Red Algae “Sarcodia suieae” Acetyl-Xylogalactan Downregulate Heat-Induced Macrophage Stress Factors Ddit3 and Hyou1 Compared to the Aquatic Animal Model of Nile Tilapia (Oreochromis niloticus) Brain Arachidonic Acid Expression
Abstract
1. Introduction
2. Results
2.1. Cellular Exeperiment
2.1.1. RAW 264.7 Macrophage Survival and Phagocytotic Activity under Heat Stress
2.1.2. RAW 264.7 Macrophage Apoptosis and Galactosidase Observation under Heat Stress
2.1.3. Intracellular Ca2+ Concentration and Mitochondrial Membrane Potential of the Pre-Treated RAW 264.7 after Heat Stress
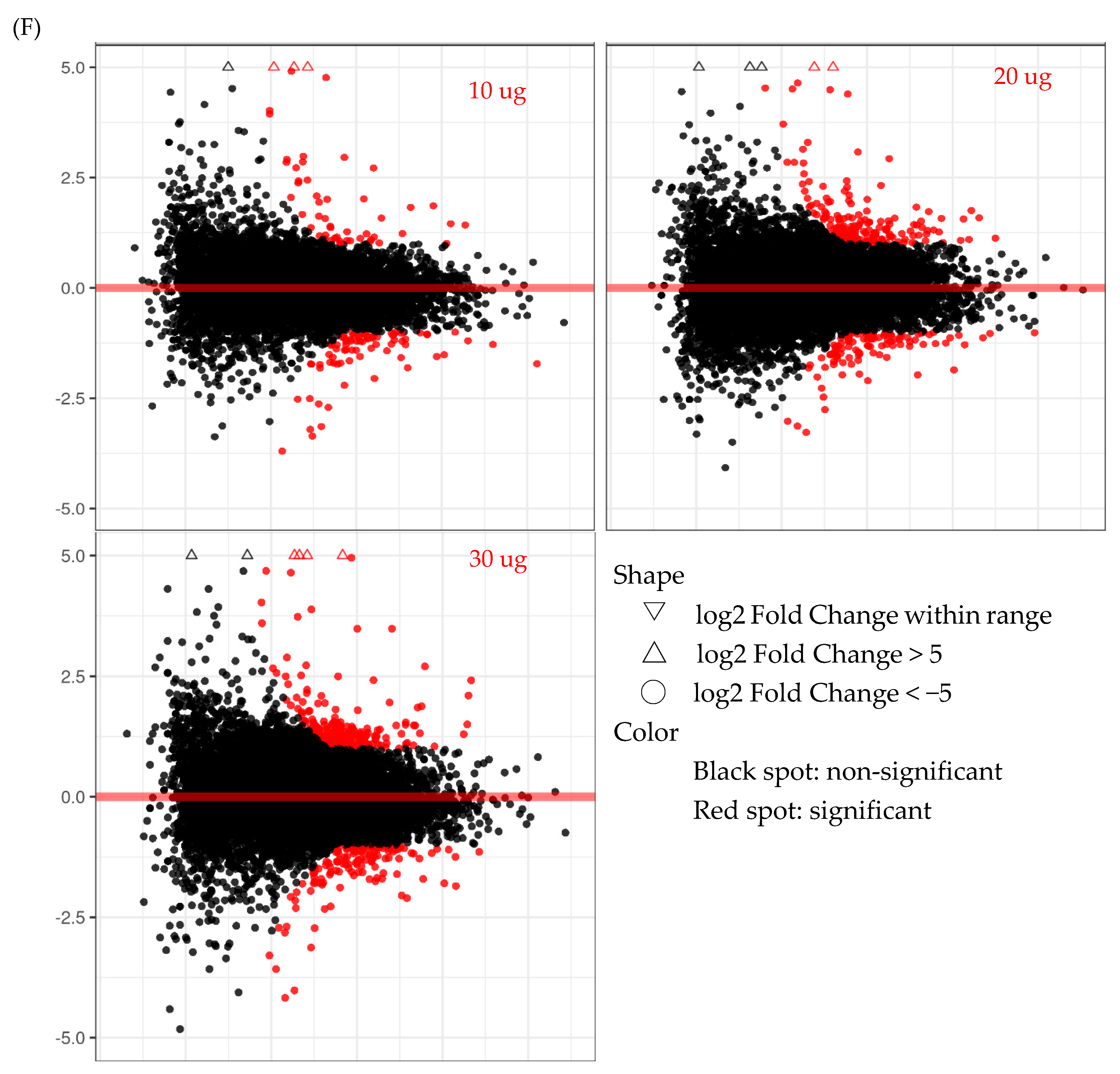
2.1.4. RNA Sequencing (Transcriptome)
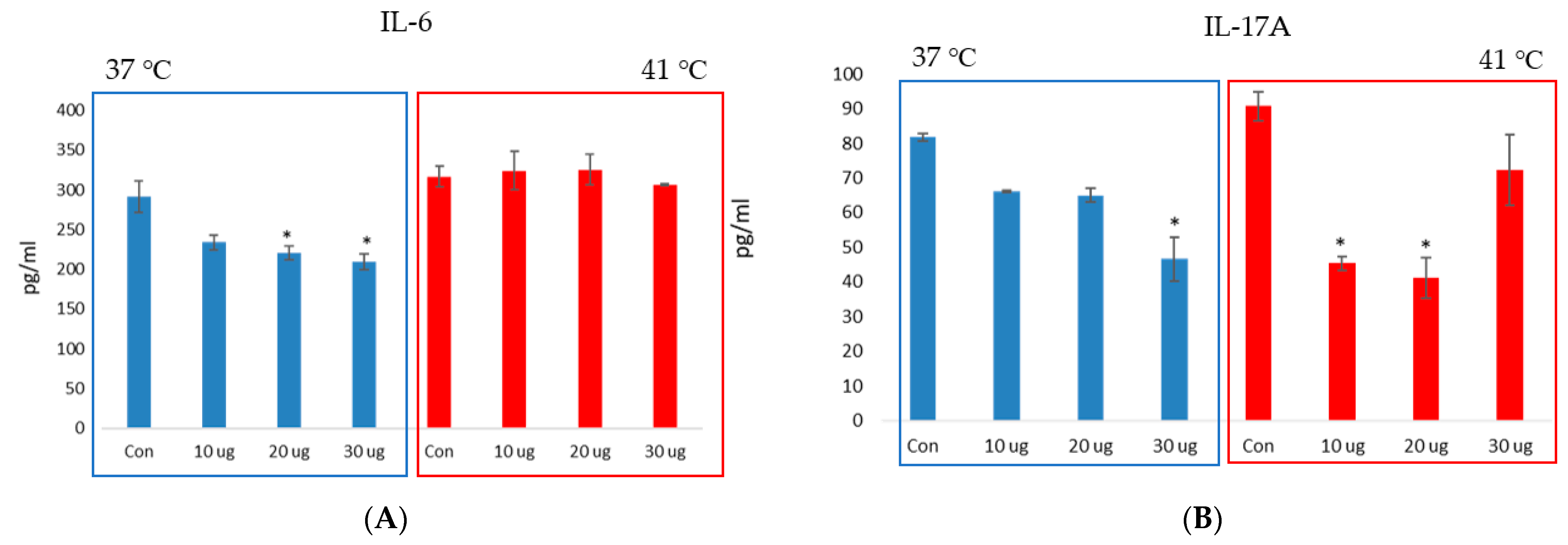
2.1.5. Cytokines IL-6 and IL-17 A Production
2.2. In Vivo Animal Expeiment
2.2.1. Blood Biochemical Analysis
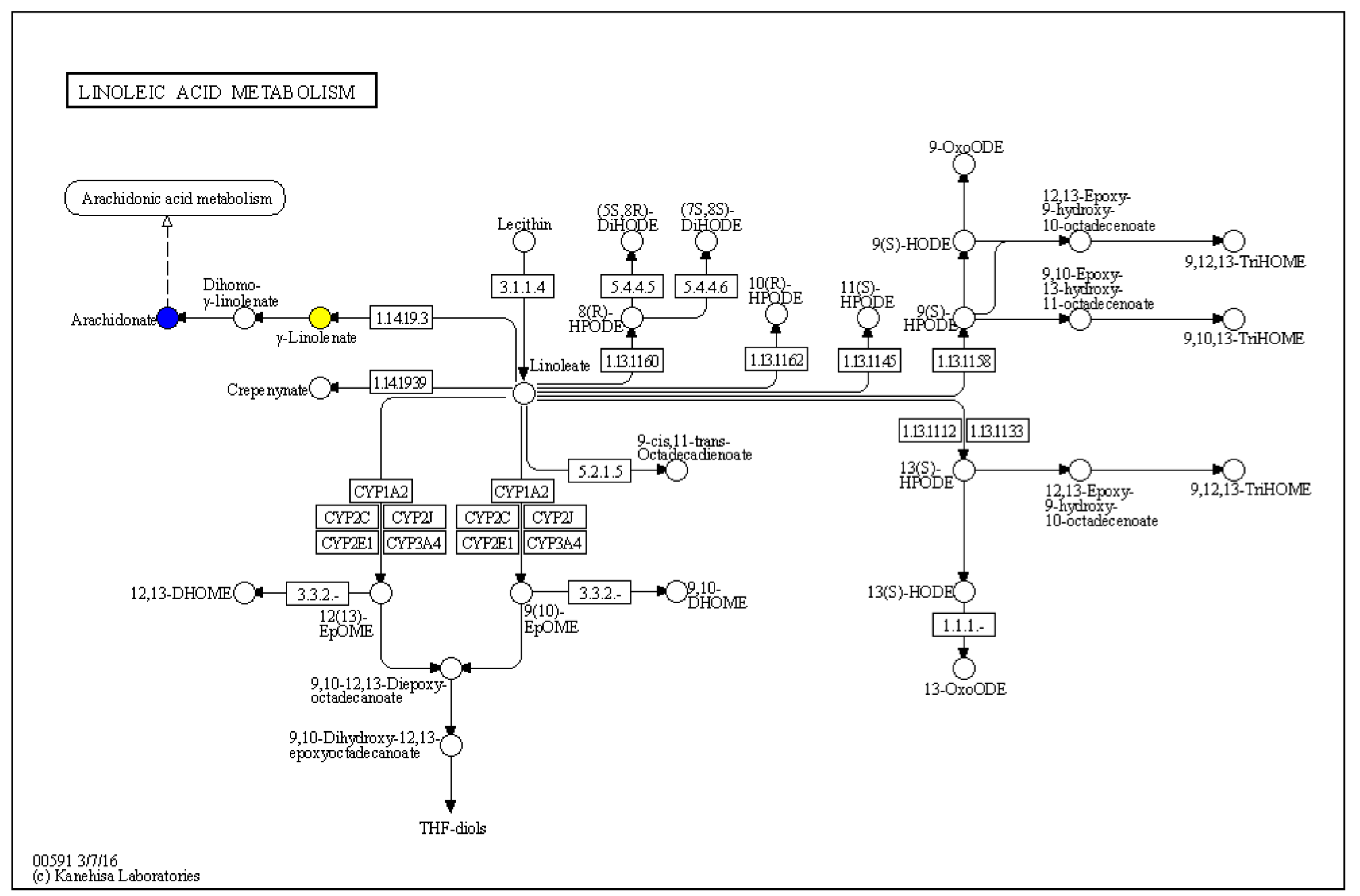
2.2.2. Brain Metabolites Analysis
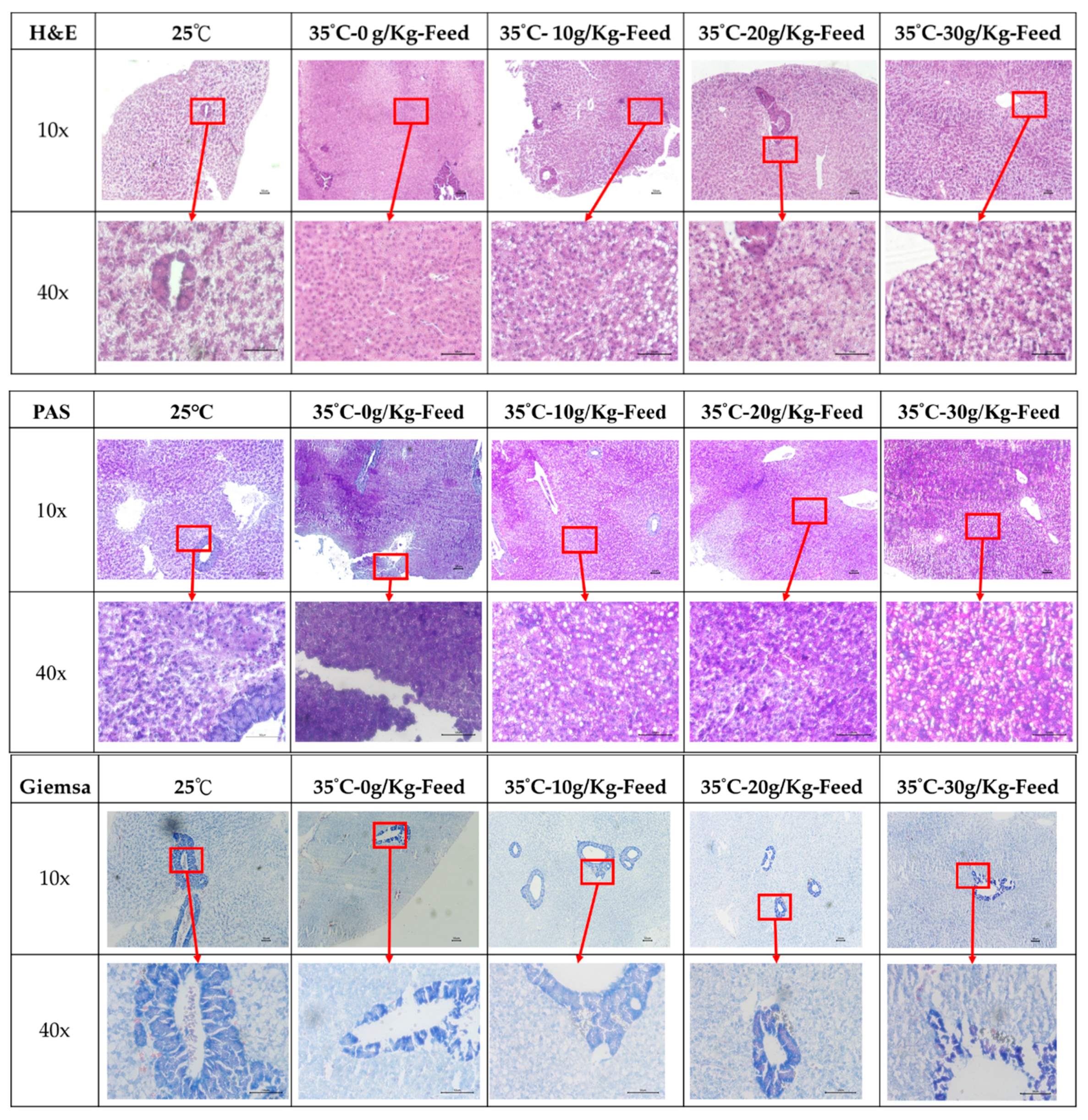
2.2.3. Histological Observation
3. Discussion
4. Materials and Methods
4.1. Sarcodia Suieae Acetyl-Xylogalactan Preparation
4.2. Cellular Findings
4.2.1. Survival of RAW 264.7 Cells under Heat Stress
4.2.2. Apoptosis of RAW 264.7 Macrophages under Heat Stress
4.2.3. Phagocytic Activity of RAW 264.7 Cells under Heat Stress
4.2.4. Observation of Galactosidase Expression in RAW 264.7 Cells under Heat Stress
4.2.5. Mitochondrial Membrane Potential Changes in RAW 264.7 Cells after Heat Stress
4.2.6. Intracellular Ca2+ Concentration in RAW 264.7 Cells after Heat Stress
4.2.7. Gene Expression
RNA Sequencing (Transcriptome)
4.2.8. Real-Time qPCR Analysis
- 95.0 °C for 15:00;
- 95.0 °C for 0:10;
- 62.0 °C for 0:30, Plate Read;
- GOTO 2, 59 more times;
- 95.0 °C for 0:10;
- Melt Curve 65.0 to 95.0 °C, Increments of 0.5 °C every 0:05;
- Plate Read.
| Gene | Forward primer | Reverse Primer |
| β-actin | CATTGCTGACAGGATGCAGAAGG | TGCTGGAAGGTGGACAGTGAGG |
| Nfkbia | GGTGACTTTGGGTGCTGAT | CTTGGTAGGTTACCCTGTTGAC |
| Ddit3 | TCCTGTCCTCAGATGAAATTGG | GCAGGGTCAAGAGTAGTGAAG |
| Gadd45a | CTGTGTGCTGGTGACGAA | GCACCCACTGATCCATGTAG |
| Akt3 | CAGAACGACCAAAGCCAAATAC | CTTCCGTCCACTCTTCTCTTTC |
| Hyou1 | CGCAAAGTCATCACCTTTAACC | GTCAGATTCTGGGAGCCAAATA |
| Cflar | CTGATTATAGGGTCCTGCTGATG | TTGCCTCTGCCTGTGTAATC |
4.2.9. Cytokines of IL-6 and IL-17A Production
4.3. In Vivo Experiment
