Human Lung Mast Cells: Therapeutic Implications in Asthma
Abstract
1. Introduction
2. Activating and Inhibitory Receptors on Human Mast Cells
3. Role of Mast Cells in Asthma
4. Mast Cell-Targeted Treatments for Bronchial Asthma
4.1. Histamine Receptors
4.2. Tryptase
4.3. Prostaglandin D2
4.4. Cysteinyl Leukotrienes
4.5. Mast Cell Cytokines and Their Receptors
4.6. Alarmins and Their Receptors
4.7. Tezepelumab
4.8. Itepekimab
4.9. Astegolimab and Etokimab
4.10. Tozorakimab
4.11. FcεRI and IgE
4.12. Intracellular Signaling Pathways
4.13. Silencing Mast Cells
4.14. Depleting Mast Cells
5. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Ehrlich, P. Beitrage sur Theorie und Praxis der Histologischen Farbung. Ph.D. Thesis, University of Leipzig, Leipzig, Germany, 1878. [Google Scholar]
- Bradding, P.; Arthur, G. Mast cells in asthma—State of the art. Clin. Exp. Allergy 2016, 46, 194–263. [Google Scholar] [CrossRef]
- Galli, S.J.; Kalesnikoff, J.; Grimbaldeston, M.A.; Piliponsky, A.M.; Williams, C.M.; Tsai, M. Mast cells as “tunable” effector and immunoregulatory cells: Recent advances. Annu. Rev. Immunol. 2005, 23, 749–786. [Google Scholar] [CrossRef]
- Rivellese, F.; Nerviani, A.; Rossi, F.W.; Marone, G.; Matucci-Cerinic, M.; de Paulis, A.; Pitzalis, C. Mast cells in rheumatoid arthritis: Friends or foes? Autoimmun. Rev. 2017, 16, 557–563. [Google Scholar] [CrossRef]
- Piliponsky, A.M.; Chen, C.C.; Grimbaldeston, M.A.; Burns-Guydish, S.M.; Hardy, J.; Kalesnikoff, J.; Contag, C.H.; Tsai, M.; Galli, S.J. Mast cell-derived TNF can exacerbate mortality during severe bacterial infections in C57BL/6-KitW-sh/W-sh mice. Am. J. Pathol. 2010, 176, 926–938. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.P.; Bot, I.; Kovanen, P.T. Mast cells in human and experimental cardiometabolic diseases. Nat. Rev. Cardiol. 2015, 12, 643–658. [Google Scholar] [CrossRef]
- Varricchi, G.; Marone, G.; Kovanen, P.T. Cardiac Mast Cells: Underappreciated Immune Cells in Cardiovascular Homeostasis and Disease. Trends Immunol. 2020, 41, 734–746. [Google Scholar] [CrossRef]
- Marone, G.; Varricchi, G.; Loffredo, S.; Galdiero, M.R.; Rivellese, F.; de Paulis, A. Are Basophils and Mast Cells Masters in HIV Infection? Int. Arch. Allergy Immunol. 2016, 171, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Varricchi, G.; Galdiero, M.R.; Loffredo, S.; Marone, G.; Iannone, R.; Marone, G.; Granata, F. Are Mast Cells MASTers in Cancer? Front. Immunol. 2017, 8, 424. [Google Scholar] [CrossRef]
- Cheng, S.; Li, Z.; Gao, R.; Xing, B.; Gao, Y.; Yang, Y.; Qin, S.; Zhang, L.; Ouyang, H.; Du, P.; et al. A pan-cancer single-cell transcriptional atlas of tumor infiltrating myeloid cells. Cell 2021, 184, 792–809.e23. [Google Scholar] [CrossRef] [PubMed]
- Varricchi, G.; Galdiero, M.R.; Marone, G.; Granata, F.; Borriello, F.; Marone, G. Controversial role of mast cells in skin cancers. Exp. Dermatol. 2017, 26, 11–17. [Google Scholar] [CrossRef]
- Dahlin, J.S.; Ekoff, M.; Grootens, J.; Lof, L.; Amini, R.M.; Hagberg, H.; Ungerstedt, J.S.; Olsson-Stromberg, U.; Nilsson, G. KIT signaling is dispensable for human mast cell progenitor development. Blood 2017, 130, 1785–1794. [Google Scholar] [CrossRef]
- Marone, G.; Borriello, F.; Varricchi, G.; Genovese, A.; Granata, F. Basophils: Historical reflections and perspectives. Chem. Immunol. Allergy 2014, 100, 172–192. [Google Scholar] [PubMed]
- Chhiba, K.D.; Hsu, C.L.; Berdnikovs, S.; Bryce, P.J. Transcriptional Heterogeneity of Mast Cells and Basophils upon Activation. J. Immunol. 2017, 198, 4868–4878. [Google Scholar] [CrossRef]
- Yamamoto, T.; Katayama, I.; Nishioka, K. Expression of stem cell factor in basal cell carcinoma. Br. J. Dermatol. 1997, 137, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.; Valent, P.; Galli, S.J. KIT as a master regulator of the mast cell lineage. J. Allergy Clin. Immunol. 2022, 149, 1845–1854. [Google Scholar] [CrossRef]
- Welle, M. Development, significance, and heterogeneity of mast cells with particular regard to the mast cell-specific proteases chymase and tryptase. J. Leukoc. Biol. 1997, 61, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Irani, A.M.; Schwartz, L.B. Human mast cell heterogeneity. Allergy Proc. 1994, 15, 303–308. [Google Scholar] [CrossRef]
- Varricchi, G.; de Paulis, A.; Marone, G.; Galli, S.J. Future Needs in Mast Cell Biology. Int. J. Mol. Sci. 2019, 20, 4397. [Google Scholar] [CrossRef]
- Derakhshan, T.; Samuchiwal, S.K.; Hallen, N.; Bankova, L.G.; Boyce, J.A.; Barrett, N.A.; Austen, K.F.; Dwyer, D.F. Lineage-specific regulation of inducible and constitutive mast cells in allergic airway inflammation. J. Exp. Med. 2021, 218, e20200321. [Google Scholar] [CrossRef]
- Dwyer, D.F.; Ordovas-Montanes, J.; Allon, S.J.; Buchheit, K.M.; Vukovic, M.; Derakhshan, T.; Feng, C.; Lai, J.; Hughes, T.K.; Nyquist, S.K.; et al. Human airway mast cells proliferate and acquire distinct inflammation-driven phenotypes during type 2 inflammation. Sci. Immunol. 2021, 6, eabb7221. [Google Scholar] [CrossRef]
- Dwyer, D.F.; Barrett, N.A.; Austen, K.F. Expression profiling of constitutive mast cells reveals a unique identity within the immune system. Nat. Immunol. 2016, 17, 878–887. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Kurotaki, D.; Osato, N.; Sato, H.; Sasaki, I.; Koizumi, S.; Wang, H.; Kaneda, C.; Nishiyama, A.; Kaisho, T.; et al. Transcription factor IRF8 plays a critical role in the development of murine basophils and mast cells. Blood 2015, 125, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Motakis, E.; Guhl, S.; Ishizu, Y.; Itoh, M.; Kawaji, H.; de Hoon, M.; Lassmann, T.; Carninci, P.; Hayashizaki, Y.; Zuberbier, T.; et al. Redefinition of the human mast cell transcriptome by deep-CAGE sequencing. Blood 2014, 123, e58–e67. [Google Scholar] [CrossRef] [PubMed]
- Plum, T.; Wang, X.; Rettel, M.; Krijgsveld, J.; Feyerabend, T.B.; Rodewald, H.R. Human Mast Cell Proteome Reveals Unique Lineage, Putative Functions, and Structural Basis for Cell Ablation. Immunity 2020, 52, 404–416.e5. [Google Scholar] [CrossRef] [PubMed]
- Reyfman, P.A.; Walter, J.M.; Joshi, N.; Anekalla, K.R.; McQuattie-Pimentel, A.C.; Chiu, S.; Fernandez, R.; Akbarpour, M.; Chen, C.I.; Ren, Z.; et al. Single-Cell Transcriptomic Analysis of Human Lung Provides Insights into the Pathobiology of Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2019, 199, 1517–1536. [Google Scholar] [CrossRef]
- Zilionis, R.; Engblom, C.; Pfirschke, C.; Savova, V.; Zemmour, D.; Saatcioglu, H.D.; Krishnan, I.; Maroni, G.; Meyerovitz, C.V.; Kerwin, C.M.; et al. Single-Cell Transcriptomics of Human and Mouse Lung Cancers Reveals Conserved Myeloid Populations across Individuals and Species. Immunity 2019, 50, 1317–1334.e10. [Google Scholar] [CrossRef]
- Cildir, G.; Toubia, J.; Yip, K.H.; Zhou, M.; Pant, H.; Hissaria, P.; Zhang, J.; Hong, W.; Robinson, N.; Grimbaldeston, M.A.; et al. Genome-wide Analyses of Chromatin State in Human Mast Cells Reveal Molecular Drivers and Mediators of Allergic and Inflammatory Diseases. Immunity 2019, 51, 949–965.e6. [Google Scholar] [CrossRef]
- Andersson, C.K.; Mori, M.; Bjermer, L.; Lofdahl, C.G.; Erjefalt, J.S. Novel site-specific mast cell subpopulations in the human lung. Thorax. 2009, 64, 297–305. [Google Scholar] [CrossRef]
- Galdiero, M.R.; Varricchi, G.; Seaf, M.; Marone, G.; Levi-Schaffer, F.; Marone, G. Bidirectional Mast Cell-Eosinophil Interactions in Inflammatory Disorders and Cancer. Front. Med. 2017, 4, 103. [Google Scholar] [CrossRef]
- Poto, R.; Quinti, I.; Marone, G.; Taglialatela, M.; de Paulis, A.; Casolaro, V.; Varricchi, G. IgG Autoantibodies Against IgE from Atopic Dermatitis Can Induce the Release of Cytokines and Proinflammatory Mediators from Basophils and Mast Cells. Front. Immunol. 2022, 13, 880412. [Google Scholar] [CrossRef]
- Genovese, A.; Borgia, G.; Bjorck, L.; Petraroli, A.; de Paulis, A.; Piazza, M.; Marone, G. Immunoglobulin superantigen protein L induces IL-4 and IL-13 secretion from human Fc epsilon RI+ cells through interaction with the kappa light chains of IgE. J. Immunol. 2003, 170, 1854–1861. [Google Scholar] [CrossRef] [PubMed]
- Florio, G.; Petraroli, A.; Patella, V.; Triggiani, M.; Marone, G. The immunoglobulin superantigen-binding site of HIV-1 gp120 activates human basophils. AIDS 2000, 14, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Leist, M.; Sunder, C.A.; Drube, S.; Zimmermann, C.; Geldmacher, A.; Metz, M.; Dudeck, A.; Maurer, M. Membrane-bound stem cell factor is the major but not only driver of fibroblast-induced murine skin mast cell differentiation. Exp. Dermatol. 2017, 26, 255–262. [Google Scholar] [CrossRef]
- Da Silva, C.A.; Reber, L.; Frossard, N. Stem cell factor expression, mast cells and inflammation in asthma. Fundam. Clin. Pharmacol. 2006, 20, 21–39. [Google Scholar] [CrossRef]
- Patella, V.; Marino, I.; Arbustini, E.; Lamparter-Schummert, B.; Verga, L.; Adt, M.; Marone, G. Stem cell factor in mast cells and increased mast cell density in idiopathic and ischemic cardiomyopathy. Circulation 1998, 97, 971–978. [Google Scholar] [CrossRef]
- de Paulis, A.; Minopoli, G.; Arbustini, E.; de Crescenzo, G.; Dal Piaz, F.; Pucci, P.; Russo, T.; Marone, G. Stem cell factor is localized in, released from, and cleaved by human mast cells. J. Immunol. 1999, 163, 2799–2808. [Google Scholar] [PubMed]
- Semlali, A.; Jacques, E.; Koussih, L.; Gounni, A.S.; Chakir, J. Thymic stromal lymphopoietin-induced human asthmatic airway epithelial cell proliferation through an IL-13-dependent pathway. J. Allergy Clin. Immunol. 2010, 125, 844–850. [Google Scholar] [CrossRef]
- Leichner, T.M.; Satake, A.; Harrison, V.S.; Tanaka, Y.; Archambault, A.S.; Kim, B.S.; Siracusa, M.C.; Leonard, W.J.; Naji, A.; Wu, G.F.; et al. Skin-derived TSLP systemically expands regulatory T cells. J. Autoimmun. 2017, 79, 39–52. [Google Scholar] [CrossRef]
- Varricchi, G.; Pecoraro, A.; Marone, G.; Criscuolo, G.; Spadaro, G.; Genovese, A.; Marone, G. Thymic Stromal Lymphopoietin Isoforms, Inflammatory Disorders, and Cancer. Front. Immunol. 2018, 9, 1595. [Google Scholar] [CrossRef]
- Siracusa, M.C.; Saenz, S.A.; Hill, D.A.; Kim, B.S.; Headley, M.B.; Doering, T.A.; Wherry, E.J.; Jessup, H.K.; Siegel, L.A.; Kambayashi, T.; et al. TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation. Nature 2011, 477, 229–233. [Google Scholar] [CrossRef]
