Five-Aminolevulinic Acid (5-ALA) Induces Heme Oxygenase-1 and Ameliorates Palmitic Acid-Induced Endoplasmic Reticulum Stress in Renal Tubules
Abstract
:1. Introduction
2. Results
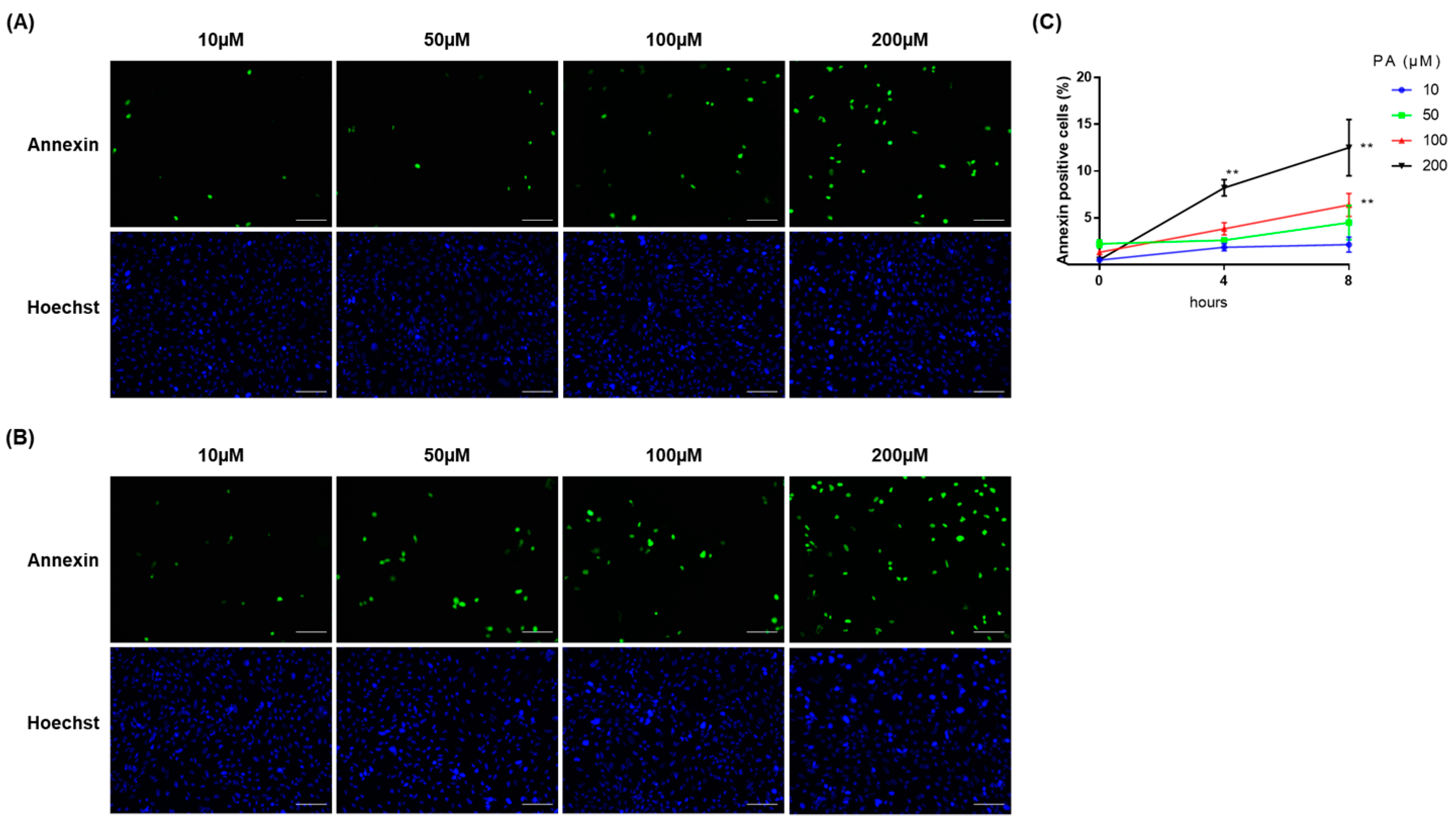
2.1. PA Induced Cellular Apoptosis in RPTECs
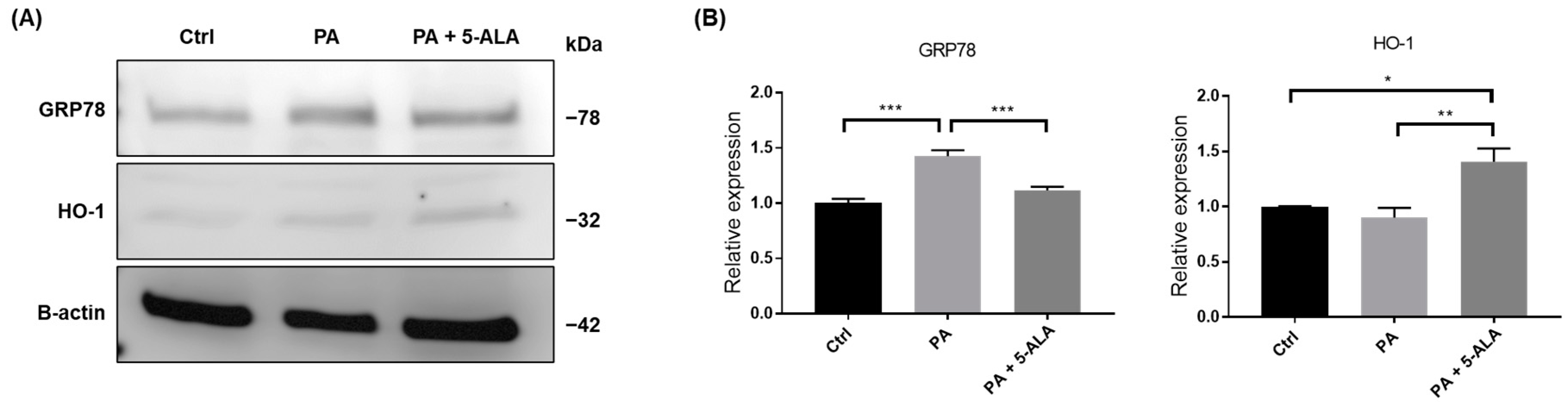
2.2. Treatment with 5-ALA Attenuated ER Stress and Induced HO-1 Expression
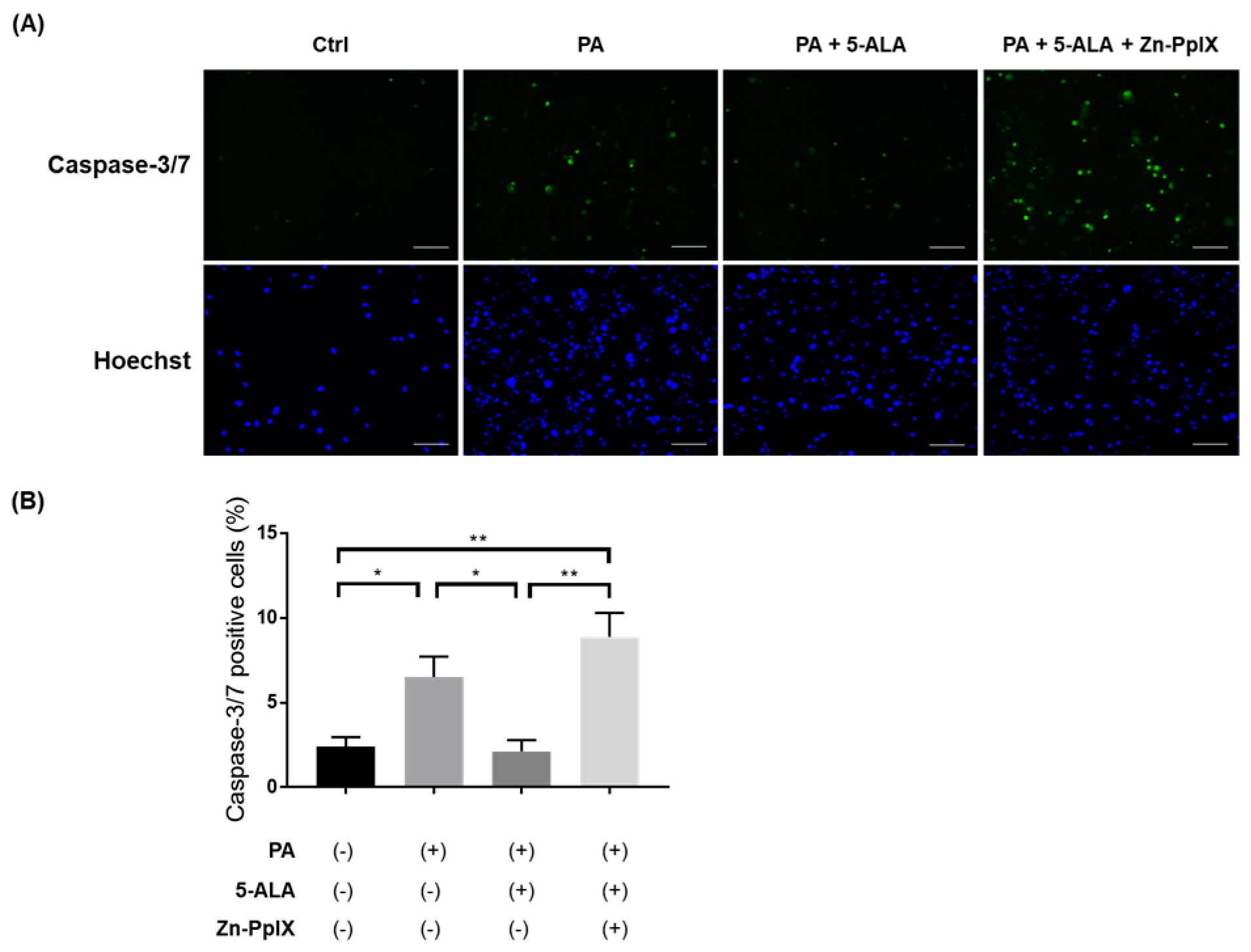
2.3. Treatment with 5-ALA Attenuated PA-Induced Caspase-3/7 Activity and Apoptosis
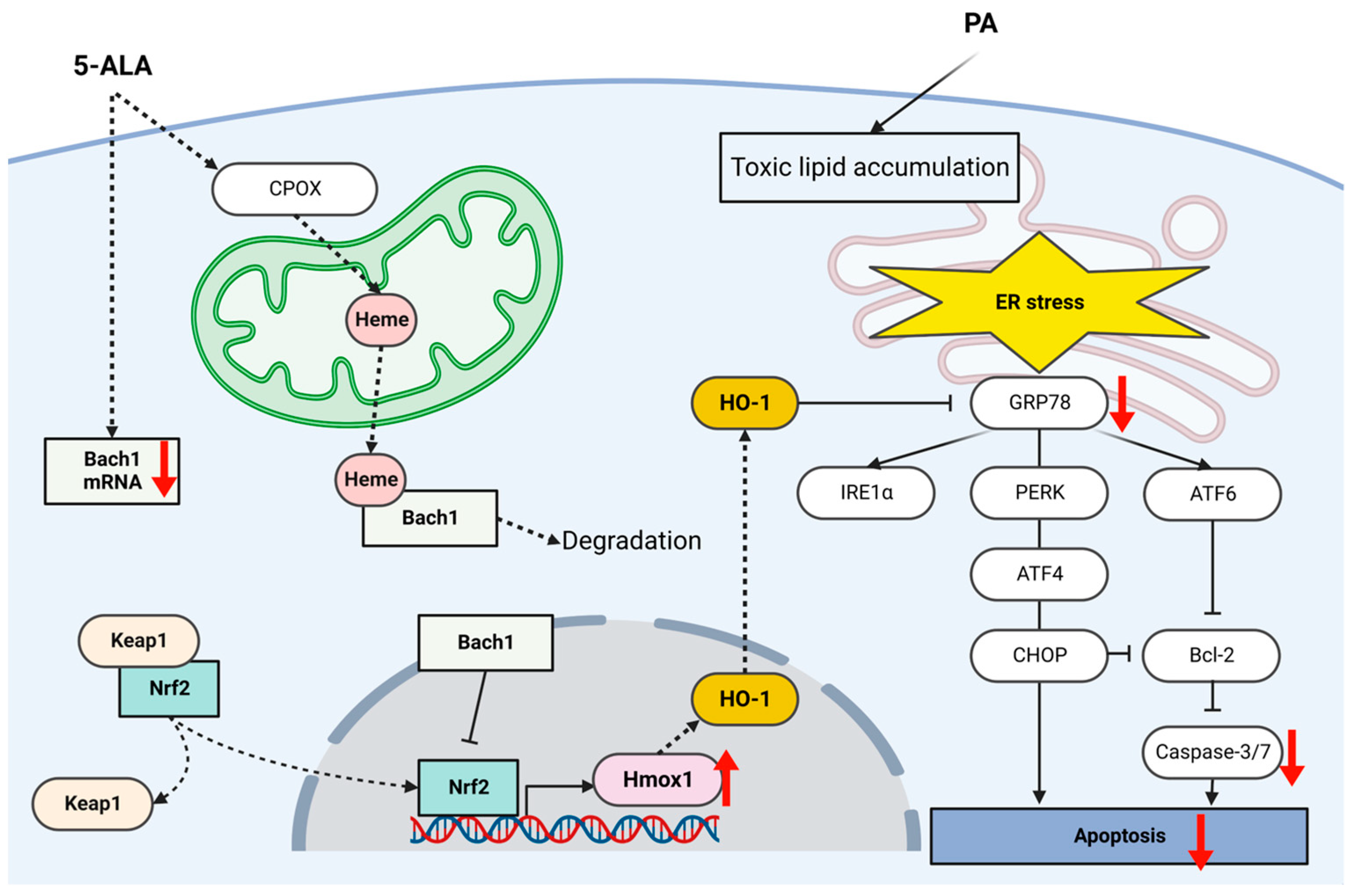
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Cell Culture
4.2. Gene Expression Analysis
4.3. Protein Expression Analysis
4.4. Quantification of Caspase Activity and Cellular Apoptosis
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iseki, K. Metabolic syndrome and chronic kidney disease: A Japanese perspective on a worldwide problem. J. Nephrol. 2008, 21, 305–312. [Google Scholar]
- Natali, A.; Pucci, G.; Boldrini, B.; Schillaci, G. Metabolic syndrome: At the crossroads of cardiorenal risk. J. Nephrol. 2009, 22, 29–38. [Google Scholar]
- Hoyas, I.; Leon-Sanz, M. Nutritional challenges in metabolic syndrome. J. Clin. Med. 2019, 8, 1301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shoji, T.; Nishizawa, Y. Chronic kidney disease as a metabolic syndrome with malnutrition—Need for strict control of risk factors. Intern. Med. 2005, 44, 179–187. [Google Scholar] [CrossRef] [Green Version]
- Nitta, K.; Goto, S.; Masakane, I.; Hanafusa, N.; Taniguchi, M.; Hasegawa, T.; Nakai, S.; Wada, A.; Hamano, T.; Hoshino, J.; et al. Annual dialysis data report for 2018, JSDT Renal Data Registry: Survey methods, facility data, incidence, prevalence, and mortality. Ren. Replace. Ther. 2020, 6, 41. [Google Scholar] [CrossRef]
