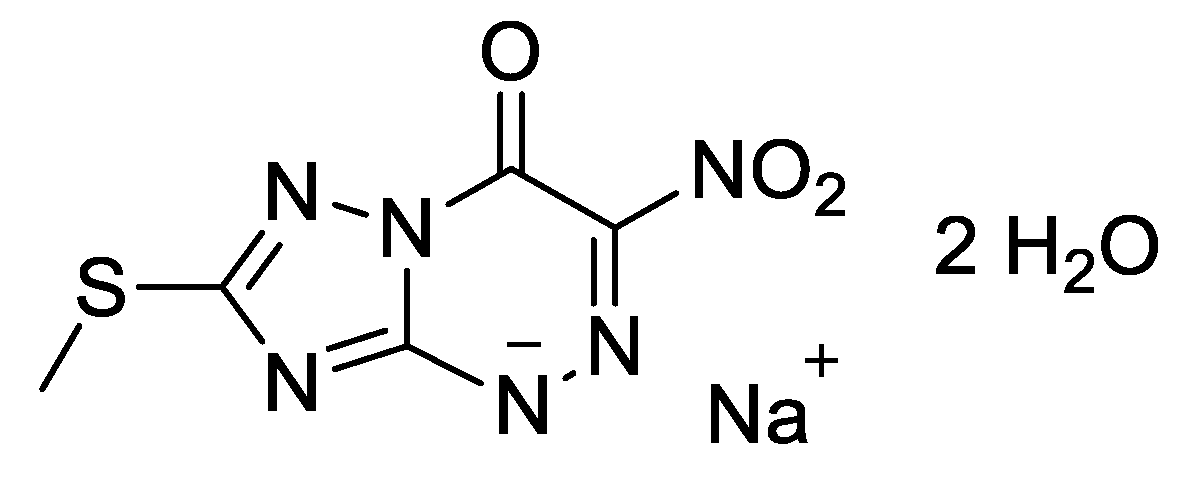
Triazavirin—A Novel Effective Antiviral Drug
Abstract
:1. Introduction
2. Approaches to Synthesis of Triazavirin and Chemical Properties of Nitroazoloazines
2.1. Synthetic Approaches
2.2. Chemical Properties of Nitroazoloazines
- ability to form stable salts on treatment with alkali metals and amines;
- N-alkylation leading to acyclic nucleosides;
- reduction of the nitro group into the corresponding amino compounds;
- oxidation of SH and S-alkyl fragments;
- destruction of the 1,2,4-triazine ring;
- nucleophilic substitution of the nitro group.
3. Pharmacological Properties of Triazavirin
3.1. Dosage Forms
3.2. Antiviral Properties of Triazavirin
3.2.1. Activity against Influenza Virus and Toxicology Profile
3.2.2. Triazavirin for Treatment of Acute Respiratory Virus Infections (ARVI) in Adults
3.2.3. Activity against Tick-Borne Encephalitis Virus
3.2.4. Activity against SARS-CoV-2
- shortens the duration of major clinical symptoms, such as fever, and reduces the frequency of complications;
- improves patients’ responses to inflammation and hypercoagulation, reduces reliance on glucocorticoids, anticoagulants, and oxygen inhalation;
- results in a higher rate of recovery of abnormal serum bilirubin, indirect bilirubin, total protein, albumin, and uric acid levels;
- reduces use of electrolyte solutions and diuretics, resulting in less damage to liver and kidney function;
3.2.5. Molecular Modeling Studies of Triazavirin against SARS-CoV-2
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lythgoe, M.P.; Middleton, P. Ongoing Clinical Trials for the Management of the COVID-19 Pandemic. Trends Pharmacol. Sci. 2020, 41, 363–382. [Google Scholar] [CrossRef] [PubMed]
- Rusinov, V.L.; Ulomskii, E.N.; Chupakhin, O.N.; Charushin, V.N. Azolo[5,1-c]-1,2,4-Triazines as a New Class of Antiviral Compounds. Russ. Chem. Bull. 2008, 57, 985–1014. [Google Scholar] [CrossRef]
- Deyeva, E.G.; Rusinov, V.L.; Charushin, V.N.; Chupakhin, O.N.; Kiselev, O.I. New antiviral drug triazavirin®: From screening to clinical trials. Razrab. Regist. Lek. Sredstv. 2014, 2, 144–151. [Google Scholar]
- Loginova, S.I.; Borisevich, S.V.; Maksimov, V.A.; Bondarev, V.P.; Kotovskaia, S.K.; Rusinov, V.L.; Charushin, V.N. Investigation of Triazavirin Antiviral Activity against Influenza A Virus (H5N1) in Cell Culture. Antibiot. Khimioterapiya 2007, 52, 18–20. [Google Scholar]
- Tikhonova, E.P.; Kuz’mina, T.Y.; Andronova, N.V.; Tyushevskaya, O.A.; Elistratova, T.A.; Kuz’min, A.E. Study of Effectiveness of Antiviral Drugs (Umifenovir, Triazavirin) against Acute Respiratory Viral Infections. Kazan Med. J. 2018, 99, 215–223. [Google Scholar] [CrossRef]
- Karpenko, I.; Deev, S.; Kiselev, O.; Charushin, V.; Rusinov, V.; Ulomsky, E.; Deeva, E.; Yanvarev, D.; Ivanov, A.; Smirnova, O.; et al. Antiviral Properties, Metabolism, and Pharmacokinetics of a Novel Azolo-1,2,4-Triazine-Derived Inhibitor of Influenza A and B Virus Replication. Antimicrob. Agents Chemother. 2010, 54, 2017–2022. [Google Scholar] [CrossRef] [Green Version]
- Kiselev, O.I.; Deeva, E.G.; Mel’nikova, T.I.; Kozeletskaia, K.N.; Kiselev, A.S.; Rusinov, V.L.; Charushin, V.N.; Chupakhin, O.N. A New Antiviral Drug Triazavirin: Results of Phase II Clinical Trial. Vopr. Virusol. 2012, 57, 9–12. [Google Scholar]
