Sensory Neurons, PIEZO Channels and PAC1 Receptors Regulate the Mechanosensitive Release of Soluble Ectonucleotidases in the Murine Urinary Bladder Lamina Propria
Abstract
1. Introduction
2. Results
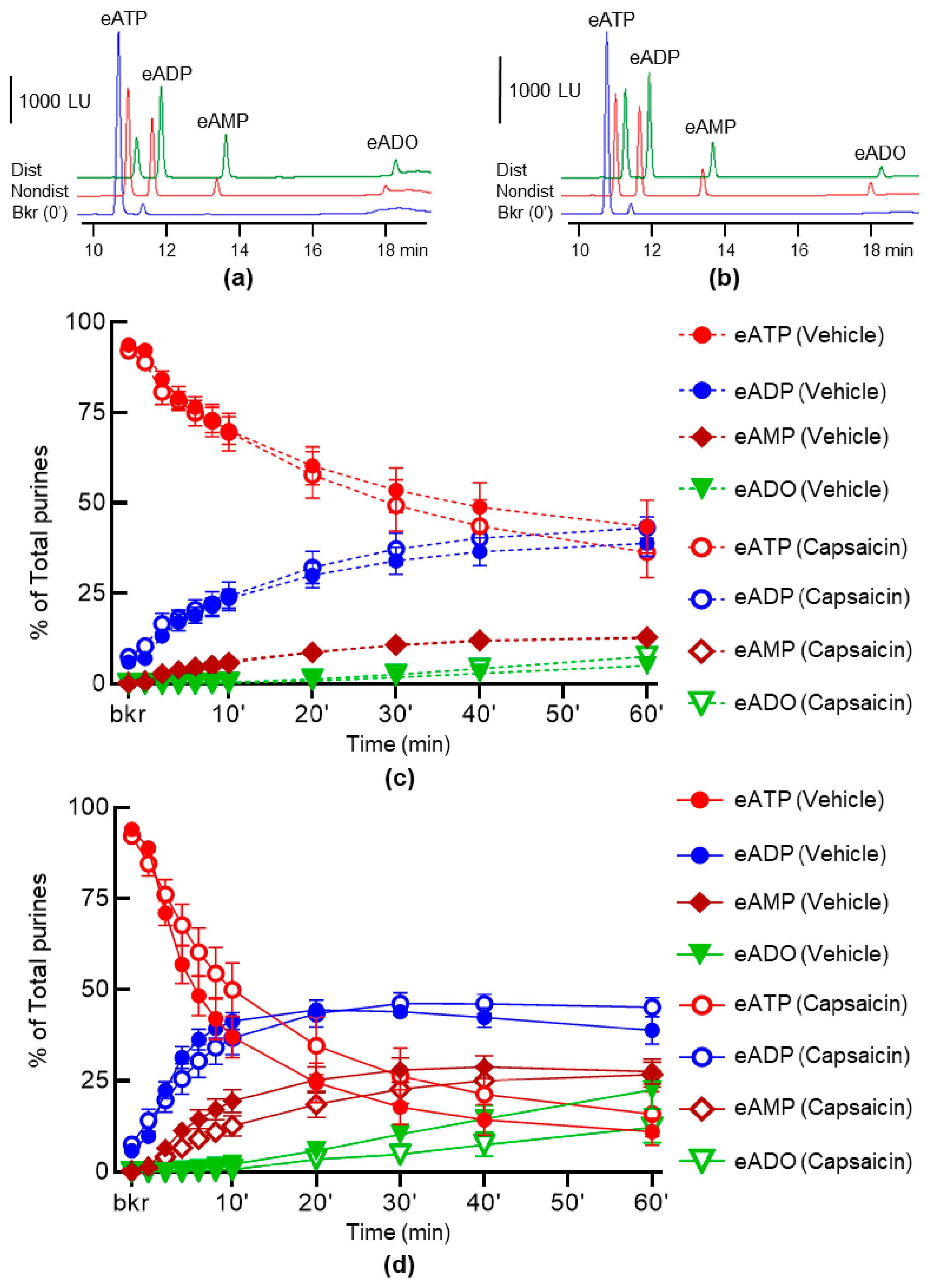
2.1. Capsaicin Does Not Alter the Spontaneous and the Distention-Induced Release of s-ENTDs in LP
2.2. Neural Blockade with Tetrodotoxin (TTX) Has No Effect on the Spontaneous Release of s-ENTDs but Increases the Distention-Induced Release of s-ENTDs in LP
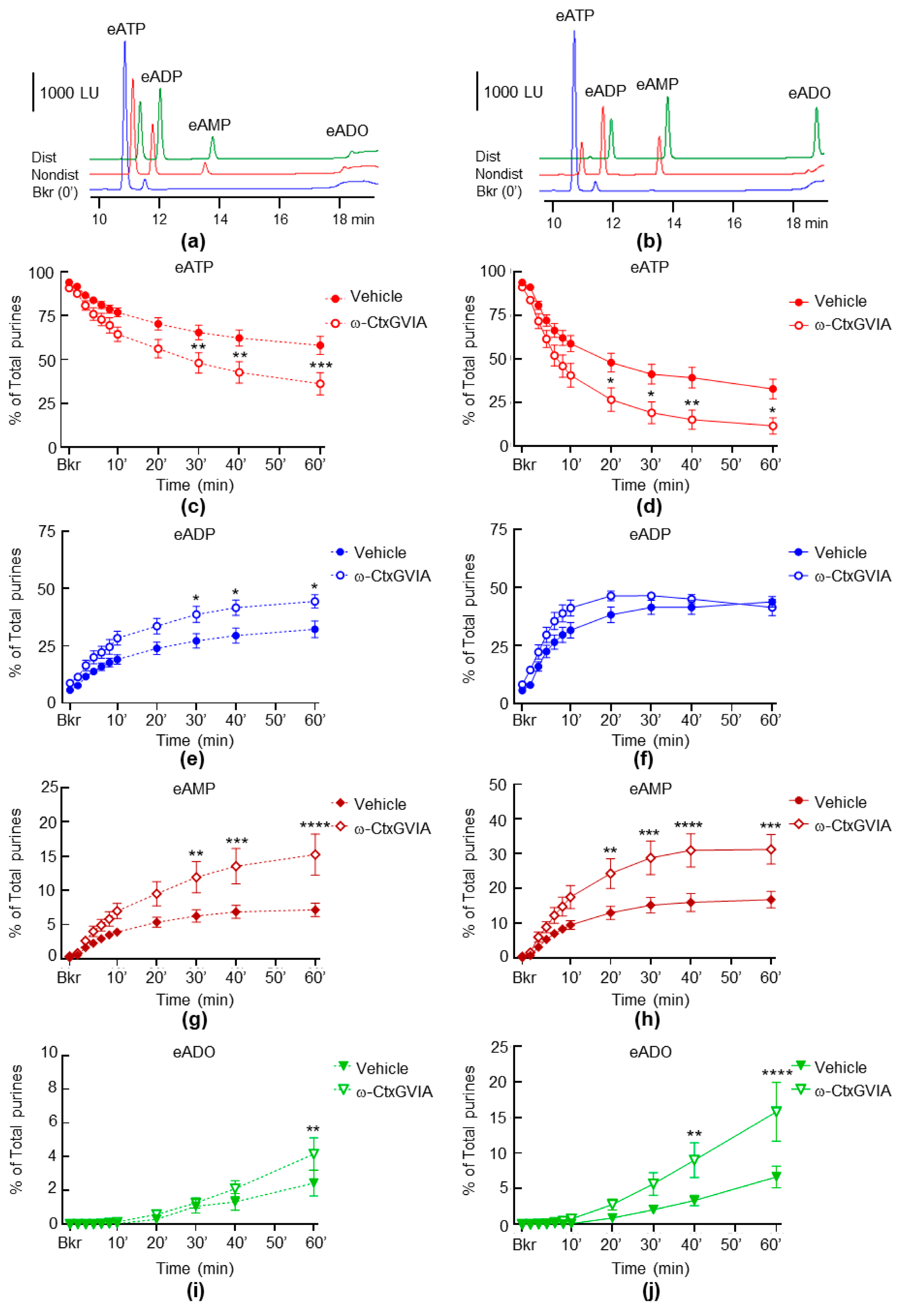
2.3. Neural Blockade with ω-Conotoxin (ω-CtxGVIA) Increases Both the Spontaneous and the Distention-Induced Release of sNTDs
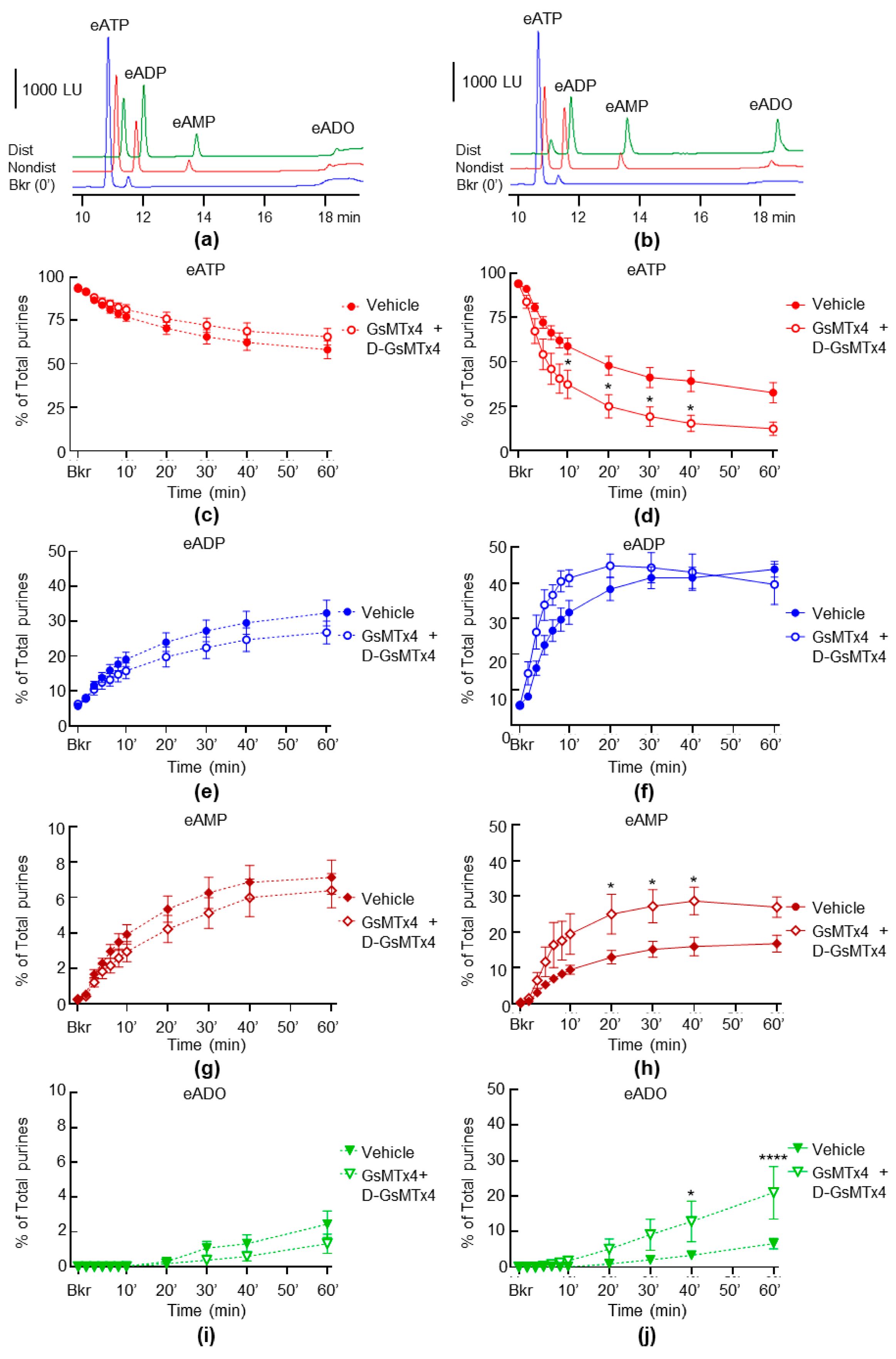
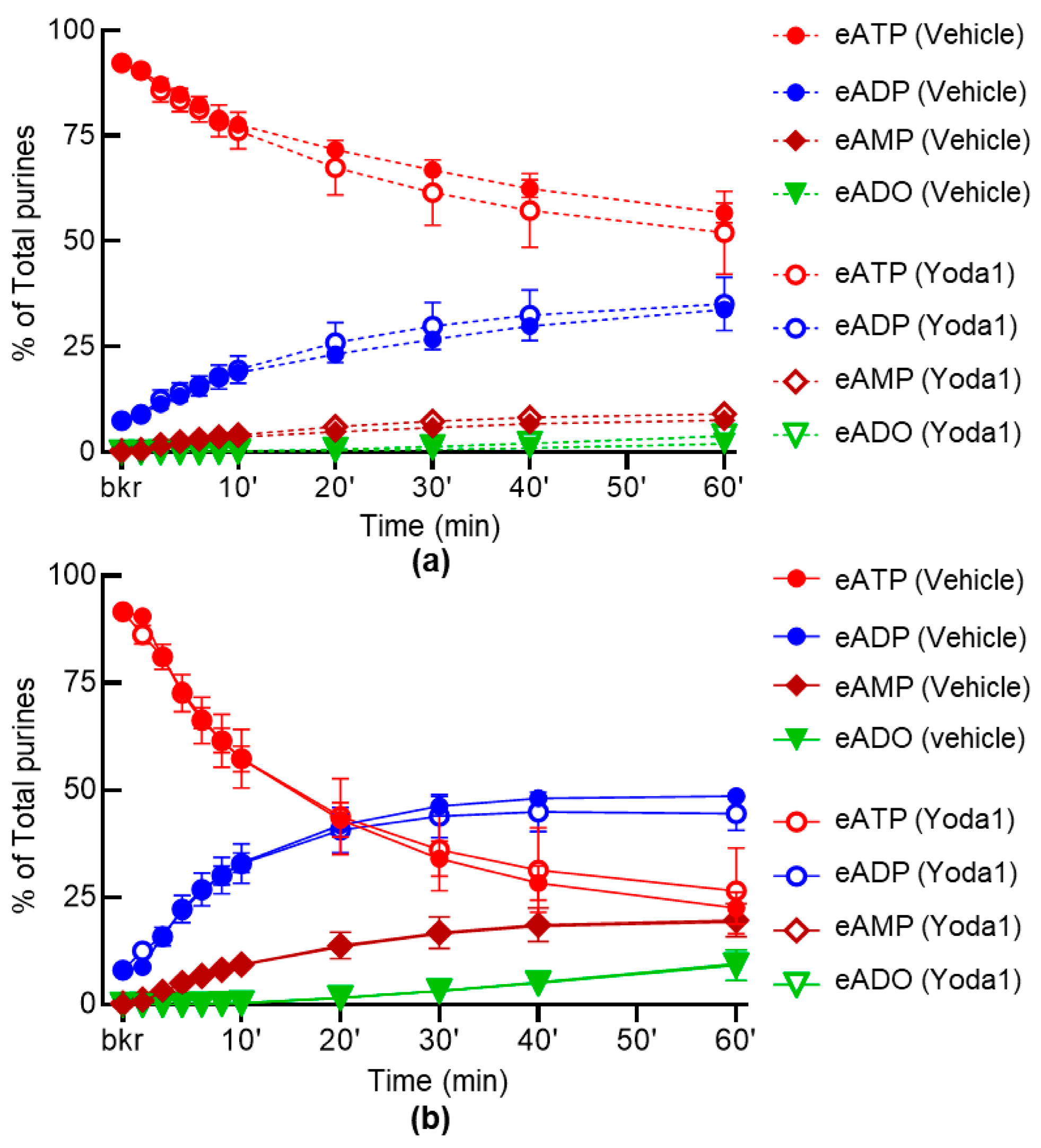
2.4. Inhibition of PIEZO Channels Has No Effect on the Spontaneous Release of s-ENTDs, but Increases the Distention-Induced Release of s-ENTDs in LP
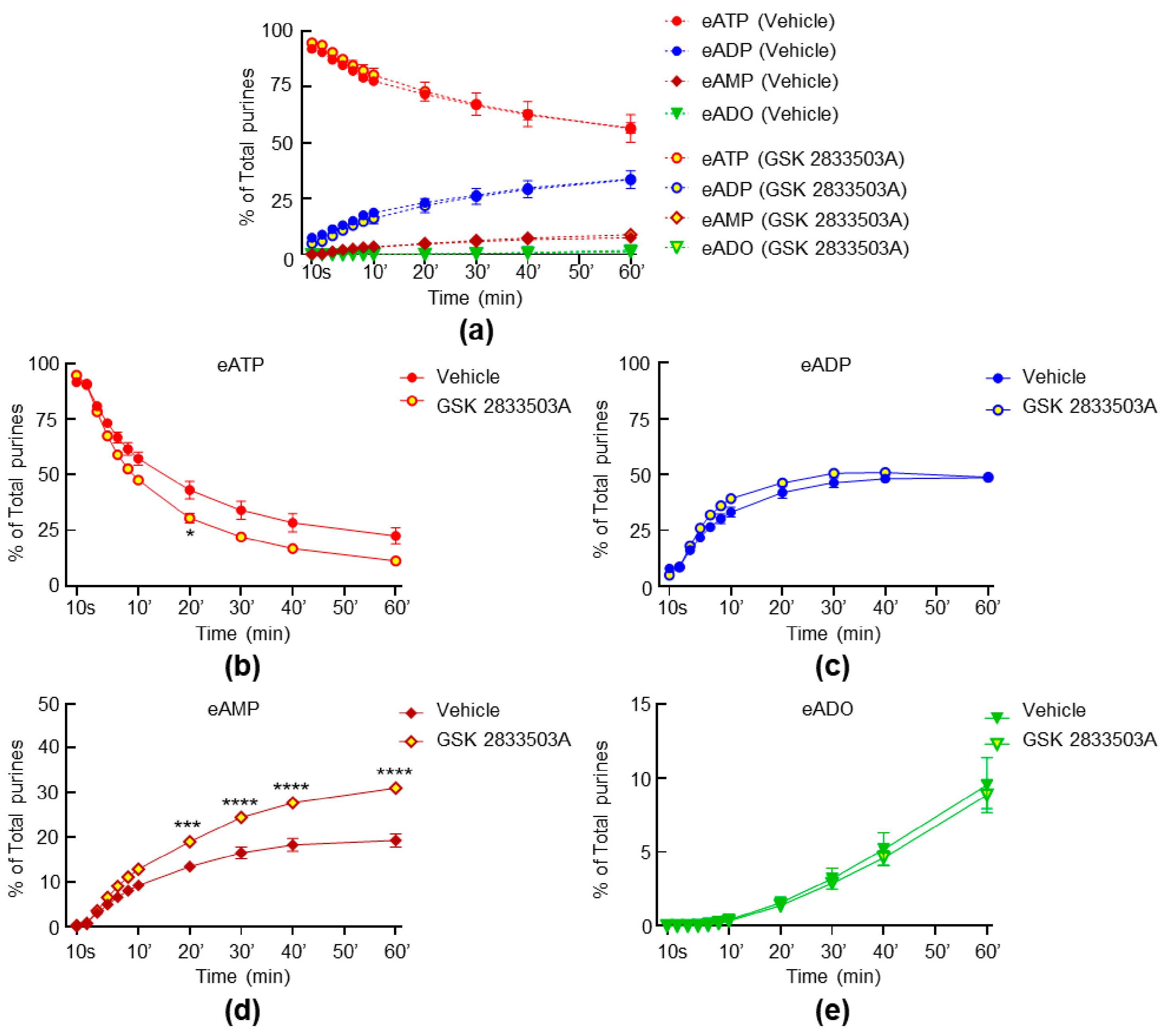
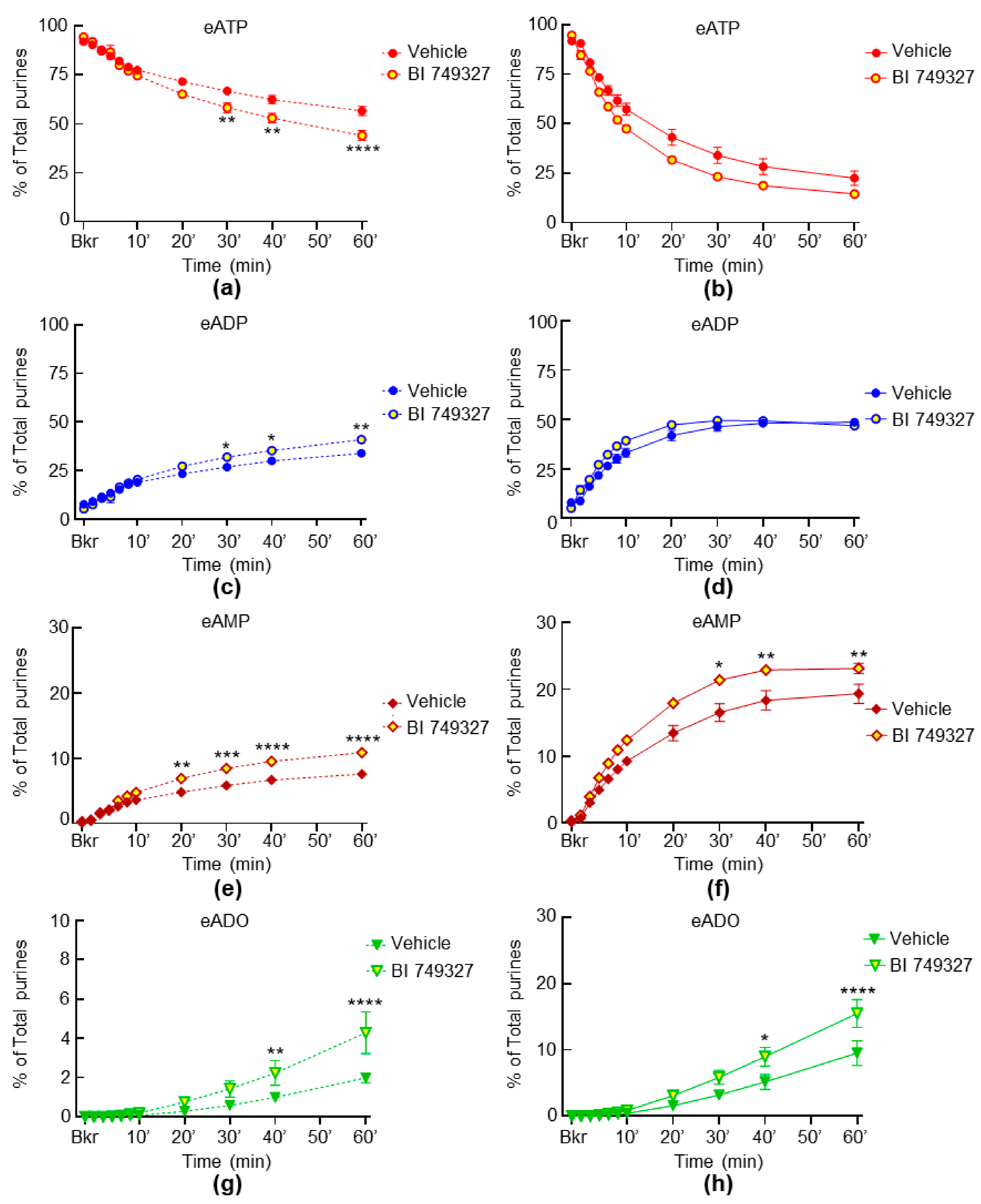
2.5. Inhibition of TRPC1 and TRPC6 Channels Have Weak Effect on the Spontaneous and Distention-Induced Release of s-ENTDs in LP
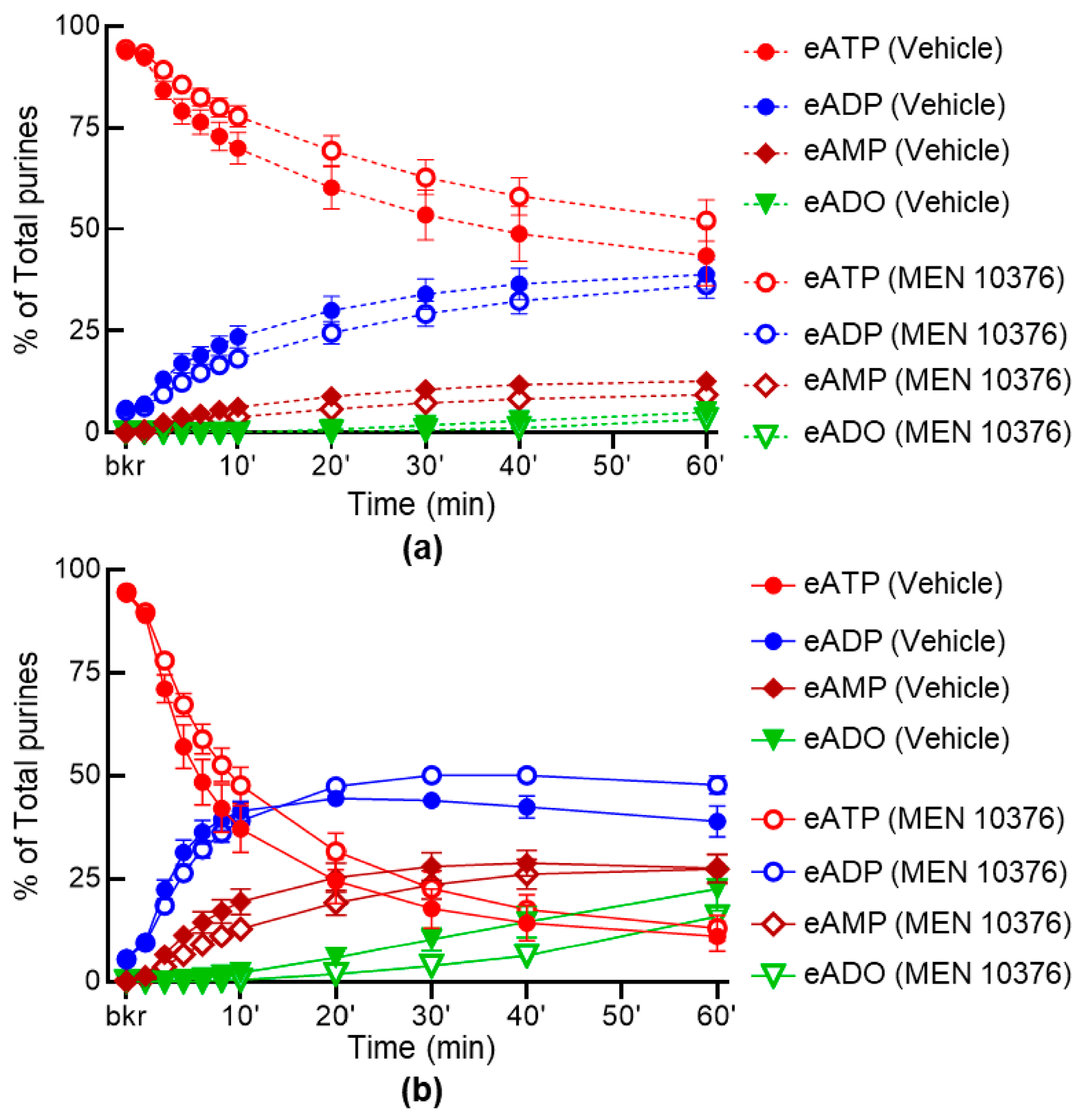
2.6. Inhibition of Receptors for Sensory Neuropeptides Affects Differentially the Release of s-ENTDs in LP
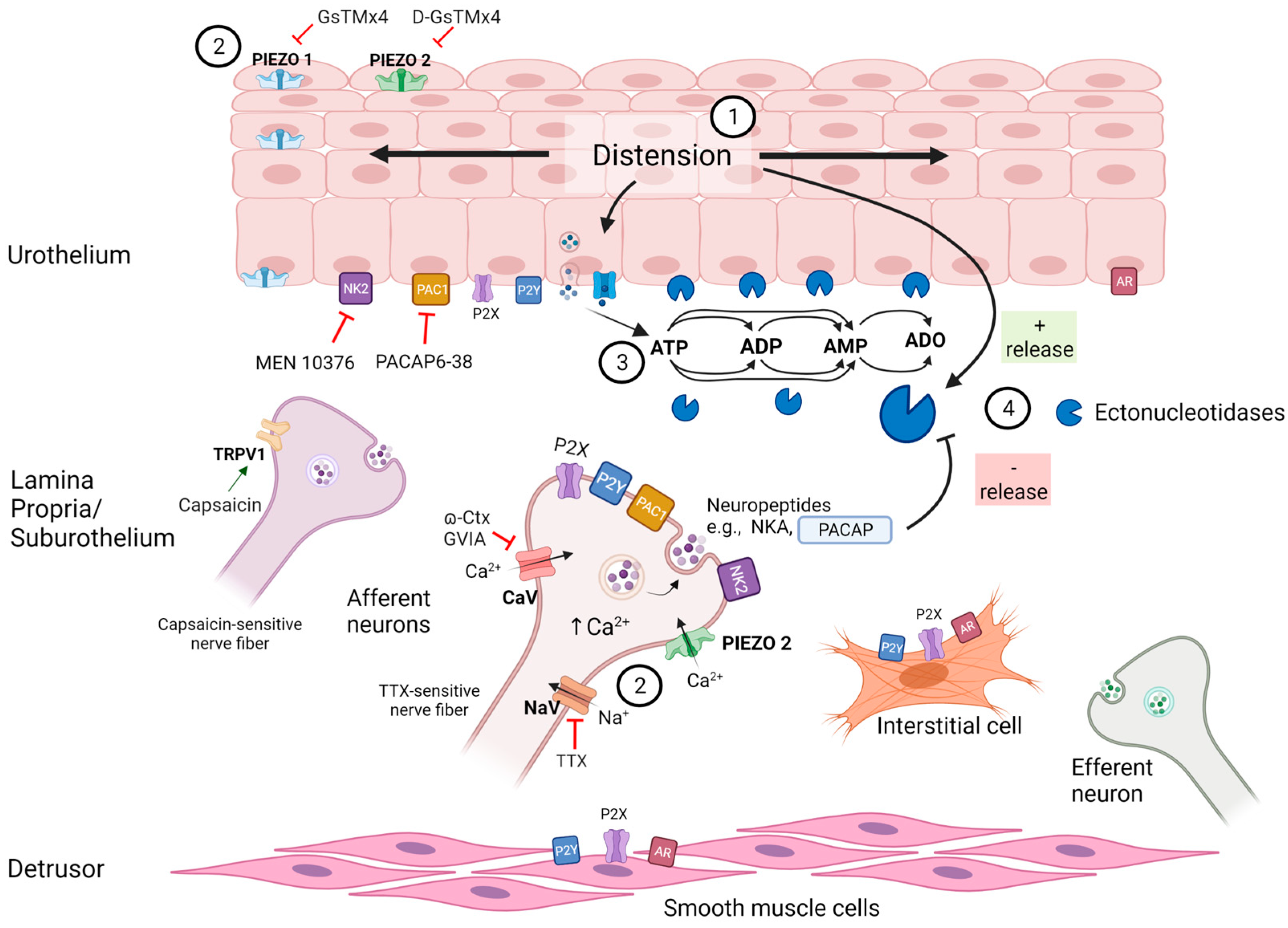
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Ex Vivo Detrusor-Free Bladder Preparation
4.3. General Experimental Protocol
4.4. Preparation of Extraluminal Samples for Measuring Soluble Nucleotidase Activities in the LP
4.5. Time-Course of ATP Hydrolysis in Concentrated ELS of Nondistended and Distended Preparations
4.6. Synthesis of 1,N6-etheno-ATP Substrate
4.7. HPLC Analysis of 1,N6-etheno-nucleotides and 1,N6-etheno-nucleosides
4.8. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burnstock, G. Purinergic signalling in the urinary tract in health and disease. Purinergic Signal. 2014, 10, 103–155. [Google Scholar] [CrossRef]
- Ferguson, D.R.; Kennedy, I.; Burton, T.J. ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes—A possible sensory mechanism? J. Physiol. 1997, 505, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Cockayne, D.A.; Hamilton, S.G.; Zhu, Q.M.; Dunn, P.M.; Zhong, Y.; Novakovic, S.; Malmberg, A.B.; Cain, G.; Berson, A.; Kassotakis, L.; et al. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature 2000, 407, 1011–1015. [Google Scholar] [CrossRef] [PubMed]
- Vlaskovska, M.; Kasakov, L.; Rong, W.; Bodin, P.; Bardini, M.; Cockayne, D.A.; Ford, A.P.; Burnstock, G. P2X3 knock-out mice reveal a major sensory role for urothelially released ATP. J. Neurosci. 2001, 21, 5670–5677. [Google Scholar] [CrossRef]
- Namasivayam, S.; Eardley, I.; Morrison, J.F. Purinergic sensory neurotransmission in the urinary bladder: An in vitro study in the rat. BJU Int. 1999, 84, 854–860. [Google Scholar] [CrossRef]