4.3.1. Blood Biochemical and IL-10 Analysis
4.3.2. LC-MS Metabolite Analysis
4.3.3. Histological Analysis and Hepatic Glycogen Observation
4.4. Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, S.H.; Kim, Y.W.; Kim, H.B.; Lee, B.J.; Lee, D.S. Anti-apoptotic Activity of Laminarin Polysaccharides and their Enzymatically Hydrolyzed Oligosaccharides from Laminaria japonica. Biotechnol. Lett. 2006, 28, 439–446. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Xu, Y.; Chen, H.; Sun, P. Extraction, Structural Characterization, and Potential Antioxidant Activity of the Polysaccharides from Four Seaweeds. Int. J. Mol. Sci. 2016, 17, 1988. [Google Scholar] [CrossRef] [PubMed]
- Portner, H.O.; Langenbuch, M.; Michaelidis, B. Synergistic effects of temperature extremes, hypoxia, and increases in CO2 on marine animals: From Earth history to global change. J. Geophys. Res. Oceans. 2005, 110. [Google Scholar] [CrossRef]
- Firmino, M.; Weis, S.N.; Souza, J.M.; Gomes, B.R.; Mól, A.R.; Mortari, M.R.; Souza, G.E.P.; Coca, G.C.; Williams, T.C.R.; Fontes, W.; et al. Label-free quantitative proteomics of rat hypothalamus under fever induced by LPS and PGE2. J. Proteom. 2018, 187, 182–199. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.S.; Repasky, E.A.; Fisher, D.T. Fever and the thermal regulation of immunity: The immune system feels the heat. Nat. Rev. Immunol. 2015, 15, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, S.; Volodina, O.; Pearce, S.C.; Gabler, N.K.; Baumgard, L.H.; Rhoads, R.P.; Selsby, J.T. Acute heat stress activated inflammatory signaling in porcine oxidative skeletal muscle. Physiol. Rep. 2017, 5, e13397. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cells 2010, 140, 805–820. [Google Scholar] [CrossRef]
- Zeng, T.; Li, J.J.; Wang, D.Q.; Li, G.Q.; Wang, G.L.; Lu, L.Z. Effects of heat stress on antioxidant defense system, inflammatory injury, and heat shock proteins of Muscovy and Pekin ducks: Evidence for differential thermal sensitivities. Cell Stress Chaperones 2014, 19, 895–901. [Google Scholar] [CrossRef]
- Montilla, S.I.R.; Johnson, T.P.; Pearce, S.C.; Gardan-Salmon, D.; Gabler, N.K.; Ross, J.W.; Rhoads, R.P.; Baumgard, L.H.; Lonergan, S.M.; Selsby, J.T. Heat stress causes oxidative stress but not inflammatory signaling in porcine skeletal muscle. Temperature 2014, 17, 42–50. [Google Scholar] [CrossRef]