- Allakhverdi, Z.; Comeau, M.R.; Jessup, H.K.; Yoon, B.R.; Brewer, A.; Chartier, S.; Paquette, N.; Ziegler, S.F.; Sarfati, M.; Delespesse, G. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J. Exp. Med. 2007, 204, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Gambardella, A.R.; Poto, R.; Tirelli, V.; Schroeder, J.T.; Marone, G.; Mattei, F.; Varricchi, G.; Schiavoni, G. Differential Effects of Alarmins on Human and Mouse Basophils. Front. Immunol. 2022, 13, 894163. [Google Scholar] [CrossRef] [PubMed]
- Shikotra, A.; Ohri, C.M.; Green, R.H.; Waller, D.A.; Bradding, P. Mast cell phenotype, TNFalpha expression and degranulation status in non-small cell lung cancer. Sci. Rep. 2016, 6, 38352. [Google Scholar] [CrossRef] [PubMed]
- Kaur, D.; Doe, C.; Woodman, L.; Heidi Wan, W.Y.; Sutcliffe, A.; Hollins, F.; Brightling, C. Mast cell-airway smooth muscle crosstalk: The role of thymic stromal lymphopoietin. Chest 2012, 142, 76–85. [Google Scholar] [CrossRef]
- Afferni, C.; Buccione, C.; Andreone, S.; Galdiero, M.R.; Varricchi, G.; Marone, G.; Mattei, F.; Schiavoni, G. The Pleiotropic Immunomodulatory Functions of IL-33 and Its Implications in Tumor Immunity. Front. Immunol. 2018, 9, 2601. [Google Scholar] [CrossRef]
- Cayrol, C.; Girard, J.P. Interleukin-33 (IL-33): A nuclear cytokine from the IL-1 family. Immunol. Rev. 2018, 281, 154–168. [Google Scholar] [CrossRef]
- Hsu, C.L.; Neilsen, C.V.; Bryce, P.J. IL-33 is produced by mast cells and regulates IgE-dependent inflammation. PLoS ONE 2010, 5, e11944. [Google Scholar] [CrossRef]
- Russi, A.E.; Ebel, M.E.; Yang, Y.; Brown, M.A. Male-specific IL-33 expression regulates sex-dimorphic EAE susceptibility. Proc. Natl. Acad. Sci. USA 2018, 115, E1520–E1529. [Google Scholar] [CrossRef]
- Iikura, M.; Suto, H.; Kajiwara, N.; Oboki, K.; Ohno, T.; Okayama, Y.; Saito, H.; Galli, S.J.; Nakae, S. IL-33 can promote survival, adhesion and cytokine production in human mast cells. Lab. Invest. 2007, 87, 971–978. [Google Scholar] [CrossRef]
- Bandara, G.; Beaven, M.A.; Olivera, A.; Gilfillan, A.M.; Metcalfe, D.D. Activated mast cells synthesize and release soluble ST2-a decoy receptor for IL-33. Eur. J. Immunol. 2015, 45, 3034–3044. [Google Scholar] [CrossRef]
- Kaur, D.; Gomez, E.; Doe, C.; Berair, R.; Woodman, L.; Saunders, R.; Hollins, F.; Rose, F.R.; Amrani, Y.; May, R.; et al. IL-33 drives airway hyper-responsiveness through IL-13-mediated mast cell: Airway smooth muscle crosstalk. Allergy 2015, 70, 556–567. [Google Scholar] [CrossRef]
- Silver, M.R.; Margulis, A.; Wood, N.; Goldman, S.J.; Kasaian, M.; Chaudhary, D. IL-33 synergizes with IgE-dependent and IgE-independent agents to promote mast cell and basophil activation. Inflamm. Res. 2010, 59, 207–218. [Google Scholar] [CrossRef]
- Joulia, R.; L’Faqihi, F.E.; Valitutti, S.; Espinosa, E. IL-33 fine tunes mast cell degranulation and chemokine production at the single-cell level. J. Allergy Clin. Immunol. 2017, 140, 497–509.e10. [Google Scholar] [CrossRef] [PubMed]
- Taracanova, A.; Alevizos, M.; Karagkouni, A.; Weng, Z.; Norwitz, E.; Conti, P.; Leeman, S.E.; Theoharides, T.C. SP and IL-33 together markedly enhance TNF synthesis and secretion from human mast cells mediated by the interaction of their receptors. Proc. Natl. Acad. Sci. USA 2017, 114, E4002–E4009. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Zhang, B.; Kempuraj, D.; Tagen, M.; Vasiadi, M.; Angelidou, A.; Alysandratos, K.D.; Kalogeromitros, D.; Asadi, S.; Stavrianeas, N.; et al. IL-33 augments substance P-induced VEGF secretion from human mast cells and is increased in psoriatic skin. Proc. Natl. Acad. Sci. USA 2010, 107, 4448–4453. [Google Scholar] [CrossRef] [PubMed]
- Rivellese, F.; Suurmond, J.; Habets, K.; Dorjee, A.L.; Ramamoorthi, N.; Townsend, M.J.; de Paulis, A.; Marone, G.; Huizinga, T.W.; Pitzalis, C.; et al. Ability of Interleukin-33- and Immune Complex-Triggered Activation of Human Mast Cells to Down-Regulate Monocyte-Mediated Immune Responses. Arthritis Rheumatol. 2015, 67, 2343–2353. [Google Scholar] [CrossRef]
- Cristinziano, L.; Poto, R.; Criscuolo, G.; Ferrara, A.L.; Galdiero, M.R.; Modestino, L.; Loffredo, S.; de Paulis, A.; Marone, G.; Spadaro, G.; et al. IL-33 and Superantigenic Activation of Human Lung Mast Cells Induce the Release of Angiogenic and Lymphangiogenic Factors. Cells 2021, 10, 145. [Google Scholar] [CrossRef]
- Igawa, S.; Di Nardo, A. Skin microbiome and mast cells. Transl. Res. 2017, 184, 68–76. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Yu, Y.Y.; Zhang, Y.R.; Zhang, W.; Yu, B. The modulatory effect of TLR2 on LL-37-induced human mast cells activation. Biochem. Biophys. Res. Commun. 2016, 470, 368–374. [Google Scholar] [CrossRef]
- Kulka, M.; Alexopoulou, L.; Flavell, R.A.; Metcalfe, D.D. Activation of mast cells by double-stranded RNA: Evidence for activation through Toll-like receptor 3. J. Allergy. Clin. Immunol. 2004, 114, 174–182. [Google Scholar] [CrossRef]
- Suurmond, J.; Rivellese, F.; Dorjee, A.L.; Bakker, A.M.; Rombouts, Y.J.; Rispens, T.; Wolbink, G.; Zaldumbide, A.; Hoeben, R.C.; Huizinga, T.W.; et al. Toll-like receptor triggering augments activation of human mast cells by anti-citrullinated protein antibodies. Ann. Rheum. Dis. 2015, 74, 1915–1923. [Google Scholar] [CrossRef] [PubMed]
- Suurmond, J.; Dorjee, A.L.; Knol, E.F.; Huizinga, T.W.; Toes, R.E. Differential TLR-induced cytokine production by human mast cells is amplified by FcvarepsilonRI triggering. Clin. Exp. Allergy 2015, 45, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, J.T. Basophils: Emerging roles in the pathogenesis of allergic disease. Immunol. Rev. 2011, 242, 144–160. [Google Scholar] [CrossRef] [PubMed]
- Patella, V.; Marino, I.; Lamparter, B.; Arbustini, E.; Adt, M.; Marone, G. Human heart mast cells. Isolation, purification, ultrastructure, and immunologic characterization. J. Immunol. 1995, 154, 2855–2865. [Google Scholar] [PubMed]
- Hofstra, C.L.; Desai, P.J.; Thurmond, R.L.; Fung-Leung, W.P. Histamine H4 receptor mediates chemotaxis and calcium mobilization of mast cells. J. Pharmacol. Exp. Ther. 2003, 305, 1212–1221. [Google Scholar] [CrossRef]
- Triggiani, M.; Gentile, M.; Secondo, A.; Granata, F.; Oriente, A.; Taglialatela, M.; Annunziato, L.; Marone, G. Histamine induces exocytosis and IL-6 production from human lung macrophages through interaction with H1 receptors. J. Immunol. 2001, 166, 4083–4091. [Google Scholar] [CrossRef]
- Levi-Schaffer, F.; Eliashar, R. Mast cell stabilizing properties of antihistamines. J. Invest. Dermatol. 2009, 129, 2549–2551. [Google Scholar] [CrossRef]
- Genovese, A.; Patella, V.; De Crescenzo, G.; De Paulis, A.; Spadaro, G.; Marone, G. Loratadine and desethoxylcarbonyl-loratadine inhibit the immunological release of mediators from human Fc epsilon RI+ cells. Clin. Exp. Allergy 1997, 27, 559–567. [Google Scholar] [CrossRef]
- Malbec, O.; Cassard, L.; Albanesi, M.; Jonsson, F.; Mancardi, D.; Chicanne, G.; Payrastre, B.; Dubreuil, P.; Vivier, E.; Daeron, M. Trans-inhibition of activation and proliferation signals by Fc receptors in mast cells and basophils. Sci. Signal 2016, 9, ra126. [Google Scholar] [CrossRef]
- Levi-Schaffer, F.; Gibbs, B.F.; Hallgren, J.; Pucillo, C.; Redegeld, F.; Siebenhaar, F.; Vitte, J.; Mezouar, S.; Michel, M.; Puzzovio, P.G.; et al. Selected recent advances in understanding the role of human mast cells in health and disease. J. Allergy Clin. Immunol. 2022, 149, 1833–1844. [Google Scholar] [CrossRef]
- Gibbs, B.F.; Sabato, V.; Bridts, C.H.; Ebo, D.G.; Ben-Zimra, M.; Levi-Schaffer, F. Expressions and inhibitory functions of CD300a receptors on purified human basophils. Exp. Dermatol. 2012, 21, 884–886. [Google Scholar] [CrossRef]
- Sabato, V.; Verweij, M.M.; Bridts, C.H.; Levi-Schaffer, F.; Gibbs, B.F.; De Clerck, L.S.; Schiavino, D.; Ebo, D.G. CD300a is expressed on human basophils and seems to inhibit IgE/FcepsilonRI-dependent anaphylactic degranulation. Cytom. B Clin. Cytom. 2012, 82, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Bachelet, I.; Munitz, A.; Moretta, A.; Moretta, L.; Levi-Schaffer, F. The inhibitory receptor IRp60 (CD300a) is expressed and functional on human mast cells. J. Immunol. 2005, 175, 7989–7995. [Google Scholar] [CrossRef]
- Bachelet, I.; Munitz, A.; Levi-Schaffer, F. Abrogation of allergic reactions by a bispecific antibody fragment linking IgE to CD300a. J. Allergy Clin. Immunol. 2006, 117, 1314–1320. [Google Scholar] [CrossRef]
- Robida, P.A.; Rische, C.H.; Morgenstern, N.B.; Janarthanam, R.; Cao, Y.; Krier-Burris, R.A.; Korver, W.; Xu, A.; Luu, T.; Schanin, J.; et al. Functional and Phenotypic Characterization of Siglec-6 on Human Mast Cells. Cells 2022, 11, 1138. [Google Scholar] [CrossRef] [PubMed]
- Hudson, S.A.; Herrmann, H.; Du, J.; Cox, P.; Haddadel, B.; Butler, B.; Crocker, P.R.; Ackerman, S.J.; Valent, P.; Bochner, B.S. Developmental, malignancy-related, and cross-species analysis of eosinophil, mast cell, and basophil siglec-8 expression. J. Clin. Immunol. 2011, 31, 1045–1053. [Google Scholar] [CrossRef]
- Mizrahi, S.; Gibbs, B.F.; Karra, L.; Ben-Zimra, M.; Levi-Schaffer, F. Siglec-7 is an inhibitory receptor on human mast cells and basophils. J. Allergy Clin. Immunol. 2014, 134, 230–233. [Google Scholar] [CrossRef]
- Yokoi, H.; Myers, A.; Matsumoto, K.; Crocker, P.R.; Saito, H.; Bochner, B.S. Alteration and acquisition of Siglecs during in vitro maturation of CD34+ progenitors into human mast cells. Allergy 2006, 61, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Korver, W.; Wong, A.; Gebremeskel, S.; Negri, G.L.; Schanin, J.; Chang, K.; Leung, J.; Benet, Z.; Luu, T.; Brock, E.C.; et al. The Inhibitory Receptor Siglec-8 Interacts With FcepsilonRI and Globally Inhibits Intracellular Signaling in Primary Mast Cells Upon Activation. Front. Immunol. 2022, 13, 833728. [Google Scholar] [CrossRef] [PubMed]
- Kikly, K.K.; Bochner, B.S.; Freeman, S.D.; Tan, K.B.; Gallagher, K.T.; D’Alessio, K.J.; Holmes, S.D.; Abrahamson, J.A.; Erickson-Miller, C.L.; Murdock, P.R.; et al. Identification of SAF-2, a novel siglec expressed on eosinophils, mast cells, and basophils. J. Allergy Clin. Immunol. 2000, 105, 1093–1100. [Google Scholar] [CrossRef]