- Loginova, S.I.; Borisevich, S.V.; Rusinov, V.L.; Ulomskiĭ, U.N.; Charushin, V.N.; Chupakhin, O.N. Toxicity of Triazavirin, a Novel Russian Antiinfluenza Chemotherapeutic. Antibiot. Khimioterapiya 2012, 57, 8–10. [Google Scholar]
- Deeva, E.G.; Kiselev, L.I.; Melnikova, T.I.; Shaldzhan, A.A.; Nekrasov, P.A.; Kiselev, A.S.; Zagorodnikova, K.A.; Charushin, V.N.; Rusinov, V.L.; Ulomsky, E.N.; et al. New antiviral drugTriazavirin. Results of phase 1 clinical trial. Epidemiol. Infect. Dis. 2013, 18, 20–26. [Google Scholar] [CrossRef]
- Rusinov, V.L.; Ulomskii, E.N.; Chupakhin, O.N.; Zubairov, M.M.; Kapustin, A.B.; Mitin, N.I.; Zhiravetskii, M.I.; Vinograd, I.A. Synthesis and Antiviral Activity of 6-Nitro-7-Oxo-4,7-Dihydroazolo-[5,1-c] [1,2,4]-Triazines. Pharm. Chem. J. 1990, 24, 646–650. [Google Scholar] [CrossRef]
- Chupakhin, O.N.; Rusinov, V.L.; Ulomskij, E.N.; Charushin, V.N.; Petrov, A.J.; Kiselev, O.I. 2-Methylthio-6-nitro-1,2,4-triazolo[5,1-c]-1,2,4-triazine-7(4H)-one Sodium Salt Dihydrate Possessing Antiviral Activity. Patent RU2294936, 10 March 2007. [Google Scholar]
- Yudin, S.M.; Magataev, V.-M.K.; Ostroumov, Y.I.; Chupakhin, O.N.; Rusinov, V.L.; Charushin, V.N. A Method for Emergency Non-Specific Prevention and Treatment of Viral Diseases of Pigs with PMWS Syndrome and Parvovirus Enteritis of Carnivores. Patent RU2337690, 10 November 2008. [Google Scholar]
- Chupakhin, O.N.; Rusinov, V.L.; Ulomskij, E.N.; Charushin, V.N.; Petrov, A.J.; Kiselev, O.I.; Artemev, G.A. Method of Obtaining Sodium Salt of 2-methylthio-6-nitro-1,2,4-triazolo[5,1-c]-1,2,4-triazin-7-one, Dihydrate. Patent RU2343154, 10 January 2009. [Google Scholar]
- Petrov, A.J. Antiviral Agent for the Prevention and Treatment of Tick-Borne Encephalitis. Patent RU2444363, 10 March 2012. [Google Scholar]
- Petrov, A.J. Antiviral Medication and Method of Prevention and Treatment of Viral Infections. Patent RU2457844, 10 August 2012. [Google Scholar]
- Kiselev, O.I.; Chupakhin, O.N.; Rusinov, V.L.; Deeva, E.G. Conjugates of 2-methylthio-6-nitro-1,2,4-triazolo[5,1-c]-1,2,4-triazin-7(4H)-one with Glutathione and Other Peptides with Antiviral Activity. Patent RU2516936, 20 May 2014. [Google Scholar]
- Malík, I.; Čižmárik, J.; Kováč, G.; Pecháčová, M.; Hudecová, L. Triazavirin Might Be the New Hope to Fight Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Ceska Slov. Farm. Cas. Ceske Farm. Spol. Slov. Farm. Spol. 2021, 70, 18–25. [Google Scholar]
- Sabitov, A.U.; Kovtun, O.P.; Batskalevich, N.A.; Lvov, N.I.; Zhdanov, K.V.; Esaulenko, E.V.; Tikhonova, E.P.; Kalinina, Y.S.; Sorokin, P.V.; Chepur, S.V.; et al. Meta-Analysis of Randomized Clinical Trials of Riamilovir Efficacy in Etiotropic Therapy of Influenza. Antibiot. Khimioterapiya 2021, 66, 58–71. [Google Scholar] [CrossRef]
- Sabitov, A.U.; Kovtun, O.P.; Batskalevich, N.A.; Maltsev, O.V.; Zhdanov, K.V.; Esaulenko, E.V.; Tikhonova, E.P.; Kalinina, Y.S.; Sorokin, P.V.; Chepur, S.V.; et al. Meta-Analysis of Randomized Controlled Clinical Trials of Riamilovir Efficacy in the Etiotropic Therapy of Acute Respiratory Viral Infection. Antibiot. Khimioterapiya 2021, 66, 48–57. [Google Scholar] [CrossRef]
