Overexpression of TcNTPDase-1 Gene Increases Infectivity in Mice Infected with Trypanosoma cruzi
Abstract
1. Introduction
2. Results
2.1. Experimental Design and Parasitological Evaluation
2.2. Assessment of Body Weight and Mortality
2.3. Histopathological Analysis of Infected Animals
2.4. Evaluation of Electrocardiographic Changes Promoted by T. cruzi Infection
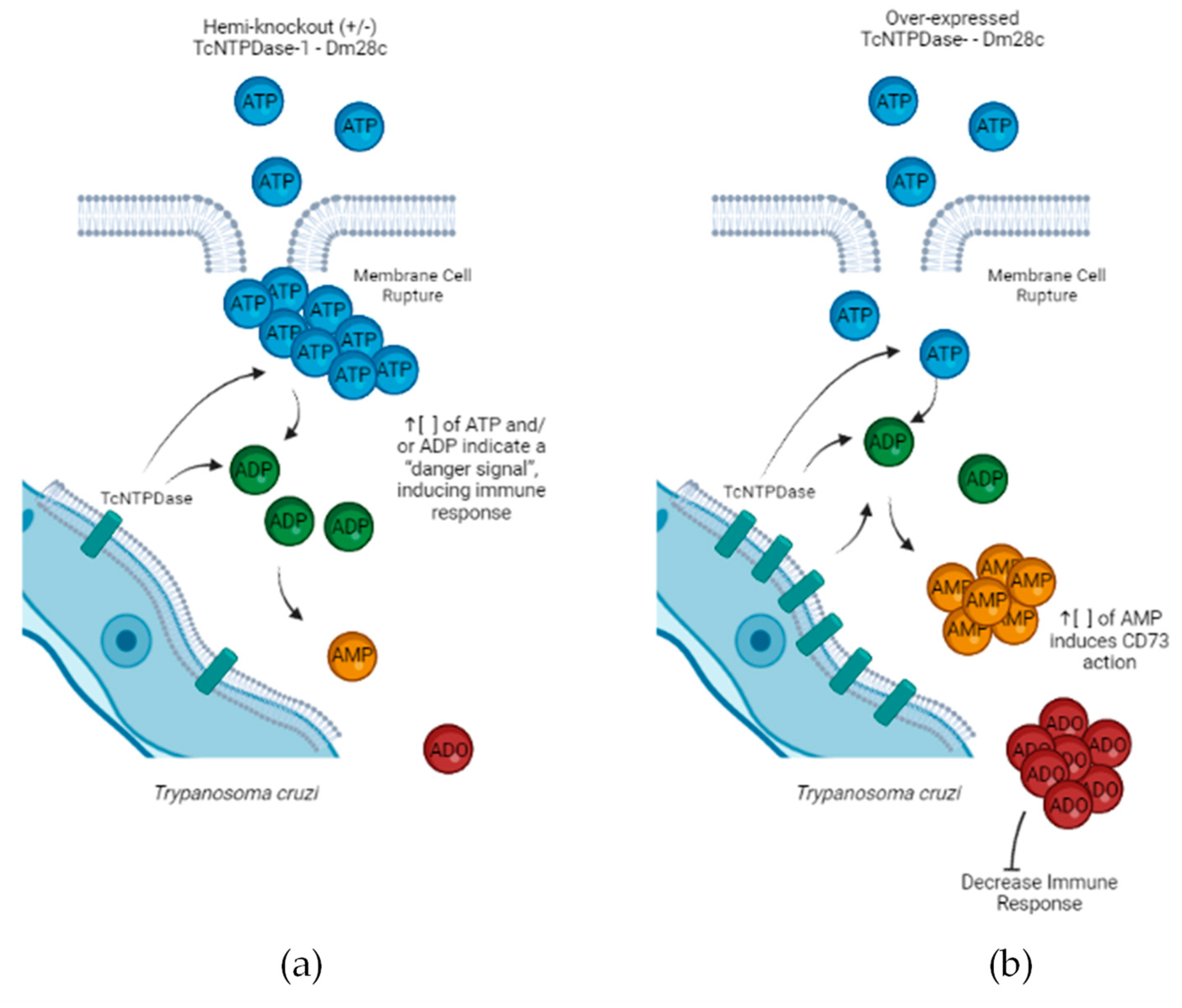
3. Discussion
4. Materials and Methods
4.1. Knockout and Overexpression of the TcNTPDase-1 Gene
4.2. In Vitro Metacyclogenesis and VERO Cells Infection
4.3. In Vivo Infection
4.3.1. Mouse Models of Acute Infection by T. cruzi
4.3.2. Parasitemia and Mortality Rates
4.3.3. Electrocardiogram (ECG) Registers
4.3.4. Histopathology and H&E
4.4. Ethics
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rassi, A.; de Rezende, J.M. American Trypanosomiasis (Chagas Disease). Infect. Dis. Clin. N. Am. 2012, 26, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Coura, J.R. Chagas disease: What is known and what is needed—A background article. Mem. Inst. Oswaldo Cruz 2007, 102, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.C.P. Globalização, iniqüidade e doença de Chagas Globalization, inequity and Chagas disease. Cad. Saude Publica 2007, 23, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Alsford, S.; Kelly, J.M.; Baker, N.; Horn, D. Genetic dissection of drug resistance in trypanosomes. Parasitology 2013, 140, 1478–1491. [Google Scholar] [CrossRef] [PubMed]
- Bern, C. Clinical therapeutics Antitrypanosomal Therapy for Chronic Chagas’ Disease. N. Engl. J. Med. 2011, 364, 2527–2534. [Google Scholar] [CrossRef]
- Morillo, C.A.; Marin-Neto, J.A.; Avezum, A.; Sosa-Estani, S.; Rassi, A., Jr.; Rosas, F.; Villena, E.; Quiroz, R.; Bonilla, R.; Britto, C.; et al. Randomized Trial of Benznidazole for Chronic Chagas’ Cardiomyopathy. N. Engl. J. Med. 2015, 373, 1295–1306. [Google Scholar] [CrossRef]
- Urbina, J.A.; Docampo, R. Specific chemotherapy of Chagas disease: Controversies and advances. Trends Parasitol. 2003, 19, 495–501. [Google Scholar] [CrossRef]
- Zimmermann, H. Extracellular metabolism of ATP and other nucleotides. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2000, 362, 299–309. [Google Scholar] [CrossRef]
- Zimmermann, H.; Braun, N.; Kegel, B.; Heine, P. New insights into molecular structure and function of ecto-nucleotidases in the nervous system. Neurochem. Int. 1998, 32, 421–425. [Google Scholar] [CrossRef]