- Kiwamoto, T.; Kawasaki, N.; Paulson, J.C.; Bochner, B.S. Siglec-8 as a drugable target to treat eosinophil and mast cell-associated conditions. Pharmacol. Ther 2012, 135, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Okayama, Y.; Kirshenbaum, A.S.; Metcalfe, D.D. Expression of a functional high-affinity IgG receptor, Fc gamma RI, on human mast cells: Up-regulation by IFN-gamma. J. Immunol. 2000, 164, 4332–4339. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Kepley, C.L.; Zhang, M.; Zhang, K.; Saxon, A. A novel human immunoglobulin Fc gamma Fc epsilon bifunctional fusion protein inhibits Fc epsilon RI-mediated degranulation. Nat. Med. 2002, 8, 518–521. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Kepley, C.L.; Zhang, K.; Terada, T.; Yamada, T.; Saxon, A. A chimeric human-cat fusion protein blocks cat-induced allergy. Nat. Med. 2005, 11, 446–449. [Google Scholar] [CrossRef]
- Zhang, K.; Kepley, C.L.; Terada, T.; Zhu, D.; Perez, H.; Saxon, A. Inhibition of allergen-specific IgE reactivity by a human Ig Fcgamma-Fcepsilon bifunctional fusion protein. J. Allergy Clin. Immunol. 2004, 114, 321–327. [Google Scholar] [CrossRef]
- Cemerski, S.; Chu, S.Y.; Moore, G.L.; Muchhal, U.S.; Desjarlais, J.R.; Szymkowski, D.E. Suppression of mast cell degranulation through a dual-targeting tandem IgE-IgG Fc domain biologic engineered to bind with high affinity to FcgammaRIIb. Immunol. Lett. 2012, 143, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Varricchi, G.; Raap, U.; Rivellese, F.; Marone, G.; Gibbs, B.F. Human mast cells and basophils-How are they similar how are they different? Immunol. Rev. 2018, 282, 8–34. [Google Scholar] [CrossRef]
- Harvima, I.T.; Nilsson, G. Mast cells as regulators of skin inflammation and immunity. Acta Derm. Venereol. 2011, 91, 644–650. [Google Scholar] [CrossRef]
- Brightling, C.E.; Bradding, P.; Symon, F.A.; Holgate, S.T.; Wardlaw, A.J.; Pavord, I.D. Mast-cell infiltration of airway smooth muscle in asthma. N. Engl. J. Med. 2002, 346, 1699–1705. [Google Scholar] [CrossRef]
- Kaur, D.; Saunders, R.; Hollins, F.; Woodman, L.; Doe, C.; Siddiqui, S.; Bradding, P.; Brightling, C. Mast cell fibroblastoid differentiation mediated by airway smooth muscle in asthma. J. Immunol. 2010, 185, 6105–6114. [Google Scholar] [CrossRef]
- Maun, H.R.; Jackman, J.K.; Choy, D.F.; Loyet, K.M.; Staton, T.L.; Jia, G.; Dressen, A.; Hackney, J.A.; Bremer, M.; Walters, B.T.; et al. An Allosteric Anti-tryptase Antibody for the Treatment of Mast Cell-Mediated Severe Asthma. Cell 2019, 179, 417–431.e19. [Google Scholar] [CrossRef] [PubMed]
- Caughey, G.H. Mast cell proteases as pharmacological targets. Eur. J. Pharmacol. 2016, 778, 44–55. [Google Scholar] [CrossRef]
- Huttunen, M.; Harvima, I.T. Mast cell tryptase and chymase in chronic leg ulcers: Chymase is potentially destructive to epithelium and is controlled by proteinase inhibitors. Br. J. Dermatol. 2005, 152, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Irani, A.M.; Goldstein, S.M.; Wintroub, B.U.; Bradford, T.; Schwartz, L.B. Human mast cell carboxypeptidase. Selective localization to MCTC cells. J. Immunol. 1991, 147, 247–253. [Google Scholar]
- Strik, M.C.; de Koning, P.J.; Kleijmeer, M.J.; Bladergroen, B.A.; Wolbink, A.M.; Griffith, J.M.; Wouters, D.; Fukuoka, Y.; Schwartz, L.B.; Hack, C.E.; et al. Human mast cells produce and release the cytotoxic lymphocyte associated protease granzyme B upon activation. Mol. Immunol. 2007, 44, 3462–3472. [Google Scholar] [CrossRef] [PubMed]
- Di Girolamo, N.; Indoh, I.; Jackson, N.; Wakefield, D.; McNeil, H.P.; Yan, W.; Geczy, C.; Arm, J.P.; Tedla, N. Human mast cell-derived gelatinase B (matrix metalloproteinase-9) is regulated by inflammatory cytokines: Role in cell migration. J. Immunol. 2006, 177, 2638–2650. [Google Scholar] [CrossRef] [PubMed]
- MacGlashan, D.W.; Peters, S.P., Jr.; Warner, J.; Lichtenstein, L.M. Characteristics of human basophil sulfidopeptide leukotriene release: Releasability defined as the ability of the basophil to respond to dimeric cross-links. J. Immunol. 1986, 136, 2231–2239. [Google Scholar]
- Austen, K.F. The cysteinyl leukotrienes: Where do they come from? What are they? Where are they going? Nat. Immunol. 2008, 9, 113–115. [Google Scholar] [CrossRef] [PubMed]
- Dahlen, S.E.; Kumlin, M. Monitoring mast cell activation by prostaglandin D2 in vivo. Thorax 2004, 59, 453–455. [Google Scholar] [CrossRef]
- Schuligoi, R.; Sturm, E.; Luschnig, P.; Konya, V.; Philipose, S.; Sedej, M.; Waldhoer, M.; Peskar, B.A.; Heinemann, A. CRTH2 and D-type prostanoid receptor antagonists as novel therapeutic agents for inflammatory diseases. Pharmacology 2010, 85, 372–382. [Google Scholar] [CrossRef]
- Triggiani, M.; Hubbard, W.C.; Chilton, F.H. Synthesis of 1-acyl-2-acetyl-sn-glycero-3-phosphocholine by an enriched preparation of the human lung mast cell. J. Immunol. 1990, 144, 4773–4780. [Google Scholar] [PubMed]
- Ohkawara, Y.; Yamauchi, K.; Tanno, Y.; Tamura, G.; Ohtani, H.; Nagura, H.; Ohkuda, K.; Takishima, T. Human lung mast cells and pulmonary macrophages produce tumor necrosis factor-alpha in sensitized lung tissue after IgE receptor triggering. Am. J. Respir. Cell Mol. Biol. 1992, 7, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Okayama, Y.; Hagaman, D.D.; Metcalfe, D.D. A comparison of mediators released or generated by IFN-gamma-treated human mast cells following aggregation of Fc gamma RI or Fc epsilon RI. J. Immunol. 2001, 166, 4705–4712. [Google Scholar] [CrossRef] [PubMed]
- Nouri-Aria, K.T.; Pilette, C.; Jacobson, M.R.; Watanabe, H.; Durham, S.R. IL-9 and c-Kit+ mast cells in allergic rhinitis during seasonal allergen exposure: Effect of immunotherapy. J. Allergy Clin. Immunol. 2005, 116, 73–79. [Google Scholar] [CrossRef]
- Ishizuka, T.; Okayama, Y.; Kobayashi, H.; Mori, M. Interleukin-3 production by mast cells from human lung. Inflammation 1999, 23, 25–35. [Google Scholar] [CrossRef]
- Okayama, Y.; Petit-Frere, C.; Kassel, O.; Semper, A.; Quint, D.; Tunon-de-Lara, M.J.; Bradding, P.; Holgate, S.T.; Church, M.K. IgE-dependent expression of mRNA for IL-4 and IL-5 in human lung mast cells. J. Immunol. 1995, 155, 1796–1808. [Google Scholar]
- Suttle, M.M.; Nilsson, G.; Snellman, E.; Harvima, I.T. Experimentally induced psoriatic lesion associates with interleukin (IL)-6 in mast cells and appearance of dermal cells expressing IL-33 and IL-6 receptor. Clin. Exp. Immunol. 2012, 169, 311–319. [Google Scholar] [CrossRef]
- Lorentz, A.; Schwengberg, S.; Sellge, G.; Manns, M.P.; Bischoff, S.C. Human intestinal mast cells are capable of producing different cytokine profiles: Role of IgE receptor cross-linking and IL-4. J. Immunol. 2000, 164, 43–48. [Google Scholar] [CrossRef]
- Burd, P.R.; Thompson, W.C.; Max, E.E.; Mills, F.C. Activated mast cells produce interleukin 13. J. Exp. Med. 1995, 181, 1373–1380. [Google Scholar] [CrossRef]
- Rumsaeng, V.; Cruikshank, W.W.; Foster, B.; Prussin, C.; Kirshenbaum, A.S.; Davis, T.A.; Kornfeld, H.; Center, D.M.; Metcalfe, D.D. Human mast cells produce the CD4+ T lymphocyte chemoattractant factor, IL-16. J. Immunol. 1997, 159, 2904–2910. [Google Scholar]
- Mashiko, S.; Bouguermouh, S.; Rubio, M.; Baba, N.; Bissonnette, R.; Sarfati, M. Human mast cells are major IL-22 producers in patients with psoriasis and atopic dermatitis. J. Allergy Clin. Immunol. 2015, 136, 351–359.e1. [Google Scholar] [CrossRef]
- Okayama, Y.; Okumura, S.; Sagara, H.; Yuki, K.; Sasaki, T.; Watanabe, N.; Fueki, M.; Sugiyama, K.; Takeda, K.; Fukuda, T.; et al. FcepsilonRI-mediated thymic stromal lymphopoietin production by interleukin-4-primed human mast cells. Eur. Respir. J. 2009, 34, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Ying, S.; O’Connor, B.; Ratoff, J.; Meng, Q.; Mallett, K.; Cousins, D.; Robinson, D.; Zhang, G.; Zhao, J.; Lee, T.H.; et al. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J. Immunol. 2005, 174, 8183–8190. [Google Scholar] [CrossRef] [PubMed]
- Shikotra, A.; Choy, D.F.; Ohri, C.M.; Doran, E.; Butler, C.; Hargadon, B.; Shelley, M.; Abbas, A.R.; Austin, C.D.; Jackman, J.; et al. Increased expression of immunoreactive thymic stromal lymphopoietin in patients with severe asthma. J. Allergy Clin. Immunol. 2012, 129, 104–111.e1-9. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.W.; Kim, D.K.; Park, M.H.; Eun, K.M.; Lee, M.; So, D.; Kong, I.G.; Mo, J.H.; Yang, M.S.; Jin, H.R.; et al. IL-25 as a novel therapeutic target in nasal polyps of patients with chronic rhinosinusitis. J. Allergy Clin. Immunol. 2015, 135, 1476–1485.e7. [Google Scholar] [CrossRef]
- Detoraki, A.; Staiano, R.I.; Granata, F.; Giannattasio, G.; Prevete, N.; de Paulis, A.; Ribatti, D.; Genovese, A.; Triggiani, M.; Marone, G. Vascular endothelial growth factors synthesized by human lung mast cells exert angiogenic effects. J. Allergy Clin. Immunol. 2009, 123, 1142–1149.e1-5. [Google Scholar] [CrossRef]
- Dvorak, A.M.; Morgan, E.S.; Weller, P.F. Ultrastructural immunolocalization of basic fibroblast growth factor to lipid bodies and secretory granules in human mast cells. Histochem. J. 2001, 33, 397–402. [Google Scholar] [CrossRef]
- Nilsson, G.; Forsberg-Nilsson, K.; Xiang, Z.; Hallbook, F.; Nilsson, K.; Metcalfe, D.D. Human mast cells express functional TrkA and are a source of nerve growth factor. Eur. J. Immunol. 1997, 27, 2295–2301. [Google Scholar] [CrossRef]
- Okumura, S.; Sagara, H.; Fukuda, T.; Saito, H.; Okayama, Y. FcepsilonRI-mediated amphiregulin production by human mast cells increases mucin gene expression in epithelial cells. J. Allergy Clin. Immunol. 2005, 115, 272–279. [Google Scholar] [CrossRef]
- Wang, S.W.; Oh, C.K.; Cho, S.H.; Hu, G.; Martin, R.; Demissie-Sanders, S.; Li, K.; Moyle, M.; Yao, Z. Amphiregulin expression in human mast cells and its effect on the primary human lung fibroblasts. J. Allergy Clin. Immunol. 2005, 115, 287–294. [Google Scholar] [CrossRef]
- Yano, K.; Yamaguchi, M.; de Mora, F.; Lantz, C.S.; Butterfield, J.H.; Costa, J.J.; Galli, S.J. Production of macrophage inflammatory protein-1alpha by human mast cells: Increased anti-IgE-dependent secretion after IgE-dependent enhancement of mast cell IgE-binding ability. Lab. Invest. 1997, 77, 185–193. [Google Scholar] [PubMed]