- Sabitov, A.U.; Belousov, V.V.; Edin, A.S.; Oleinichenko, E.V.; Gladunova, E.P.; Tikhonova, E.P.; Kuzmina, T.Y.; Kalinina, Y.S.; Sorokin, P.V. Practical Experience of Using Riamilovir in Treatment of Patients with Moderate COVID-19. Antibiot. Khimioterapiya 2020, 65, 27–30. [Google Scholar] [CrossRef]
- Kasyanenko, K.V.; Maltsev, O.V.; Kozlov, K.V.; Lapikov, I.I.; Lvov, N.I.; Sukachev, V.S.; Zhdanov, K.V.; Sorokin, P.V.; Ratnikova, A.K. Clinical Efficiency and Safety of Riamilovir for Treating Patients with SARS-CoV-2 Infection. Antibiot. Khimioterapiya 2021, 65, 16–21. [Google Scholar] [CrossRef]
- Sabitov, A.U.; Sorokin, P.V.; Dashutina, S.U. The Efficacy and Safety of Riamilovir in the Treatment of Patients with COVID-19. Antibiot. Khimioterapiya 2021, 66, 35–37. [Google Scholar] [CrossRef]
- Zykov, K.A.; Sinitsyn, E.A.; Rvacheva, A.V.; Bogatyreva, A.O.; Zykova, A.A.; Shapovalenko, T.V. Rationale for a New Outpatient Drug Therapy Algorithm in COVID-19 Patients Based on the Principle of «Multi-Hit» Approach. Antibiot. Khimioterapiya 2021, 66, 49–61. [Google Scholar] [CrossRef]
- Sabitov, A.U.; Sorokin, P.V.; Dashutina, S.Y. Experience of the Preventive Use of the Drug Riamilovir in the Foci of Coronavirus Infection (COVID-19). Ter. Arkhiv 2021, 93, 435–439. [Google Scholar] [CrossRef]
- Kasyanenko, K.V.; Kozlov, K.V.; Maltsev, O.V.; Lapikov, I.I.; Gordienko, V.V.; Sharabhanov, V.V.; Sorokin, P.V.; Zhdanov, K.V. Evaluation of the Effectiveness of Riamilovir in the Complex Therapy of Patients with COVID-19. Ter. Arkhiv 2021, 93, 290–294. [Google Scholar] [CrossRef]
- Wu, X.; Yu, K.; Wang, Y.; Xu, W.; Ma, H.; Hou, Y.; Li, Y.; Cai, B.; Zhu, L.; Zhang, M.; et al. The Efficacy and Safety of Triazavirin for COVID-19: A Trial Protocol. Engineering 2020, 6, 1199–1204. [Google Scholar] [CrossRef]
- Wu, X.; Yu, K.; Wang, Y.; Xu, W.; Ma, H.; Hou, Y.; Li, Y.; Cai, B.; Zhu, L.; Zhang, M.; et al. Efficacy and Safety of Triazavirin Therapy for Coronavirus Disease 2019: A Pilot Randomized Controlled Trial. Engineering 2020, 6, 1185–1191. [Google Scholar] [CrossRef]
- Rusinov, V.L.; Petrov, A.Y.; Postovskii, I.Y. Synthesis of nitro derivatives of azolo[5,1-c][1,2,4]triazine. Chem. Heterocycl. Compd. 1980, 9, 1283–1285. [Google Scholar]
- Sorokin, P.V.; Thorne, D.E. Anti-Viral Drug. Patent WO2017144708, 31 August 2017. [Google Scholar]
- Voinkov, E.K.; Drokin, R.A.; Ulomskiy, E.N.; Slepukhin, P.A.; Rusinov, V.L.; Chupakhin, O.N. Crystal Structure of Medicinal Product Triazavirin. J. Chem. Crystallogr. 2019, 49, 213–218. [Google Scholar] [CrossRef]
- Shestakova, T.S.; Khalymbadzha, I.A.; Deev, S.L.; Eltsov, O.S.; Rusinov, V.L.; Shenkarev, Z.O.; Arseniev, A.S.; Chupakhin, O.N. Synthesis of the [2H,15N]-Labeled Antiviral Drug “Triazavirine”. Russ. Chem. Bull. 2011, 60, 729–732. [Google Scholar] [CrossRef]
- Shestakova, T.S.; Deev, S.L.; Khalymbadzha, I.A.; Rusinov, V.L.; Paramonov, A.S.; Arseniev, A.S.; Shenkarev, Z.O.; Charushin, V.N.; Chupakhin, O.N. Antiviral Drug Triazavirin, Selectively Labeled with 2H, 13C, and 15N Stable Isotopes. Synthesis and Properties. Chem. Heterocycl. Compd. 2021, 57, 479–482. [Google Scholar] [CrossRef]
- Egorova, L.G.; Petrov, A.Y.; Rusinov, V.L. NH Acidities of 7-Oxo-4,7-Dihydropyrazolo- and 1,2,4-Triazolo[5,1-c][1,2,4]Triazines. Chem. Heterocycl. Compd. 1984, 20, 564–566. [Google Scholar] [CrossRef]