- Zimmermann, H.; Braun, N. Extracellular metabolism of nucleotides in the nervous system. J. Auton. Pharmacol. 1996, 16, 397–400. [Google Scholar] [CrossRef]
- Zimmermann, H. Ectonucleotidases: Some recent developments and a note on nomenclature. Drug Dev. Res. 2001, 52, 44–56. [Google Scholar] [CrossRef]
- Di Virgilio, F. Purines, purinergic receptors, and cancer. Cancer Res. 2012, 72, 5441–5447. [Google Scholar] [CrossRef] [PubMed]
- Handa, M.; Guidotti, G. Purification and Cloning of a Soluble ATP-Diphosphohydrolase (Apyrase) from Potato Tubers (Solanum tuberosum). Biochem. Biophys. Res. Commun. 1996, 218, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, E.G.; Ferreira, S.T.; de Carvalho, T.M.; De Souza, W.; Kettlun, A.M.; Mancilla, M.; Valenzuela, M.A.; Verjovski-Almeida, S. Partial purification and immunohistochemical localization of ATP diphosphohydrolase from Schistosoma mansoni: Immunological cross-reactivities with potato apyrase and Toxoplasma gondii nucleoside triphosphate hydrolase. J. Biol. Chem. 1996, 271, 22139–22145. [Google Scholar] [CrossRef]
- Sansom, F.; Robson, S.C.; Hartland, E.L. Possible Effects of Microbial Ecto-Nucleoside Triphosphate Diphosphohydrolases on Host-Pathogen Interactions. Microbiol. Mol. Biol. Rev. 2008, 72, 765–781. [Google Scholar] [CrossRef]
- Zebisch, M.; Strä, N. Structural Insight into Signal Conversion and Inactivation by NTPDase2 in Purinergic Signaling. Proc. Natl. Acad. Sci. USA 2008, 105, 6882–6887. [Google Scholar] [CrossRef]
- Vivian, J.; Riedmaier, P.; Ge, H.; Le Nours, J.; Sansom, F.; Wilce, M.C.; Byres, E.; Dias, M.; Schmidberger, J.W.; Cowan, P.; et al. Crystal Structure of a Legionella pneumophila Ecto-Triphosphate Diphosphohydrolase, A Structural and Functional Homolog of the Eukaryotic NTPDases. Structure 2010, 18, 228–238. [Google Scholar] [CrossRef]
- Kukulski, F.; Lévesque, S.A.; Lavoie, G.; Lecka, J.; Bigonnesse, F.; Knowles, A.F.; Robson, S.C.; Kirley, T.L.; Sévigny, J. Comparative hydrolysis of P2 receptor agonists by NTPDases 1, 2, 3 and 8. Purinergic Signal 2005, 1, 193–204. [Google Scholar] [CrossRef]
- Robson, S.C.; Sévigny, J.; Zimmermann, H. The E-NTPDase family of ectonucleotidases: Structure function relationships and pathophysiological significance. Purinergic Signal. 2006, 2, 409–430. [Google Scholar] [CrossRef]
