Non-Metal-Doped Porous Carbon Nitride Nanostructures for Photocatalytic Green Hydrogen Production
Abstract
1. Introduction
Novelty and Focus Review
2. H2 as Fuel Strategy
Solar-Driven H2 Production
- Photoelectrochemical H2O splitting driven by quantum dots or semiconductor (i.e., electrodes using a photoelectrochemical cell) to convert light energy into H2 chemical energy. Photoelectrochemical systems could be based on semiconductors or dyes and using dissolved metal complexes.
- The photobiological process includes the production of H2 from biological systems (i.e., algae and bacteria using sunlight driven by the initial absorption of light by the pigments in algae while the enzymes in the cell act as catalysts to promote H2 or O2 production). Both photoelectrochemical and photobiological approaches should be improved significantly to meet large-scale applications because current solar-to-H2 systems’ efficiencies are less than 1%.
- Thermochemical cycles for generating high temperature from solar light to produce H2, which can achieve efficiencies higher than 40%. However, it needs a concentrated solar receiver/reactors able to generate a high temperature of nearly 800 °C.
3. Fundamentals of HER
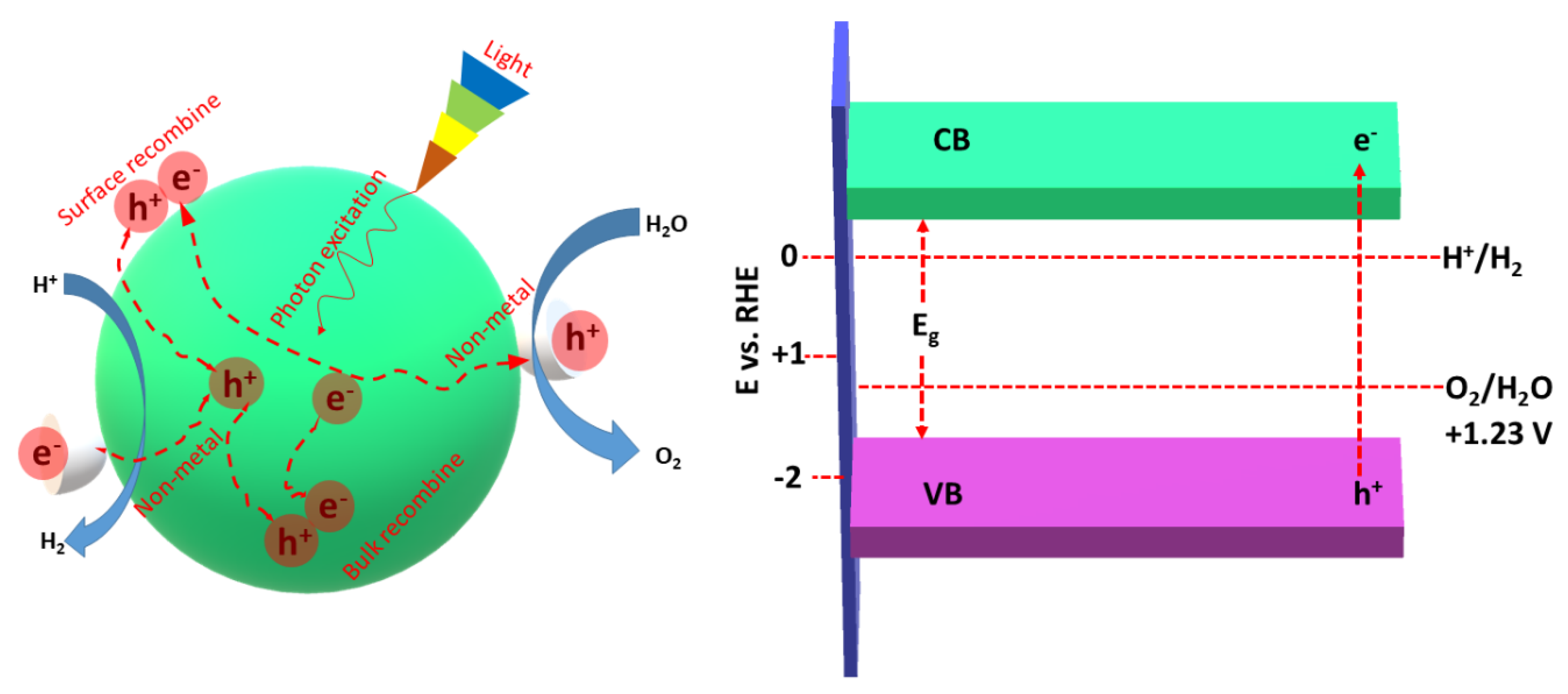
3.1. Photocatalytic HER Mechanism
- (I)
- The isolation of the photoexcited carriers into free carriers followed by migration to the active sites of gCN.
- (II)
- The initiation of a reduction reaction comprising these charges to produce H2 on the surface of gCN with the assistance of e− in the CB. The HER reaction in different electrolytes is shown in Equations (1) and (2)
3.2. Electrocatalytic HER Mechanism
- (I)
- A Volmer reaction step that includes a discharge step to allow reduction of protons on the M* and subsequent proton adsorption on M* of gCNs to form gCN-M*Hads (Equation (4));
- (II)
- A Heyrovsky reaction step that involves electrochemical desorption to desorb H2 from the M* via the proton/electron transfer and regenerate of M* Equation (5);
- (III)
- A Tafel reaction step that includes the coupling of two adsorbed protons to release H2 and the regeneration of M* (Equation (6)).
3.3. HER Measurements and Calculations
4. Role of Non-Metal Dopants
4.1. Integration of Heteroatoms
4.2. Non-Metal-Doping Configuration and Effects
5. Heteroatom-Doped Porous Carbon Nitride
5.1. Mono Heteroatom Doped Porous Carbon Nitride
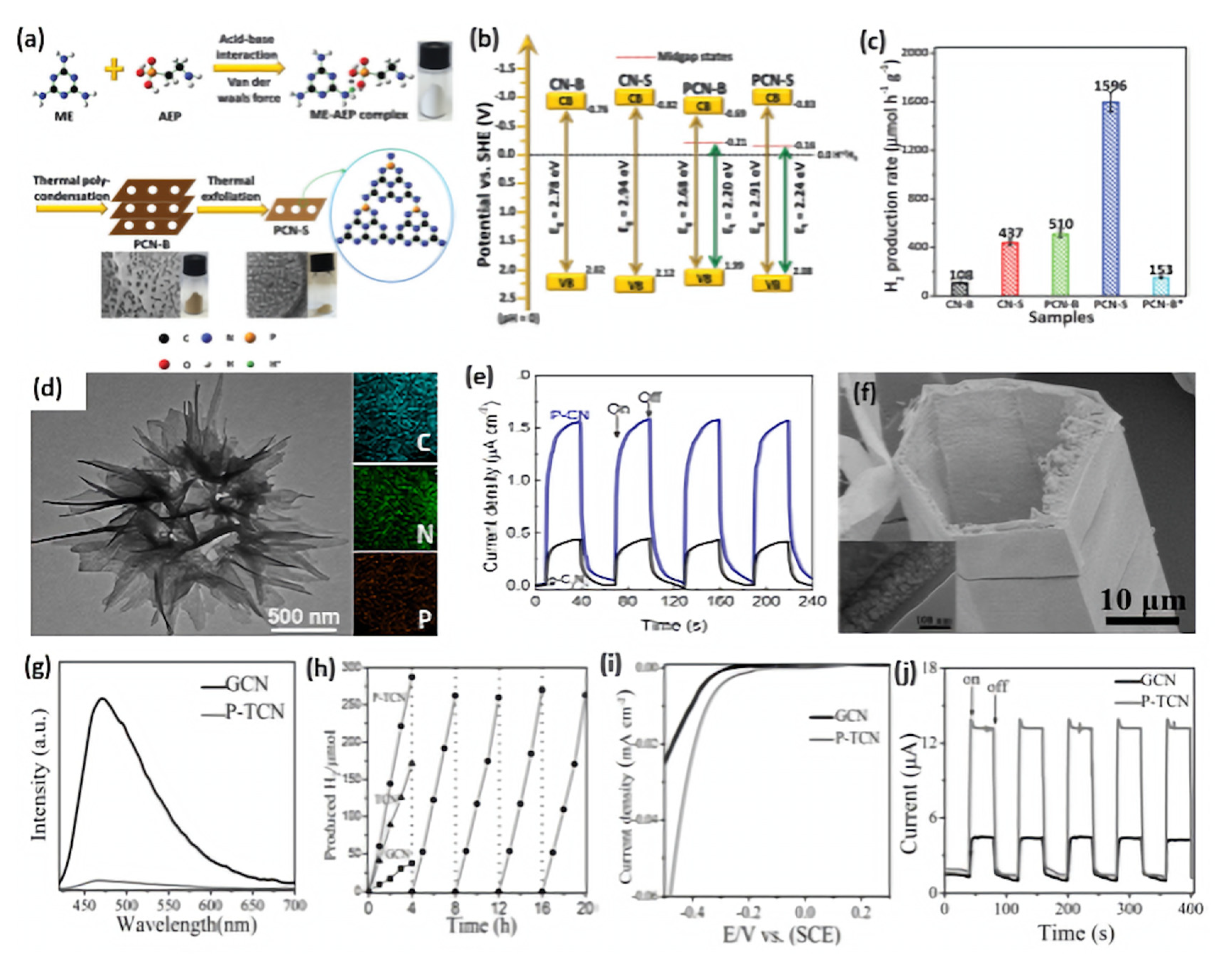
5.1.1. Phosphorus Doping
5.1.2. Sulfur Doping
5.1.3. Boron Doping
5.1.4. Oxygen Doping
5.1.5. Carbon Doping
5.1.6. Nitrogen Doping
5.1.7. Halogen Doping
5.2. Binary Heteroatom-Doped Porous Carbon Nitride
5.3. Ternary Heteroatom-Doped Porous Carbon Nitride
6. Conclusions and Prospective
- Previous porous, doped gCNs in the form of 2D nanosheets and other porous nanostructures are rarely reported or not yet reported. Porous multidimensional doped gCN (i.e., nanoflower, nanodendrite, yolk–shell, and nanocage) and one-dimensional (i.e., nanowires, nanotubes, nanorods, and nanotubes) morphologies are imminent with their impressive characteristics (i.e., high electrical conductivity, great surface area, abundant defects, massive active/accessible active sites, stabilization of metal/non-metal atoms, and maximized atomic utilization) [163]. These merits can endow the HER activity and the durability of doped porous gCNs. Such porous nanostructures could be synthesized using multiple nitrogen-rich carbon precursors containing non-metal elements (i.e., melamine, thiourea, cyanuric acid, and cyanimide) and changing the preparation conditions (i.e., annealing environment, templates, and solvent type) [28,163]. Meanwhile, the reported g-doped porous gCNs are powder, which cannot be used directly in electrolysis and require several steps to be used as a cathode. This could be realized via the in situ fabrication of gCNs on solid carbon-cloth sheets or metal hydroxide/oxide substrate that could be used as a cathode for the HER.
- Particular attention should be paid to developing facile, one-step, and eco-friendly methods to fabricate g-C3N4 with various morphologies. Recently, our group developed a simple, template-free, one-pot approach for the fabrication of porous one-dimensional gCN nanostructures (i.e., wires, fibers, tubes, and rods) in situ, doped with various metals (i.e., Au, Pd, Pt, Cu, and their combinations) with high surface area and outstanding catalytic properties for CO oxidation [3,4,5,164]. The same tactics can be extended to prepare other gCN structures with various single-atom metals, dopants, and nanoparticles for CO2 reduction. Single-atom-impeded g-C3N4 for CO2 reduction is not studied enough. g-C3N4 comprises a triazine or heptazine skeleton that can accommodate various single-metal atoms to maximize atom utilization; minimize attrition; reduce deactivation; and enhance CO2 activity, selectivity, and durability.
- Both experimental and theoretical calculations/simulations (i.e., DFT and artificial intelligence) could be coupled to understand the effect of non-metal dopants on the physicochemical properties of porous gCN nanostructures and their catalytic/photocatalytic activities and mechanisms.
- The relatively high overpotential, low current densities, and inferior long-term stability are critical barriers in gCNs for HERs, which cannot meet practical requirements (i.e., current density up to several ambers and durability for several weeks or months). This could be solved using noble metal dopants in the formation of heterojunction structures with porous metal oxynitride [165,166], multimetallic nanocrystals [12,167,168], MXenes [2,169,170,171,172], MOF [10,173,174], graphene [175], and graphdiyne [176] to augment solar light harvesting and charge carrier separation during the HER.