- Chupakhin, O.N.; Rusinov, V.L.; Ulomskij, E.N.; Savateev, K.V.; Borisov, S.S.; Novikova, N.A.; Loginova, S.J.; Borisevich, S.V.; Sorokin, P.V. 2-Methylsulphanyl-6-nitro-oxo-1,2,4-triazolo[5,1-c][1,2,4]triazinide l-argininiumdihydrate, Possessing Antiviral Activity, Method of Its Obtaining and Application for Prevention and Treatment of West Nile Fever. Patent RU2536874, 27 December 2014. [Google Scholar]
- Chupakhin, O.N.; Rusinov, V.L.; Ulomskij, E.N.; Savateev, K.V.; Borisov, S.S.; Novikova, N.A.; Loginova, S.J.; Borisevich, S.V.; Sorokin, P.V. 2-Methylsulphanyl-6-nitro-7-oxo-1,2,4-triazolo[5,1-c][1,2,4]triazinide l-arginine Dihydrate Having Antiviral Activity, Method for Producing the Same and Use in Prophylaxis and Treatment of West Nile Virus. Patent EA026688, 31 May 2017. [Google Scholar]
- Chupakhin, O.N.; Rusinov, V.L.; Ulomsky, E.N.; Savateev, K.V.; Fedotov, V.V.; Borisov, S.S.; Novirova, N.A.; Loginova, N.A.; Borisevich, S.V.; Sorokin, P.V. 2-Methylsulphanyl-6-Nitro-7-oxo-1,2,4-triazolo[5,1-c][1,2,4]triazinide L-arginine Dehydrate Active Toward West Nile Virus. Patent US9790227, 17 October 2017. [Google Scholar]
- Chupakhin, O.N.; Charushin, V.N.; Kotovskaya, S.K.; Rusinov, V.L.; Ulomsky, E.N.; Bykov, V.N.; Stepanov, A.V.; Chepur, S.V.; Lebedeva, I.K.; Tsikarishvili, G.V.; et al. Azoloazinium Salts of Fluoroquinolones with Antibacterial Antiviral Activity. Patent RU2547835, 10 April 2015. [Google Scholar]
- Blazhennikova, I.V.; Kurpyakova, A.F.; Bykov, V.N.; Geibo, D.S.; Nikiforov, A.S.; Stepanov, A.V.; Charushin, V.N.; Chupakhin, O.N.; Kotovskaya, S.K.; Rusinov, V.L. Experimental comparison of the pharmacokinetics of triazavirin and levofloxacin, as well as a conjugate based on them. Eksperimental’naya Klin. Farmakol. 2015, 78, 34–38. [Google Scholar] [CrossRef] [Green Version]
- Rusinov, V.L.; Ulomskii, E.N.; Klyuev, N.A.; Orlov, V.M.; Shorshnev, S.V.; Chupakhin, O.N. Nitroazines. 19. Prototropic Tautomerism in 6-Nitro-7-Oxo-4,7-Dihydro-1,2,4-Triazolo[5,1-c][1,2,4]Triazines. Chem. Heterocycl. Compd. 1992, 28, 1331–1334. [Google Scholar] [CrossRef]
- Ulomskii, E.N.; Rusinov, V.L.; Chupakhin, O.N.; Rusinov, G.L.; Chernyshev, A.I.; Aleksandrov, G.G. Nitroazines. 7. Alkylation of 6-Nitro-7-Oxo-4,7-Dihydroazolo[5,1-c][1,2,4]-Triazines and Identification of the Products. Chem. Heterocycl. Compd. 1987, 23, 1236–1243. [Google Scholar] [CrossRef]
- Rusinov, V.L.; Chupakhin, O.N.; Deev, S.L.; Shestakova, T.S.; Ulomskii, E.N.; Rusinova, L.I.; Kiselev, O.I.; Deeva, E.G. Synthesis and Antiviral Activity of Nucleoside Analogs Based on 1,2,4-Triazolo[3,2-c][1,2,4]Triazin-7-Ones. Russ. Chem. Bull. 2010, 59, 136–143. [Google Scholar] [CrossRef]
- Chupakhin, O.N.; Kisilev, O.I.; Rusinov, V.L.; Charushin, V.N.; Tugova, N.P.; Medvedera, N.R.; Ulomskii, E.N.; Evans, D.M.; Dyeva, E.G. Small Molecule Havingantiviral Properties. Patent WO2013122575, 22 August 2013. [Google Scholar]
- Kiselev, O.I.; Rusinov, V.L.; Ulomsky, E.N.; Medvedeva, N.R.; Sapozhnikova, I.M.; Charushin, V.N.; Chupakhin, O.N.; Danilenko, D.; Deeva, E.G. Small molecules having antiviral properties. Patent WO2018035509, 22 February 2018. [Google Scholar]
- Ulomskii, E.N.; Deev, S.L.; Tkachev, A.V.; Moiseev, I.K.; Rusinov, V.L. Adamantylation of 3-Nitro-and 3-Ethoxycarbonyl-1,2,4-Triazolo[5,1-c]-1,2,4-Triazin-4-Ones. Russ. J. Org. Chem. 2002, 38, 272–280. [Google Scholar] [CrossRef]