- Atkinson, B.; Dwyer, K.; Enjyoji, K.; Robson, S.C. Ecto-nucleotidases of the CD39/NTPDase family modulate platelet activation and thrombus formation: Potential as therapeutic targets. Blood Cells Mol. Dis. 2006, 36, 217–222. [Google Scholar] [CrossRef]
- Bours, M.; Swennen, E.; Di Virgilio, F.; Cronstein, B.; Dagnelie, P. Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol. Ther. 2006, 112, 358–404. [Google Scholar] [CrossRef]
- La Sala, A.; Ferrari, D.; Di Virgilio, F.; Idzko, M.; Norgauer, J.; Girolomoni, G. Alerting and tuning the immune response by extracellular nucleotides. J. Leukoc. Biol. 2003, 73, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Deaglio, S.; Dwyer, K.M.; Gao, W.; Friedman, D.; Usheva, A.; Erat, A.; Chen, J.-F.; Enjyoji, K.; Linden, J.; Oukka, M.; et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med. 2007, 204, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Plesner, L. Ecto-ATPases: Identities and Functions. Int. Rev. Cytol. 1995, 158, 141–214. [Google Scholar] [CrossRef]
- Fietto, J.L.; DeMarco, R.; Nascimento, I.P.; Castro, I.M.; Carvalho, T.M.; de Souza, W.; Bahia, M.T.; Alves, M.J.; Verjovski-Almeida, S. Characterization and immunolocalization of an NTP diphosphohydrolase of Trypanosoma cruzi. Biochem. Biophys. Res. Commun. 2004, 316, 454–460. [Google Scholar] [CrossRef]
- Paes-Vieira, L.; Gomes-Vieira, A.L.; Meyer-Fernandes, J.R. NTPDase activities: Possible roles on Leishmania spp. infectivity and virulence. Cell Biol. Int. 2018, 42, 670–682. [Google Scholar] [CrossRef]
- De Souza, M.C.; de Assis, E.A.; Gomes, R.S.; da Silva, E.D.A.M.; Melo, M.N.; Fietto, J.L.R.; Afonso, L.C.C. The influence of ecto-nucleotidases on Leishmania amazonensis infection and immune response in C57B/6 mice. Acta Trop. 2010, 115, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Basombrío, M.; Segura, M.A.; Gómez, L.; Padilla, M. Studies on the virulence and attenuation of Trypanosoma cruzi using immunodeficient animals. Mem. Do Inst. Oswaldo Cruz 2000, 95, 175–178. [Google Scholar] [CrossRef][Green Version]
- Contreras, V.T.; De Lima, A.R.; Zorrilla, G. Trypanosoma cruzi: Maintenance in Culture Modify Gene and Antigenic Expression of Metacyclic Trypomastigotes. Mem. Inst. Oswaldo Cruz 1998, 93, 753–760. [Google Scholar] [CrossRef]