- Melillo, R.M.; Guarino, V.; Avilla, E.; Galdiero, M.R.; Liotti, F.; Prevete, N.; Rossi, F.W.; Basolo, F.; Ugolini, C.; de Paulis, A.; et al. Mast cells have a protumorigenic role in human thyroid cancer. Oncogene 2010, 29, 6203–6215. [Google Scholar] [CrossRef] [PubMed]
- Visciano, C.; Liotti, F.; Prevete, N.; Cali, G.; Franco, R.; Collina, F.; de Paulis, A.; Marone, G.; Santoro, M.; Melillo, R.M. Mast cells induce epithelial-to-mesenchymal transition and stem cell features in human thyroid cancer cells through an IL-8-Akt-Slug pathway. Oncogene 2015, 34, 5175–5186. [Google Scholar] [CrossRef] [PubMed]
- Granata, F.; Frattini, A.; Loffredo, S.; Staiano, R.I.; Petraroli, A.; Ribatti, D.; Oslund, R.; Gelb, M.H.; Lambeau, G.; Marone, G.; et al. Production of vascular endothelial growth factors from human lung macrophages induced by group IIA and group X secreted phospholipases A2. J. Immunol. 2010, 184, 5232–5241. [Google Scholar] [CrossRef]
- de Paulis, A.; Prevete, N.; Fiorentino, I.; Rossi, F.W.; Staibano, S.; Montuori, N.; Ragno, P.; Longobardi, A.; Liccardo, B.; Genovese, A.; et al. Expression and functions of the vascular endothelial growth factors and their receptors in human basophils. J. Immunol. 2006, 177, 7322–7331. [Google Scholar] [CrossRef]
- Loffredo, S.; Borriello, F.; Iannone, R.; Ferrara, A.L.; Galdiero, M.R.; Gigantino, V.; Esposito, P.; Varricchi, G.; Lambeau, G.; Cassatella, M.A.; et al. Group V Secreted Phospholipase A2 Induces the Release of Proangiogenic and Antiangiogenic Factors by Human Neutrophils. Front. Immunol. 2017, 8, 443. [Google Scholar] [CrossRef]
- Poto, R.; Cristinziano, L.; Modestino, L.; de Paulis, A.; Marone, G.; Loffredo, S.; Galdiero, M.R.; Varricchi, G. Neutrophil Extracellular Traps, Angiogenesis and Cancer. Biomedicines 2022, 10, 431. [Google Scholar] [CrossRef]
- Thomas, M.; Augustin, H.G. The role of the Angiopoietins in vascular morphogenesis. Angiogenesis 2009, 12, 125–137. [Google Scholar] [CrossRef]
- Loffredo, S.; Bova, M.; Suffritti, C.; Borriello, F.; Zanichelli, A.; Petraroli, A.; Varricchi, G.; Triggiani, M.; Cicardi, M.; Marone, G. Elevated plasma levels of vascular permeability factors in C1 inhibitor-deficient hereditary angioedema. Allergy 2016, 71, 989–996. [Google Scholar] [CrossRef]
- Varricchi, G.; Loffredo, S.; Bencivenga, L.; Ferrara, A.L.; Gambino, G.; Ferrara, N.; de Paulis, A.; Marone, G.; Rengo, G. Angiopoietins, Vascular Endothelial Growth Factors and Secretory Phospholipase A2 in Ischemic and Non-Ischemic Heart Failure. J. Clin. Med. 2020, 9, 1928. [Google Scholar] [CrossRef]
- Varricchi, G.; Poto, R.; Ferrara, A.L.; Gambino, G.; Marone, G.; Rengo, G.; Loffredo, S.; Bencivenga, L. Angiopoietins, vascular endothelial growth factors and secretory phospholipase A2 in heart failure patients with preserved ejection fraction. Eur. J. Intern. Med. 2022, 22, S0953. [Google Scholar] [CrossRef] [PubMed]
- Duah, E.; Teegala, L.R.; Kondeti, V.; Adapala, R.K.; Keshamouni, V.G.; Kanaoka, Y.; Austen, K.F.; Thodeti, C.K.; Paruchuri, S. Cysteinyl leukotriene 2 receptor promotes endothelial permeability, tumor angiogenesis, and metastasis. Proc. Natl. Acad. Sci. USA 2019, 116, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Patella, V.; Florio, G.; Petraroli, A.; Marone, G. HIV-1 gp120 induces IL-4 and IL-13 release from human Fc epsilon RI+ cells through interaction with the VH3 region of IgE. J. Immunol. 2000, 164, 589–595. [Google Scholar] [CrossRef]
- Yang, W.; Kaur, D.; Okayama, Y.; Ito, A.; Wardlaw, A.J.; Brightling, C.E.; Bradding, P. Human lung mast cells adhere to human airway smooth muscle, in part, via tumor suppressor in lung cancer-1. J. Immunol. 2006, 176, 1238–1243. [Google Scholar] [CrossRef]
- Hollins, F.; Kaur, D.; Yang, W.; Cruse, G.; Saunders, R.; Sutcliffe, A.; Berger, P.; Ito, A.; Brightling, C.E.; Bradding, P. Human airway smooth muscle promotes human lung mast cell survival, proliferation, and constitutive activation: Cooperative roles for CADM1, stem cell factor, and IL-6. J. Immunol. 2008, 181, 2772–2780. [Google Scholar] [CrossRef] [PubMed]
- Salomonsson, M.; Malinovschi, A.; Kalm-Stephens, P.; Dahlin, J.S.; Janson, C.; Alving, K.; Hallgren, J. Circulating mast cell progenitors correlate with reduced lung function in allergic asthma. Clin. Exp. Allergy 2019, 49, 874–882. [Google Scholar] [CrossRef]
- Schleimer, R.P.; MacGlashan, D.W.; Peters, S.P., Jr.; Pinckard, R.N.; Adkinson, N.F.; Lichtenstein, L.M., Jr. Characterization of inflammatory mediator release from purified human lung mast cells. Am. Rev. Respir. Dis. 1986, 133, 614–617. [Google Scholar]
- Murray, J.J.; Tonnel, A.B.; Brash, A.R.; Roberts, L.J.; Gosset, P., 2nd; Workman, R.; Capron, A.; Oates, J.A. Release of prostaglandin D2 into human airways during acute antigen challenge. N. Engl. J. Med. 1986, 315, 800–804. [Google Scholar] [CrossRef]
- Casale, T.B.; Wood, D.; Richerson, H.B.; Zehr, B.; Zavala, D.; Hunninghake, G.W. Direct evidence of a role for mast cells in the pathogenesis of antigen-induced bronchoconstriction. J. Clin. Invest. 1987, 80, 1507–1511. [Google Scholar] [CrossRef]
- Wenzel, S.E.; Fowler, A.A.; Schwartz, L.B., 3rd. Activation of pulmonary mast cells by bronchoalveolar allergen challenge. In vivo release of histamine and tryptase in atopic subjects with and without asthma. Am. Rev. Respir. Dis. 1988, 137, 1002–1008. [Google Scholar]
- Varricchi, G.; Ferri, S.; Pepys, J.; Poto, R.; Spadaro, G.; Nappi, E.; Paoletti, G.; Virchow, J.C.; Heffler, E.; Canonica, W.G. Biologics and airway remodeling in severe asthma. Allergy, 2022; early view. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, M.; Takahashi, M.; Aoike, N. Expression of vascular endothelial growth factor, basic fibroblast growth factor, and angiogenin immunoreactivity in asthmatic airways and its relationship to angiogenesis. J. Allergy. Clin. Immunol. 2001, 107, 295–301. [Google Scholar] [CrossRef]
- Chetta, A.; Zanini, A.; Foresi, A.; Del Donno, M.; Castagnaro, A.; D’Ippolito, R.; Baraldo, S.; Testi, R.; Saetta, M.; Olivieri, D. Vascular component of airway remodeling in asthma is reduced by high dose of fluticasone. Am. J. Respir. Crit. Care Med. 2003, 167, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Detoraki, A.; Granata, F.; Staibano, S.; Rossi, F.W.; Marone, G.; Genovese, A. Angiogenesis and lymphangiogenesis in bronchial asthma. Allergy 2010, 65, 946–958. [Google Scholar] [CrossRef] [PubMed]
- Varricchi, G.; Loffredo, S.; Borriello, F.; Pecoraro, A.; Rivellese, F.; Genovese, A.; Spadaro, G.; Marone, G. Superantigenic Activation of Human Cardiac Mast Cells. Int. J. Mol. Sci. 2019, 20, 1828. [Google Scholar] [CrossRef] [PubMed]
- Altman, M.C.; Lai, Y.; Nolin, J.D.; Long, S.; Chen, C.C.; Piliponsky, A.M.; Altemeier, W.A.; Larmore, M.; Frevert, C.W.; Mulligan, M.S.; et al. Airway epithelium-shifted mast cell infiltration regulates asthmatic inflammation via IL-33 signaling. J. Clin. Invest. 2019, 129, 4979–4991. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Spanos, C.; Pang, X.; Alferes, L.; Ligris, K.; Letourneau, R.; Rozniecki, J.J.; Webster, E.; Chrousos, G.P. Stress-induced intracranial mast cell degranulation: A corticotropin-releasing hormone-mediated effect. Endocrinology 1995, 136, 5745–5750. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Cetrulo, C.L.; Theoharides, T.C. Corticotropin-releasing hormone induces vascular endothelial growth factor release from human mast cells via the cAMP/protein kinase A/p38 mitogen-activated protein kinase pathway. Mol. Pharmacol. 2006, 69, 998–1006. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Enakuaa, S.; Sismanopoulos, N.; Asadi, S.; Papadimas, E.C.; Angelidou, A.; Alysandratos, K.D. Contribution of stress to asthma worsening through mast cell activation. Ann. Allergy Asthma Immunol. 2012, 109, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Varricchi, G.; Rossi, F.W.; Galdiero, M.R.; Granata, F.; Criscuolo, G.; Spadaro, G.; de Paulis, A.; Marone, G. Physiological Roles of Mast Cells: Collegium Internationale Allergologicum Update 2019. Int. Arch. Allergy Immunol. 2019, 179, 247–261. [Google Scholar] [CrossRef]
- Zuberbier, T.; Aberer, W.; Asero, R.; Abdul Latiff, A.H.; Baker, D.; Ballmer-Weber, B.; Bernstein, J.A.; Bindslev-Jensen, C.; Brzoza, Z.; Buense Bedrikow, R.; et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy 2018, 73, 1393–1414. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, J.; Schunemann, H.J.; Togias, A.; Bachert, C.; Erhola, M.; Hellings, P.W.; Klimek, L.; Pfaar, O.; Wallace, D.; Ansotegui, I.; et al. Next-generation Allergic Rhinitis and Its Impact on Asthma (ARIA) guidelines for allergic rhinitis based on Grading of Recommendations Assessment, Development and Evaluation (GRADE) and real-world evidence. J. Allergy Clin. Immunol. 2020, 145, 70–80.e3. [Google Scholar] [CrossRef] [PubMed]
- Kay, L.J.; Suvarna, S.K.; Peachell, P.T. Histamine H4 receptor mediates chemotaxis of human lung mast cells. Eur. J. Pharmacol. 2018, 837, 38–44. [Google Scholar] [CrossRef]
- Murata, Y.; Song, M.; Kikuchi, H.; Hisamichi, K.; Xu, X.L.; Greenspan, A.; Kato, M.; Chiou, C.F.; Kato, T.; Guzzo, C.; et al. Phase 2a, randomized, double-blind, placebo-controlled, multicenter, parallel-group study of a H4 R-antagonist (JNJ-39758979) in Japanese adults with moderate atopic dermatitis. J. Dermatol. 2015, 42, 129–139. [Google Scholar] [CrossRef]
- Kollmeier, A.P.; Barnathan, E.S.; O’Brien, C.; Chen, B.; Xia, Y.K.; Zhou, B.; Loza, M.J.; Silkoff, P.E.; Ge, M.; Thurmond, R.L. A phase 2a study of toreforant, a histamine H4 receptor antagonist, in eosinophilic asthma. Ann. Allergy Asthma. Immunol. 2018, 121, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Werfel, T.; Layton, G.; Yeadon, M.; Whitlock, L.; Osterloh, I.; Jimenez, P.; Liu, W.; Lynch, V.; Asher, A.; Tsianakas, A.; et al. Efficacy and safety of the histamine H4 receptor antagonist ZPL-3893787 in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2019, 143, 1830–1837. [Google Scholar] [CrossRef]
- Maun, H.R.; Vij, R.; Walters, B.T.; Morando, A.; Jackman, J.K.; Wu, P.; Estevez, A.; Chen, X.; Franke, Y.; Lipari, M.T.; et al. Bivalent antibody pliers inhibit beta-tryptase by an allosteric mechanism dependent on the IgG hinge. Nat. Commun. 2020, 11, 6435. [Google Scholar] [CrossRef]
