. Acidic Condensation of BODIPYs with Aldehydes: A Quick and Versatile Route to Alkenyl-BODIPYs and C(sp(3) )-Connected DYEmers. Chemistry 2016, 22, 10320–10325. [Google Scholar] [CrossRef]
- Bröring, M.; Krüger, R.; Link, S.; Kleeberg, C.; Köhler, S.; Xie, X.; Ventura, B.; Flamigni, L. Bis(BF2)-2,2′-bidipyrrins (BisBODIPYs): Highly fluorescent BODIPY dimers with large stokes shifts. Chemistry 2008, 14, 2976–2983. [Google Scholar] [CrossRef]
- Ventura, B.; Marconi, G.; Bröring, M.; Krüger, R.; Flamigni, L. Bis(BF 2 )-2,2′-bidipyrrins, a class of BODIPY dyes with new spectroscopic and photophysical properties. New J. Chem. 2009, 33, 428–438. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Yang, X.; Xu, B. PET-based bisBODIPY photosensitizers for highly efficient excited triplet state and singlet oxygen generation: Tuning photosensitizing ability by dihedral angles. Phys. Chem. Chem. Phys. 2017, 19, 24792–24804. [Google Scholar] [CrossRef]
- Antina, L.A.; Kalyagin, A.A.; Ksenofontov, A.A.; Pavelyev, R.S.; Lodochnikova, O.A.; Islamov, D.R.; Antina, E.V.; Berezin, M.B. Effect of polar protic solvents on the photophysical properties of bis(BODIPY) dyes. J. Mol. Liq. 2021, 337, 116416. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: New York, NY, USA, 2006; ISBN 978-0-387-46312-4. [Google Scholar]
- Lee, S.-C.; Heo, J.; Woo, H.C.; Lee, J.-A.; Seo, Y.H.; Lee, C.-L.; Kim, S.; Kwon, O.-P. Fluorescent Molecular Rotors for Viscosity Sensors. Chemistry 2018, 24, 13706–13718. [Google Scholar] [CrossRef]
- Haidekker, M.A.; Brady, T.P.; Lichlyter, D.; Theodorakis, E.A. Effects of solvent polarity and solvent viscosity on the fluorescent properties of molecular rotors and related probes. Bioorganic Chem. 2005, 33, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Georges, J. Fluorescence quantum yield of rhodamine 6G in ethanol as a function of concentration using thermal lens spectrometry. Chem. Phys. Lett. 1996, 260, 115–118. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Chai, J.-D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [Green Version]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Riddick, J.A.; Bunger, W.B.; Sakano, T.K. Organic solvents: Physical properties and methods of purification, 4th ed.; Riddick, J.A., Bunger, W.B., Sakano, T.K., Eds.; Wiley: New York, NY, USA; Chichester, UK, 1986; ISBN 978-0471084679. [Google Scholar]

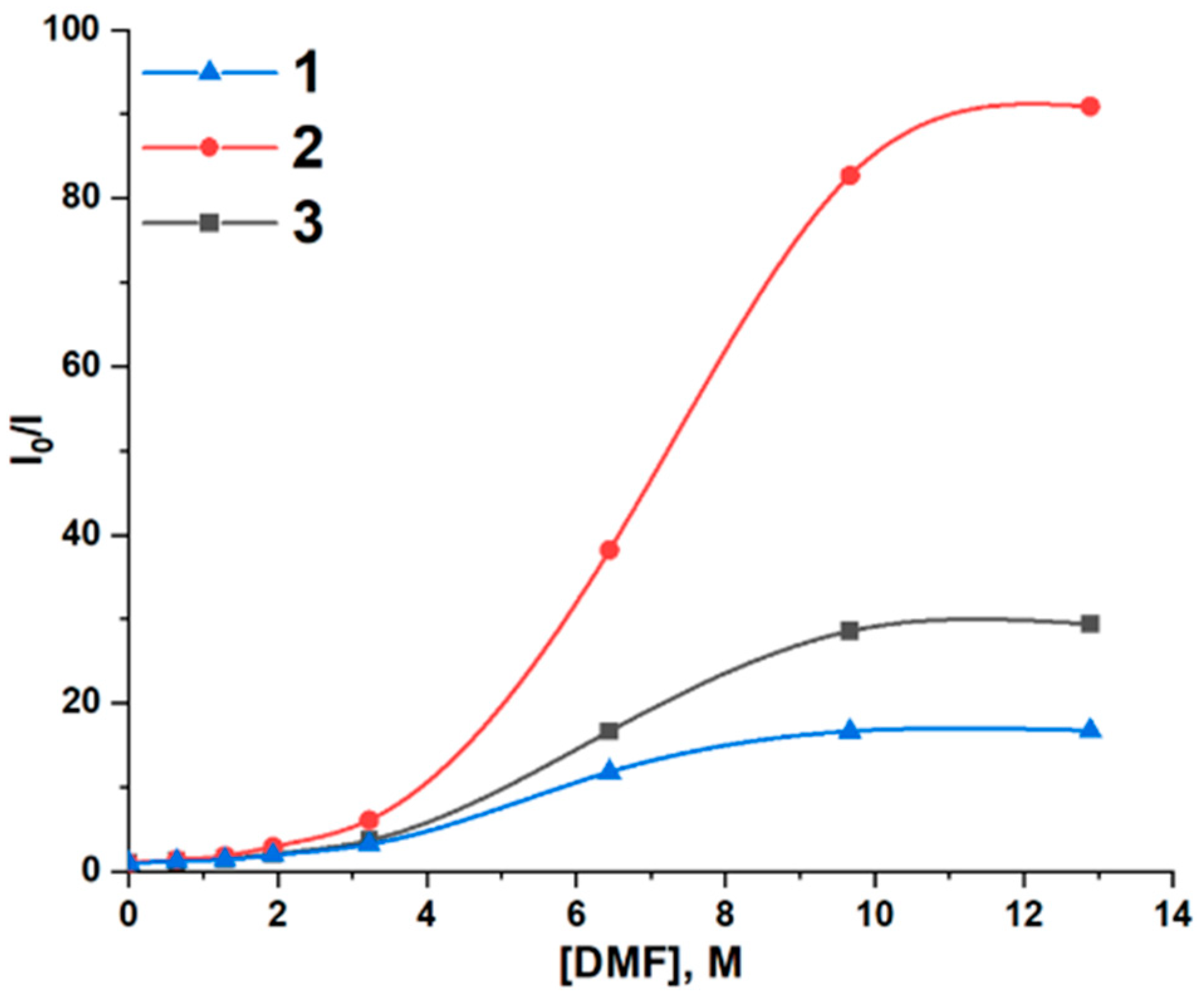


| Luminophores | |||
|---|---|---|---|
| 1 | 2 | 3 | |
| Solv | KSV, M−1; (lgK) | ||
| Acetone | 0.048 (2.7) | 0.095 (4.2) | 0.056 (3.5) |
| DMF | 0.699 (4.7) | 1.593 (5.0) | 0.845 (4.8) |
| DMSO | 1.913 (5.1) | 4.211 (5.4) | 2.590 (5.2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalyagin, A.; Antina, L.; Ksenofontov, A.; Antina, E.; Berezin, M. Solvent-Dependent Fluorescence Properties of CH2-bis(BODIPY)s. Int. J. Mol. Sci. 2022, 23, 14402. https://doi.org/10.3390/ijms232214402
Kalyagin A, Antina L, Ksenofontov A, Antina E, Berezin M. Solvent-Dependent Fluorescence Properties of CH2-bis(BODIPY)s. International Journal of Molecular Sciences. 2022; 23(22):14402. https://doi.org/10.3390/ijms232214402
Chicago/Turabian StyleKalyagin, Alexander, Lubov Antina, Alexander Ksenofontov, Elena Antina, and Mikhail Berezin. 2022. "Solvent-Dependent Fluorescence Properties of CH2-bis(BODIPY)s" International Journal of Molecular Sciences 23, no. 22: 14402. https://doi.org/10.3390/ijms232214402
APA StyleKalyagin, A., Antina, L., Ksenofontov, A., Antina, E., & Berezin, M. (2022). Solvent-Dependent Fluorescence Properties of CH2-bis(BODIPY)s. International Journal of Molecular Sciences, 23(22), 14402. https://doi.org/10.3390/ijms232214402