- Meyer-Fernandes, J.R.; Saad-Nehme, J.; Peres-Sampaio, C.E.; Belmont-Firpo, R.; Bisaggio, D.F.R.; Couto, L.C.D.; De Souza, A.L.F.; Lopes, A.H.S.C.; Souto-Padrón, T. A Mg-dependent ecto-ATPase is increased in the infective stages of Trypanosoma cruzi. Parasitol. Res. 2004, 93, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Silva-Gomes, N.L.; Ennes-Vidal, V.; Carolo, J.C.F.; Batista, M.M.; Soeiro, M.N.; Menna-Barreto, R.; Moreira, O.C. Nucleoside triphosphate diphosphohydrolase1 (TcNTPDase-1) gene expression is increased due to heat shock and in infective forms of Trypanosoma cruzi. Parasit. Vectors 2014, 7, 463. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cohn, C.; Gottlieb, M. The acquisition of purines by trypanosomatids. Parasitol. Today 1997, 13, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Paes-Vieira, L.; Gomes-Vieira, A.L.; Meyer-Fernandes, J.R. E-NTPDases: Possible Roles on Host-Parasite Interactions and Therapeutic Opportunities. Front. Cell. Infect. Microbiol. 2021, 11, 1119. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Fernandes, J.R.; Cosentino-Gomes, D.; Vieira, D.P.; Lopes, A.H. Ecto-nucleoside triphosphate diphosphohydrolase activities in trypanosomatids: Possible roles in infection, virulence and purine recycling. Open Parasitol. J. 2010, 4, 116–119. [Google Scholar] [CrossRef][Green Version]
- Fonseca, F.V.; de Souza, A.L.F.; Mariano, A.C.; Entringer, P.F.; Gondim, K.C.; Meyer-Fernandes, J.R. Trypanosoma rangeli: Characterization of a Mg-dependent ecto ATP-diphosphohydrolase activity. Exp. Parasitol. 2006, 112, 76–84. [Google Scholar] [CrossRef]
- Ennes-Vidal, V.; Castro RO, S.; Britto, C.; Barrabin, H.; D’Avila-Levy, C.M.; Moreira, O.C. CrATP interferes in the promastigote-macrophage interaction in Leishmania amazonensis infection. Parasitology 2011, 138, 960–968. [Google Scholar] [CrossRef]
- Santos, R.F.; Pôssa, M.A.S.; Bastos, M.S.; Guedes, P.M.M.; Almeida, M.R.; DeMarco, R.; Verjovski-Almeida, S.; Bahia, M.T.; Fietto, J.L.R. Influence of Ecto-Nucleoside Triphosphate Diphosphohydrolase Activity on Trypanosoma cruzi Infectivity and Virulence. PLoS Negl. Trop. Dis. 2009, 3, e387. [Google Scholar] [CrossRef]
- Silva-Gomes, N.L.; Rampazzo, R.; Moreira, C.M.D.N.; Porcino, G.N.; Dos Santos, C.M.B.; Krieger, M.A.; Vasconcelos, E.G.; Fragoso, S.P.; Moreira, O.C. Knocking Down TcNTPDase-1 Gene Reduces in vitro Infectivity of Trypanosoma cruzi. Front. Microbiol. 2020, 11, 434. [Google Scholar] [CrossRef]
- Leite, A.L.J.; de Oliveira, D.S.; Mota, L.W.R.; Carvalho, L.C.F.; Zimmermann, F.F.; de Paiva, N.C.N.; Vieira, P.M.D.A.; de Lana, M.; Afonso, L.C.C.; Talvani, A. Ectonucleotidases from trypomastigotes from different sources and various genetic backgrounds of Trypanosoma cruzi potentiate their infectivity and host inflammation. Cytokine 2020, 136, 155255. [Google Scholar] [CrossRef]