- Rong, W.; Spyer, K.M.; Burnstock, G. Activation and sensitisation of low and high threshold afferent fibres mediated by P2X receptors in the mouse urinary bladder. J. Physiol. 2002, 541, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Maynard, J.P.; Sfanos, K.S. P2 purinergic receptor dysregulation in urologic disease. Purinergic Signal. 2022, 18, 267–287. [Google Scholar] [CrossRef]
- Zimmermann, H.; Zebisch, M.; Strater, N. Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal. 2012, 8, 437–502. [Google Scholar] [CrossRef]
- Aresta Branco, M.S.L.; Gutierrez Cruz, A.; Dayton, J.; Perrino, B.A.; Mutafova-Yambolieva, V.N. Mechanosensitive Hydrolysis of ATP and ADP in Lamina Propria of the Murine Bladder by Membrane-Bound and Soluble Nucleotidases. Front. Physiol. 2022, 13, 918100. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Mansfield, K.J.; Allen, W.; Chess-Williams, R.; Burcher, E.; Moore, K.H. ATP during early bladder stretch is important for urgency in detrusor overactivity patients. Biomed. Res. Int. 2014, 2014, 204604. [Google Scholar] [CrossRef]
- Durnin, L.; Hayoz, S.; Corrigan, R.D.; Yanez, A.; Koh, S.D.; Mutafova-Yambolieva, V.N. Urothelial purine release during filling of murine and primate bladders. Am. J. Physiol. Renal Physiol. 2016, 311, F708–F716. [Google Scholar] [CrossRef] [PubMed]
- Todorov, L.D.; Mihaylova-Todorova, S.; Westfall, T.D.; Sneddon, P.; Kennedy, C.; Bjur, R.A.; Westfall, D.P. Neuronal release of soluble nucleotidases and their role in neurotransmitter inactivation. Nature 1997, 387, 76–79. [Google Scholar] [CrossRef]
- Apodaca, G.; Balestreire, E.; Birder, L.A. The uroepithelial-associated sensory web. Kidney Int. 2007, 72, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Dalghi, M.G.; Montalbetti, N.; Carattino, M.D.; Apodaca, G. The Urothelium: Life in a Liquid Environment. Physiol. Rev. 2020, 100, 1621–1705. [Google Scholar] [CrossRef]
- de Groat, W.C.; Yoshimura, N. Afferent nerve regulation of bladder function in health and disease. In Sensory Nerves; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 91–138. [Google Scholar]
- Zagorodnyuk, V.P.; Brookes, S.J.; Spencer, N.J.; Gregory, S. Mechanotransduction and chemosensitivity of two major classes of bladder afferents with endings in the vicinity to the urothelium. J. Physiol. 2009, 587, 3523–3538. [Google Scholar] [CrossRef]
- Birder, L.; Andersson, K.E. Urothelial signaling. Physiol. Rev. 2013, 93, 653–680. [Google Scholar] [CrossRef]
- de Groat, W.C.; Griffiths, D.; Yoshimura, N. Neural control of the lower urinary tract. Compr. Physiol. 2015, 5, 327–396. [Google Scholar] [PubMed]
- Dalghi, M.G.; Clayton, D.R.; Ruiz, W.G.; Al-Bataineh, M.M.; Satlin, L.M.; Kleyman, T.R.; Ricke, W.A.; Carattino, M.D.; Apodaca, G. Expression and distribution of PIEZO1 in the mouse urinary tract. Am. J. Physiol. Renal Physiol. 2019, 317, F303–F321. [Google Scholar] [CrossRef] [PubMed]
- Marshall, K.L.; Saade, D.; Ghitani, N.; Coombs, A.M.; Szczot, M.; Keller, J.; Ogata, T.; Daou, I.; Stowers, L.T.; Bönnemann, C.G.; et al. PIEZO2 in sensory neurons and urothelial cells coordinates urination. Nature 2020, 588, 290–295. [Google Scholar] [CrossRef]