- The safety of H2 storage tanks should be considered because in the case of unexpected accidents, the H2 tank becomes a bomb. Defeating these barriers requires using high-pressure vessels made of fiber-based composites that can afford a high pressure of up to 700 bar and subsequently can improve cold or cryo-compressed hydrogen storage along with boosting H2 density and using novel, durable, low-cost materials for H2 adsorption [18,46,47,48]. Moreover, using novel adsorbents for H2 storage is safer than tanks, but needs more efforts to decrease operation conditions (i.e., pressure) and enhance storage capacity.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eid, K.; Sliem, M.H.; Al-Ejji, M.; Abdullah, A.M.; Harfouche, M.; Varma, R.S. Hierarchical Porous Carbon Nitride-Crumpled Nanosheet-Embedded Copper Single Atoms: An Efficient Catalyst for Carbon Monoxide Oxidation. ACS Appl. Mater. Interfaces 2022, 14, 40749–40760. [Google Scholar] [CrossRef]
- Eid, K.; Lu, Q.; Abdel-Azeim, S.; Soliman, A.; Abdullah, A.M.; Abdelgwad, A.M.; Forbes, R.P.; Ozoemena, K.I.; Varma, R.S.; Shibl, M.F. Highly exfoliated Ti3C2Tx MXene nanosheets atomically doped with Cu for efficient electrochemical CO2 reduction: An experimental and theoretical study. J. Mater. Chem. A 2022, 10, 1965–1975. [Google Scholar] [CrossRef]
- Eid, K.; Sliem, M.H.; Jlassi, K.; Eldesoky, A.S.; Abdo, G.G.; Al-Qaradawi, S.Y.; Sharaf, M.A.; Abdullah, A.M.; Elzatahry, A.A. Precise fabrication of porous one-dimensional gC3N4 nanotubes doped with Pd and Cu atoms for efficient CO oxidation and CO2 reduction. Inorg. Chem. Commun. 2019, 107, 107460. [Google Scholar] [CrossRef]
- Eid, K.; Sliem, M.H.; Al-Kandari, H.; Sharaf, M.A.; Abdullah, A.M. Rational synthesis of porous graphitic-like carbon nitride nanotubes codoped with Au and Pd as an efficient catalyst for carbon monoxide oxidation. Langmuir 2019, 35, 3421–3431. [Google Scholar] [CrossRef]
- Eid, K.; Sliem, M.H.; Eldesoky, A.S.; Al-Kandari, H.; Abdullah, A.M. Rational synthesis of one-dimensional carbon nitride-based nanofibers atomically doped with Au/Pd for efficient carbon monoxide oxidation. Int. J. Hydrogen Energy 2019, 44, 17943–17953. [Google Scholar] [CrossRef]
- Lu, Q.; Eid, K.; Li, W. Heteroatom-Doped Porous Carbon-Based Nanostructures for Electrochemical CO2 Reduction. Nanomaterials 2022, 12, 2379. [Google Scholar] [CrossRef]
- Abdu, H.I.; Eid, K.; Abdullah, A.M.; Sliem, M.H.; Elzatahry, A.; Lu, X. Dry ice-mediated rational synthesis of edge-carboxylated crumpled graphene nanosheets for selective and prompt hydrolysis of cellulose and eucalyptus lignocellulose under ambient reaction conditions. Green Chem. 2020, 22, 5437–5446. [Google Scholar] [CrossRef]
- Li, C.; Eid, K.; Wang, H.; Deng, Y.; Lu, S.; Li, X.; Wang, L.; Gu, H. One-pot synthesis of bimetallic PdCu nanoframes as an efficient catalyst for the methanol oxidation reaction. New J. Chem. 2018, 42, 798–801. [Google Scholar] [CrossRef]
- Lu, Q.; Li, J.; Eid, K.; Gu, X.; Wan, Z.; Li, W.; Al-Hajri, R.S.; Abdullah, A.M. Facile one-step aqueous-phase synthesis of porous PtBi nanosponges for efficient electrochemical methanol oxidation with a high CO tolerance. J. Electroanal. Chem. 2022, 916, 116361. [Google Scholar] [CrossRef]
- Ahsan, M.A.; He, T.; Eid, K.; Abdullah, A.M.; Sanad, M.F.; Aldalbahi, A.; Alvarado-Tenorio, B.; Du, A.; Puente Santiago, A.R.; Noveron, J.C. Controlling the Interfacial Charge Polarization of MOF-Derived 0D–2D vdW Architectures as a Unique Strategy for Bifunctional Oxygen Electrocatalysis. ACS Appl. Mater. Interfaces 2022, 14, 3919–3929. [Google Scholar] [CrossRef]
- Eid, K.; Ahmad, Y.H.; AlQaradawi, S.Y.; Allam, N.K. Rational design of porous binary Pt-based nanodendrites as efficient catalysts for direct glucose fuel cells over a wide pH range. Catal. Sci. Technol. 2017, 7, 2819–2827. [Google Scholar] [CrossRef]
- Wang, H.; Yin, S.; Eid, K.; Li, Y.; Xu, Y.; Li, X.; Xue, H.; Wang, L. Fabrication of mesoporous cage-bell Pt nanoarchitectonics as efficient catalyst for oxygen reduction reaction. ACS Sustain. Chem. Eng. 2018, 6, 11768–11774. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, H.; Eid, K.; Wang, L. Nanoparticle in Nanocage: Au@ Porous Pt Yolk-Shell Nanoelectrocatalysts. Part. Part. Syst. Charact. 2015, 32, 863–868. [Google Scholar] [CrossRef]
- Ahmed, A.; Al-Amin, A.Q.; Ambrose, A.F.; Saidur, R. Hydrogen fuel and transport system: A sustainable and environmental future. Int. J. Hydrogen Energy 2016, 41, 1369–1380. [Google Scholar] [CrossRef]
- Manoharan, Y.; Hosseini, S.E.; Butler, B.; Alzhahrani, H.; Senior, B.T.F.; Ashuri, T.; Krohn, J. Hydrogen fuel cell vehicles; current status and future prospect. Appl. Sci. 2019, 9, 2296. [Google Scholar] [CrossRef]
- Gamal, A.; Eid, K.; Abdullah, A.M. Engineering of Pt-based nanostructures for efficient dry (CO2) reforming: Strategy and mechanism for rich-hydrogen production. Int. J. Hydrogen Energy 2021, 47, 5901–5928. [Google Scholar] [CrossRef]
- Gamal, A.; Eid, K.; El-Naas, M.H.; Kumar, D.; Kumar, A. Catalytic methane decomposition to carbon nanostructures and COx-free hydrogen: A mini-review. Nanomaterials 2021, 11, 1226. [Google Scholar] [CrossRef]
- Tarhan, C.; Çil, M.A. A study on hydrogen, the clean energy of the future: Hydrogen storage methods. J. Energy Storage 2021, 40, 102676. [Google Scholar] [CrossRef]
- Olabi, A.; Abdelghafar, A.A.; Baroutaji, A.; Sayed, E.T.; Alami, A.H.; Rezk, H.; Abdelkareem, M.A. Large-vscale hydrogen production and storage technologies: Current status and future directions. Int. J. Hydrogen Energy 2021, 46, 23498–23528. [Google Scholar] [CrossRef]
- Dawood, F.; Anda, M.; Shafiullah, G. Hydrogen production for energy: An overview. Int. J. Hydrogen Energy 2020, 45, 3847–3869. [Google Scholar]
- Acar, C.; Dincer, I. Review and evaluation of hydrogen production options for better environment. J. Clean. Prod. 2019, 218, 835–849. [Google Scholar] [CrossRef]
- Zhao, W.; Chen, Z.; Yang, X.; Qian, X.; Liu, C.; Zhou, D.; Sun, T.; Zhang, M.; Wei, G.; Dissanayake, P.D.; et al. Recent advances in photocatalytic hydrogen evolution with high-performance catalysts without precious metals. Renew. Sustain. Energy Rev. 2020, 132, 110040. [Google Scholar] [CrossRef]
- Zhu, J.; Hu, L.; Zhao, P.; Lee, L.Y.S.; Wong, K.-Y. Recent Advances in Electrocatalytic Hydrogen Evolution Using Nanoparticles. Chem. Rev. 2020, 120, 851–918. [Google Scholar] [CrossRef] [PubMed]
- Wan, D.; Yan, B.; Chen, J.; Wu, S.; Hong, J.; Song, D.; Zhao, X.; Chi, X.; Zeng, S.; Huang, Z.; et al. New Family of Plasmonic Photocatalysts without Noble Metals. Chem. Mater. 2019, 31, 2320–2327. [Google Scholar] [CrossRef]
- Wu, H.; Feng, C.; Zhang, L.; Zhang, J.; Wilkinson, D.P. Non-noble Metal Electrocatalysts for the Hydrogen Evolution Reaction in Water Electrolysis. Electrochem. Energy Rev. 2021, 4, 473–507. [Google Scholar] [CrossRef]
- Guo, J.; Zhou, J.; Sun, Z.; Wang, M.; Zou, X.; Mao, H.; Yan, F. Enhanced photocatalytic and antibacterial activity of acridinium-grafted g-C3N4 with broad-spectrum light absorption for antimicrobial photocatalytic therapy. Acta Biomater. 2022, 146, 370–384. [Google Scholar] [CrossRef]
- Shi, L.; Liang, L.; Wang, F.; Ma, J.; Sun, J. Polycondensation of guanidine hydrochloride into a graphitic carbon nitride semiconductor with a large surface area as a visible light photocatalyst. Catal. Sci. Technol. 2014, 4, 3235–3243. [Google Scholar] [CrossRef]
- Lu, Q.; Eid, K.; Li, W.; Abdullah, A.M.; Xu, G.; Varma, R.S. Engineering graphitic carbon nitride (gC3N4) for catalytic reduction of CO2 to fuels and chemicals: Strategy and mechanism. Green Chem. 2021, 23, 5394–5428. [Google Scholar] [CrossRef]
- Zhao, G.; Huang, X.; Wang, X.; Wang, X. Progress in catalyst exploration for heterogeneous CO2reduction and utilization: A critical review. J. Mater. Chem. A 2017, 5, 21625–21649. [Google Scholar] [CrossRef]
- Wen, J.; Xie, J.; Chen, X.; Li, X. A review on g-C3N4-based photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Ong, W.J.; Tan, L.L.; Ng, Y.H.; Yong, S.T.; Chai, S.P. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer To Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, J.; Jiang, L. Photocatalytic hydrogen evolution based on carbon nitride and organic semiconductors. Nanotechnology 2022, 33, 322001. [Google Scholar] [CrossRef] [PubMed]
- Dolai, S.; Bhunia, S.K.; Kluson, P.; Stavarek, P.; Pittermannova, A. Solvent-Assisted Synthesis of Supramolecular-Assembled Graphitic Carbon Nitride for Visible Light Induced Hydrogen Evolution–A Review. ChemCatChem 2022, 14, e202101299. [Google Scholar] [CrossRef]
- Prasad, C.; Tang, H.; Liu, Q.; Bahadur, I.; Karlapudi, S.; Jiang, Y. A latest overview on photocatalytic application of g-C3N4 based nanostructured materials for hydrogen production. Int. J. Hydrogen Energy 2020, 45, 337–379. [Google Scholar] [CrossRef]
- Rhimi, B.; Wang, C.; Bahnemann, D.W. Latest progress in g-C3N4 based heterojunctions for hydrogen production via photocatalytic water splitting: A mini review. J. Phys. Energy 2020, 2, 042003. [Google Scholar] [CrossRef]
- Wang, T.; Tian, B.; Han, B.; Ma, D.; Sun, M.; Hanif, A.; Xia, D.; Shang, J. Recent advances on porous materials for synergetic adsorption and photocatalysis. Energy Environ. Mater. 2021, 5, 711–730. [Google Scholar] [CrossRef]
- Su, K.; Deng, S.; Li, L.; Qin, Q.; Yang, J.; Chen, Y.; Zhang, S.; Chen, J. gC3N4 Derived Materials for Photocatalytic Hydrogen Production: A Mini Review on Design Strategies. J. Renew. Mater. 2022, 10, 653. [Google Scholar] [CrossRef]
- Phoon, B.L.; Ong, C.C.; Lee, K.-C.; Pan, G.-T.; Leo, B.F.; Chong, S.; Pan, K.-L. Co-Doped, Tri-Doped, and Rare-Earth-Doped g-C3N4 for Photocatalytic Applications: State-of-the-Art. Catalysts 2022, 12, 586. [Google Scholar] [CrossRef]
- Jin, H.; Liu, X.; Chen, S.; Vasileff, A.; Li, L.; Jiao, Y.; Song, L.; Zheng, Y.; Qiao, S.-Z. Heteroatom-doped transition metal electrocatalysts for hydrogen evolution reaction. ACS Energy Lett. 2019, 4, 805–810. [Google Scholar] [CrossRef]
- Shcherban, N. Preparation, physicochemical properties, and functional characteristics of carbon nitride: A review. Theor. Exp. Chem. 2016, 52, 265–284. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Ma, T.; Zhang, Y.; Huang, H. 2D graphitic carbon nitride for energy conversion and storage. Adv. Funct. Mater. 2021, 31, 2102540. [Google Scholar] [CrossRef]
- Hou, H.; Shao, G.; Yang, W. Recent advances in gC3N4-based photocatalysts incorporated by MXenes and their derivatives. J. Mater. Chem. A 2021, 9, 13722–13745. [Google Scholar] [CrossRef]