- Quinteiro-Filho, W.M.; Gomes, A.V.S.; Pinheiro, M.L.; Ribeiro, A.; Ferraz-De-Paula, V.; Astolfi-Ferreira, C.S.; Ferreira, A.J.P.; Palermo-Neto, J. Heat stress impairs performance and induces intestinal inflammation in broiler chickens infected with Salmonella enteritidis. Avian Pathol. 2012, 41, 421–427. [Google Scholar] [CrossRef]
- Furusawa, Y.; Yamanouchi, Y.; Iizumi, T.; Zhao, Q.L.; Mitsuhashi, Y.; Morita, A.; Enomoto, A.; Tabuchi, Y.; Kondo, T. Checkpoint kinase 2 is dispensable for regulation of the p53 response but is required for G2/M arrest and cell survival in cells with p53 defects under heat stress. Apoptosis 2017, 22, 1225–1234. [Google Scholar] [CrossRef]
- Ganesan, S.; Pearce, S.C.; Gabler, N.K.; Baumgard, L.H.; Rhoads, R.P.; Selsby, J.T. Short-term heat stress results in increased apoptotic signaling and autophagy in oxidative skeletal muscle in Sus scrofa. J. Therm. Biol. 2018, 72, 73–80. [Google Scholar] [CrossRef]
- Ganesan, S.; Summers, C.M.; Pearce, S.C.; Gabler, N.K.; Valentine, R.J.; Baumgard, L.H.; Rhoads, R.P.; Selsby, J.T. Short-term heat stress causes altered intracellular signaling in oxidative skeletal muscle. J. Anim. Sci. 2017, 95, 2438–2451. [Google Scholar] [CrossRef]
- Park, S.; Lim, Y.; Lee, D.; Elvira, R.; Lee, J.M.; Lee, M.R.; Han, J. Modulation of protein synthesis by eIF2α Phosphorylation protects cell from heat stress-mediated apoptosis. Cells 2018, 7, 254. [Google Scholar] [CrossRef]
- Sarkar, S.A.; Kutlu, B.; Velmurugan, K.; Kizaka-Kondoh, S.; Lee, C.E.; Wong, R.; Valentine, A.; Davidson, H.W.; Hutton, J.C.; Pugazhenthi, S. Cytokine-mediated induction of anti-apoptotic genes that are linked to nuclear factor kappa-B (NF-κB) signalling in human islets and in a mouse beta cell line. Diabetologia 2009, 52, 1092–1101. [Google Scholar] [CrossRef][Green Version]
- Chae, J.J.; Komarow, H.D.; Cheng, J.; Wood, G.; Raben, N.; Liu, P.P.; Kastner, D.L. Targeted disruption of pyrin, the FMF protein, causes heightened sensitivity to endotoxin and a defect in macrophage apoptosis. Mol. Cell 2003, 11, 591–604. [Google Scholar] [CrossRef]
- Wu, T.M.; Nan, F.H.; Chen, K.C.; Wu, Y.S. Sarcodia suieae acetyl-xylogalactan regulate RAW 264.7 macrophage NF-kappa B activation and IL-1 beta cytokine production in macrophage polarization. Sci. Rep. 2019, 9, 19627. [Google Scholar] [CrossRef]
