Glucagon-like Peptide-1 Receptor in the Human Hypothalamus Is Associated with Body Mass Index and Colocalizes with the Anorexigenic Neuropeptide Nucleobindin-2/Nesfatin-1
Abstract
:1. Introduction
2. Results
2.1. Distribution of GLP-1R Immunoreactivity in the Human Hypothalamus Is Different between Normal Weight and Overweight or Obese Subjects
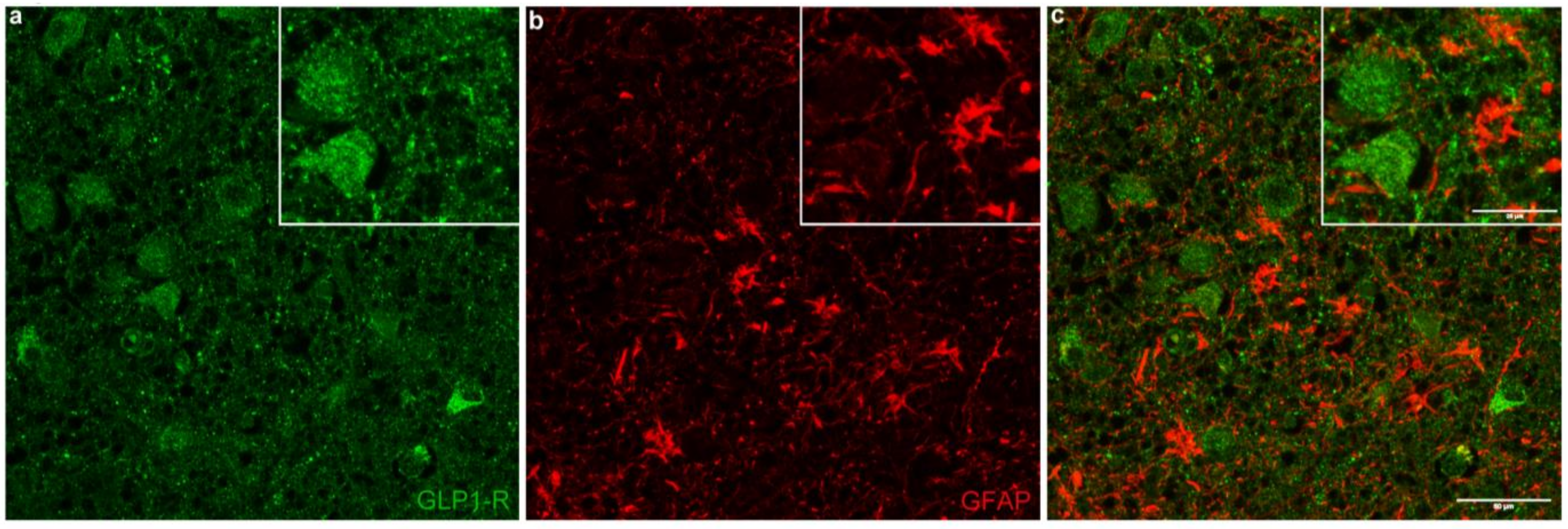
2.2. GLP-1R Colocalizes with the Anorexigenic and Antiobesogenic Neuropeptide NUCB2/Nesfatin-1 but Not with the Astrocytic Marker GFAP
3. Discussion
4. Materials and Methods
4.1. Tissue Collection
4.2. Histochemistry
4.3. Immunohistochemistry
4.4. Double Immunofluorescence Labeling
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andersen, A.; Lund, A.; Knop, F.K.; Vilsbøll, T. Glucagon-like peptide 1 in health and disease. Nat. Rev. Endocrinol. 2018, 14, 390–403. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.D.; Finan, B.; Bloom, S.R.; D’Alessio, D.; Drucker, D.J.; Flatt, P.R.; Fritsche, A.; Gribble, F.; Grill, H.J.; Habener, J.F.; et al. Glucagon-like peptide 1 (GLP-1). Mol. Metab. 2019, 30, 72–130. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, E.; Martinez, M.D.; Roncero, I.; Chowen, J.A.; Garcia-Cuartero, B.; Gispert, J.D.; Sanz, C.; Vazquez, P.; Maldonado, A.; De Caceres, J.; et al. The expression of GLP-1 receptor mRNA and protein allows the effect of GLP-1 on glucose metabolism in the human hypothalamus and brainstem. J. Neurochem. 2005, 92, 798–806. [Google Scholar] [CrossRef]
- Ten Kulve, J.S.; van Bloemendaal, L.; Balesar, R.; IJzerman, R.G.; Swaab, D.F.; Diamant, M.; la Fleur, S.E.; Alkemade, A. Decreased Hypothalamic Glucagon-Like Peptide-1 Receptor Expression in Type 2 Diabetes Patients. J. Clin. Endocrinol. Metab. 2016, 101, 2122–2129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farr, O.M.; Sofopoulos, M.; Tsoukas, M.A.; Dincer, F.; Thakkar, B.; Sahin-Efe, A.; Filippaios, A.; Bowers, J.; Srnka, A.; Gavrieli, A.; et al. GLP-1 receptors exist in the parietal cortex, hypothalamus and medulla of human brains and the GLP-1 analogue liraglutide alters brain activity related to highly desirable food cues in individuals with diabetes: A crossover, randomised, placebo-controlled trial. Diabetologia 2016, 59, 954–965. [Google Scholar]