- Abe, J.O.; Popoola, A.; Ajenifuja, E.; Popoola, O. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrogen Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Zohuri, B. Hydrogen Energy: Challenges and Solutions for a Cleaner Future; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Rivard, E.; Trudeau, M.; Zaghib, K. Hydrogen storage for mobility: A review. Materials 2019, 12, 1973. [Google Scholar] [CrossRef]
- Kaur, M.; Pal, K. Review on hydrogen storage materials and methods from an electrochemical viewpoint. J. Energy Storage 2019, 23, 234–249. [Google Scholar] [CrossRef]
- Cousins, K.; Zhang, R. Highly porous organic polymers for hydrogen fuel storage. Polymers 2019, 11, 690. [Google Scholar] [CrossRef]
- Zhao, D.; Weijie, Y.; Kaifu, H.; Leon, S. Thermodynamics and kinetics tuning of LiBH4 for hydrogen storage. Prog. Chem. 2021, 33, 1586. [Google Scholar]
- Ding, Z.; Li, S.; Zhou, Y.; Chen, Z.; Yang, W.; Ma, W.; Shaw, L. LiBH4 for hydrogen storage-new perspectives. Nano Mater. Sci. 2020, 2, 109–119. [Google Scholar] [CrossRef]
- Ding, Z.; Shaw, L. Enhancement of hydrogen desorption from nanocomposite prepared by ball milling MgH2 with in situ aerosol spraying LiBH4. ACS Sustain. Chem. Eng. 2019, 7, 15064–15072. [Google Scholar] [CrossRef]
- Ding, Z.; Lu, Y.; Li, L.; Shaw, L. High reversible capacity hydrogen storage through Nano-LiBH4+ Nano-MgH2 system. Energy Storage Mater. 2019, 20, 24–35. [Google Scholar] [CrossRef]
- Ding, Z.; Wu, P.; Shaw, L. Solid-state hydrogen desorption of 2 MgH2+ LiBH4 nano-mixture: A kinetics mechanism study. J. Alloys Compd. 2019, 806, 350–360. [Google Scholar] [CrossRef]
- Ding, Z.; Chen, Z.; Ma, T.; Lu, C.-T.; Ma, W.; Shaw, L. Predicting the hydrogen release ability of LiBH4-based mixtures by ensemble machine learning. Energy Storage Mater. 2020, 27, 466–477. [Google Scholar] [CrossRef]
- Ding, Z.; Zhao, X.; Shaw, L.L. Reaction between LiBH4 and MgH2 induced by high-energy ball milling. J. Power Sources 2015, 293, 236–245. [Google Scholar] [CrossRef]
- Ding, Z.; Li, H.; Shaw, L. New insights into the solid-state hydrogen storage of nanostructured LiBH4-MgH2 system. Chem. Eng. J. 2020, 385, 123856. [Google Scholar] [CrossRef]
- Chen, Z.; Keene, S.; Gaieck, W.; Phun, G.S.; Stinson, R.; Stinson, W.D.; Wang, Y.; Barrera, L.; Chen, Z.; Mayer, M. Optimization of Z-Scheme Photocatalytic Reactors for Solar Water Splitting. In ECS Meeting Abstracts; IOP Publishing: Bristol, UK, 2022; p. 1553. [Google Scholar]
- Pivovar, B.S.; Ruth, M.F.; Myers, D.J.; Dinh, H.N. Hydrogen: Targeting $1/kg in 1 Decade. Electrochem. Soc. Interface 2021, 30, 61. [Google Scholar] [CrossRef]
- Scheffe, J.R.; Haussener, S.; Patzke, G.R. Solar Hydrogen Production. Energy Technol. 2022, 10, 2101021. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, X.; Pan, Y.; Liang, J.; Zeng, G.; Wu, Z.; Wang, H. Doping of graphitic carbon nitride for photocatalysis: A reveiw. Appl. Catal. B Environ. 2017, 217, 388–406. [Google Scholar] [CrossRef]
- Xiao, N.; Li, S.; Li, X.; Ge, L.; Gao, Y.; Li, N. The roles and mechanism of cocatalysts in photocatalytic water splitting to produce hydrogen. Chin. J. Catal. 2020, 41, 642–671. [Google Scholar] [CrossRef]
- McCrory, C.C.; Jung, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking heterogeneous electrocatalysts for the oxygen evolution reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, M.; Xu, W.; Wu, X.; Jiang, J. Catalytically active carbon from cattail fibers for electrochemical reduction reaction. Front. Chem. 2019, 7, 786. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Xiao, P.; Li, H.; Carabineiro, S.A. Graphitic carbon nitride: Synthesis, properties, and applications in catalysis. ACS Appl. Mater. Interfaces 2014, 6, 16449–16465. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Li, Z.; Huang, Z.H.; Kang, F.; Yang, Q.H. Holey graphitic carbon nitride nanosheets with carbon vacancies for highly improved photocatalytic hydrogen production. Adv. Funct. Mater. 2015, 25, 6885–6892. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Antonietti, M. Polymeric Graphitic Carbon Nitride as a Heterogeneous Organocatalyst: From Photochemistry to Multipurpose Catalysis to Sustainable Chemistry. Angew. Chem. Int. Ed. 2012, 51, 68–89. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhou, Y.; Li, Z.; Yan, S.; Wang, N.; Zou, Z. High-yield synthesis of millimetre-long, semiconducting carbon nitride nanotubes with intense photoluminescence emission and reproducible photoconductivity. Nanoscale 2012, 4, 3687–3692. [Google Scholar] [CrossRef]
- Ding, F.; Yang, D.; Tong, Z.; Nan, Y.; Wang, Y.; Zou, X.; Jiang, Z. Graphitic carbon nitride-based nanocomposites as visible-light driven photocatalysts for environmental purification. Environ. Sci. Nano 2017, 4, 1455–1469. [Google Scholar] [CrossRef]
- Lai, J.; Li, S.; Wu, F.; Saqib, M.; Luque, R.; Xu, G. Unprecedented metal-free 3D porous carbonaceous electrodes for full water splitting. Energy Environ. Sci. 2016, 9, 1210–1214. [Google Scholar] [CrossRef]
- Zhao, J.; Gilani, M.R.H.S.; Liu, Z.; Luque, R.; Xu, G. Facile surfactant-free synthesis of polybenzoxazine-based polymer and nitrogen-doped carbon nanospheres. Polym. Chem. 2018, 9, 4324–4331. [Google Scholar] [CrossRef]
- Wei, D.; Liu, Y.; Wang, Y.; Zhang, H.; Huang, L.; Yu, G. Synthesis of N-doped graphene by chemical vapor deposition and its electrical properties. Nano Lett. 2009, 9, 1752–1758. [Google Scholar] [CrossRef]
- Li, X.; Fan, L.; Li, Z.; Wang, K.; Zhong, M.; Wei, J.; Wu, D.; Zhu, H. Boron doping of graphene for graphene–silicon p–n junction solar cells. Adv. Energy Mater. 2012, 2, 425–429. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, Z.; Xu, J.; Liu, Q.; Xu, H.; Tang, H.; Li, G.; Jiang, Y.; Qu, F.; Lin, Z. Probing supramolecular assembly and charge carrier dynamics toward enhanced photocatalytic hydrogen evolution in 2D graphitic carbon nitride nanosheets. Appl. Catal. B Environ. 2019, 256, 117867. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Liu, J.; Han, B.; Hu, X.; Yang, F.; Xu, Z.; Li, Y.; Jia, S.; Li, Z. Carbon quantum dot implanted graphite carbon nitride nanotubes: Excellent charge separation and enhanced photocatalytic hydrogen evolution. Angew. Chem. 2018, 130, 5867–5873. [Google Scholar] [CrossRef]
- Huo, T.; Ba, G.; Deng, Q.; Yu, F.; Wang, G.; Li, H.; Hou, W. A dual strategy for synthesizing carbon/defect comodified polymeric carbon nitride porous nanotubes with boosted photocatalytic hydrogen evolution and synchronous contaminant degradation. Appl. Catal. B Environ. 2021, 287, 119995. [Google Scholar] [CrossRef]
- Zhu, Y.-P.; Ren, T.-Z.; Yuan, Z.-Y. Mesoporous phosphorus-doped g-C3N4 nanostructured flowers with superior photocatalytic hydrogen evolution performance. ACS Appl. Mater. Interfaces 2015, 7, 16850–16856. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, H.; Sun, H.; Liu, S.; Tade, M.O.; Wang, S.; Jin, W. Recent advances in non-metal modification of graphitic carbon nitride for photocatalysis: A historic review. Catal. Sci. Technol. 2016, 6, 7002–7023. [Google Scholar] [CrossRef]
- Mun, S.J.; Park, S.-J. Graphitic carbon nitride materials for photocatalytic hydrogen production via water splitting: A short review. Catalysts 2019, 9, 805. [Google Scholar] [CrossRef]
- Naseri, A.; Samadi, M.; Pourjavadi, A.; Moshfegh, A.Z.; Ramakrishna, S. Graphitic carbon nitride (gC3N4)-based photocatalysts for solar hydrogen generation: Recent advances and future development directions. J. Mater. Chem. A 2017, 5, 23406–23433. [Google Scholar] [CrossRef]
- Jiang, H.; Li, Y.; Wang, D.; Hong, X.; Liang, B. Recent advances in heteroatom doped graphitic carbon nitride (g-C3N4) and g-C3N4/metal oxide composite photocatalysts. Curr. Org. Chem. 2020, 24, 673–693. [Google Scholar] [CrossRef]
- Ye, L.; Wang, D.; Chen, S. Fabrication and enhanced photoelectrochemical performance of MoS2/S-doped g-C3N4 heterojunction film. ACS Appl. Mater. Interfaces 2016, 8, 5280–5289. [Google Scholar] [CrossRef]
- Wang, J.; Han, W.Q. A review of heteroatom doped materials for advanced lithium–sulfur batteries. Adv. Funct. Mater. 2022, 32, 2107166. [Google Scholar] [CrossRef]
- Zhang, H.; Tian, W.; Duan, X.; Sun, H.; Shen, Y.; Shao, G.; Wang, S. Functional carbon nitride materials for water oxidation: From heteroatom doping to interface engineering. Nanoscale 2020, 12, 6937–6952. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; He, W.; Ye, J.; Gao, X.; Wang, D.; Song, J. Polymeric Carbon Nitride-Derived Photocatalysts for Water Splitting and Nitrogen Fixation. Small 2021, 17, 2005149. [Google Scholar] [CrossRef]
- Belgacem, A.B.; Hinkov, I.; Yahia, S.B.; Brinza, O.; Farhat, S. Arc discharge boron nitrogen doping of carbon nanotubes. Mater. Today Commun. 2016, 8, 183–195. [Google Scholar] [CrossRef]
- Ma, L.; Hu, S.; Li, P.; Wang, Q.; Ma, H.; Li, W. In situ synthesis of sulfur doped carbon nitride with enhanced photocatalytic performance using DBD plasma treatment under H2S atmosphere. J. Phys. Chem. Solids 2018, 118, 166–171. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Y.; Wang, X. Two-dimensional covalent carbon nitride nanosheets: Synthesis, functionalization, and applications. Energy Environ. Sci. 2015, 8, 3092–3108. [Google Scholar] [CrossRef]
- Han, Q.; Hu, C.; Zhao, F.; Zhang, Z.; Chen, N.; Qu, L. One-step preparation of iodine-doped graphitic carbon nitride nanosheets as efficient photocatalysts for visible light water splitting. J. Mater. Chem. A 2015, 3, 4612–4619. [Google Scholar] [CrossRef]
- Ran, J.; Ma, T.Y.; Gao, G.; Du, X.-W.; Qiao, S.Z. Porous P-doped graphitic carbon nitride nanosheets for synergistically enhanced visible-light photocatalytic H2 production. Energy Environ. Sci. 2015, 8, 3708–3717. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, L.; Liu, J.; Fan, X.; Wang, B.; Wang, M.; Ren, W.; Wang, J.; Li, M.; Shi, J. Brand new P-doped gC3N4: Enhanced photocatalytic activity for H2 evolution and Rhodamine B degradation under visible light. J. Mater. Chem. A 2015, 3, 3862–3867. [Google Scholar] [CrossRef]
- Guo, S.; Deng, Z.; Li, M.; Jiang, B.; Tian, C.; Pan, Q.; Fu, H. Phosphorus-doped carbon nitride tubes with a layered micro-nanostructure for enhanced visible-light photocatalytic hydrogen evolution. Angew. Chem. 2016, 128, 1862–1866. [Google Scholar] [CrossRef]
- Guo, S.; Tang, Y.; Xie, Y.; Tian, C.; Feng, Q.; Zhou, W.; Jiang, B. P-doped tubular g-C3N4 with surface carbon defects: Universal synthesis and enhanced visible-light photocatalytic hydrogen production. Appl. Catal. B Environ. 2017, 218, 664–671. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; Shi, R.; Bao, S.; Wang, J.; Amini, A.; Chandrashekar, B.N.; Cheng, C. Phosphorous doped graphitic-C3N4 hierarchical architecture for hydrogen production from water under visible light. Mater. Today Energy 2017, 5, 91–98. [Google Scholar] [CrossRef]