- Rizzuto, R.; Pinton, P.; Ferrari, D.; Chami, M.; Szabadkai, G.; Magalhaes, P.J.; Virgilio, F.D.; Pozzan, T. Calcium and apoptosis: Facts and hypotheses. Oncogene 2003, 22, 8619–8627. [Google Scholar] [CrossRef]
- Zhou, D.; Yin, D.; Xiao, F.; Hao, J. Expressions of senescence-associated β-galactosidase and senescence marker protein-30 are associated with lens epithelial cell apoptosis. Med. Sci. Monit. 2015, 21, 3728–3735. [Google Scholar] [CrossRef]
- Zhang, X.; Ren, A.; Li, M.J.; Cao, P.F.; Chen, T.X.; Zhang, G.; Shi, L.; Jiang, A.L.; Zhao, M.W. Heat stress modulates mycelium growth, heat shock protein expression, ganoderic acid biosynthesis, and hyphal branching of Ganoderma lucidum via cytosolic Ca2+. Appl. Environ. Microbiol. 2016, 82, 4112–4125. [Google Scholar] [CrossRef]
- Slawinska, A.; Hsieh, J.; Schmidt, C.J.; Lamont, S.J. Heat stress and lipopolysaccharide stimulation of chicken macrophage-like cell line activates expression of distinct sets of genes. PLoS ONE 2016, 11, e0164575. [Google Scholar] [CrossRef] [PubMed]
- Moine, L.; De-Barboza, G.D.; Pérez, A.; Benedetto, M.; De-Talamoni, N.T. Glutamine protects intestinal calcium absorption against oxidative stress and apoptosis. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2017, 212, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Catozzi, C.; Ñvila, G.; Zamarian, V.; Pravettoni, D.; Sala, G.; Ceciliani, F.; Lacetera, N.; Lecchi, C. In-vitro effect of heat stress on bovine monocytes lifespan and polarization. Immunobiology 2020, 225, 151888. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Meng, X.W.; Ma, J.; Liu, H.; Song, S.Y.; Chen, Q.C.; Liu, H.Y.; Zhang, J.; Song, N.; Ji, F.H.; et al. Dexmedetomidine protects H9c2 cardiomyocytes against oxygen-glucose deprivation/reoxygenation-induced intracellular calcium overload and apoptosis through regulating FKBP12. 6/RyR2 signaling. Drug Des. Dev. Ther. 2019, 2, 3137–3149. [Google Scholar] [CrossRef]
- Li, L.; Su, Z.; Zou, Z.; Tan, H.; Cai, D.; Su, L.; Gu, Z. Ser46 phosphorylation of p53 is an essential event in prolyl-isomerase Pin1-mediated p53-independent apoptosis in response to heat stress. Cell Death Dis. 2019, 10, 96. [Google Scholar] [CrossRef]