- Reiner, D.J.; Mietlicki-Baase, E.G.; McGrath, L.E.; Zimmer, D.J.; Bence, K.K.; Sousa, G.L.; Konanur, V.R.; Krawczyk, J.; Burk, D.H.; Kanoski, S.E.; et al. Astrocytes Regulate GLP-1 Receptor-Mediated Effects on Energy Balance. J. Neurosci. 2016, 36, 3531–3540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chowen, J.A.; de Fonseca, F.R.; Alvarez, E.; Navarro, M.; García-Segura, L.M.; Blázquez, E. Increased glucagon-like peptide-1 receptor expression in glia after mechanical lesion of the rat brain. Neuropeptides 1999, 33, 212–215. [Google Scholar] [CrossRef] [Green Version]
- Iwai, T.; Ito, S.; Tanimitsu, K.; Udagawa, S.; Oka, J. Glucagon-like peptide-1 inhibits LPS-induced IL-1beta production in cultured rat astrocytes. Neurosci. Res. 2006, 55, 352–360. [Google Scholar] [CrossRef]
- Bułdak, Ł.; Machnik, G.; Skudrzyk, E.; Bołdys, A.; Okopień, B. The impact of exenatide (a GLP-1 agonist) on markers of inflammation and oxidative stress in normal human astrocytes subjected to various glycemic conditions. Exp. Ther. Med. 2019, 17, 2861–2869. [Google Scholar] [CrossRef] [Green Version]
- Graham, D.L.; Durai, H.H.; Trammell, T.S.; Noble, B.L.; Mortlock, D.P.; Galli, A.; Stanwood, G.D. A novel mouse model of glucagon-like peptide-1 receptor expression: A look at the brain. J. Comp. Neurol. 2020, 528, 2445–2470. [Google Scholar] [CrossRef]
- Van Bloemendaal, L.; IJzerman, R.G.; Ten Kulve, J.S.; Barkhof, F.; Konrad, R.J.; Drent, M.L.; Veltman, D.J.; Diamant, M. GLP-1 receptor activation modulates appetite- and reward-related brain areas in humans. Diabetes 2014, 63, 4186–4196. [Google Scholar] [CrossRef] [PubMed]
- Plamboeck, A.; Veedfald, S.; Deacon, C.; Hartmann, B.; Wettergren, A.; Svendsen, L.B.; Meisner, S.; Hovendal, C.; Vilsbøll, T.; Knop, F.K.; et al. The effect of exogenous GLP-1 on food intake is lost in male truncally vagotomized subjects with pyloroplasty. Am. J. Physiol.-Gastrointest. Liver Physiol. 2013, 304, G1117–G1127. [Google Scholar] [CrossRef] [PubMed]
- Oh-I, S.; Shimizu, H.; Satoh, T.; Okada, S.; Adachi, S.; Inoue, K.; Eguchi, H.; Yamamoto, M.; Imaki, T.; Hashimoto, K.; et al. Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature 2006, 443, 709–712. [Google Scholar] [CrossRef] [PubMed]
- Stengel, A.; Goebel, M.; Taché, Y. Nesfatin-1: A novel inhibitory regulator of food intake and body weight. Obes. Rev. 2011, 12, 261–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dore, R.; Levata, L.; Lehnert, H.; Schulz, C. Nesfatin-1: Functions and physiology of a novel regulatory peptide. J. Endocrinol. 2017, 232, R45–R65. [Google Scholar] [CrossRef] [Green Version]