- Sansom, F.M. The role of the NTPDase enzyme family in parasites: What do we know, and where to from here? Parasitology 2012, 139, 963–980. [Google Scholar] [CrossRef]
- Silvério, J.C.; Pereira, I.R.; Cipitelli, M.D.C.; Vinagre, N.F.; Rodrigues, M.M.; Gazzinelli, R.T.; Lannes-Vieira, J. CD8+ T-Cells Expressing Interferon Gamma or Perforin Play Antagonistic Roles in Heart Injury in Experimental Trypanosoma cruzi-Elicited Cardiomyopathy. PLoS Pathog. 2012, 8, e1002645. [Google Scholar] [CrossRef]
- Pereira, I.R.; Vilar-Pereira, G.; Da Silva, A.A.; Lannes-Vieira, J. Severity of chronic experimental Chagas’ heart disease parallels tumour necrosis factor and nitric oxide levels in the serum: Models of mild and severe disease. Mem. Inst. Oswaldo Cruz 2014, 109, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Neto, J.R.D.C.; da Costa, A.W.F.; Braga, Y.L.L.; Lucio, F.H.; Martins, A.L.M.D.S.; dos Reis, M.A.; de Oliveira, F.A.; Celes, M.R.N.; da Silva, M.V.; Oliveira, M.A.P.; et al. The Colombian Strain of Trypanosoma cruzi Induces a Proinflammatory Profile, Neuronal Death, and Collagen Deposition in the Intestine of C57BL/6 Mice Both during the Acute and Early Chronic Phase. Mediat. Inflamm. 2022, 2022, 7641357. [Google Scholar] [CrossRef]
- Lewis, M.D.; Francisco, A.F.; Taylor, M.C.; Kelly, J.M. A New Experimental Model for Assessing Drug Efficacy against Trypanosoma cruzi Infection Based on Highly Sensitive In Vivo Imaging. SLAS Discov. Adv. Sci. Drug Discov. 2015, 20, 36–43. [Google Scholar] [CrossRef]
- Hennig, M.; Ewering, L.; Pyschny, S.; Shimoyama, S.; Olecka, M.; Ewald, D.; Magarin, M.; Uebing, A.; Thierfelder, L.; Jux, C.; et al. Dietary protein restriction throughout intrauterine and postnatal life results in potentially beneficial myocardial tissue remodeling in the adult mouse heart. Sci. Rep. 2019, 9, 15126. [Google Scholar] [CrossRef] [PubMed]
- Chagas, C. Nova tripanozomiaze humana: Estudos sobre a morfolojia e o ciclo evolutivo do Schizotrypanum cruzi n. gen., n. sp., ajente etiolojico de nova entidade morbida do homem. Mem. Inst. Oswaldo Cruz. 1909, 1, 159–218. [Google Scholar] [CrossRef]
- Pérez-Molina, J.A.; Molina, I. Chagas disease. Lancet 2018, 391, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Savio, L.E.B.; Coutinho-Silva, R. Immunomodulatory effects of P2X7 receptor in intracellular parasite infections. Curr. Opin. Pharmacol. 2019, 47, 53–58. [Google Scholar] [CrossRef]