- Heppner, T.J.; Hennig, G.W.; Nelson, M.T.; May, V.; Vizzard, M.A. PACAP38-Mediated Bladder Afferent Nerve Activity Hyperexcitability and Ca(2+) Activity in Urothelial Cells from Mice. J. Mol. Neurosci. 2019, 68, 348–356. [Google Scholar] [CrossRef]
- Grundy, L.; Chess-Williams, R.; Brierley, S.M.; Mills, K.; Moore, K.H.; Mansfield, K.; Rose’Meyer, R.; Sellers, D.J.; Grundy, D. NKA enhances bladder afferent mechanosensitivity via urothelial and detrusor activation. Am. J. Physiol. Renal Physiol. 2018, 315, F1174–F1185. [Google Scholar] [CrossRef] [PubMed]
- Girard, B.M.; Wolf-Johnston, A.; Braas, K.M.; Birder, L.A.; May, V.; Vizzard, M.A. PACAP-mediated ATP release from rat urothelium and regulation of PACAP/VIP and receptor mRNA in micturition pathways after cyclophosphamide (CYP)-induced cystitis. J. Mol. Neurosci. 2008, 36, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Cinar, E.; Zhou, S.; DeCourcey, J.; Wang, Y.; Waugh, R.E.; Wan, J. Piezo1 regulates mechanotransductive release of ATP from human RBCs. Proc. Natl. Acad. Sci. USA 2015, 112, 11783–11788. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Chen, Z.; Liu, L.; Ding, N.; Wen, J.; Liu, J.; Wang, W.; Ge, N.; Zu, S.; Song, W.; et al. Functional Expression of Transient Receptor Potential and Piezo1 Channels in Cultured Interstitial Cells of Human-Bladder Lamina Propria. Front. Physiol. 2021, 12, 762847. [Google Scholar] [CrossRef]
- Bahns, E.; Halsband, U.; Jänig, W. Responses of sacral visceral afferents from the lower urinary tract, colon and anus to mechanical stimulation. Pflügers Arch. 1987, 410, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Daly, D.M.; Collins, V.M.; Chapple, C.R.; Grundy, D. The afferent system and its role in lower urinary tract dysfunction. Curr. Opin. Urol. 2011, 21, 268–274. [Google Scholar] [CrossRef]
- Arms, L.; Vizzard, M.A. Neuropeptides in lower urinary tract function. In Urinary Tract; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2011; pp. 395–423. [Google Scholar] [CrossRef]
- Fry, C.H.; McCloskey, K.D. Purinergic signalling in the urinary bladder—When function becomes dysfunction. Auton. Neurosci. 2021, 235, 102852. [Google Scholar] [CrossRef]
- Silva-Ramos, M.; Silva, I.; Oliveira, O.; Ferreira, S.; Reis, M.J.; Oliveira, J.C.; Correia-de-Sa, P. Urinary ATP may be a dynamic biomarker of detrusor overactivity in women with overactive bladder syndrome. PLoS ONE 2013, 8, e64696. [Google Scholar] [CrossRef]
- Sun, Y.; Keay, S.; De Deyne, P.G.; Chai, T.C. Augmented stretch activated adenosine triphosphate release from bladder uroepithelial cells in patients with interstitial cystitis. J. Urol. 2001, 166, 1951–1956. [Google Scholar] [CrossRef]
- Taidi, Z.; Mansfield, K.J.; Bates, L.; Sana-Ur-Rehman, H.; Liu, L. Purinergic P2X7 receptors as therapeutic targets in interstitial cystitis/bladder pain syndrome; key role of ATP signaling in inflammation. Bladder 2019, 6, e38. [Google Scholar] [CrossRef]