- Marone, G.; Galdiero, M.R.; Pecoraro, A.; Pucino, V.; Criscuolo, G.; Triassi, M.; Varricchi, G. Prostaglandin D2 receptor antagonists in allergic disorders: Safety, efficacy, and future perspectives. Expert. Opin. Investig. Drugs 2019, 28, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Brightling, C.E.; Gaga, M.; Inoue, H.; Li, J.; Maspero, J.; Wenzel, S.; Maitra, S.; Lawrence, D.; Brockhaus, F.; Lehmann, T.; et al. Effectiveness of fevipiprant in reducing exacerbations in patients with severe asthma (LUSTER-1 and LUSTER-2): Two phase 3 randomised controlled trials. Lancet Respir. Med. 2021, 9, 43–56. [Google Scholar] [CrossRef]
- Asano, K.; Sagara, H.; Ichinose, M.; Hirata, M.; Nakajima, A.; Ortega, H.; Tohda, Y. A Phase 2a Study of DP2 Antagonist GB001 for Asthma. J. Allergy Clin. Immunol. Pract. 2020, 8, 1275–1283.e1. [Google Scholar] [CrossRef]
- American Lung Association Asthma Clinical Research, C.; Peters, S.P.; Anthonisen, N.; Castro, M.; Holbrook, J.T.; Irvin, C.G.; Smith, L.J.; Wise, R.A. Randomized comparison of strategies for reducing treatment in mild persistent asthma. N. Engl. J. Med. 2007, 356, 2027–2039. [Google Scholar]
- Wollenberg, A.; Barbarot, S.; Bieber, T.; Christen-Zaech, S.; Deleuran, M.; Fink-Wagner, A.; Gieler, U.; Girolomoni, G.; Lau, S.; Muraro, A.; et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: Part II. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 850–878. [Google Scholar] [CrossRef] [PubMed]
- Hanania, N.A.; Korenblat, P.; Chapman, K.R.; Bateman, E.D.; Kopecky, P.; Paggiaro, P.; Yokoyama, A.; Olsson, J.; Gray, S.; Holweg, C.T.; et al. Efficacy and safety of lebrikizumab in patients with uncontrolled asthma (LAVOLTA I and LAVOLTA II): Replicate, phase 3, randomised, double-blind, placebo-controlled trials. Lancet Respir. Med. 2016, 4, 781–796. [Google Scholar] [CrossRef]
- Panettieri, R.A.; Sjobring, U., Jr.; Peterffy, A.; Wessman, P.; Bowen, K.; Piper, E.; Colice, G.; Brightling, C.E. Tralokinumab for severe, uncontrolled asthma (STRATOS 1 and STRATOS 2): Two randomised, double-blind, placebo-controlled, phase 3 clinical trials. Lancet Respir. Med. 2018, 6, 511–525. [Google Scholar] [CrossRef]
- Brightling, C.E.; Chanez, P.; Leigh, R.; O’Byrne, P.M.; Korn, S.; She, D.; May, R.D.; Streicher, K.; Ranade, K.; Piper, E. Efficacy and safety of tralokinumab in patients with severe uncontrolled asthma: A randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Respir. Med. 2015, 3, 692–701. [Google Scholar] [CrossRef]
- Corren, J.; Lemanske, R.F.; Hanania, N.A.; Korenblat, P.E.; Parsey, M.V.; Arron, J.R.; Harris, J.M.; Scheerens, H.; Wu, L.C.; Su, Z.; et al. Lebrikizumab treatment in adults with asthma. N. Engl. J. Med. 2011, 365, 1088–1098. [Google Scholar] [CrossRef]
- Marone, G.; Granata, F.; Pucino, V.; Pecoraro, A.; Heffler, E.; Loffredo, S.; Scadding, G.W.; Varricchi, G. The Intriguing Role of Interleukin 13 in the Pathophysiology of Asthma. Front. Pharmacol. 2019, 10, 1387. [Google Scholar] [CrossRef]
- Wenzel, S.; Ford, L.; Pearlman, D.; Spector, S.; Sher, L.; Skobieranda, F.; Wang, L.; Kirkesseli, S.; Rocklin, R.; Bock, B.; et al. Dupilumab in persistent asthma with elevated eosinophil levels. N. Engl. J. Med. 2013, 368, 2455–2466. [Google Scholar] [CrossRef]
- Wenzel, S.; Castro, M.; Corren, J.; Maspero, J.; Wang, L.; Zhang, B.; Pirozzi, G.; Sutherland, E.R.; Evans, R.R.; Joish, V.N.; et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting beta2 agonist: A randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet 2016, 388, 31–44. [Google Scholar] [CrossRef]
- Castro, M.; Corren, J.; Pavord, I.D.; Maspero, J.; Wenzel, S.; Rabe, K.F.; Busse, W.W.; Ford, L.; Sher, L.; FitzGerald, J.M.; et al. Dupilumab Efficacy and Safety in Moderate-to-Severe Uncontrolled Asthma. N. Engl. J. Med. 2018, 378, 2486–2496. [Google Scholar] [CrossRef]
- Ortega, H.G.; Liu, M.C.; Pavord, I.D.; Brusselle, G.G.; FitzGerald, J.M.; Chetta, A.; Humbert, M.; Katz, L.E.; Keene, O.N.; Yancey, S.W.; et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N. Engl. J. Med. 2014, 371, 1198–1207. [Google Scholar] [CrossRef]
- Detoraki, A.; Tremante, E.; D’Amato, M.; Calabrese, C.; Casella, C.; Maniscalco, M.; Poto, R.; Brancaccio, R.; Boccia, M.; Martino, M.; et al. Mepolizumab improves sino-nasal symptoms and asthma control in severe eosinophilic asthma patients with chronic rhinosinusitis and nasal polyps: A 12-month real-life study. Ther. Adv. Respir. Dis. 2021, 15, 17534666211009398. [Google Scholar] [CrossRef]
- Castro, M.; Zangrilli, J.; Wechsler, M.E.; Bateman, E.D.; Brusselle, G.G.; Bardin, P.; Murphy, K.; Maspero, J.F.; O’Brien, C.; Korn, S. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: Results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir. Med. 2015, 3, 355–366. [Google Scholar] [CrossRef]
- Harrison, T.W.; Chanez, P.; Menzella, F.; Canonica, G.W.; Louis, R.; Cosio, B.G.; Lugogo, N.L.; Mohan, A.; Burden, A.; McDermott, L.; et al. Onset of effect and impact on health-related quality of life, exacerbation rate, lung function, and nasal polyposis symptoms for patients with severe eosinophilic asthma treated with benralizumab (ANDHI): A randomised, controlled, phase 3b trial. Lancet Respir. Med. 2021, 9, 260–274. [Google Scholar] [CrossRef]
- GINA. Global Strategy for Asthma Management and Prevention; Global Initiative for Asthma: Fontana, WI, USA, 2021. [Google Scholar]
- Castro, M.; Wenzel, S.E.; Bleecker, E.R.; Pizzichini, E.; Kuna, P.; Busse, W.W.; Gossage, D.L.; Ward, C.K.; Wu, Y.; Wang, B.; et al. Benralizumab, an anti-interleukin 5 receptor alpha monoclonal antibody, versus placebo for uncontrolled eosinophilic asthma: A phase 2b randomised dose-ranging study. Lancet Respir. Med. 2014, 2, 879–890. [Google Scholar] [CrossRef]
- Haldar, P.; Brightling, C.E.; Hargadon, B.; Gupta, S.; Monteiro, W.; Sousa, A.; Marshall, R.P.; Bradding, P.; Green, R.H.; Wardlaw, A.J.; et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N. Engl. J. Med. 2009, 360, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, S.E.; Barnes, P.J.; Bleecker, E.R.; Bousquet, J.; Busse, W.; Dahlen, S.E.; Holgate, S.T.; Meyers, D.A.; Rabe, K.F.; Antczak, A.; et al. A randomized, double-blind, placebo-controlled study of tumor necrosis factor-alpha blockade in severe persistent asthma. Am. J. Respir. Crit. Care Med. 2009, 179, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Busse, W.W.; Holgate, S.; Kerwin, E.; Chon, Y.; Feng, J.; Lin, J.; Lin, S.L. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am. J. Respir. Crit. Care Med. 2013, 188, 1294–1302. [Google Scholar] [CrossRef]
- Hobo, A.; Harada, K.; Maeda, T.; Uchiyama, M.; Irisawa, R.; Yamazaki, M.; Tsuboi, R. IL-17-positive mast cell infiltration in the lesional skin of lichen planopilaris: Possible role of mast cells in inducing inflammation and dermal fibrosis in cicatricial alopecia. Exp. Dermatol. 2020, 29, 273–277. [Google Scholar] [CrossRef]
- Brightling, C.E.; Nair, P.; Cousins, D.J.; Louis, R.; Singh, D. Risankizumab in Severe Asthma—A Phase 2a, Placebo-Controlled Trial. N. Engl. J. Med. 2021, 385, 1669–1679. [Google Scholar] [CrossRef]
- Whetstone, C.E.; Ranjbar, M.; Omer, H.; Cusack, R.P.; Gauvreau, G.M. The Role of Airway Epithelial Cell Alarmins in Asthma. Cells 2022, 11, 1105. [Google Scholar] [CrossRef] [PubMed]
- Ebina-Shibuya, R.; Leonard, W.J. Role of thymic stromal lymphopoietin in allergy and beyond. Nat. Rev. Immunol. 2022, 1, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Marone, G.; Spadaro, G.; Braile, M.; Poto, R.; Criscuolo, G.; Pahima, H.; Loffredo, S.; Levi-Schaffer, F.; Varricchi, G. Tezepelumab: A novel biological therapy for the treatment of severe uncontrolled asthma. Expert. Opin. Investig. Drugs 2019, 28, 931–940. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Lv, Z.; Li, Y.; Chen, Y.; Huang, K.; Corrigan, C.J.; Ying, S. Elevated Expression of IL-33 and TSLP in the Airways of Human Asthmatics In Vivo: A Potential Biomarker of Severe Refractory Disease. J. Immunol. 2018, 200, 2253–2262. [Google Scholar] [CrossRef]
- Ko, H.K.; Cheng, S.L.; Lin, C.H.; Lin, S.H.; Hsiao, Y.H.; Su, K.C.; Yu, C.J.; Wang, H.C.; Sheu, C.C.; Chiu, K.C.; et al. Blood tryptase and thymic stromal lymphopoietin levels predict the risk of exacerbation in severe asthma. Sci. Rep. 2021, 11, 8425. [Google Scholar] [CrossRef] [PubMed]
- Corren, J.; Parnes, J.R.; Wang, L.; Mo, M.; Roseti, S.L.; Griffiths, J.M.; van der Merwe, R. Tezepelumab in Adults with Uncontrolled Asthma. N. Engl. J. Med. 2017, 377, 936–946. [Google Scholar] [CrossRef]
- Menzies-Gow, A.; Corren, J.; Bourdin, A.; Chupp, G.; Israel, E.; Wechsler, M.E.; Brightling, C.E.; Griffiths, J.M.; Hellqvist, A.; Bowen, K.; et al. Tezepelumab in Adults and Adolescents with Severe, Uncontrolled Asthma. N. Engl. J. Med. 2021, 384, 1800–1809. [Google Scholar] [CrossRef]
- Diver, S.; Khalfaoui, L.; Emson, C.; Wenzel, S.E.; Menzies-Gow, A.; Wechsler, M.E.; Johnston, J.; Molfino, N.; Parnes, J.R.; Megally, A.; et al. Effect of tezepelumab on airway inflammatory cells, remodelling, and hyperresponsiveness in patients with moderate-to-severe uncontrolled asthma (CASCADE): A double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir. Med. 2021, 9, 1299–1312. [Google Scholar] [CrossRef]
- Sverrild, A.; Hansen, S.; Hvidtfeldt, M.; Clausson, C.M.; Cozzolino, O.; Cerps, S.; Uller, L.; Backer, V.; Erjefalt, J.; Porsbjerg, C. The effect of tezepelumab on airway hyperresponsiveness to mannitol in asthma (UPSTREAM). Eur. Respir. J. 2022, 59, 2101296. [Google Scholar] [CrossRef]
- Cao, L.; Liu, F.; Liu, Y.; Liu, T.; Wu, J.; Zhao, J.; Wang, J.; Li, S.; Xu, J.; Dong, L. TSLP promotes asthmatic airway remodeling via p38-STAT3 signaling pathway in human lung fibroblast. Exp. Lung. Res. 2018, 44, 288–301. [Google Scholar] [CrossRef]
- Braile, M.; Fiorelli, A.; Sorriento, D.; Di Crescenzo, R.M.; Galdiero, M.R.; Marone, G.; Santini, M.; Varricchi, G.; Loffredo, S. Human Lung-Resident Macrophages Express and Are Targets of Thymic Stromal Lymphopoietin in the Tumor Microenvironment. Cells 2021, 10, 2012. [Google Scholar] [CrossRef] [PubMed]