- Nakaar, V.; Beckers, C.; Polotsky, V.; Joiner, K. Basis for substrate specificity of the Toxoplasma gondii nucleoside triphosphate hydrolase. Mol. Biochem. Parasitol. 1998, 97, 209–220. [Google Scholar] [CrossRef]
- Jesus, J.B.; Lopes, A.H.; Meyer-Fernandes, J.R. Characterization of an ecto-ATPase of Tritrichomonas foetus. Vet. Parasitol. 2002, 103, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Berrêdo-Pinho, M.; Peres-Sampaio, C.; Chrispim, P.; Belmont-Firpo, R.; Lemos, A.; Martiny, A.; Vannier-Santos, M.; Meyer-Fernandes, J. A Mg-Dependent Ecto-ATPase in Leishmania amazonensis and Its Possible Role in Adenosine Acquisition and Virulence. Arch. Biochem. Biophys. 2001, 391, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Zingales, B.; Andrade, S.G.; Briones, M.R.S.; A Campbell, D.; Chiari, E.; Fernandes, O.; Guhl, F.; Lages-Silva, E.; Macedo, A.M.; Machado, C.R.; et al. A new consensus for Trypanosoma cruzi intraspecific nomenclature: Second revision meeting recommends TcI to TcVI. Mem. Do Inst. Oswaldo Cruz 2009, 104, 1051–1054. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, L.M.D.; Gollob, K.J.; Zingales, B.; Dutra, W.O. Pathogen diversity, immunity, and the fate of infections: Lessons learned from Trypanosoma cruzi human–host interactions. Lancet Microbe 2022, 3, e711–e722. [Google Scholar] [CrossRef]
- Duz, A.L.C.; Vieira, P.M.D.A.; Roatt, B.; Aguiar-Soares, R.D.O.; Cardoso, J.M.D.O.; De Oliveira, F.C.B.; Reis, L.E.S.; Tafuri, W.L.; Veloso, V.M.; Reis, A.B.; et al. The TcI and TcII Trypanosoma cruzi experimental infections induce distinct immune responses and cardiac fibrosis in dogs. Mem. Inst. Oswaldo Cruz 2014, 109, 1005–1013. [Google Scholar] [CrossRef]
- Da Silva, D.R.; de Castro, S.L.; Alves, M.C.d.S.; Batista, W.d.S.; de Oliveira, G.M. Acute experimental Trypanosoma cruzi infection: Establishing a murine model that utilises non-invasive measurements of disease parameters. Mem. Inst. Oswaldo Cruz 2012, 107, 211–216. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marinho, C.R.F.; Regina D’impé, M.; Lima, R.; Grisotto, M.G.; Alvarez, J.M. Influence of Acute-Phase Parasite Load on Pathology, Parasitism, and Activation of the Immune System at the Late Chronic Phase of Chagas’ Disease. Infect. Immun. 1999, 67, 308–318. [Google Scholar] [CrossRef]
