Transformation of Corn Stover into Furan Aldehydes by One-Pot Reaction with Acidic Lithium Bromide Solution
Abstract
1. Introduction
2. Results and Discussion
2.1. Production of Furfural and 5-HMF during Biochar Production from Corn Stover
2.2. Conversion of Corn Stover to Furfural by Inhibiting the Humins Formation
2.3. Changing the Reaction Conditions for High Yield of Furan Aldehydes
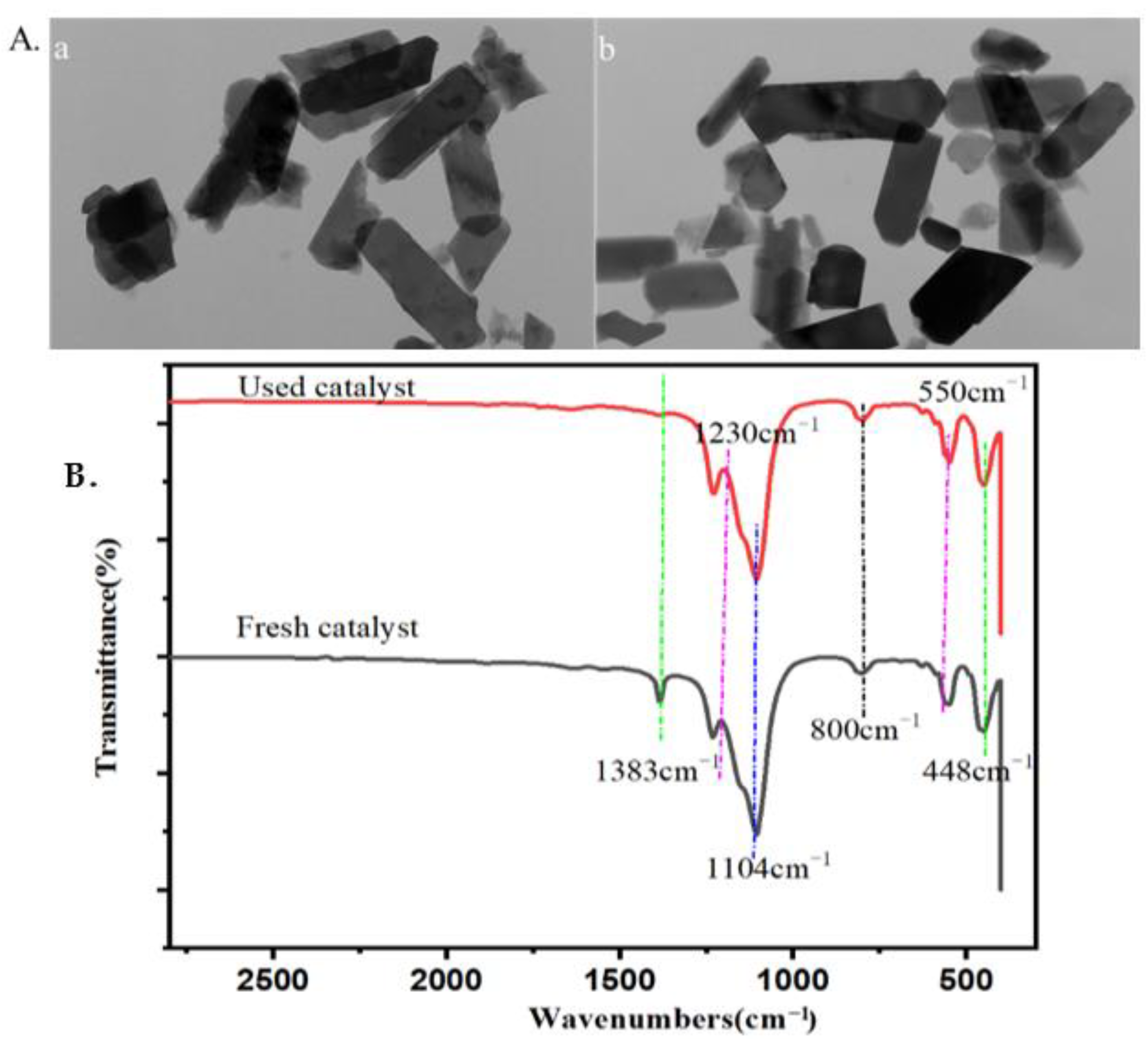
2.4. The Properties and Reusability of Catalyst and Lignin after Reaction
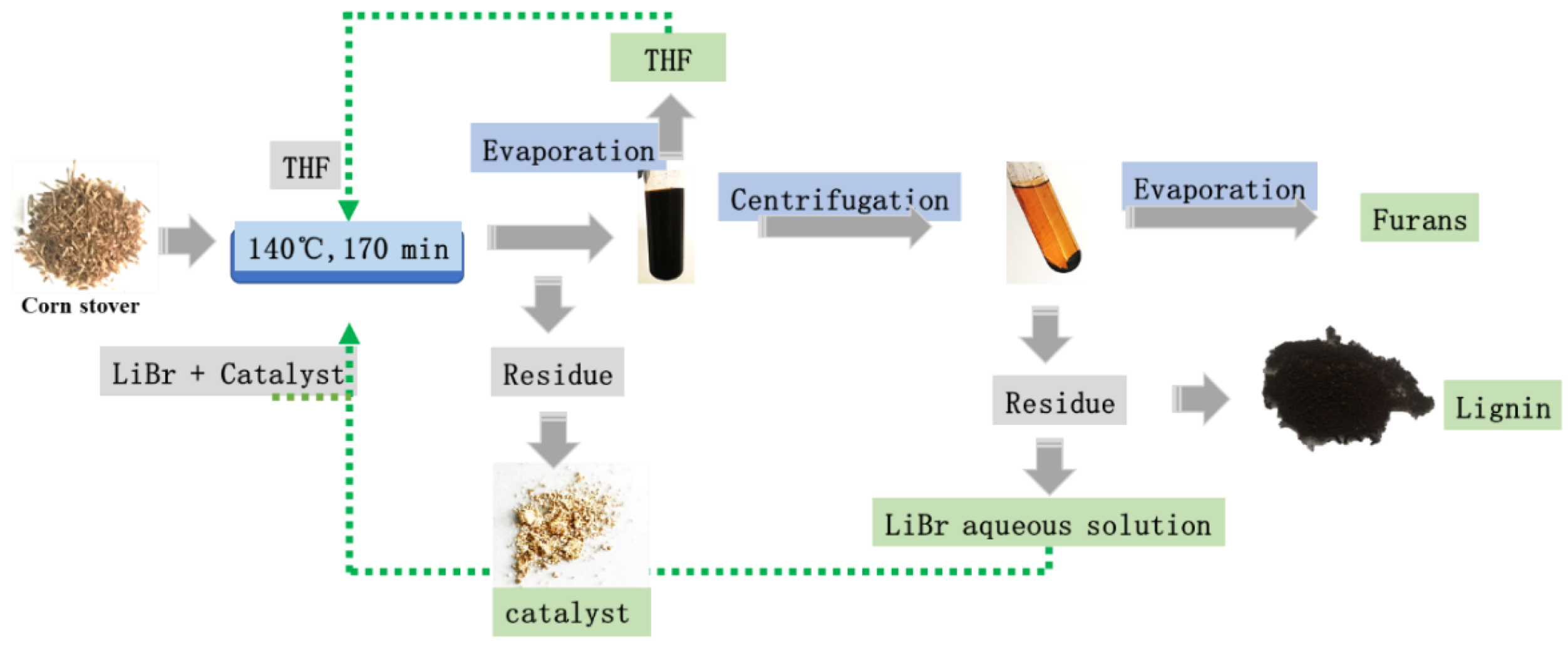
2.5. Integrated Protocol to Apply the Method in Biorefinery
3. Materials and Methods
3.1. Materials
3.2. The Conversion of Corn Stover to Furfural and 5-HMF by Acidic LiBr Treatment
3.3. Analyzation of the Products
3.4. Analysis Method
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kang, S.; Fu, J.; Zhang, G. From lignocellulosic biomass to levulinic acid: A review on acid-catalyzed hydrolysis. Renew. Sustain. Energy Rev. 2018, 94, 340–362. [Google Scholar] [CrossRef]
- Waal, J.; Jong, E.D. Chemocatalytic Processes for the Production of Bio-Based Chemicals from Carbohydrates. In Producing Fuels and Fine Chemicals from Biomass Using Nanomaterials; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Zunita, M.; Wahyuningrum, D.; Buchari; Bundjali, B.; Wenten, I.G.; Boopathy, R. The performance of 1,3-dipropyl-2-(2-propoxyphenyl)-4,5-diphenylimidazolium iodide based ionic liquid for biomass conversion into levulinic acid and formic acid. Bioresour. Technol. 2020, 315, 123864. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, R.; Liu, Q.; Liang, M.; Yang, J.; Lu, S.; Li, G.; Lu, L.; Si, C. Valorization of corn stover into furfural and levulinic acid over SAPO-18 zeolites: Effect of Brønsted to Lewis acid sites ratios. Ind. Crop. Prod. 2019, 141, 111759. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, H.; Wang, P.; Li, Y. Production of furfural from xylose, xylan and corncob in gamma-valerolactone using FeCl3·6H2O as catalyst. Bioresour. Technol. 2014, 151, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Singhvi, M.; Kim, B.S. Lignin valorization using biological approach. Biotechnol. Appl. Biochem. 2021, 68, 459–468. [Google Scholar] [CrossRef]
