3D Printing in Regenerative Medicine: Technologies and Resources Utilized
Abstract
:1. Introduction
2. 3D Bioprinting Techniques
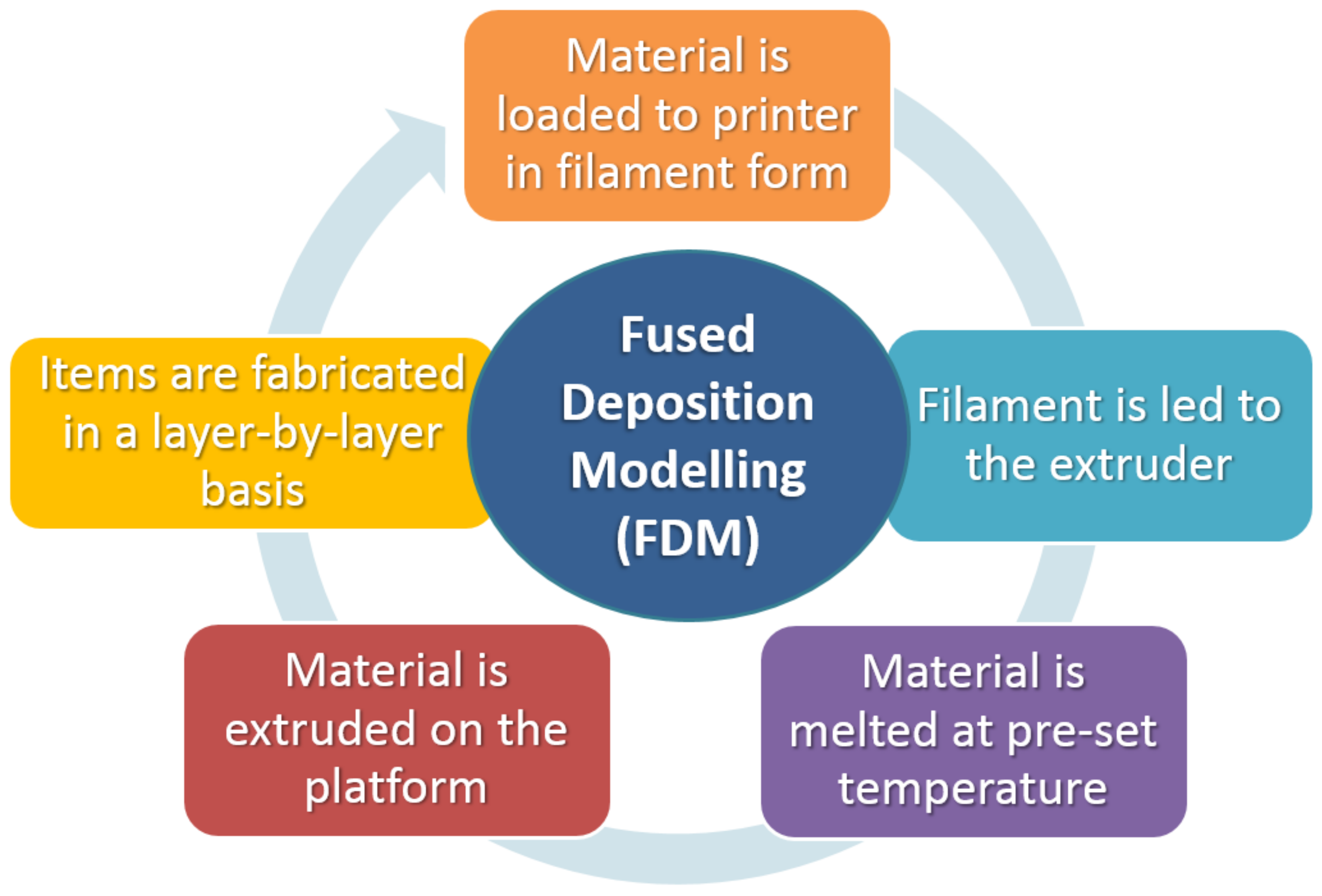
2.1. Fused Deposition Modeling (FDM)
Materials Compatible with the FDM Process
2.2. Stereolithography (SLA)
Materials Compatible with SLA Process
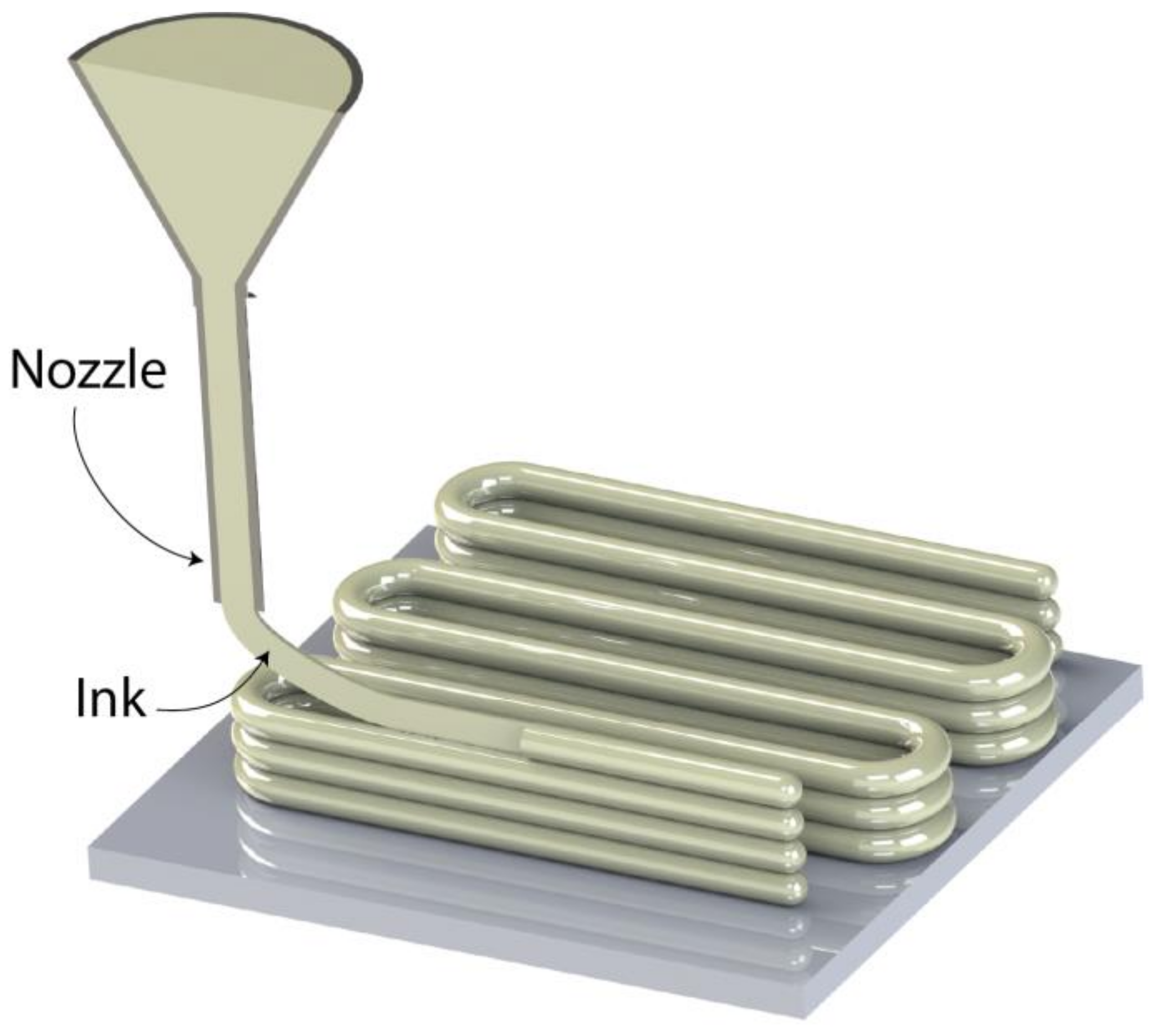
2.3. Direct Ink Writing (DIW)
2.4. Laser-Guided Direct Writing (LGDW)
Materials Suitable for DIW & LGDW
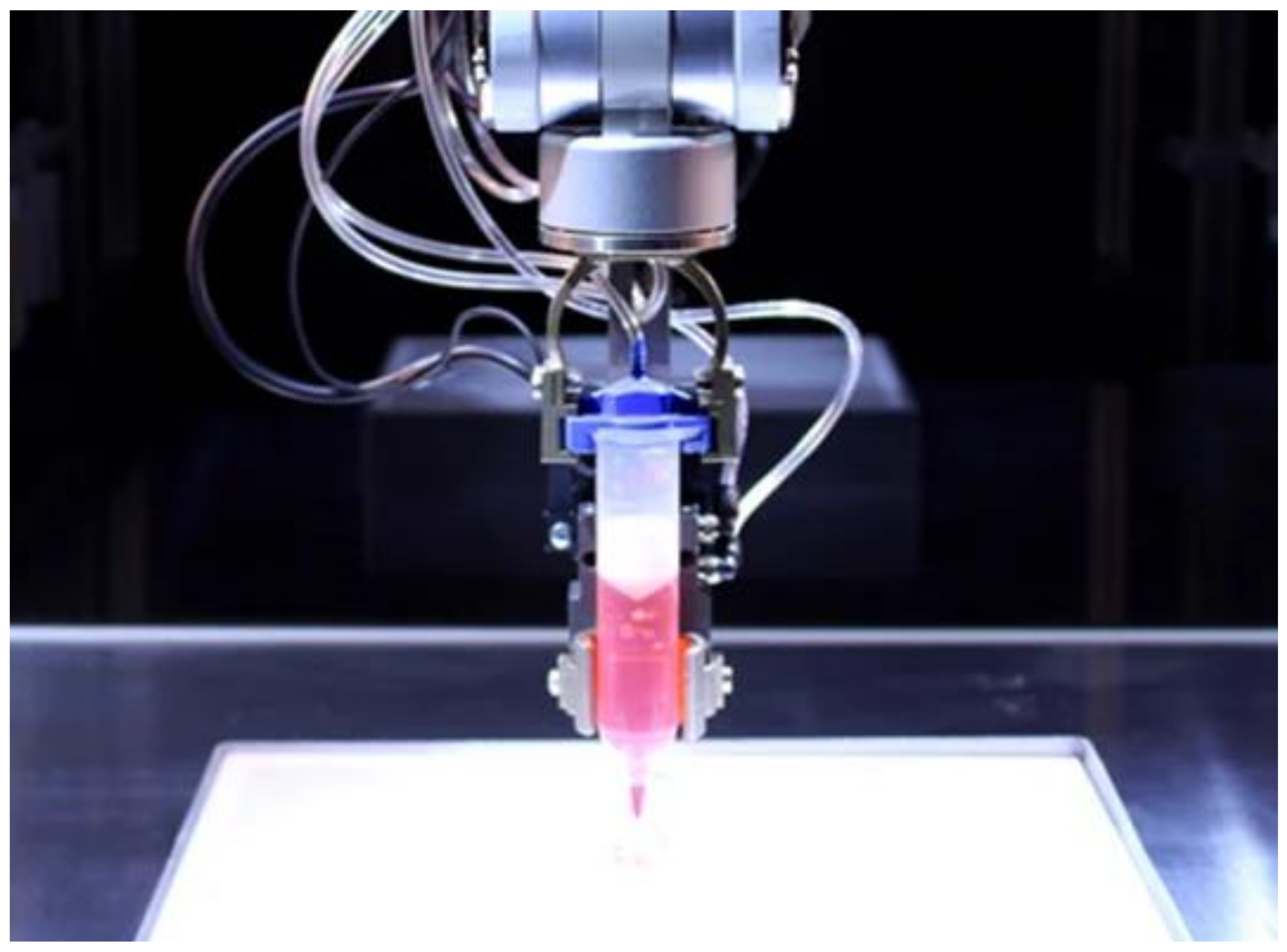
2.5. Inkjet Bioprinting
3. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bielenstein, J.; Radenković, M.; Najman, S.; Liu, L.; Ren, Y.; Cai, B.; Beuer, F.; Rimashevskiy, D.; Schnettler, R.; Alkildani, S.; et al. In Vivo Analysis of the Regeneration Capacity and Immune Response to Xenogeneic and Synthetic Bone Substitute Materials. Int. J. Mol. Sci. 2022, 23, 10636. [Google Scholar] [CrossRef] [PubMed]