- Vazquez, B.P.; Vazquez, T.P.; Miguel, C.B.; Rodrigues, W.F.; Mendes, M.T.; De Oliveira, C.J.F.; Chica, J.E.L. Inflammatory responses and intestinal injury development during acute Trypanosoma cruzi infection are associated with the parasite load. Parasites Vectors 2015, 8, 206. [Google Scholar] [CrossRef] [PubMed]
- Chatelain, E.; Konar, N. Translational challenges of animal models in Chagas disease drug development: A review. Drug Des. Dev. Ther. 2015, 9, 4807–4823. [Google Scholar] [CrossRef]
- Santi-Rocca, J.; Fernandez-Cortes, F.; Chillón-Marinas, C.; González-Rubio, M.L.; Martin, D.; Gironès, N.; Fresno, M. A multi-parametric analysis of Trypanosoma cruzi infection: Common pathophysiologic patterns beyond extreme heterogeneity of host responses. Sci. Rep. 2017, 7, 8893. [Google Scholar] [CrossRef] [PubMed]
- Rosshart, S.P.; Vassallo, B.G.; Angeletti, D.; Hutchinson, D.S.; Morgan, A.P.; Takeda, K.; Hickman, H.D.; McCulloch, J.A.; Badger, J.H.; Ajami, N.J.; et al. Wild Mouse Gut Microbiota Promotes Host Fitness and Improves Disease Resistance. Cell 2017, 171, 1015–1028.e13. [Google Scholar] [CrossRef] [PubMed]
- Enriquez, J.; Mims, B.M.D.; Trasti, S.; Furr, K.L.; Grisham, M.B. Genomic, microbial and environmental standardization in animal experimentation limiting immunological discovery. BMC Immunol. 2020, 21, 50. [Google Scholar] [CrossRef]
- Casellas, J. Inbred mouse strains and genetic stability: A review. Animal 2011, 5, 1–7. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bonaldo, M.C.; Souto-Padron, T.; De Souza, W.; Goldenberg, S. Cell-substrate adhesion during Trypanosoma cruzi differentiation. J. Cell Biol. 1988, 106, 1349–1358. [Google Scholar] [CrossRef]
- Medina-Acosta, E.; Cross, G.A.M. Rapid isolation of DNA from trypanosomatid protozoa using a simple ‘mini-prep’ procedure. Mol. Biochem. Parasitol. 1993, 59, 327–329. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.Y.; A Buck, G. Expression of an exogenous gene in Trypanosoma cruzi epimastigotes. Mol. Biochem. Parasitol. 1991, 44, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Contreras, V.T.; Salles, J.M.; Thomas, N.; Morel, C.M.; Goldenberg, S. In vitro differentiation of Trypanosoma cruzi under chemically defined conditions. Mol. Biochem. Parasitol. 1985, 16, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Hasler, M.; de Oliveira, G.M.; da Gama, A.N.; Fiuza, L.F.D.A.; Fesser, A.F.; Cal, M.; Rocchetti, R.; Peres, R.B.; Guan, X.L.; Kaiser, M.; et al. Combination with Tomatidine Improves the Potency of Posaconazole Against Trypanosoma cruzi. Front. Cell. Infect. Microbiol. 2021, 11, 617917. [Google Scholar] [CrossRef]