- Psilopanagioti, A.; Nikou, S.; Papadaki, H. Nucleobindin-2/nesfatin-1 in the human hypothalamus is reduced in obese subjects and colocalizes with oxytocin, vasopressin, melanin-concentrating hormone, and cocaine- and amphetamine-regulated transcript. Neuroendocrinology 2019, 108, 190–200. [Google Scholar] [CrossRef]
- Kras, K.; Muszyński, S.; Tomaszewska, E.; Arciszewski, M.B. Minireview: Peripheral Nesfatin-1 in Regulation of the Gut Activity-15 Years since the Discovery. Animals 2022, 12, 101. [Google Scholar] [CrossRef]
- Kmiecik, A.M.; Dzięgiel, P.; Podhorska-Okołów, M. Nucleobindin-2/Nesfatin-1-A New Cancer Related Molecule? Int. J. Mol. Sci. 2021, 22, 8313. [Google Scholar] [CrossRef]
- Katsurada, K.; Maejima, Y.; Nakata, M.; Kodaira, M.; Suyama, S.; Iwasaki, Y.; Kario, K.; Yada, T. Endogenous GLP-1 acts on paraventricular nucleus to suppress feeding: Projection from nucleus tractus solitarius and activation of corticotropin-releasing hormone, nesfatin-1 and oxytocin neurons. Biochem. Biophys. Res. Commun. 2014, 451, 276–281. [Google Scholar] [CrossRef]
- Katsurada, K.; Yada, T. Neural effects of gut- and brain-derived glucagon-like peptide-1 and its receptor agonist. J. Diabetes Investig. 2016, 7 (Suppl. S1), 64–69. [Google Scholar] [CrossRef] [Green Version]
- Saito, R.; So, M.; Motojima, Y.; Matsuura, T.; Yoshimura, M.; Hashimoto, H.; Yamamoto, Y.; Kusuhara, K.; Ueta, Y. Activation of Nesfatin-1-Containing Neurones in the Hypothalamus and Brainstem by Peripheral Administration of Anorectic Hormones and Suppression of Feeding via Central Nesfatin-1 in Rats. J. Neuroendocrinol. 2016, 28. [Google Scholar] [CrossRef]
- Brown, E.; Wilding, J.P.H.; Barber, T.M.; Alam, U.; Cuthbertson, D.J. Weight loss variability with SGLT2 inhibitors and GLP-1 receptor agonists in type 2 diabetes mellitus and obesity: Mechanistic possibilities. Obes. Rev. 2019, 20, 816–828. [Google Scholar] [CrossRef] [Green Version]
- Swaab, D.F. Part I: Nuclei of the human hypothalamus: Cytoarchitecture, chemoarchitecture, functional neuroanatomy, topographic neuropathology. In Handbook of Clinical Neurology. The Human Hypothalamus: Basic and Clinical Aspects; Aminoff, M.J., Boller, F., Swaab, D.F., Eds.; Elsevier: Amsterdam, The Netherlands, 2003; Volume 79, pp. 3–59. [Google Scholar]
- Göke, R.; Larsen, P.J.; Mikkelsen, J.D.; Sheikh, S.P. Distribution of GLP-1 binding sites in the rat brain: Evidence that exendin-4 is a ligand of brain GLP-1 binding sites. Eur. J. Neurosci. 1995, 7, 2294–2300. [Google Scholar] [CrossRef]