- Kowalewicz, K.; Vorndran, E.; Feichtner, F.; Waselau, A.-C.; Brueckner, M.; Meyer-Lindenberg, A. In-Vivo Degradation Behavior and Osseointegration of 3D Powder-Printed Calcium Magnesium Phosphate Cement Scaffolds. Materials 2021, 14, 946. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Qi, J.; Liu, J.; Wang, H.; Liu, Y.; Feng, Y.; Xu, G. The Ability of Biodegradable Thermosensitive Hydrogel Composite Calcium-Silicon-Based Bioactive Bone Cement in Promoting Osteogenesis and Repairing Rabbit Distal Femoral Defects. Polymers 2022, 14, 3852. [Google Scholar] [CrossRef] [PubMed]
- Kantaros, A.; Chatzidai, N.; Karalekas, D. 3D printing-assisted design of scaffold structures. Int. J. Adv. Manuf. Technol. 2016, 82, 559–571. [Google Scholar] [CrossRef]
- Guillotin, B.; Guillemot, F. Cell patterning technologies for organotypic tissue fabrication. Trends Biotechnol. 2011, 29, 183–190. [Google Scholar] [CrossRef]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Wakitani, S.; Imoto, K.; Yamamoto, T.; Saito, M.; Murata, N.; Yoneda, M. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthr. Cartil. 2002, 10, 199–206. [Google Scholar] [CrossRef] [Green Version]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [Green Version]
- Ikada, Y. Challenges in tissue engineering. J. R. Soc. Interface 2006, 3, 589–601. [Google Scholar] [CrossRef]
- Atala, A. Wake Forest Innovations. Available online: https://www.wakeforestinnovations.com/experts/anthony-atala-md/ (accessed on 13 September 2022).