- Ulomskii, E.N.; Tsoi, E.V.; Rusinov, V.L.; Chupakhin, O.N.; Kalb, G.L.; Sosonkin, I.M. Reduction of Nitro Derivatives of Azolo[5,1-c][1,2,4]Triazines. Chem. Heterocycl. Compd. 1992, 28, 570–573. [Google Scholar] [CrossRef]
- Niles, J.C.; Wishnok, J.S.; Tannenbaum, S.R. Peroxynitrite-Induced Oxidation and Nitration Products of Guanine and 8-Oxoguanine: Structures and Mechanisms of Product Formation. Nitric Oxide 2006, 14, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Medvedeva, N.R.; Sapozhnikova, I.S.; Rusinov, V.L.; Ulomsky, E.N. The Redox Transformations and Nucleophilic Replacements as Possible Metabolic Reactions of the Drug “Triazaverin”. The Chemical Modeling of the Metabolic Processes. Chim. Techno Acta 2015, 2, 234–247. [Google Scholar] [CrossRef] [Green Version]
- Rusinov, V.L.; Sapozhnikova, I.M.; Ulomskii, E.N.; Medvedeva, N.R.; Egorov, V.V.; Kiselev, O.I.; Deeva, E.G.; Vasin, A.V.; Chupakhin, O.N. Nucleophilic Substitution of Nitro Group in Nitrotriazolotriazines as a Model of Potential Interaction with Cysteine-Containing Proteins. Chem. Heterocycl. Compd. 2015, 51, 275–280. [Google Scholar] [CrossRef]
- Ulomskiy, E.N.; Lyapustin, D.N.; Mukhin, E.M.; Voinkov, E.K.; Fedotov, V.V.; Savateev, K.V.; Eltsov, O.S.; Gorbunov, E.B.; Drokin, R.A.; Rusinov, V.L.; et al. ANRORC Process in 1-Alkylazolo[5,1-c][1,2,4]Triazin-4(1H)-Ones. Chem. Heterocycl. Compd. 2018, 54, 63–69. [Google Scholar] [CrossRef]
- Rusinov, V.L.; Ulomskii, E.N.; Kozhevnikov, D.N.; Chupakhin, O.N.; Aleksandrov, G.G. Nitroazines. XXVI. Hydrolytic destruction of azoloanneled nitro-1,2,4-triazines. Russ. J. Org. Chem. 1996, 32, 738–744. [Google Scholar]
- Rusinov, V.L.; Ulomskii, E.N.; Chupakhin, O.N.; Petrov, A.Y.; Sharonov, E.A. Nitroazines. 9. Characteristic Features of Nucleophilic Substitution of the Nitro Group in Dihydroazolo[5,1-c] [1,2,4]Triazines. Chem. Heterocycl. Compd. 1989, 25, 209–213. [Google Scholar] [CrossRef]
- Petrov, A.Y.; Sorokin, P.V.; Shablokova, A.S. Tabletted Antiviral Agent and Method for Preparing It. Patent RU2446802, 27 January 2012. [Google Scholar]
- Petrov, A.Y.; Sorokin, P.V.; Shablakova, A.S. Encapsulated Antiviral Medication and Its Production Method. Patent RU2451514, 27 May 2012. [Google Scholar]
- Shablakova, A.S.; Chupakhin, O.N.; Petrov, A.Y.; Charushin, V.N.; Rusinov, V.L.; Glavatskikh, S.A.; Ulomsky, E.N. Development of Solid Dosage Forms of an Antiviral Agent. Izv. Vuzov Chem. Chem. Technol. Ser. 2013, 56, 102–104. [Google Scholar]
- Kiselev, O.I.; Chupakhin, O.N.; Rusinov, V.L.; Charushin, V.N.; Deeva, E.G.; Ulomsky, E.N. Injection Solution for Treating Viral Diseases Specified in H1N1, H3N2, H5N1 Influenza, Tick-Borne Encephalitis and West Nile Fever. Patent RU2574007, 27 January 2016. [Google Scholar]
- Kozhikhova, K.V.; Ivantsova, M.N.; Tokareva, M.I.; Shulepov, I.D.; Tretiyakov, A.V.; Shaidarov, L.V.; Rusinov, V.L.; Mironov, M.A. Preparation of Chitosan-Coated Liposomes as a Novel Carrier System for the Antiviral Drug Triazavirin. Pharm. Dev. Technol. 2018, 23, 334–342. [Google Scholar] [CrossRef]
- Mironov, M.A.; Tokareva, M.I.; Ivantsova, M.N.; Sorokin, P.V.; Rusinov, V.L.; Matern, A.I.; Charushin, V.N.; Chupakhin, O.N.; Mironov, M.A. Liposomal Composition and Method of Its Preparation. Patent RU2514000, 27 April 2014. [Google Scholar]