- Merchenthaler, I.; Lane, M.; Shughrue, P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J. Comp. Neurol. 1999, 403, 261–280. [Google Scholar] [CrossRef]
- Richards, P.; Parker, H.E.; Adriaenssens, A.E.; Hodgson, J.M.; Cork, S.C.; Trapp, S.; Gribble, F.M.; Reimann, F. Identification and characterization of GLP-1 receptor-expressing cells using a new transgenic mouse model. Diabetes 2014, 63, 1224–1233. [Google Scholar] [CrossRef] [Green Version]
- Cork, S.C.; Richards, J.E.; Holt, M.K.; Gribble, F.M.; Reimann, F.; Trapp, S. Distribution and characterisation of Glucagon-like peptide-1 receptor expressing cells in the mouse brain. Mol. Metab. 2015, 4, 718–731. [Google Scholar] [CrossRef] [Green Version]
- Shugrue, P.J.; Lane, M.; Merchenthaler, I. Glucagon-like peptide-1 receptor (GLP1-R) mRNA in the rat hypothalamus. Endocrinology 1996, 137, 5159–5162. [Google Scholar] [CrossRef] [Green Version]
- Jensen, C.B.; Pyke, C.; Rasch, M.G.; Dahl, A.B.; Knudsen, L.B.; Secher, A. Characterization of the Glucagonlike Peptide-1 Receptor in Male Mouse Brain Using a Novel Antibody and In Situ Hybridization. Endocrinology 2018, 159, 665–675. [Google Scholar] [CrossRef] [Green Version]
- Heppner, K.M.; Kirigiti, M.; Secher, A.; Paulsen, S.J.; Buckingham, R.; Pyke, C.; Knudsen, L.B.; Vrang, N.; Grove, K.L. Expression and distribution of glucagon-like peptide-1 receptor mRNA, protein and binding in the male nonhuman primate (Macaca mulatta) brain. Endocrinology 2015, 156, 255–267. [Google Scholar] [CrossRef] [Green Version]
- Van Bloemendaal, L.; Ten Kulve, J.S.; la Fleur, S.E.; Ijzerman, R.G.; Diamant, M. Effects of glucagon-like peptide 1 on appetite and body weight: Focus on the CNS. J. Endocrinol. 2014, 221, T1–T16. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.; François, M.; Huesing, C.; Münzberg, H. The Hypothalamic Preoptic Area and Body Weight Control. Neuroendocrinology 2018, 106, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Lebow, M.A.; Chen, A. Overshadowed by the amygdala: The bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Mol. Psychiatry 2016, 21, 450–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vann, S.D.; Aggleton, J.P. The mammillary bodies: Two memory systems in one? Nat. Rev. Neurosci. 2004, 5, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Tsujino, N.; Sakurai, T. Role of orexin in modulating arousal, feeding, and motivation. Front. Behav. Neurosci. 2013, 7, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernardis, L.L.; Bellinger, L.L. The lateral hypothalamic area revisited: Ingestive behavior. Neurosci. Biobehav. Rev. 1996, 20, 189–287. [Google Scholar] [CrossRef]