- Mutafova-Yambolieva, V.N.; Durnin, L. The purinergic neurotransmitter revisited: A single substance or multiple players? Pharmacol. Ther. 2014, 144, 162–191. [Google Scholar] [CrossRef] [PubMed]
- Durnin, L.; Kwok, B.; Kukadia, P.; McAvera, R.; Corrigan, R.D.; Ward, S.M.; Zhang, Y.; Chen, Q.; Koh, S.D.; Sanders, K.M.; et al. An ex vivo bladder model with detrusor smooth muscle removed to analyse biologically active mediators released from the suburothelium. J. Physiol. 2019, 597, 1467–1485. [Google Scholar] [CrossRef] [PubMed]
- Breen, L.T.; Smyth, L.M.; Yamboliev, I.A.; Mutafova-Yambolieva, V.N. β-NAD is a novel nucleotide released on stimulation of nerve terminals in human urinary bladder detrusor muscle. Am. J. Physiol. Renal Physiol. 2006, 290, F486–F495. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez Cruz, A.; Aresta Branco, M.S.L.; Perrino, B.A.; Sanders, K.M.; Mutafova-Yambolieva, V.N. Urinary ATP Levels Are Controlled by Nucleotidases Released from the Urothelium in a Regulated Manner. Metabolites 2022, 13, 30. [Google Scholar] [CrossRef] [PubMed]
- Schueth, A.; Spronck, B.; van Zandvoort, M.; van Koeveringe, G.A. Computer-assisted three-dimensional tracking of sensory innervation in the murine bladder mucosa with two-photon microscopy. J. Chem. Neuroanat. 2017, 85, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Heppner, T.J.; Hennig, G.W.; Nelson, M.T.; Herrera, G.M. Afferent nerve activity in a mouse model increases with faster bladder filling rates in vitro, but voiding behavior remains unaltered in vivo. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2022, 323, R682–R693. [Google Scholar] [CrossRef]
- Jackson, E.K.; Gillespie, D.G.; Cheng, D.; Mi, Z.; Menshikova, E.V. Characterization of the N(6)-etheno-bridge method to assess extracellular metabolism of adenine nucleotides: Detection of a possible role for purine nucleoside phosphorylase in adenosine metabolism. Purinergic Signal. 2020, 16, 187–211. [Google Scholar] [CrossRef]
- Andersson, K.E. Bladder activation: Afferent mechanisms. Urology 2002, 59, 43–50. [Google Scholar] [CrossRef]
- Chai, T.C.; Russo, A.; Yu, S.; Lu, M. Mucosal signaling in the bladder. Auton. Neurosci. 2016, 200, 49–56. [Google Scholar] [CrossRef]
- Grundy, L.; Erickson, A.; Caldwell, A.; Garcia-Caraballo, S.; Rychkov, G.; Harrington, A.; Brierley, S.M. Tetrodotoxin-sensitive voltage-gated sodium channels regulate bladder afferent responses to distension. Pain 2018, 159, 2573–2584. [Google Scholar] [CrossRef]
- Olivera, B.M.; Miljanich, G.P.; Ramachandran, J.; Adams, M.E. Calcium Channel Diversity and Neurotransmitter Release: The -Conotoxins and -Agatoxins. Annu. Rev. Biochem. 1994, 63, 823–867. [Google Scholar] [CrossRef]
- Li, X.; Hu, J.; Zhao, X.; Li, J.; Chen, Y. Piezo channels in the urinary system. Exp. Mol. Med. 2022, 54, 697–710. [Google Scholar] [CrossRef] [PubMed]