- Daigeler, A.; Brenzelm, C.; Bulut, D.; Geisler, A.; Hilgert, C.; Lehnhardt, M.; Steinau, H.U.; Flier, A.; Steinstraesser, L.; Klein-Hitpass Mittelkötte, U.; et al. TRAIL and Taurolidine induce apoptosis and decrease proliferation in human fibrosarcoma. J. Exp. Clin. Cancer Res. 2008, 27, 82. [Google Scholar] [CrossRef]
- Li, T.; Su, L.; Liu, X.; Zhang, Y.; Liu, X. DDIT3 and KAT2A proteins regulate TNFRSF10A and TNFRSF10B expression in endoplasmic reticulum stress-mediated apoptosis in human lung cancer cells. J. Biol. Chem. 2015, 290, 11108–11118. [Google Scholar] [CrossRef]
- Lurlaro, R.; Muñoz-Pinedo, C. Cell death induced by endoplasmic reticulum stress. FEBS J. 2016, 283, 2640–2652. [Google Scholar] [CrossRef]
- Syc-Mazurek, S.B.; Fernandes, K.A.; Wilson, M.P.; Shrager, P.; Libby, R.T. Together JUN and DDIT3 (CHOP) control retinal ganglion cell death after axonal injury. Mol. Neurodegener. 2017, 12, 71. [Google Scholar] [CrossRef]
- He, M.X.; He, Y.W. CFLAR/c-FLIPL: A star in the autophagy, apoptosis and necroptosis alliance. Autophagy 2013, 9, 791–793. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, Y.; Nakabayashi, O.; Nakano, H. FLIP the Switch: Regulation of Apoptosis and Necroptosis by cFLIP. Int. J. Mol. Sci. 2015, 16, 30321–30341. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Y.; Zhang, A.; Ma, J.; Wang, Z.; Zhang, X. Targeting of miR-20a against CFLAR to potentiate TRAIL-induced apoptotic sensitivity in HepG2 cells. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 2087–2097. [Google Scholar] [PubMed]
- Choi, J.A.; Lim, Y.J.; Cho, S.N.; Lee, J.H.; Jeong, J.A.; Kim, E.J.; Park, J.B.; Kim, S.H.; Park, H.S.; Kim, H.J.; et al. Mycobacterial HBHA induces endoplasmic reticulum stress-mediated apoptosis through the generation of reactive oxygen species and cytosolic Ca 2+ in murine macrophage RAW 264.7 cells. Cell Death Dis. 2013, 4, e957. [Google Scholar] [CrossRef] [PubMed]
- Orosz, L.; Papanicolaou, E.G.; Seprényi, G.; Megyeri, K. IL-17A and IL-17F induce autophagy in RAW 264.7 macrophages. Biomed. Pharmacother. 2016, 77, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Abdelrhman, A.M.; Ashour, M.; Al-Zahaby, M.A.; Sharawy, Z.Z.; Nazmi, H.; Zaki, M.A.; Ahmed, N.H.; Ahmed, S.R.; El-Haroun, E.; Van Doan, H.; et al. Effect of polysaccharides derived from brown macroalgae Sargassum dentifolium on growth performance, serum biochemical, digestive histology and enzyme activity of hybrid red tilapia. Aquac. Rep. 2022, 25, 101212. [Google Scholar] [CrossRef]