- Xing, R.; Qi, W.; Huber, G.W. Production of furfural and carboxylic acids from waste aqueous hemicellulose solutions from the pulp and paper and cellulosic ethanol industries. Energy Environ. Sci. 2011, 4, 2193–2205. [Google Scholar] [CrossRef]
- Mansilla, H.D.; Baeza, J.; Urzúa, S.; Maturana, G.; Villaseñor, J.; Durán, N. Acid-catalysed hydrolysis of rice hull: Evaluation of furfural production. Bioresour. Technol. 1998, 66, 189–193. [Google Scholar] [CrossRef]
- Cai, C.M.; Nagane, N.; Kumar, R.; Wyman, C.E. Coupling metal halides with a co-solvent to produce furfural and 5-HMF at high yields directly from lignocellulosic biomass as an integrated biofuels strategy. Green Chem. 2014, 16, 3819–3829. [Google Scholar] [CrossRef]
- Cai, C.M.; Zhang, T.; Kumar, R.; Wyman, C.E. THF co-solvent enhances hydrocarbon fuel precursor yields from lignocellulosic biomass. Green Chem. 2013, 15, 3140–3145. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, C.; Lin, Q.; Cheng, B.; Kong, F.; Li, H.; Ren, J. Solid acid-induced hydrothermal treatment of bagasse for production of furfural and levulinic acid by a two-step process. Ind. Crops Prod. 2018, 123, 118–127. [Google Scholar] [CrossRef]
- Gao, H.; Liu, H.; Pang, B.; Yu, G.; Du, J.; Zhang, Y.; Wang, H.; Mu, X. Production of furfural from waste aqueous hemicellulose solution of hardwood over ZSM-5 zeolite. Bioresour. Technol. 2014, 172, 453–456. [Google Scholar] [CrossRef]
- Bhat, N.S.; Vinod, N.; Onkarappa, S.B.; Dutta, S. Hydrochloric acid-catalyzed coproduction of furfural and 5-(chloromethyl)furfural assisted by a phase transfer catalyst. Carbohydr. Res. 2020, 496, 108105. [Google Scholar] [CrossRef]
- Singhvi, M.S.; Gokhale, D.V. Lignocellulosic biomass: Hurdles and challenges in its valorization. Appl. Microbiol. Biotechnol. 2019, 103, 9305–9320. [Google Scholar] [CrossRef]
- Fang, X.; Wang, Z.; Song, W.; Li, S.; Lin, W. Preparation of furans from catalytic conversion of corn stover in H2O–THF co-solvent system—The effects of acids combined with alkali metal cations. J. Taiwan Inst. Chem. Eng. 2019, 97, 105–111. [Google Scholar] [CrossRef]