- Min, S.H.; Yoon, J.-H.; Moon, S.J.; Hahn, S.; Cho, Y.M. Combination of sodium-glucose cotransporter 2 inhibitor and dipeptidyl peptidase-4 inhibitor in type 2 diabetes: A systematic review with meta-analysis. Sci. Rep. 2018, 8, 4466. [Google Scholar] [CrossRef] [PubMed]
- Hozumi, K.; Sugawara, K.; Ishihara, T.; Ishihara, N.; Ogawa, W. Effects of imeglimin on mitochondrial function, AMPK activity, and gene expression in hepatocytes. Sci. Rep. 2023, 13, 746. [Google Scholar] [CrossRef] [PubMed]
- Takata, T.; Isomoto, H. Pleiotropic effects of sodium-glucose Cotransporter-2 inhibitors: Renoprotective mechanisms beyond glycemic control. Int. J. Mol. Sci. 2021, 22, 4374. [Google Scholar] [CrossRef]
- Heo, C.U.; Choi, C.I. Current progress in pharmacogenetics of second-line antidiabetic medications: Towards precision medicine for Type 2 diabetes. J. Clin. Med. 2019, 8, 393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, H.R.; Kang, D.; Sinn, D.H.; Gu, S.; Cho, S.J.; Lee, J.E.; Huh, W.; Paik, S.W.; Ryu, S.; Chang, Y.; et al. Nonalcoholic fatty liver disease accelerates kidney function decline in patients with chronic kidney disease: A cohort study. Sci. Rep. 2018, 8, 4718. [Google Scholar] [CrossRef] [Green Version]
- Takaki, A.; Kawai, D.; Yamamoto, K. Multiple hits, including oxidative stress, as pathogenesis and treatment target in non-alcoholic steatohepatitis (NASH). Int. J. Mol. Sci. 2013, 14, 20704–20728. [Google Scholar] [CrossRef] [Green Version]
- Nagahara, R.; Matono, T.; Sugihara, T.; Matsuki, Y.; Yamane, M.; Okamoto, T.; Miyoshi, K.; Nagahara, T.; Okano, J.I.; Koda, M.; et al. Gene expression analysis of the activating factor 3/nuclear Protein 1 axis in a non-alcoholic steatohepatitis mouse model. Yonago Acta Med. 2019, 62, 36–46. [Google Scholar] [CrossRef] [Green Version]