- Adel, M.; Pourgholam, R.; Zorriehzahra, J.; Ghiasi, M. Hemato–Immunological and biochemical parameters, skin antibacterial activity, and survival in rainbow trout (Oncorhynchus mykiss) following the diet supplemented with Mentha piperita against Yersinia ruckeri. Fish Shellfish Immunol. 2016, 55, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.K.; Choi, K.H.; Oh, J.N.; Kim, S.H.; Lee, M.; Jeong, J.; Choe, G.C.; Lee, C.K. Linoleic acid reduces apoptosis via NF-κB during the in vitro development of induced parthenogenic porcine embryos. Theriogenology 2022, 187, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Pearman, A.T.; Zimmerman, G.A.; McIntyre, T.M.; Prescott, S.M. Intracellular unesterified arachidonic acid signals apoptosis. PNAS 2000, 97, 11280–11285. [Google Scholar] [CrossRef]
- Penzo, D.; Petronilli, V.; Angelin, A.; Cusan, C.; Colonna, R.; Scorrano, L.; Pagano, F.; Prato, M.; Di Lisa, F.; Bernardi, P. Arachidonic acid released by phospholipase A2 activation triggers Ca2+-dependent apoptosis through the mitochondrial pathway. J. Biol. Chem. 2004, 279, 25219–25225. [Google Scholar] [CrossRef]
- Zhang, F.Y.; Li, R.Z.; Xu, C.; Fan, X.X.; Li, J.X.; Meng, W.Y.; Wang, X.R.; Liang, T.L.; Guan, X.X.; Pan, H.D.; et al. Emodin induces apoptosis and suppresses non-small-cell lung cancer growth via downregulation of sPLA2-IIa. Phytomedicine 2022, 95, 153786. [Google Scholar] [CrossRef] [PubMed]
- Caro, A.A.; Cederbaum, A.I. Role of cytochrome P450 in phospholipase A2-and arachidonic acid-mediated cytotoxicity. Free Radic. Biol. Med. 2006, 40, 364–375. [Google Scholar] [CrossRef] [PubMed]

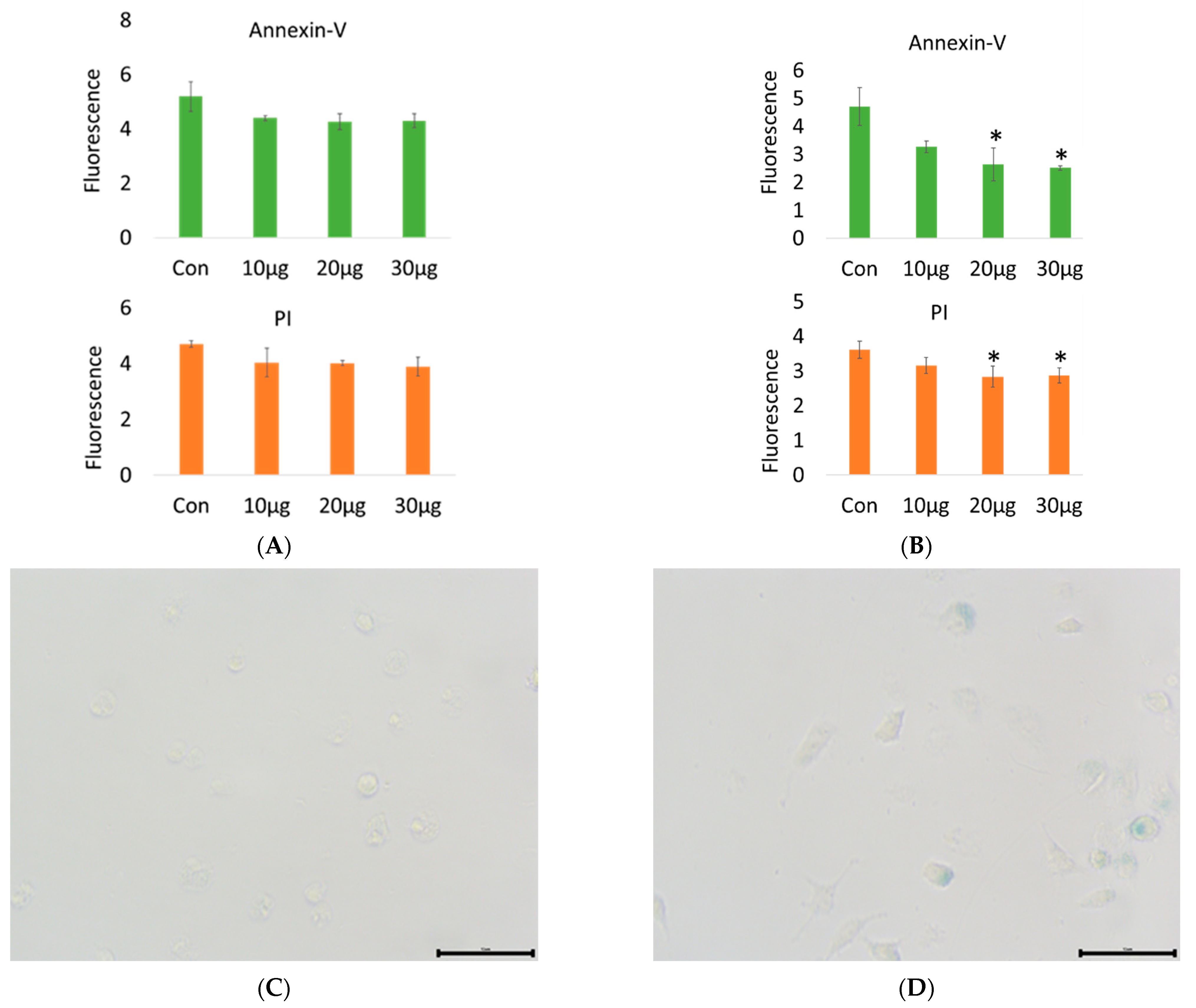
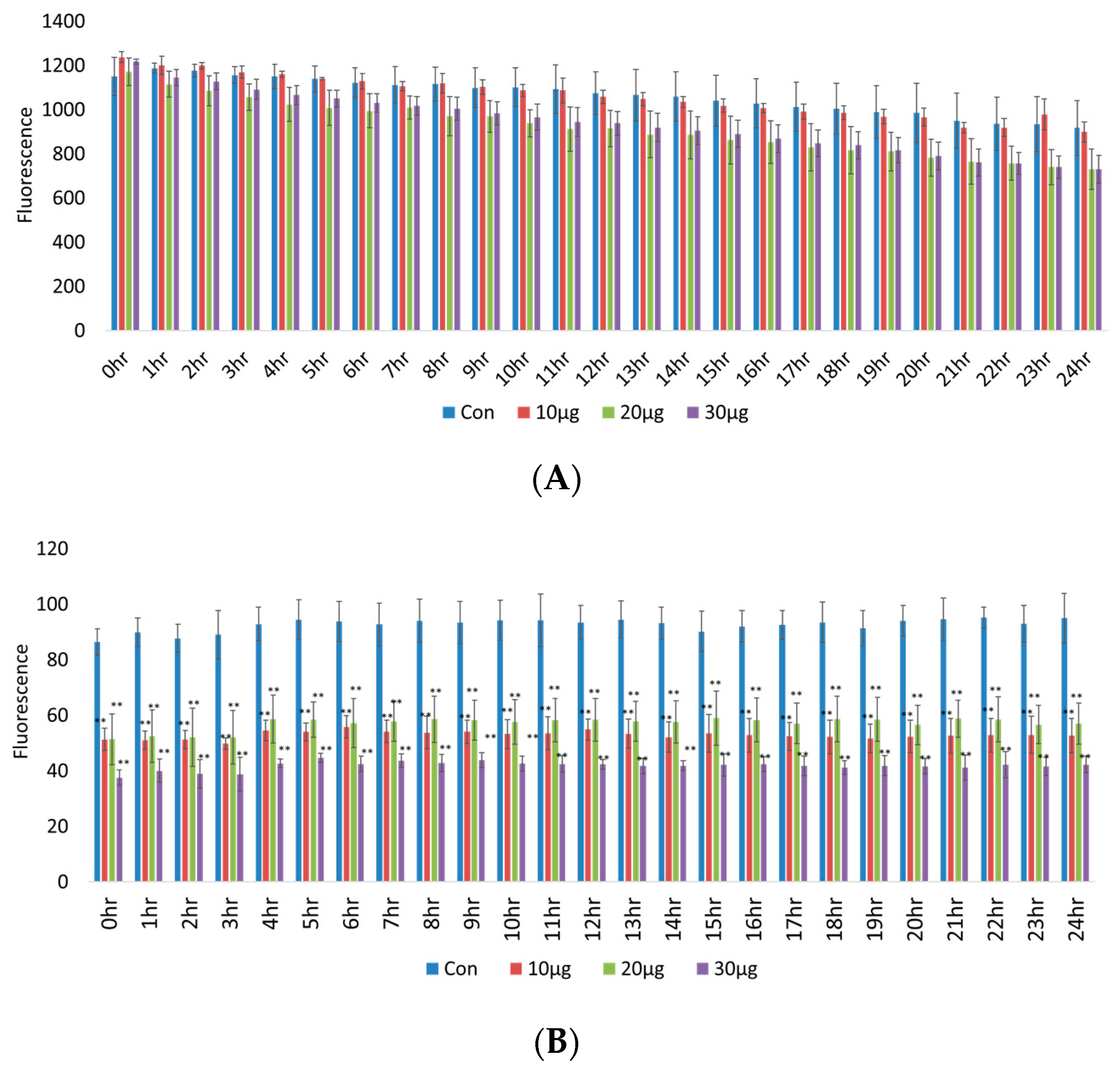
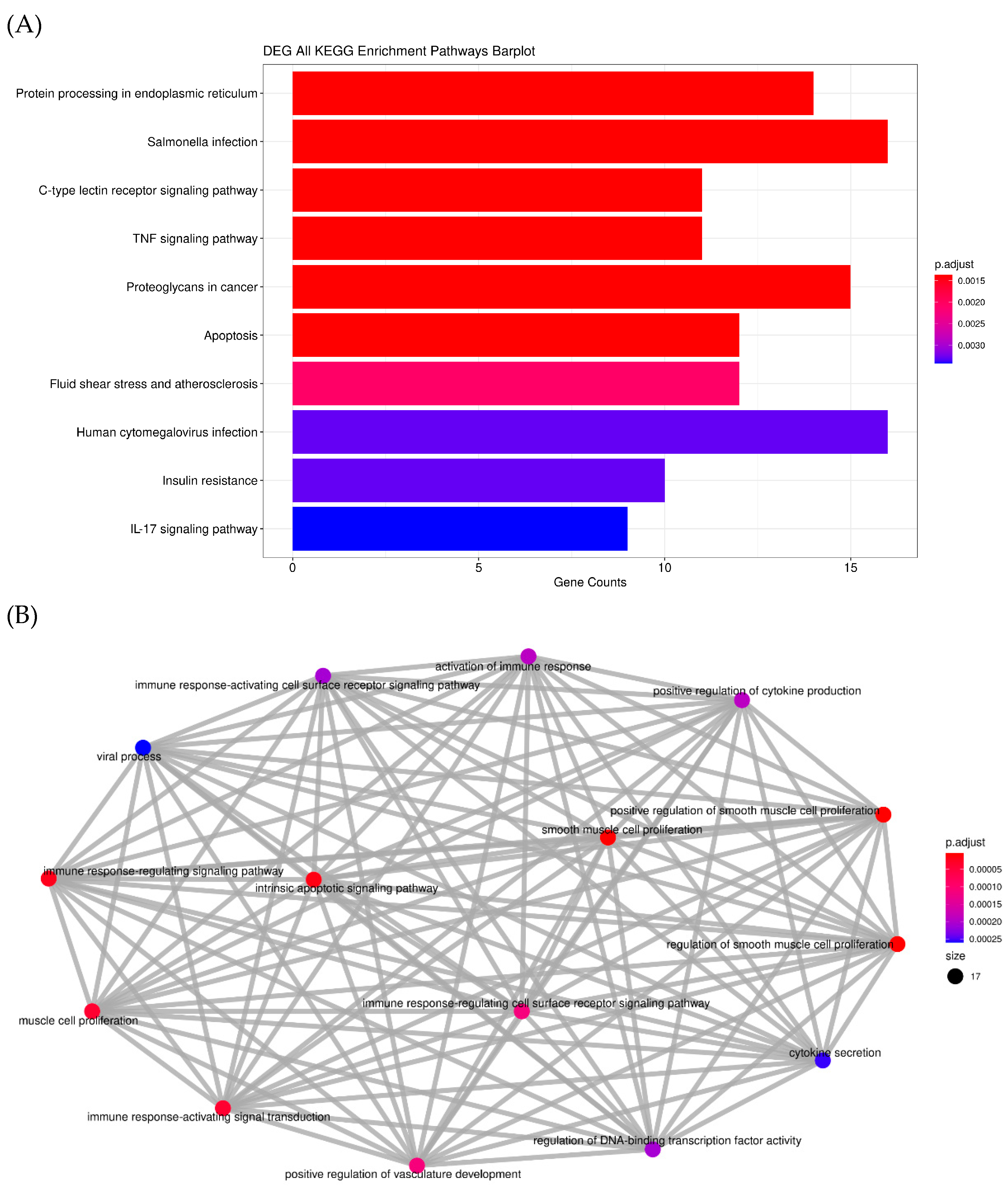
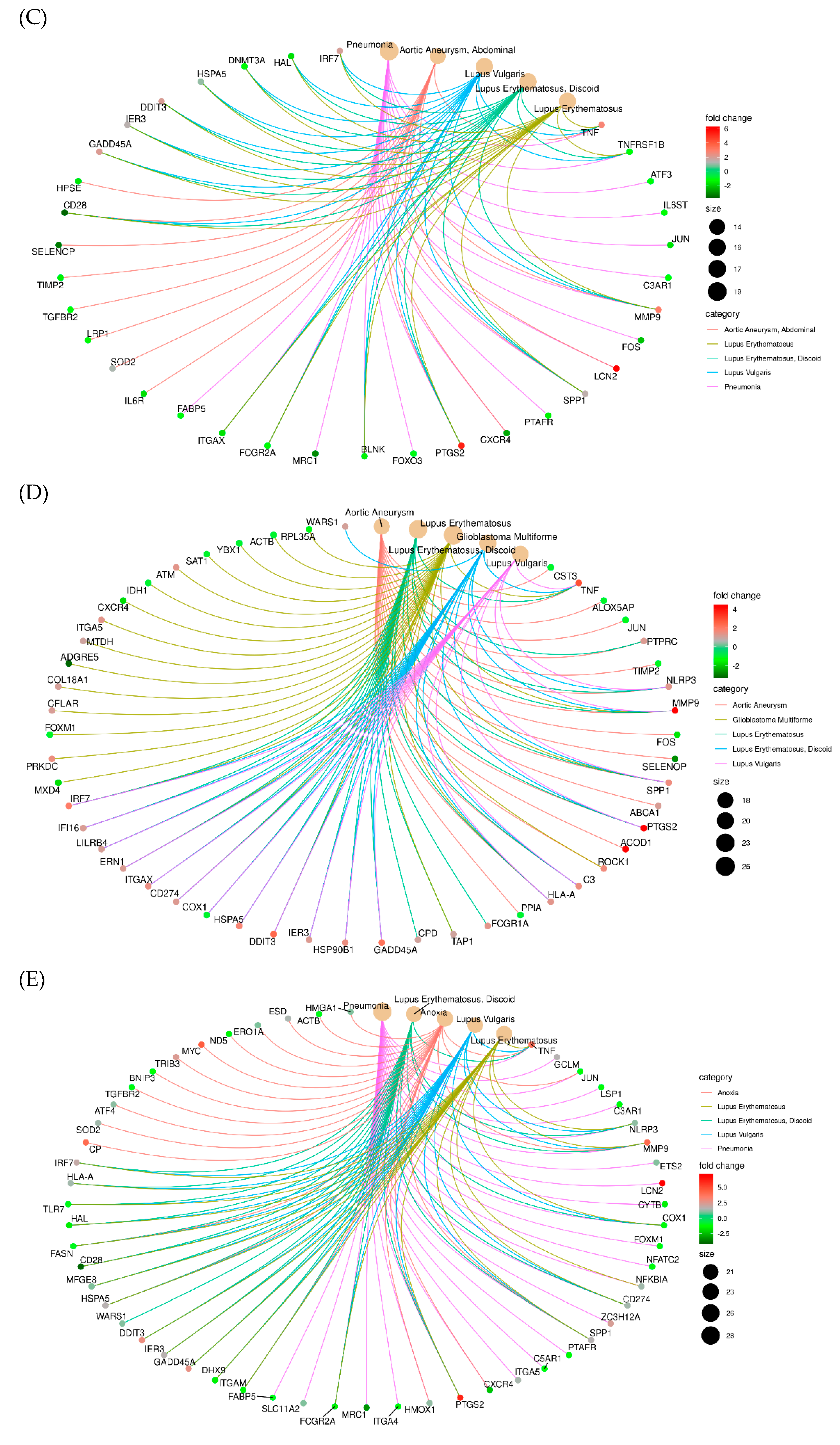

| Item | Glu | T-Cho | BUN | T-Bil | GOT | GPT | T-Pro | Alb |
| Unit | mg/dL | mg/dL | mg/dL | mg/dL | IU/L | IU/L | g/dL | g/dL |
| 25 °C | 318 ± 46.81 | 115 ± 4.16 | 5 ± 0 | 13.5 ± 0.10 | 56 ± 14.19 | 22 ± 11.14 | 10.87 ± 0.23 | 3.02 ± 0.26 |
| 35 °C—0 g/Kg-Feed | 333 ± 8.33 | 120 ± 2.00 | 5 ± 0 | 14.07 ± 0.15 | 51 ± 11.53 | 11 ± 1.15 | 9.67 ± 2.31 | 2.87 ± 0.76 |