- Stuber, G.D.; Wise, R.A. Lateral Hypothalamic Circuits for Feeding and Reward. Nat. Neurosci. 2016, 19, 198–205. [Google Scholar] [CrossRef] [Green Version]
- Schick, R.R.; Zimmermann, J.P.; vorm Walde, T.; Schusdziarra, V. Peptides that regulate food intake: Glucagon-like peptide 1-(7-36) amide acts at lateral and medial hypothalamic sites to suppress feeding in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, R1427–R1435. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Eghbali, M.; Ou, J.; Lu, R.; Toro, L.; Stefani, E. Quantitative determination of spatial protein-protein correlations in fluorescence confocal microscopy. Biophys. J. 2010, 98, 493–504. [Google Scholar] [CrossRef] [Green Version]
- Vargas-Soria, M.; Carranza-Naval, M.J.; Del Marco, A.; Garcia-Alloza, M. Role of liraglutide in Alzheimer’s disease pathology. Alzheimers Res. Ther. 2021, 13, 112. [Google Scholar] [CrossRef]
- Arendt, T.; Bigl, V.; Tennstedt, A.; Arendt, A. Neuronal loss in different parts of the nucleus basalis is related to neuritic plaque formation in cortical target areas in Alzheimer’s disease. Neuroscience 1985, 14, 1–14. [Google Scholar] [CrossRef]
- Salcedo, I.; Tweedie, D.; Li, Y.; Greig, N.H. Neuroprotective and neurotrophic actions of glucagon-like peptide-1: An emerging opportunity to treat neurodegenerative and cerebrovascular disorders. Br. J. Pharmacol. 2012, 166, 1586–1599. [Google Scholar] [CrossRef]
- Dong, D.; Xie, J.; Wang, J. Neuroprotective Effects of Brain-Gut Peptides: A Potential Therapy for Parkinson’s Disease. Neurosci. Bull. 2019, 35, 1085–1096. [Google Scholar] [CrossRef]
- Arabacı Tamer, S.; Koyuncuoğlu, T.; Karagöz Köroğlu, A.; Akakın, D.; Yüksel, M.; Yeğen, B.Ç. Nesfatin-1 ameliorates oxidative brain damage and memory impairment in rats induced with a single acute epileptic seizure. Life Sci. 2022, 294, 120376. [Google Scholar] [CrossRef]
- Kim, D.S.; Choi, H.I.; Wang, Y.; Luo, Y.; Hoffer, B.J.; Greig, N.H. A New Treatment Strategy for Parkinson’s Disease through the Gut-Brain Axis: The Glucagon-Like Peptide-1 Receptor Pathway. Cell Transplant. 2017, 26, 1560–1571. [Google Scholar] [CrossRef] [Green Version]