- Hosokawa, K.; Takata, T.; Sugihara, T.; Matono, T.; Koda, M.; Kanda, T.; Taniguchi, S.; Ida, A.; Mae, Y.; Yamamoto, M.; et al. Ipragliflozin ameliorates endoplasmic reticulum stress and apoptosis through preventing ectopic lipid deposition in renal tubules. Int. J. Mol. Sci. 2019, 21, 190. [Google Scholar] [CrossRef] [Green Version]
- Hamada, S.; Takata, T.; Yamada, K.; Yamamoto, M.; Mae, Y.; Iyama, T.; Ikeda, S.; Kanda, T.; Sugihara, T.; Isomoto, H. Steatosis is involved in the progression of kidney disease in a high-fat-diet-induced non-alcoholic steatohepatitis mouse model. PLoS ONE 2022, 17, e0265461. [Google Scholar] [CrossRef]
- Kinoshita, H.; Kanda, T.; Takata, T.; Sugihara, T.; Mae, Y.; Yamashita, T.; Onoyama, T.; Takeda, Y.; Isomoto, H. Oligopeptide Transporter-1 is associated with fluorescence intensity of 5-aminolevulinic acid-based photodynamic diagnosis in pancreatic cancer cells. Yonago Acta Med. 2020, 63, 154–162. [Google Scholar] [CrossRef]
- Harada, Y.; Murayama, Y.; Takamatsu, T.; Otsuji, E.; Tanaka, H. 5-aminolevulinic acid-induced protoporphyrin IX fluorescence imaging for tumor detection: Recent advances and challenges. Int. J. Mol. Sci. 2022, 23, 6478. [Google Scholar] [CrossRef]
- Yang, X.; Palasuberniam, P.; Kraus, D.; Chen, B. Aminolevulinic acid-based tumor detection and therapy: Molecular mechanisms and strategies for enhancement. Int. J. Mol. Sci. 2015, 16, 25865–25880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeda, T.A.; Sasai, M.; Adachi, Y.; Ohnishi, K.; Fujisawa, J.I.; Izawa, S.; Taketani, S. Potential role of heme metabolism in the inducible expression of heme oxygenase-1. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1813–1824. [Google Scholar] [CrossRef] [PubMed]