- Murthy, S.E.; Loud, M.C.; Daou, I.; Marshall, K.L.; Schwaller, F.; Kühnemund, J.; Francisco, A.G.; Keenan, W.T.; Dubin, A.E.; Lewin, G.R.; et al. The mechanosensitive ion channel Piezo2 mediates sensitivity to mechanical pain in mice. Sci. Transl. Med. 2018, 10, eaat9897. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.Z.; Marshall, K.L.; Min, S.; Daou, I.; Chapleau, M.W.; Abboud, F.M.; Liberles, S.D.; Patapoutian, A. PIEZOs mediate neuronal sensing of blood pressure and the baroreceptor reflex. Science 2018, 362, 464–467. [Google Scholar] [CrossRef]
- Dalghi, M.G.; Ruiz, W.G.; Clayton, D.R.; Montalbetti, N.; Daugherty, S.L.; Beckel, J.M.; Carattino, M.D.; Apodaca, G. Functional roles for PIEZO1 and PIEZO2 in urothelial mechanotransduction and lower urinary tract interoception. JCI Insight 2021, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Coste, B.; Mathur, J.; Schmidt, M.; Earley, T.J.; Ranade, S.; Petrus, M.J.; Dubin, A.E.; Patapoutian, A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 2010, 330, 55–60. [Google Scholar] [CrossRef]
- Noguri, T.; Hatakeyama, D.; Kitahashi, T.; Oka, K.; Ito, E. Profile of dorsal root ganglion neurons: Study of oxytocin expression. Mol. Brain 2022, 15, 44. [Google Scholar] [CrossRef]
- Miyamoto, T.; Mochizuki, T.; Nakagomi, H.; Kira, S.; Watanabe, M.; Takayama, Y.; Suzuki, Y.; Koizumi, S.; Takeda, M.; Tominaga, M. Functional role for Piezo1 in stretch-evoked Ca(2+) influx and ATP release in urothelial cell cultures. J. Biol. Chem. 2014, 289, 16565–16575. [Google Scholar] [CrossRef]
- Suchyna, T.M.; Johnson, J.H.; Hamer, K.; Leykam, J.F.; Gage, D.A.; Clemo, H.F.; Baumgarten, C.M.; Sachs, F. Identification of a peptide toxin from Grammostola spatulata spider venom that blocks cation-selective stretch-activated channels. J. Gen. Physiol. 2000, 115, 583–598. [Google Scholar] [CrossRef]
- Bae, C.; Sachs, F.; Gottlieb, P.A. The mechanosensitive ion channel Piezo1 is inhibited by the peptide GsMTx4. Biochemistry 2011, 50, 6295–6300. [Google Scholar] [CrossRef]
- Suchyna, T.M. Piezo channels and GsMTx4: Two milestones in our understanding of excitatory mechanosensitive channels and their role in pathology. Prog. Biophys. Mol. Biol. 2017, 130, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Kerstein, P.C.; Jacques-Fricke, B.T.; Rengifo, J.; Mogen, B.J.; Williams, J.C.; Gottlieb, P.A.; Sachs, F.; Gomez, T.M. Mechanosensitive TRPC1 channels promote calpain proteolysis of talin to regulate spinal axon outgrowth. J. Neurosci. 2013, 33, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Spassova, M.A.; Hewavitharana, T.; Xu, W.; Soboloff, J.; Gill, D.L. A common mechanism underlies stretch activation and receptor activation of TRPC6 channels. Proc. Natl. Acad. Sci. USA 2006, 103, 16586–16591. [Google Scholar] [CrossRef]
- Bowman, C.L.; Gottlieb, P.A.; Suchyna, T.M.; Murphy, Y.K.; Sachs, F. Mechanosensitive ion channels and the peptide inhibitor GsMTx-4: History, properties, mechanisms and pharmacology. Toxicon 2007, 49, 249–270. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, P.A.; Sachs, F. Piezo1: Properties of a cation selective mechanical channel. Channels 2012, 6, 214–219. [Google Scholar] [CrossRef]