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Atala, A.; Bauer, S.B.; Soker, S.; Yoo, J.J.; Retik, A.B. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet 2006, 367, 1241–1246. [Google Scholar] [CrossRef]
- Elliott, M.J.; De Coppi, P.; Speggiorin, S.; Roebuck, D.; Butler, C.R.; Samuel, E.; Crowley, C.; McLaren, C.; Fierens, A.; Vondrys, D.; et al. Stem-cell-based, tissue engineered tracheal replacement in a child: A 2-year follow-up study. Lancet 2012, 380, 994–1000. [Google Scholar] [CrossRef] [Green Version]
- Dong, L.; Wang, S.-J.; Zhao, X.-R.; Zhu, Y.-F.; Yu-Fang, Z. 3D-Printed Poly(ε-caprolactone) Scaffold Integrated with Cell-laden Chitosan Hydrogels for Bone Tissue Engineering. Sci. Rep. 2017, 7, 13412. [Google Scholar] [CrossRef] [Green Version]
- Nicodemus, G.D.; Bryant, S.J. Cell Encapsulation in Biodegradable Hydrogels for Tissue Engineering Applications. Tissue Eng. Part B Rev. 2008, 14, 149–165. [Google Scholar] [CrossRef]
- Kantaros, A.; Piromalis, D. Fabricating Lattice Structures via 3D Printing: The Case of Porous Bio-Engineered Scaffolds. Appl. Mech. 2021, 2, 289–302. [Google Scholar] [CrossRef]
- Moroni, L.; de Wijn, J.; van Blitterswijk, C. 3D fiber-deposited scaffolds for tissue engineering: Influence of pores geometry and architecture on dynamic mechanical properties. Biomaterials 2006, 27, 974–985. [Google Scholar] [CrossRef]
- Amirkhani, S.; Bagheri, R.; Yazdi, A.Z. Effect of pore geometry and loading direction on deformation mechanism of rapid prototyped scaffolds. Acta Mater. 2012, 60, 2778–2789. [Google Scholar] [CrossRef]
- Leong, K.F.; Cheah, C.M.; Chua, C.K. Solid freeform fabrication of three-dimensional scaffolds for engineering replacement tissues and organs. Biomaterials 2003, 24, 2363–2378. [Google Scholar] [CrossRef]
- Eshraghi, S.; Das, S. Mechanical and microstructural properties of polycaprolactone scaffolds with one-dimensional, two-dimensional, and three-dimensional orthogonally oriented porous architectures produced by selective laser sintering. Acta Biomater. 2010, 6, 2467–2476. [Google Scholar] [CrossRef] [Green Version]
- Miranda, P.; Pajares, A.; Guiberteau, F. Finite element modeling as a tool for predicting the fracture behavior of robocast scaffolds. Acta Biomater. 2008, 4, 1715–1724. [Google Scholar] [CrossRef]
- Miranda, P.; Saiz, E.; Gryn, K.; Tomsia, A.P. Sintering and robocasting of β-tricalcium phosphate scaffolds for orthopaedic applications. Acta Biomater. 2006, 2, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Dong, C.; Zhang, X.; Shi, L.; Chen, Z.; Xu, Y.; Cai, H. Preparation and characterization of nanosilver-doped porous hydroxyapatite scaffolds. Ceram. Int. 2015, 41, 1671–1676. [Google Scholar] [CrossRef]
- Rodriguez, G.; Dias, J.; D’Ávila, M.A.; Bártolo, P. Influence of Hydroxyapatite on Extruded 3D Scaffolds. Procedia Eng. 2013, 59, 263–269. [Google Scholar] [CrossRef]
- Shuai, C.; Mao, Z.; Lu, H.; Nie, Y.; Hu, H.; Peng, S. Fabrication of porous polyvinyl alcohol scaffold for bone tissue engineering via selective laser sintering. Biofabrication 2013, 5, 015014. [Google Scholar] [CrossRef] [PubMed]