- Takeda, T.A.; Mu, A.; Tai, T.T.; Kitajima, S.; Taketani, S. Continuous de novo biosynthesis of haem and its rapid turnover to bilirubin are necessary for cytoprotection against cell damage. Sci. Rep. 2015, 5, 10488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maamoun, H.; Zachariah, M.; McVey, J.H.; Green, F.R.; Agouni, A. Heme oxygenase (HO)-1 induction prevents endoplasmic reticulum stress-mediated endothelial cell death and impaired angiogenic capacity. Biochem. Pharmacol. 2017, 127, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Takaki, H.; Akazawa, Y.; Kido, Y.; Morishita, M.; Honda, T.; Shibata, H.; Miuma, S.; Miyaaki, H.; Taura, N.; Kondo, H.; et al. Hepatitis C virus infection increases c-Jun N-terminal kinase (JNK) phosphorylation and accentuates hepatocyte lipoapoptosis. Med. Sci. Monit. 2017, 23, 4526–4532. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, T.; Sugihara, T.; Kanda, T.; Takata, T.; Isomoto, H. 5-aminolevulinic acid attenuates glucose-regulated Protein 78 expression and hepatocyte lipoapoptosis via heme Oxygenase-1 induction. Int. J. Mol. Sci. 2021, 22, 11405. [Google Scholar] [CrossRef]
- Yamamoto, T.; Takabatake, Y.; Takahashi, A.; Kimura, T.; Namba, T.; Matsuda, J.; Minami, S.; Kamiori, J.; Matsui, I.; Matsusaka, T.; et al. High-fat diet-induced lysosomal dysfunction and impaired autophagic flux contribute to lipotoxicity in the kidney. J. Am. Soc. Nephrol. 2017, 28, 1534–1551. [Google Scholar] [CrossRef] [Green Version]
- Mendez-Sanchez, N.; Cruz-Ramon, V.C.; Ramirez-Perez, O.L.; Hwang, J.P.; Barranco-Fragoso, B.; Cordova-Gallardo, J. New aspects of lipotoxicity in nonalcoholic steatohepatitis. Int. J. Mol. Sci. 2018, 19, 2034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.Y.; Cho, Y.R.; Kim, H.J.; Higashimori, T.; Danton, C.; Lee, M.K.; Dey, A.; Rothermel, B.; Kim, Y.B.; Kalinowski, A.; et al. Unraveling the temporal pattern of diet-induced insulin resistance in individual organs and cardiac dysfunction in C57BL/6 mice. Diabetes 2005, 54, 