- Teng, X.; Si, Z.; Li, S.; Yang, Y.; Wang, Z.; Li, G.; Zhao, J.; Cai, D.; Qin, P. Tin-loaded sulfonated rape pollen for efficient catalytic production of furfural from corn stover. Ind. Crop. Prod. 2020, 151, 112481. [Google Scholar] [CrossRef]
- Shen, T.; Hu, Y.; Hu, R.; Zhuang, W.; Li, M.; Niu, H.; Xu, H.; Zhu, C.; Ying, H. Continuous production of furfural from pulp prehydrolysate in a vaporization reactor. Ind. Crop. Prod. 2020, 153, 112565. [Google Scholar] [CrossRef]
- Yang, X.; Li, N.; Lin, X.; Pan, X.; Zhou, Y. Selective Cleavage of the Aryl Ether Bonds in Lignin for Depolymerization by Acidic Lithium Bromide Molten Salt Hydrate under Mild Conditions. J. Agric. Food Chem. 2016, 64, 8379–8387. [Google Scholar] [CrossRef]
- Lu, X.; Liu, X.; Zhang, W.; Wang, X.; Wang, S.; Xia, T. The residue from the acidic concentrated lithium bromide treated crop residue as biochar to remove Cr (VI). Bioresour. Technol. 2020, 296, 122348. [Google Scholar] [CrossRef]
- Li, H.; Dong, X.; da Silva, E.B.; de Oliveira, L.M.; Chen, Y.; Ma, L.Q. Mechanisms of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere 2017, 178, 466–478. [Google Scholar] [CrossRef]
- Lu, X.; Li, C.; Zhang, S.; Wang, X.; Zhang, W.; Wang, S.; Xia, T. Enzymatic sugar production from elephant grass and reed straw through pretreatments and hydrolysis with addition of thioredoxin-His-S. Biotechnol. Biofuels 2019, 12, 297. [Google Scholar] [CrossRef]
- Lu, X.; Chen, J.; Lu, J.; Wang, S.; Xia, T. Monosaccharides and carbon nanosphere obtained by acidic concentrated LiBr treatment of raw crop residues via optimizing the synthesis process. Bioresour. Technol. 2020, 310, 123522. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Xu, Z.-Y.; Dong, J.-J.; Li, H.; Han, R.-Z.; Xu, G.-C.; Ni, Y. Composite coal fly ash solid acid catalyst in synergy with chloride for biphasic preparation of furfural from corn stover hydrolysate. Bioresour. Technol. 2019, 293, 122065. [Google Scholar] [CrossRef] [PubMed]