- BioMed. Harbour BioMed Announces First Subject Dosed in Phase I Study of Next-Gen Anti-TSLP Fully Human Monoclonal Antibody; BioMed: Southfield, MI, USA, 2022. [Google Scholar]
- Gauvreau, G.M.; Hohlfeld, J.; Boulet, L.-P.; Cockcroft, D.; Davis, B.; FitzGerald, J.M.; Korn, S.; Kornmann, O.; Leigh, R.; Mayers, I.; et al. Late Breaking Abstract—Efficacy of CSJ117 on allergen-induced asthmatic responses in mild atopic asthma patients. Eur. Respir. J. 2020, 56, 3690. [Google Scholar]
- Wang, W.; Li, Y.; Lv, Z.; Chen, Y.; Li, Y.; Huang, K.; Corrigan, C.J.; Ying, S. Bronchial Allergen Challenge of Patients with Atopic Asthma Triggers an Alarmin (IL-33, TSLP, and IL-25) Response in the Airways Epithelium and Submucosa. J. Immunol. 2018, 201, 2221–2231. [Google Scholar] [CrossRef] [PubMed]
- Gasiuniene, E.; Janulaityte, I.; Zemeckiene, Z.; Barkauskiene, D.; Sitkauskiene, B. Elevated levels of interleukin-33 are associated with allergic and eosinophilic asthma. Scand. J. Immunol. 2019, 89, e12724. [Google Scholar] [CrossRef]
- Corrigan, C.J.; Wang, W.; Meng, Q.; Fang, C.; Eid, G.; Caballero, M.R.; Lv, Z.; An, Y.; Wang, Y.H.; Liu, Y.J.; et al. Allergen-induced expression of IL-25 and IL-25 receptor in atopic asthmatic airways and late-phase cutaneous responses. J. Allergy Clin. Immunol. 2011, 128, 116–124. [Google Scholar] [CrossRef]
- Beale, J.; Jayaraman, A.; Jackson, D.J.; Macintyre, J.D.R.; Edwards, M.R.; Walton, R.P.; Zhu, J.; Man Ching, Y.; Shamji, B.; Edwards, M.; et al. Rhinovirus-induced IL-25 in asthma exacerbation drives type 2 immunity and allergic pulmonary inflammation. Sci. Transl. Med. 2014, 6, 256ra134. [Google Scholar] [CrossRef]
- Wechsler, M.E.; Ruddy, M.K.; Pavord, I.D.; Israel, E.; Rabe, K.F.; Ford, L.B.; Maspero, J.F.; Abdulai, R.M.; Hu, C.C.; Martincova, R.; et al. Efficacy and Safety of Itepekimab in Patients with Moderate-to-Severe Asthma. N. Engl. J. Med. 2021, 385, 1656–1668. [Google Scholar] [CrossRef]
- Chinthrajah, S.; Cao, S.; Liu, C.; Lyu, S.C.; Sindher, S.B.; Long, A.; Sampath, V.; Petroni, D.; Londei, M.; Nadeau, K.C. Phase 2a randomized, placebo-controlled study of anti-IL-33 in peanut allergy. JCI Insight 2019, 4, e131347. [Google Scholar] [CrossRef]
- Kelsen, S.G.; Agache, I.O.; Soong, W.; Israel, E.; Chupp, G.L.; Cheung, D.S.; Theess, W.; Yang, X.; Staton, T.L.; Choy, D.F.; et al. Astegolimab (anti-ST2) efficacy and safety in adults with severe asthma: A randomized clinical trial. J. Allergy Clin. Immunol. 2021, 148, 790–798. [Google Scholar] [CrossRef]
- Bagnasco, D.; Ferrando, M.; Varricchi, G.; Puggioni, F.; Passalacqua, G.; Canonica, G.W. Anti-Interleukin 5 (IL-5) and IL-5Ra Biological Drugs: Efficacy, Safety, and Future Perspectives in Severe Eosinophilic Asthma. Front. Med. 2017, 4, 135. [Google Scholar] [CrossRef]
- Ortega, H.; Liu, M.C.; Pavord, I.D. Mepolizumab treatment in patients with severe eosinophilic asthma. N. Engl. J. Med 2015, 372, 1777. [Google Scholar] [CrossRef]
- Pavord, I.D.; Korn, S.; Howarth, P.; Bleecker, E.R.; Buhl, R.; Keene, O.N.; Ortega, H.; Chanez, P. Mepolizumab for severe eosinophilic asthma (DREAM): A multicentre, double-blind, placebo-controlled trial. Lancet 2012, 380, 651–659. [Google Scholar] [CrossRef]
- Chupp, G.L.; Bradford, E.S.; Albers, F.C.; Bratton, D.J.; Wang-Jairaj, J.; Nelsen, L.M.; Trevor, J.L.; Magnan, A.; Ten Brinke, A. Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): A randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trial. Lancet Respir. Med. 2017, 5, 390–400. [Google Scholar] [CrossRef]
- Bel, E.H.; Wenzel, S.E.; Thompson, P.J.; Prazma, C.M.; Keene, O.N.; Yancey, S.W.; Ortega, H.G.; Pavord, I.D. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N. Engl. J. Med. 2014, 371, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, J.A.; Virchow, J.C.; Murphy, K.; Maspero, J.F.; Jacobs, J.; Adir, Y.; Humbert, M.; Castro, M.; Marsteller, D.A.; McElhattan, J.; et al. Effect of fixed-dose subcutaneous reslizumab on asthma exacerbations in patients with severe uncontrolled asthma and corticosteroid sparing in patients with oral corticosteroid-dependent asthma: Results from two phase 3, randomised, double-blind, placebo-controlled trials. Lancet Respir. Med. 2020, 8, 461–474. [Google Scholar] [PubMed]
- Varricchi, G.; Senna, G.; Loffredo, S.; Bagnasco, D.; Ferrando, M.; Canonica, G.W. Reslizumab and Eosinophilic Asthma: One Step Closer to Precision Medicine? Front. Immunol. 2017, 8, 242. [Google Scholar] [CrossRef] [PubMed]
- Bleecker, E.R.; FitzGerald, J.M.; Chanez, P.; Papi, A.; Weinstein, S.F.; Barker, P.; Sproule, S.; Gilmartin, G.; Aurivillius, M.; Werkstrom, V.; et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting beta2-agonists (SIROCCO): A randomised, multicentre, placebo-controlled phase 3 trial. Lancet 2016, 388, 2115–2127. [Google Scholar] [CrossRef]
- Ferguson, G.T.; FitzGerald, J.M.; Bleecker, E.R.; Laviolette, M.; Bernstein, D.; LaForce, C.; Mansfield, L.; Barker, P.; Wu, Y.; Jison, M.; et al. Benralizumab for patients with mild to moderate, persistent asthma (BISE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir. Med. 2017, 5, 568–576. [Google Scholar] [CrossRef]
- Casale, T.B.; Luskin, A.T.; Busse, W.; Zeiger, R.S.; Trzaskoma, B.; Yang, M.; Griffin, N.M.; Chipps, B.E. Omalizumab Effectiveness by Biomarker Status in Patients with Asthma: Evidence from PROSPERO, A Prospective Real-World Study. J. Allergy Clin. Immunol. Pract. 2019, 7, 156–164.e1. [Google Scholar] [CrossRef]
- Gasser, P.; Tarchevskaya, S.S.; Guntern, P.; Brigger, D.; Ruppli, R.; Zbaren, N.; Kleinboelting, S.; Heusser, C.; Jardetzky, T.S.; Eggel, A. The mechanistic and functional profile of the therapeutic anti-IgE antibody ligelizumab differs from omalizumab. Nat. Commun. 2020, 11, 165. [Google Scholar] [CrossRef]
- Braithwaite, I.E.; Cai, F.; Tom, J.A.; Galanter, J.M.; Owen, R.P.; Zhu, R.; Williams, M.; McGregor, A.G.; Eliahu, A.; Durk, M.R.; et al. Inhaled JAK inhibitor GDC-0214 reduces exhaled nitric oxide in patients with mild asthma: A randomized, controlled, proof-of-activity trial. J. Allergy Clin. Immunol. 2021, 148, 783–789. [Google Scholar] [CrossRef]
- Schanin, J.; Luu, T.; Korver, W.; Brock, E.C.; Benet, Z.; Xu, A.; Chang, K.; Wong, A.; Butuci, M.; McEwen, L.M.; et al. An Agonistic Monoclonal Antibody Against Siglec-6 Selectively Inhibits and Reduces Human Tissue Mast Cells; EAACI: Hamburg, Germany, 2022. [Google Scholar]
- Wilkinson, T.; Dixon, R.; Page, C.; Carroll, M.; Griffiths, G.; Ho, L.P.; De Soyza, A.; Felton, T.; Lewis, K.E.; Phekoo, K.; et al. ACCORD: A Multicentre, Seamless, Phase 2 Adaptive Randomisation Platform Study to Assess the Efficacy and Safety of Multiple Candidate Agents for the Treatment of COVID-19 in Hospitalised Patients: A structured summary of a study protocol for a randomised controlled trial. Trials 2020, 21, 691. [Google Scholar] [PubMed]
- Ballantyne, S.J.; Barlow, J.L.; Jolin, H.E.; Nath, P.; Williams, A.S.; Chung, K.F.; Sturton, G.; Wong, S.H.; McKenzie, A.N. Blocking IL-25 prevents airway hyperresponsiveness in allergic asthma. J. Allergy Clin. Immunol. 2007, 120, 1324–1331. [Google Scholar] [CrossRef] [PubMed]
- Busse, W.W.; Morgan, W.J.; Gergen, P.J.; Mitchell, H.E.; Gern, J.E.; Liu, A.H.; Gruchalla, R.S.; Kattan, M.; Teach, S.J.; Pongracic, J.A.; et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N. Engl. J. Med. 2011, 364, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Beck, L.A.; Marcotte, G.V.; MacGlashan, D.; Togias, A.; Saini, S. Omalizumab-induced reductions in mast cell Fce psilon RI expression and function. J. Allergy Clin. Immunol. 2004, 114, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Hanania, N.A.; Alpan, O.; Hamilos, D.L.; Condemi, J.J.; Reyes-Rivera, I.; Zhu, J.; Rosen, K.E.; Eisner, M.D.; Wong, D.A.; Busse, W. Omalizumab in severe allergic asthma inadequately controlled with standard therapy: A randomized trial. Ann. Intern. Med. 2011, 154, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Normansell, R.; Walker, S.; Milan, S.J.; Walters, E.H.; Nair, P. Omalizumab for asthma in adults and children. Cochrane Database Syst. Rev. 2014, 13, CD003559. [Google Scholar] [CrossRef]
- Hew, M.; Gillman, A.; Sutherland, M.; Wark, P.; Bowden, J.; Guo, M.; Reddel, H.K.; Jenkins, C.; Marks, G.B.; Thien, F.; et al. Real-life effectiveness of omalizumab in severe allergic asthma above the recommended dosing range criteria. Clin. Exp. Allergy 2016, 46, 1407–1415. [Google Scholar] [CrossRef]
- Alhossan, A.; Lee, C.S.; MacDonald, K.; Abraham, I. “Real-life” Effectiveness Studies of Omalizumab in Adult Patients with Severe Allergic Asthma: Meta-analysis. J. Allergy Clin. Immunol. Pract. 2017, 5, 1362–1370.e2. [Google Scholar] [CrossRef]
- Esquivel, A.; Busse, W.W.; Calatroni, A.; Togias, A.G.; Grindle, K.G.; Bochkov, Y.A.; Gruchalla, R.S.; Kattan, M.; Kercsmar, C.M.; Khurana Hershey, G.; et al. Effects of Omalizumab on Rhinovirus Infections, Illnesses, and Exacerbations of Asthma. Am. J. Respir. Crit. Care Med. 2017, 196, 985–992. [Google Scholar] [CrossRef]
- Pelaia, C.; Calabrese, C.; Terracciano, R.; de Blasio, F.; Vatrella, A.; Pelaia, G. Omalizumab, the first available antibody for biological treatment of severe asthma: More than a decade of real-life effectiveness. Ther. Adv. Respir. Dis. 2018, 12, 1753466618810192. [Google Scholar] [CrossRef] [PubMed]
- Pennington, L.F.; Tarchevskaya, S.; Brigger, D.; Sathiyamoorthy, K.; Graham, M.T.; Nadeau, K.C.; Eggel, A.; Jardetzky, T.S. Structural basis of omalizumab therapy and omalizumab-mediated IgE exchange. Nat. Commun. 2016, 7, 11610. [Google Scholar] [CrossRef] [PubMed]
- Cruse, G.; Yin, Y.; Fukuyama, T.; Desai, A.; Arthur, G.K.; Baumer, W.; Beaven, M.A.; Metcalfe, D.D. Exon skipping of FcepsilonRIbeta eliminates expression of the high-affinity IgE receptor in mast cells with therapeutic potential for allergy. Proc. Natl. Acad. Sci. USA 2016, 113, 14115–14120. [Google Scholar] [CrossRef] [PubMed]
- Kambayashi, T.; Koretzky, G.A. Proximal signaling events in Fc epsilon RI-mediated mast cell activation. J. Allergy Clin. Immunol 2007, 119, 544–552. [Google Scholar] [CrossRef]