3530–3540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rinaldi, A.; Lazareth, H.; Poindessous, V.; Nemazanyy, I.; Sampaio, J.L.; Malpetti, D.; Bignon, Y.; Naesens, M.; Rebant, M.; Anglicheau, D.; et al. Impaired fatty acid metabolism perpetuates lipotoxicity along the transition to chronic kidney injury. JCI Insight 2022, 7, e161783. [Google Scholar] [CrossRef]
- Drosatos, K.; Schulze, P.C. Cardiac lipotoxicity: Molecular pathways and therapeutic implications. Curr. Heart Fail. Rep. 2013, 10, 109–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Miao, L.; Zhang, H.; Wu, G.; Zhang, Z.; Lv, J. Chlorogenic acid against palmitic acid in endoplasmic reticulum stress-mediated apoptosis resulting in protective effect of primary rat hepatocytes. Lipids Health Dis. 2018, 17, 270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maekawa, H.; Inagi, R. Stress Signal Network between Hypoxia and ER Stress in Chronic Kidney Disease. Front. Physiol. 2017, 8, 74. [Google Scholar] [CrossRef]
- Takata, T.; Isomoto, H. The versatile role of uromodulin in renal homeostasis and its relevance in chronic kidney disease. Intern. Med. 2023. [Google Scholar] [CrossRef]
- Chitraju, C.; Mejhert, N.; Haas, J.T.; Diaz-Ramirez, L.G.; Grueter, C.A.; Imbriglio, J.E.; Pinto, S.; Koliwad, S.K.; Walther, T.C.; Farese, R.V. Triglyceride synthesis by DGAT1 protects adipocytes from lipid-induced ER stress during lipolysis. Cell. Metab. 2017, 26, 407–418.e3. [Google Scholar] [CrossRef]
- Gáll, T.; Balla, G.; Balla, J. Heme, heme oxygenase, and endoplasmic reticulum stress—A new insight into the pathophysiology of vascular diseases. Int. J. Mol. Sci. 2019, 20, 3675. [Google Scholar] [CrossRef] [Green Version]