- Alcaino, C.; Knutson, K.; Gottlieb, P.A.; Farrugia, G.; Beyder, A. Mechanosensitive ion channel Piezo2 is inhibited by D-GsMTx4. Channels 2017, 11, 245–253. [Google Scholar] [CrossRef]
- Lee, W.; Leddy, H.A.; Chen, Y.; Lee, S.H.; Zelenski, N.A.; McNulty, A.L.; Wu, J.; Beicker, K.N.; Coles, J.; Zauscher, S.; et al. Synergy between Piezo1 and Piezo2 channels confers high-strain mechanosensitivity to articular cartilage. Proc. Natl. Acad. Sci. USA 2014, 111, E5114–E5122. [Google Scholar] [CrossRef]
- Syeda, R.; Xu, J.; Dubin, A.E.; Coste, B.; Mathur, J.; Huynh, T.; Matzen, J.; Lao, J.; Tully, D.C.; Engels, I.H.; et al. Chemical activation of the mechanotransduction channel Piezo1. Elife 2015, 4, e07369. [Google Scholar] [CrossRef]
- Kress, M.; Karasek, J.; Ferrer-Montiel, A.V.; Scherbakov, N.; Haberberger, R.V. TRPC channels and diacylglycerol dependent calcium signaling in rat sensory neurons. Histochem. Cell Biol. 2008, 130, 655–667. [Google Scholar] [CrossRef]
- Maroto, R.; Raso, A.; Wood, T.G.; Kurosky, A.; Martinac, B.; Hamill, O.P. TRPC1 forms the stretch-activated cation channel in vertebrate cells. Nat Cell Biol. 2005, 7, 179–185. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, B.; Zhao, J.; Wang, Q.; An, F.; Hu, X.; Yang, Z.; Xu, J.; Tan, M.; Li, L. Increased Piezo1 channel activity in interstitial Cajal-like cells induces bladder hyperactivity by functionally interacting with NCX1 in rats with cyclophosphamide-induced cystitis. Exp. Mol. Med. 2018, 50, 1–16. [Google Scholar] [CrossRef]
- Michishita, M.; Yano, K.; Tomita, K.I.; Matsuzaki, O.; Kasahara, K.I. Piezo1 expression increases in rat bladder after partial bladder outlet obstruction. Life Sci. 2016, 166, 1–7. [Google Scholar] [CrossRef]
- Prakasam, H.S.; Herrington, H.; Roppolo, J.R.; Jackson, E.K.; Apodaca, G. Modulation of bladder function by luminal adenosine turnover and A1 receptor activation. Am. J. Physiol. Renal Physiol. 2012, 303, F279–F292. [Google Scholar] [CrossRef] [PubMed]
- Kitta, T.; Chancellor, M.B.; de Groat, W.C.; Kuno, S.; Nonomura, K.; Yoshimura, N. Roles of adenosine A1 and A2A receptors in the control of micturition in rats. Neurourol. Urodyn. 2014, 33, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.A.; Santer, R.M. Distribution and changes with age of calcitonin gene-related peptide- and substance P-immunoreactive nerves of the rat urinary bladder and lumbosacral sensory neurons. Eur. J. Morphol. 2002, 40, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Merrill, L.; Gonzalez, E.J.; Girard, B.M.; Vizzard, M.A. Receptors, channels, and signalling in the urothelial sensory system in the bladder. Nat. Rev. Urol. 2016, 13, 193–204. [Google Scholar] [CrossRef]
- Rahnama’i, M.S.; Biallosterski, B.T.; Van Kerrebroeck, P.E.; van Koeveringe, G.A.; Gillespie, J.I.; de Wachter, S.G. Distribution and sub-types of afferent fibre in the mouse urinary bladder. J. Chem. Neuroanat. 2017, 79, 1–11. [Google Scholar] [CrossRef]