| 35 °C—10 g/Kg-Feed | 381 ± 7.81 | 127 ± 10.07 | 5 ± 0 | 15.23 ± 0.38 * | 95 ± 11.50 | 28 ± 12.77 | 11 ± 0.00 | 3.27 ± 0.21 |
| 35 °C—20 g/Kg-Feed | 279 ± 57.36 | 132 ± 19.35 | 5 ± 0 | 13.13 ± 1.10 | 101 ± 34.43 | 30 ± 7.51 | 9.8 ± 1.51 | 2.93 ± 0.55 |
| 35 °C—30 g/Kg-Feed | 324 ± 28.10 | 124 ± 14.93 | 5 ± 0 | 13.8 ± 0.10 | 47 ± 4.93 | 13 ± 3.00 | 10.87 ± 0.23 | 3.27 ± 0.15 |
| Item | Ca | TG | UA | LDH | Alb/T-Pro | CPK | ALP | Mg |
| Unit | mg/dL | mg/dL | mg/dL | IU/L | Ratio | IU/L | IU/L | mg/dL |
| 25 ℃ | 20 ± 0.00 | 112 ± 61.61 | 20 ± 0.00 | 4000 ± 0.00 | 0.29 ± 0.02 | 2000 ± 0 | 79.67 ± 11.55 | 3.13 ± 0.15 |
| 35 °C—0 g/Kg-Feed | 20 ± 0.00 | 70 ± 21.08 | 20 ± 0.00 | 4000 ± 0.00 | 0.30 ± 0.01 | 2000 ± 0 | 86.33 ± 16.86 | 3.30 ± 0 |
| 35 °C—10 g/Kg-Feed | 20 ± 0.00 | 75 ± 10.82 | 20 ± 0.00 | 4000 ± 0.00 | 0.30 ± 0.02 | 1765 ± 407.03 | 91 ± 21.79 | 3.67 ± 0.51 |
| 35 °C—20 g/Kg-Feed | 20 ± 0.69 | 122 ± 7.57 | 20 ± 0.00 | 3169 ± 1439.33 | 0.30 ± 0.01 | 2000 ± 0 | 68 ± 16.70 | 3.47 ± 0.21 |
| 35 °C—30 g/Kg-Feed | 20 ± 0.00 | 106 ± 42.93 | 20 ± 0.00 | 4000 ± 0.00 | 0.30 ± 0.66 | 1993 ± 10.97 | 113 ± 35.93 | 3.73 ± 0.49 |
| Item | HDL-c | Amy | IP | GGT | Cre | FRA | IL-10 | |
| Unit | mg/dL | IU/L | mg/dL | IU/L | mg/dL | umol/L | ng/L | |
| 25 °C | 108 ± 8.89 | 18.33 ± 0.58 | 16.5 ± 0.53 | 10.67 ± 1.15 | 0.3 ± 0 | 88 ± 4 | ND. | |
| 35 °C—0 g/Kg-Feed | 129 ± 8.08 | 10 ± 0 * | 16.1 ± 0.82 | 10 ± 0 | 0.3 ± 0 | 84 ± 7.51 | ND. | |
| 35 °C—10 g/Kg-Feed | 100 ± 17.90 | 10 ± 0 * | 19.3 ± 1.21 | 10 ± 0 | 0.33 ± 0.06 | 87 ± 3.61 | ND. | |
| 35 °C—20 g/Kg-Feed | 116 ± 9.29 | 16.33 ± 3.79 | 14.33 ± 2.12 | 10 ± 0 | 0.3 ± 0 | 87 ± 4.93 | ND. | |
| 35 °C—30 g/Kg-Feed | 102 ± 8.96 | 12.33 ± 4.04 | 17.27 ± 3.37 | 12 ± 1.73 | 0.3 ± 0 | 91 ± 8.50 | ND. |
| 35 °C—0 g/Kg-Feed vs. 25 °C | |||||
| featureID | CpdName | Score | KEGGID | pvalue | log2foldchange |
| FT0730 | Ginkgolide A | 0.61611 | 0.027372015 | −1.290974201 | |
| FT0213 | 2-Hydroxyethanesulfonate | 0.99537 | C05123 | 0.015537504 | −0.799873206 |
| FT0013 | Phosphoenolpyruvic acid | 0.99915 | C00074 | 0.012554802 | 1.155737014 |
| 35 °C—10 g/Kg-Feed vs. 25 °C | |||||
| featureID | CpdName | Score | KEGGID | pvalue | log2foldchange |
| FT0213 | 2-Hydroxyethanesulfonate | 0.99537 | C05123 | 0.02122731 | −0.552585532 |