- Valiulin, S.V.; Onischuk, A.A.; Dubtsov, S.N.; Baklanov, A.M.; An’kov, S.V.; Plokhotnichenko, M.E.; Tolstikova, T.G.; Dultseva, G.G.; Rusinov, V.L.; Charushin, V.N.; et al. Aerosol Inhalation Delivery of Triazavirin in Mice: Outlooks for Advanced Therapy Against Novel Viral Infections. J. Pharm. Sci. 2021, 110, 1316–1322. [Google Scholar] [CrossRef] [PubMed]
- Valiulin, S.V.; Onischuk, A.A.; Baklanov, A.M.; Dubtsov, S.N.; Dultseva, G.G.; An’kov, S.V.; Tolstikova, T.G.; Rusinov, V.L.; Charushin, V.N. An Integrated Aerosol Setup for Therapeutics and Toxicological Testing: Generation Techniques and Measurement Instrumentation. Measurement 2021, 181, 109659. [Google Scholar] [CrossRef]
- Kasianenko, K.V.; Lvov, N.I.; Maltsev, O.V.; Zhdanov, K.V. Nucleoside Analogues for the Treatment of Influenza: History and Experience. J. Infectology 2019, 11, 20–26. [Google Scholar] [CrossRef] [Green Version]
- Chupakhin, O.N.; Charushin, V.N.; Rusinov, V.L. Scientific Foundations for the Creation of Antiviral and Antibacterial Preparations. Her. Russ. Acad. Sci. 2016, 86, 206–212. [Google Scholar] [CrossRef]
- Loginova, S.I.; Borisevich, S.V.; Maksimov, V.A.; Bondarev, V.P.; Kotovskaia, S.K.; Rusinov, V.L.; Charushin, V.N.; Chupakhin, O.N. [Therapeutic Efficacy of Triazavirin, a Novel Russian Chemotherapeutic, against Influenza Virus A (H5N1)]. Antibiot. Chemoterapy 2011, 56, 10–12. [Google Scholar]
- Ratnikova, L.I. Application of a New Domestic Antiviral Drug (Triazavirin) in Etiotropictreatment of Influenza. Eksperimental’naya Klin. Farmakol. 2018, 81, 24–27. [Google Scholar] [CrossRef]
- Sologub, T.V.; Tokin, I.I.; Midikari, A.S.; Tsvetkov, V.V. A Comparative Efficacy and Safety of Using Antiviral Drugs in Therapy of Patients with Influenza. Infekc. Bolezn. 2017, 15, 40–47. [Google Scholar] [CrossRef]
- Leneva, I.A.; Falynskova, I.N.; Makhmudova, N.R.; Glubokova, E.A.; Kartasheva, N.P.; Leonova, E.I.; Mikhailova, N.A.; Shestakova, I.V. Effect of Triazavirine on the Outcome of a Lethal Influenza Infection and Secondary Bacterial Pneumonia Following Influenza in Mice. Microbiol. Indep. Res. J. 2017, 4, 52–57. [Google Scholar] [CrossRef] [Green Version]
- Tokin, I.I.; Tsvetkov, V.V.; Golobokov, G.S. Comparative Clinical and Economic Evaluation of Two Alternative Antiviral Therapy Regimens for Influenza Patients. J. Infectology 2018, 10, 110–116. [Google Scholar] [CrossRef]
- Emel’yanova, A.N.; Tikhonova, E.P.; Kuz’mina, T.Y.; Emel’yanov, A.S.; Chuprova, G.A.; Epifantseva, N.V.; Klimovich, K.I.; Radyukin, N.O.; Radyukina, E.O.; Yurchuk, S.V.; et al. A Evaluation of the efficacy of antiviral treatment of influenza a (subtype H1N1) during 2017–2018 and 2018–2019 epidemic seasons. Eksperimental’naya Klin. Farmakol. 2020, 83, 23–27. [Google Scholar] [CrossRef]
- Tokin, I.I.; Zubkova, T.G.; Drozdova, Y.V.; Lioznov, D.A. Experience of Etiotropic Therapy of Acute Respiratory Viral Infection with Domestic Antiviral Drug. Infekc. Bolezn. 2019, 17, 13–17. [Google Scholar] [CrossRef]
- Lioznov, D.A.; Tokin, I.I.; Zubkova, T.G.; Sorokin, P.V. The Practice of Using a Domestic Antiviral Drug in the Etiotropic Therapy of Acute Respiratory Viral Infection. Ter. Arkhiv 2020, 92, 160–164. [Google Scholar] [CrossRef]