- Wieding, J.; Jonitz, A.; Bader, R. The Effect of Structural Design on Mechanical Properties and Cellular Response of Additive Manufactured Titanium Scaffolds. Materials 2012, 5, 1336–1347. [Google Scholar] [CrossRef]
- Serra, T.; Planell, J.; Navarro, M. High-resolution PLA-based composite scaffolds via 3-D printing technology. Acta Biomater. 2013, 9, 5521–5530. [Google Scholar] [CrossRef]
- Cox, S.C.; Thornby, J.A.; Gibbons, G.J.; Williams, M.A.; Mallick, K.K. 3D printing of porous hydroxyapatite scaffolds intended for use in bone tissue engineering applications. Mater. Sci. Eng. C 2015, 47, 237–247. [Google Scholar] [CrossRef]
- Inzana, J.A.; Olvera, D.; Fuller, S.M.; Kelly, J.P.; Graeve, O.A.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials 2014, 35, 4026–4034. [Google Scholar] [CrossRef] [Green Version]
- Elomaa, L.; Teixeira, S.; Hakala, R.; Korhonen, H.; Grijpma, D.W.; Seppälä, J.V. Preparation of poly(ε-caprolactone)-based tissue engineering scaffolds by stereolithography. Acta Biomater. 2011, 7, 3850–3856. [Google Scholar] [CrossRef]
- Gauvin, R.; Chen, Y.-C.; Lee, J.W.; Soman, P.; Zorlutuna, P.; Nichol, J.W.; Bae, H.; Chen, S.; Khademhosseini, A. Microfabrication of complex porous tissue engineering scaffolds using 3D projection stereolithography. Biomaterials 2012, 33, 3824–3834. [Google Scholar] [CrossRef]
- Kantaros, A.; Karalekas, D. Fiber Bragg grating based investigation of residual strains in ABS parts fabricated by fused deposition modeling process. Mater. Des. 2013, 50, 44–50. [Google Scholar] [CrossRef]
- Kantaros, A.; Piromalis, D. Employing a Low-Cost Desktop 3D Printer: Challenges, and How to Overcome Them by Tuning Key Process Parameters. Int. J. Mech. Appl. 2021, 10, 11–19. [Google Scholar] [CrossRef]
- Kantaros, A.; Diegel, O.; Piromalis, D.; Tsaramirsis, G.; Khadidos, A.O.; Khadidos, A.O.; Qudus Khan, F.; Jan, S. 3D printing: Making an innovative technology widely accessible through makerspaces and outsourced services. Mater. Today Proc. 2022, 49, 2712–2723. [Google Scholar] [CrossRef]
- Kantaros, A.; Karalekas, D. FBG Based IN situ Characterization of Residual Strains in FDM Process. In Residual Stress, Thermomechanics & Infrared Imaging, Hybrid Techniques and Inverse Problems; Conference Proceedings of the Society for Experimental Mechanics Series; Springer: Cham, Switzerland, 2014; Volume 8. [Google Scholar] [CrossRef]
- Tsaramirsis, G.; Kantaros, A.; Al-Darraji, I.; Piromalis, D.; Apostolopoulos, C.; Pavlopoulou, A.; Alrammal, M.; Ismail, Z.; Buhari, S.M.; Stojmenovic, M.; et al. A Modern Approach towards an Industry 4.0 Model: From Driving Technologies to Management. J. Sens. 2022, 2022, 5023011. [Google Scholar] [CrossRef]
- Kantaros, A.; Giannatsis, J.; Karalekas, D. A novel strategy for the incorporation of optical sensors in Fused Deposition Modeling parts. In Proceedings of the International Conference on Advanced Manufacturing Engineering and Technologies, Stockolm, Sweden, 27–30 October 2013; Universitets Service US AB, KTH Royal Institite of Technology: Stockholm, Sweden, 2013. ISBN 978-91-7501-893-5. Available online: https://www.researchgate.net/publication/269631461_A_novel_strategy_for_the_incorporation_of_optical_sensors_in_FDM_parts (accessed on 10 November 2022).