| FT0730 | Ginkgolide A | 0.61611 | 0.031505868 | −1.258174039 | |
| 35 °C—20 g/Kg-Feed vs. 25 °C | |||||
| featureID | CpdName | Score | KEGGID | pvalue | log2foldchange |
| FT0730 | Ginkgolide A | 0.61611 | 0.012228984 | −2.056046835 | |
| FT1562 | Arachidonic acid | 0.99801 | C00219 | 0.017941797 | −0.883800718 |
| 35 °C—30 g/Kg-Feed vs. 25 °C | |||||
| featureID | CpdName | Score | KEGGID | pvalue | log2foldchange |
| FT0507 | S-Lactoylglutathione | 0.70373 | C03451 | 0.043343983 | −0.754247254 |
| FT1562 | Arachidonic acid | 0.99801 | C00219 | 0.039958685 | −0.950275021 |
| FT0730 | Ginkgolide A | 0.61611 | 0.015836172 | −2.033361388 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, P.-K.; Wang, K.-T.; Nan, F.-H.; Wu, T.-M.; Wu, Y.-S. Red Algae “Sarcodia suieae” Acetyl-Xylogalactan Downregulate Heat-Induced Macrophage Stress Factors Ddit3 and Hyou1 Compared to the Aquatic Animal Model of Nile Tilapia (Oreochromis niloticus) Brain Arachidonic Acid Expression. Int. J. Mol. Sci. 2022, 23, 14662. https://doi.org/10.3390/ijms232314662
Pan P-K, Wang K-T, Nan F-H, Wu T-M, Wu Y-S. Red Algae “Sarcodia suieae” Acetyl-Xylogalactan Downregulate Heat-Induced Macrophage Stress Factors Ddit3 and Hyou1 Compared to the Aquatic Animal Model of Nile Tilapia (Oreochromis niloticus) Brain Arachidonic Acid Expression. International Journal of Molecular Sciences. 2022; 23(23):14662. https://doi.org/10.3390/ijms232314662
Chicago/Turabian StylePan, Po-Kai, Kuang-Teng Wang, Fan-Hua Nan, Tsung-Meng Wu, and Yu-Sheng Wu. 2022. "Red Algae “Sarcodia suieae” Acetyl-Xylogalactan Downregulate Heat-Induced Macrophage Stress Factors Ddit3 and Hyou1 Compared to the Aquatic Animal Model of Nile Tilapia (Oreochromis niloticus) Brain Arachidonic Acid Expression" International Journal of Molecular Sciences 23, no. 23: 14662. https://doi.org/10.3390/ijms232314662
APA StylePan, P.-K., Wang, K.-T., Nan, F.-H., Wu, T.-M., & Wu, Y.-S. (2022). Red Algae “Sarcodia suieae” Acetyl-Xylogalactan Downregulate Heat-Induced Macrophage Stress Factors Ddit3 and Hyou1 Compared to the Aquatic Animal Model of Nile Tilapia (Oreochromis niloticus) Brain Arachidonic Acid Expression. International Journal of Molecular Sciences, 23(23), 14662. https://doi.org/10.3390/ijms232314662