- Villoutreix, B.O.; Laconde, G.; Lagorce, D.; Martineau, P.; Miteva, M.A.; Dariavach, P. Tyrosine kinase syk non-enzymatic inhibitors and potential anti-allergic drug-like compounds discovered by virtual and in vitro screening. PLoS ONE 2011, 6, e21117. [Google Scholar] [CrossRef]
- Hayashi, H.; Kaneko, R.; Demizu, S.; Akasaka, D.; Tayama, M.; Harada, T.; Irie, H.; Ogino, Y.; Fujino, N.; Sasaki, E. TAS05567, a Novel Potent and Selective Spleen Tyrosine Kinase Inhibitor, Abrogates Immunoglobulin-Mediated Autoimmune and Allergic Reactions in Rodent Models. J. Pharmacol. Exp. Ther. 2018, 366, 84–95. [Google Scholar] [CrossRef]
- Ramis, I.; Otal, R.; Carreno, C.; Domenech, A.; Eichhorn, P.; Orellana, A.; Maldonado, M.; De Alba, J.; Prats, N.; Fernandez, J.C.; et al. A novel inhaled Syk inhibitor blocks mast cell degranulation and early asthmatic response. Pharmacol. Res. 2015, 99, 116–124. [Google Scholar] [CrossRef]
- Tabeling, C.; Herbert, J.; Hocke, A.C.; Lamb, D.J.; Wollin, S.L.; Erb, K.J.; Boiarina, E.; Movassagh, H.; Scheffel, J.; Doehn, J.M.; et al. Spleen tyrosine kinase inhibition blocks airway constriction and protects from Th2-induced airway inflammation and remodeling. Allergy 2017, 72, 1061–1072. [Google Scholar] [CrossRef]
- Strich, J.R.; Ramos-Benitez, M.J.; Randazzo, D.; Stein, S.R.; Babyak, A.; Davey, R.T.; Suffredini, A.F.; Childs, R.W.; Chertow, D.S. Fostamatinib Inhibits Neutrophils Extracellular Traps Induced by COVID-19 Patient Plasma: A Potential Therapeutic. J. Infect. Dis. 2021, 223, 981–984. [Google Scholar] [CrossRef]
- Koziol-White, C.J.; Jia, Y.; Baltus, G.A.; Cooper, P.R.; Zaller, D.M.; Crackower, M.A.; Sirkowski, E.E.; Smock, S.; Northrup, A.B.; Himes, B.E.; et al. Inhibition of spleen tyrosine kinase attenuates IgE-mediated airway contraction and mediator release in human precision cut lung slices. Br. J. Pharmacol. 2016, 173, 3080–3087. [Google Scholar] [CrossRef]
- Meltzer, E.O.; Berkowitz, R.B.; Grossbard, E.B. An intranasal Syk-kinase inhibitor (R112) improves the symptoms of seasonal allergic rhinitis in a park environment. J. Allergy Clin. Immunol. 2005, 115, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Guyer, B.J.; Shimamoto, S.R.; Bradhurst, A.L.; Grossbard, E.B.; Dreskin, S.C.; Nelson, H.S. Mast cell inhibitor R112 is well tolerated and affects prostaglandin D2 but not other mediators, symptoms, or nasal volumes in a nasal challenge model of allergic rhinitis. Allergy Asthma Proc. 2006, 27, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Awan, F.T.; Addison, D.; Alfraih, F.; Baratta, S.J.; Campos, R.N.; Cugliari, M.S.; Goh, Y.T.; Ionin, V.A.; Mundnich, S.; Sverdlov, A.L.; et al. International consensus statement on the management of cardiovascular risk of Bruton’s tyrosine kinase inhibitors in CLL. Blood Adv. 2022, 6, 5516–5525. [Google Scholar] [CrossRef]
- Dispenza, M.C.; Krier-Burris, R.A.; Chhiba, K.D.; Undem, B.J.; Robida, P.A.; Bochner, B.S. Bruton’s tyrosine kinase inhibition effectively protects against human IgE-mediated anaphylaxis. J. Clin. Invest. 2020, 130, 4759–4770. [Google Scholar] [CrossRef]
- Dispenza, M.C.; Pongracic, J.A.; Singh, A.M.; Bochner, B.S. Short-term ibrutinib therapy suppresses skin test responses and eliminates IgE-mediated basophil activation in adults with peanut or tree nut allergy. J. Allergy Clin. Immunol. 2018, 141, 1914–1916.e7. [Google Scholar] [CrossRef] [PubMed]
- Morita, H.; Matsumoto, K.; Saito, H. Biologics for allergic and immunologic diseases. J. Allergy Clin. Immunol. 2022, 150, 766–777. [Google Scholar] [CrossRef] [PubMed]
- Youngblood, B.A.; Brock, E.C.; Leung, J.; Falahati, R.; Bryce, P.J.; Bright, J.; Williams, J.; Shultz, L.D.; Greiner, D.L.; Brehm, M.A.; et al. AK002, a Humanized Sialic Acid-Binding Immunoglobulin-Like Lectin-8 Antibody that Induces Antibody-Dependent Cell-Mediated Cytotoxicity against Human Eosinophils and Inhibits Mast Cell-Mediated Anaphylaxis in Mice. Int. Arch. Allergy Immunol. 2019, 180, 91–102. [Google Scholar] [CrossRef]
- Kerr, S.C.; Gonzalez, J.R.; Schanin, J.; Peters, M.C.; Lambrecht, B.N.; Brock, E.C.; Charbit, A.; Ansel, K.M.; Youngblood, B.A.; Fahy, J.V. An anti-siglec-8 antibody depletes sputum eosinophils from asthmatic subjects and inhibits lung mast cells. Clin. Exp. Allergy 2020, 50, 904–914. [Google Scholar] [CrossRef]
- Schanin, J.; Gebremeskel, S.; Korver, W.; Falahati, R.; Butuci, M.; Haw, T.J.; Nair, P.M.; Liu, G.; Hansbro, N.G.; Hansbro, P.M.; et al. A monoclonal antibody to Siglec-8 suppresses non-allergic airway inflammation and inhibits IgE-independent mast cell activation. Mucosal Immunol. 2021, 14, 366–376. [Google Scholar] [CrossRef]
- Dellon, E.S.; Peterson, K.A.; Murray, J.A.; Falk, G.W.; Gonsalves, N.; Chehade, M.; Genta, R.M.; Leung, J.; Khoury, P.; Klion, A.D.; et al. Anti-Siglec-8 Antibody for Eosinophilic Gastritis and Duodenitis. N. Engl. J. Med. 2020, 383, 1624–1634. [Google Scholar] [CrossRef]
- Bhasin, S.; Gill, T.M.; Reuben, D.B.; Latham, N.K.; Ganz, D.A.; Greene, E.J.; Dziura, J.; Basaria, S.; Gurwitz, J.H.; Dykes, P.C.; et al. A Randomized Trial of a Multifactorial Strategy to Prevent Serious Fall Injuries. N. Engl. J. Med. 2020, 383, 129–140. [Google Scholar] [CrossRef]
- Shehata, Y.; Sheikh, A. Farming, childhood allergy, and unpasteurised milk A review of; “Which aspects of the farming lifestyle explain the inwerse association with childhood allergy.” Perkin MR, Strachan DP. Prim. Care Respir. J. 2007, 16, 59–60. [Google Scholar]
- Bachelet, I.; Munitz, A.; Berent-Maoz, B.; Mankuta, D.; Levi-Schaffer, F. Suppression of normal and malignant kit signaling by a bispecific antibody linking kit with CD300a. J. Immunol. 2008, 180, 6064–6069. [Google Scholar] [CrossRef] [PubMed]
- Eggel, A.; Buschor, P.; Baumann, M.J.; Amstutz, P.; Stadler, B.M.; Vogel, M. Inhibition of ongoing allergic reactions using a novel anti-IgE DARPin-Fc fusion protein. Allergy 2011, 66, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Eggel, A.; Tarchevskaya, S.S.; Vogel, M.; Prinz, H.; Jardetzky, T.S. Accelerated disassembly of IgE-receptor complexes by a disruptive macromolecular inhibitor. Nature 2012, 491, 613–617. [Google Scholar] [CrossRef]
- Arock, M.; Hoermann, G.; Sotlar, K.; Hermine, O.; Sperr, W.R.; Hartmann, K.; Brockow, K.; Akin, C.; Triggiani, M.; Broesby-Olsen, S.; et al. Clinical impact and proposed application of molecular markers, genetic variants, and cytogenetic analysis in mast cell neoplasms: Status 2022. J. Allergy Clin. Immunol. 2022, 149, 1855–1865. [Google Scholar] [CrossRef]
- Arock, M.; Sotlar, K.; Akin, C.; Broesby-Olsen, S.; Hoermann, G.; Escribano, L.; Kristensen, T.K.; Kluin-Nelemans, H.C.; Hermine, O.; Dubreuil, P.; et al. KIT mutation analysis in mast cell neoplasms: Recommendations of the European Competence Network on Mastocytosis. Leukemia 2015, 29, 1223–1232. [Google Scholar] [CrossRef]
- Valent, P.; Akin, C.; Bonadonna, P.; Hartmann, K.; Brockow, K.; Niedoszytko, M.; Nedoszytko, B.; Siebenhaar, F.; Sperr, W.R.; Oude Elberink, J.N.G.; et al. Proposed Diagnostic Algorithm for Patients with Suspected Mast Cell Activation Syndrome. J. Allergy Clin. Immunol. Pract. 2019, 7, 1125–1133. [Google Scholar] [CrossRef]
- Fonseca, W.; Rasky, A.J.; Ptaschinski, C.; Morris, S.H.; Best, S.K.K.; Phillips, M.; Malinczak, C.A.; Lukacs, N.W. Group 2 innate lymphoid cells (ILC2) are regulated by stem cell factor during chronic asthmatic disease. Mucosal Immunol. 2019, 12, 445–456. [Google Scholar] [CrossRef]
- Ptaschinski, C.; Rasky, A.J.; Fonseca, W.; Lukacs, N.W. Stem Cell Factor Neutralization Protects From Severe Anaphylaxis in a Murine Model of Food Allergy. Front. Immunol. 2021, 12, 604192. [Google Scholar] [CrossRef]
- Brandt, E.B.; Strait, R.T.; Hershko, D.; Wang, Q.; Muntel, E.E.; Scribner, T.A.; Zimmermann, N.; Finkelman, F.D.; Rothenberg, M.E. Mast cells are required for experimental oral allergen-induced diarrhea. J. Clin. Invest. 2003, 112, 1666–1677. [Google Scholar]
- Terhorst-Molawi, D.; Hawro, T.; Grekowitz, E.; Kiefer, L.; Metz, M.; Alvarado, D.; Hawthorne, T.; Merchant, K.; Crew, L.; Crowley, E.; et al. The Anti-KIT Antibody, CDX-0159, Reduces Mast Cell Numbers and Circulating Tryptase and Improves Disease Control in Patients with Chronic Inducible Urticaria (Cindu). J. Allergy Clin. Immunol. 2022, 149, AB178. [Google Scholar] [CrossRef]
- Rasky, A.; Habiel, D.M.; Morris, S.; Schaller, M.; Moore, B.B.; Phan, S.; Kunkel, S.L.; Phillips, M.; Hogaboam, C.; Lukacs, N.W. Inhibition of the stem cell factor 248 isoform attenuates the development of pulmonary remodeling disease. Am. J. Physiol. Lung. Cell Mol. Physiol. 2020, 318, L200–L211. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.; Khan, D.A.; Elieh Ali Komi, D.; Kaplan, A.P. Biologics for the Use in Chronic Spontaneous Urticaria: When and Which. J. Allergy Clin. Immunol. Pract. 2021, 9, 1067–1078. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, D.; Maurer, M.; Gedrich, R.; Seibel, S.B.; Murphy, M.B.; Crew, L.; Goldstein, J.; Crocker, A.; Vitale, L.A.; Morani, P.A.; et al. Anti-KIT monoclonal antibody CDX-0159 induces profound and durable mast cell suppression in a healthy volunteer study. Allergy 2022, 77, 2393–2403. [Google Scholar] [CrossRef]
- Valent, P.; Akin, C.; Hartmann, K.; Reiter, A.; Gotlib, J.; Sotlar, K.; Sperr, W.R.; Degenfeld-Schonburg, L.; Smiljkovic, D.; Triggiani, M.; et al. Drug-induced mast cell eradication: A novel approach to treat mast cell activation disorders? J. Allergy Clin. Immunol. 2022, 149, 1866–1874. [Google Scholar] [CrossRef] [PubMed]
- Kolkhir, P.; Elieh-Ali-Komi, D.; Metz, M.; Siebenhaar, F.; Maurer, M. Understanding human mast cells: Lesson from therapies for allergic and non-allergic diseases. Nat. Rev. Immunol. 2022, 22, 294–308. [Google Scholar] [CrossRef]
- Cahill, K.N.; Katz, H.R.; Cui, J.; Lai, J.; Kazani, S.; Crosby-Thompson, A.; Garofalo, D.; Castro, M.; Jarjour, N.; DiMango, E.; et al. KIT Inhibition by Imatinib in Patients with Severe Refractory Asthma. N. Engl. J. Med. 2017, 376, 1911–1920. [Google Scholar] [CrossRef]
- Chanez, P.; Israel, E.; Davidescu, L.; Ursol, G.; Korzh, O.; Deshmukh, V.; Kuryk, L.; Nortje, M.-M.; Godlevska, O.; Devouassoux, G.; et al. Abstract: Masitinib significantly decreases the rate of asthma exacerbations in patients with severe asthma uncontrolled by oral corticosteroids: A phase 3 multicenter study. Am. J. Respir. Crit. Care Med. 2020, 201, A4210. [Google Scholar]