- Gallo, J.M.R.; Alonso, D.M.; Mellmer, M.A.; Dumesic, J.A. Production and upgrading of 5-hydroxymethylfurfural using heterogeneous catalysts and biomass-derived solvents. Green Chem. 2013, 15, 85–90. [Google Scholar] [CrossRef]
- Dai, L.; Zhu, W.; Lu, J.; Kong, F.; Si, C.; Ni, Y. A lignin-containing cellulose hydrogel for lignin fractionation. Green Chem. 2019, 21, 5222–5230. [Google Scholar] [CrossRef]
- Deng, W.; Kennedy, J.R.; Tsilomelekis, G.; Zheng, W.; Nikolakis, V. Cellulose Hydrolysis in Acidified LiBr Molten Salt Hydrate Media. Ind. Eng. Chem. Res. 2015, 54, 5226–5236. [Google Scholar] [CrossRef]
- Azadi, P.; Carrasquillo-Flores, R.; Pagan-Torres, Y.J.; Guerbuez, E.I.; Farnood, R.; Dumesic, J.A. Catalytic conversion of biomass using solvents derived from lignin. Green Chem. 2012, 14, 1573–1576. [Google Scholar] [CrossRef]
- Fulmer, E.I.; Christensen, L.M.; Hixon, R.M.; Foster, R.L. The Production of Furfural from Xylose Solutions by Means of Hydrochloric Acid–Sodium Chloride Systems. J. Phys. Chem. 1936, 40, 133–141. [Google Scholar] [CrossRef]
- Dai, L.; Wang, Y.; Zou, X.; Chen, Z.; Liu, H.; Ni, Y. Ultrasensitive Physical, Bio, and Chemical Sensors Derived from 1-, 2-, and 3-D Nanocellulosic Materials. Small 2020, 16, 1906567. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, W.; Lu, Y.; Zhang, T.; Jameel, H.; Chang, H.M.; Ma, L. Production of furfural from xylose and corn stover catalyzed by a novel porous carbon solid acid in γ-valerolactone. RSC Adv. 2017, 7, 29916–29924. [Google Scholar] [CrossRef]
- Zhang, T.; Li, W.; Xu, Z.; Liu, Q.; Ma, Q.; Jameel, H.; Chang, H.-m.; Ma, L. Catalytic conversion of xylose and corn stalk into furfural over carbon solid acid catalyst in γ-valerolactone. Bioresour. Technol. 2016, 209, 108–114. [Google Scholar] [CrossRef]
- Li, X.; Lu, X.; Liang, M.; Xu, R.; Yu, Z.; Duan, B.; Lu, L.; Si, C. Conversion of waste lignocellulose to furfural using sulfonated carbon microspheres as catalyst. Waste Manag. 2020, 108, 119–126. [Google Scholar] [CrossRef]
- Zuo, M.; Jia, W.; Feng, Y.; Zeng, X.; Tang, X.; Sun, Y.; Lin, L. Effective selectivity conversion of glucose to furan chemicals in the aqueous deep eutectic solvent. Renew. Energy 2021, 164, 23–33. [Google Scholar] [CrossRef]