- Jwa, S.-J.; Won, J.-M.; Kim, D.-H.; Kim, K.-B.; Lee, J.-B.; Heo, M.; Shim, K.-S.; Jo, H.-S.; Lee, W.-J.; Roh, T.-S.; et al. Breast Tissue Restoration after the Partial Mastectomy Using Polycaprolactone Scaffold. Polymers 2022, 14, 3817. [Google Scholar] [CrossRef]
- Liparoti, S.; Mottola, S.; Viscusi, G.; Belvedere, R.; Petrella, A.; Gorrasi, G.; Pantani, R.; De Marco, I. Production of Mesoglycan/PCL Based Composites through Supercritical Impregnation. Molecules 2022, 27, 5800. [Google Scholar] [CrossRef]
- Ayran, M.; Dirican, A.Y.; Saatcioglu, E.; Ulag, S.; Sahin, A.; Aksu, B.; Croitoru, A.-M.; Ficai, D.; Gunduz, O.; Ficai, A. 3D-Printed PCL Scaffolds Combined with Juglone for Skin Tissue Engineering. Bioengineering 2022, 9, 427. [Google Scholar] [CrossRef]
- Kantaros, A.; Laskaris, N.; Piromalis, D.; Ganetsos, T. Manufacturing Zero-Waste COVID-19 Personal Protection Equipment: A Case Study of Utilizing 3D Printing While Employing Waste Material Recycling. Circ. Econ. Sust. 2021, 1, 851–869. [Google Scholar] [CrossRef]
- Dorovskikh, S.I.; Vikulova, E.S.; Sergeevichev, D.S.; Guselnikova, T.Y.; Zheravin, A.A.; Nasimov, D.A.; Vasilieva, M.B.; Chepeleva, E.V.; Saprykin, A.I.; Basova, T.V.; et al. Biological Studies of New Implant Materials Based on Carbon and Polymer Carriers with Film Heterostructures Containing Noble Metals. Biomedicines 2022, 10, 2230. [Google Scholar] [CrossRef]
- Chua, C.K.; Leong, K.F.; Tan, K.H.; Wiria, F.E.; Cheah, C.M. Development of tissue scaffolds using selective laser sintering of polyvinyl alcohol/hydroxyapatite biocomposite for craniofacial and joint defects. J. Mater. Sci. Mater. Med. 2004, 15, 1113–1121. [Google Scholar] [CrossRef]
- Oka, M.; Ushio, K.; Kumar, P.; Ikeuchi, K.; Hyon, S.H.; Nakamura, T.; Fujita, H. Development of artificial articular cartilage. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2000, 214, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Cestari, F.; Yang, Y.; Wilbig, J.; Günster, J.; Motta, A.; Sglavo, V.M. Powder 3D Printing of Bone Scaffolds with Uniform and Gradient Pore Sizes Using Cuttlebone-Derived Calcium Phosphate and Glass-Ceramic. Materials 2022, 15, 5139. [Google Scholar] [CrossRef] [PubMed]
- Jo, B.W.; Song, C.S. Thermoplastics and Photopolymer Desktop 3D Printing System Selection Criteria Based on Technical Specifications and Performances for Instructional Applications. Technologies 2021, 9, 91. [Google Scholar] [CrossRef]
- Rasheed, S.; Lughmani, W.A.; Obeidi, M.A.; Brabazon, D.; Ahad, I.U. Additive Manufacturing of Bone Scaffolds Using PolyJet and Stereolithography Techniques. Appl. Sci. 2021, 11, 7336. [Google Scholar] [CrossRef]
- Formlabs, Guide to Stereolithography (SLA) 3D Printing. Available online: https://formlabs.com/blog/ultimate-guide-to-stereolithography-sla-3d-printing/ (accessed on 16 November 2022).