- Luo, D.; Guo, Y.; Cheng, Y.; Zhao, J.; Wang, Y.; Rong, J. Natural product celastrol suppressed macrophage M1 polarization against inflammation in diet-induced obese mice via regulating Nrf2/HO-1, MAP kinase and NF-κB pathways. Aging 2017, 9, 2069–2082. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.; Han, Y.; Hu, Y.; Wu, X.; Wang, Y.; Zhang, X.; Fu, J.; Zou, X.; Zhang, J.; Chen, X.; et al. Targeting HO-1 by epigallocatechin-3-gallate reduces contrast-induced renal injury via anti-oxidative stress and anti-inflammation pathways. PLoS ONE 2016, 11, e0149032. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Zhu, P.; Fujino, M.; Isaka, Y.; Ito, H.; Takahashi, K.; Nakajima, M.; Tanaka, T.; Zhuang, J.; Li, X.K. 5-aminolaevulinic acid (ALA), enhances heme oxygenase (HO)-1 expression and attenuates tubulointerstitial fibrosis and renal apoptosis in chronic cyclosporine nephropathy. Biochem. Biophys. Res. Commun. 2019, 508, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lin, M.; Zhang, L.; Huang, S.; Hu, C.; Zheng, L.; Li, L.; Zhang, C.; Yang, C.; Long, Y.; et al. Cyclic helix B peptide protects HK-2 cells from oxidative stress by inhibiting ER stress and activating Nrf2 signalling and autophagy. Mol. Med. Rep. 2017, 16, 8055–8061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uchida, A.; Kidokoro, K.; Sogawa, Y.; Itano, S.; Nagasu, H.; Satoh, M.; Sasaki, T.; Kashihara, N. 5-aminolevulinic acid exerts renoprotective effect via Nrf2 activation in murine rhabdomyolysis-induced acute kidney injury. Nephrology 2019, 24, 28–38. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Sun, Y.; Sun, X.; Zhao, H.; Yao, M.; Hou, L.; Jiang, L. Up-regulation of HO-1 by Nrf2 activation protects against palmitic acid-induced ROS increase in human neuroblastoma BE(2)-M17 cells. Nutr. Res. 2018, 52, 80–86. [Google Scholar] [CrossRef]