- Cao, S.; Huang, Q.; Zhu, B.; Yu, J. Trace-level phosphorus and sodium co-doping of g-C3N4 for enhanced photocatalytic H2 production. J. Power Sources 2017, 351, 151–159. [Google Scholar] [CrossRef]
- Fang, H.-B.; Zhang, X.-H.; Wu, J.; Li, N.; Zheng, Y.-Z.; Tao, X. Fragmented phosphorus-doped graphitic carbon nitride nanoflakes with broad sub-bandgap absorption for highly efficient visible-light photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2018, 225, 397–405. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, J.; He, B.-b.; Wang, H.-w.; Wang, R.; Gong, Y.-s. In-situ construction of coral-like porous P-doped g-C3N4 tubes with hybrid 1D/2D architecture and high efficient photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2019, 241, 159–166. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, Y.; Wang, Y.; Hu, S.; Wang, B.; Liao, Q.; Li, H.; Bao, J.; Ge, G.; Jia, S. Three-dimensional flower-like phosphorus-doped gC3N4 with a high surface area for visible-light photocatalytic hydrogen evolution. J. Mater. Chem. A 2018, 6, 16485–16494. [Google Scholar] [CrossRef]
- Sun, Y.-j.; He, J.-y.; Zhang, D.; Wang, X.-j.; Zhao, J.; Liu, R.-h.; Li, F.-t. Simultaneous construction of dual-site phosphorus modified g-C3N4 and its synergistic mechanism for enhanced visible-light photocatalytic hydrogen evolution. Appl. Surf. Sci. 2020, 517, 146192. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, J.; Cao, J.; Lv, C.; Huang, G.; Zhang, G.; Xu, Y.; Zhang, S.; Meng, P.; Zhan, T. Phosphorus-doped polymeric carbon nitride nanosheets for enhanced photocatalytic hydrogen production. APL Mater. 2020, 8, 041108. [Google Scholar] [CrossRef]
- Yu, D.; Jia, T.; Deng, Z.; Wei, Q.; Wang, K.; Chen, L.; Wang, P.; Cui, J. One-Dimensional P-Doped Graphitic Carbon Nitride Tube: Facile Synthesis, Effect of Doping Concentration, and Enhanced Mechanism for Photocatalytic Hydrogen Evolution. Nanomaterials 2022, 12, 1759. [Google Scholar] [CrossRef]
- Li, B.; Si, Y.; Fang, Q.; Shi, Y.; Huang, W.-Q.; Hu, W.; Pan, A.; Fan, X.; Huang, G.-F. Hierarchical self-assembly of well-defined Louver-like P-doped carbon nitride nanowire arrays with highly efficient hydrogen evolution. Nano-Micro Lett. 2020, 12, 1–16. [Google Scholar] [CrossRef]
- Zhang, F.; Li, J.; Wang, H.; Li, Y.; Liu, Y.; Qian, Q.; Jin, X.; Wang, X.; Zhang, J.; Zhang, G. Realizing synergistic effect of electronic modulation and nanostructure engineering over graphitic carbon nitride for highly efficient visible-light H2 production coupled with benzyl alcohol oxidation. Appl. Catal. B Environ. 2020, 269, 118772. [Google Scholar] [CrossRef]
- Zhan, X.; Zhao, Y.; Zhou, G.; Yu, J.; Wang, H.; Shi, H. Oxygen-containing groups and P doped porous carbon nitride nanosheets towards enhanced photocatalytic activity. Chemosphere 2022, 287, 132399. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhang, H.; Liu, H.; Zheng, X.; Zou, W.; Dong, L.; Qi, L. Enhanced activity of visible-light photocatalytic H2 evolution of sulfur-doped g-C3N4 photocatalyst via nanoparticle metal Ni as cocatalyst. Appl. Catal. B Environ. 2018, 235, 66–74. [Google Scholar] [CrossRef]
- Wang, H.; Bian, Y.; Hu, J.; Dai, L. Highly crystalline sulfur-doped carbon nitride as photocatalyst for efficient visible-light hydrogen generation. Appl. Catal. B Environ. 2018, 238, 592–598. [Google Scholar] [CrossRef]
- Zhou, Y.; Lv, W.; Zhu, B.; Tong, F.; Pan, J.; Bai, J.; Zhou, Q.; Qin, H. Template-free one-step synthesis of g-C3N4 nanosheets with simultaneous porous network and S-doping for remarkable visible-light-driven hydrogen evolution. ACS Sustain. Chem. Eng. 2019, 7, 5801–5807. [Google Scholar] [CrossRef]
- Bi, J.; Zhu, L.; Wu, J.; Xu, Y.; Wang, Z.; Zhang, X.; Han, Y. Optimizing electronic structure and charge transport of sulfur/potassium co-doped graphitic carbon nitride with efficient photocatalytic hydrogen evolution performance. Appl. Organomet. Chem. 2019, 33, e5163. [Google Scholar] [CrossRef]
- Lv, H.; Huang, Y.; Koodali, R.T.; Liu, G.; Zeng, Y.; Meng, Q.; Yuan, M. Synthesis of sulfur-doped 2D graphitic carbon nitride nanosheets for efficient photocatalytic degradation of phenol and hydrogen evolution. ACS Appl. Mater. Interfaces 2020, 12, 12656–12667. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, C.; Xue, Z.; Zhang, C.; Qin, J.; Liu, R. Spatial separation of charge carriers via heterogeneous structural defects in graphitic carbon nitride for photocatalytic hydrogen evolution. ACS Appl. Nano Mater. 2020, 3, 4428–4436. [Google Scholar] [CrossRef]
- Luo, L.; Gong, Z.; Ma, J.; Wang, K.; Zhu, H.; Li, K.; Xiong, L.; Guo, X.; Tang, J. Ultrathin sulfur-doped holey carbon nitride nanosheets with superior photocatalytic hydrogen production from water. Appl. Catal. B Environ. 2021, 284, 119742. [Google Scholar] [CrossRef]
- Bai, J.; Zhou, P.; Xu, P.; Deng, Y.; Zhou, Q. Synergy of dopants and porous structures in graphitic carbon nitride for efficient photocatalytic H2 evolution. Ceram. Int. 2021, 47, 4043–4048. [Google Scholar] [CrossRef]
- Fei, T.; Qin, C.; Zhang, Y.; Dong, G.; Wang, Y.; Zhou, Y.; Cui, M. A 3D peony-like sulfur-doped carbon nitride synthesized by self-assembly for efficient photocatalytic hydrogen production. Int. J. Hydrogen Energy 2021, 46, 20481–20491. [Google Scholar] [CrossRef]
- Hong, J.; Xia, X.; Wang, Y.; Xu, R. Mesoporous carbon nitride with in situ sulfur doping for enhanced photocatalytic hydrogen evolution from water under visible light. J. Mater. Chem. 2012, 22, 15006–15012. [Google Scholar] [CrossRef]
- Feng, L.-L.; Zou, Y.; Li, C.; Gao, S.; Zhou, L.-J.; Sun, Q.; Fan, M.; Wang, H.; Wang, D.; Li, G.-D. Nanoporous sulfur-doped graphitic carbon nitride microrods: A durable catalyst for visible-light-driven H2 evolution. Int. J. Hydrogen Energy 2014, 39, 15373–15379. [Google Scholar] [CrossRef]
- Gu, Q.; Liu, J.; Gao, Z.; Xue, C. Homogenous boron-doping in self-sensitized carbon nitride for enhanced visible-light photocatalytic activity. Chem.—Asian J. 2016, 11, 3169–3173. [Google Scholar] [CrossRef]
- Xing, W.; Chen, G.; Li, C.; Han, Z.; Hu, Y.; Meng, Q. Doping effect of non-metal group in porous ultrathin gC3N4 nanosheets towards synergistically improved photocatalytic hydrogen evolution. Nanoscale 2018, 10, 5239–5245. [Google Scholar] [CrossRef]
- Bao, H.; Wang, L.; Li, G.; Zhou, L.; Xu, Y.; Liu, Z.; Wu, M. Carrier engineering of carbon nitride boosts visible-light photocatalytic hydrogen evolution. Carbon 2021, 179, 80–88. [Google Scholar] [CrossRef]
- Mahvelati-Shamsabadi, T.; Fattahimoghaddam, H.; Lee, B.-K.; Ryu, H.; Jang, J. Caesium sites coordinated in Boron-doped porous and wrinkled graphitic carbon nitride nanosheets for efficient charge carrier separation and Transfer: Photocatalytic H2 and H2O2 production. Chem. Eng. J. 2021, 423, 130067. [Google Scholar] [CrossRef]
- Qi, K.; Cui, N.; Zhang, M.; Ma, Y.; Wang, G.; Zhao, Z.; Khataee, A. Ionic liquid-assisted synthesis of porous boron-doped graphitic carbon nitride for photocatalytic hydrogen production. Chemosphere 2021, 272, 129953. [Google Scholar] [CrossRef] [PubMed]
- Chi, X.; Liu, F.; Gao, Y.; Song, J.; Guan, R.; Yuan, H. An efficient B/Na co-doped porous g-C3N4 nanosheets photocatalyst with enhanced photocatalytic hydrogen evolution and degradation of tetracycline under visible light. Appl. Surf. Sci. 2022, 576, 151837. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y.; Cheng, S.; Zhao, X.; Zhang, J.; Ao, Z.; Zhao, C.; Li, B.; Wang, S.; Wang, S. Nitrogen defects/boron dopants engineered tubular carbon nitride for efficient tetracycline hydrochloride photodegradation and hydrogen evolution. Appl. Catal. B Environ. 2022, 303, 120932. [Google Scholar] [CrossRef]
- Li, J.; Shen, B.; Hong, Z.; Lin, B.; Gao, B.; Chen, Y. A facile approach to synthesize novel oxygen-doped g-C3N4 with superior visible-light photoreactivity. Chem. Commun. 2012, 48, 12017–12019. [Google Scholar] [CrossRef]
- Huang, Z.-F.; Song, J.; Pan, L.; Wang, Z.; Zhang, X.; Zou, J.-J.; Mi, W.; Zhang, X.; Wang, L. Carbon nitride with simultaneous porous network and O-doping for efficient solar-energy-driven hydrogen evolution. Nano Energy 2015, 12, 646–656. [Google Scholar] [CrossRef]
- Guo, S.; Zhu, Y.; Yan, Y.; Min, Y.; Fan, J.; Xu, Q. Holey structured graphitic carbon nitride thin sheets with edge oxygen doping via photo-Fenton reaction with enhanced photocatalytic activity. Appl. Catal. B Environ. 2016, 185, 315–321. [Google Scholar] [CrossRef]
- Wang, C.; Fan, H.; Ren, X.; Ma, J.; Fang, J.; Wang, W. Hydrothermally induced O-doping and porous structure of graphitic carbon nitride with highly ordered architecture and dramatically enhanced photocatalytic property. ChemSusChem 2018, 11, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-W.; Gong, S.; Mahmood, N.; Pan, L.; Zhang, X.; Zou, J.-J. Oxygen-doped nanoporous carbon nitride via water-based homogeneous supramolecular assembly for photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2018, 221, 9–16. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun, Z.; Tang, C.; Zhou, Y.; Zeng, L.; Huang, L. Enhancement of photocatalytic hydrogen evolution activity of porous oxygen doped g-C3N4 with nitrogen defects induced by changing electron transition. Appl. Catal. B Environ. 2019, 240, 30–38. [Google Scholar] [CrossRef]
- Song, P.; Liang, S.; Cui, J.; Ren, D.; Duan, R.; Yang, Q.; Sun, S. Purposefully designing novel hydroxylated and carbonylated melamine towards the synthesis of targeted porous oxygen-doped gC3N4 nanosheets for highly enhanced photocatalytic hydrogen production. Catal. Sci. Technol. 2019, 9, 5150–5159. [Google Scholar] [CrossRef]
- Huang, J.; Wang, H.; Yu, H.; Zhang, Q.; Cao, Y.; Peng, F. Oxygen doping in graphitic carbon nitride for enhanced photocatalytic hydrogen evolution. ChemSusChem 2020, 13, 5041–5049. [Google Scholar] [CrossRef]
- Wei, H.; Zhang, Y.; Zhang, G.; Cui, J.; Wang, Y.; Qin, Y.; Zhang, X.; Tan, H.H.; Liu, J.; Wu, Y. In situ W/O Co-doped hollow carbon nitride tubular structures with enhanced visible-light-driven photocatalytic performance for hydrogen evolution. Int. J. Hydrogen Energy 2021, 46, 234–246. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Y.; Tan, H.; Sun, H.; Shang, Q.; Zhao, X.; Qiu, T.; Li, Y. Foamer-derived bulk nitrogen defects and oxygen-doped porous carbon nitride with greatly extended visible-light response and efficient photocatalytic activity. ACS Appl. Mater. Interfaces 2021, 13, 23866–23876. [Google Scholar] [CrossRef]
- Wu, Y.; Xiong, P.; Wu, J.; Huang, Z.; Sun, J.; Liu, Q.; Cheng, X.; Yang, J.; Zhu, J.; Zhou, Y. Band engineering and morphology control of oxygen-incorporated graphitic carbon nitride porous nanosheets for highly efficient photocatalytic hydrogen evolution. Nano-Micro Lett. 2021, 13, 48. [Google Scholar] [CrossRef]