- Yang, T.; Li, W.; Ogunbiyi, A.T.; An, S. Efficient catalytic conversion of corn stover to furfural and 5-hydromethylfurfural using glucosamine hydrochloride derived carbon solid acid in Upsilon-valerolactone. Ind. Crop. Prod. 2021, 161, 113173. [Google Scholar] [CrossRef]
- Lai, F.; Yan, F.; Wang, P.; Li, C.; Shen, X.; Zhang, Z. Efficient conversion of carbohydrates and biomass into furan compounds by chitin/Ag co-modified H3PW12O40 catalysts. J. Clean. Prod. 2021, 316, 128243. [Google Scholar] [CrossRef]
- Lu, X.; Zheng, X.; Li, X.; Zhao, J. Adsorption and mechanism of cellulase enzymes onto lignin isolated from corn stover pretreated with liquid hot water. Biotechnol. Biofuels 2016, 9, 118. [Google Scholar] [CrossRef]
- Li, N.; Li, Y.; Geun, Y.C.; Yang, X.; Lin, X.; John, R.; Pan, X. An uncondensed lignin depolymerized in the solid state and isolated from lignocellulosic biomass: A mechanistic study. Green Chem. 2018, 20, 4224–4235. [Google Scholar] [CrossRef]
- Ma, H.; Yang, J.; Gao, X.; Liu, Z.; Liu, X.; Xu, Z. Removal of chromium (VI) from water by porous carbon derived from corn straw: Influencing factors, regeneration and mechanism. J. Hazard. Mater. 2019, 369, 550–560. [Google Scholar] [CrossRef]
- Pandey, K.K. A study of chemical structure of soft and hardwood and wood polymers by FTIR spectroscopy. J. Appl. Polym. Sci. 1999, 71, 1969–1975. [Google Scholar] [CrossRef]
- Savage, P.E.; Levine, R.B.; Huelsman, C.M. Chapter 8: Hydrothermal Processing of Biomass. In Thermochemical Conversion of Biomass to Liquid Fuels and Chemicals; Royal Society of Chemistry: London, UK, 2010. [Google Scholar]
- Liu, Q.; Ma, Q.; Sabnis, S.; Zheng, W.; Vlachos, D.G.; Fan, W.; Li, W.; Ma, L. Production of high-yield short-chain oligomers from cellulose via selective hydrolysis in molten salt hydrates and separation. Green Chem. 2019, 21, 5030–5038. [Google Scholar] [CrossRef]
- Yoo, C.G.; Zhang, S.; Pan, X. Effective conversion of biomass into bromomethylfurfural, furfural, and depolymerized lignin in lithium bromide molten salt hydrate of a biphasic system. RSC Adv. 2016, 7, 300–308. [Google Scholar] [CrossRef]