- Alam, J.; Cook, J.L. How many transcription factors does it take to turn on the heme oxygenase-1 gene? Am. J. Respir. Cell Mol. Biol. 2007, 36, 166–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naito, Y.; Takagi, T.; Higashimura, Y. Heme oxygenase-1 and anti-inflammatory M2 macrophages. Arch. Biochem. Biophys. 2014, 564, 83–88. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, K.; Sun, J.; Taketani, S.; Nakajima, O.; Nishitani, C.; Sassa, S.; Hayashi, N.; Yamamoto, M.; Shibahara, S.; Fujita, H.; et al. Heme mediates derepression of Maf recognition element through direct binding to transcription repressor Bach1. EMBO J. 2001, 20, 2835–2843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zenke-Kawasaki, Y.; Dohi, Y.; Katoh, Y.; Ikura, T.; Ikura, M.; Asahara, T.; Tokunaga, F.; Iwai, K.; Igarashi, K. Heme induces ubiquitination and degradation of the transcription factor Bach1. Mol. Cell. Biol. 2007, 27, 6962–6971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gáll, T.; Pethő, D.; Nagy, A.; Hendrik, Z.; Méhes, G.; Potor, L.; Gram, M.; Åkerström, B.; Smith, A.; Nagy, P.; et al. Heme induces endoplasmic reticulum stress (HIER stress) in human aortic smooth muscle cells. Front. Physiol. 2018, 9, 1595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takata, T.; Hamada, S.; Mae, Y.; Iyama, T.; Ogihara, R.; Seno, M.; Nakamura, K.; Takata, M.; Sugihara, T.; Isomoto, H. Uromodulin regulates murine aquaporin-2 activity via thick ascending limb-collecting duct cross-talk during water deprivation. Int. J. Mol. Sci. 2022, 23, 9410. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar]
- Li, J.; Cui, J.; Li, Z.; Fu, X.; Li, J.; Li, H.; Wang, S.; Zhang, M. ORP8 induces apoptosis by releasing cytochrome c from mitochondria in non–small cell lung cancer. Oncol. Rep. 2020, 43, 1516–1524. [Google Scholar] [CrossRef]



| Gene Product | Accession ID | Forward Primer (5′ to 3′) | Reverse Primer (5′ to 3′) |
|---|---|---|---|
| GRP78 | NM_005347.5 | CGTGGAATGACCCGTCTGTG | CCAGCGTCTTTGGTTGC |
| HMOX1 | NM_002133.3 | GCCAGCAACAAAGTGCAAG | GAGTGTAAGGACCCATCGGA |