- Hofmann, M.C.; Whited, B.M.; Mitchell, J.; Vogt, W.C.; Criswell, T.; Rylander, C.; Rylander, M.N.; Soker, S.; Wang, G.; Xu, Y. Scanning-fiber-based imaging method for tissue engineering. J. Biomed. Opt. 2012, 7, 066010. [Google Scholar] [CrossRef]
- Lin, Y.M.; Boccaccini, A.R.; Polak, J.M.; Bishop, A.E.; Maquet, V. Biocompatibility of poly-DL-lactic acid (PDLLA) for lung tissue engineering. J. Biomater. Appl. 2006, 21, 109–118. [Google Scholar] [CrossRef]
- Mack, B.C.; Wright, K.W.; Davis, M.E. A biodegradable filament for controlled drug delivery. J. Control. Release 2009, 139, 205–211. [Google Scholar] [CrossRef]
- Lin, F.; Wang, X.; Wang, Y.; Yang, Y.; Li, Y. Preparation and biocompatibility of electrospinning PDLLA/β-TCP/collagen for peripheral nerve regeneration. RSC Adv. 2017, 7, 41593–41602. [Google Scholar] [CrossRef] [Green Version]
- Cai, Z.; Wan, Y.; Becker, L.M.; Long, Y.; Dean, D. Poly(propylene fumarate)-based materials: Synthesis, functionalization, properties, device fabrication and biomedical applications. Biomaterials 2019, 208, 45–71. [Google Scholar] [CrossRef]
- Kondiah, P.J.; Kondiah, P.P.D.; Choonara, Y.E.; Marimuthu, T.; Pillay, V. A 3D Bioprinted Pseudo-Bone Drug Delivery Scaffold for Bone Tissue Engineering. Pharmaceutics 2020, 12, 166. [Google Scholar] [CrossRef] [Green Version]
- Mamaghani, K.R.; Naghib, S.M.; Zahedi, A.; Rahmanian, M.; Mozafari, M. GelMa/PEGDA containing graphene oxide as an IPN hydrogel with superior mechanical performance. Mater. Today Proc. 2018, 5, 15790–15799. [Google Scholar] [CrossRef]
- Martinez-Garcia, F.D.; van Dongen, J.A.; Burgess, J.K.; Harmsen, M.C. Matrix Metalloproteases from Adipose Tissue-Derived Stromal Cells Are Spatiotemporally Regulated by Hydrogel Mechanics in a 3D Microenvironment. Bioengineering 2022, 9, 340. [Google Scholar] [CrossRef]
- Jiang, F.; Zhou, M.; Drummer, D. Effects of Fumed Silica on Thixotropic Behavior and Processing Window by UV-Assisted Direct Ink Writing. Polymers 2022, 14, 3107. [Google Scholar] [CrossRef]
- Washington State University, Manufacturing Processes and Machinery Lab, Fundamentals of Direct-Ink-Writing. Available online: https://labs.wsu.edu/mpml/projects/ (accessed on 16 November 2022).
- Bunea, A.-I.; del Castillo Iniesta, N.; Droumpali, A.; Wetzel, A.E.; Engay, E.; Taboryski, R. Micro 3D Printing by Two-Photon Polymerization: Configurations and Parameters for the Nanoscribe System. Micro 2021, 1, 164–180. [Google Scholar] [CrossRef]
- Tamo, A.K.; Tran, T.A.; Doench, I.; Jahangir, S.; Lall, A.; David, L.; Peniche-Covas, C.; Walther, A.; Osorio-Madrazo, A. 3D Printing of Cellulase-Laden Cellulose Nanofiber/Chitosan Hydrogel Composites: Towards Tissue Engineering Functional Biomaterials with Enzyme-Mediated Biodegradation. Materials 2022, 15, 6039. [Google Scholar] [CrossRef]
- Rioux, Y.; Fradette, J.; Maciel, Y.; Bégin-Drolet, A.; Ruel, J. Biofabrication of Sodium Alginate Hydrogel Scaffolds for Heart Valve Tissue Engineering. Int. J. Mol. Sci. 2022, 23, 8567. [Google Scholar] [CrossRef]
- Ramezani, H.; Mohammad Mirjamali, S.; He, Y. Simulations of Extrusion 3D Printing of Chitosan Hydrogels. Appl. Sci. 2022, 12, 7530. [Google Scholar] [CrossRef]
- Teixeira, M.C.; Lameirinhas, N.S.; Carvalho, J.P.F.; Valente, B.F.A.; Luís, J.; Pires, L.; Oliveira, H.; Oliveira, M.; Silvestre, A.J.D.; Vilela, C.; et al. Alginate-Lysozyme Nanofibers Hydrogels with Improved Rheological Behavior, Printability and Biological Properties for 3D Bioprinting Applications. Nanomaterials 2022, 12, 2190. [Google Scholar] [CrossRef]