- Humbert, M.; de Blay, F.; Garcia, G.; Prud’homme, A.; Leroyer, C.; Magnan, A.; Tunon-de-Lara, J.M.; Pison, C.; Aubier, M.; Charpin, D.; et al. Masitinib, a c-kit/PDGF receptor tyrosine kinase inhibitor, improves disease control in severe corticosteroid-dependent asthmatics. Allergy 2009, 64, 1194–1201. [Google Scholar] [CrossRef]
- Lee-Fowler, T.M.; Guntur, V.; Dodam, J.; Cohn, L.A.; DeClue, A.E.; Reinero, C.R. The tyrosine kinase inhibitor masitinib blunts airway inflammation and improves associated lung mechanics in a feline model of chronic allergic asthma. Int. Arch. Allergy Immunol. 2012, 158, 369–374. [Google Scholar] [CrossRef]
- Vaali, K.; Lappalainen, J.; Lin, A.H.; Mayranpaa, M.I.; Kovanen, P.T.; Berstad, A.; Eklund, K.K. Imatinib mesylate alleviates diarrhea in a mouse model of intestinal allergy. Neurogastroenterol. Motil. 2012, 24, e325–e335. [Google Scholar] [CrossRef]
- Jung, S.H.; Sun, X.; Ryu, W.S.; Yang, B.S. Topical administration of the pan-Src kinase inhibitors, dasatinib and LCB 03-0110, prevents allergic contact dermatitis in mice. Br. J. Dermatol. 2013, 168, 112–119. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.L.; Magalhaes, R.F.; Branco, V.C.; Silva, J.D.; Cruz, F.F.; Marques, P.S.; Ferreira, T.P.; Morales, M.M.; Martins, M.A.; Olsen, P.C.; et al. The tyrosine kinase inhibitor dasatinib reduces lung inflammation and remodelling in experimental allergic asthma. Br. J. Pharmacol. 2016, 173, 1236–1247. [Google Scholar] [CrossRef] [PubMed]
- Gotlib, J.; Reiter, A.; Radia, D.H.; Deininger, M.W.; George, T.I.; Panse, J.; Vannucchi, A.M.; Platzbecker, U.; Alvarez-Twose, I.; Mital, A.; et al. Efficacy and safety of avapritinib in advanced systemic mastocytosis: Interim analysis of the phase 2 PATHFINDER trial. Nat. Med. 2021, 27, 2192–2199. [Google Scholar] [CrossRef]
- DeAngelo, D.J.; Radia, D.H.; George, T.I.; Robinson, W.A.; Quiery, A.T.; Drummond, M.W.; Bose, P.; Hexner, E.O.; Winton, E.F.; Horny, H.P.; et al. Safety and efficacy of avapritinib in advanced systemic mastocytosis: The phase 1 EXPLORER trial. Nat. Med. 2021, 27, 2183–2191. [Google Scholar] [CrossRef]
- Kneidinger, M.; Schmidt, U.; Rix, U.; Gleixner, K.V.; Vales, A.; Baumgartner, C.; Lupinek, C.; Weghofer, M.; Bennett, K.L.; Herrmann, H.; et al. The effects of dasatinib on IgE receptor-dependent activation and histamine release in human basophils. Blood 2008, 111, 3097–3107. [Google Scholar] [CrossRef]
- Peter, B.; Winter, G.E.; Blatt, K.; Bennett, K.L.; Stefanzl, G.; Rix, U.; Eisenwort, G.; Hadzijusufovic, E.; Gridling, M.; Dutreix, C.; et al. Target interaction profiling of midostaurin and its metabolites in neoplastic mast cells predicts distinct effects on activation and growth. Leukemia 2016, 30, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Krauth, M.T.; Mirkina, I.; Herrmann, H.; Baumgartner, C.; Kneidinger, M.; Valent, P. Midostaurin (PKC412) inhibits immunoglobulin E-dependent activation and mediator release in human blood basophils and mast cells. Clin. Exp. Allergy 2009, 39, 1711–1720. [Google Scholar] [CrossRef]
- Savage, J.H.; Courneya, J.P.; Sterba, P.M.; Macglashan, D.W.; Saini, S.S.; Wood, R.A. Kinetics of mast cell, basophil, and oral food challenge responses in omalizumab-treated adults with peanut allergy. J. Allergy Clin. Immunol. 2012, 130, 1123–1129.e2. [Google Scholar] [CrossRef]
- Ishizaka, K.; Ishizaka, T.; Hornbrook, M.M. Physico-chemical properties of human reaginic antibody. IV. Presence of a unique immunoglobulin as a carrier of reaginic activity. J. Immunol. 1966, 97, 75–85. [Google Scholar] [PubMed]
- Johansson, S.G.; Bennich, H. Immunological studies of an atypical (myeloma) immunoglobulin. Immunology 1967, 13, 381–394. [Google Scholar] [PubMed]
- Varricchi, G.; Pecoraro, A.; Loffredo, S.; Poto, R.; Rivellese, F.; Genovese, A.; Marone, G.; Spadaro, G. Heterogeneity of Human Mast Cells With Respect to MRGPRX2 Receptor Expression and Function. Front. Cell Neurosci. 2019, 13, 299. [Google Scholar] [CrossRef] [PubMed]
- Vivanco Gonzalez, N.; Oliveria, J.P.; Tebaykin, D.; Ivison, G.T.; Mukai, K.; Tsai, M.M.; Borges, L.; Nadeau, K.C.; Galli, S.J.; Tsai, A.G.; et al. Mass Cytometry Phenotyping of Human Granulocytes Reveals Novel Basophil Functional Heterogeneity. iScience 2020, 23, 101724. [Google Scholar] [CrossRef] [PubMed]
- Derakhshan, T.; Boyce, J.A.; Dwyer, D.F. Defining mast cell differentiation and heterogeneity through single-cell transcriptomics analysis. J. Allergy Clin. Immunol. 2022, 150, 739–747. [Google Scholar] [CrossRef]
- Heijink, I.H.; Kuchibhotla, V.N.S.; Roffel, M.P.; Maes, T.; Knight, D.A.; Sayers, I.; Nawijn, M.C. Epithelial cell dysfunction, a major driver of asthma development. Allergy 2020, 75, 1902–1917. [Google Scholar] [CrossRef]
- Cohen, M.; Giladi, A.; Gorki, A.D.; Solodkin, D.G.; Zada, M.; Hladik, A.; Miklosi, A.; Salame, T.M.; Halpern, K.B.; David, E.; et al. Lung Single-Cell Signaling Interaction Map Reveals Basophil Role in Macrophage Imprinting. Cell 2018, 175, 1031–1044.e18. [Google Scholar] [CrossRef]
- Aegerter, H.; Lambrecht, B.N.; Jakubzick, C.V. Biology of lung macrophages in health and disease. Immunity 2022, 55, 1564–1580. [Google Scholar] [CrossRef]
- Balestrieri, B.; Granata, F.; Loffredo, S.; Petraroli, A.; Scalia, G.; Morabito, P.; Cardamone, C.; Varricchi, G.; Triggiani, M. Phenotypic and Functional Heterogeneity of Low-Density and High-Density Human Lung Macrophages. Biomedicines 2021, 9, 505. [Google Scholar] [CrossRef]
- MacGlashan, D.; Saini, S., Jr.; Schroeder, J.T. Response of peripheral blood basophils in subjects with chronic spontaneous urticaria during treatment with omalizumab. J. Allergy Clin. Immunol. 2021, 147, 2295–2304.e12. [Google Scholar] [CrossRef]
- Lommatzsch, M.; Marchewski, H.; Schwefel, G.; Stoll, P.; Virchow, J.C.; Bratke, K. Benralizumab strongly reduces blood basophils in severe eosinophilic asthma. Clin. Exp. Allergy 2020, 50, 1267–1269. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Gaudenzio, N.; Tsai, M. Mast Cells in Inflammation and Disease: Recent Progress and Ongoing Concerns. Annu. Rev. Immunol. 2020, 38, 49–77. [Google Scholar] [CrossRef] [PubMed]

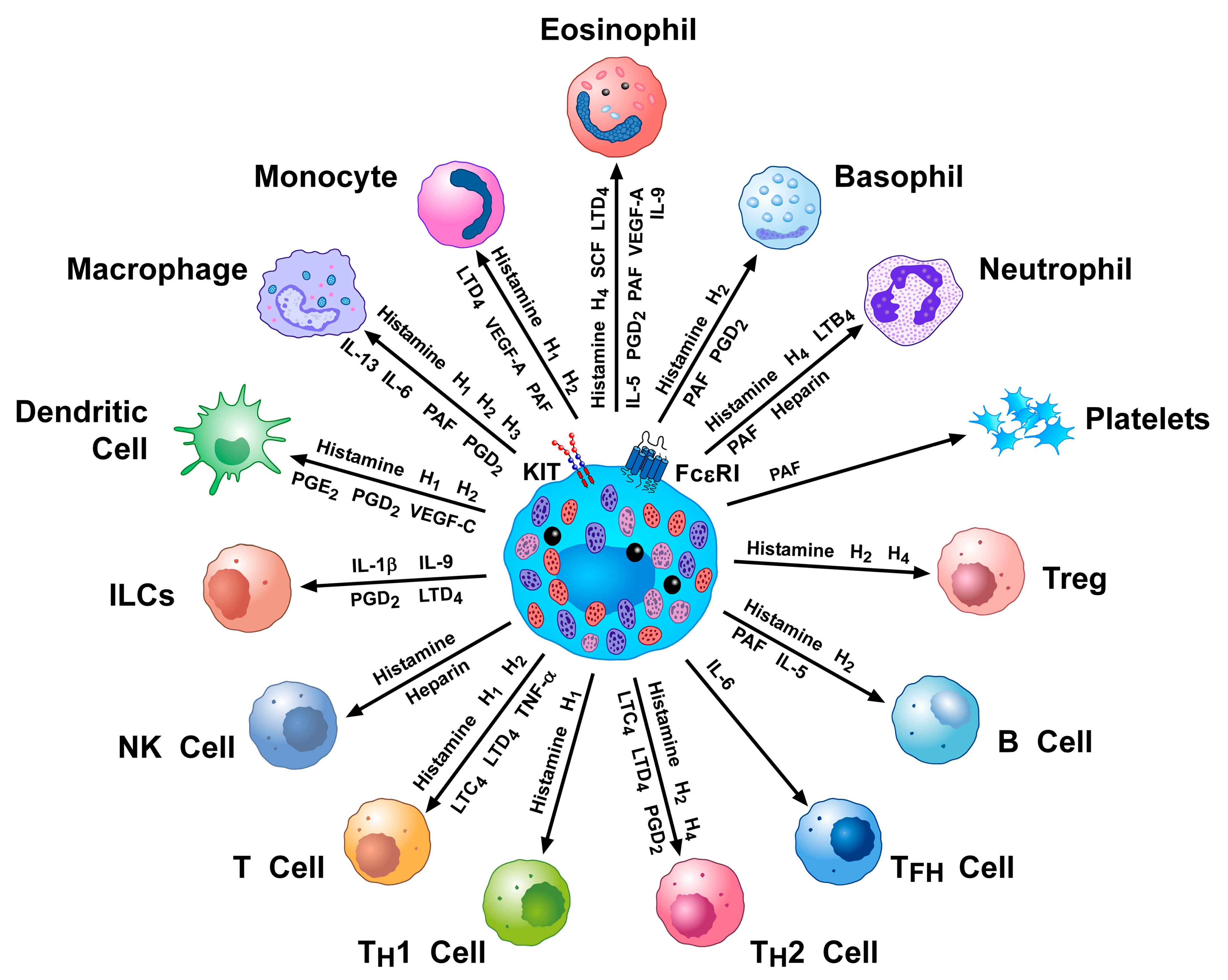
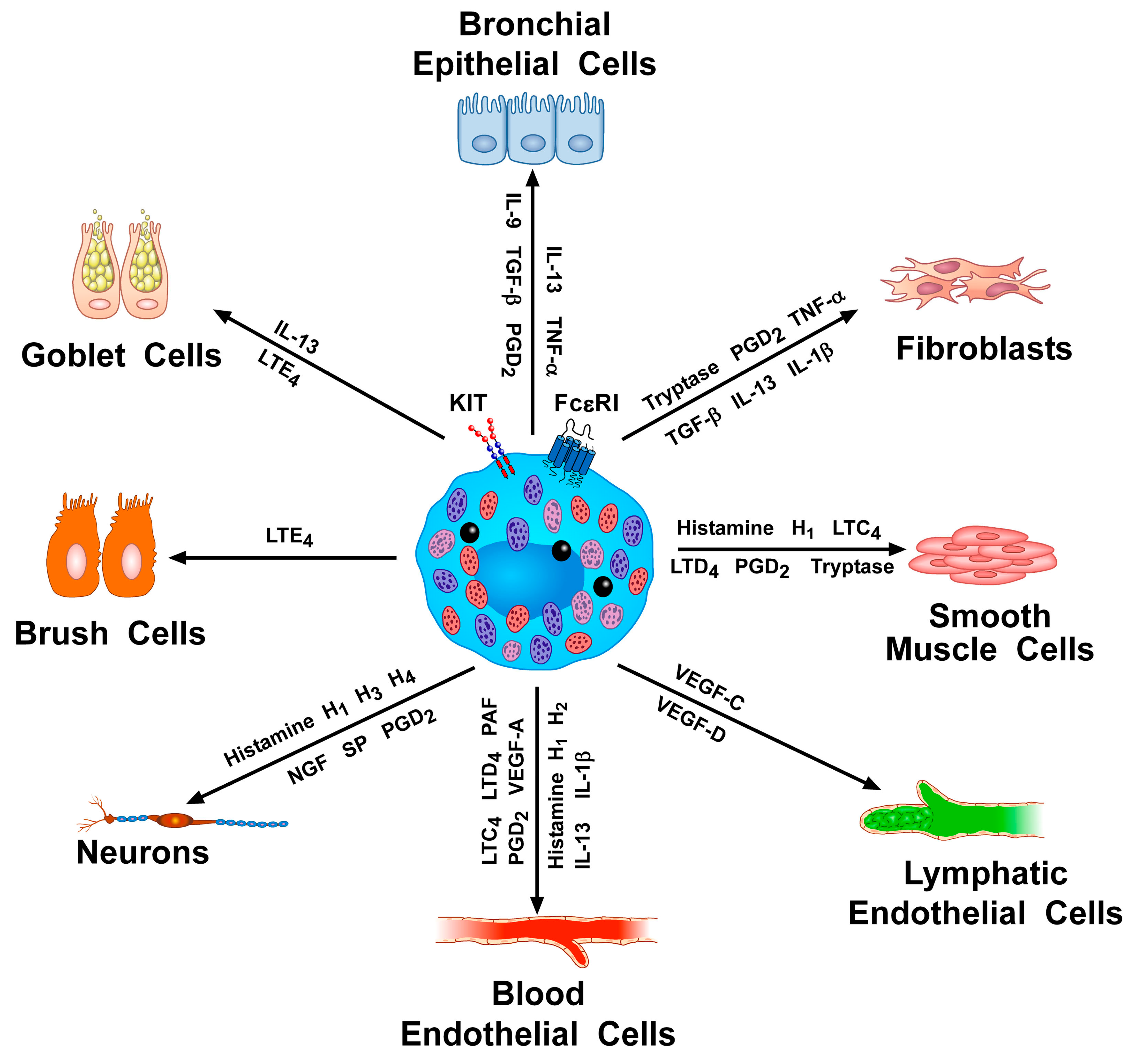
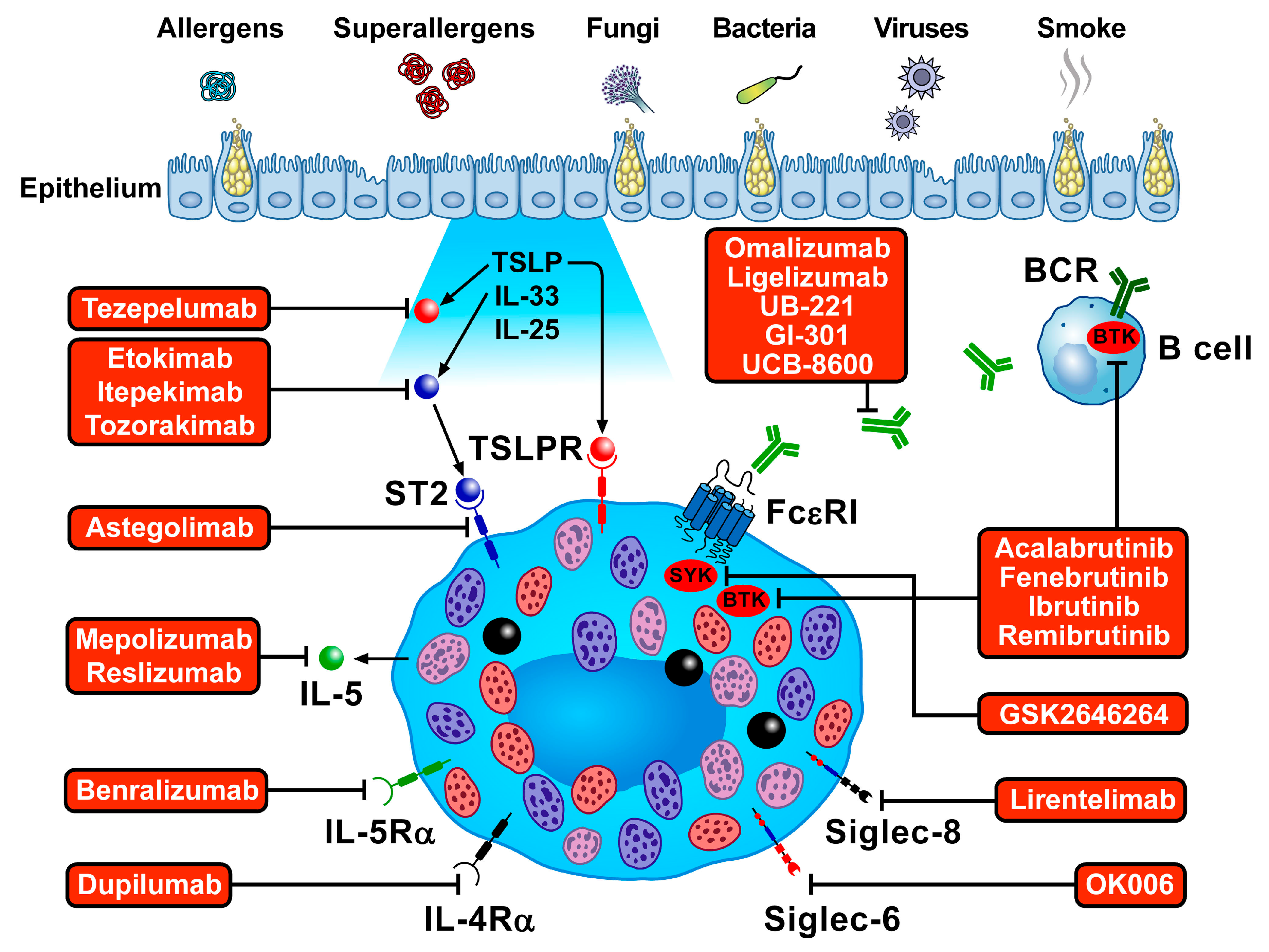
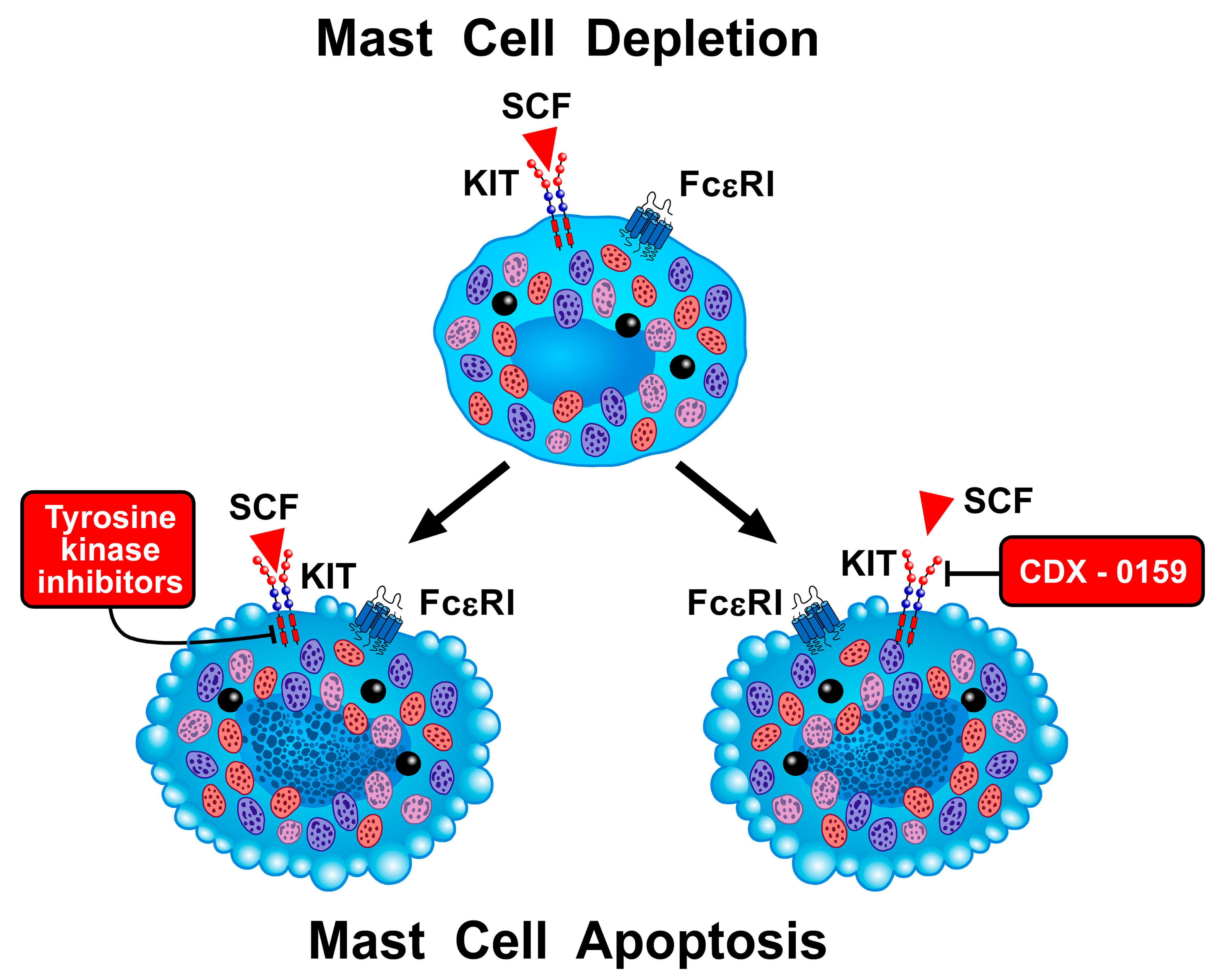
| Mediator | Properties | References |
|---|---|---|
| Preformed | ||
| Histamine | Preformed in cytoplasmic granules of human mast cells (≅3 pg/cell) and basophils (≅1 pg/cell). | [64,65] |
| β-tryptase | A tetrameric serine protease, abundant in secretory granules of human mast cells. Tryptase+ mast cells are increased in asthmatics bronchial tissue, and their numbers correlate with airway hyperresponsiveness. Tryptase concentrations in bronchoalveolar lavage (BAL) fluid correlates with asthma severity. | [89,90,91,92] |
| Chymase | A chymotrypsin-like serine protease stored in human mast cell secretory granules causes matrix destruction and inflammation. | [93,94] |
| Cathepsin G | A serine protease that controls the functional state of immune cells. | [93] |
| Carboxypeptidase A3 | It cleaves several proteins. | [95] |
| Granzyme B | A protease involved in the induction of target cell death. | [96] |
| Matrix metalloproteinases (MMPs) | A family of extracellular proteinases. | [97] |
| Lipid Mediators | ||
| Cysteinyl leukotriene C4 (LTC4) | Human lung mast cells (HLMCs) synthesize LTC4, a potent bronchoconstrictor acting through the activation of cysteinyl leukotriene receptor 1 (CysLTR1) and CysLTR2. | [98,99] |
| Prostaglandin D2 (PGD2) | It activates the CRTh2 receptor on several immune cells. | [100,101] |
| Platelet-activating factor (PAF) | A phospholipid with proinflammatory and vasoactive effects. | [102] |
| Cytokines | ||
| Stem cell factor (SCF) | They exert several proinflammatory and immunomodulatory effects. | [37] |
| TNF-α | [103] | |
| IL-1β | [104] | |
| IL-3 | [105,106] | |
| IL-5 | [107] | |
| IL-6 | [108] | |
| IL-9 | [109] | |
| IL-10 | [57] | |
| IL-11 | [108] | |
| IL-13 | [109,110] | |
| IL-16 | [111] | |
| IL-22 | [112] | |
| Thymic stromal lymphopoietin (TSLP) | [113,114,115] | |
| IL-25/IL-17E | [116] | |
| Granulocyte-macrophage colony-stimulating factor (GM-CSF) | [107] | |
| Vascular endothelial growth factor (VEGF) | [117] | |
| Fibroblast growth factor 2 (FGF-2) | [118] | |
| Nerve growth factor (NGF) | [119] | |
| Amphiregulin | [120,121] | |
| Chemokines | ||
| CXCL8/IL-8, CCL1/I-309, CCL2/MCP-1, CCL3/MIP-1α, CXCL1/GRO-α, CXCL10/IP-10 | They exert several chemotactic and proinflammatory effects. | [122,123,124] |
| Angiogenic factors | ||
| VEGF-A | The main angiogenic factor released by HLMCs. VEGFs released by macrophages, basophils, and neutrophils contribute to mast cell infiltration in bronchial asthma. | [56,117,125,126,127,128] |
| Angiopoietins (ANGPT1 and ANGPT2) | ANGPTs are involved in blood vessel formation and are released by HLMCs. | [129,130,131,132] |
| LTC4, LTD4 | Non-canonical angiogenic factors. | [133] |
| Lymphangiogenic factors | ||
| VEGF-C, VEGF-D | The main lymphangiogenic factor released by HLMCs. | [58] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poto, R.; Criscuolo, G.; Marone, G.; Brightling, C.E.; Varricchi, G. Human Lung Mast Cells: Therapeutic Implications in Asthma. Int. J. Mol. Sci. 2022, 23, 14466. https://doi.org/10.3390/ijms232214466
Poto R, Criscuolo G, Marone G, Brightling CE, Varricchi G. Human Lung Mast Cells: Therapeutic Implications in Asthma. International Journal of Molecular Sciences. 2022; 23(22):14466. https://doi.org/10.3390/ijms232214466
Chicago/Turabian StylePoto, Remo, Gjada Criscuolo, Gianni Marone, Chris E. Brightling, and Gilda Varricchi. 2022. "Human Lung Mast Cells: Therapeutic Implications in Asthma" International Journal of Molecular Sciences 23, no. 22: 14466. https://doi.org/10.3390/ijms232214466
APA StylePoto, R., Criscuolo, G., Marone, G., Brightling, C. E., & Varricchi, G. (2022). Human Lung Mast Cells: Therapeutic Implications in Asthma. International Journal of Molecular Sciences, 23(22), 14466. https://doi.org/10.3390/ijms232214466