- Verevshchikov, V.K.; Shemyakina, E.K.; Sabitov, A.U.; Batskalevich, N.A. Modern Etiotropic Therapy of Influenza and ARVI in Adult Patients with Premorbid Pathology. Antibiot. Khimioter. 2018, 63, 47–50. [Google Scholar]
- Verevshchikov, V.K.; Shemyakina, E.K.; Sabitov, A.U.; Khamanova, Y.B. The Possibilities of Etiotropic Therapy for Influenza and ARVI, Taking into Account the Period of Hospitalization and the Risk of Developing Secondary Complications. Antibiot. Khimioter. 2019, 64, 15–19. [Google Scholar] [CrossRef]
- Loginova, S.I.; Borisevich, S.V.; Rusinov, V.L.; Ulomskiĭ, U.N.; Charushin, V.N.; Chupakhin, O.N. Investigation of Triazavirin Antiviral Activity against Tick-Borne Encephalitis Pathogen in Cell Culture. Antibiot. Chemoterapy 2014, 59, 3–5. [Google Scholar]
- Loginova, S.Y.; Borisevich, S.V.; Rusinov, V.L.; Ulomsky, E.N.; Charushin, V.N.; Chupakhin, O.N.; Sorokin, P.V. Investigation of Prophylactic Efficacy of Triazavirin Against Experimental Forest-Spring Encephalitis on Albino Mice. Antibiot. Chemoterapy 2015, 60, 8–11. [Google Scholar]
- Loginova, S.Y.; Borisevich, S.V.; Rusinov, V.L.; Ulomsky, E.N.; Charushin, V.N.; Chupakhin, O.N.; Sorokin, P.V. Investigation of Therapeutic Efficacy of Triazavirin Against Experimental Forest-Spring Encephalitis on Albino Mice. Antibiot. Chemoterapy 2015, 60, 11–13. [Google Scholar]
- Tikhonova, E.P.; Kuz’mina, T.Y.; Anisimova, A.A.; Kalinina, Y.S. On the possibilities of using triazavirin in the complex treatment of tick-borne viral encephalitis in adults. Eksperimental’naya Klin. Farmakol. 2018, 81, 21–25. [Google Scholar] [CrossRef]
- Chepur, S.V.; Smirnova, A.V.; Kirienko, A.N.; Myasnikova, I.A.; Kanevsky, B.A.; Sorokin, P.V. Study of Riamilovir Activity Against SARS-CoV-2 Infection in Syrian Hamsters. Antibiot. Khimioterapiya 2021, 66, 13–19. [Google Scholar] [CrossRef]
- Yang, Z.-W.; Zhao, Y.-Z.; Zang, Y.-J.; Wang, H.; Zhu, X.; Meng, L.-J.; Yuan, X.-H.; Zhang, L.; Zhang, S.-L. Rapid Structure-Based Screening Informs Potential Agents for Coronavirus Disease (COVID-19) Outbreak. Chin. Phys. Lett. 2020, 37, 058701. [Google Scholar] [CrossRef]
- Almasi, F.; Mohammadipanah, F. Hypothetical Targets and Plausible Drugs of Coronavirus Infection Caused by SARS-CoV-2. Transbound. Emerg. Dis. 2021, 68, 318–332. [Google Scholar] [CrossRef] [PubMed]
- Naydenova, K.; Muir, K.W.; Wu, L.-F.; Zhang, Z.; Coscia, F.; Peet, M.J.; Castro-Hartmann, P.; Qian, P.; Sader, K.; Dent, K.; et al. Structure of the SARS-CoV-2 RNA-Dependent RNA Polymerase in the Presence of Favipiravir-RTP. Proc. Natl. Acad. Sci. USA 2021, 118, e2021946118. [Google Scholar] [CrossRef] [PubMed]
- Kabinger, F.; Stiller, C.; Schmitzová, J.; Dienemann, C.; Kokic, G.; Hillen, H.S.; Hobartner, C.; Cramer, P. Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis. Nat. Struct. Mol. Biol. 2021, 28, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Celik, I.; Erol, M.; Duzgun, Z. In silico evaluation of potential inhibitory activity of remdesivir, favipiravir, ribavirin and galidesivir active forms on SARS-CoV-2 RNA polymerase. Mol. Divers. 2022, 26, 279–292. [Google Scholar] [CrossRef]