- Tschöp, M.; DiMarchi, R. Single-Molecule Combinatorial Therapeutics for Treating Obesity and Diabetes. Diabetes 2017, 66, 1766–1769. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.H.; Lee, H.W.; Choi, H.J. GLP-1 Based Combination Therapy for Obesity and Diabetes. J. Obes. Metab. Syndr. 2017, 26, 155–160. [Google Scholar] [CrossRef] [Green Version]
- Psilopanagioti, A.; Makrygianni, M.; Nikou, S.; Logotheti, S.; Papadaki, H. Nucleobindin 2/nesfatin-1 expression and colocalisation with neuropeptide Y and cocaine- and amphetamine-regulated transcript in the human brainstem. J. Neuroendocrinol. 2020, 32, e12899. [Google Scholar] [CrossRef]
- Zhou, D.; Chen, Y.-W.; Zhao, Z.-H.; Yang, R.-X.; Xin, F.-Z.; Liu, X.-L.; Pan, Q.; Zhou, H.; Fan, J.-G. Sodium butyrate reduces high-fat diet-induced non-alcoholic steatohepatitis through upregulation of hepatic GLP-1R expression. Exp. Mol. Med. 2018, 50, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Timper, K.; del Río-Martín, A.; Cremer, A.L.; Bremser, S.; Alber, J.; Giavalisco, P.; Varela, L.; Heilinger, C.; Nolte, H.; Trifunovic, A.; et al. GLP-1 Receptor Signaling in Astrocytes Regulates Fatty Acid Oxidation, Mitochondrial Integrity, and Function. Cell Metab. 2020, 31, 1189–1205.e13. [Google Scholar] [CrossRef]
- Rocha-Ferreira, E.; Poupon, L.; Zelco, A.; Leverin, A.L.; Nair, S.; Jonsdotter, A.; Carlsson, Y.; Thornton, C.; Hagberg, H.; Rahim, A.A. Neuroprotective exendin-4 enhances hypothermia therapy in a model of hypoxic-ischaemic encephalopathy. Brain 2018, 141, 2925–2942. [Google Scholar] [CrossRef] [Green Version]
- Mai, J.; Majtanik, M.; Paxinos, G. Part 2: Myelo- and cytoarchitectonic atlas of the human brain in stereotaxic (MNI) space. In Atlas of the Human Brain, 4th ed.; Mai, J., Majtanik, M., Paxinos, G., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2015; pp. 181–233, 427–431. [Google Scholar]
- Xing, L.; McDonald, H.; Da Fonte, D.F.; Gutierrez-Villagomez, J.M.; Trudeau, V.L. Dopamine D1 receptor activation regulates the expression of the estrogen synthesis gene aromatase B in radial glial cells. Front. Neurosci. 2015, 9, 310. [Google Scholar] [CrossRef] [PubMed]
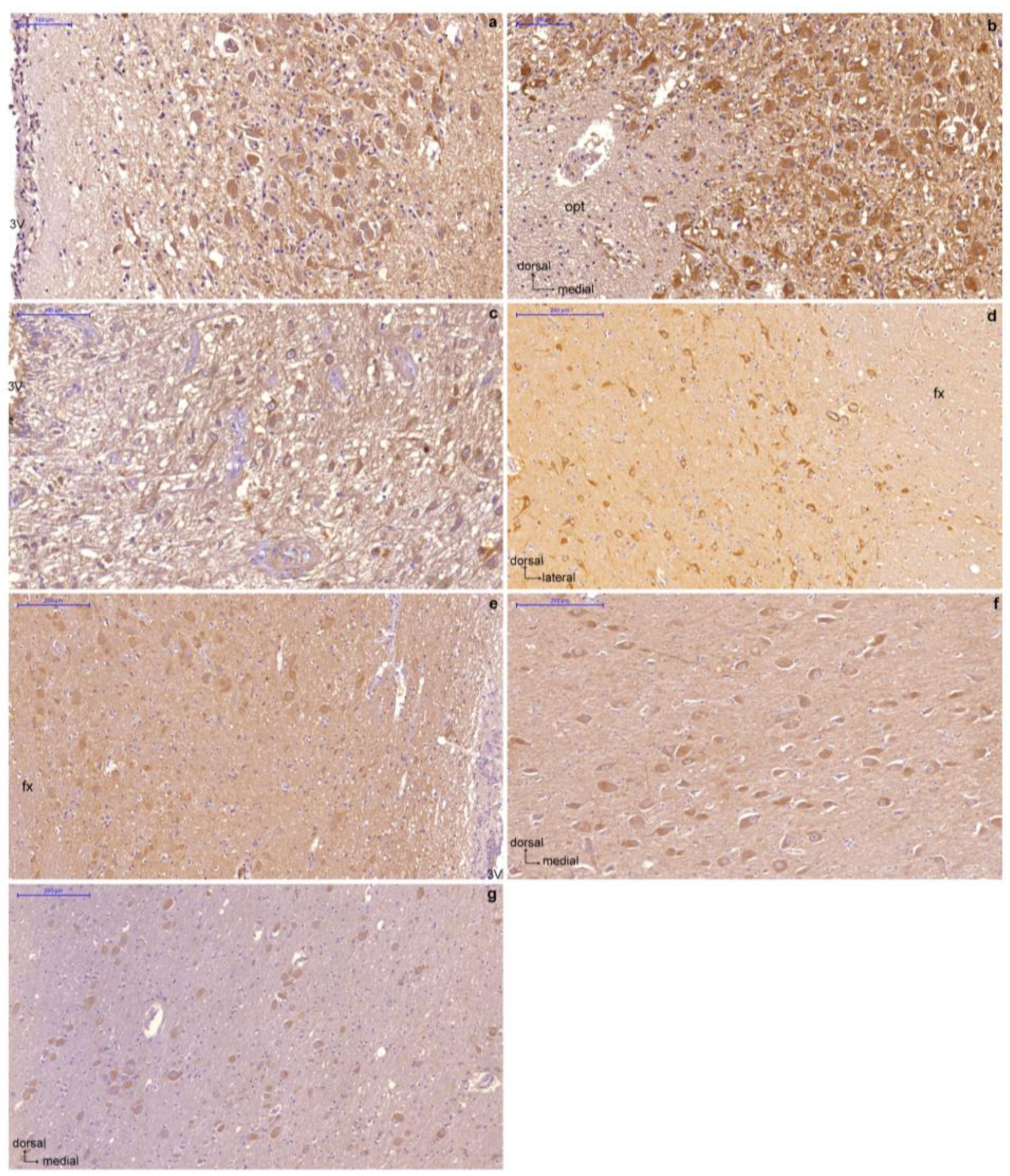
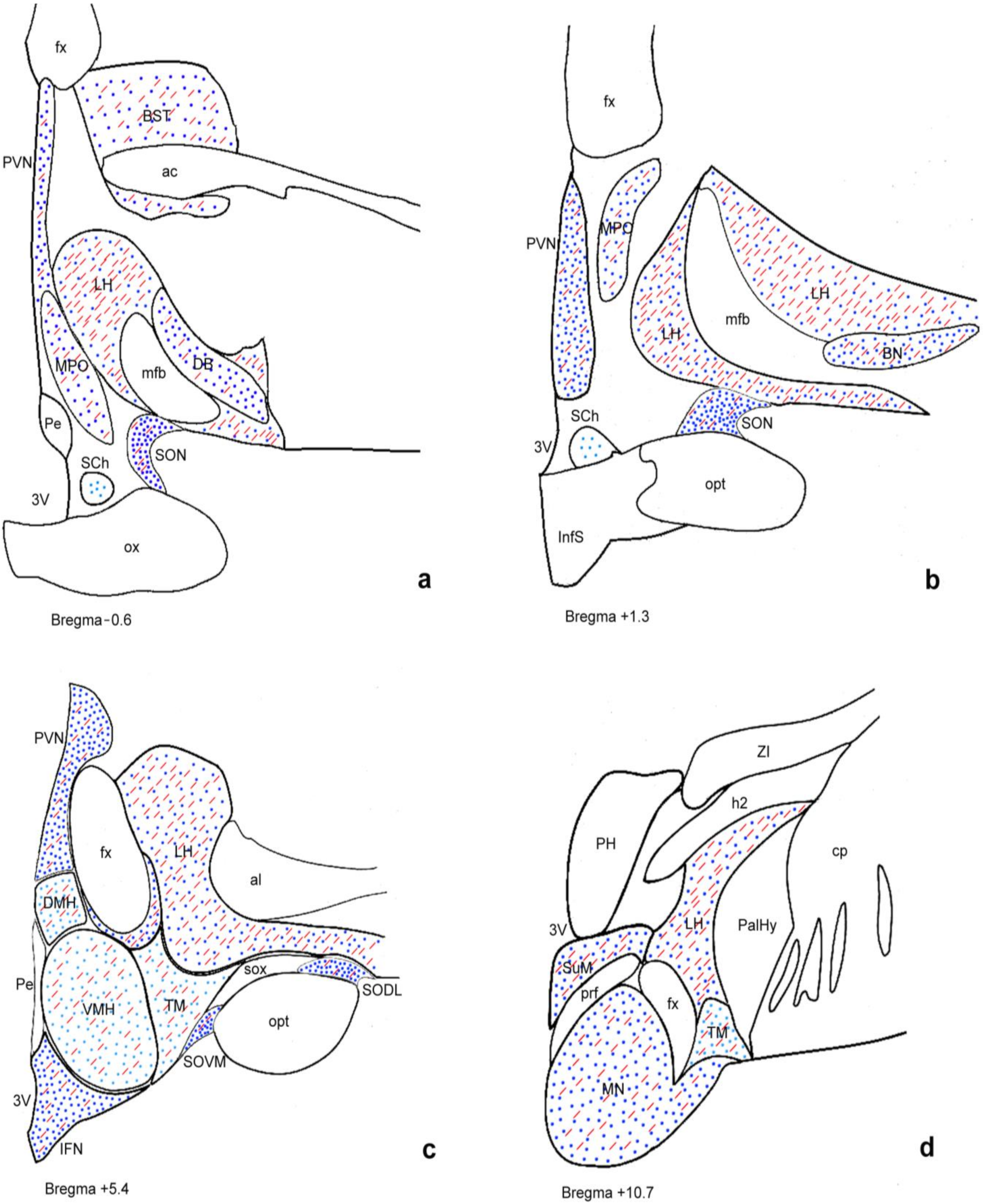
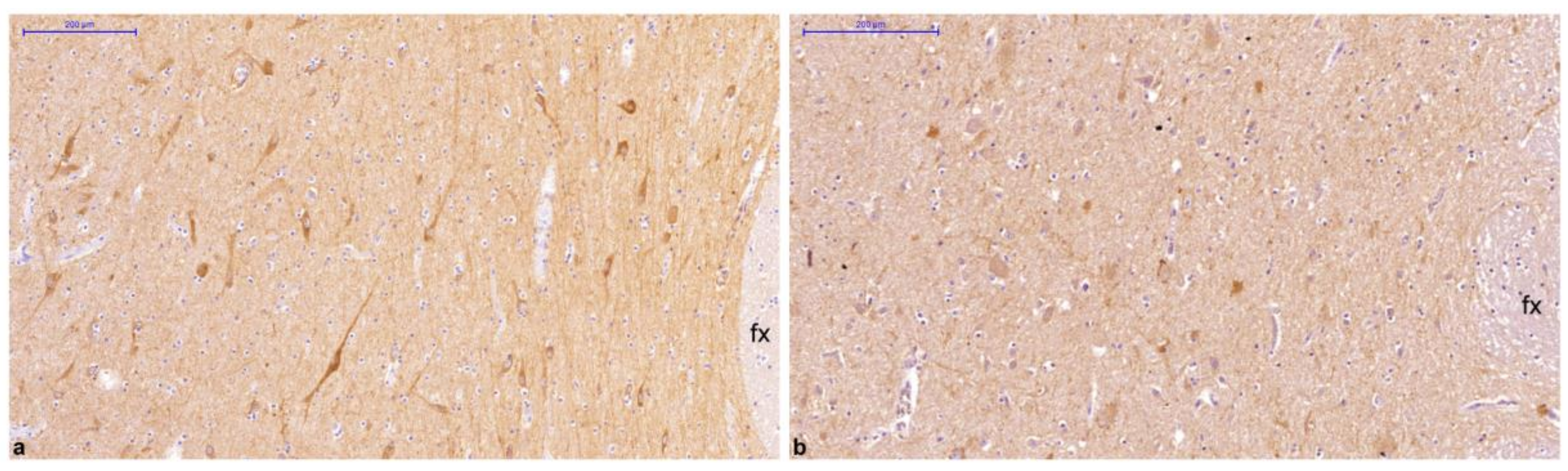
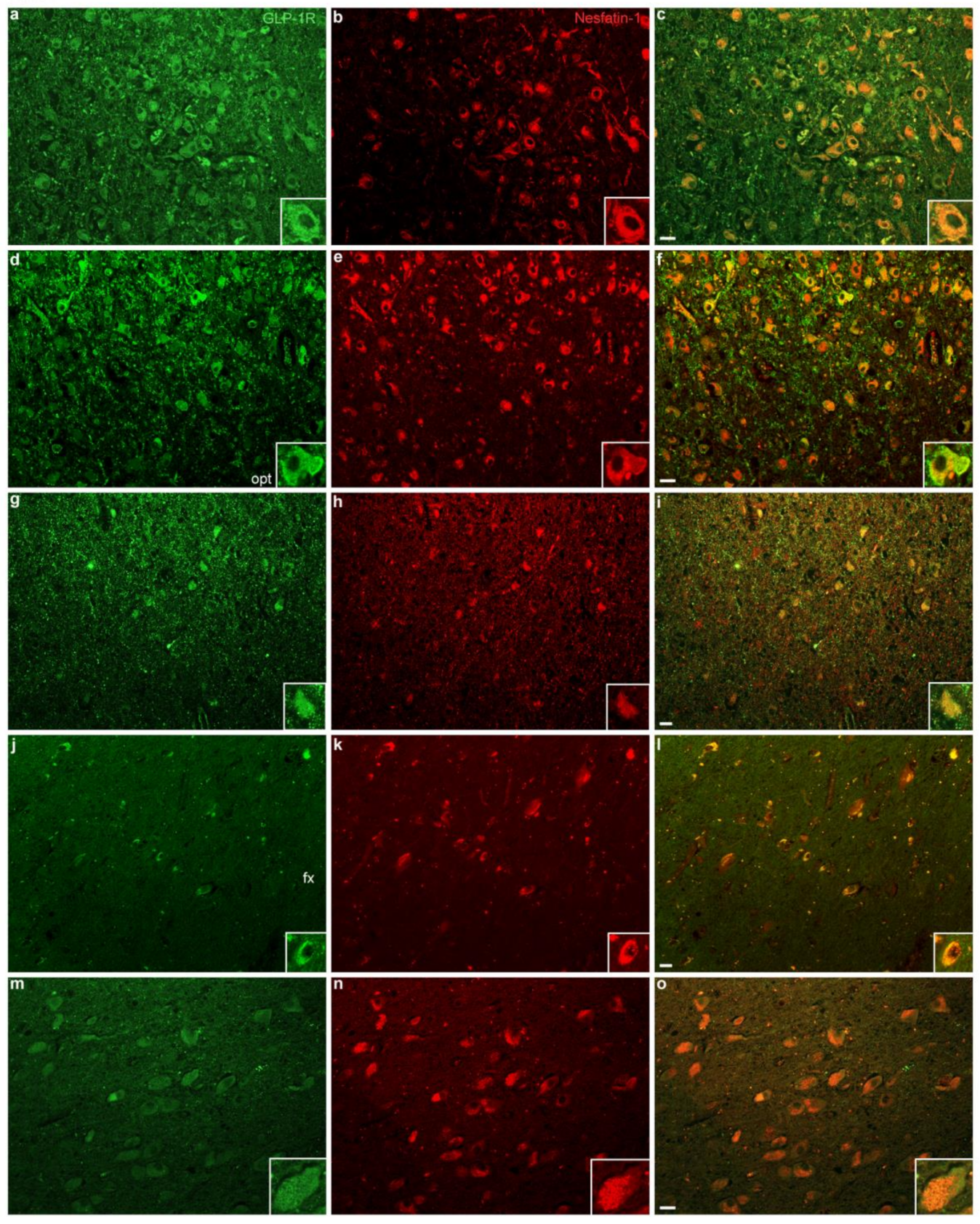
| Case | Sex | Age (Years) | BMI (kg/m2) | PMI (Hours) | Cause of Death, Medical History |
|---|---|---|---|---|---|
| 1 | M | 30 | 21.5 | 10 | Homicide |
| 2 | F | 65 | 21.6 | 24 | Automobile accident |