- Guo, Y.; Cheng, C.; Huo, T.; Ren, Y.; Liu, X. Highly effective flame retardant lignin/polyacrylonitrile composite prepared via solution blending and phosphorylation. Polym. Degrad. Stab. 2020, 181, 109362. [Google Scholar] [CrossRef]

| Substrate Loading (%) | LiBr (%)/Volume | HCl (M) | Catalyst (%) | Organic Phase | Temperature (°C) | Time (min) | Furfural (%) | 5-HMF (%) |
|---|---|---|---|---|---|---|---|---|
| 10 | 60/10 mL | 0.1 | - | - | 140 | 120 | 0.91 | 3.41 |
| 10 | 60/10 mL | 0.5 | - | - | 140 | 150 | 1.72 | 0.00 |
| Substrate Loading (wt%) | LiBr (g/mL)/Volume | HCl (M) | Catalyst (%) | Organic Phase/Volume | Temperature (℃) | Time (min) | Furfural (%) | 5-HMF (%) |
|---|---|---|---|---|---|---|---|---|
| 10 | 1.2/5 mL | 0.1 | DMF/5 mL | 140 | 120 | - | - | |
| 10 | 1.2/5 mL | 0.1 | ZSM-5 | DMF/5 mL | 140 | 120 | 4.36 | 3.05 |
| 10 | 1.2/5 mL | 0.5 | DMF/5 mL | 140 | 150 | 31.17 | 17.15 | |
| 10 | 1.2/5 mL | 0.5 | ZSM-5 | DMF/5 mL | 140 | 150 | 36.00 | 19.40 |
| Test | Substrate Loading (wt %) | LiBr (g/mL)/Volume | HCl (M) | Catalyst (%) | Organic Phase/Volume | Temp (℃) | Time (min) | Furfural (%) | 5-HMF (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1-1 | 10 | 1.2/4 mL | 0.5 | ZSM-5 | DMF/5 mL | 140 | 150 | 1.47 | 0.44 |
| 2-1 | 10 | 1.2/3 mL | 0.5 | ZSM-5 | DMF/5 mL | 140 | 150 | 1.04 | 0 |
| 3-1 | 10 | 1.2/2.5 mL | 0.5 | ZSM-5 | DMF/5 mL | 140 | 150 | 0.99 | 0 |
| 4-1 | 10 | 1.2/1.5 mL | 0.5 | ZSM-5 | DMF/5 mL | 140 | 150 | 0.98 | 0 |
| 1-2 | 5 | 1.2/5 mL | 0.5 | ZSM-5 | DMF/5 mL | 140 | 150 | 35.49 | 22 |
| 2-2 | 20 | 1.2/5 mL | 0.5 | ZSM-5 | DMF/5 mL | 140 | 150 | 19.65 | 9.01 |
| 1-3 | 10 | 1.2/5 mL | 0.5 | ZSM-5 | GVL/ 5 mL | 140 | 150 | 2.31 | 0 |
| 2-3 | 10 | 1.2/5 mL | 0.5 | ZSM-5 | THF/5 mL | 140 | 150 | 38.1 | 19.64 |
| 3-3 | 10 | 1.2/5 mL | 0.5 | ZSM-5 | DMSO/5 mL | 140 | 150 | 1.29 | 0.18 |
| 1-4 | 10 | 1.2/5 mL | 0.5 | ZSM-5 | THF/10 mL | 140 | 150 | 62.81 | 29.46 |
| 2-4 | 10 | 1.2/5 mL | 0.5 | ZSM-5 | THF/15 mL | 140 | 150 | 74.49 | 34.94 |
| 3-4 | 10 | 1.2/5 mL | 0.5 | ZSM-5 | THF/20 mL | 140 | 150 | 66.68 | 27.17 |