- Wen, J.; Zhang, S.; Liu, Y.; Zhai, Y. Formic acid assisted Fabrication of Oxygen-doped Rod-like Carbon Nitride with Improved Photocatalytic Hydrogen Evolution. J. Colloid Interface Sci. 2022, 624, 338–347. [Google Scholar] [CrossRef]
- Song, T.; Hou, L.; Long, B.; Ali, A.; Deng, G.-J. Constructing ultralong hollow chain-ball-like carbon nitride implanted with oxygen for superior visible-light photocatalytic hydrogen production. J. Alloys Compd. 2021, 857, 157609. [Google Scholar] [CrossRef]
- Li, J.; Wu, D.; Iocozzia, J.; Du, H.; Liu, X.; Yuan, Y.; Zhou, W.; Li, Z.; Xue, Z.; Lin, Z. Achieving efficient incorporation of Π-electrons into graphitic carbon nitride for markedly improved hydrogen generation. Angew. Chem. 2019, 131, 2007–2011. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Fan, T.-T.; Yu, X.; Wu, Q.-L.; Zhu, Q.-H.; Zhang, L.-Z.; Li, J.-H.; Fang, W.-P.; Yi, X.-D. Gradual carbon doping of graphitic carbon nitride towards metal-free visible light photocatalytic hydrogen evolution. J. Mater. Chem. A 2018, 6, 15310–15319. [Google Scholar] [CrossRef]
- Jiang, Y.; Lin, Z.; Zhang, Y.; Lai, Y.; Liang, D.; Yang, C. Facile synthesis of porous C-doped C3N4: Fast charge separation and enhanced photocatalytic hydrogen evolution. New J. Chem. 2020, 44, 17891–17898. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, L.; Liu, J.; Zhou, J.; Jiao, Y.; Xiao, X.; Zhao, C.; Zhou, Y.; Ye, S.; Jiang, B. Porous Carbon Nitride Thin Strip: Precise Carbon Doping Regulating Delocalized π-Electron Induces Elevated Photocatalytic Hydrogen Evolution. Small 2021, 17, 2006622. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, S.; Zhang, C.; Fang, J.; Xie, L.; Zhou, Y.; Zhuo, S. Hollow tubular carbon doping graphitic carbon nitride with adjustable structure for highly enhanced photocatalytic hydrogen production. Carbon 2021, 182, 287–296. [Google Scholar] [CrossRef]
- Fang, J.; Fan, H.; Li, M.; Long, C. Nitrogen self-doped graphitic carbon nitride as efficient visible light photocatalyst for hydrogen evolution. J. Mater. Chem. A 2015, 3, 13819–13826. [Google Scholar] [CrossRef]
- Xu, F.; Mo, Z.; Yan, J.; Fu, J.; Song, Y.; El-Alami, W.; Wu, X.; Li, H.; Xu, H. Nitrogen-rich graphitic carbon nitride nanotubes for photocatalytic hydrogen evolution with simultaneous contaminant degradation. J. Colloid Interface Sci. 2020, 560, 555–564. [Google Scholar] [CrossRef]
- Guo, F.; Wang, L.; Sun, H.; Li, M.; Shi, W. High-efficiency photocatalytic water splitting by a N-doped porous gC3N4 nanosheet polymer photocatalyst derived from urea and N, N-dimethylformamide. Inorg. Chem. Front. 2020, 7, 1770–1779. [Google Scholar] [CrossRef]
- Liang, L.; Cong, Y.; Yao, L.; Wang, F.; Shi, L. One step to prepare Cl doped porous defect modified g-C3N4 with improved visible-light photocatalytic performance for H2 production and rhodamine B degradation. Mater. Res. Express 2018, 5, 115510. [Google Scholar] [CrossRef]
- Peng, X.; Li, J.; Liu, X.; Yi, L.; Cai, P.; Wen, Z. Cl-doped carbon nitride nanostrips for remarkably improving visible-light photocatalytic hydrogen production. Int. J. Hydrogen Energy 2021, 46, 28591–28601. [Google Scholar] [CrossRef]
- Gao, Y.; Hou, F.; Hu, S.; Wu, B.; Jiang, B. Synchronization iodine surface modification and lattice doping porous carbon nitride for photocatalytic hydrogen production. Appl. Surf. Sci. 2019, 481, 1089–1095. [Google Scholar] [CrossRef]
- Lan, Z.-A.; Zhang, G.; Wang, X. A facile synthesis of Br-modified g-C3N4 semiconductors for photoredox water splitting. Appl. Catal. B Environ. 2016, 192, 116–125. [Google Scholar] [CrossRef]
- Hu, S.; Ma, L.; You, J.; Li, F.; Fan, Z.; Lu, G.; Liu, D.; Gui, J. Enhanced visible light photocatalytic performance of g-C3N4 photocatalysts co-doped with iron and phosphorus. Appl. Surf. Sci. 2014, 311, 164–171. [Google Scholar] [CrossRef]
- Fan, Q.; Liu, J.; Yu, Y.; Zuo, S.; Li, B. A simple fabrication for sulfur doped graphitic carbon nitride porous rods with excellent photocatalytic activity degrading RhB dye. Appl. Surf. Sci. 2017, 391, 360–368. [Google Scholar] [CrossRef]
- Wang, H.; Yang, C.; Li, M.; Chen, F.; Cui, Y. Enhanced photocatalytic hydrogen production of restructured B/F codoped g-C3N4 via post-thermal treatment. Mater. Lett. 2018, 212, 319–322. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, H.; Yang, C.; Li, M.; Zhao, Y.; Chen, F. Post-activation of in situ BF codoped g-C3N4 for enhanced photocatalytic H2 evolution. Appl. Surf. Sci. 2018, 441, 621–630. [Google Scholar] [CrossRef]
- Du, J.; Li, S.; Du, Z.; Meng, S.; Li, B. Boron/oxygen-codoped graphitic carbon nitride nanomesh for efficient photocatalytic hydrogen evolution. Chem. Eng. J. 2021, 407, 127114. [Google Scholar] [CrossRef]
- Babu, P.; Mohanty, S.; Naik, B.; Parida, K. Synergistic effects of boron and sulfur Co-doping into graphitic carbon nitride framework for enhanced photocatalytic activity in visible light driven hydrogen generation. ACS Appl. Energy Mater. 2018, 1, 5936–5947. [Google Scholar] [CrossRef]
- Yang, C.; Teng, W.; Song, Y.; Cui, Y. CI codoped porous g-C3N4 for superior photocatalytic hydrogen evolution. Chin. J. Catal. 2018, 39, 1615–1624. [Google Scholar] [CrossRef]
- Wang, H.; Wang, B.; Bian, Y.; Dai, L. Enhancing photocatalytic activity of graphitic carbon nitride by codoping with P and C for efficient hydrogen generation. ACS Appl. Mater. Interfaces 2017, 9, 21730–21737. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Meng, X.; Li, L.; Sun, T. P, S Co-doped g-C3N4 isotype heterojunction composites for high-efficiency photocatalytic H2 evolution. J. Alloys Compd. 2020, 827, 154259. [Google Scholar] [CrossRef]
- Liu, K.; Ma, J.; Yang, X.; Liu, Z.; Li, X.; Zhang, J.; Cui, R.; Sun, R. Phosphorus/oxygen co-doping in hollow-tube-shaped carbon nitride for efficient simultaneous visible-light-driven water splitting and biorefinery. Chem. Eng. J. 2022, 437, 135232. [Google Scholar] [CrossRef]
- Jiang, Y.; Fang, S.; Cao, C.; Hong, E.; Zeng, L.; Yang, W.; Huang, L.; Yang, C. Enhanced light harvesting and charge separation of carbon and oxygen co-doped carbon nitride as excellent photocatalyst for hydrogen evolution reaction. J. Colloid Interface Sci. 2022, 612, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Bai, C.; Wang, Z.; She, P.; Sun, H.; Lu, G.; Liang, S.; Liu, Z. Phosphorus–Oxygen-Codoped Graphitic Carbon Nitride for Enhanced Hydrogen Evolution and Photocatalytic Degradation under Visible Light Irradiation. ACS Appl. Energy Mater. 2022, 5, 5774–5784. [Google Scholar] [CrossRef]
- Jiao, Y.; Liu, M.; Qin, J.; Li, Y.; Wang, J.; He, Z.; Li, Z. Sulfur/phosphorus doping-mediated morphology transformation of carbon nitride from rods to porous microtubes with superior photocatalytic activity. J. Colloid Interface Sci. 2022, 608, 1432–1440. [Google Scholar] [CrossRef]
- Li, J.; Qi, Y.; Mei, Y.; Ma, S.; Li, Q.; Xin, B.; Yao, T.; Wu, J. Construction of phosphorus-doped carbon nitride/phosphorus and sulfur co-doped carbon nitride isotype heterojunction and their enhanced photoactivity. J. Colloid Interface Sci. 2020, 566, 495–504. [Google Scholar] [CrossRef]
- Liu, Q.; Shen, J.; Yu, X.; Yang, X.; Liu, W.; Yang, J.; Tang, H.; Xu, H.; Li, H.; Li, Y. Unveiling the origin of boosted photocatalytic hydrogen evolution in simultaneously (S, P, O)-Codoped and exfoliated ultrathin g-C3N4 nanosheets. Appl. Catal. B Environ. 2019, 248, 84–94. [Google Scholar] [CrossRef]
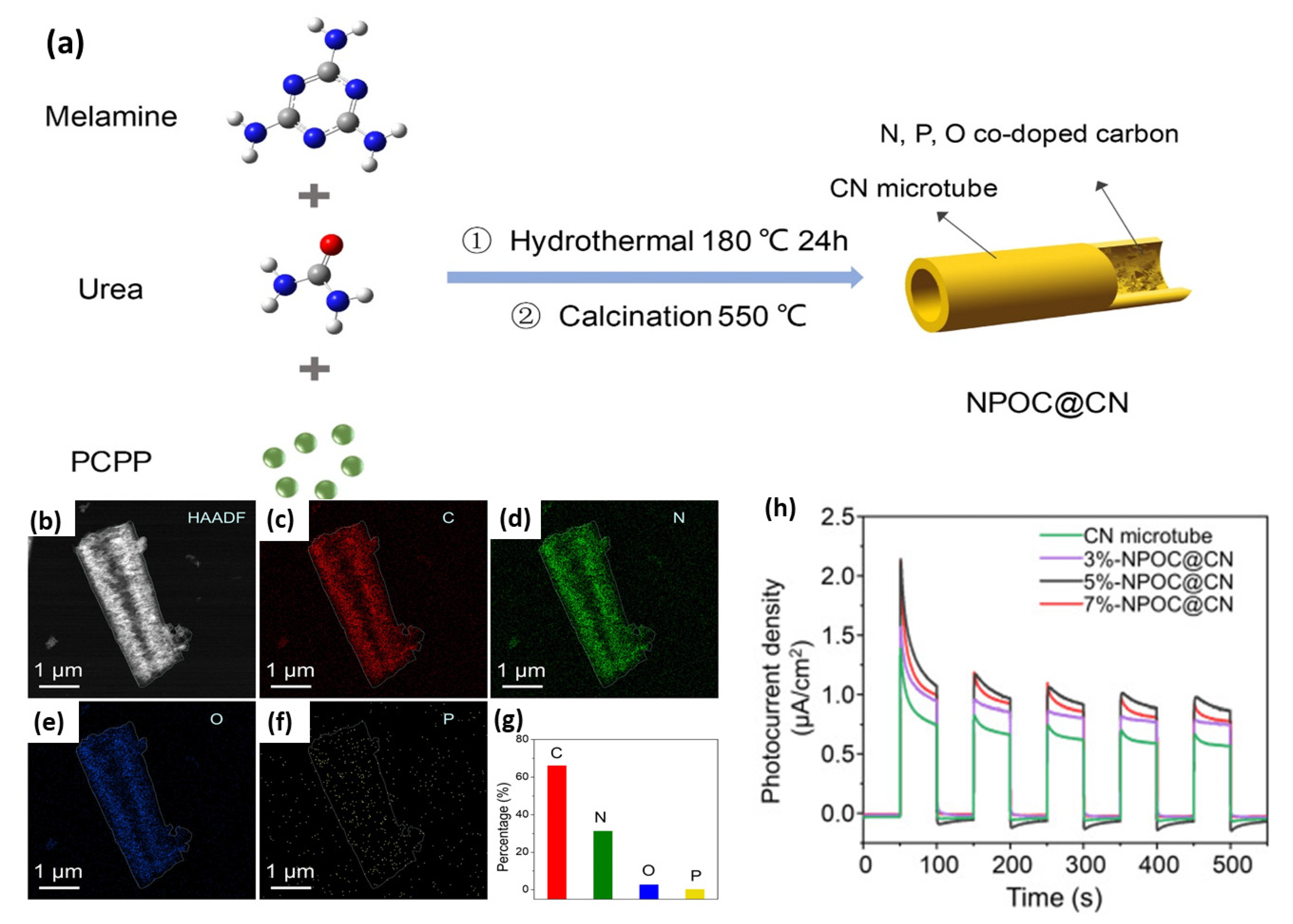
- Jiang, L.; Guo, Y.; Pan, J.; Zhao, J.; Ling, Y.; Xie, Y.; Zhou, Y.; Zhao, J. N, P, O co-doped carbon filling into carbon nitride microtubes to promote photocatalytic hydrogen production. Sci. Total Environ. 2022, 809, 151114. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Zhang, H.; Xiang, Q. Porous graphitic carbon nitride for solar photocatalytic applications. Nanoscale Horiz. 2020, 5, 765–786. [Google Scholar] [CrossRef] [PubMed]
- Eid, K.; Sliem, M.H.; Abdullah, A.M. Unraveling template-free fabrication of carbon nitride nanorods codoped with Pt and Pd for efficient electrochemical and photoelectrochemical carbon monoxide oxidation at room temperature. Nanoscale 2019, 11, 11755–11764. [Google Scholar] [CrossRef] [PubMed]
- Eid, K.; Sliem, M.H.; Abdullah, A.M. Tailoring the defects of sub-100 nm multipodal titanium nitride/oxynitride nanotubes for efficient water splitting performance. Nanoscale Adv. 2021, 3, 5016–5026. [Google Scholar] [CrossRef] [PubMed]
- Eid, K.; Soliman, K.A.; Abdulmalik, D.; Mitoraj, D.; Sleim, M.H.; Liedke, M.O.; El-Sayed, H.A.; AlJaber, A.S.; Al-Qaradawi, I.Y.; Reyes, O.M. Tailored fabrication of iridium nanoparticle-sensitized titanium oxynitride nanotubes for solar-driven water splitting: Experimental insights on the photocatalytic–activity–defects relationship. Catal. Sci. Technol. 2020, 10, 801–809. [Google Scholar] [CrossRef]
- Abualrejal, M.M.; Eid, K.; Tian, R.; Liu, L.; Chen, H.; Abdullah, A.M.; Wang, Z. Rational synthesis of three-dimensional core–double shell upconversion nanodendrites with ultrabright luminescence for bioimaging application. Chem. Sci. 2019, 10, 7591–7599. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Eid, K.; Abdullah, A.M.; Niu, W.; Wang, C.; Lan, Y.; Elzatahry, A.A.; Xu, G. Unveiling one-pot template-free fabrication of exquisite multidimensional PtNi multicube nanoarchitectonics for the efficient electrochemical oxidation of ethanol and methanol with a great tolerance for CO. ACS Appl. Mater. Interfaces 2020, 12, 31309–31318. [Google Scholar] [CrossRef]
- Ibrahim, Y.; Meslam, M.; Eid, K.; Salah, B.; Abdullah, A.M.; Ozoemena, K.I.; Elzatahry, A.; Sharaf, M.A.; Sillanpää, M. A review of MXenes as emergent materials for dye removal from wastewater. Sep. Purif. Technol. 2022, 282, 120083. [Google Scholar] [CrossRef]
- Salah, B.; Eid, K.; Abdelgwad, A.M.; Ibrahim, Y.; Abdullah, A.M.; Hassan, M.K.; Ozoemena, K.I. Titanium Carbide (Ti3C2Tx) MXene Ornamented with Palladium Nanoparticles for Electrochemical CO Oxidation. Electroanalysis 2022, 34, 677–683. [Google Scholar] [CrossRef]
- Ibrahim, Y.; Mohamed, A.; Abdelgawad, A.M.; Eid, K.; Abdullah, A.M.; Elzatahry, A. The recent advances in the mechanical properties of self-standing two-dimensional MXene-based nanostructures: Deep insights into the supercapacitor. Nanomaterials 2020, 10, 1916. [Google Scholar] [CrossRef]
- Ibrahim, Y.; Kassab, A.; Eid, K.; Abdullah, A.M.; Ozoemena, K.I.; Elzatahry, A. Unveiling fabrication and environmental remediation of MXene-based nanoarchitectures in toxic metals removal from wastewater: Strategy and mechanism. Nanomaterials 2020, 10, 885. [Google Scholar] [CrossRef]
- Ipadeola, A.K.; Eid, K.; Abdullah, A.M.; Al-Hajri, R.S.; Ozoemena, K.I. Pd/Ni-metal–organic framework-derived porous carbon nanosheets for efficient CO oxidation over a wide pH range. Nanoscale Adv. 2022, 4, 5044–5055. [Google Scholar] [CrossRef]