| NRF2 | NM_006164.5 | CAGCGACGGAAAGAGTATGA | TGGGCAACCTGGGAGTAG |
| BACH1 | NM_206866.3 | CTCAGCCTTAATGACCAGCGG | GCCTACGATTCTTGAGTGGAAG |
| NQO1 | NM_001025434.2 | GTGATATTCCAGAGTAAGAAGGCAG | ATTCTCCAGGCGTTTCTTCCAT |
| SLC7A11 | XM_054349496.1 | GGTGGTGTGTTTGCTGTC | GCTGGTAGAGGAGTGTGC |
| ACTB | NM_001101.5 | CATGTACGTTGCTATCCAGGC | CTCCTTAATGTCACGCACGAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamada, S.; Mae, Y.; Takata, T.; Hanada, H.; Kubo, M.; Taniguchi, S.; Iyama, T.; Sugihara, T.; Isomoto, H. Five-Aminolevulinic Acid (5-ALA) Induces Heme Oxygenase-1 and Ameliorates Palmitic Acid-Induced Endoplasmic Reticulum Stress in Renal Tubules. Int. J. Mol. Sci. 2023, 24, 10151. https://doi.org/10.3390/ijms241210151
Hamada S, Mae Y, Takata T, Hanada H, Kubo M, Taniguchi S, Iyama T, Sugihara T, Isomoto H. Five-Aminolevulinic Acid (5-ALA) Induces Heme Oxygenase-1 and Ameliorates Palmitic Acid-Induced Endoplasmic Reticulum Stress in Renal Tubules. International Journal of Molecular Sciences. 2023; 24(12):10151. https://doi.org/10.3390/ijms241210151
Chicago/Turabian StyleHamada, Shintaro, Yukari Mae, Tomoaki Takata, Hinako Hanada, Misaki Kubo, Sosuke Taniguchi, Takuji Iyama, Takaaki Sugihara, and Hajime Isomoto. 2023. "Five-Aminolevulinic Acid (5-ALA) Induces Heme Oxygenase-1 and Ameliorates Palmitic Acid-Induced Endoplasmic Reticulum Stress in Renal Tubules" International Journal of Molecular Sciences 24, no. 12: 10151. https://doi.org/10.3390/ijms241210151
APA StyleHamada, S., Mae, Y., Takata, T., Hanada, H., Kubo, M., Taniguchi, S., Iyama, T., Sugihara, T., & Isomoto, H. (2023). Five-Aminolevulinic Acid (5-ALA) Induces Heme Oxygenase-1 and Ameliorates Palmitic Acid-Induced Endoplasmic Reticulum Stress in Renal Tubules. International Journal of Molecular Sciences, 24(12), 10151. https://doi.org/10.3390/ijms241210151