- Shahab, S.; Sheikhi, M. Triazavirin—Potential Inhibitor for 2019-NCoV Coronavirus M Protease: A DFT Study. Curr. Mol. Med. 2021, 21, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.A.A.; Abdelrahman, A.H.M.; Allemailem, K.S.; Almatroudi, A.; Moustafa, M.F.; Hegazy, M.-E.F. In Silico Evaluation of Prospective Anti-COVID-19 Drug Candidates as Potential SARS-CoV-2 Main Protease Inhibitors. Protein J. 2021, 40, 296–309. [Google Scholar] [CrossRef]
- Ercan, S.; Çınar, E. A Molecular Docking Study of Potential Inhibitors and Repurposed Drugs against SARS-CoV-2 Main Protease Enzyme. J. Indian Chem. Soc. 2021, 98, 100041. [Google Scholar] [CrossRef]
- Chtita, S.; Belhassan, A.; Aouidate, A.; Belaidi, S.; Bouachrine, M.; Lakhlifi, T. Discovery of Potent SARS-CoV-2 Inhibitors from Approved Antiviral Drugs via Docking and Virtual Screening. Comb. Chem. High Throughput Screen. 2021, 24, 441–454. [Google Scholar] [CrossRef]
- Acute Respiratory Viral Infections (ARVI) in Adults. Available online: https://cr.minzdrav.gov.ru/recomend/724_1 (accessed on 14 November 2022). (In Russian)
























| Group | Elimination Rate Constant, ke, Copies/g × Days | Elimination Half-Life, τ1/2, Days |
|---|---|---|
| Control | 0.31 | 2.21 |
| Therapy with Riamilovir, 20 mg/kg, intraperitoneal, daily from 3 to 7 days after infection | 0.57 | 1.22 |
| Parameter | Number of Patients n = 69 | Riamilovir Group (Number of Patients n = 34) | Ribavirin + Umifenovir Group (Number of Patients n = 35) |
|---|---|---|---|
| Age, years (M ± SD) | 36 ± 3 | 39 ± 3 | 32 ± 3 |
| Male, (%) | 55 (79.7) | 25 (73.5) | 30 (85.7) |
| Body temperature, °C (M ± SD) | 38.0 ± 0.8 | 38.0 ± 0.6 | 37.9 ± 0.9 |
| Leukocytes (×109/L), (M ± SD) | 7.28 ± 1.35 | 5.76 ± 0.85 | 8.81 ± 1.54 |
| Lymphocytes (×109/L), (M ± SD) | 1.6 ± 0.04 | 1.3 ± 0.02 | 2.08 ± 0.01 |
| Thrombocytes (×109/L), (M ± SD) | 224 ± 13.45 | 228.39 ± 15.01 | 219.61 ± 11.74 |
| Aspartate transaminase (IU/L), (M ± SD) | 29.26 ± 4.27 | 28.36 ± 3.12 | 30.21 ± 5.41 |
| Alanine transaminase (IU/L), (M ± SD) | 29.37 ± 3.24 | 32.19 ± 4.34 | 26.54 ± 2.14 |
| Number of Patients | Discharged by the 14th Day of Hospitalization | Not Discharged by the 14th Day of Hospitalization | Negative PCR Test after 7 Days of Hospitalization | Positive PCR Test after 7 Days of Hospitalization |
|---|---|---|---|---|
| Riamilovir | 21 | 13 | 26 | 8 |
| Ribavirin + Umifenovir | 11 | 24 | 10 | 25 |
| Overall | 32 | 37 | 36 | 33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chupakhin, O.N.; Rusinov, V.L.; Varaksin, M.V.; Ulomskiy, E.N.; Savateev, K.V.; Butorin, I.I.; Du, W.; Sun, Z.; Charushin, V.N. Triazavirin—A Novel Effective Antiviral Drug. Int. J. Mol. Sci. 2022, 23, 14537. https://doi.org/10.3390/ijms232314537
Chupakhin ON, Rusinov VL, Varaksin MV, Ulomskiy EN, Savateev KV, Butorin II, Du W, Sun Z, Charushin VN. Triazavirin—A Novel Effective Antiviral Drug. International Journal of Molecular Sciences. 2022; 23(23):14537. https://doi.org/10.3390/ijms232314537
Chicago/Turabian StyleChupakhin, Oleg N., Vladimir L. Rusinov, Mikhail V. Varaksin, Evgeny N. Ulomskiy, Konstantin V. Savateev, Ilya I. Butorin, Weijie Du, Zhiyong Sun, and Valery N. Charushin. 2022. "Triazavirin—A Novel Effective Antiviral Drug" International Journal of Molecular Sciences 23, no. 23: 14537. https://doi.org/10.3390/ijms232314537