| 3 | M | 66 | 23.0 | 16 | Cardiac tamponade, myocardial infarction, hypertension |
| 4 | M | 53 | 23.0 | 10 | Myocardial infarction |
| 5 | M | 40 | 23.3 | 24 | Myocardial infarction |
| 6 | M | 57 | 23.9 | 12 | Myocardial infarction |
| 7 | F | 27 | 24.2 | 11 | Automobile accident |
| 8 | M | 31 | 24.8 | 24 | Dilated cardiomyopathy |
| 9 | F | 55 | 24.9 | 24 | Myocardial infarction |
| 10 | F | 56 | 24.9 | 11 | Myocardial infarction |
| 11 | M | 24 | 24.9 | 5 | Automobile accident |
| 12 | M | 64 | 27.7 | 17 | Myocardial infarction |
| 13 | M | 37 | 27.8 | 3 | Automobile accident, hypertension |
| 14 | F | 58 | 29.0 | 24 | Automobile accident |
| 15 | F | 77 | 29.1 | 12 | Automobile accident, hypertension |
| 16 | M | 86 | 29.1 | 15 | Gastric cancer/hemorrhage |
| 17 | F | 22 | 29.3 | 8 | Automobile accident |
| 18 | M | 60 | 29.4 | 24 | Automobile accident |
| 19 | F | 64 | 29.4 | 3 | Cor pulmonale |
| 20 | M | 58 | 30.5 | 24 | Myocardial infarction |
| 21 | M | 63 | 30.7 | 12 | Gastric hemorrhage, alcoholism |
| 22 | M | 56 | 31.6 | 12 | Aspiration pneumonia, hypertension |
| 23 | M | 39 | 31.7 | 2 | Myocardial infarction |
| 24 | M | 59 | 32.4 | 12 | Myocardial infarction |
| 25 | M | 27 | 32.4 | 24 | Myocardial infarction, familial hypercholesterolemia |
| 26 | M | 42 | 33.7 | 6 | Myocardial infarction |
| 27 | M | 58 | 35.5 | 10 | Electrocution |
| 28 | M | 54 | 46.3 | 12 | Myocardial infarction |
| Cases (n) | GLP-1R Immunoexpression Number of Cases (Percentage) | |||
|---|---|---|---|---|
| +++ | ++ | + | ||
| Normal BMI | 11 | 5 (46%) | 4 (36%) | 2 (18%) |
| Non-normal BMI * | 17 | 1 (6%) | 8 (47%) | 8 (47%) |
| Kendall’s Tau-b Correlation Coefficient | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BMI | PVN | SON | IFN | LH | PeF | VMH | DMH | TM | MPO | SCh | MN | BN | DB | BST | |
| BMI | |||||||||||||||
| τb | 1.00 | −0.134 | −0.178 | −0.034 | −0.347 * | −0.246 | 0.092 | −0.041 | 0.087 | −0.055 | −0.242 | 0.048 | −0.252 | −0.110 | −0.058 |
| p | 0.395 | 0.268 | 0.831 | 0.024 | 0.109 | 0.557 | 0.796 | 0.647 | 0.720 | 0.531 | 0.765 | 0.119 | 0.506 | 0.743 | |
| Case | BMI (kg/m2) | PVN | SON | IFN | LH | BN | |
|---|---|---|---|---|---|---|---|
| Normal weight group | 2 | 21.6 | 81.2 | 94.7 | 95.2 | 85.6 | 95.1 |
| 4 | 23.0 | 99.0 | 99.0 | n/a | 98.7 | 97.0 | |
| 6 | 23.9 | 94.7 | 95.0 | 99.1 | n/a | n/a | |
| 9 | 24.9 | 88.9 | 93.8 | 81.9 | 98.5 | 99.0 | |
| Mean value ± SEM | 23.4 ± 0.7 | 91.0 ± 3.9 | 95.6 ± 1.2 | 92.1 ± 5.2 | 94.3 ± 4.3 | 97.0 ± 1.1 | |