| 1-5 | 10 | 1.2/5 mL | 0.5 | ZSM-5 | DMF/5 mL | 140 | 170 | 39.5 | 17.35 |
| 2-5 | 10 | 1.2/5 mL | 0.5 | ZSM-5 | DMF/5 mL | 140 | 200 | 50.26 | 15.56 |
| 3-5 | 10 | 1.2/5 mL | 0.5 | ZSM-5 | DMF/5 mL | 140 | 220 | 33.6 | 9.86 |
| 6 | 10 | 1.2/5 mL | 0.5 | ZSM-5 | THF/15 mL | 140 | 200 | 100 | 40.71 |
| 7 | 10 | 1.2/5 mL | 0.5 | Reused ZSM-5 | THF/15 mL | 140 | 200 | 100 | 40.68 |
| System | Catalyst | Solid Loading (wt%) | Temp (°C) | Time (min) | 5-HMF (%) | Furfural (%) | Ref |
|---|---|---|---|---|---|---|---|
| LiBr-H2O-THF | ZSM-5 | 10 | 140 | 200 | 40.7 | 100.0 | This paper |
| GVL-H2O | SO3H-NG-C | 1.3 | 190 | 80 | 17.0 | 53.0 | [34] |
| H2O-MIBK | Ch15-AgPW | 3.3 | 170 | 180 | 25.6 | 72.2 | [34] |
| Toluene-NaCl-DMSO | SO42−/Sn-TRP | 2 | 190 | 180 | 33.2 | 77.8 | [34] |
| GVL-H2O | C-Co-S | 1 | 170 | 30 | - | ~70 | [34] |
| GVL-H2O | SAPO-18 | 2.5 | 205 | 40 | - | 95.1 | [34] |
| Catalyst | BET Surface Area (m2/g) | Pore Size (nm) | Total Acid Content (μmol/g) |
|---|---|---|---|
| Before | 310 | 4.11 | 378 |
| After | 308 | 4.05 | 370 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, M.; Xin, Q.; Sun, W.; Xiao, J.; Lu, X. Transformation of Corn Stover into Furan Aldehydes by One-Pot Reaction with Acidic Lithium Bromide Solution. Int. J. Mol. Sci. 2022, 23, 14901. https://doi.org/10.3390/ijms232314901
Gao M, Xin Q, Sun W, Xiao J, Lu X. Transformation of Corn Stover into Furan Aldehydes by One-Pot Reaction with Acidic Lithium Bromide Solution. International Journal of Molecular Sciences. 2022; 23(23):14901. https://doi.org/10.3390/ijms232314901
Chicago/Turabian StyleGao, Meixiang, Qi Xin, Wan Sun, Jiaqi Xiao, and Xianqin Lu. 2022. "Transformation of Corn Stover into Furan Aldehydes by One-Pot Reaction with Acidic Lithium Bromide Solution" International Journal of Molecular Sciences 23, no. 23: 14901. https://doi.org/10.3390/ijms232314901
APA StyleGao, M., Xin, Q., Sun, W., Xiao, J., & Lu, X. (2022). Transformation of Corn Stover into Furan Aldehydes by One-Pot Reaction with Acidic Lithium Bromide Solution. International Journal of Molecular Sciences, 23(23), 14901. https://doi.org/10.3390/ijms232314901