- Lecci, A.; Giuliani, S.; Tramontana, M.; Santicioli, P.; Criscuoli, M.; Dion, S.; Maggi, C.A. Bladder distension and activation of the efferent function of sensory fibres: Similarities with the effect of capsaicin. Br. J. Pharmacol. 1998, 124, 259–266. [Google Scholar] [CrossRef]
- Shaker, H.S.; Tu, L.M.; Kalfopoulos, M.; Hassouna, M.; Dion, S.; Elhilali, M. Hyperreflexia of the urinary bladder: Possible role of the efferent function of the capsaicin sensitive primary afferents. J. Urol. 1998, 160, 2232–2239. [Google Scholar] [CrossRef]
- Maggi, C.A.; Giuliani, S.; Ballati, L.; Lecci, A.; Manzini, S.; Patacchini, R.; Renzetti, A.R.; Rovero, P.; Quartara, L.; Giachetti, A. In vivo evidence for tachykininergic transmission using a new NK-2 receptor-selective antagonist, MEN 10,376. J. Pharmacol. Exp. Ther. 1991, 257, 1172–1178. [Google Scholar]
- Heppner, T.J.; Tykocki, N.R.; Hill-Eubanks, D.; Nelson, M.T. Transient contractions of urinary bladder smooth muscle are drivers of afferent nerve activity during filling. J. Gen. Physiol. 2016, 147, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Sculptoreanu, A.; Artim, D.E.; de Groat, W.C. Neurokinins inhibit low threshold inactivating K+ currents in capsaicin responsive DRG neurons. Exp. Neurol. 2009, 219, 562–573. [Google Scholar] [CrossRef]
- Liao, C.; May, V.; Li, J. PAC1 Receptors: Shapeshifters in Motion. J. Mol. Neurosci. 2019, 68, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Shivers, B.D.; Görcs, T.J.; Gottschall, P.E.; Arimura, A. Two high affinity binding sites for pituitary adenylate cyclase-activating polypeptide have different tissue distributions. Endocrinology 1991, 128, 3055–3065. [Google Scholar] [CrossRef]
- Hernández, M.; Barahona, M.V.; Recio, P.; Benedito, S.; Martínez, A.C.; Rivera, L.; García-Sacristán, A.; Prieto, D.; Orensanz, L.M. Neuronal and smooth muscle receptors involved in the PACAP- and VIP-induced relaxations of the pig urinary bladder neck. Br. J. Pharmacol. 2006, 149, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Ojala, J.; Tooke, K.; Hsiang, H.; Girard, B.M.; May, V.; Vizzard, M.A. PACAP/PAC1 Expression and Function in Micturition Pathways. J. Mol. Neurosci. 2019, 68, 357–367. [Google Scholar] [CrossRef] [PubMed]
- May, V.; Vizzard, M.A. Bladder dysfunction and altered somatic sensitivity in PACAP−/− mice. J. Urol. 2010, 183, 772–779. [Google Scholar] [CrossRef]
- Girard, B.M.; Campbell, S.E.; Beca, K.I.; Perkins, M.; Hsiang, H.; May, V.; Vizzard, M.A. Intrabladder PAC1 Receptor Antagonist, PACAP(6-38), Reduces Urinary Bladder Frequency and Pelvic Sensitivity in Mice Exposed to Repeated Variate Stress (RVS). J. Mol. Neurosci. 2021, 71, 1575–1588. [Google Scholar] [CrossRef]
- Durnin, L.; Corrigan, R.D.; Sanders, K.M.; Mutafova-Yambolieva, V.N. A Decentralized (Ex Vivo) Murine Bladder Model with the Detrusor Muscle Removed for Direct Access to the Suburothelium during Bladder Filling. J. Vis. Exp. 2019, 153, e60344. [Google Scholar] [CrossRef]