| Non-normal weight group | 14 | 29.0 | 94.5 | 93.8 | 85.5 | 99.0 | 90.4 |
| 18 | 29.4 | 99.9 | 99.4 | 99.0 | 99.5 | 99.0 | |
| 23 | 31.7 | 85.2 | 98.9 | 95.5 | 98.7 | 91.7 | |
| 25 | 32.4 | 86.0 | 99.0 | 71.4 | 99.0 | 99.0 | |
| Mean value ± SEM | 30.6 ± 0.8 | 91.4 ± 3.5 | 97.8 ± 1.3 | 87.9 ± 6.2 | 99.0 ± 0.2 | 95.0 ± 2.3 | |
| Total Mean value ± SEM | 27.0 ± 1.5 | 91.2 ± 2.4 | 96.7 ± 0.9 | 89.7 ± 3.9 | 97.0 ± 1.9 | 95.9 ± 1.4 |
| Case | BMI (kg/m2) | PVN | SON | IFN | LH | BN | |
|---|---|---|---|---|---|---|---|
| Normal weight group | 2 | 21.6 | 60.6 | 72.2 | 61.0 | 63.6 | 72.9 |
| 4 | 23.0 | 62.9 | 46.6 | n/a | 70.3 | 77.5 | |
| 6 | 23.9 | 55.2 | 68.4 | 82.0 | n/a | n/a | |
| 9 | 24.9 | 58.1 | 42.3 | 60.1 | 64.7 | 71.8 | |
| Mean value ± SEM | 23.4 ± 0.7 | 59.2 ± 1.7 | 57.4 ± 7.6 | 67.7 ± 7.2 | 66.2 ± 2.1 | 74.1 ± 1.7 | |
| Non-normal weight group | 14 | 29.0 | 62.2 | 75.0 | 69.4 | 29.5 | n/a |
| 18 | 29.4 | 39.2 | 53.5 | 52.5 | 62.1 | 89.1 | |
| 23 | 31.7 | 50.0 | 67.7 | 51.3 | 75.5 | 98.0 | |
| 25 | 32.4 | 52.2 | 56.5 | 58.8 | 38.3 | 42.7 | |
| Mean value ± SEM | 30.6 ± 0.8 | 50.9 ± 4.7 | 63.2 ± 5.0 | 58.0 ± 4.1 | 51.4 ± 10.6 | 76.6 ± 17.0 | |
| Total Mean value ± SEM | 27.0 ± 1.5 | 55.1 ± 2.8 | 60.3 ± 4.3 | 62.2 ± 4.0 | 57.7 ± 6.5 | 75.3 ± 7.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Psilopanagioti, A.; Nikou, S.; Logotheti, S.; Arbi, M.; Chartoumpekis, D.V.; Papadaki, H. Glucagon-like Peptide-1 Receptor in the Human Hypothalamus Is Associated with Body Mass Index and Colocalizes with the Anorexigenic Neuropeptide Nucleobindin-2/Nesfatin-1. Int. J. Mol. Sci. 2022, 23, 14899. https://doi.org/10.3390/ijms232314899
Psilopanagioti A, Nikou S, Logotheti S, Arbi M, Chartoumpekis DV, Papadaki H. Glucagon-like Peptide-1 Receptor in the Human Hypothalamus Is Associated with Body Mass Index and Colocalizes with the Anorexigenic Neuropeptide Nucleobindin-2/Nesfatin-1. International Journal of Molecular Sciences. 2022; 23(23):14899. https://doi.org/10.3390/ijms232314899
Chicago/Turabian StylePsilopanagioti, Aristea, Sofia Nikou, Souzana Logotheti, Marina Arbi, Dionysios V. Chartoumpekis, and Helen Papadaki. 2022. "Glucagon-like Peptide-1 Receptor in the Human Hypothalamus Is Associated with Body Mass Index and Colocalizes with the Anorexigenic Neuropeptide Nucleobindin-2/Nesfatin-1" International Journal of Molecular Sciences 23, no. 23: 14899. https://doi.org/10.3390/ijms232314899