- Ipadeola, A.K.; Eid, K.; Abdullah, A.M.; Ozoemena, K.I. Pd-Nanoparticles Embedded Metal–Organic Framework-Derived Hierarchical Porous Carbon Nanosheets as Efficient Electrocatalysts for Carbon Monoxide Oxidation in Different Electrolytes. Langmuir 2022, 38, 11109–11120. [Google Scholar] [CrossRef] [PubMed]
- Nemati, F.; Rezaie, M.; Tabesh, H.; Eid, K.; Xu, G.; Ganjali, M.R.; Hosseini, M.; Karaman, C.; Erk, N.; Show, P.-L. Cerium functionalized graphene nano-structures and their applications; A review. Environ. Res. 2022, 208, 112685. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Chen, Z.; Zhao, J. Metal-free graphdiyne doped with sp-hybridized boron and nitrogen atoms at acetylenic sites for high-efficiency electroreduction of CO2 to CH4 and C2H4. J. Mater. Chem. A 2019, 7, 4026–4035. [Google Scholar] [CrossRef]






| Title | Focus | Ref. |
|---|---|---|
| Non-metal-Doped Porous Carbon Nitride Nanostructures for Photocatalytic Green Hydrogen Production | Rational design of heteroatoms (i.e., B, N, S, P, F, and O) doped porous carbon nitride for the photocatalytic HER. The effect of mono, binary, and ternary dopants on photocatalytic HER, and their fundamentals and mechanisms are discussed. H2 energy and storage in addition to HER fundamentals and calculation are also discussed. The current challenges and possible solutions for the synthesis of active gCN photocatalysts for green HERs are also emphasized. | This work |
| Photocatalytic hydrogen evolution based on carbon nitride and organic semiconductors | Organic (i.e., carbon nitride, linear polymers, conjugated porous polymers, and small molecules) for a photocatalytic HER. | [33] |
| Solvent-Assisted Synthesis of Supramolecular-Assembled Graphitic Carbon Nitride for Visible Light Induced Hydrogen Evolution—A Review | Solvent (i.e., water, DMSO, and water–chloroform) assisted the supramolecular-assembled carbon nitride via hydrogen bonding and hydrogen–halogen interaction. This is in addition to tunable characteristics/properties of photocatalytic HERs. | [34] |
| A latest overview on photocatalytic application of g-C3N4 based nanostructured materials for hydrogen production | g-C3N4 nanosheets supported metals (i.e., transition metals, non-metals, noble, and semiconductor), and carbon materials (i.e., graphene and carbon nanotubes, and carbon dots) for photocatalytic HER. | [35] |
| Preparation, Physicochemical Properties, and Functional Characteristics of Carbon Nitride: a Review | Emphasizing various approaches for preparation, and functionalization of porous carbon nitride and their properties that could enhance the photocatalysis, catalysis, and adsorption applications. | [41] |
| gC3N4 Derived Materials for Photocatalytic Hydrogen Production: A Mini Review on Design Strategies | Highlighting the recent advance in doped g-C3N4 with metals/non-metals (i.e., Ag, Ni, Mo, F, B, and S) and formation of heterojunction with semiconductors (i.e., TiO2, ZnO, MCoS2), metal-organic framework, graphdiyne for photocatalytic HERs, and their mechanisms. | [38] |
| Latest progress in g-C3N4 based heterojunctions for hydrogen production via photocatalytic water splitting: a mini review | The fabrication of g-C3N4-based heterojunctions (i.e., type-II, Z-scheme, S-scheme and Schottky) with transition metal oxide/sulfide (i.e., Co2P, FeOx, CuS, Cu2O, Ni) noble-metals (i.e., Ag, Au, Pt, and Pd), non-metals (i.e., B, F, S, and W), semiconductors (ZnO, ZrO2, and Mo2S), and carbon materials (graphene, carbon, nanotubes, and carbon dots) for photocatalytic HERs. | [36] |
| 2D Graphitic Carbon Nitride for Energy Conversion and Storage | The preparation (i.e., thermal oxidation etching, chemical exfoliation, ultrasonication-assisted liquid phase exfoliation, chemical vapor deposition) of energy production (i.e., photo-/electrocatalytic HER, CO2 reduction, and oxygen evolution/reduction) and energy storage (i.e., alkali-metal ion, lithium-metal, lithium-sulfur batteries, metal-air batteries, and supercapacitors). | [42] |
| Recent advances on porous materials for synergetic adsorption and photocatalysis | Focus on the fabrication of porous g-C3N4, metal oxides/sulfides (i.e., ZnS, SnS2, BiS3), metal-organic frameworks (i.e., ZIF, MIL, and PCN) for photocatalytic HERs, and photocatalytic adsorbents (TiO2-actvated carbon, activated carbon-MoS2, and biochar-TiO2). | [37] |
| Co-Doped, Tri-Doped, and Rare-Earth-Doped g-C3N4 for Photocatalytic Applications: State-of-the-Art | Unravelling the effects of co-doping, tri-doping, and rare-earth-doping of g-C3N4 with non-metals (i.e., P, F, N, I, S, Cl) and metals (Ag, Fe, Co, and Pt) on photocatalytic water splitting and dye degradation. | [39] |
| Recent advances in g-C3N4-based photocatalysts incorporated by MXenes and their derivatives | The fabrication of g-C3N4/2D MXenes (I.e., Ti3C2Tx, Nb2CTx) and their derivatives for environmental and energy applications (i.e., photocatalytic HER, generation, CO2 conversion, pollutant degradation, N2 fixation, and H2O2 production). | [43] |
| Name | Equation | Equation No. |
|---|---|---|
| HER in an aqueous solution of acidic electrolyte | ||
| Volmer reaction step | gCNs-M* + H3O+ + e− → gCNs-M*Hads +H2O | (4) |
| Heyrovsky reaction step | gCNs-M*Hads + H3O+ + e− → gCNs-M* + H2 +H2O | (5) |
| Tafel reaction step | gCNs-M*Hads + gCNs-M*Hads → 2gCNs-M* + H2 | (6) |
| HER in an aqueous solution of alkaline electrolyte | ||
| Volmer reaction step | gCNs-M* + H2O + e− → gCNs-M*Hads + OH− | (7) |
| Heyrovsky reaction step | gCNs-M*Hads + H2O + e− → gCNs-M* + H2 + OH | (8) |
| Tafel reaction step | gCNs-M*Hads + gCNs-M*Hads → 2gCNs-M* + H2 | (9) |
| Name | Equation | Equation No. |
|---|---|---|
| Quantum efficiency (QE) | QE (%) = | (10) |
| Current density (J) | J = I/A | (11) |
| Over potential (η) | η = E − Eo | (12) |
| Turnover frequency (TOF) | TOF = JA/2Fm | (13) |
| Reduction current density vs. scan rate | Slope = 2nFAΓ0/4RT | (14) |
| The turnover frequency (TOF) | TOF = J × NA/(F × n × Γ) | (15) |
| Energy efficiency (Eefficiency) | Eefficiency = [Eeq/Eeq + η] × EFaradic | (16) |
| Quantum yield | QY= [2.nx.NA.h.c/till.I.A.λ] | (17) |
| Electrochemical active surface area (ECSA) | ECSA = CDL/Cs | (18) |
| Double-layer capacitance (Cdl) | Cdl = (Δj)/2dVb | (19) |
| Incident photon to current conversion efficiency | IPCE (%) = [(1240 × J)/(λ × Io)] × 100 | (20) |
| Doping | Advantages | Disadvantages |
|---|---|---|
| N | Generates abundant defects and active sites Increases electronic conductivity Promotes ion adsorption Earth-abundant and inexpensive Modulates the Fermi level, bandgap, and localized electronic state Eases the generation of electron–hole pairs and delays their recombination Enhances light absorption | Not durable at elevated temperatures Does not enlarge interlay distance Uncontrolled doping sites and concentration Cumbersome process High operating temperature |
| S | Expands interlayer distance Induces reduction reaction Promotes ion adsorption/diffusion Modulates the Fermi level, bandgap, and localized electronic state Facilitates the generation of electron–hole pairs and delays their recombination Enhances light absorption | Leads to structural deformation Uncontrolled doping sites and concentration High operating temperature Slow preparation process |
| P | Enlarges interlayer distance Enhances ion adsorption/diffusion Upsurges geometric distortion Alters the Fermi level, bandgap, and localized electronic state Induces creation of electron–hole pairs and prevents their quick recombination Enhances light absorption | Causes large structural distortion Uncontrolled doping sites and concentration High operating temperature Slow preparation process |
| B | Generates massive in-plane defect Enhances ion adsorption/diffusion Modulates the Fermi level, bandgap, localized electronic state, and spin density Induces creation of electron–hole pairs and prevents their quick recombination Enhances light absorption | Difficult to prepare Forms high-energy trap Uncontrolled doping sites and concentration |
| F | Enlarges interlayer distance Enhances ion adsorption/diffusion Enhances electronic conductivity Suppresses the John–Teller effect Modulates the Fermi level, bandgap, and localized electronic state | Causes large structural deformation upon cycling Excess doping causes rapid capacity disappearance Hazardous precursors Uncontrolled doping sites and concentration |
| Methods | Precursors | Doping | Advantages and Disadvantages |
|---|---|---|---|
| Thermal annealing | Boric trioxide Boron trichloride Boron trioxide Ammonia Diammonium hydrogen Urea Hydrogen sulfide Diaminodiphenyl sulfone Dibenzyl sulfide Sulfur powder Hexachlorocyclotriphosphazene Phosphoric acid Diammonium phosphate Hexafluorophosphate Ammonium fluoride Ammonium chloride Ammonium bromide | B B B N N N S S S S P P P P F Cl Br | Simple, one-pot, feasible for various precursors (i.e., gases, liquids, and solids), tunable doping. Limitations: high operation temperature and energy consumption |
| Physical vapor deposition (PVD) or chemical vapor deposition (CVD) | Boric acid Phenylboronic acid Ammonia Iodine Pyrimidine | B B N I N | Allows simultaneous growth of doped gCNs with controllable doping Limitations: complex process, energy consumption, requires special laboratory equipment, and generates waste gases Limitations: high cost, inferior yield, and feasible for low ranges of precursors |
| Ball milling | Ammonia sulfur powder Ammonium fluoride Ammonium chloride Ammonium bromide | N S F Cl Br | Low-cost, facile, and scalable process Limitations: doping only at edges Limitations: uncontrolled doping process |
| Bottom-up synthesis | Boron tribromide Lithium nitride Pentachloropyridine Thiourea | B N N S | Highly productive, solution-based, need mild conditions Limitations: inevitable high oxygen content and uncontrollable doping |
| Wet chemical method | Hydrazine Ammonium thiocyanate Hydrogen fluoride Hydrogen iodide Ammonium chloride Ammonium bromide Boric acid | N S&N F I Cl Br B | Inexpensive, low energy consumption, solution-based, productive, easy process, and feasible for wide ranges of precursors Limitations: low-doping content and uncontrollable doping |
| Plasma | N2 Cl2 Hydrogen sulfide | N Cl D | Quick process and inferior power consumption Limitations: low yield and feasible for specific precursors |
| Arc-discharge | NH3 Pyrrole Boron trioxide | N N B | Productive and quick process Limitation: high energy consumption (i.e., voltage and current) Limitation: inferior and uncontrollable doping content |
| Photocatalysts | Doping Element | Morphology | Synthetic Method | Co-Catalyst | Light Source | H2 Evolution Rate (μmol h−1g−1) | Apparent Quantum Efficiency | Durability | Ref |