- Bobalova, J.; Bobal, P.; Mutafova-Yambolieva, V.N. High-Performance Liquid Chromatographic Technique for Detection of a Fluorescent Analogue of ADP-Ribose in Isolated Blood Vessel Preparations. Anal. Biochem. 2002, 305, 269–276. [Google Scholar] [CrossRef]
- Levitt, B.; Head, R.J.; Westfall, D.P. High-pressure liquid chromatographic-fluorometric detection of adenosine and adenine nucleotides: Application to endogenous content and electrically induced release of adenyl purines in guinea pig vas deferens. Anal. Biochem. 1984, 137, 93–100. [Google Scholar] [CrossRef] [PubMed]


| Drug | Mechanism of Action | Working Concentration (µM) | Vehicle | Vendor |
|---|---|---|---|---|
| BI-749327 | TRPC6 inhibitor | 1 | DMSO 0.2% | MedChemExpress, Monmouth Junction, NJ, USA |
| Capsaicin | TRPV1 channel agonist | 1 | DMSO 0.1% | Tocris Biosciences, Minneapolis, MN, USA |
| ω-CtxGVIA | Cav2.2 (N-type) Ca2+ channel inhibitor | 0.1 | KBS | Tocris Biosciences |
| D-GsMTx4 | PIEZO inhibitor | 1 | KBS | Tocris Biosciences |
| GSK 1702934A | TRPC3/6 activator | 3 | DMSO 0.2% | Tocris Biosciences |
| GSK 2833503A | TRPC3/6 inhibitor | 1 | DMSO 0.2% | Tocris Biosciences |
| GsMTx4 | PIEZO inhibitor | 1 | KBS | Tocris Biosciences |
| MEN 10376 | NK2 receptor antagonist | 10 | KBS | Cayman Chemicals, Ann Arbor, MI, USA |
| TTX | Fast Na+ channel inhibitor | 0.5 | KBS | Alomone Labs, Jerusalem, Israel |
| PACAP6-38 | PAC1 receptor antagonist | 0.3 | KBS | Tocris Biosciences |
| Pico145 | TRPC1,4,5 inhibitor | 1 | DMSO 0.2% | MedChemExpress |
| Yoda1 | PIEZO1 activator | 40 | DMSO 0.2% | Tocris Biosciences |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aresta Branco, M.S.L.; Gutierrez Cruz, A.; Borhani Peikani, M.; Mutafova-Yambolieva, V.N. Sensory Neurons, PIEZO Channels and PAC1 Receptors Regulate the Mechanosensitive Release of Soluble Ectonucleotidases in the Murine Urinary Bladder Lamina Propria. Int. J. Mol. Sci. 2023, 24, 7322. https://doi.org/10.3390/ijms24087322
Aresta Branco MSL, Gutierrez Cruz A, Borhani Peikani M, Mutafova-Yambolieva VN. Sensory Neurons, PIEZO Channels and PAC1 Receptors Regulate the Mechanosensitive Release of Soluble Ectonucleotidases in the Murine Urinary Bladder Lamina Propria. International Journal of Molecular Sciences. 2023; 24(8):7322. https://doi.org/10.3390/ijms24087322
Chicago/Turabian StyleAresta Branco, Mafalda S. L., Alejandro Gutierrez Cruz, Mahsa Borhani Peikani, and Violeta N. Mutafova-Yambolieva. 2023. "Sensory Neurons, PIEZO Channels and PAC1 Receptors Regulate the Mechanosensitive Release of Soluble Ectonucleotidases in the Murine Urinary Bladder Lamina Propria" International Journal of Molecular Sciences 24, no. 8: 7322. https://doi.org/10.3390/ijms24087322
APA StyleAresta Branco, M. S. L., Gutierrez Cruz, A., Borhani Peikani, M., & Mutafova-Yambolieva, V. N. (2023). Sensory Neurons, PIEZO Channels and PAC1 Receptors Regulate the Mechanosensitive Release of Soluble Ectonucleotidases in the Murine Urinary Bladder Lamina Propria. International Journal of Molecular Sciences, 24(8), 7322. https://doi.org/10.3390/ijms24087322