- Duan, B. State-of-the-Art Review of 3D Bioprinting for Cardiovascular Tissue Engineering. Ann. Biomed. Eng. 2017, 45, 195–209. [Google Scholar] [CrossRef]
- Sauty, B.; Santesarti, G.; Fleischhammer, T.; Lindner, P.; Lavrentieva, A.; Pepelanova, I.; Marino, M. Enabling Technologies for Obtaining Desired Stiffness Gradients in GelMA Hydrogels Constructs. Macromol. Chem. Phys. 2021, 223, 2100326. [Google Scholar] [CrossRef]
- Piao, Y.; You, H.; Xu, T.; Bei, H.; Piwko, I.Z.; Kwan, Y.Y.; Zhao, X. Biomedical applications of gelatin methacryloyl hydrogels. Eng. Regen. 2021, 2, 47–56. [Google Scholar] [CrossRef]
- Mohd, N.; Razali, M.; Ghazali, M.J.; Abu Kasim, N.H. Current Advances of Three-Dimensional Bioprinting Application in Dentistry: A Scoping Review. Materials 2022, 15, 6398. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Heikal, L.; Ferns, G.; Ghezzi, P.; Nokhodchi, A.; Maniruzzaman, M. 3D Bioprinting of Novel Biocompatible Scaffolds for Endothelial Cell Repair. Polymers 2019, 11, 1924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, X.; Li, G.; Liu, T.; Gu, M. Experimental Study of the Jetting Behavior of High-Viscosity Nanosilver Inks in Inkjet-Based 3D Printing. Nanomaterials 2022, 12, 3076. [Google Scholar] [CrossRef]
- 3D Natives. Is 3D Bioprinting the Future of Tailor-Made Medicine? Available online: https://www.3dnatives.com/en/future-3d-bioprinting1805201741/#! (accessed on 16 November 2022).
- Tofan, T.; Borodinas, S.; Kačianauskas, R.; Jasevičius, R. Modeling 3D Droplet Movement Using a Drop-on-Demand Inkjet Printhead Model. Processes 2022, 10, 1467. [Google Scholar] [CrossRef]
- Han, X.; Sharma, N.; Xu, Z.; Scheideler, L.; Geis-Gerstorfer, J.; Rupp, F.; Thieringer, F.M.; Spintzyk, S. An In Vitro Study of Osteoblast Response on Fused-Filament Fabrication 3D Printed PEEK for Dental and Cranio-Maxillofacial Implants. J. Clin. Med. 2019, 8, 771. [Google Scholar] [CrossRef] [Green Version]
- Told, R.; Ujfalusi, Z.; Pentek, A.; Kerenyi, M.; Banfai, K.; Vizi, A.; Szabo, P.; Melegh, S.; Bovari-Biri, J.; Pongracz, E.J.; et al. A state-of-the-art guide to the sterilization of thermoplastic polymers and resin materials used in the additive manufacturing of medical devices. Mater. Des. 2022, 223, 111119. [Google Scholar] [CrossRef]
- Rynio, P.; Galant, K.; Wójcik, Ł.; Grygorcewicz, B.; Kazimierczak, A.; Falkowski, A.; Gutowski, P.; Dołęgowska, B.; Kawa, M. Effects of Sterilization Methods on Different 3D Printable Materials for Templates of Physician-Modified Aortic Stent Grafts Used in Vascular Surgery—A Preliminary Study. Int. J. Mol. Sci. 2022, 23, 3539. [Google Scholar] [CrossRef]


| FDM Process | SLA Process | Direct Ink Writing & Laser-Guided Direct Writing Processes |
|---|---|---|
| Polycaprolactone (PCL) Polylactic acid (PLA) Polyether ether ketone (PEEK) Poly-vinyl alcohol (PVA) | Poly(D,L-lactide) (PDLLA) Polypropylene fumarate (PPF) PEGDA & GelMA inks | Hydrogel inks Gelatin-methacryloyl (GelMA) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kantaros, A. 3D Printing in Regenerative Medicine: Technologies and Resources Utilized. Int. J. Mol. Sci. 2022, 23, 14621. https://doi.org/10.3390/ijms232314621
Kantaros A. 3D Printing in Regenerative Medicine: Technologies and Resources Utilized. International Journal of Molecular Sciences. 2022; 23(23):14621. https://doi.org/10.3390/ijms232314621
Chicago/Turabian StyleKantaros, Antreas. 2022. "3D Printing in Regenerative Medicine: Technologies and Resources Utilized" International Journal of Molecular Sciences 23, no. 23: 14621. https://doi.org/10.3390/ijms232314621