|---|---|---|---|---|---|---|---|---|---|
| P-CN | P | Mesoporous nanostructured flowers | Template-free co-condensation method | 3 wt% Pt | 300 W Xeon arc lamp | 2082 | No obvious attenuation of H2 evolution rate after illumination of 16 h | [76] | |
| PCN-S | P | Porous nanosheets | Thermal polycondensation of melamine-2-aminoethylphosphonic acid complex, followed by thermal exfoliation | 1 wt% Pt | 300 W Xe arc lamp with a UV-cutoff filter (>400 nm) | 1596 | 3.56% at 420 nm | [89] | |
| P10-550 | P | Layered platelet-like morphology | Thermally induced copolymerization route using hexachlorocyclotriphosphazene as P source and guanidinium hydrochloride as g-C3N4 precursor. (10 wt%P, calcination temperature = 550 °C) | 3 wt% Pt | 300 W xenon lamp with a 420 nm cutoff filter | 506 | The hydrogen amount is still comparable to that of first cycle after five cycles | [90] | |
| P-TCN | P | Hexagonal tubes with micro-nanostructure | Pyrolysis of the melamine–cyanuric acid supramolecular precursor formed by phosphorous acid-assisted hydrothermal method | 1 wt% Pt | 300 W Xeon arc lamp with bandpass filter (365, 420, 450, 520, and 600 nm) | 670 | 5.68% at 420 nm | No noticeable deterioration after irradiation for 20 h | [91] |
| CN-SP | P | Tubular g-C3N4 with surface carbon defects | Thermal polymerization of a supramolecular precursor formed under pyrophosphate–assisted hydrothermal process | 1 wt% Pt | 300 W Xe arc lamp with a ≥420 nm cutoff filter | 570 | [92] | ||
| P-CNRs | P | Macro/mesoporous g-C3N4 micro-rods | Direct calcination of reflux-treated ethylene diphosphonic acid–melamine complex fiber network | 3 wt% Pt | 300 W Xe arc lamp with a ≥420 nm cutoff filter | 4960 | No obvious decay after irradiation for 20 h | [93] | |
| P0.01 | P, Na | Porous multi-layer nanosheets | Polymerization of the mixed precursors of melamine and sodium tripolyphosphate | 1 wt% Pt | 350 W Xe arc lamp | 3820 | No decrease in H2 production rate after irradiation for 12 h | [94] | |
| PCNNFs | P | Fragmented nanoflakes | First P-doping via using phytic acid biomass as P source and urea as C3N4 precursor, followed by posttreatment | 3 wt% Pt | 300 W Xe arc lamp with a >420 nm cutoff filter | 15,921 | 6.74% at 420 nm; 0.24% at 600 nm | No obvious decay in photocatalytic H2 production under irradiation for 50 h | [95] |
| PCNT | P | Hierarchical coral-like porous tubes | Pyrolysis and freeze-drying using dicyandiamide as carbon nitride source and phytic acid as P source | 3 wt% Pt | 300 W Xe arc lamp with a ≥420 nm cutoff filter | 2020 | 4.32% at 420 nm; 3.58% at 450 nm; 1.28% at 500 nm | H2 production rate kept almost same after 10 h reaction | [96] |
| PCN1.5 | P | Flower-like structure consisting of multitudinous nanosheets | Template-free and thermal copolymerization route using phosphoric acid as P source and cyanuric acid–melamine complex as supramolecular precursor | 3 wt% Pt | 300 W Xe lamp with a 400 nm UV-light cutoff filter | 5128 | Only about 7.3% attenuation was observed after visible light illumination of 16 h | [97] | |
| PCN-50 | P | Platelet-like surface | Polymerization of urea and NH4H2PO2 at 570 °C for 3 h | 1 wt% Pt | 300 W Xe lamp with a 400 nm UV-light cutoff filter | ~9167 | Photocatalytic performance was maintained through 20 h of cycling experiments | [98] | |
| PCN(1.6) | P | Nearly transparent nanosheets agglomerate | The calcination of polymeric carbon nitride formed by urea condensation and amorphous phosphorus | 3 wt% Pt | 300 W Xe arc lamp as simulated sunlight (>300 nm) or with a 420 nm cutoff filter | 8707 and 5720 under the simulated solar light and visible light | [99] | ||
| P-CNTS | P | Tubular structure with a large number of pores in the walls | Pre-hydrothermal and calcination under a nitrogen atmosphere | 1 wt% Pt | 300 W Xe lamp with a 420 nm UV-light cutoff filter | 2749.3 | The amount of produced hydrogen slightly decreased after three cycles of tests | [100] | |
| L-PCN-1.0 | P | Louver-like nanowire arrays | Supramolecular self-assembly of melamine–cyanuric acid | 1 wt% Pt | 300 W Xe arc lamp with a ≥420 nm cutoff filter | 1872.9 | 6.93% at 420 ± 15 nm | The hydrogen production has no noticeable deactivation over four cycles | [101] |
| PCN-HMS | P | Hierarchical mesoporous microspheres | Supramolecular chemistry-mediated one-pot strategy | 1 wt% Pt | 300 W Xe lamp with an ultraviolet cut-off filter (λ ≥ 420 nm) | 1820 | [102] | ||
| PO-CN | P | Porous ultrathin nanosheets | Two-step thermal treatment | 3 wt% Pt | 300 W Xe lamp with a 420 nm cutoff filter | 997.7 | [103] | ||
| NiSCN | S, Ni | Nanosheets | High-temperature thermal polymerization of urea and benzyl disulfide | 5 wt% Ni | 300 W Xe arc lamp with a cutoff filter (λ > 420 nm) | 2021.3 | 2.51% at 420 nm | The H2 production rate decreases a little after four cycles for 20 h | [104] |
| MTCN-6 | S | Rectangular rods | Self-assembly of melamine with tri-thiocyanuric acid, followed by calcination | 1 wt% Pt | 300 W Xe arc lamp with a cutoff filter (λ > 420 nm) | 1511.2 | 3.9% at 420 nm | No obvious decrease in H2 generation rate over five cycles | [105] |
| PCNS-2 | S | Ultrathin nanosheets with porous networks | The polymerization of thiourea and NH4Cl at 550 °C for 3 h | 300 W Xe arc lamp with a cutoff filter (λ > 420 nm) | ~367 | No obvious decrease of H2 evolution rate within four cycles | [106] | ||
| CN-0.20%Dx-25 | S,K | Needle-like nanorods | Condensation of thiourea and dithiooxamide followed by post-treatment in molten salt | Pt | 300 W Xe arc lamp with a cutoff filter (λ > 420 nm) | 1962.10 | Obvious decrease in the photocatalytic H2 evolution performance due to K leaching | [107] | |
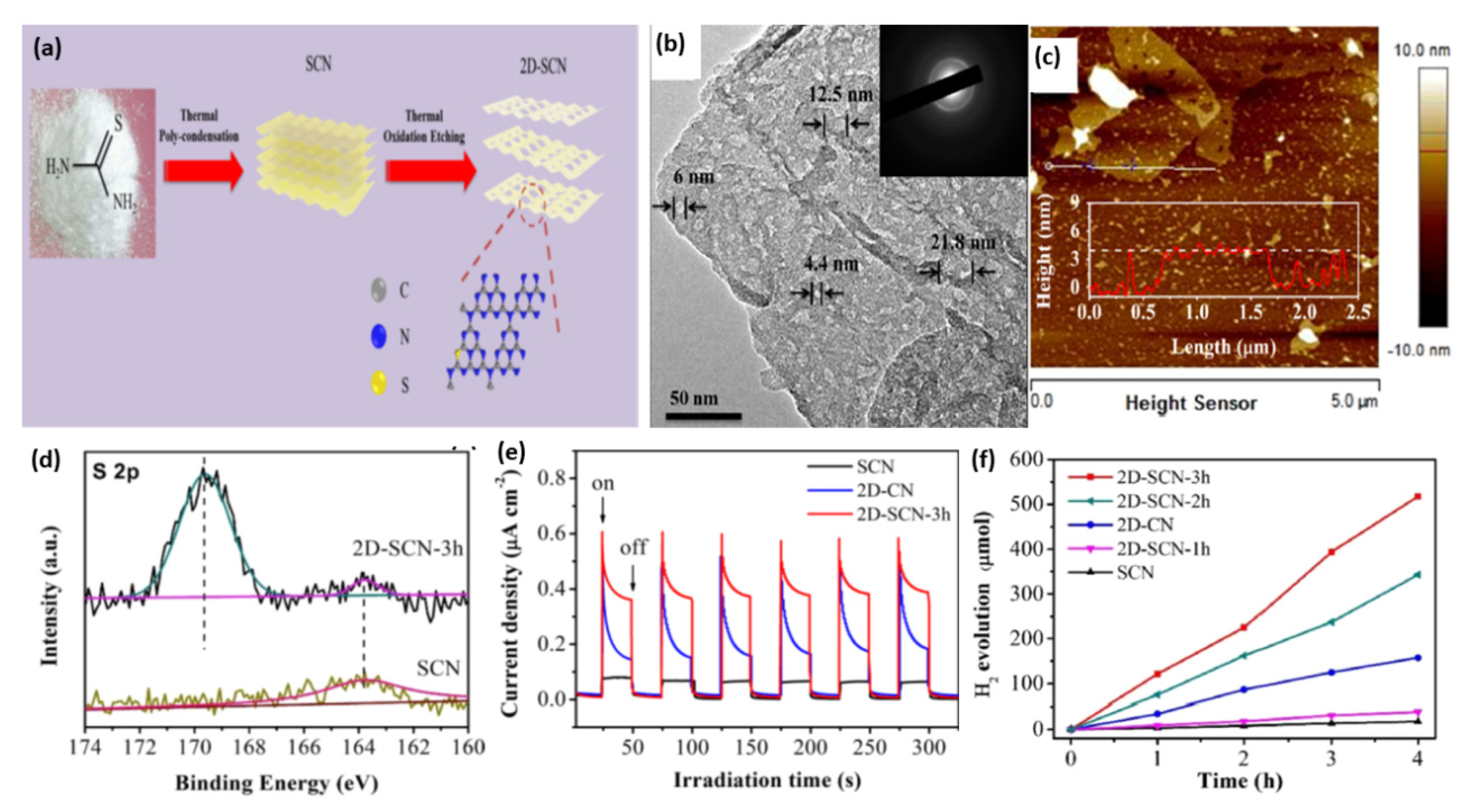
| 2D-SCN | S | Nanosheets | Polycondensation of thiourea, followed by thermal oxidative treatment | 1 wt% Pt | 140 W Xe lamp | 8493 | 8.35% at 420 nm | The hydrogen evolution activity was maintained after 36 h of continuous irradiation | [108] |
| S/g-C3+xN4+y | S, cyano group | Porous leaf with irregular shape | Treating pristine g-C3N4 nanosheets under acetonitrile and hydrogen sulfide atmosphere | 3 wt% Pt | 300 W Xe lamp with a UV light filter (λ > 420 nm) | 1901 | 33.5% and 13.1% at 405 and 420 nm | The amount of produced hydrogen was decreased in first three cycles, but returned to the previous high level after the re-addition of TEOA sacrificial agent | [109] |
| S-CN(0.1) | S | Holey nanosheets | One-step thermolysis of thiocyanuric acid | 3 wt% Pt | 300 W Xe lamp with a UV light filter (λ > 420 nm) | 6225.4 | 10% at 420 ± 10 nm | The photocatalytic HER stabilizes at ca. 6200 μmol h−1g−1 under five cycles of reuse | [110] |
| PCNS | S | Layered structure | One-step auxiliary thermal polycondensation of melamine and ammonium persulfate | 2 wt% Pt | 300 W Xenon lamp with a 420 nm UV-cutoff | 58,680 | No obvious decrease of H2 production after three cycles | [111] | |
| SCN1.0 | S | Peony-like morphology | Thermal condensation of cyanuric acid–melamine–trithiocyanuric acid complex under N2 atmosphere | 3 wt% Pt | 300 W Xe lamp | 11,354 | 13.69% at 420 nm | Only about 3.2% attenuation of photocatalytic hydrogen production after four cycles | [112] |
| mpgCNS | S | Mesoporous nanosheet | Pyrolysis of thiourea using SiO2 nanoparticles as the hard template | 3 wt% Pt | 300 W Xe lamp with a 420 nm cutoff filter | 1360 | 5.3% at 420 nm | 10% activity drop over the photoreaction for 72 h with evacuation at every 12 h | [113] |
| CN-MT | S | Nanoporous microrods | Thermal condensation of melamine-trithiocyanuric acid supramolecular cocrystal under N2 atmosphere | 1 wt% Pt | 500 W Xe lamp with a 400 nm filter | 5000 | No loss of catalytic activity after the catalytic H2 evolution for 60 h | [114] | |
| SCN-HMS | S | Mesoporous microspheres | Supramolecular chemistry-mediated one-pot strategy | 1 wt% Pt | 300W Xe lamp with an ultraviolet cutoff filter (λ ≥ 420 nm) | 2230 | 3.8% at 420 nm | No obvious decrease was observed for the H2 evolution rate even after four cycles | [102] |
| B-SSCN | B | Microsphere | One-step solvothermal method by using cyanuric chloride and cyanuric acid as precursors and ammonia borane as B source | 3 wt% Pt | 300 W Xenon lamp with a UV cutoff filter (λ > 420 nm) | 910 | ~1.15% at 420 nm | The H2 evolution rate was well preserved after four test cycles over 4 days and no structure change after reaction | [115] |
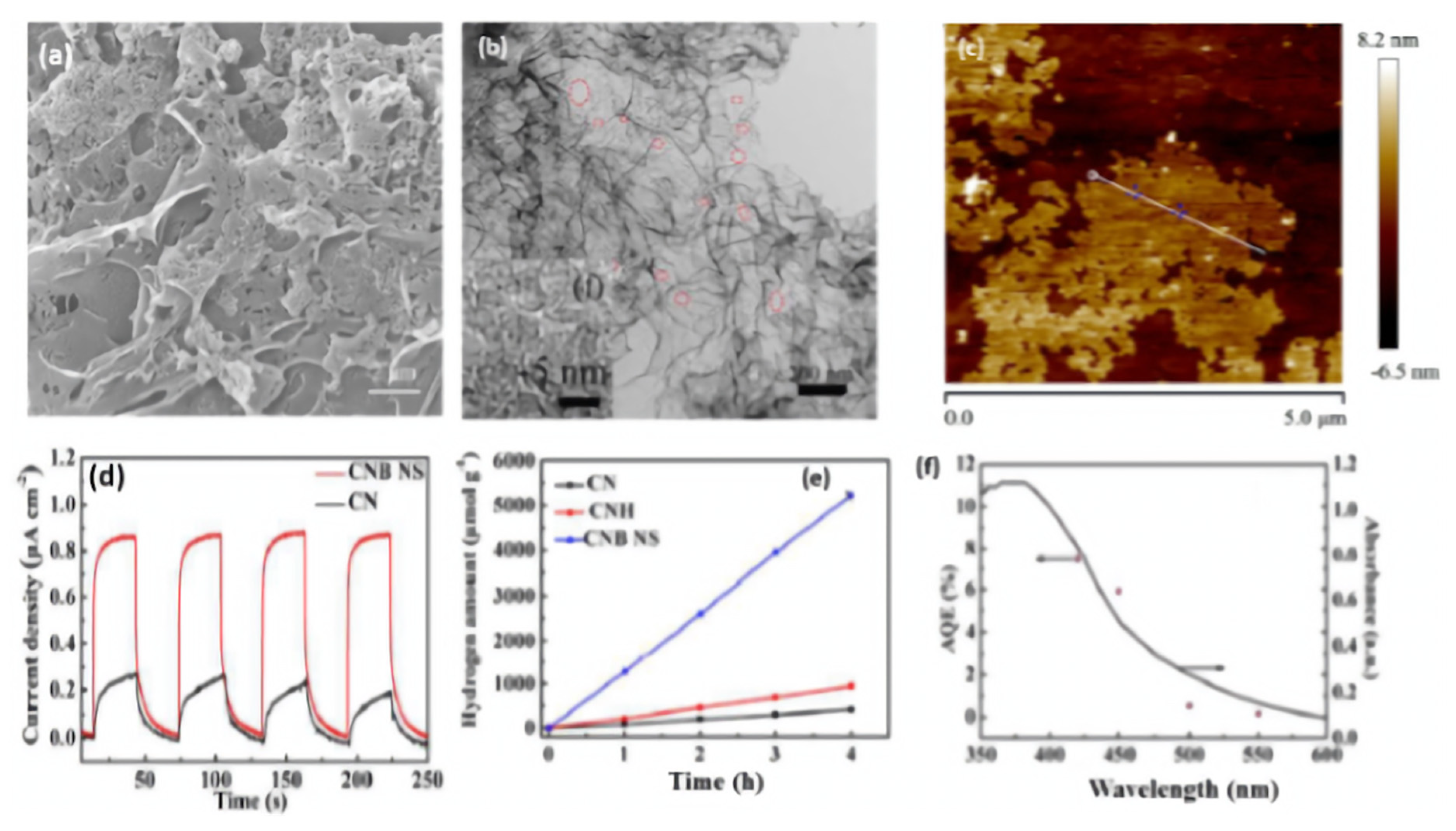
| CNB NS | B | Porous ultrathin nanosheet | Reforming and thermal condensation of barbituric acid and melamine | 3 wt% Pt | 300 W Xe lamp with a UV cutoff filter (λ > 400 nm) | 1323.25 | 7.45% at 420 nm | The amount of H2 production increase steadily with extended the reaction time and no significant deactivation is observed after five cycles | [116] |
| B-CNNT | B | Ordered nanotubes | Hydrothermal and calcination of melamine and boric acid | Pt | 300 W Xenon lamp with a UV cutoff filter (λ > 420 nm) | 22,100 | 7.33% at 420 nm | The H2 production increases steadily with time and retain stability | [117] |
| B, CsCN-Ns | B, Cs | Porous and wrinkled nanosheets | Recrystallization of melamine in water in the presence of boric acid and CsCl followed by calcination and thermal etching | 3 wt% Pt | Xenon lamp with a cutoff filter (λ ≥ 420 nm) and IR filter | 1120 | The H2 production rate was stable in five successive cycles | [118] | |
| B/g-C3N4 | B | Nanosheets | The pyrolysis of urea and 1-ethyl-3-methylimidazolium tetrafluoroborate | 0 | 350 W Xenon lamp with a UV cutoff filter (λ > 365 nm) | 901 | The photocatalytic activity remains unchanged after three reaction cycles | [119] | |
| PNCN-BNa-3 | B, Na | Porous nanosheets | Controlling the heating rate and thermal posttreatment using melamine nitrate as precursor, sodium borohydride as B source and Na source | 1.2 wt% Pt | 10 W white LED lamp (λ > 420 nm) with the color temperature of 6500 K | 5971.51 | 9.39% at 430 nm | Even after five cycles of photocatalytic test, the hydrogen generation activity is not significantly reduced | [120] |
| D-TCN450 | B | Hollow tube | Self-supramolecular reaction and NaBH4 thermal reduction approach | 3 wt% Pt | 300 W Xe lamp with a 420 nm cutoff filter | 789.2 | [121] | ||
| O-doped g-C3N4 | O | Irregular porous structure with hierarchical edges | Hydrothermal treatment of g-C3N4 with H2O2 at 140 °C for 10 h | 1.2 wt% Pt | 300W Xe arc lamp with a UV-cutoff filter (λ < 420 nm) | 375 | The H2 evolution remains stable in the recycling three runs | [122] | |
| MCN | O | Porous network composed by nanosheets | Condensation of supramolecular aggregates formed by H2O2-treated melamine | 3 wt% Pt | 300 W Xenon lamp with a 420 nm filter | 1204 | 7.8% at 420 nm | A stable HER rate within 25 h | [123] |