- Dos Santos, P.V.; Roffê, E.; Santiago, H.C.; Torres, R.A.; Marino, A.P.M.; Paiva, C.N.; Silva, A.A.; Gazzinelli, R.T.; Lannes-Vieira, J. Prevalence of CD8+αβ T cells in Trypanosoma cruzi-elicited myocarditis is associated with acquisition of CD62LLowLFA-1HighVLA-4High activation phenotype and expression of IFN-γ-inducible adhesion and chemoattractant molecules. Microbes Infect. 2001, 3, 971–984. [Google Scholar] [CrossRef] [PubMed]



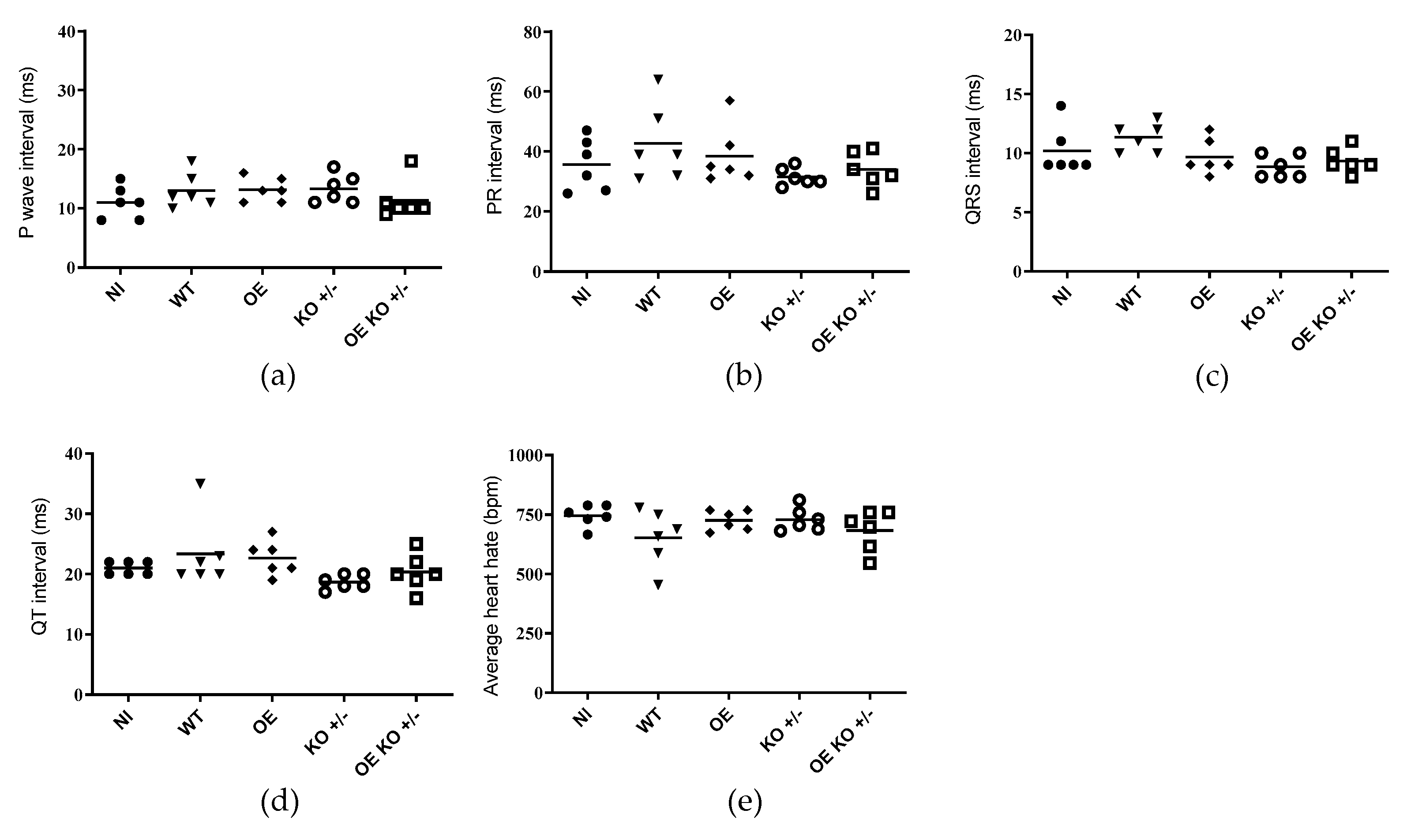
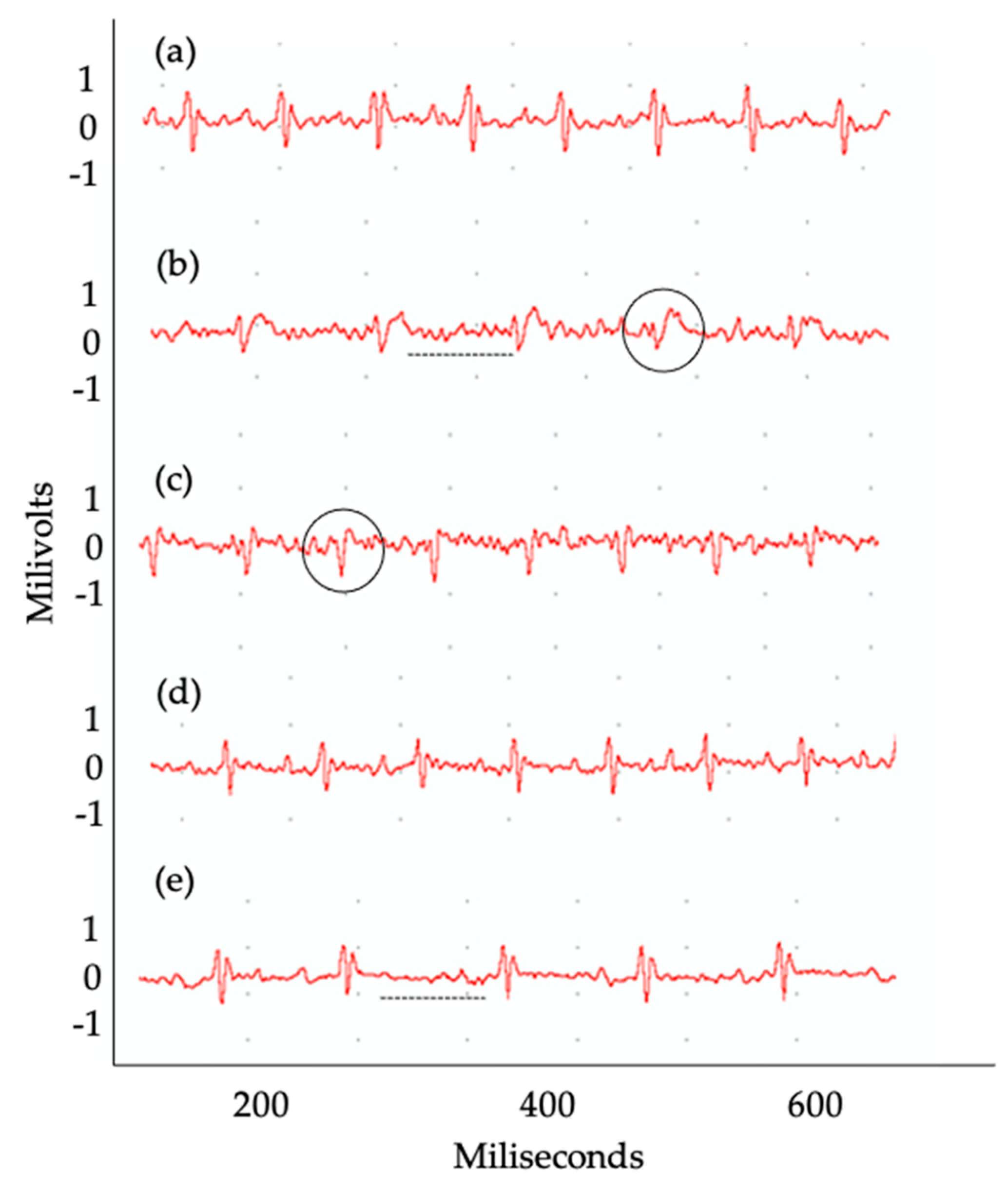
| Groups | Arrythmias | Animals Affected (%) |
|---|---|---|
| Non-infected (NI) | Absent | 0 |
| WT | AVB/SB | 33 |
| OE | AVB | 17 |
| KO +/− | Absent | 0 |
| OE KO +/− | SB | 17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva-Gomes, N.L.d.; Ruivo, L.A.d.S.; Moreira, C.; Meuser-Batista, M.; Silva, C.F.d.; Batista, D.d.G.J.; Fragoso, S.; Oliveira, G.M.d.; Soeiro, M.d.N.C.; Moreira, O.C. Overexpression of TcNTPDase-1 Gene Increases Infectivity in Mice Infected with Trypanosoma cruzi. Int. J. Mol. Sci. 2022, 23, 14661. https://doi.org/10.3390/ijms232314661
Silva-Gomes NLd, Ruivo LAdS, Moreira C, Meuser-Batista M, Silva CFd, Batista DdGJ, Fragoso S, Oliveira GMd, Soeiro MdNC, Moreira OC. Overexpression of TcNTPDase-1 Gene Increases Infectivity in Mice Infected with Trypanosoma cruzi. International Journal of Molecular Sciences. 2022; 23(23):14661. https://doi.org/10.3390/ijms232314661
Chicago/Turabian StyleSilva-Gomes, Natália Lins da, Leonardo Alexandre de Souza Ruivo, Claudia Moreira, Marcelo Meuser-Batista, Cristiane França da Silva, Denise da Gama Jaen Batista, Stênio Fragoso, Gabriel Melo de Oliveira, Maria de Nazaré Correia Soeiro, and Otacilio C. Moreira. 2022. "Overexpression of TcNTPDase-1 Gene Increases Infectivity in Mice Infected with Trypanosoma cruzi" International Journal of Molecular Sciences 23, no. 23: 14661. https://doi.org/10.3390/ijms232314661
APA StyleSilva-Gomes, N. L. d., Ruivo, L. A. d. S., Moreira, C., Meuser-Batista, M., Silva, C. F. d., Batista, D. d. G. J., Fragoso, S., Oliveira, G. M. d., Soeiro, M. d. N. C., & Moreira, O. C. (2022). Overexpression of TcNTPDase-1 Gene Increases Infectivity in Mice Infected with Trypanosoma cruzi. International Journal of Molecular Sciences, 23(23), 14661. https://doi.org/10.3390/ijms232314661