| HS-g-C3N4-O | O | Holey thin sheets | Using photo-Fenton reaction in the presence of Fe3+/Fe2+ and H2O2 | 5 wt% Pt | 300W Xe lamp with 420 nm filter | 6752 | The H2 evolution rate is quite stable under continuous irradiation of 26 h | [124] | |
| P-CNO | O | Porous nanosheet with highly ordered architecture | Heating the hydrothermally treated dicyandiamide at 550 °C for 2 h | 1 wt% Pt | 300 W Xeon lamp with a UV cutoff filter (λ > 400 nm) | 1748.6 | 7.2% at 420 nm | The hydrogen production performance shows no trend of deactivation even after 15 h | [125] |
| GCN-4 | O | Three-dimensional porous nanosheets | Water-based homogeneous supramolecular assembly | 3 wt% Pt | 300 W Xeon-lamp equipped with a 420 nm-cutoff filter | 1968 | 10.3% at 420 nm | A stable HER rate after six cycling trips | [126] |
| p-CN2 | O | Loose and porous layers | A simple co-pyrolysis of dicyandiamide and ammonium persulphate | 3 wt% Pt | 300 W Xeon lamp with a UV cutoff filter (λ > 420 nm) | 395.96 | 0.79% at 420 nm | The photocatalytic H2 production activity is well retained after four successive cycles while the phase structures are not changed | [127] |
| POCN | O | Nanosheet | Thermal polymerization reaction of melamine and ethanol | 1 wt% Pt | 300 W Xe lamp equipped with a 420 nm cutoff filter | 1286 | 12.06% at 420 nm | The HER is no apparent attenuation after four cycles | [128] |
| CN3 | O | Numerous macropores and mesopores with an assembling flake | Pyrolyzing H2SO4 and HNO3 modified melamine precursors | 3 wt% Pt | 300 W Xe arc lamp with an AM 1.5 optical filter | 3700 | 20.88% at 420 nm | No significant decline of H2 production is observed after five runs within 5 h | [129] |
| W,O/g-C3N4 | W/O | Hollow tubular structure | One-step polycondensation of ammonium metatungstate hydrate and melamine | 1 wt% Pt | 300 W Xe lamp with a cutoff filter (λ > 400 nm) | 403.57 | [130] | ||
| U/AC0.5 | O | Loose and rich bread-like porous structure | Thermal polymerization of urea and foaming agent azodicarbonamide | 3 wt% Pt | 300 W Xe lamp equipped with a 420 nm cutoff filter | 4470 | 13.0% at 400 nm | No significant decrease in photocatalytic performance after five cycles | [131] |
| OCN-3 | O | Hollow and monolayered nanosheet | Multiple thermal treatments under the N2/O2 atmosphere | 3 wt% Pt | 300 W Xenon lamp with an optical filter (λ > 420 nm) | 3519.6 | 26.96% at 400 nm | Only 10.4% activity loss after 20 h | [132] |
| FCN15 | O | Rod | Calcinating supramolecular precursors prepared from acid (or alkali) and melamine | 3 wt% Pt | 300 W Xenon lamp | 12,766 | 9.4% at 420 nm | The FT-IR and Raman spectra did not change significantly after 16 h cycle test | [133] |
| OCNT | O | Ultralong hollow chain-ball | Facile supramolecular self-assembly route | 2 wt% Pt | 300 W Xe lamp equipped with a 420 and 510 nm cutoff filter | 5470 | 9.4% at 420 nm and 2.1% at 510 nm | The hydrogen production rate did not attenuate after six consecutive photocatalytic reactions | [134] |
| m-CN-0.067 | C | Nanosheet | Copolymerizing barbituric acid with melamine via microwave-assisted heating | 0.5 wt% Pt | 300 W Xe lamp equipped with a 420 nm cutoff filter | 2500 | They offer slightly reduced H2 generation during the course of 15 h visible light irradiation | [135] | |
| C-rich g-C3N4 | C | Nanosheet | A hydrothermal–conjugate–copolymerization strategy | 0 | 300 W Xe lamp equipped with a 420 nm cutoff filter | 125.1 | 6.8% at 420 nm | No noticeable deterioration of stability activity is observed after three cycles test | [136] |
| CDCN-20 | C | Thinner nanosheet enriched with many small holes | Co-polymerization of dicyandiamide with acrylamide | 3 wt% Pt | 300 W Xe lamp with a 420 nm filter | 1266.8 | 10.14% at 420 nm | No apparent decrease in photocatalytic activity is observed after four cycling test | [137] |
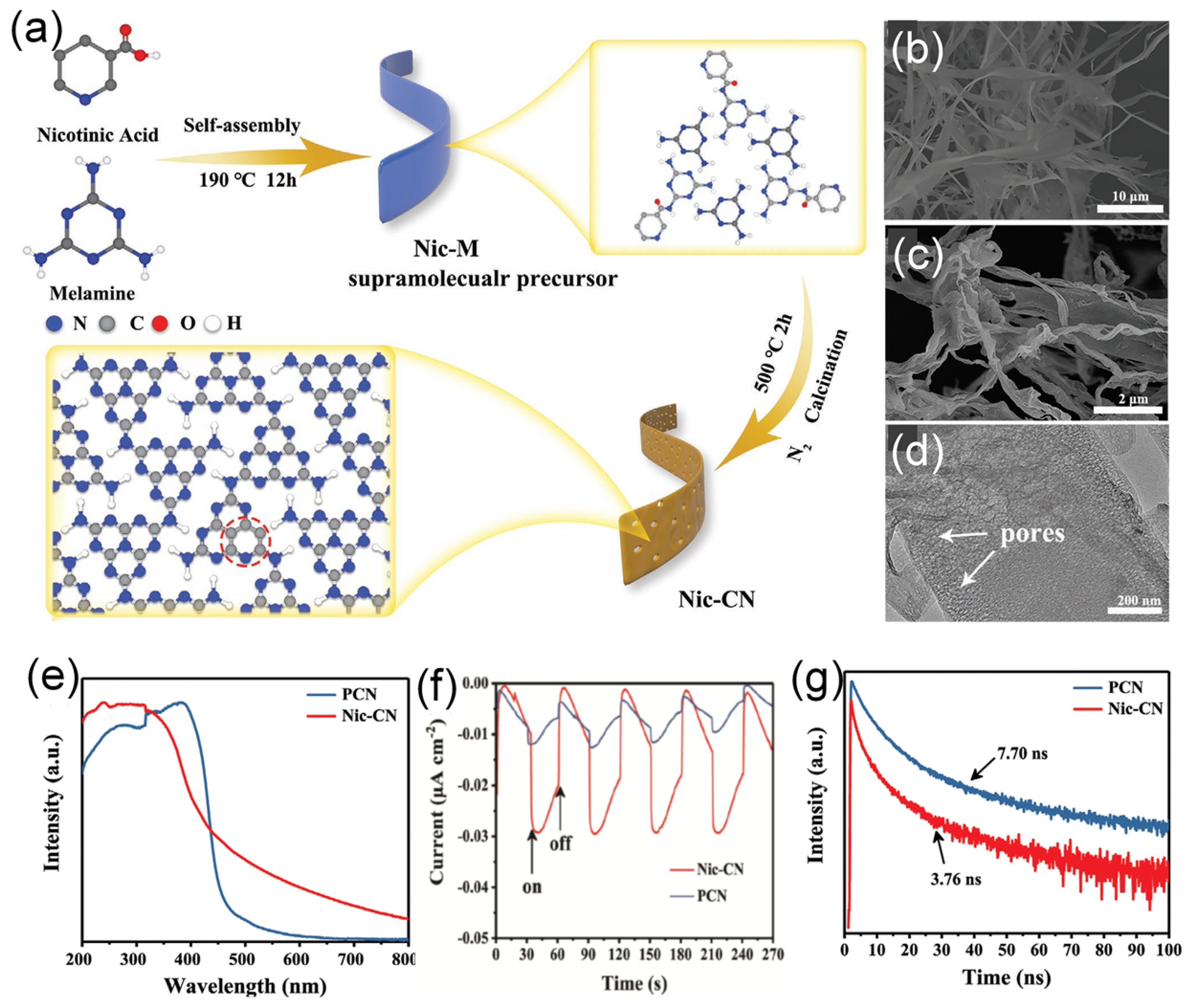
| Nic-CN | C | 1D thin, porous strip-like structure | Calcination of strip-like supramolecular precursor formed by organic molecular self-assembly of melamine and nicotinic acid | 1 wt% Pt | 300 W Xe lamp (λ > 420 nm) | 6310 | 6.8% at 420 nm | No obvious attenuation in hydrogen evolution after 5 times of cycling | [138] |
| CN-40 | C | Hollow tubular structure | Hydrothermal calcination method using melamine and sodium alginate as precursors | 3 wt% Pt | 300 W Xe lamp with a 420 or 400 nm cutoff filter | 1210.3 | 3.16% at 420 nm | More than 56% of original performance can be remained after four runs of reaction | [139] |
| C3N4+X | N | Nanosheet | Co-thermal condensation of precursor with nitrogen-rich additive | 3 wt% Pt | 300 W Xeon lamp with a 400 nm cutoff filter | 553.5 | The amount of produced hydrogen increased linearly with the consecutive irradiation time | [140] | |
| CNNTs | N | Hollow nanotube | Supermolecule self-assembly method | 3 wt% Pt | 300 W Xe lamp (λ > 420 nm) | 18,060 | 12.55% at 420 nm | The photocatalytic hydrogen evolution kept stable over four cycles | [141] |
| CNU-DMF | N | Porous nanosheets | One-step thermal copolymerization of urea and N,N-dimethylformamide | 3 wt% Pt | 300 W Xe lamp (λ > 400 nm) | 5268 | 11.4% at 420 nm | No obvious decrease after four cycles of reaction within 16 h | [142] |
| Cl-pdg-CN-M-3 | Cl | Accumulation of thin sheets | Pyrolysis of the mixture of melamine and NH4Cl | 3 wt% Pt | 300 W Xe lamp with a 400 nm cutoff filter | 833 | The H2 produced increased steadily with irradiation time lengthened in each run without noticeable deactivation | [143] | |
| Cl-p-C3N4 | Cl | Ultrathin nanostrips | Calcination of melamine and tetrachloroterephthalonitrile in an inert atmosphere | 1 wt% Pt | 300 W Xenon lamp with a 420 nm cutoff filter | 5976 | 8.91% at 420 nm | The H2 production rate has no apparent inactivation after four cycles | [144] |
| CNI | I | Loose and porous structure | Calcination of self-assembly precursors prepared from urea and ammonium iodide | 1 wt% Pt | 300 W Xe lamp equipped with a 420 nm cutoff filter | 3800 | 3.3% at 420 nm | Excellent cycle stability in photocatalytic hydrogen production | [145] |
| CNU-Br0.1 | Br | Layered platelet-like and curl-like thin nanosheet | A facile co-condensation strategy by using urea and ammonia bromine as starting materials | 3 wt% Pt | 300 W Xe-lamp equipped with an appropriate long pass cutoff filter | 240 | No activity decrease was seen after four consecutive cycles’ reaction | [146] |
| Photocatalysts | Dopants | Morphology | Synthetic Method | Co-Catalyst | Light Source | H2 Evolution Rate (μmol h−1g−1) | Apparent Quantum Efficiency | Durability | Refs |
|---|---|---|---|---|---|---|---|---|---|
| r-CN-B/F | B, F | Dense aggregated microstructures comprising irregular nanosheets | Post-thermal treatment of B/F co-doped carbon nitride obtained from direct condensation using ionic liquid as dopant | 3 wt% Pt | Visible light irradiation (λ > 400 nm) | 6870 | High H2 evolution remains in the consecutive four runs | [149] | |
| p-CN-BF | B, F | Small particles composed of porous nanosheets | In situ B and F co-doping using [Emin]BF4 as dopants followed by post-calcination in air | 3 wt% Pt | 300 W Xe arc lamp | 7020 | Only slight decrease observed in H2 evolution after several cycles | [150] | |
| BO-C3N4 | B,O | Porous nanomesh | Two-step doping and etching | 3 wt% Pt | 300 W Xe arc lamp equipped with a cutoff filter (λ ≥ 420 nm) | 9751 | 8.1% at 420 nm | There was no obvious deactivation over 20 h | [151] |
| CNBS | B,S | Nanosheets composed of nanoholes and rupture | Co-pyrolysis of boric acid, thiourea and melamine in the muffle furnace | 1 wt% Pt | 150 W Xenon lamp with a 420 nm cutoff filter | 2660 | No decrease in hydrogen production rate during long-time photocatalytic measurement up to five runs | [152] | |
| CNIN0.2 | C,I | Unconsolidated porous accumulation | In situ co-doping with iodized ionic liquid followed by post-thermal treatment in air | 3 wt% Pt | 300 W xenon-lamp with appropriate cutoff filter | 3364 | Almost negligible deactivation could be observed after four consecutive cycles | [153] | |
| CPCN-1* | P,C | Granular morphology | Self-assembly melamine with phytic acid, followed by hydrothermal treatment | 1 wt% Pt | 300W Xe lamp | 1493.3 | 2.14% at 420 nm | The H2 generation rate recovered to initial value over three cycles | [154] |
| PSCN | P,S | Layered structure | One-step high-temperature polymerization | 3 wt% Pt | 300 W Xenon lamp with an UV-cutoff filter > 400 nm | 1969 | The hydrogen evolution was not apparently attenuated following three cycles’ running | [155] | |
| PACN | P,O | Spiral nanotube | Phytic acid-assisted supramolecular self-assembly method | Pt | 5 W LED lights | 6437.65 | PACN exhibited excellent recycling stability in four runs and maintained 95.20% of the primitive value of hydrogen evolution after four runs | [156] | |
| CNB | C,O | Wrinkled nanosheets | Hydrothermal method using dicyandiamide, cyanuric acid and cyanobenzene as precursors | 3 wt% Pt | 300 W Xe-lamp with a 420 nm cutoff filter | 2595.4 | 16.6% at 420 nm | The photocatalytic activity decreased slightly after five cycles and no noticeable change of microstructure could be found | [157] |
| POCN-10 | P,O | Nanosheet | One-step thermal copolymerization of melamine and ammonium polyphosphate | 5 wt% Pt | 300 W Xenon lamp equipped with a 400 nm cutoff filter | 1588 | No apparent decline of H2 evolution activity by cycling three times in 12 h | [158] | |
| SPCN0.1 | S,P | Porous microtube | Using melamine and ammonium dihydrogen phosphate as precursors | 3 wt% Pt | 300 W Xe lamp equipped with optical cutoff filter (λ > 420 nm) | 4200.3 | 10.3% at 420 nm | The performance did not significantly decrease after 12 h of experiment | [159] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Q.; Abdelgawad, A.; Li, J.; Eid, K. Non-Metal-Doped Porous Carbon Nitride Nanostructures for Photocatalytic Green Hydrogen Production. Int. J. Mol. Sci. 2022, 23, 15129. https://doi.org/10.3390/ijms232315129
Lu Q, Abdelgawad A, Li J, Eid K. Non-Metal-Doped Porous Carbon Nitride Nanostructures for Photocatalytic Green Hydrogen Production. International Journal of Molecular Sciences. 2022; 23(23):15129. https://doi.org/10.3390/ijms232315129
Chicago/Turabian StyleLu, Qingqing, Ahmed Abdelgawad, Jiaojiao Li, and Kamel Eid. 2022. "Non-Metal-Doped Porous Carbon Nitride Nanostructures for Photocatalytic Green Hydrogen Production" International Journal of Molecular Sciences 23, no. 23: 15129. https://doi.org/10.3390/ijms232315129
APA StyleLu, Q., Abdelgawad, A., Li, J., & Eid, K. (2022). Non-Metal-Doped Porous Carbon Nitride Nanostructures for Photocatalytic Green Hydrogen Production. International Journal of Molecular Sciences, 23(23), 15129. https://doi.org/10.3390/ijms232315129